Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
2.2. Glass Characterization
3. Results and Discussion
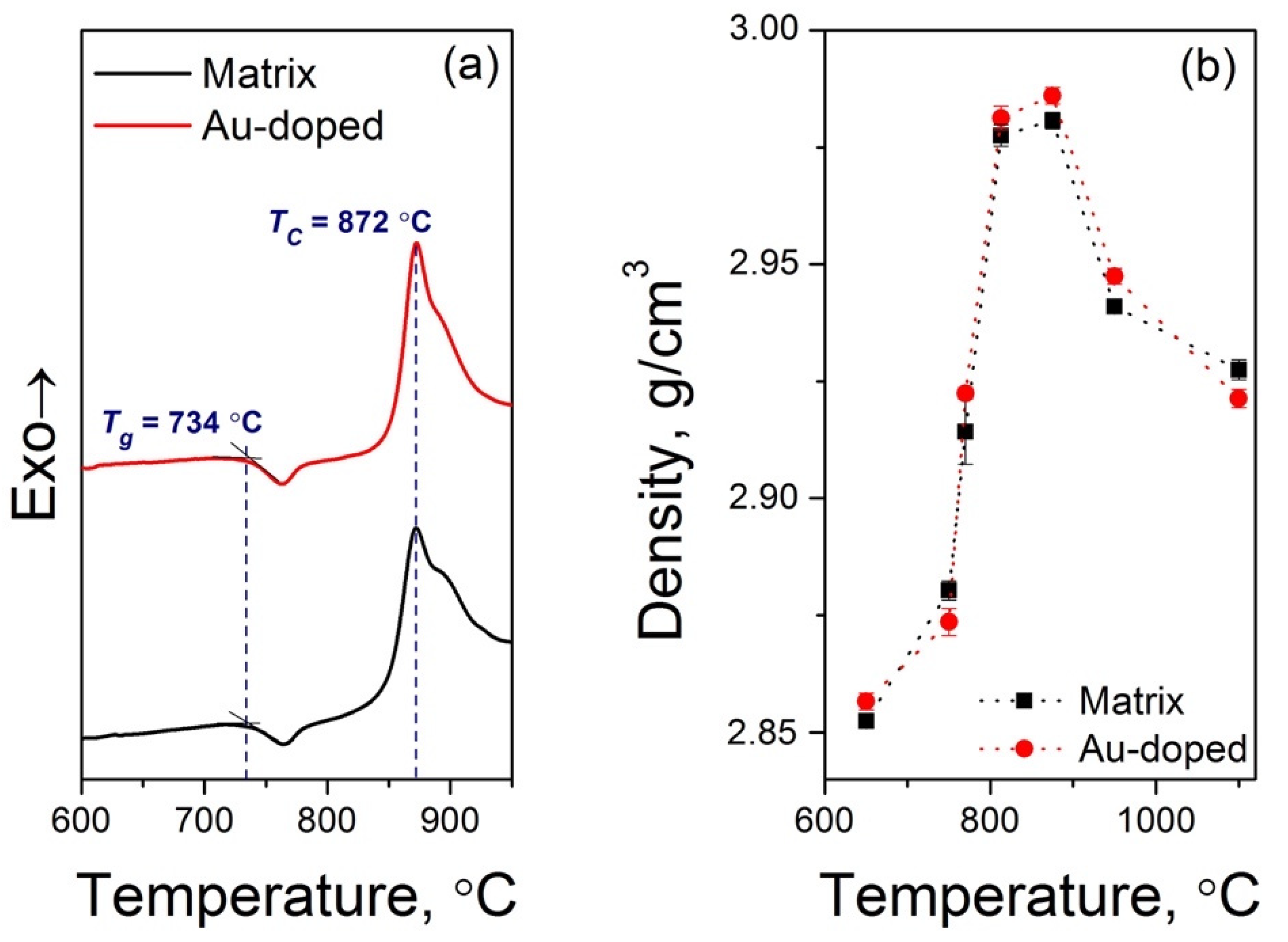
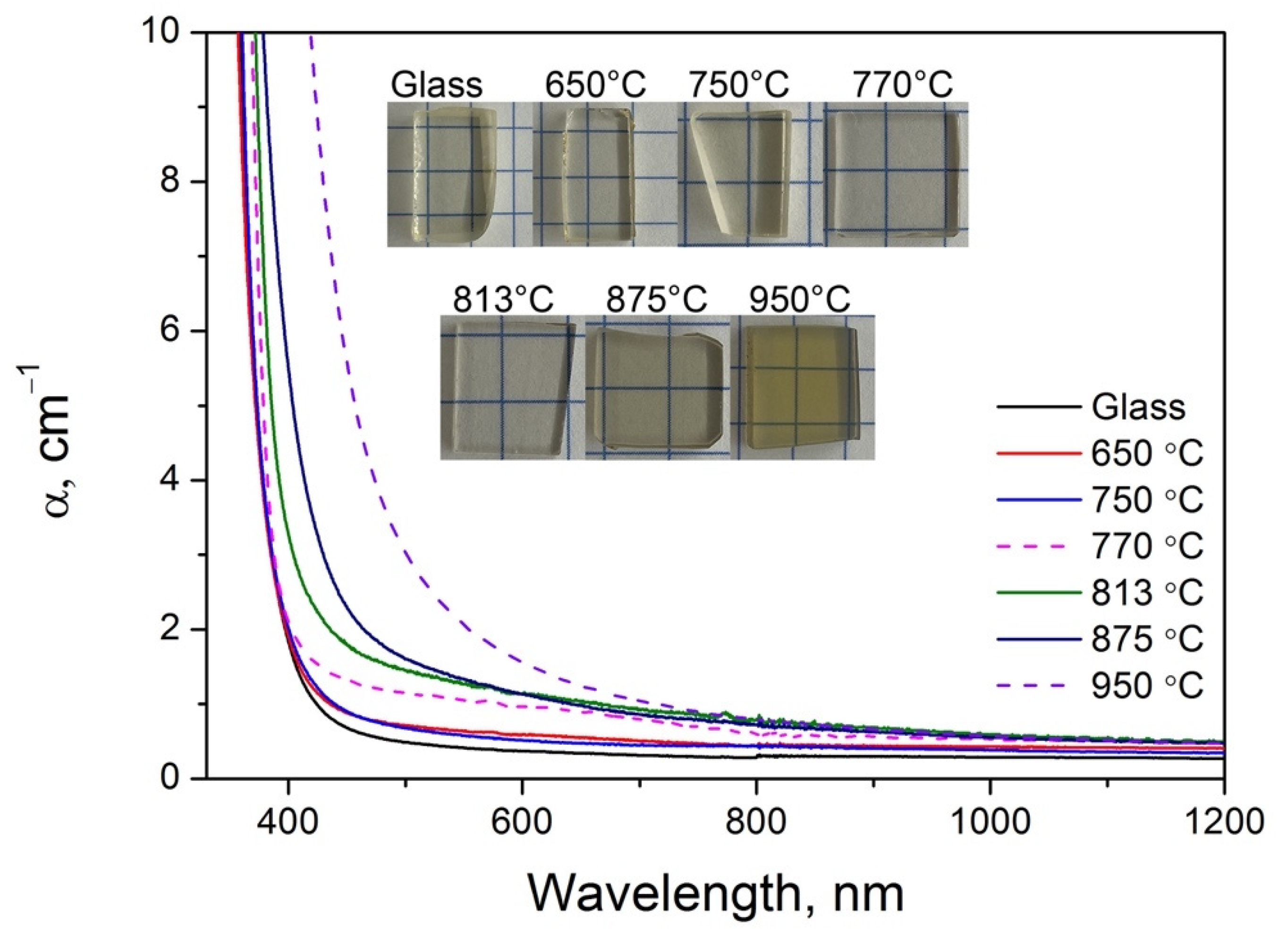
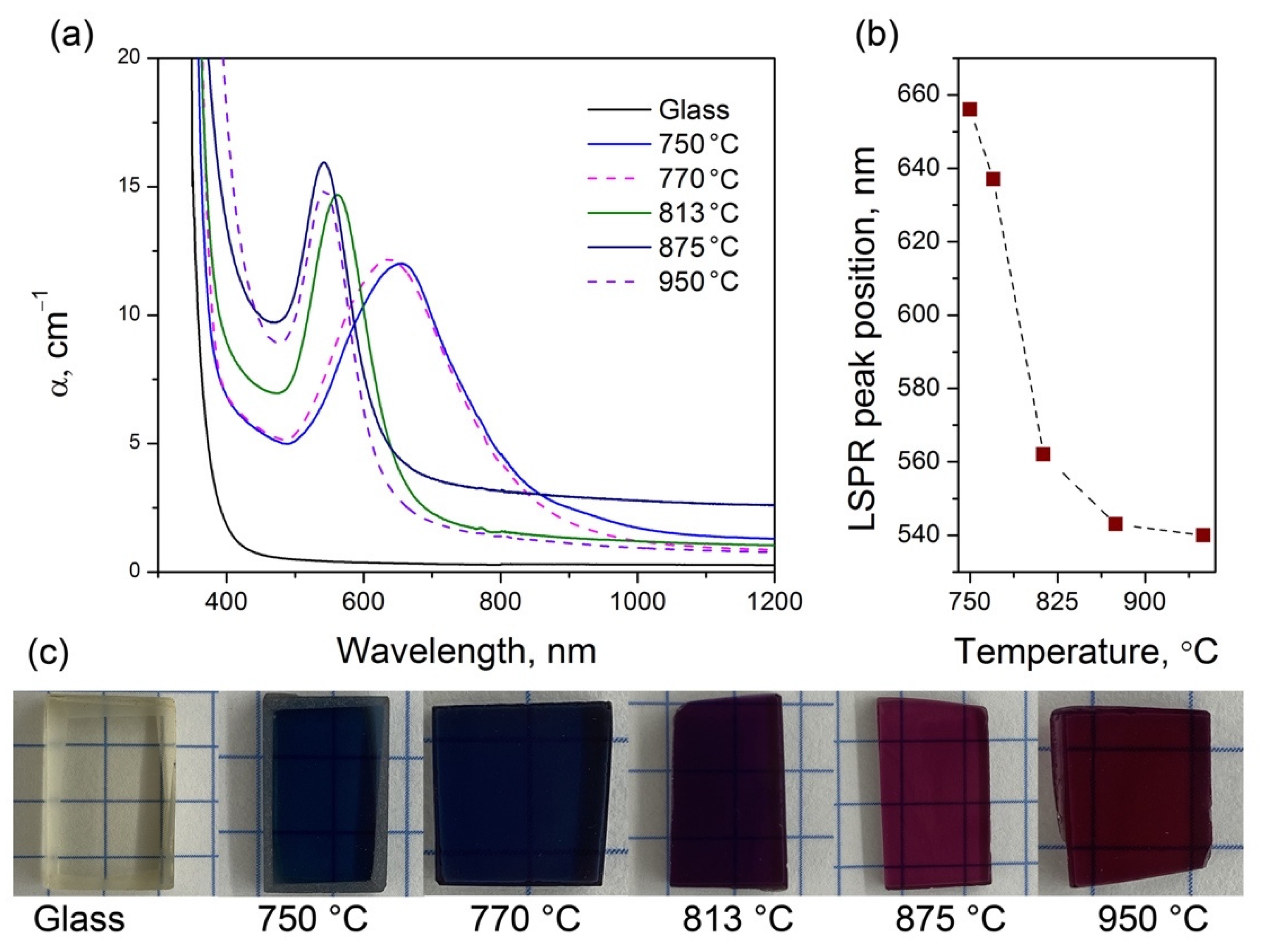
3.1. Physicochemical Properties
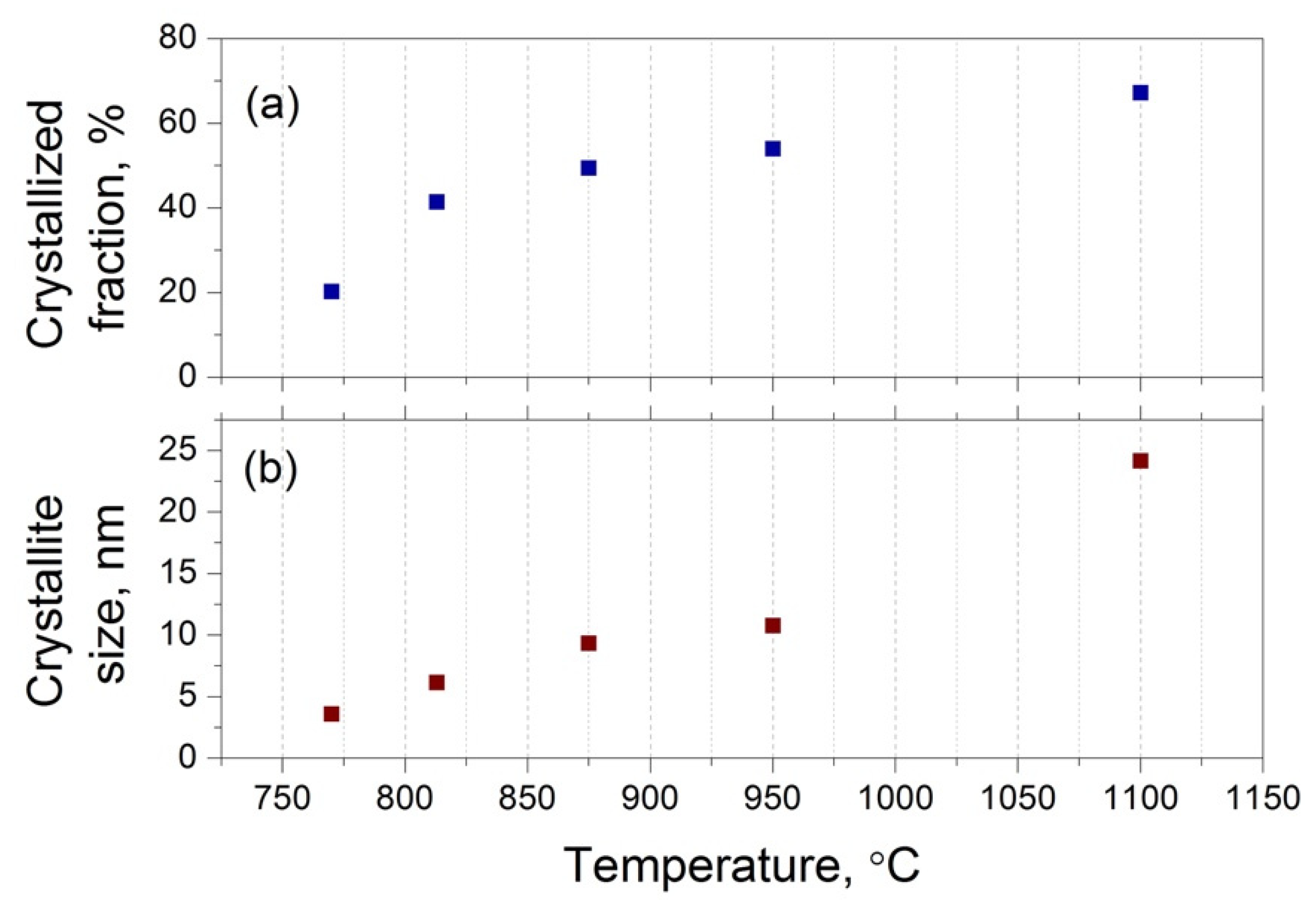
3.2. XRD Studies
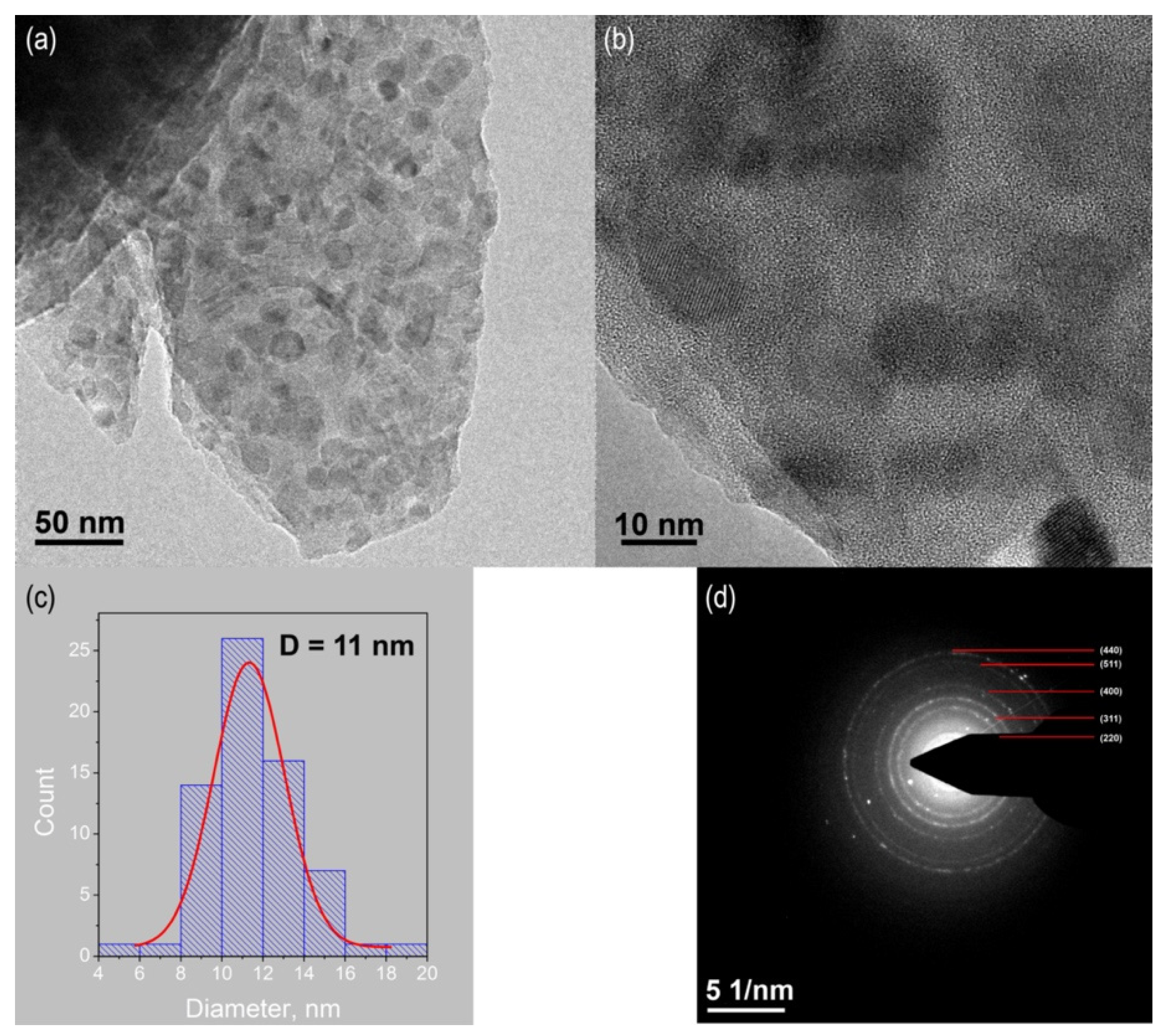
3.3. TEM/HRTEM Studies
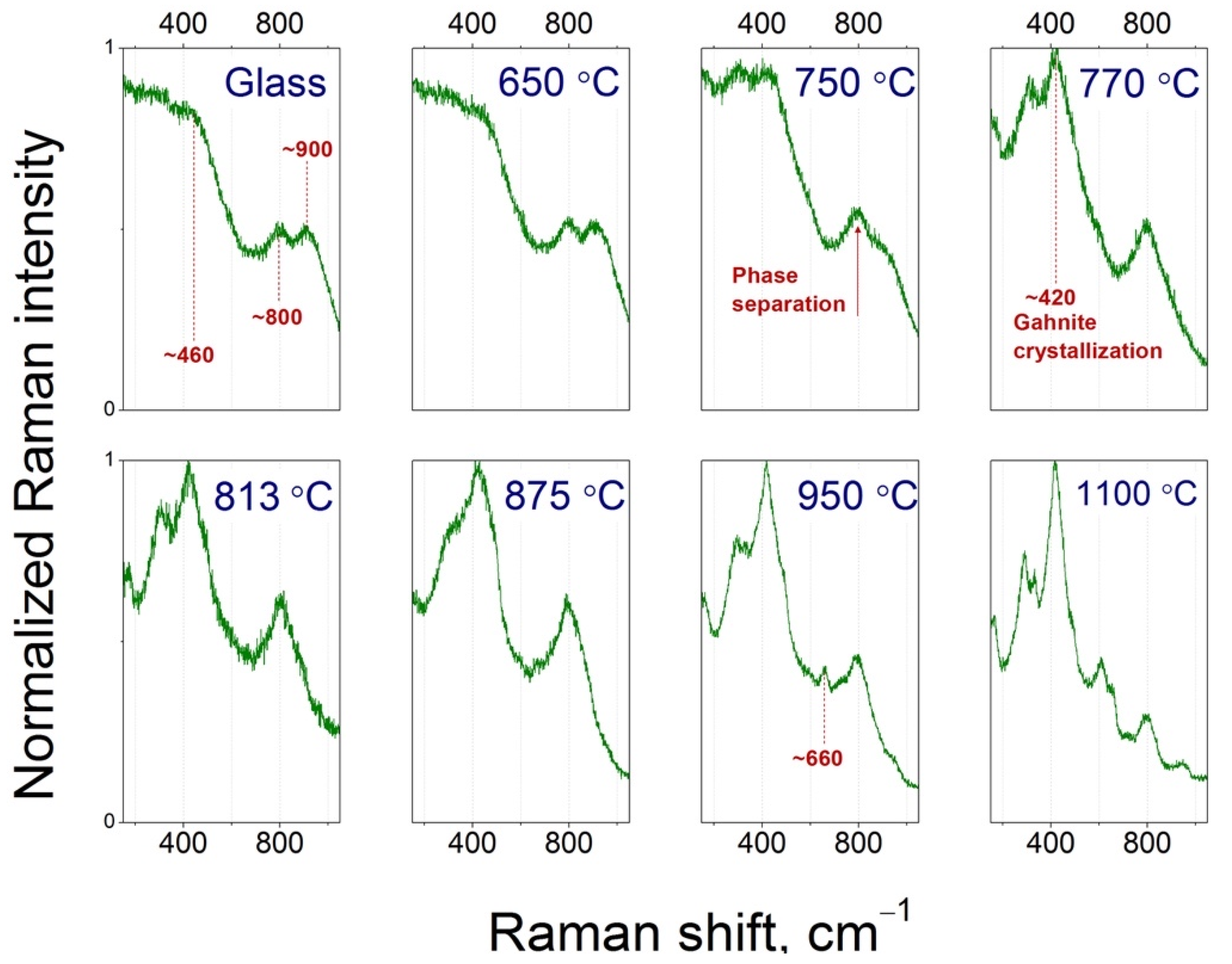
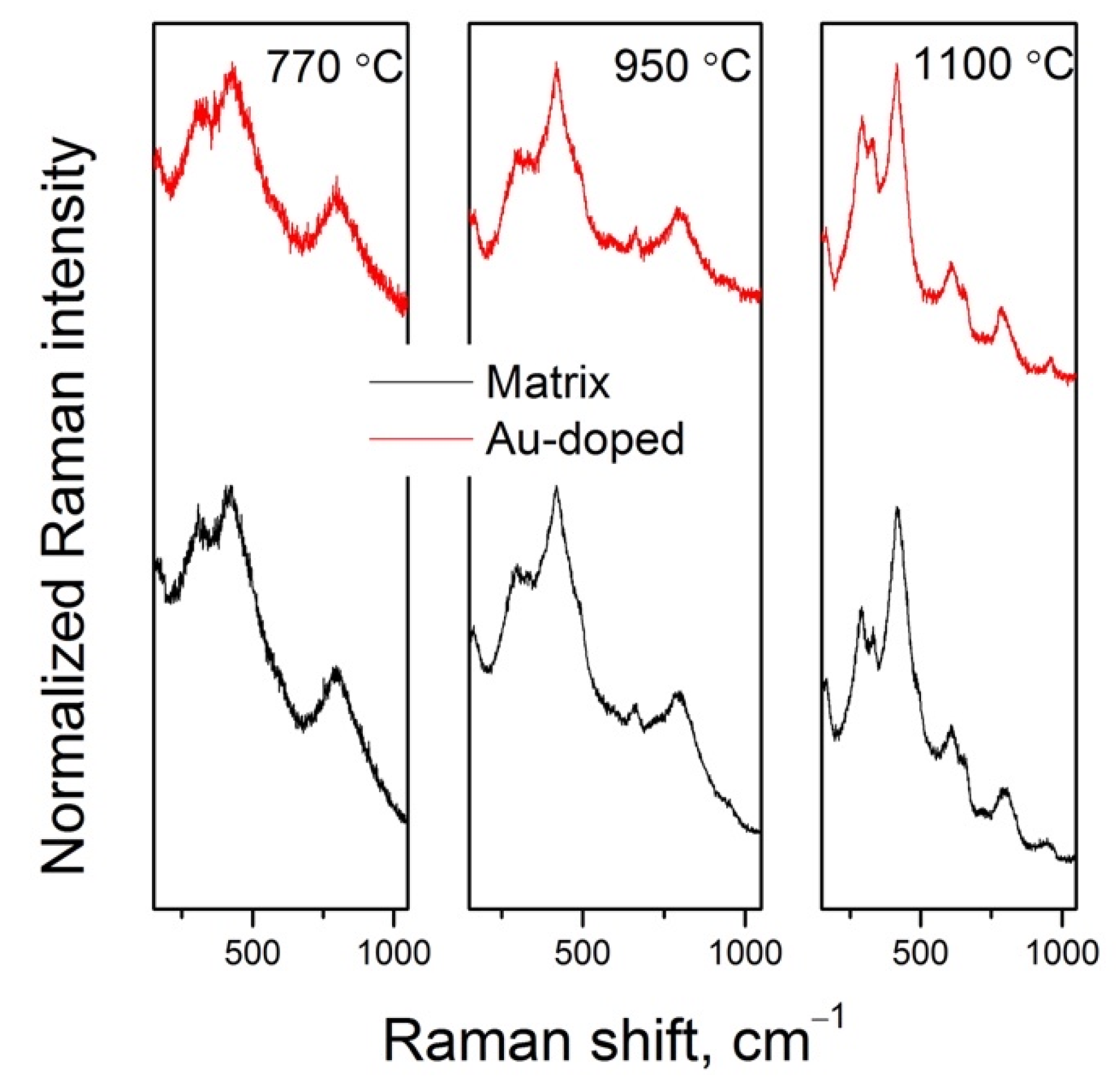
3.4. Raman Spectra
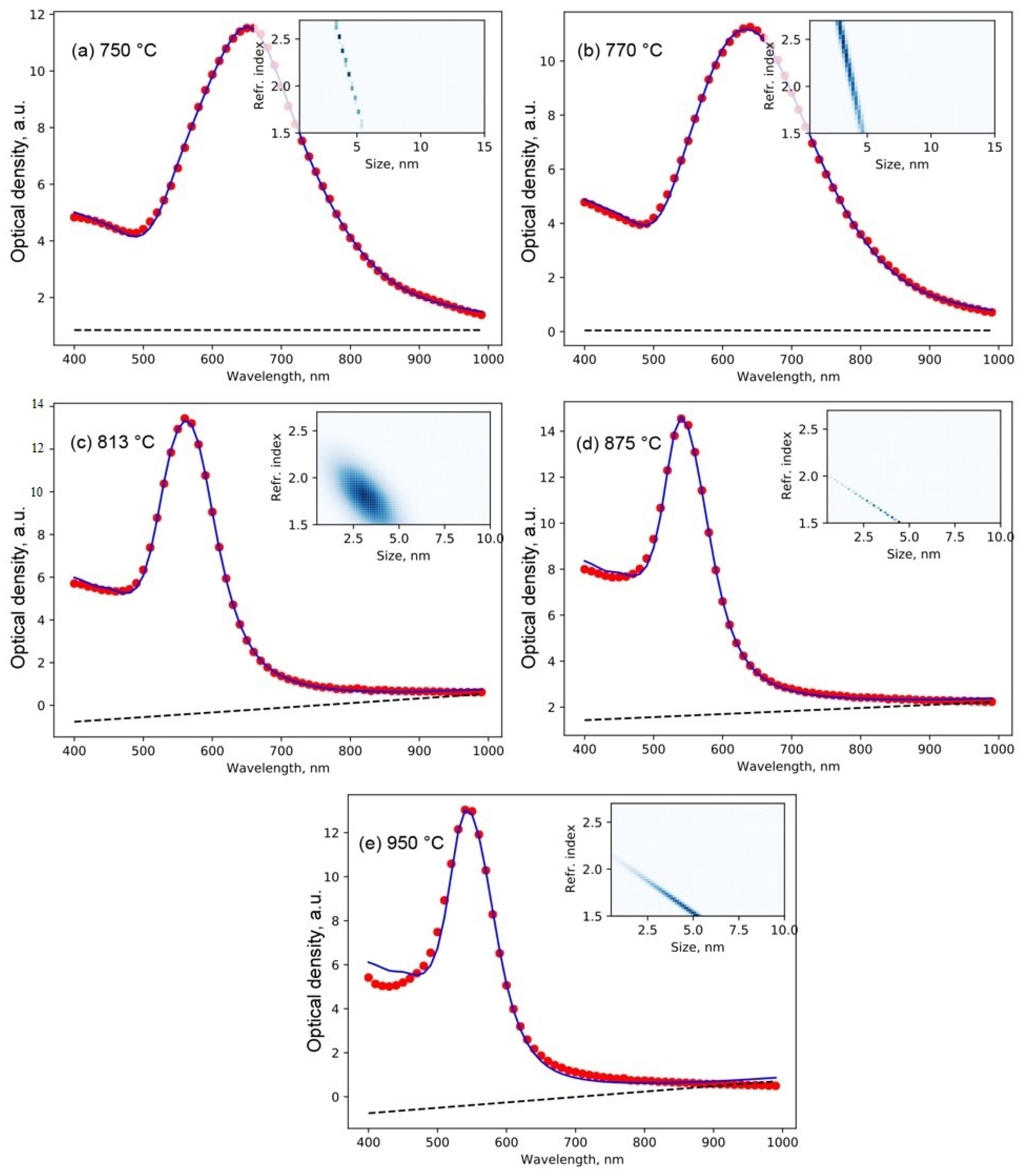
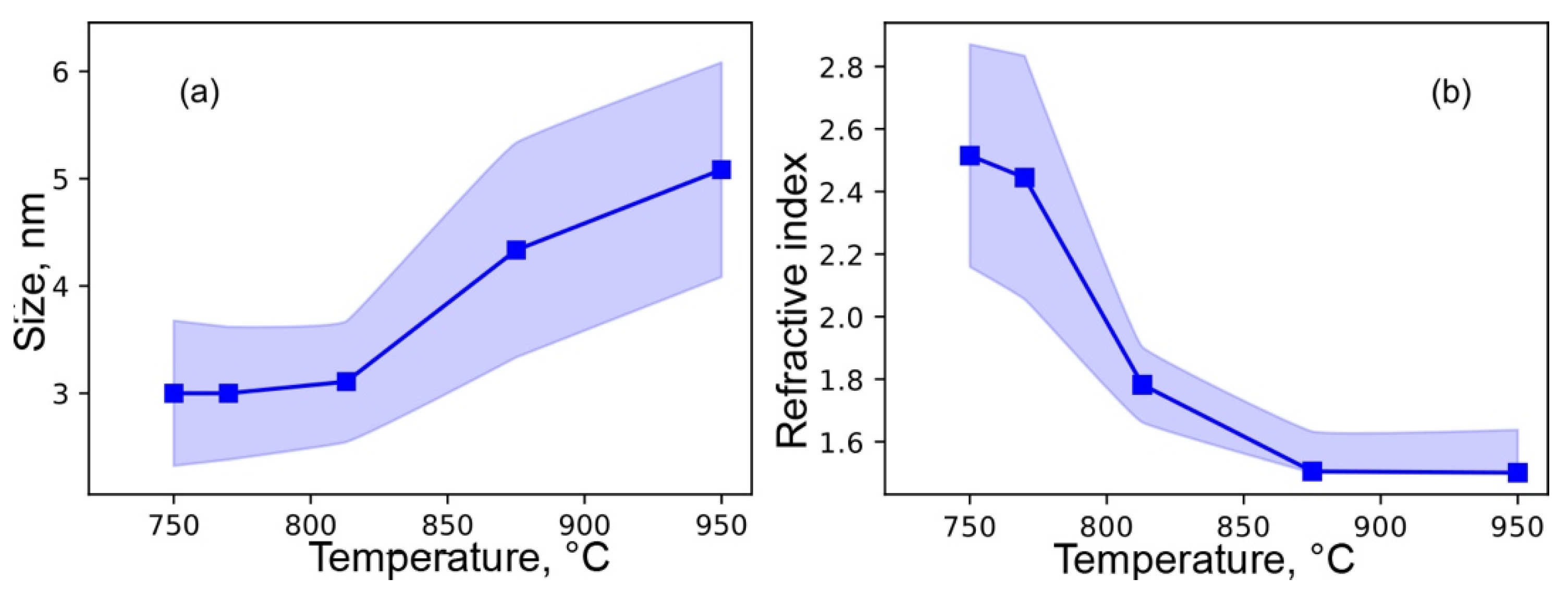
3.5. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soukoulis, C.M.; Wegener, M. Past Achievements and Future Challenges in the Development of Three-Dimensional Photonic Metamaterials. Nat. Photonics 2011, 5, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, T.; Honma, T. Optical Active Nano-Glass-Ceramics. Int. J. Appl. Glass Sci. 2013, 4, 125–135. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials. Materials 2018, 11, 212. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhou, J.; Zhou, S.; Yue, Y.; Qiu, J. Transparent Glass-Ceramics Functionalized by Dispersed Crystals. Prog. Mater. Sci. 2018, 97, 38–96. [Google Scholar] [CrossRef]
- Veber, A.; Lu, Z.; Vermillac, M.; Pigeonneau, F.; Blanc, W.; Petit, L. Nano-Structured Optical Fibers Made of Glass-Ceramics, and Phase Separated and Metallic Particle-Containing Glasses. Fibers 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Golubev, N.V.; Ignat’eva, E.S.; Mashinsky, V.M.; Kozlova, E.O.; Sigaev, V.N.; Monguzzi, A.; Paleari, A.; Lorenzi, R. Pre-Crystallization Heat Treatment and Infrared Luminescence Enhancement in Ni2+-Doped Transparent Glass-Ceramics. J. Non-Cryst. Solids 2019, 515, 42–49. [Google Scholar] [CrossRef]
- Lorenzi, R.; Golubev, N.V.; Ignat’eva, E.S.; Sigaev, V.N.; Ferrara, C.; Acciarri, M.; Vanacore, G.M.; Paleari, A. Defect-Assisted Photocatalytic Activity of Glass-Embedded Gallium Oxide Nanocrystals. J. Colloid Interface Sci. 2022, 608, 2830–2838. [Google Scholar] [CrossRef]
- Venkateswaran, C.; Sreemoolanadhan, H.; Vaish, R. Lithium Aluminosilicate (LAS) Glass-Ceramics: A Review of Recent Progress. Int. Mater. Rev. 2021, 1–38. [Google Scholar] [CrossRef]
- Shakhgil’dyan, G.Y.; Savinkov, V.I.; Shakhgil’dyan, A.Y.; Alekseev, R.O.; Naumov, A.S.; Lopatkina, E.V.; Sigaev, V.N. Effect of Sitallization Conditions on the Hardness of Transparent Sitalls in the System ZnO–MgO–Al2O3–SiO2. Glass Ceram. 2021, 77, 426–428. [Google Scholar] [CrossRef]
- Sant’Ana Gallo, L.; Célarié, F.; Bettini, J.; Rodrigues, A.C.M.; Rouxel, T.; Zanotto, E.D. Fracture Toughness and Hardness of Transparent MgO–Al2O3–SiO2 Glass-Ceramics. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Gan, L.; Zhang, J.; Wang, P. Structure/Property Nonlinear Variation Induced by Gamma Ray Irradiation of Boroaluminosilicate Transparent Glass Ceramic Containing Gahnite Nanocrystallite. J. Non-Cryst. Solids 2022, 578, 121346. [Google Scholar] [CrossRef]
- Chakraborty, P. Metal Nanoclusters in Glasses as Non-Linear Photonic Materials. J. Mater. Sci. 1998, 33, 2235–2249. [Google Scholar] [CrossRef]
- Savinkov, V.I.; Shakhgil’Dyan, G.Y.; Paleari, A.; Sigaev, V.N. Synthesis of Optically Uniform Glasses Containing Gold Nanoparticles: Spectral and Nonlinear Optical Properties. Glass Ceram. (Engl. Transl. Steklo Keram.) 2013, 70, 143–148. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Lipatiev, A.S.; Fedotov, S.S.; Vetchinnikov, M.P.; Lotarev, S.V.; Sigaev, V.N. Microstructure and Optical Properties of Tracks with Precipitated Silver Nanoparticles and Clusters Inscribed by the Laser Irradiation in Phosphate Glass. Ceram. Int. 2021, 47, 14320–14329. [Google Scholar] [CrossRef]
- Shakhgil’dyan, G.Y.; Lipat’ev, A.S.; Vetchinnikov, M.P.; Popova, V.V.; Lotarev, S.V.; Sigaev, V.N. Femtosecond Laser Modification of Zinc-Phosphate Glasses with High Silver Oxide Content. Glass Ceram. (Engl. Transl. Steklo Keram.) 2017, 73, 420–422. [Google Scholar] [CrossRef]
- Lipat’ev, A.; Shakhgil’dyan, G.; Lipat’eva, T.; Lotarev, S.; Fedotov, S.; Vetchinnikov, M.; Ignat’eva, E.; Golubev, N.; Sigaev, V.; Kazanskii, P. Formation of Luminescent and Birefringent Microregions in Phosphate Glass Containing Silver. Glass Ceram. 2016, 73, 277–282. [Google Scholar] [CrossRef]
- Drexhage, K.H. Influence of a Dielectric Interface on Fluorescence Decay Time. J. Lumin. 1970, 1–2, 693–701. [Google Scholar] [CrossRef]
- Shakhgil’dyan, G.Y.; Ziyatdinova, M.Z.; Kovgar, V.V.; Lotarev, S.V.; Sigaev, V.N.; Prusova, I.V. Effect of Gold Nanoparticles on the Spectral Luminescence Properties of Eu3+-Doped Phosphate Glass. Glass Ceram. (Engl. Transl. Steklo I Keram.) 2019, 76, 121–125. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Ziyatdinova, M.Z.; Vetchinnikov, M.P.; Lotarev, S.V.; Savinkov, V.I.; Presnyakova, N.N.; Lopatina, E.V.; Vilkovisky, G.A.; Sigaev, V.N. Thermally-Induced Precipitation of Gold Nanoparticles in Phosphate Glass: Effect on the Optical Properties of Er3+ Ions. J. Non-Cryst. Solids 2020, 550, 120408. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Smith, S. Gold-Assisted Enhancement of the Luminescence of Mn2+ Ions Induced by Silicon in Phosphate Glass. Phys. Lett. A 2020, 384, 126776. [Google Scholar] [CrossRef]
- Danmallam, I.M.; Ghoshal, S.K.; Ariffin, R.; Bulus, I. Europium Luminescence in Silver and Gold Nanoparticles Co-Embedded Phosphate Glasses: Judd-Ofelt Calculation. Opt. Mater. 2020, 105, 109889. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated Definition of Glass-Ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Sakamoto, A.; Yamamoto, S. Glass-Ceramics: Engineering Principles and Applications. Int. J. Appl. Glass Sci. 2010, 1, 237–247. [Google Scholar] [CrossRef]
- Stookey, S.D. Catalyzed Crystallization of Glass in Theory and Practice. Ind. Eng. Chem. 1959, 51, 805–808. [Google Scholar] [CrossRef]
- Garai, M.; Sasmal, N.; Molla, A.R.; Tarafder, A.; Karmakar, B. Effects of In-Situ Generated Coinage Nanometals on Crystallization and Microstructure of Fluorophlogopite Mica Containing Glass‒Ceramics. J. Mater. Sci. Technol. 2015, 31, 110–119. [Google Scholar] [CrossRef]
- Garai, M.; Murthy, T.S.R.C.; Karmakar, B. Microstructural Characterization and Wear Properties of Silver and Gold Nanoparticle Doped K-Mg-Al-Si-O-F Glass-Ceramics. Ceram. Int. 2018, 44, 22308–22317. [Google Scholar] [CrossRef]
- Garai, M.; Reka, A.A.; Karmakar, B.; Molla, A.R. Microstructure–Mechanical Properties of Ag0/Au0 Doped K–Mg–Al–Si–O–F Glass-Ceramics. RSC Adv. 2021, 11, 11415–11424. [Google Scholar] [CrossRef]
- Kochetkov, D.A.; Nikonorov, N.V.; Sycheva, G.A.; Tsekhomskii, V.A. The Effect of Gold Nanoparticles on Crystallization Processes in Photostructured Lithium-Silicate Glass. Glass Phys. Chem. 2013, 39, 351–357. [Google Scholar] [CrossRef]
- Thieme, C.; Kracker, M.; Patzig, C.; Thieme, K.; Rüssel, C.; Höche, T. The Acceleration of Crystal Growth of Gold-Doped Glasses within the System BaO/SrO/ZnO/SiO2. J. Eur. Ceram. Soc. 2019, 39, 554–562. [Google Scholar] [CrossRef]
- Garai, M.; Molla, A.R.; Reka, A.A.; Karmakar, B. Wide Thermal Expansion in Ag0/Au0 Nanoparticle Doped SiO2-MgO-Al2O3-B2O3-K2O-MgF2 Glass-Ceramics. Mater. Today Proc. 2021, 50, 134–138. [Google Scholar] [CrossRef]
- Kracker, M.; Thieme, C.; Thieme, K.; Patzig, C.; Berthold, L.; Höche, T.; Rüssel, C. Redox Effects and Formation of Gold Nanoparticles for the Nucleation of Low Thermal Expansion Phases from BaO/SrO/ZnO/SiO2 Glasses. RSC Adv. 2018, 8, 6267–6277. [Google Scholar] [CrossRef] [Green Version]
- Shakhgildyan, G.; Avakyan, L.; Ziyatdinova, M.; Atroshchenko, G.; Presnyakova, N.; Vetchinnikov, M.; Lipatiev, A.; Bugaev, L.; Sigaev, V. Tuning the Plasmon Resonance of Gold Nanoparticles in Phase-Separated Glass via the Local Refractive Index Change. J. Non-Cryst. Solids 2021, 566, 120893. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Basyrova, L.; Bukina, V.; Balabanov, S.; Belyaev, A.; Drobotenko, V.; Dymshits, O.; Alekseeva, I.; Tsenter, M.; Zapalova, S.; Khubetsov, A.; et al. Synthesis, Structure and Spectroscopy of Fe2+:MgAl2O4 Transparent Ceramics and Glass-Ceramics. J. Lumin. 2021, 236, 118090. [Google Scholar] [CrossRef]
- Loiko, P.A.; Dymshits, O.S.; Skoptsov, N.A.; Malyarevich, A.M.; Zhilin, A.A.; Alekseeva, I.P.; Tsenter, M.Y.; Bogdanov, K.V.; Mateos, X.; Yumashev, K.V. Crystallization and Nonlinear Optical Properties of Transparent Glass-Ceramics with Co:Mg(Al,Ga)2O4 Nanocrystals for Saturable Absorbers of Lasers at 1.6–1.7. Mm. J. Phys. Chem. Solids 2017, 103, 132–141. [Google Scholar] [CrossRef]
- Kurajica, S.; Šipušić, J.; Zupancic, M.; Brautović, I.; Albrecht, M. ZnO-Al2O3-SiO2 Glass Ceramics: Influence of Composition on Crystal Phases, Crystallite Size and Appearance. J. Non-Cryst. Solids 2020, 553, 120481. [Google Scholar] [CrossRef]
- Golubkov, V.V.; Dymshits, O.S.; Petrov, V.I.; Shashkin, A.V.; Tsenter, M.Y.; Zhilin, A.A.; Kang, U. Small-Angle X-Ray Scattering and Low-Frequency Raman Scattering Study of Liquid Phase Separation and Crystallization in Titania-Containing Glasses of the ZnO–Al2O3–SiO2 System. J. Non-Cryst. Solids 2005, 351, 711–721. [Google Scholar] [CrossRef]
- Loshmanov, A.A.; Sigaev, V.N.; Khodakovskaya, R.Y.; Pavlushkin, N.M.; Yamzin, I.I. Small-Angle Neutron Scattering on Silica Glasses Containing Titania. J. Appl. Crystallogr. 1974, 7, 207–210. [Google Scholar] [CrossRef]
- Dymshits, O.S.; Zhilin, A.A.; Petrov, V.I.; Tsenter, M.Y.; Chuvaeva, T.I.; Shashkin, A.V.; Golubkov, V.V.; Kang, U.; Lee, K.-H. A Raman Spectroscopic Study of Phase Transformations in Titanium-Containing Magnesium Aluminosilicate Glasses. Glass Phys. Chem. 2002, 28, 66–78. [Google Scholar] [CrossRef]
- Kreibig, U.; Genzel, L. Optical Absorption of Small Metallic Particles. Surf. Sci. 1985, 156, 678–700. [Google Scholar] [CrossRef]
- Tong, Y.L. The Multivariate Normal Distribution; Springer: New York, NY, USA, 1990; ISBN 978-1-4613-9657-4. [Google Scholar]
- Avakyan, L.A.; Heinz, M.; Skidanenko, A.V.; Yablunovski, K.A.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; Bugaev, L.A. Insight on Agglomerates of Gold Nanoparticles in Glass Based on Surface Plasmon Resonance Spectrum: Study by Multi-Spheres T-Matrix Method. J. Phys. Condens. Matter 2018, 30, 045901. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhgildyan, G.; Durymanov, V.; Ziyatdinova, M.; Atroshchenko, G.; Golubev, N.; Trifonov, A.; Chereuta, O.; Avakyan, L.; Bugaev, L.; Sigaev, V. Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System. Crystals 2022, 12, 287. https://doi.org/10.3390/cryst12020287
Shakhgildyan G, Durymanov V, Ziyatdinova M, Atroshchenko G, Golubev N, Trifonov A, Chereuta O, Avakyan L, Bugaev L, Sigaev V. Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System. Crystals. 2022; 12(2):287. https://doi.org/10.3390/cryst12020287
Chicago/Turabian StyleShakhgildyan, Georgiy, Veniamin Durymanov, Mariam Ziyatdinova, Grigoriy Atroshchenko, Nikita Golubev, Alexey Trifonov, Olga Chereuta, Leon Avakyan, Lusegen Bugaev, and Vladimir Sigaev. 2022. "Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System" Crystals 12, no. 2: 287. https://doi.org/10.3390/cryst12020287
APA StyleShakhgildyan, G., Durymanov, V., Ziyatdinova, M., Atroshchenko, G., Golubev, N., Trifonov, A., Chereuta, O., Avakyan, L., Bugaev, L., & Sigaev, V. (2022). Effect of Gold Nanoparticles on the Crystallization and Optical Properties of Glass in ZnO-MgO-Al2O3-SiO2 System. Crystals, 12(2), 287. https://doi.org/10.3390/cryst12020287






