Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials
Abstract
:1. Introduction
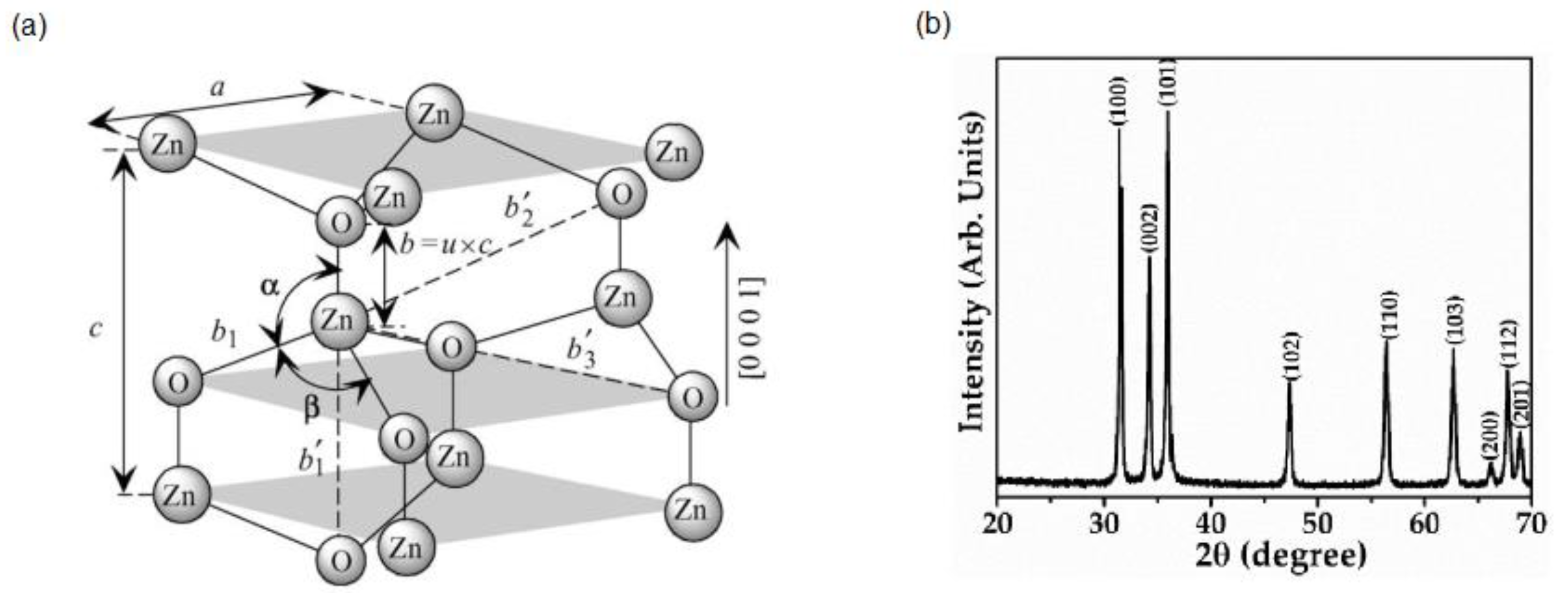
2. Zinc Oxide (ZnO)
3. Enhancing the Thermoelectric Performance of ZnO
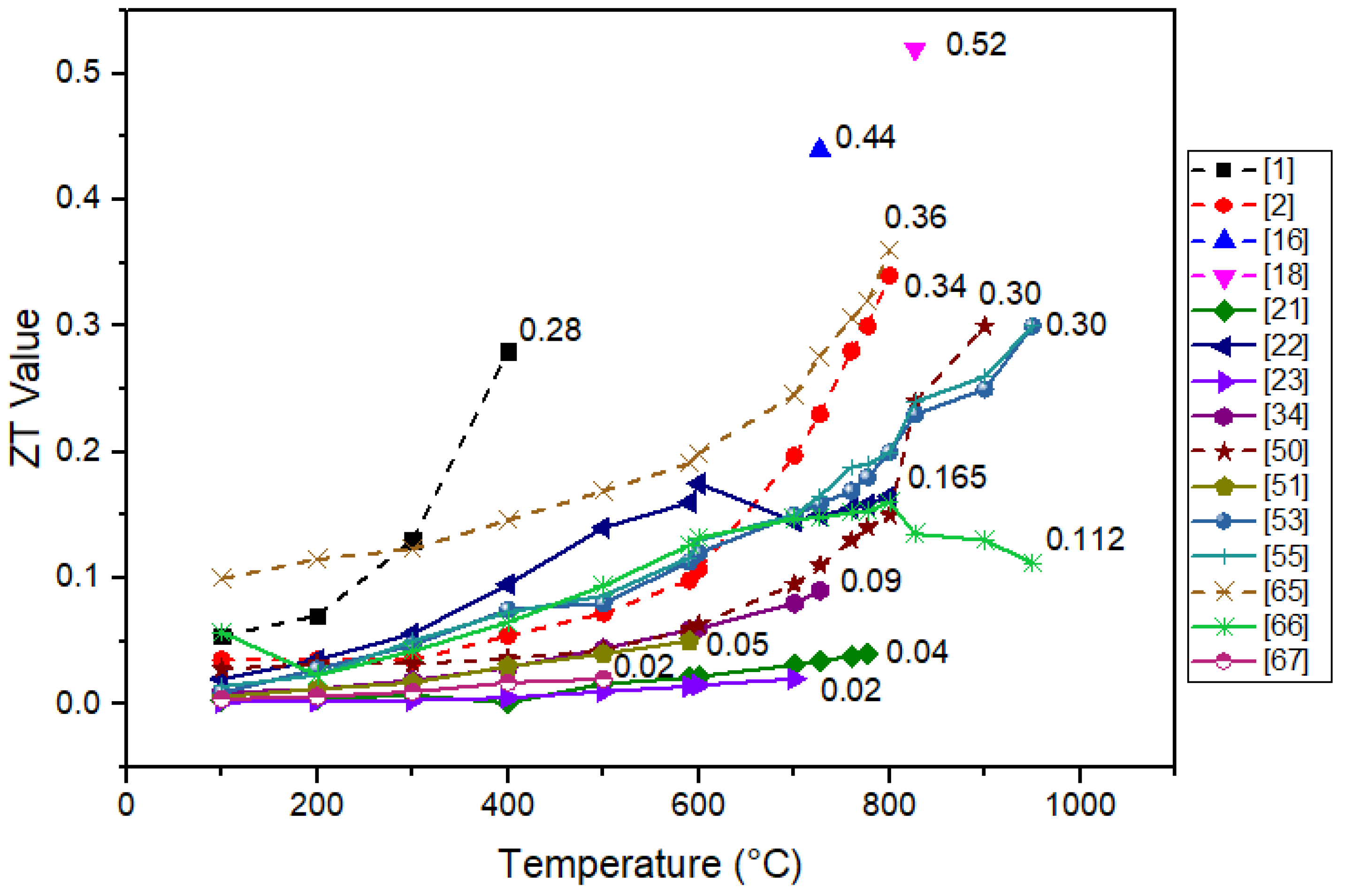
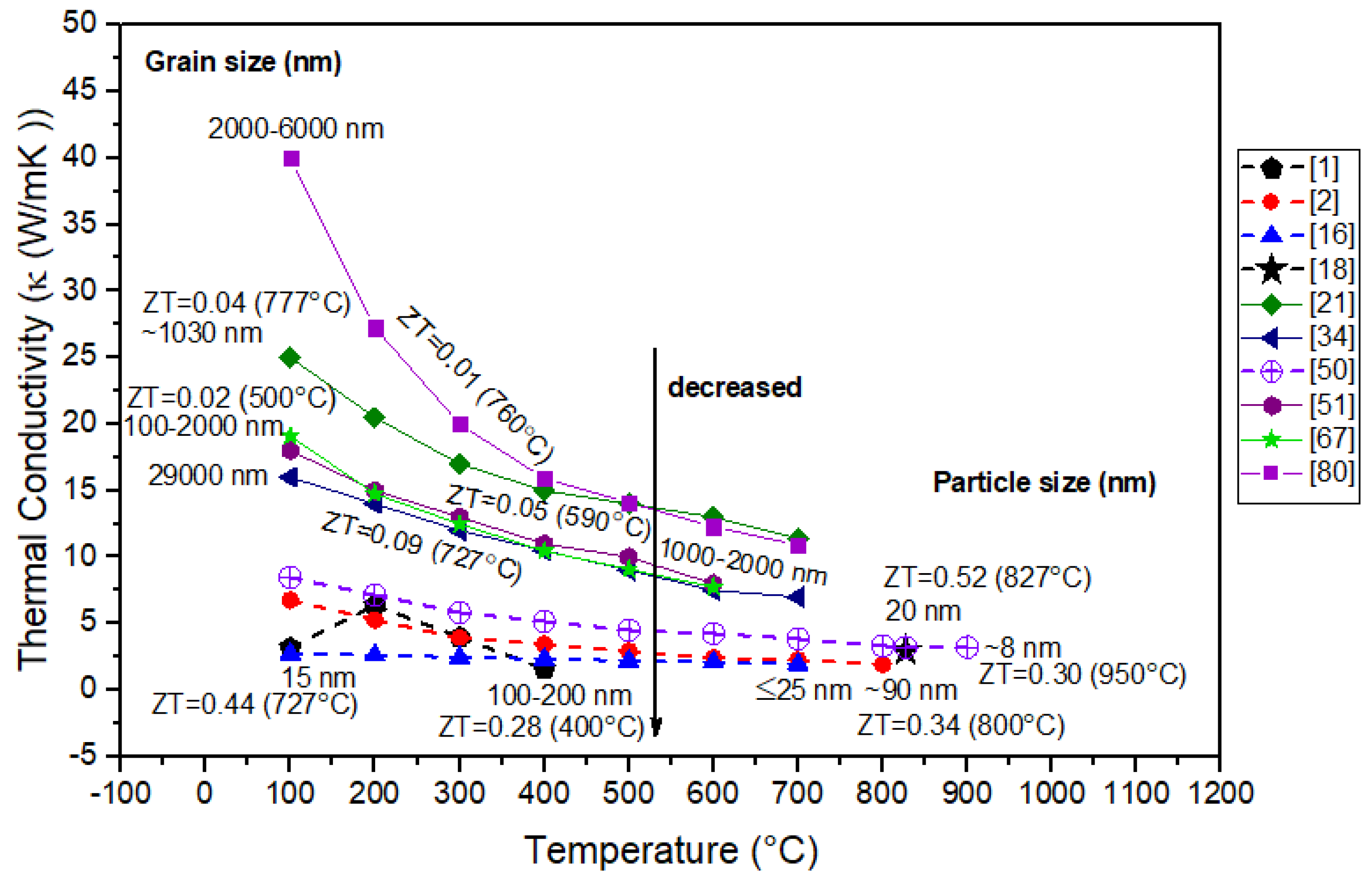
3.1. Nanostructure Approach
3.2. Doping Approach
3.2.1. Al-doped ZnO
3.2.2. Ga-doped ZnO
3.2.3. Ni-doped ZnO
3.2.4. Bi-doped ZnO
3.2.5. Sn-doped ZnO
4. Concluding Remarks
5. Way Forward
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jantrasee, S.; Moontragoon, P.; Pinitsoontorn, S. Thermoelectric properties of Al-doped ZnO: Experiment and simulation. J. Semicond. 2016, 37, 092002-1–092002-8. [Google Scholar] [CrossRef]
- Nam, W.H.; Lim, Y.S.; Choi, S.-M.; Seo, W.-S.; Lee, J.Y. High-temperature charge transport and thermoelectric properties of a degenerately Al-doped ZnO nanocomposite. J. Mater. Chem. 2012, 22, 14633–14638. [Google Scholar] [CrossRef]
- Ihns, M. Structural Engineering of Ca3Co4O9 Thermoelectric Thin Films. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2013. [Google Scholar]
- Zaferani, S.H.; Jafarian, M.; Vashaee, D.; Ghomashchi, R. Thermal Management Systems and Waste Heat Recycling by Thermoelectric Generators—An Overview. Energies 2021, 14, 5646. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide materials for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 2012, 32, 525–540. [Google Scholar] [CrossRef]
- Zhu, B.B.; Li, D.; Zhang, T.S.; Luo, Y.B.; Donelson, R.; Zhang, T.; Zheng, Y.; Du, C.F.; Wei, L.; Hng, H.H. The improvement of thermoelectric property of bulk ZnO via ZnS addition: Influence of intrinsic defects. Ceram. Int. 2018, 44, 6461–6465. [Google Scholar] [CrossRef]
- Funahashi, R.; Barbier, T.; Combe, E. Thermoelectric materials for middle and high temperature ranges. J. Mater. Res. 2015, 30, 2544–2557. [Google Scholar] [CrossRef]
- Koumoto, K.; Wang, Y.; Zhang, R.; Kosuga, A.; Funahashi, R. Oxide Thermoelectric Materials: A Nanostructuring Approach. Annu. Rev. Mater. Res. 2010, 40, 363–394. [Google Scholar] [CrossRef]
- Jouharaa, H.; Zabnienska-Góra, A.; Khordehgaha, N.; Doraghia, Q.; Ahmada, L.; Normana, L.; Axcella, B.; Wrobela, L.; Daid, S. Thermoelectric generator (TEG) technologies and applications. Int. J. Thermofluids. 2021, 9, 100063. [Google Scholar] [CrossRef]
- Huang, S.; Ning, S.; Xiong, R. First-Principles Study of Silicon–Tin Alloys as a High-Temperature Thermoelectric Material. Materials 2022, 15, 4107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, X.; Wang, J.; Jiao, S.; Xue, D. Multifunctional inorganic nanomaterials for energy applications. Nanoscale 2020, 12, 14–42. [Google Scholar] [CrossRef]
- Ohtaki, M. Recent aspects of oxide thermoelectric materials for power generation from mid-to-high temperature heat source. J. Ceram. Soc. Jpn. 2011, 119, 770–775. [Google Scholar] [CrossRef]
- Lourdes, M.G. Preparation and Characterisation of Nanostructured Bulk Bi2Te3 Thermoelectric Materials Using Ultrasound Milling. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2016. [Google Scholar]
- Yin, Y.; Tudu, B.; Tiwari, A. Recent advances in oxide thermoelectric materials and modules. Vacuum 2017, 146, 356–374. [Google Scholar] [CrossRef]
- Sulaiman, S.; Izman, S.; Uday, M.B.; Omar, M.F. Review on grain size effects on thermal conductivity in ZnO thermoelectric materials. RSC Adv. 2022, 12, 5428–5438. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Peleckis, G.; Wang, X.; Siegel, R.W.; Borca-tasciuc, T.; Dou, S.X.; Ramanath, G. Al-Doped Zinc Oxide Nanocomposites with Enhanced Thermoelectric Properties. Nano. Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Sudin, I.; Noor, A.M.; Rajoo, S.; Uday, M.B.; Obayes, N.H.; Omar, M.F. A review of thermoelectric ZnO nanostructured ceramics for energy recovery. Int. J. Eng. Technol. 2018, 7, 27–30. [Google Scholar] [CrossRef]
- Biswas, S.; Singh, S.; Singh, S.; Chattopadhyay, S.; Silva, K.K.H.D.; Yoshimura, M.; Mitra, J.; Kamble, V.B. Selective Enhancement in Phonon Scattering Leads to a High Thermoelectric Figure-of-Merit in Graphene Oxide-Encapsulated ZnO Nanocomposites. ACS Appl. Mater. Interfaces 2021, 13, 23771–23786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, X.; Zhao, L.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Wiff, J.P.; Kinemuchi, Y.; Kaga, H.; Ito, C.; Watari, K. Correlations between thermoelectric properties and effective mass caused by lattice distortion in Al-doped ZnO ceramics. J. Eur. Ceram. Soc. 2008, 29, 1413–1418. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, X.J.; Hng, H.H.; Ma, J. Characterization of Al-doped ZnO thermoelectric materials prepared by RF plasma powder processing and hot press sintering. Ceram. Int. 2009, 35, 3067–3072. [Google Scholar] [CrossRef]
- Qu, X.; Wang, W.; Lv, S.; Jia, D. Thermoelectric properties and electronic structure of Al-doped ZnO. Solid State Commun. 2011, 151, 332–336. [Google Scholar] [CrossRef]
- Koresh, I.; Amouyal, Y. Effects of microstructure evolution on transport properties of thermoelectric nickel-doped zinc oxide. J. Eur. Ceram. Soc. 2017, 37, 3541–3550. [Google Scholar] [CrossRef]
- Liu, W.; Hu, J.; Zhang, S.; Deng, M.; Han, C.-G.; Liu, Y. New trends, strategies and opportunities in thermoelectric materials: A perspective. Mater. Today Phys. 2017, 1, 50–60. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.; Kim, S. Thermoelectric characteristics of glass fibers coated with ZnO and Al-doped ZnO. Mater. Res. Bull. 2017, 96, 246–249. [Google Scholar] [CrossRef]
- Kedia, S.K.; Singh, A.; Chaudhary, S. Design, development, and testing of a thermopower measurement system by studying the electron transport properties on indium and nitrogen co-doped sputtered ZnO films. Measurement 2018, 117, 49–56. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Y.; Lu, X.; Zhu, H.; Han, X.; Chen, G.; Ren, Z. Routes for high-performance thermoelectric materials. Mater. Today 2018, 21, 974–988. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, L.; Li, C.; Qi, N.; Chen, Z.; Su, X.; Tang, X. Significant Enhancement in the Thermoelectric Performance of Aluminum-Doped ZnO Tuned by Pore Structure. ACS Appl. Mater. Interfaces 2020, 12, 51669–51678. [Google Scholar] [CrossRef]
- Wu, N. Development and Processing of p-Type Oxide Thermoelectric Materials. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2014. [Google Scholar]
- Tritt, T.M.; Subramanian, M. Thermoelectric Materials, Phenomena, and Applications: A Bird’ s Eye View. MRS Bull. 2006, 31, 188–198. [Google Scholar] [CrossRef]
- Markov, M.; Rezaei, S.E.; Sadeghi, S.N.; Esfarjani, K.; Zebarjadi, M. Thermoelectric properties of semimetals. Phys. Rev. Mater. 2019, 3, 095401. [Google Scholar] [CrossRef]
- Kim, H.J.; Skuza, J.R.; Park, Y.; King, G.C.; Choi, S.H.; Nagavalli, A. System to Measure Thermal Conductivity and Seebeck Coefficient for Thermoelectrics. NasaTM 2012, 217791, LF99-15831. [Google Scholar]
- Yamaguchi, H.; Chonan, Y.; Oda, M.; Komiyama, T.; Aoyama, T.; Sugiyama, S. Thermoelectric properties of ZnO ceramics Co-doped with Al and transition metals. J. Electron. Mater. 2011, 40, 723–727. [Google Scholar] [CrossRef]
- Colder, H.; Guilmeau, E.; Harnois, C.; Marinel, S.; Retoux, R.; Savary, E. Preparation of Ni-doped ZnO ceramics for thermoelectric applications. J. Eur. Ceram. Soc. 2011, 31, 2957–2963. [Google Scholar] [CrossRef]
- Brintha, S.R.; Ajitha, M. Synthesis and characterization of ZnO nanoparticles via aqueous solution, sol-gel and hydrothermal methods. J. Appl. Chem. 2015, 8, 66–72. [Google Scholar] [CrossRef]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol-Gel Synthesis of Zinc Oxide (ZnO) Nanoparticles: Study of Structural and Optical Properties. J. Sci. Islamic Repub. Iran 2015, 26, 281–285. [Google Scholar]
- Wu, Z.-H.; Xie, H.-Q.; Zhai, Y.-B. Preparation and Thermoelectric Properties of Co-Doped ZnO Synthesized by Sol–Gel. J. Nanosci. Nanotechnol. 2015, 15, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Seong, J.K.; Kwon, Y.; Nahm, S.; Cho, W.S. Influence of SnO2addition on the thermoelectric properties of Zn1-xSnxO (0.01 ≤ x ≤ 0.05). Mater. Res. Bull. 2008, 43, 54–61. [Google Scholar] [CrossRef]
- Kumar, M.; Sahu, S.S. Zinc Oxide Nanostructures Synthesized by Oxidization of Zinc. Master’s Thesis, National Institute of Technology Rourkela, Rourkela, India, 2010. [Google Scholar]
- Ilican, S.; Gorgun, K.; Aksoy, S.; Caglar, Y.; Caglar, M. Fabrication of p-Si/n-ZnO: Al heterojunction diode and determination of electrical parameters. J. Mol. Struct. 2018, 1156, 675–683. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, N.; Salah, N.; Alshahrie, A.; Koumoto, K. Microwave Irradiation to Produce High Performance Thermoelectric Material Based on Al Doped ZnO Nanostructures. Crystals 2020, 10, 610. [Google Scholar] [CrossRef]
- Patel, N. Characterization of Electrical Performance of Aluminum-Doped Zinc Oxide Pellets. DePaul Discov. 2014, 3, 6. [Google Scholar]
- Feng, Q.; Shi, X.; Xing, Y.; Li, T.; Li, F.; Pan, D.; Liang, H. Thermoelectric microgenerators using a single large-scale Sb doped ZnO microwires. J. Alloy. Compd. 2018, 739, 298–304. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 978-3-527-40813-9. [Google Scholar]
- Park, K.; Seong, J.K.; Kim, G.H. NiO added Zn1-xNixO (0 ≤ x ≤ 0.05) for thermoelectric power generation. J. Alloy. Compd. 2009, 473, 423–427. [Google Scholar] [CrossRef]
- Janotti, A.; Walle, C.G.V.D. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc OxideNanomaterials: Reactants, Process Parametersand Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Saini, M.; Singh, M.; Chhillar, N.; Dehiya, B.S.; Kishor, K.; Alharthi, F.A.; Al-Zaqri, N.; Alghamdi, A.A. Micro-Plasma Assisted Synthesis of ZnO Nanosheets for theEfficient Removal of Cr6+ from the Aqueous Solution. Crystals 2021, 11, 2. [Google Scholar] [CrossRef]
- Han, L. High Temperature Thermoelectric Properties of ZnO Based Materials. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2014. [Google Scholar]
- Zhang, L.; Tosho, T.; Okinaka, N.; Akiyama, T. Thermoelectric Properties of Solution Combustion Synthesized Al-Doped ZnO. Mater. Trans. 2008, 49, 2868–2874. [Google Scholar] [CrossRef]
- Sayari, A.; El Mir, L. Structural and optical characterization of Ni and Al co-doped ZnO nanopowders synthesized via the sol-gel process. Kona Powder Part. J. 2015, 32, 154–162. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohtaki, M.; Eguchi, K.; Arai, H. Thermoelectric properties of Al-doped ZnO as a promising oxide material for high- temperature thermoelectric conversion. J. Mater. Chem. 1997, 7, 85–90. [Google Scholar] [CrossRef]
- Virtudazo, R.V.R.; Srinivasan, B.; Guo, Q.; Wu, R.; Takei, T.; Shimasaki, Y.; Wada, H.; Kuroda, K.; Bernik, S.; Mori, T. Improvement in the thermoelectric properties of porous networked Al-doped ZnO nanostructured materials synthesized via an alternative interfacial reaction and low-pressure SPS processing. Inorg. Chem. Front. 2020, 7, 4118–4132. [Google Scholar] [CrossRef]
- Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of (Zn1-xAlx)O. J. Appl. Phys. 1996, 79, 1816–1818. [Google Scholar] [CrossRef]
- Prasad, K.S.; Rao, A.; Bhardwaj, R.; Johri, K.K.; Chang, C.; Kuo, Y. Spark plasma sintering technique: An alternative method to enhance ZT values of Sb doped Cu2SnSe3. J. Mater. Sci. Mater. Electron. 2018, 29, 13200–13208. [Google Scholar] [CrossRef]
- Hedge, G.S.; Prabhu, A.N.; Huang, R.Y.; Kuo, Y.K. Reduction in thermal conductivity and electrical resistivity of indium and tellurium co-doped bismuth selenide thermoelectric system. J. Mater. Sci. Mater. Electron. 2020, 31, 19511–19525. [Google Scholar] [CrossRef]
- Neeli, G.; Behara, D.K.; Kumar, M.K. State of the Art Review on Thermoelectric Materials. Int. J. Sci. Res. 2016, 5, 1833–1844. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Muthusamy, O.; Singh, S.; Hirata, K.; Kuga, K.; Harish, S.K.; Shimomura, M.; Adachi, M.; Yamamoto, Y.; Matsunami, M.; Takeuchi, T. Synergetic Enhancement of the Power Factor and Suppression of Lattice Thermal Conductivity via Electronic Structure Modification and Nanostructuring on a Ni- and B-Codoped p-Type Si–Ge Alloy for Thermoelectric Application. ACS Appl. Electron. Mater. 2021, 3, 5621–5631. [Google Scholar] [CrossRef]
- Kavita; Gupta, V.; Ranjeet. Structural and morphological properties of nanostructured Bi2Te3 with Mn-doping for thermoelectric applications. Mater. Today Proc. 2021, 54, 820–826. [Google Scholar] [CrossRef]
- Li, D.H.; He, S.F.; Chen, J.; Jiang, C.Y.; Yang, C. Solid-state Chemical Reaction Synthesis and Characterization of Lanthanum Tartrate Nanocrystallites under Ultrasonication Spectra. IOP Conf. Ser. Mater. Sci. Eng. 2017, 242, 012023. [Google Scholar] [CrossRef]
- Yahya, N. Carbon and Oxide Nanostructures: Synthesis, Characterisation and Applications; Springer Link: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-14673-2. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Zhang, D.-B.; Li, H.-Z.; Zhang, B.-P.; Liang, D.; Xia, M. Hybrid-structured ZnO thermoelectric materials with high carrier mobility and reduced thermal conductivity. RSC Adv. 2017, 7, 10855–10864. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohtaki, M.; Eguchi, K.; Arai, H. Thermoelectric properties of ZnO Doped with The Group 13 Elements. In Proceedings of the 16th International Conference on Thermoelectrics, Dresden, Germany, 26–29 August 1997; pp. 240–243. [Google Scholar] [CrossRef]
- Cai, K.F.; Müller, E.; Drašar, C.; Mrotzek, A. Preparation and thermoelectric properties of Al-doped ZnO ceramics. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2003, 104, 45–48. [Google Scholar] [CrossRef]
- Callaway, J. Model for Lattice Thermal Conductivity at Low Temperatures. Phys. Rev. 1959, 113, 1046–1051. [Google Scholar] [CrossRef]
- Bui, C.T.; Sow, C.H.; Li, B.; Thong, J.T.L. Diameter-Dependent Thermal Transport in Individual ZnO Nanowires and its Correlation with Surface Coating and Defects. Small 2012, 8, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Kinemuchi, Y.; Nakano, H.; Mikami, M.; Kobayashi, K.; Watari, K.; Hotta, Y. Enhanced boundary-scattering of electrons and phonons in nanograined zinc oxide. J. Appl. Phys. 2010, 108, 053721. [Google Scholar] [CrossRef]
- Luu, S.D.N.; Duong, T.A.; Phan, T.B. Effect of dopants and nanostructuring on the thermoelectric properties of ZnO materials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 023001. [Google Scholar] [CrossRef]
- Wu, X.; Lee, J.; Varshney, V.; Wohlwend, J.L.; Roy, A.K.; Luo, T. Thermal Conductivity of Wurtzite Zinc-Oxide from First-Principles Lattice Dynamics—A Comparative Study with Gallium Nitride. Sci. Rep. 2016, 6, 22504. [Google Scholar] [CrossRef] [PubMed]
- Finn, P.A.; Asker, C.; Wan, K.; Bilotti, E.; Fenwick, O.; Nielsen, C.B. Thermoelectric Materials: Current Status and Future Challenges. Front. Electron. Mater. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Prasad, R.; Bhame, S.D. Review on texturization effects in thermoelectric oxides. Mater. Renew. Sustain. Energy. 2020, 9, 3. [Google Scholar] [CrossRef]
- Han, C.; Li, Z.; Dou, S. Recent progress in thermoelectric materials. Chin. Sci. Bull. 2014, 59, 2073–2091. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Mahmoudian, M.R.; Darroudi, M.; Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 2013, 24, 618–624. [Google Scholar] [CrossRef]
- Takashiri, M.; Miyazaki, K.; Tanaka, S.; Kurosaki, J.; Nagai, D.; Tsukamoto, H. Effect of grain size on thermoelectric properties of n -type nanocrystalline bismuth-telluride based thin films. J. Appl. Phys. 2008, 104, 084302. [Google Scholar] [CrossRef]
- Liang, X. Structure and Thermoelectric Properties of ZnO Based Materials Structure and Thermoelectric Properties of ZnO. Ph.D. Thesis, Havard University, Cambridge, MA, USA, 2013. [Google Scholar]
- Zhang, X.; Zhao, L.-D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, L.; Wang, C.; Wang, Y. Theoretical and experimental investigations of the thermoelectric properties of Al-, Bi- and Sn-doped ZnO. Mater. Sci. Semicond. Proces. 2017, 66, 247–252. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B.; Ye, D.; Liu, Y.; Li, S. Enhanced Al/Ni co-doping and power factor in textured ZnO thermoelectric ceramics prepared by hydrothermal synthesis and spark plasma sintering. J. Alloys Compd. 2016, 656, 784–792. [Google Scholar] [CrossRef]
- Matiullah; Wang, C.L.; Su, W.B.; Zaman, A.; Ullah, I.; Zhai, J.Z.; Liu, D.K. Effects of sintering atmospheres on thermoelectric properties, phase, microstructure and lattice parameters c/a ratio of Al, Ga dual doped ZnO ceramics sintered at high temperature. J. Mater. Sci. Mater. Electron. 2018, 29, 9555–9563. [Google Scholar] [CrossRef]
- Han, L.; Nong, N.V.; Hung, L.T.; Holgate, T.; Pryds, N.; Ohtaki, M.; Linderotha, S. The influence of alpha- and gamma-Al2O3 phases on the thermoelectric properties of Al-doped ZnO. J. Alloys Compd. 2013, 555, 291–296. [Google Scholar] [CrossRef]
- Ohtaki, M.; Araki, K.; Yamamoto, K. High thermoelectric performance of dually doped ZnO ceramics. J. Electron. Mater. 2009, 38, 1234–1238. [Google Scholar] [CrossRef]
- Wang, M.; Lee, K.E.; Hahn, S.H.; Kim, E.J.; Kim, S.; Chung, J.S.; Shin, E.W.; Park, C. Optical and photoluminescent properties of sol-gel Al-doped ZnO thin films. Mater. Lett. 2007, 61, 1118–1121. [Google Scholar] [CrossRef]
- Maciá-Barber, E. Thermoelectric Materials Advances and Applications; Jenny Stanford Publishing: United Square, Singapore, 2015. [Google Scholar]
- Jood, P.; Peleckis, G.; Wang, X.; Dou, S.X. Effect of gallium doping and ball milling process on the thermoelectric performance of n-type ZnO. J. Mater. Res. 2012, 27, 2278–2285. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Choi, S.; Park, J. Studies of thermoelectric transport properties of atomic layer deposited gallium-doped ZnO. Ceram. Int. 2017, 43, 7784–7788. [Google Scholar] [CrossRef]
| Composition/Synthesis Method | Thermoelectric Properties | Ref |
|---|---|---|
| (Zn0.99Al0.01)O/Solution combustion | ZT = 0.05, S = ~−148 μV/K, σ = ~21,000 S/m, PF = ~48,000 W/mK2, κ = 8 W/mK at 590 °C | [51] |
| ZnO/RF plasma powder | ZT = ~0.007, S = ~−386 μV/K, σ = ~369 S/m, PF = ~0.55 × 10−4 W/mK2, κ = 8 W/mK at 777 °C | [21] |
| (Zn0.99Al0.01)O/RF plasma powder | ZT = 0.04, S = −68 μV/K, σ = ~86,505 S/m, PF = ~4 × 10−4 W/mK2, κ = 10.6 W/mK at 777 °C | |
| (Zn0.98Al0.02)O/Solid-state reaction | ZT = 0.3, S = −180 μV/K, σ = 40,000 S/m, κ = 5.4 W/mK at 1000 °C | [55] |
| ZnO/Nylon-lined ball mill | ZT = 0.028, S = −318 μV/K, σ = ~2037 S/m, PF = ~2.06 × 10−4 W/mK2, κ = 8 W/mK at 800 °C | [53,66] |
| (Zn0.98Al0.02)O/Nylon-lined ball mill | ZT = 0.3, S = −182 μV/K, σ = ~39,971 S/m, PF = ~13.24 × 10−4 W/mK2, κ = 5 W/mK at 1000 °C | |
| (Zn0.98Al0.02)O/Solution method | ZT = 0.34, S = ~−148 μV/K, σ = ~29,000 S/m, κ = ~2 W/mK at 800 °C | [2] |
| (Zn0.98Al0.02)O/Forced hydrolysis method | ZT = ~0.3, S = ~−205 μV/K, σ = ~18,798 S/m, κ = ~3.2 W/mK at 950 °C | [50] |
| ZnO/Solid-state reaction | ZT = ~0.009, S = ~−107 μV/K, σ = ~2500 S/m, κ = ~3.1 W/mK at 700 °C | [80] |
| (Zn0.98Al0.02)O/Solid-state reaction | ZT = ~0.031, S = ~−133 μV/K, σ = 5400 S/m, κ = ~3 W/mK at 700 °C | |
| (Zn0.975Al0.025)O/microwave synthesis | ZT = 0.44, S = ~−300 μV/K, σ = ~10,000 S/m κ = ~2 W/mK at 727 °C | [16] |
| ZnO/Sol-gel method | ZT = ~0.007, S = −240 μV/K, σ = ~1563 S/m, κ = ~9.8 W/mK at 500 °C | [67] |
| (Zn0.97Al0.03)O/Sol-gel method | ZT = ~0.02, S = ~−81 μV/K, σ = ~37,500 S/m, κ = ~9.5 W/mK at 500 °C | |
| ZnO/Chemical co-deposition method | ZT = ~0.009, S = ~−250 μV/K, σ = ~1333 S/m, κ = ~8.3 W/mK at 600 °C | [22] |
| (Zn0.97Al0.03)O/Chemical co-deposition method | ZT = 0.15, S = ~−240 μV/K, σ = ~15,000 S/m, κ = ~5 W/mK at 600 °C |
| Composition/Synthesis Method | Thermoelectric Properties | Ref |
|---|---|---|
| ZnO/Nylon-lined ball mill | ZT = ~0.076, S = ~−430 μV/K, σ = ~1622 S/m, PF = ~3 × 10−4 W/mK2, κ = 5 W/mK at 1000 °C | [66] |
| (Zn0.98Ga0.02)O/Nylon-lined ball mill | ZT = ~0.13, S = ~−180 μV/K, σ = ~16,600 S/m, PF = ~5.38 × 10−4 W/mK2, κ = 5 W/mK at 1000 °C | |
| (Zn0.995Ga0.005)O/high energy wet milling | ZT = ~0.0022, S = ~−133 μV/K, σ = ~10,989 S/m, κ = 26.9 W/mK at 27 °C | [87] |
| ZnO/Wet chemistry gel combustion method | ZT = ~0.0022, S = ~−312.5 μV/K, σ = ~197 S/m, κ = ~9.4 W/mK at 800 °C | [78] |
| (Zn0.98Ga0.02)O/Wet chemistry gel combustion method | ZT = ~0.026, S = ~−562.5 μV/K, σ = ~268 S/m, κ = ~3.5 W/mK at 800 °C | |
| (Zn0.99Ga0.01)O/Atomic layer deposition | S = 60 μV/K, σ = ~180,832 S/m, PF = ~6.6 × 10−4 W/mK2 | [88] |
| Composition/Synthesis Method | Thermoelectric Properties | Ref |
|---|---|---|
| ZnO/Precipitation | ZT = 0.002, S = ~−517 μV/K, σ = ~50 S/m, κ = 8 W/mK at 700 °C | [23] |
| (Zn0.97Ni0.03)O/Precipitation | ZT = 0.02, S = ~−306 μV/K, σ = ~1790 S/m, κ = 8 W/mK at 700 °C | |
| ZnO/Tape casting method | PF = 0.62 × 10−4 W/mK2, S = ~−203 μV/K, σ = ~1500 S/m at 800 °C | [46] |
| (Zn0.97Ni0.03)O/Tape casting method | PF = 17.6 × 10−4 W/mK2, S = −503 μV/K, σ = 6970 S/m at 800 °C | |
| ZnO/Liquid route synthesis | ZT = 0.0034, S = ~−310 μV/K, σ = ~250 S/m, PF = ~0.24 × 10−4 W/mK2, κ = 7 W/mK at 727 °C | [34] |
| (Zn0.97Ni0.03)O/Liquid route synthesis | ZT = 0.09, S = ~−420 μV/K, σ = ~3401 S/m, PF = 6 × 10−4 W/mK2, κ = 6.5 W/mK at 727 °C |
| Composition/Synthesis Method | Thermoelectric Properties | Ref |
|---|---|---|
| ZnO/Solid-state reaction | ZT = ~0.009, S = ~−107 μV/K, σ = ~2500 S/m, κ = ~3.1 W/mK at 700 °C | [80] |
| (Zn0.98Bi0.02)O/Solid-state reaction | ZT = 0.006, S = −533 μV/K, σ = ~230 S/m, κ = 10.5 W/mK at 700 °C |
| Composition/Synthesis Method | Thermoelectric Properties | Ref |
|---|---|---|
| (Zn0.99Sn0.01)O/Solid-state reaction | PF = 1.25 × 10−3 W/mK2, S = ~−160 μV/K, σ = ~15 S/m at 800 °C | [38] |
| ZnO/Solid-state reaction | ZT = ~0.009, S = ~−107 μV/K, σ = ~2500 S/m, κ = ~3.1 W/mK at 700 °C | [80] |
| (Zn0.98Sn0.02)O/Solid-state reaction | ZT = 0.012, S = −93 μV/K, σ = 900 S/m, κ = 7 W/mK at 700 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, S.; Sudin, I.; Al-Naib, U.M.B.; Omar, M.F. Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials. Crystals 2022, 12, 1076. https://doi.org/10.3390/cryst12081076
Sulaiman S, Sudin I, Al-Naib UMB, Omar MF. Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials. Crystals. 2022; 12(8):1076. https://doi.org/10.3390/cryst12081076
Chicago/Turabian StyleSulaiman, Suraya, Izman Sudin, Uday M. Basheer Al-Naib, and Muhammad Firdaus Omar. 2022. "Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials" Crystals 12, no. 8: 1076. https://doi.org/10.3390/cryst12081076
APA StyleSulaiman, S., Sudin, I., Al-Naib, U. M. B., & Omar, M. F. (2022). Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials. Crystals, 12(8), 1076. https://doi.org/10.3390/cryst12081076






