Extremely Fast and Efficient Removal of Congo Red Using Cationic-Incorporated Hydroxyapatite Nanoparticles (HAp: X (X = Fe, Ni, Zn, Co, and Ag))
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of HAp and Ion-Doped HAp Nanoparticles
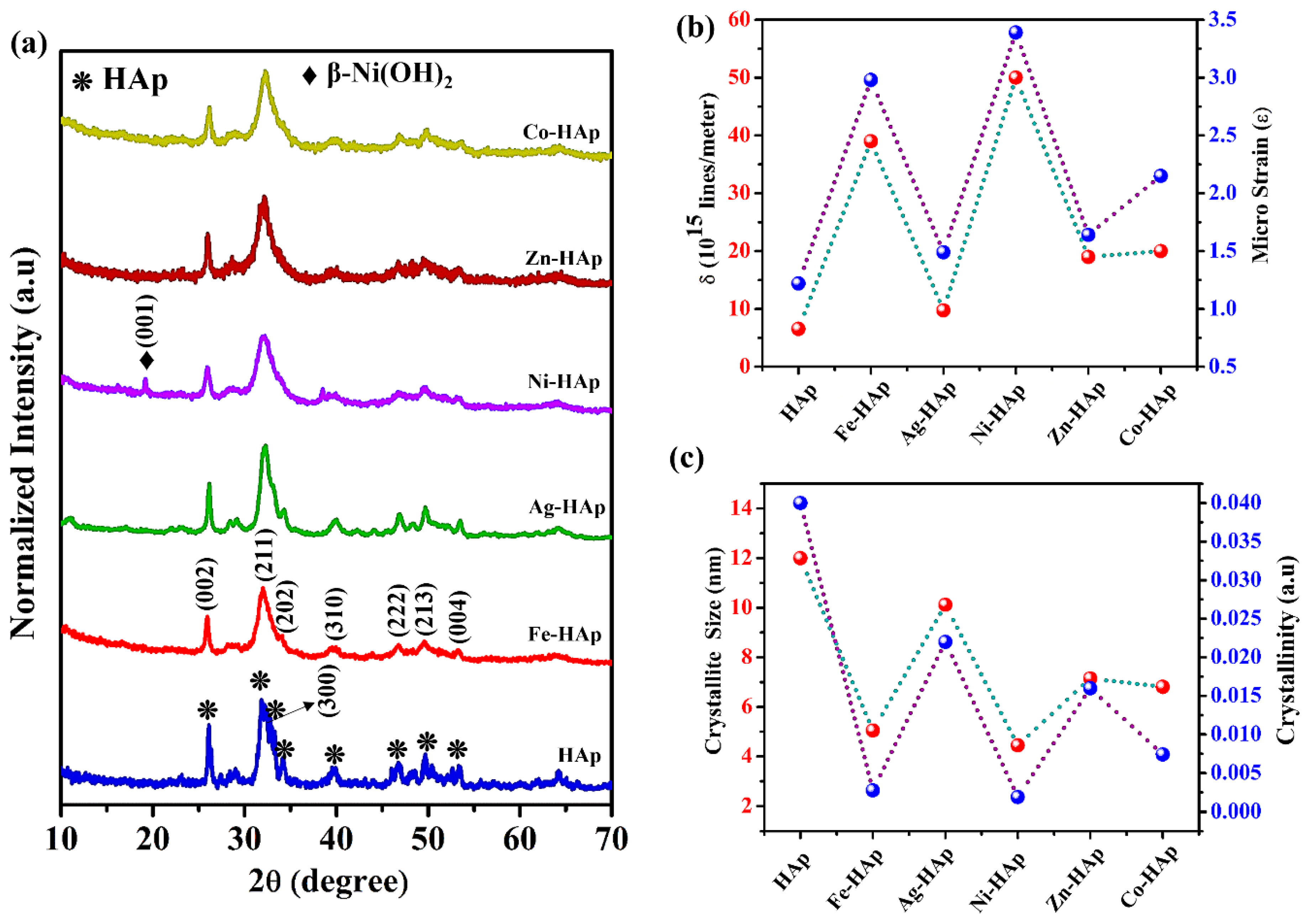
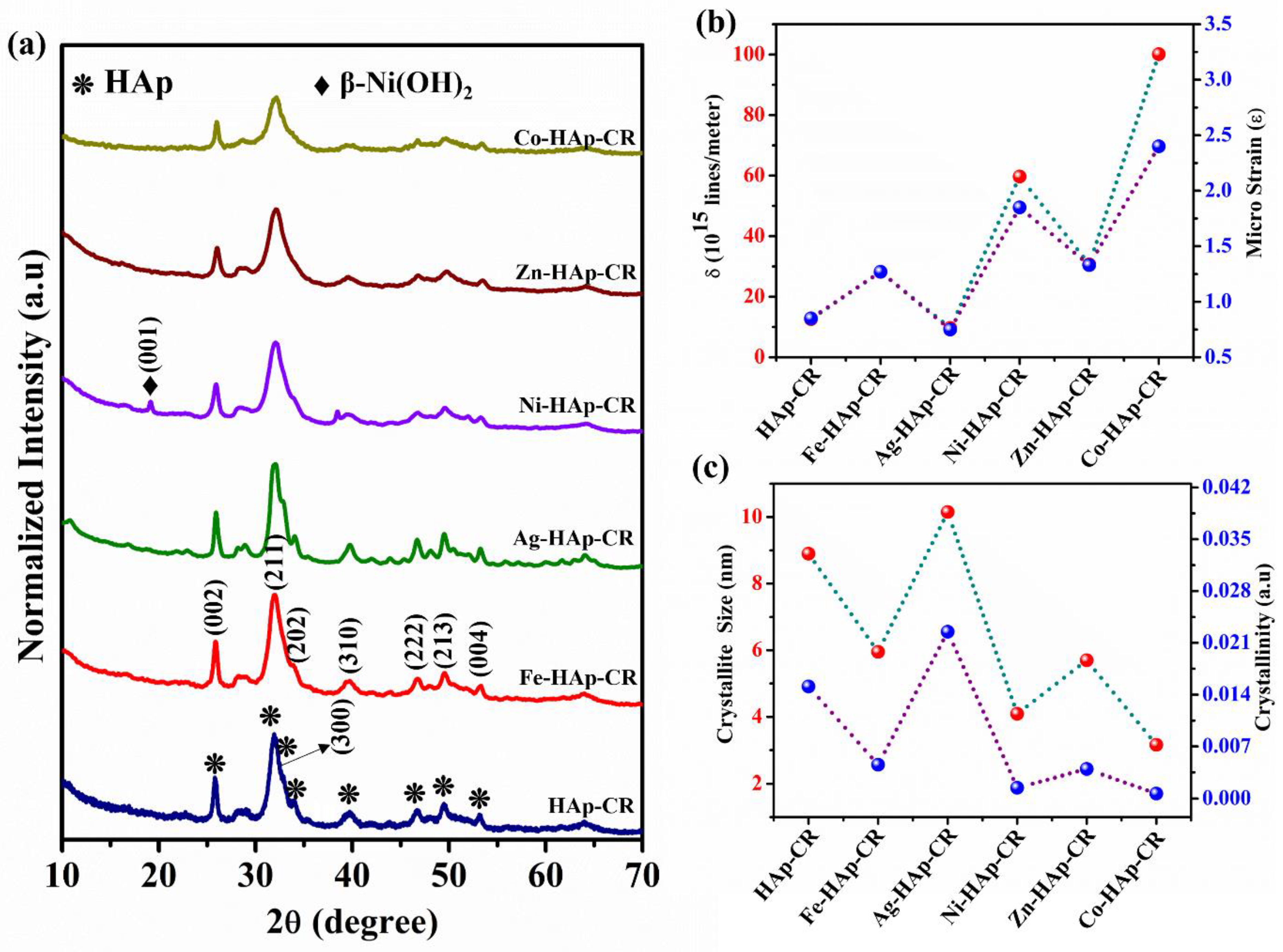
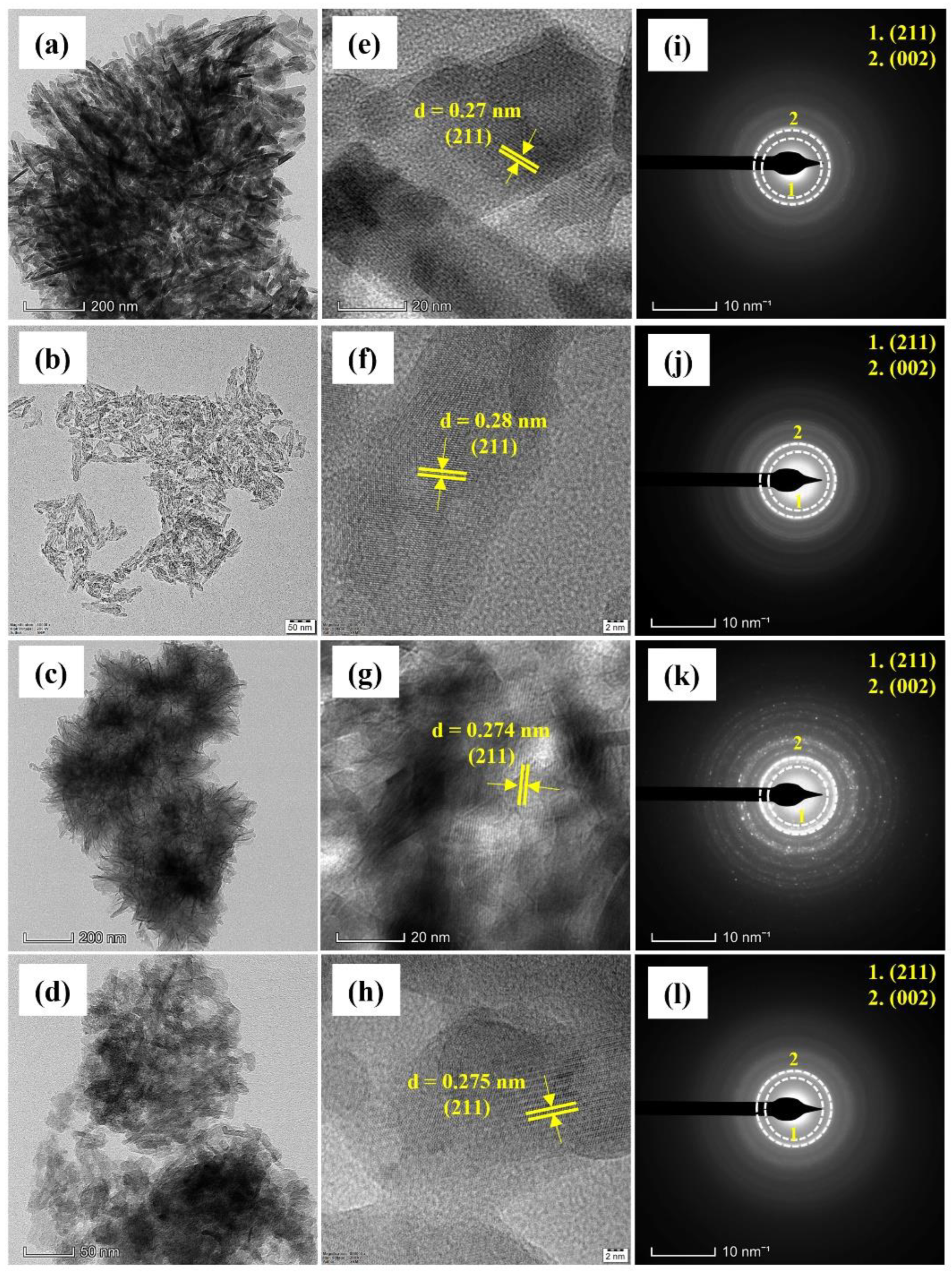
2.2. Characterization
2.3. Batch Adsorption Study
3. Results and Discussion
3.1. Adsorption of CR
3.1.1. Effect of Contact Time and Kinetic Isotherm
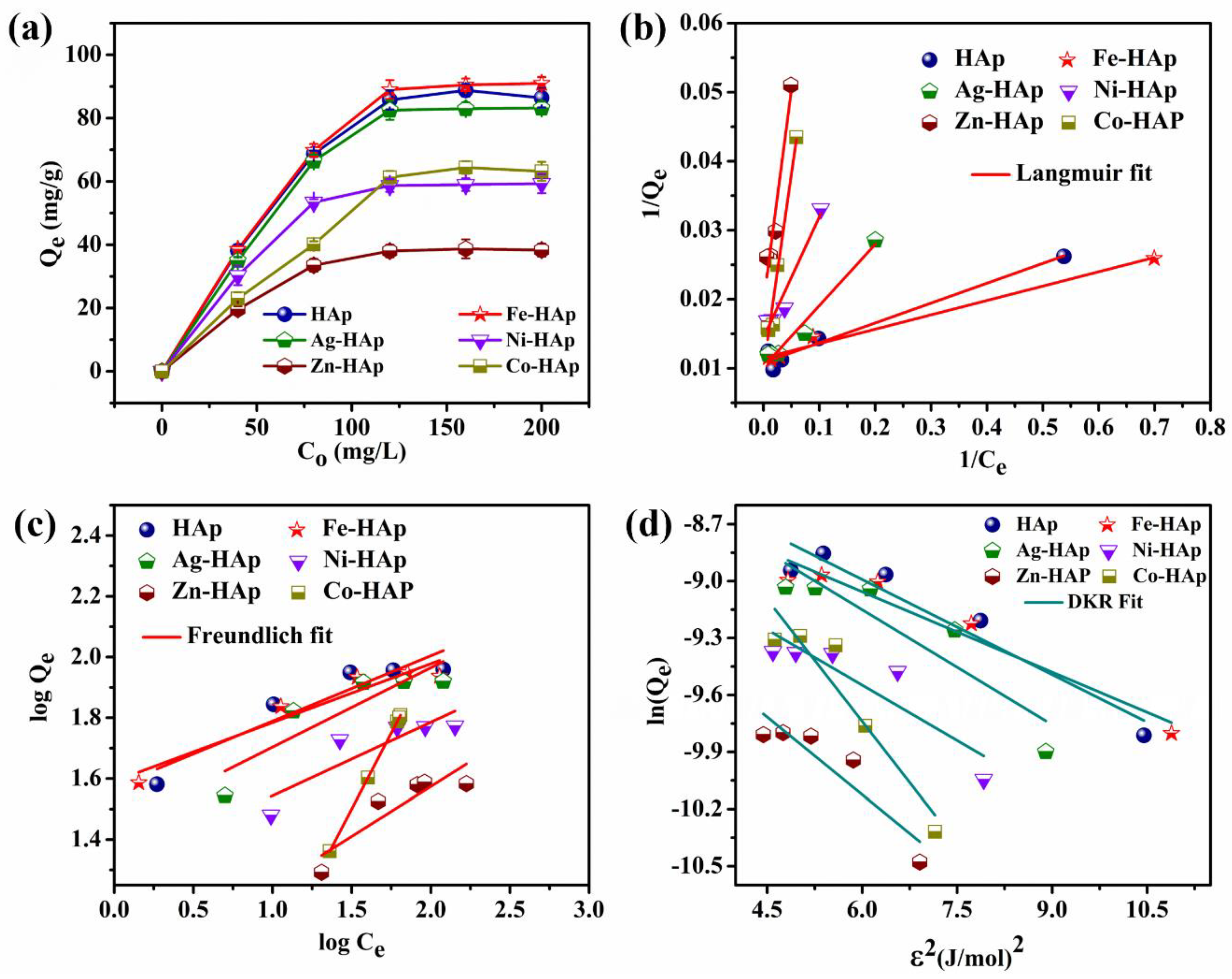
3.1.2. Effect of Initial Concentration and Adsorption Isotherm
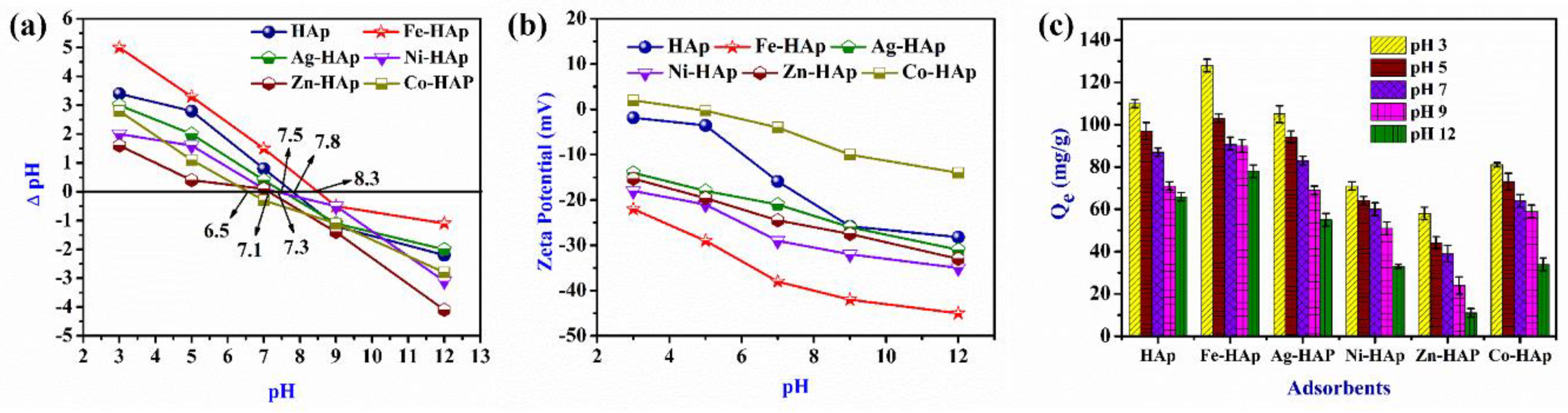
3.1.3. Effect of pH
3.1.4. Effect of Temperature
3.2. Regeneration
3.3. Structural Analysis after CR Adsorption
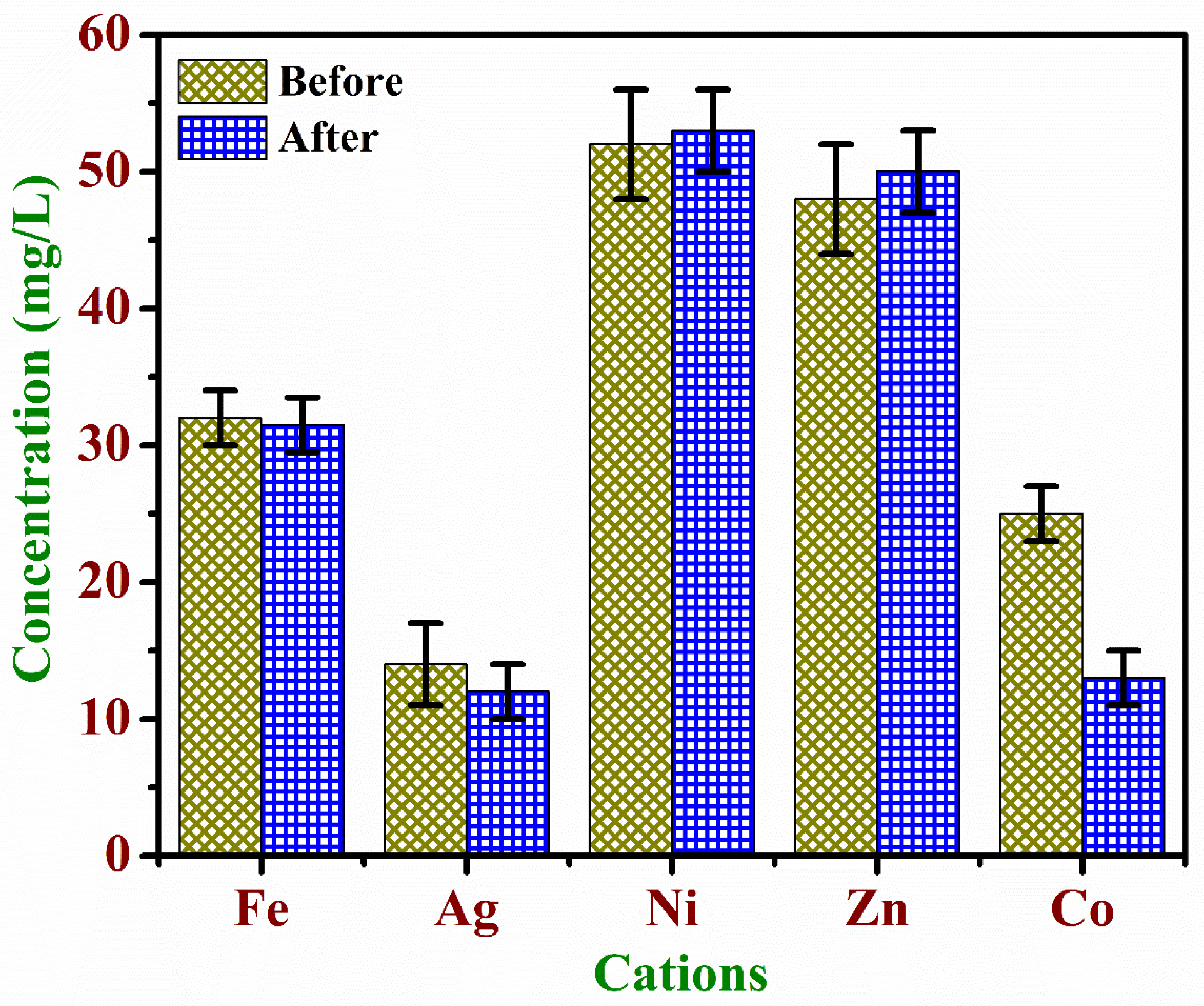
3.4. Quantification of Dopant Cations for before and after CR Adsorption
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Wang, N.; Zhang, H.; Baeyens, J. Adsorption of Congo Red Dye on FexCo3−xO4 Nanoparticles. J. Environ. Manag. 2019, 238, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sathiyavimal, S.; Vasantharaj, S.; Shanmugavel, M.; Manikandan, E.; Nguyen-Tri, P.; Brindhadevi, K.; Pugazhendhi, A. Facile Synthesis and Characterization of Hydroxyapatite from Fish Bones: Photocatalytic Degradation of Industrial Dyes (Crystal Violet and Congo Red). Prog. Org. Coatings 2020, 148, 105890. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, R.; Wu, P.; Wu, L. Removal of Congo Red Dye from Aqueous Solution with Hydroxyapatite/Chitosan Composite. Chem. Eng. J. 2012, 211–212, 336–342. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive Amputation of Hazardous Azo Dye Congo Red from Wastewater: A Critical Review. Environ. Sci. Pollut. Res. 2016, 23, 14810–14853. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an Anionic Dye (Congo Red) from Aqueous Solutions by Pine Bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Khan, A.; Bhoi, R.; Saharan, V.K.; George, S. Synthesis of Titanium Doped Hydroxyapatite Using Waste Marble Powder for the Degradation of Congo Red Dye in Wastewater. Mater. Today Proc. 2022, 57, 1645–1653. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Kampoosaen, C.; Kengnok, O. Adsorption Characteristics of Congo Red on Carbonized Leonardite. J. Clean. Prod. 2016, 134, 506–514. [Google Scholar] [CrossRef]
- Azeez, L.; Adebisi, S.A.; Adejumo, A.L.; Busari, H.K.; Aremu, H.K.; Olabode, O.A.; Awolola, O. Adsorptive Properties of Rod-Shaped Silver Nanoparticles-Functionalized Biogenic Hydroxyapatite for Remediating Methylene Blue and Congo Red. Inorg. Chem. Commun. 2022, 142, 109655. [Google Scholar] [CrossRef]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of Rice Husk for the Adsorption of Congo Red from Aqueous Solution in Column Mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef]
- Foroughi-Dahr, M.; Abolghasemi, H.; Esmaili, M.; Shojamoradi, A.; Fatoorehchi, H. Adsorption Characteristics of Congo Red from Aqueous Solution onto Tea Waste. Chem. Eng. Commun. 2015, 202, 181–193. [Google Scholar] [CrossRef]
- Ghanavati Nasab, S.; Semnani, A.; Teimouri, A.; Kahkesh, H.; Momeni Isfahani, T.; Habibollahi, S. Removal of Congo Red from Aqueous Solution by Hydroxyapatite Nanoparticles Loaded on Zein as an Efficient and Green Adsorbent: Response Surface Methodology and Artificial Neural Network-Genetic Algorithm. J. Polym. Environ. 2018, 26, 3677–3697. [Google Scholar] [CrossRef]
- Lian, L.; Guo, L.; Guo, C. Adsorption of Congo Red from Aqueous Solutions onto Ca-Bentonite. J. Hazard. Mater. 2009, 161, 126–131. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo Dye Adsorption on an Industrial Waste-Transformed Hydroxyapatite Adsorbent: Kinetics, Isotherms, Mechanism and Regeneration Studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- Zhang, Z.; Moghaddam, L.; O’Hara, I.M.; Doherty, W.O.S. Congo Red Adsorption by Ball-Milled Sugarcane Bagasse. Chem. Eng. J. 2011, 178, 122–128. [Google Scholar] [CrossRef]
- Rajendran, S.; Yadav, V.K.; Gacem, A.; Algethami, J.S.; Alqahtani, M.S.; Aldakheel, F.M.; Binshaya, A.S.; Alharthi, N.S.; Khan, I.A.; Islam, S. Functionalized Microbial Consortia with Silver-Doped Hydroxyapatite (Ag@HAp) Nanostructures for Removal of RO84 from Industrial Effluent. Crystals. 2022, 12, 970. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, I.V.; Rosseeva, E.V.; Zhuravlev, A.V. Refinement of apatite atomic structure of albid tissue of Late Devon conodont. Crystallogr. Rep. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Karalkeviciene, R.; Raudonyte-Svirbutaviciene, E.; Gaidukevic, J.; Zarkov, A.; Kareiva, A. Solvothermal Synthesis of Calcium-Deficient Hydroxyapatite via Hydrolysis of α -Tricalcium Phosphate in Different Aqueous-Organic Media. Crystals 2022, 12, 253. [Google Scholar] [CrossRef]
- Vidotto, M.; Grego, T.; Petrović, B.; Somers, N.; Antonić Jelić, T.; Kralj, D.; Matijaković Mlinarić, N.; Leriche, A.; Dutour Sikirić, M.; Erceg, I.; et al. A Comparative EPR Study of Non-Substituted and Mg-Substituted Hydroxyapatite Behaviour in Model Media and during Accelerated Ageing. Crystals 2022, 12, 297. [Google Scholar] [CrossRef]
- Marincaș, L.; Turdean, G.L.; Toșa, M.; Kovács, Z.; Kovács, B.; Barabás, R.; Farkas, N.I.; Bizo, L. Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine. Crystals 2021, 11, 1124. [Google Scholar] [CrossRef]
- Thakur, D.; Yeh, S.C.; Cheng, R.H.; Loke, S.S.; Wei, H.H.; Cheng, P.Y.; Lai, Y.C.; Chen, H.Y.; Huang, Y.R.; Ding, S.W. Varying Synthesis Conditions and Comprehensive Characterization of Fluorine-Doped Hydroxyapatite Nanocrystals in a Simulated Body Fluid. Crystals 2022, 12, 139. [Google Scholar] [CrossRef]
- Ashok, M.; Meenakshi Sundaram, N.; Narayana Kalkura, S. Crystallization of Hydroxyapatite at Physiological Temperature. Mater. Lett. 2003, 57, 2066–2070. [Google Scholar] [CrossRef]
- Kolanthai, E.; Ganesan, K.; Epple, M.; Kalkura, S.N. Synthesis of Nanosized Hydroxyapatite/Agarose Powders for Bone Filler and Drug Delivery Application. Mater. Today Commun. 2016, 8, 31–40. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Miao, X.; Li, Y.; Jiang, L.; Pan, K.; Tian, G.; Ren, Z.; Wang, G.; Fu, H. A New Layered Photocathode with Porous NiO Nanosheets: An Effective Candidate for p-Type Dye-Sensitized Solar Cells. Chem.—Asian J. 2013, 8, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Kettaf, S.; Guellati, O.; Harat, A.; Kennaz, H.; Momodu, D.; Dangbegnon, J.; Manyala, N.; Guerioune, M. Electrochemical Measurements of Synthesized Nanostructured β-Ni(OH)2 Using Hydrothermal Process and Activated Carbon Based Nanoelectroactive Materials. SN Appl. Sci. 2019, 1, 34. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Ramesh Babu, R.; Sethuraman, K.; Moorthy Babu, S. Hydrothermally Grown ZnO Nanoparticles for Effective Photocatalytic Activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 2–9. [Google Scholar] [CrossRef]
- Kamal, H.; Hezma, A.M. Spectroscopic Investigation and Magnetic Study of Iron, Manganese, Copper and Cobalt-Doped Hydroxyapatite Nanopowders. Phys. Sci. Int. J. 2015, 7, 137–151. [Google Scholar] [CrossRef]
- Li, Z.Y.; Lam, W.M.; Yang, C.; Xu, B.; Ni, G.X.; Abbah, S.A.; Cheung, K.M.C.; Luk, K.D.K.; Lu, W.W. Chemical Composition, Crystal Size and Lattice Structural Changes after Incorporation of Strontium into Biomimetic Apatite. Biomaterials 2007, 28, 1452–1460. [Google Scholar] [CrossRef]
- Mercado, D.F.; Magnacca, G.; Malandrino, M.; Rubert, A.; Montoneri, E.; Celi, L.; Bianco Prevot, A.; Gonzalez, M.C. Paramagnetic Iron-Doped Hydroxyapatite Nanoparticles with Improved Metal Sorption Properties. A Bioorganic Substrates-Mediated Synthesis. ACS Appl. Mater. Interfaces 2014, 6, 3937–3946. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, Characterization and Thermal Behavior of Apatitic Tricalcium Phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Sarath Chandra, V.; Baskar, G.; Suganthi, R.V.; Elayaraja, K.; Ahymah Joshy, M.I.; Sofi Beaula, W.; Mythili, R.; Venkatraman, G.; Narayana Kalkura, S. Blood Compatibility of Iron-Doped Nanosize Hydroxyapatite and Its Drug Release. ACS Appl. Mater. Interfaces 2012, 4, 1200–1210. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Yang, X.; Ding, H.; Wang, D.; Ma, D.; Feng, Q. Synthesis of Mesoporous Carbon Nitride by Molten Salt-Assisted Silica Aerogel for Rhodamine B Adsorption and Photocatalytic Degradation. J. Mater. Sci. 2021, 56, 11248–11265. [Google Scholar] [CrossRef]
- Nayak, B.; Samant, A.; Patel, R.; Misra, P.K. Comprehensive Understanding of the Kinetics and Mechanism of Fluoride Removal over a Potent Nanocrystalline Hydroxyapatite Surface. ACS Omega 2017, 2, 8118–8128. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, I.S.; Elfiky, E.M. Auto-Combustion Synthesis and Characterization of Iron Oxide Nanoparticles (α-Fe2O3) for Removal of Lead Ions from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2021, 31, 384–396. [Google Scholar] [CrossRef]
- Ghaedi, M.; Biyareh, M.N.; Kokhdan, S.N.; Shamsaldini, S.; Sahraei, R.; Daneshfar, A.; Shahriyar, S. Comparison of the Efficiency of Palladium and Silver Nanoparticles Loaded on Activated Carbon and Zinc Oxide Nanorods Loaded on Activated Carbon as New Adsorbents for Removal of Congo Red from Aqueous Solution: Kinetic and Isotherm Study. Mater. Sci. Eng. C. 2012, 32, 725–734. [Google Scholar] [CrossRef]
- Su, Y.; Cui, M.; Zhu, J.; Wu, Y.; Wei, Y.; Bian, S. A Facile Synthesis of Nanosheet-Structured Barium Carbonate Spheres with a Superior Adsorption Capacity for Cr(VI) Removal. J. Mater. Sci. 2018, 53, 4078–4088. [Google Scholar] [CrossRef]
- Rabiee Faradonbeh, M.; Dadkhah, A.A.; Rashidi, A.; Tasharofi, S.; Mansourkhani, F. Newly MOF-Graphene Hybrid Nanoadsorbent for Removal of Ni(II) from Aqueous Phase. J. Inorg. Organomet. Polym. Mater. 2018, 28, 829–836. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, M.A.; Arévalo-Niño, K.; Quintero-Zapata, I.; Castro-González, I.; Almaguer-Cantú, V. Cr(VI) Adsorption from Aqueous Solution by Fungal Bioremediation Based Using Rhizopus Sp. J. Environ. Manag. 2019, 251, 109595. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Y.; Liu, N.; Wu, Y. Iron Manganese Oxide Modified Multi-Walled Carbon Nanotube as Efficient Adsorbent for Removal of Organic Dyes: Performance, Kinetics and Mechanism Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4027–4042. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from Water by Adsorption onto Activated Carbon Prepared from Coir Pith, an Agricultural Solid Waste. Dye. Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Parvin, S.; Biswas, B.K.; Rahman, M.A.; Rahman, M.H.; Anik, M.S.; Uddin, M.R. Study on Adsorption of Congo Red onto Chemically Modified Egg Shell Membrane. Chemosphere 2019, 236, 124326. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tavallali, H.; Sharifi, M.; Kokhdan, S.N.; Asghari, A. Preparation of Low Cost Activated Carbon from Myrtus Communis and Pomegranate and Their Efficient Application for Removal of Congo Red from Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 107–114. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Azadirachta Indica Leaf Powder as an Effective Biosorbent for Dyes: A Case Study with Aqueous Congo Red Solutions. J. Environ. Manag. 2004, 71, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of Nickel(II) Ions onto Sargassum wightii: Application of Two-Parameter and Three-Parameter Isotherm Models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical Basis for the Dubinin-Radushkevitch (D-R) Adsorption Isotherm Equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Sekar, S.; Panchu, S.E.; Kolanthai, E.; Rajaram, V.; Subbaraya, N.K. Enhanced Stability of Hydroxyapatite/Sodium Alginate Nanocomposite for Effective Fluoride Adsorption. Mater. Today Proc. 2022, 58, 909–917. [Google Scholar] [CrossRef]
- Sekar, S.; Panchu, S.E.; Kolanthai, E.; Subbaraya, N.K. Enhanced Fluoride Adsorption and Regeneration Efficiency of Cross-linker-free Mesoporous Hydroxyapatite/Chitosan Nanocomposites. Res. Chem. Intermed. 2022, 48, 4857–4882. [Google Scholar] [CrossRef]
- Panchu, S.E.; Sekar, S.; Rajaram, V.; Kolanthai, E.; Panchu, S.J.; Swart, H.C.; Narayana Kalkura, S. Enriching Trace Level Adsorption Affinity of As3+ Ion Using Hydrothermally Synthesized Iron-Doped Hydroxyapatite Nanorods. J. Inorg. Organomet. Polym. Mater. 2022, 32, 47–62. [Google Scholar] [CrossRef]
- Panchu, S.E.; Sekar, S.; Kolanthai, E.; Rajaram, V.; Subbaraya, N.K. Bioremediation: Removal of Fluoride and Methylene Blue from Water Using Eco-Friendly Bio-Adsorbents. Mater. Today Proc. 2022, 58, 871–881. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic Study and Equilibrium Isotherm Analysis of Congo Red Adsorption by Clay Materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Guan, Y.; Cao, W.; Wang, X.; Marchetti, A.; Tu, Y. Hydroxyapatite Nano-Rods for the Fast Removal of Congo Red Dye from Aqueous Solution. Mater. Res. Express. 2018, 5, 065053. [Google Scholar] [CrossRef]


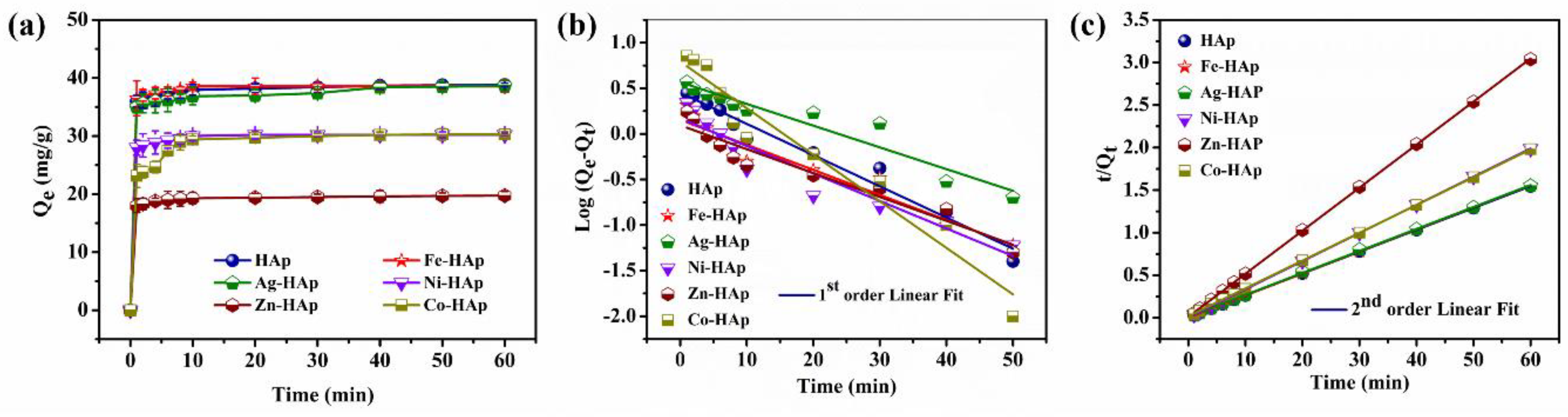

| Adsorbent | Qexp (mg/g) | First-Order Kinetics | Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg/g) | K1 (min−1) | R2 | Qm (mg/g) | K2 (mg/g·min−1) | R2 | ||
| HAp | 38.52 | 2.83 | 0.0342 | 0.9659 | 38.29 | 0.0259 | 0.9999 |
| Fe-HAp | 38.86 | 1.42 | 0.0274 | 0.9213 | 38.92 | 0.0253 | 0.9999 |
| Ag-HAp | 38.67 | 3.69 | 0.0238 | 0.9162 | 38.45 | 0.0257 | 0.9997 |
| Ni-HAp | 30.21 | 1.47 | 0.0301 | 0.9061 | 30.21 | 0.0310 | 0.9999 |
| Zn-HAp | 19.75 | 1.24 | 0.0261 | 0.9236 | 19.49 | 0.0505 | 0.9999 |
| Co-HAp | 30.3 | 6.20 | 0.0510 | 0.9393 | 30.86 | 0.0326 | 0.9999 |
| Adsorbents | pH | Time (min) | Qe (mg/g) | No of Cycles/RCE% | Ref. |
|---|---|---|---|---|---|
| Ag NPs-functionalized hydroxyapatite | 2 | 90 | 48 | 3/80 | [8] |
| Tea Waste | 7 | 720 | 23 | -- | [10] |
| Ca-bentonite | 7 | 480 | 85 | -- | [12] |
| B93-HAp | 5.5 | 20 | 122 | 6/64 | [13] |
| Sugarcane bagasse | 7 | 1440 | 38 | -- | [14] |
| Silver nanoparticles loaded on activated carbon (Ag-NPs-AC) | 4–7 | 14 | 67 | -- | [35] |
| Activated carbon | 8 | 40 | 7 | -- | [41] |
| Egg shell membrane | 4.5 | 180 | 117 | -- | [42] |
| Activated carbon-Pomegranante (AC-PG) | 7 | 90 | 19 | -- | [43] |
| Neem leaf powder (NLP) | 6.7 | 300 | 72 | -- | [44] |
| HAp | 7 | 1 | 87 | 7/1 | This work |
| Fe-HAp | 91 | 7/92 | |||
| Ag-HAp | 83 | 7/42 | |||
| Ni-HAp | 60 | 7/30 | |||
| Zn-HAp | 39 | 7/3 | |||
| Co-HAp | 64 | 7/16 |
| Isotherm | Adsorbents | ||||||
| HAp | Fe-HAp | Ag-HAp | Ni-HAp | Zn-HAp | Co-HAp | ||
| Qexp (mg/g) | 86 | 91 | 83 | 59 | 38 | 63 | |
| Langmuir | Qm (mg/g) | 86.95 | 92.59 | 85.03 | 59.44 | 39.28 | 64.23 |
| KL (mg/L) | 0.0287 | 0.0208 | 0.0886 | 0.1749 | 0.6176 | 0.5625 | |
| RL | 0.1483 | 0.1937 | 0.0534 | 0.0277 | 0.0081 | 0.0088 | |
| R2 | 0.9769 | 0.9835 | 0.9893 | 0.9883 | 0.9799 | 0.9869 | |
| Freundlich | KF (mg/g) | 37.15 | 38.90 | 27.54 | 19.95 | 8.12 | 1.00 |
| 1/n | 4.65 | 5.18 | 3.83 | 4.14 | 3.05 | 9.45 | |
| R2 | 0.84447 | 0.88927 | 0.72296 | 0.70921 | 0.73481 | 1 | |
| DKR | Qm (mg/g) | 79.89 | 84.53 | 76.12 | 58.91 | 37.73 | 59.99 |
| β (mol/J)2 | −1.67 × 10−9 | −1.41 × 10−9 | −2.02 × 10−9 | −1.94 × 10−9 | −2.71 × 10−9 | −4.27 × 10−9 | |
| E (kJ/mol) | 17.6 | 18.8 | 15.8 | 16.2 | 13.6 | 10.9 | |
| R2 | 0.9039 | 0.9263 | 0.7677 | 0.7467 | 0.7958 | 0.8610 | |
| Adsorbents | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 303 K | 313 K | 323 K | 333 K | |||
| HAp | −16.44 | −16.36 | −16.21 | −16.08 | 266 | −2.218 |
| Fe-HAp | −16.94 | −17.18 | −17.29 | −17.38 | 189 | −0.526 |
| Ag-HAp | −16.53 | −16.53 | −16.33 | −16.27 | 202 | −0.122 |
| Ni-HAp | −15.18 | −15.24 | −15.41 | −15.58 | 186 | −0.111 |
| Zn-HAP | −13.94 | −13.89 | −13.59 | −12.84 | 277 | −3.608 |
| Co-HAp | −15.52 | −15.31 | −14.98 | −14.81 | 260 | −2.463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchu, S.E.; Sekar, S.; Kolanthai, E.; Sridharan, M.B.; Subbaraya, N.K. Extremely Fast and Efficient Removal of Congo Red Using Cationic-Incorporated Hydroxyapatite Nanoparticles (HAp: X (X = Fe, Ni, Zn, Co, and Ag)). Crystals 2023, 13, 209. https://doi.org/10.3390/cryst13020209
Panchu SE, Sekar S, Kolanthai E, Sridharan MB, Subbaraya NK. Extremely Fast and Efficient Removal of Congo Red Using Cationic-Incorporated Hydroxyapatite Nanoparticles (HAp: X (X = Fe, Ni, Zn, Co, and Ag)). Crystals. 2023; 13(2):209. https://doi.org/10.3390/cryst13020209
Chicago/Turabian StylePanchu, Sandeep Eswaran, Saranya Sekar, Elayaraja Kolanthai, Moorthy Babu Sridharan, and Narayana Kalkura Subbaraya. 2023. "Extremely Fast and Efficient Removal of Congo Red Using Cationic-Incorporated Hydroxyapatite Nanoparticles (HAp: X (X = Fe, Ni, Zn, Co, and Ag))" Crystals 13, no. 2: 209. https://doi.org/10.3390/cryst13020209
APA StylePanchu, S. E., Sekar, S., Kolanthai, E., Sridharan, M. B., & Subbaraya, N. K. (2023). Extremely Fast and Efficient Removal of Congo Red Using Cationic-Incorporated Hydroxyapatite Nanoparticles (HAp: X (X = Fe, Ni, Zn, Co, and Ag)). Crystals, 13(2), 209. https://doi.org/10.3390/cryst13020209







