1. Introduction
Biomineralization occurs when morphology, growth, composition, chemical bonding, and local electronic and atomic structures of crystalline phase are governed by cellular effects in a specific organism. The mechanisms controlling biomineralization in bone tissues remain weakly understood. Their research should cut across many interdisciplinary problems and go through different disciplines. The convergence of various approaches and mindsets derived from biological, medical, physical, chemical, and engineering sciences is extremely needed [
1].
Being a living substance, bone structures monitor, adapt, and accommodate to various external conditions. In particular, these structures track the distribution of mechanical loads in the skeleton. According to J. Wolff [
2], the spatial organization of bone structures is determined during the execution of locomotors body functions against the gravity force. Is this paradigm applicable to bone nanostructures? The electron microscopic studies [
3,
4,
5,
6] demonstrate a correspondence between the directions of collagen fibrils in the mineralized bone and the force lines that refer to the primary load at each point of the skeleton space. The transverse orientation of collagen fibrils is more common in the cortex that experiences compression, whereas their longitudinal orientation predominates in areas that are primarily stretched [
3,
4,
5,
6]. The oriented organic molecules of the extracellular matrix of the calcified tissue of vertebrates playing an important role in the structuring of the apatite phase [
6,
7,
8,
9,
10,
11] initiate biologically necessary substitutions and vacancies through the aqueous medium [
11,
12,
13,
14] and form the necessary biochemical environment in the mineralization area [
6,
15,
16,
17].
The impact of mechanical loads, organic molecules, and water on the nanostructure of mineralized bone attracts high attention. A lot of effort has been invested to understand the interplay of the hard hydroxyapatite (HAP, Ca
10(PO
4)
6(OH)
2) with the flexible collagen fibers, which makes bone more elastic and firmer than the crystal and collagen taken separately [
6,
7,
8,
16,
17,
18,
19]. The main morphological models [
6,
7,
8,
9,
10] are successful in achieving a qualitative description of the hierarchical organization of the skeleton starting from its macro to nano level. However, a quantitative description, especially at the nanoscale, faces great difficulties because the relationships between the upper and lower hierarchical levels have not been studied.
Transmission electron microscopy and X-ray diffraction (XRD) widely used to explore bone structures make evident that coplanar and mosaic assemblies of the nanocrystallites of HAP (NHAP) form the mineral matrix of bone [
6,
7,
8,
17]. According to Newman and Newman [
10], NHAPs are separated one from the other by hydrated nanolayers of a saturated aqueous solution containing mainly the OH
−, [PO
4]
3−, and [CO
3]
2− anions and Ca
2+ cations. According to [
19], the effective NHAP in bones is a rectangular block with dimensions ≈ 20 × 7.5 × 3.5 nm
3. Such a nanoblock contains about 4⋅10
4 atoms. The thickness of the hydrated layer is ≈ 2.5 nm. The NHAPs assemblies generally reproduce the helix shape of collagen fibrils [
6].
To bridge the local electronic and atomic structures of the bone with its higher-lying hierarchical levels, the 3D superlattice (3DSL) of “black-nanoboxes-in-muddy-waters” model was suggested [
19]. This model takes into account both the qualitative structure-functional organization of mineralized bone and the quantum-mechanical peculiarities of electron waves propagation in 3D superlattices assembled from the NHAPs. The 3DSL model predicts a distinct HAP-to-bone redshift of valence bands [
19] and provides a useful tool for controlling the impact of upper-lying hierarchical levels on the atomic-molecular architecture of mineralized bone. Regrettably, the successful application of the model requires the quantification of local electronic and atomic structures and their relationships with the specifics of the organism.
Recently, there has been an explosive interest in mineralized bone regeneration [
20]. The regeneration is tightly related to the use of both new synthetic biomaterials (see, e.g., [
21,
22,
23,
24]) and new methods for controlling the organic—inorganic interactions [
25] in native bone. The experimental studies [
25,
26,
27,
28,
29] make evident that the atomic-molecular architecture of native bone cannot be considered as a fixed structure. The short-, long- and super-range order parameters of NHAPs assemblies are subjects of complicated site- [
25,
26] and age-dependent [
27,
28,
29] deviations. These parameters are “fingerprints” of the biological state of mineralized bone and can be used to monitor its development under biogenic or pathogenic conditions.
Specifically, the analysis of concurrent age-related changes in electrons binding energies, degrees of crystallinity, sizes of crystallites, Ca
2+ deficiency, and HAP lattice constants in bone has shown that the age effects are closely related to charge heterogeneity in the mineral matrix [
27]. Thus, mineralized bone is a kind of electric battery composed from nanometric cells formed from negatively charged NHAPs embedded into positively charged intercrystallite water. In contrast, strong site-dependent deviations of structural and spectroscopic parameters are detected in the femur in osteoarthritis (OA) damaged knee compartments [
25,
26]. According to [
25], the site-dependence is motivated by the coupling of mineral and organic matrices. Under normal stress conditions, the sacrificial bonds [
30] block the destruction of collagen fibers [
30,
31]. However, in cases of abnormal stress, when fibers break, their fragments start to interact with NHAPs, producing new non-apatite chemical states. Moreover, the observation of the restoration of calcium bonds and crystallinity in OA-damaged areas [
25] provides a new look at the interplay between biochemical and biomechanical processes accompanying biomineralization under pathogenic conditions. However, the revealed site-dependencies cannot be regarded as quantitative patterns due to low statistics in [
25].
In the present work, mineralized bone is examined to understand the nanomechanisms producing its spatiotemporal changes under pathogenic (OA) conditions. High resolution XRD and X-ray photoelectron spectroscopic (XPS) studies of the human femur in intact and OA-affected areas were carried out. XRD studies make it possible to observe the distortion of the crystal structure depending on the spatial distribution of mechanical loads in the skeleton, whereas XPS studies track changes in the chemical bonds in the bone.
The choice of the bone samples, their preparation, and the experimental techniques are described in
Section 2. Site-dependent changes in stoichiometry, crystallites sizes, crystallographic lattice constants, degrees of crystallinity, Ca
2+ 2p
3/2,1/2 electron-binding energies (BEs) are reported and examined in
Section 3. The structural and spectroscopic characteristics of mineralized bone under OA conditions were measured for 10 specimens in the identical experimental conditions. The most substantial deviations are revealed near the epicenter of excessive mechanical loads but not inside it. Statistical analysis of the experimental results generally confirms the non-Wolffian patterns in the atomic-molecular architecture under abnormal stress conditions, which were reported in [
25]. To understand their nature, the chronobiological concept (
Section 3.4) is applied. The identified deviations are primarily associated with catalytic reactions occurring at the organic—inorganic interface and with NHAP carbonization. The peculiarities of these reactions at the interface are due to the coupling of NHAPs with collagen fragments. Perspectives in the experimental and theoretical research of biomineralization under pathogenic conditions are discussed in
Section 3 too.
3. Results and Discussions
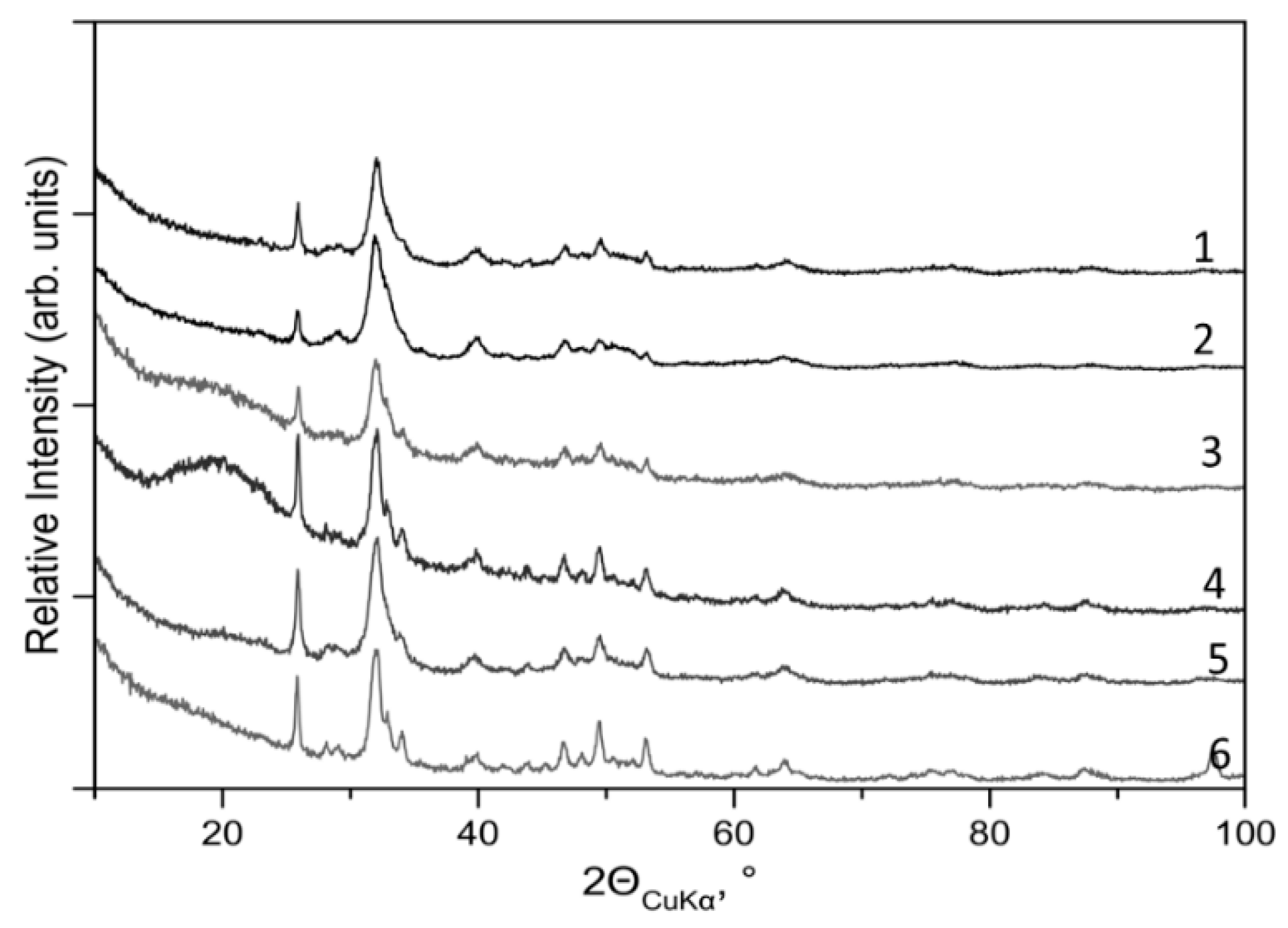
To illustrate the site- and patient-dependences of X-ray diffraction and photoemission measurements, sets of XRD patterns and Ca 2p
3/2,1/2−1 PE spectra of the specimens are shown in
Figure 2 and
Figure 3, respectively. These experimental data were used to extract structural and spectroscopic parameters of the atomic-molecular architecture in the different areas. The extracted site-dependent changes of crystallinity, lattice constants
a and
c, and population of apatite Ca
2+ states are presented in
Figure 4a–c. In the plots to display the spatiotemporal changes, a chronobiological order
1→
3→
2 is used. It refers to the transitions from healthy bone with a full-layer cartilage (area
1) to its partially damage (area
2) and, then, to its full loss (area
2). This arrangement is discussed in more detail in
Section 3.4.
3.1. XRD Data Analyses
No other crystal phases excepting hexagonal HAP (space group P6
3/m) are revealed in the bone samples. The XRD data analyses make evident the distinct site- and patient-dependent deviations of degree
D of crystallinity, linear size
L of NHAPs, and crystallographic lattice constants
a and
c. The site-dependent changes in
D,
a and
c, are respectively displayed in
Figure 4a,b.
3.1.1. Crystallinity
Examining
Figure 4a, we observe a noticeable spread of the degrees of crystallinity, which specifies both the site- and patient-dependent variations. The average degree 〈
D〉 for each area is marked with a cross. The dotted line traces its site-dependent behavior. As it was mentioned, above the degrees are arranged in the figure in chronobiological order
1→
3→
2. To get a more complete picture, the degrees of crystallinity detected far from the frontal areas on the distal side (
4) are also shown.
On the distal side, the degree 〈
D〉 is maximum (90%) and does not practically vary when moving along it. An opposite situation is realized on the proximal side, in which the degrees vary significantly. The lowest 〈
D〉 (≈42%) is observed in the vicinities of the sclerotic bone (area
3). 〈
D〉 is equal to 65% in
1 and to 85% in
2. These variations agree reasonably with those reported in [
25]. Moving in the direction from
1 to
3 and, then, to
2, we first fall into the region of hard destructions, and then relocate to the region, where the degree of crystallinity increases, pointing to the remineralization (restoration) of the apatite phase. Despite the general resemblance of the site-dependent changes to those reported in [
25], there are substantial difference as the 〈
D〉 does not display such strong destruction (amorphization) in
3. The measured standard deviations of crystallinity σ
D are equal ≈23% in areas
1–
3 and ≈9% in
4.
3.1.2. Lattice Constants
The site-dependence of the lattice constants
a and
c and their HAP-to-bone differences (Δ
a and Δ
c) are plotted in
Figure 4b. The differences are defined as
where 〈
a〉 and 〈
c〉 are the averaged values of the constants. The lattice constants, HAP-to-bone differences, and standard deviations in the areas are illustrated in
Table 1. Examining these data, the
c-stretching (Δ
c < 0) in bone is clearly visible for all areas. We hypothesize that
c-stretching is a general phenomenon in bone under OA conditions. This stretch is a distinct effect as the standard deviation σ
c < |Δ
c|. Another behavior is detected for the lattice constant
a. It is substantially compressed on the distal side (Δ
a > 0) and close to the stoichiometric HAP value on the proximal side, where the standard deviation σ
a is greater than |Δ
a|. The volume
V of HAP crystal cells in bone is enlarged on the proximal side but stressed on the distal side. Volume versus location variations associated with the stoichiometric HAP (
Rvol) is shown in
Table 1 (last column).
The variations of the lattice constant
c attracted our attention. Comparing them with the variations of crystallinity in
Figure 4a, we see their close resemblance. Both the greatest stretch of the constant and the biggest depress of
D occur in the intermediate area
3. Therefore, we can assume that the uniaxial stretching of the crystal cell and the reduction of crystallinity are interrelated events in bone mineral under OA conditions. It is known [
37,
38,
39] that
c-stretching in biogenic HAP is usually motivated by the replacement of
by
. It is also known that the constant
c increases nearly linearly with increasing concentrations (
w) of
[
37]. Then, taking into account both the linear
w(
c) dependence [
37] and the average values of
c from
Table 1, we computed the concentrations of carbonate ions in various areas of the studied specimens. These concentrations are shown in
Table 2. Considering them, we see that the concentration of
on the proximal side is higher than that on the distal one, and the maximum of carbonization is at
3. The extracted site-dependency
w is shown with the dashed line in
Figure 4b.
3.1.3. Sizes of Crystallites
The site dependence of the linear size of NHAPs is not discussed here. We note that the average size 〈L〉 is ≈20 nm on the distal side, ≈15 nm and ≈11 nm on the proximal side in the intact and sclerotic areas, respectively. In 3, the size variations are very significant. Obviously, more statistics are needed to study the dependence. Tentatively, this uncertainty can be assigned to the low crystallinity in 3.
3.2. XPS Data Analyses
The Ca
2+ 2p
3/2,1/2−1 PE spectra of the HAP and CHAP reference compounds and native bone samples are exhibited in
Figure 3. The distinct energy shifts and line shapes distortions are clearly visible. We draw attention to the downward shift of the Ca
2+ 2p
3/2,1/2−1 PE lines in the bone specimens compared to those in the reference minerals. To document the energy shifts and the line shape distortions, the measured spectra were examined using the Voigt fit analysis.
According to this analysis, the Ca 2p−13/2 and 2p−11/2 PE lines shapes corresponding to HAP and CHAP, as well as to intact areas on the proximal side and over all areas on the distal side are precisely reproduced with the single-Voigt-function approximation. The Ca 2p3/2 and 2p1/2 spin-orbit components are split ~3.55 eV; the Gaussian width completely dominates in the full-width-at-half-maximum of the individual Ca2+ 2p3/2−1 and 2p1/2−1 components, and their relative intensity is close to 2:1. The simple composition of the spin-orbit split components allows us to assign them to the PE emission from the Ca2+ apatite states. In bone tissues, they are denoted via Ca2+(A), the corresponding Ca2+ 2p3/2 BE is disposed in the range 347.1–347.0 eV. Thus, the apatite-like calcium dominates (its population PA is nearly 100%) in the intact area and on the distal side of the saw-cuts. Another situation is realized in OA-damaged areas, where the single-Voigt-function approximation does not work. At least three components must be included to reproduce the PE signals from the areas. In addition to Ca2+(A), the measured Ca2+ 2p3/2,1/2 PE lines contain the PE fluxes outgoing from non-apatite calcium states denoted as Ca2+(X) and Ca2+(Y). The Ca2+(X) 2p3/2 BE is disposed at ≈346.5 eV, and the Ca2+(Y) 2p3/2 BE is disposed at 348.2–348.0 eV.
Figure 4c demonstrates the decompositions of the Ca
2+ 2p
3/2 photoelectron signals emitted from the different areas in the specimens. It can be seen that areas
1 and
4 are predominantly populated by Ca
2+(A), whereas its population decreases in areas
2 and
3. The average population
PA is 57.5% in
2 and 22% in
3. Our measurements show that Ca
2+(X) plays an important role in the areas since its population
PX is 36,5% in
2 and 73% in
3. As for Ca
2+(Y), its part is nearly 5–6%. In more than half of the bone samples, Ca
2+(Y) was not practically visible. The average site-dependent population of Ca
2+(A) is shown with a dashed line in
Figure 4c. The standard deviations of
PA are less than 5% in areas 1 and 4, ≈29% in
2 and ≈18% in
3.
The accurate assignment of the non-apatite calcium bonds in the OA areas is a difficult problem. Note that the Ca
2+2p
3/2 BEs in HAP, α- and β-tricalcium phosphates (TCP), amorphous calcium phosphate (ACP), octacalcium phosphate, and brushite vary in a narrow range of 347.0–347.3 eV [
40,
41]. These BEs are presented in
Table 3. The calcium—phosphate bonds are not considered as a main source of non-apatite Ca
2+(X) and Ca
2+(Y) states, the Bes of which are located far from the indicated range. In CaCO
3 polymorphs, the relevant BEs vary in a wider range. According to [
40], the Ca
2+2p
3/2 BE is 346.5 eV in calcite and 347.9 eV in aragonite. Thus, the Ca
2+(X) can be tentatively assigned with calcium ions with calcite-like bonds [
25]. Since in mineralized bone there are no crystal phases except for HAP, amorphous calcium carbonate is a plausible candidate for the role of Ca
2+(X). Regrettably, its effect in bone is less studied than ACP (see, [
42,
43,
44]).
The comparison of Ca
2+ 2p
−13/2 BEs in calcium phosphates and carbonates in
Table 3 shows that the carbonization gives rise to the Ca
2+ 2p
−13/2 Bes compared to HAP. The energy positions of the Ca
2+ 2p
−13/2,1/2 PE lines in CHAP (curve 5 in
Figure 3a) are lower than those in HAP (curve 1). Thus, we infer that the replacement of
by
is not a source of Ca
2+(X) in XPS, as accounting for this carbonization does not compensate the contribution of Ca
2+(X) to the PE lines composition in
Figure 4c.
The similarity of the site-dependent variations in PA, D, and lattice constant c indicates the important role of chemical reactions occurring at the interface between damaged cartilage and NHAPS, as well as their close relationship with the Wolff’s biomechanics in the formation and orientation of bone nanostructures in subchondral bone under pathogenic conditions.
3.3. Coefficients of Variation
The coefficient of variations
ρj for crystallinity, populations of Ca
2+(A), and stretching of the lattice constant
c was examined too. The coefficient defined as
(where the numerator is a standard deviation of
j and denominator is the averaged value of
j) shows the degree of variability in relation to the sample mean.
Figure 4d presents the site-dependence of the coefficients for
D, Δ
c, and
PA. It is seen that they vary non-monotonously and come through extremums at
3. In contrast to
ρD and
ρP, which demonstrate maximal variability in this area
, the coefficient
ρΔc points to the minimal variability of Δ
c in it. This specificity confirms our assignment of the
c-stretching with the concentration of
in NHAPs. Since the concentration in the vicinities of the sclerosis areas approaches the saturation limit (see,
Table 2), the depress of
ρΔc in
3 is expected. As for
ρΔa not plotted in the figure, the differences Δ
a demonstrate an extremely high variability. The only exception is the distal side, where
ρΔa ≈ 73%. We emphasize that the site-dependence of the standard deviations of
D,
c, and
PA and their mean values differ substantially.
3.4. Chronobiological Concept
The systematic and site-dependent changes in crystallinity, lattice constants, carbonization, and electron BEs of the human femur under pathogenic (OA) conditions are revealed. For all studied samples, the HAP-to-bone changes are maximum when scanning along the proximal side, vary substantially when moving in depth to the distal side, and demonstrate minor deviations by monitoring the distal side. The most intense reduction in crystallinity, stretching of the HAP lattice constant c, growth of carbonization, and rupture of apatite chemical bonds take place on the proximal side in the vicinities of the sclerotic bone (area 3). Specifically, inside the sclerosis area where the mechanical loads are maximum, the values of c, D, w, and PA approach those in healthy bone (in our case, these are the values in areas 1 and 4). This site-dependence is quite unexpected from the standpoint of Wolff’s paradigm, according to which the strongest deviations should be expected in the sclerosis areas but not in their vicinities. This finding shows that the nature of the spatial changes in mineralized bone under OA conditions is primarily related to biochemical reactions occurring at the organic—inorganic interface. It is these reactions at the interface that generate the greatest changes in 3 and produce the non-apatite calcium states in areas 2 and 3.
These non-apatite states not inherent in healthy bone are a trigger of the complex reconstruction of the atomic-molecular architecture of bone in the setting of OA. For a better understanding of the mechanisms of reconstruction, we consider the site-dependent changes as spatiotemporal. The structural and spectroscopic characteristics in the areas 1, 2, and 3 can be associated with different degrees of OA development. Hence, the site-dependencies are closely related to temporal changes in the bone samples, and one can speak of their spatiotemporal changes.
To quantify the temporal changes in native bones, we consider their characteristics on the nanoscale as functions of a continuous parameter, introduced as
≡
t/T, where
t and
T are the time counted from the onset of the disease and time-period up to full loss of cartilage, respectively. Thus,
is the dimensionless parameter that characterizes the totality of changes in the atomic-molecular architecture of the bone occurred by a given point in time. Thus, the dependencies of
D, Δ
c, Δ
a,
ω, and
PA on
τ is a consequence of the hierarchical organization of the mineralized bone and reflects its emergent properties. To specify the temporal changes in these observable characteristics, we turn to the Hellmann–Feynman theorem (see, e.g., [
46])
Here, is a Hamiltonian of the biological system that depends on , is an eigenfunction of the Hamiltonian, and is the corresponding eigenvalue. The function is determined by the stationary Schrödinger equation. Thus, we can distinguish the parametrical dependence of the characteristics on from the time (t) dependence due to , where r and R are the electronic and nuclear variables in the skeleton frame. Such distinguishing becomes possible as the biological system is in a non-equilibrium state.
Taking into account the parametrical dependence, we consider the spatial changes in the sequence intact area
1 → vicinities of the sclerosis area
2 → sclerosis area
3 in
Figure 4a–c as the corresponding temporal changes in
D(
τ), Δ
c(
τ), Δ
a(
τ),
ω(
τ), and
PA(
τ) as
increases from 0 to 1. Looking at the graphs, we see that the most dramatic destruction of the atomic-molecular architecture of mineralized bone occurs upon its contact with the damaged collagen fibers. After their complete removal in area
2, bone regeneration becomes the prevailing trend. Thus, we can assume that the opposition of catalytic processes caused by OA and cellular effects determines the atomic-molecular architecture in
3, and the resumption of the biomineralization process controls it in
2.
4. Conclusions
The distinct site-dependent deviations in crystallinity, HAP lattice constants, carbonization, core-electron Bes, and calcium bonds in mineralized bone under OA conditions were revealed. The quantitative values of these characteristics and their standard deviations in each area were measured. The strongest OA-induced distortions were concentrated on the proximal side in the vicinities of sclerotic bone, where the erased cartilage come into contact with the mineral matrix. These distortions consist primarily in reduction of crystallinity, stretching of the lattice constant c, and dominance of new chemical states that are not characteristic of HAP. This site-dependence of the structural and spectroscopic characteristics of mineralized bone under OA conditions is a general phenomenon that was detected in all samples.
There are two main mechanisms leading to the distortions. The first is associated with (i) the replacement of by , the concentration of which approaches saturation limit in the vicinities of the sclerosis area, and with (ii) the increase in catalytic reactions provoking the breaking of calcium bonds in HAP at the mineral-collagen interface. The second mechanism is related with cellular effects resulting in the restoration of mineralized bone within the sclerosis area as after the loss of cartilage tissue, the characteristics of the atomic-molecular architecture in 2 approach those of healthy bone.
It is stated that the atomic-molecular architecture of the mineralized phase varies under the action of mechanical loads, but also depending on its localization in the bone space. This architecture on the proximal side differs significantly from that on the distal side. In particular, on the distal side we see the substantial increase of crystallinity, sharp shortening of the lattice constant a, as well as a strong reduction in the concentration of compared to the corresponding values within the intact area on the proximal side.
Finally, we note that fine tuning of the ion exchange processes in native bone aimed at its treatment and restoration is impossible without a thorough study of the mechanisms of biomineralization under both biogenic and pathogenic conditions. Further experimental and theoretical investigations are required to (i) identify the non-apatite calcium bonds in the OA-damaged areas, (ii) establish a relationship between chemical reactions at the interface, changes in crystallinity, and crystal lattice parameters, and (iii) understand their interplay with cellular effects in a specific organism. We believe that further studies of the atomic-molecular architecture of bone tissue will make it possible to understand the relationship between biomineralization and age, as well as the peculiarities of its spatiotemporal changes under other pathogenic (e.g., osteoporosis and osteonecrosis) conditions. In particular, higher resolution 3D mapping of bone distortions along the proximal surface and in depth seems to be extremely important for this purpose.