A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Their Abbreviations
2.2. Reagents
2.3. Synthesis
2.4. Composition
2.5. Pb Sorption from the Simulated Model Solution
2.6. Pb Sorption from the Dust Leaching Solution (Model Solution)
2.7. Pb Sorption from the Dust Leaching Solution (Real Solution)
2.8. Raman Spectroscopy
2.9. Powder and Single-Crystal X-ray Diffraction
3. Results
3.1. Composition
3.2. Pb Sorption from the Simulated Model Solution
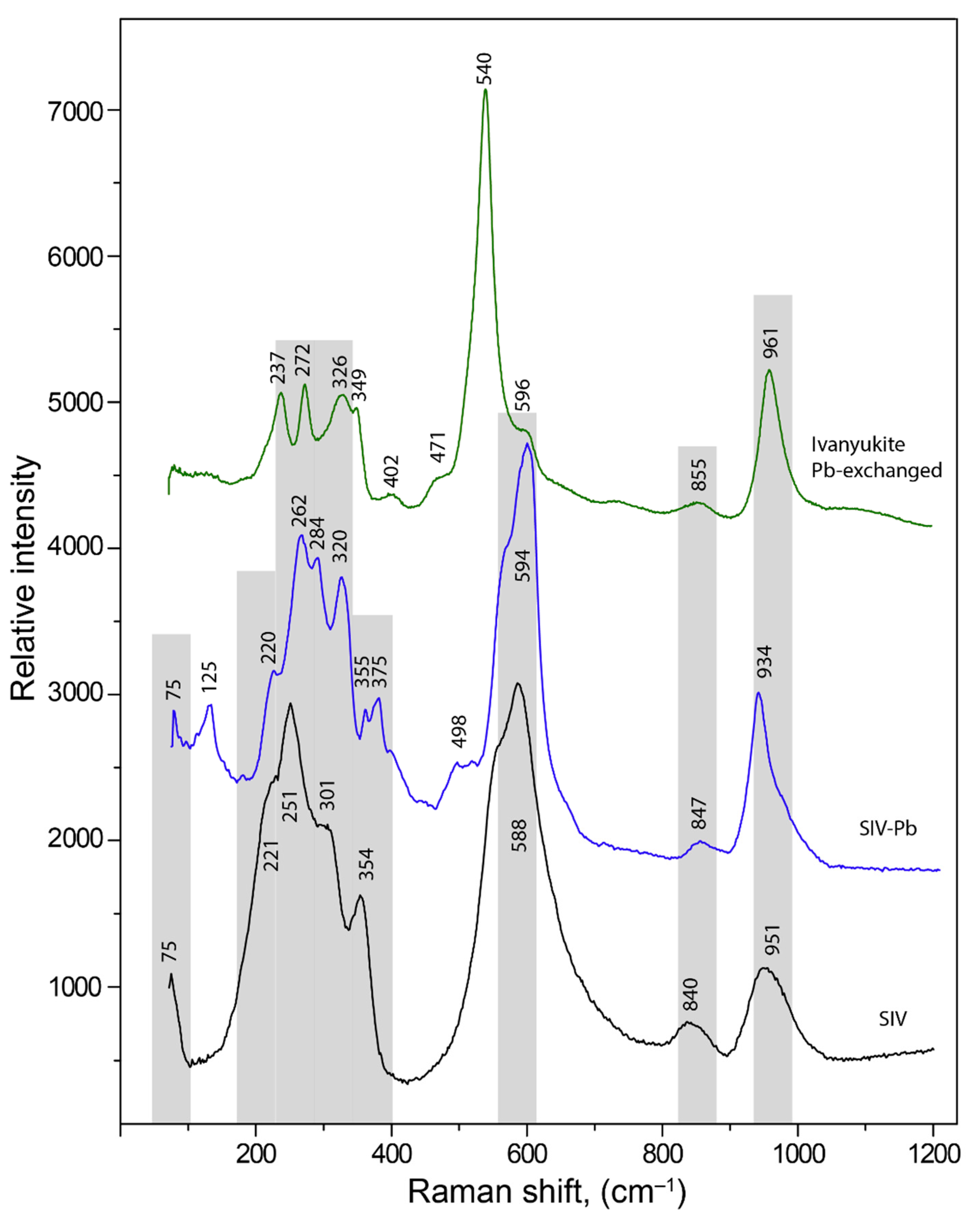
3.3. Raman Spectroscopy
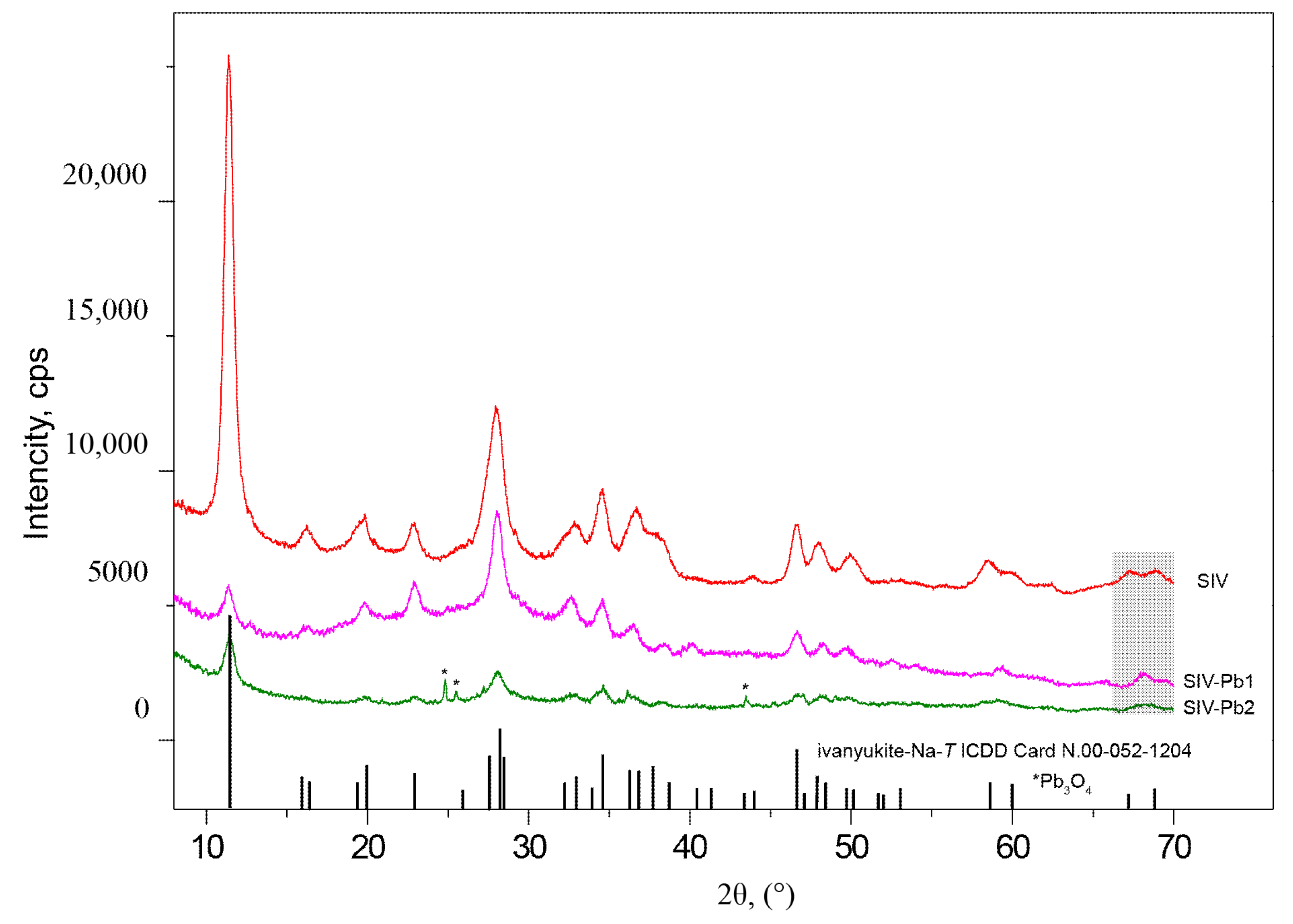
3.4. Powder Diffraction
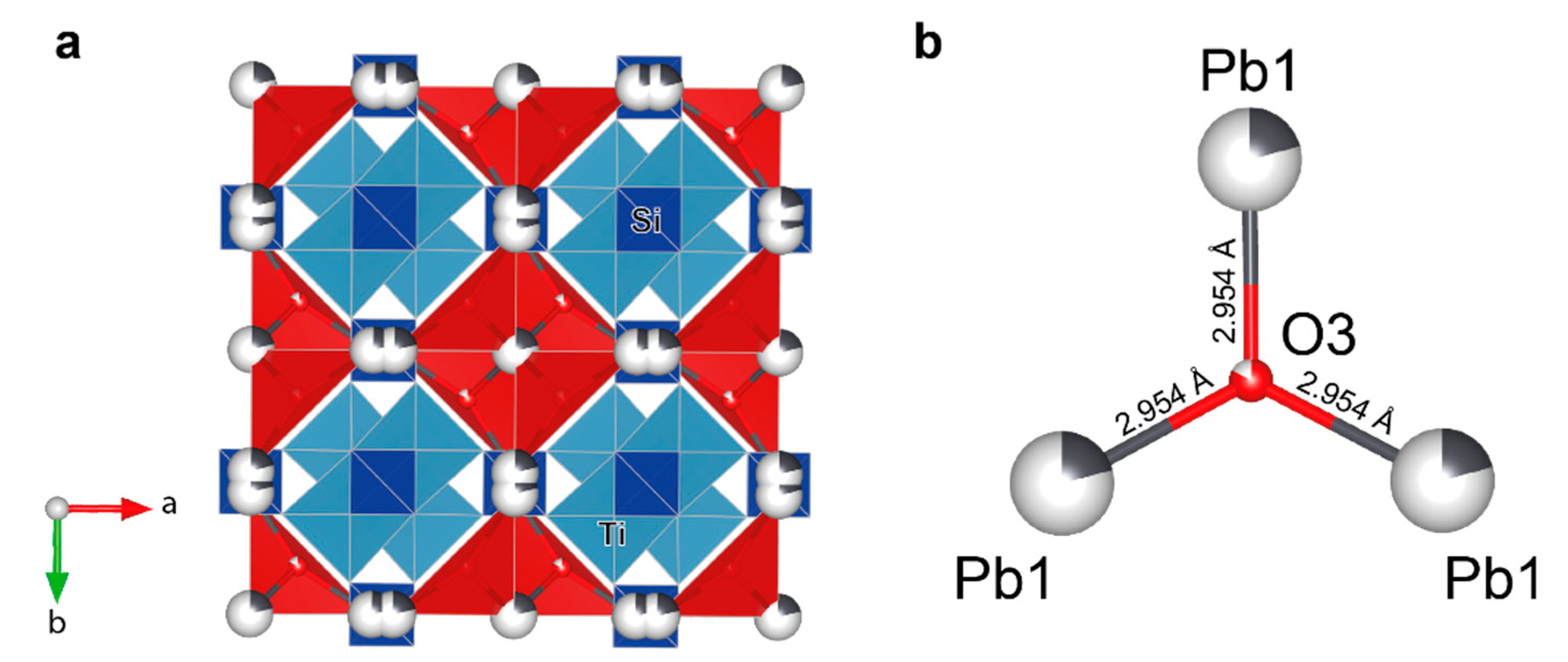
3.5. Single-Crystal XRD
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yakovenchuk, V.N.; Nikolaev, A.P.; Selivanova, E.A.; Pakhomovsky, Y.A.; Korchak, J.A.; Spiridonova, D.V.; Zalkind, O.A.; Krivovichev, S.V. Ivanyukite-Na-T, ivanyukite-Na-C, ivanyukite-K, and ivanyukite-Cu: New microporous titanosilicates from the Khibiny massif (Kola Peninsula, Russia) and crystal structure of ivanyukite-Na-T. Am. Mineral. 2009, 94, 1450–1458. [Google Scholar] [CrossRef]
- Popa, K.; Pavel, C.C.; Bilba, N.; Cecal, A. Purification of waste waters containing 60Co2+, 115mCd2+ and 203Hg2+ radioactive ions by ETS-4 titanosilicate. J. Radioanal. Nucl. Chem. 2006, 269, 155–160. [Google Scholar] [CrossRef]
- Kalashnikova, G.O.; Zhitova, E.S.; Selivanova, E.A.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Kasikov, A.G.; Drogobuzhskaya, S.V.; Elizarova, I.R.; Kiselev, Y.G.; et al. The new method for obtaining titanosilicate AM-4 and its decationated form: Crystal chemistry, properties and advanced areas of application. Microporous Mesoporous Mater. 2021, 313, 110787. [Google Scholar] [CrossRef]
- Cruciani, G.; De Luca, P.; Nastro, A.; Pattison, P. Rietveld refinement of the zorite structure of ETS-4 molecular sieves. Microporous Mesoporous Mater. 1998, 21, 143–153. [Google Scholar] [CrossRef]
- Anthony, R.G.; Dosch, R.G.; Gu, D.; Philip, C.V. Use of silicotitanates for removing cesium and strontium from defense waste. Ind. Eng. Chem. Res. 1994, 33, 2702–2705. [Google Scholar] [CrossRef]
- Men’shikov, Y.P.; Sokolova, E.V.; Egorov-Tismenko, Y.K.; Khomyakov, A.P.; Polezhaeva, L.I. Sitinakite, Na2KTi4Si2O13(OH)·4H2O—A new mineral. Zap. RMO 1992, 121, 94–99. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Kalashnikova, G.O.; Nikolaev, A.I.; Perovskiy, I.А.; Bazai, A.V.; Yakovenchuk, V.N.; Bocharov, V.N.; Kabanova, N.A.; Krivovichev, S.V. Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study. Minerals 2022, 12, 248. [Google Scholar] [CrossRef]
- Chapman, D.M.; Roe, A.L. Synthesis, characterization and crystal chemistry of microporous titanium-silicate materials. Zeolites 1990, 10, 730–737. [Google Scholar] [CrossRef]
- Dadachov, M.; Rocha, J.; Ferreira, A.; Lin, Z.; Anderson, M. Ab initio structure determination of layered sodium titanium silicate containing edge-sharing titanate chains (AM-4) Na3(Na,H)Ti2O2[Si2O6]2·2H2O. Chem. Commun. 1997, 3, 2371–2372. [Google Scholar] [CrossRef]
- Britvin, S.N.; Gerasimova, L.G.; Ivanyuk, G.Y.; Kalashnikova, G.O.; Krzhizhanovskaya, M.G.; Krivovivhev, S.V.; Mararitsa, V.F.; Nikolaev, A.I.; Oginova, O.A.; Panteleev, V.N.; et al. Application of titanium-containing sorbents for treating liquid radioactive waste with the subsequent conservation of radionuclides in Synroc-type titanate ceramics. Theor. Found. Chem. Eng. 2016, 50, 598–606. [Google Scholar] [CrossRef]
- Gerasimova, L.; Nikolaev, A.; Maslova, M.; Shchukina, E.; Samburov, G.; Yakovenchuk, V.; Ivanyuk, G. Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis. Minerals 2018, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Gerasimova, L.G.; Nikolaev, A.I.; Shchukina, E.S.; Maslova, M.V. Hydrothermal synthesis of framed titanosilicates with a structure of ivanyukite mineral. Дoклaды Aкaдeмuu нayк 2019, 487, 289–292. [Google Scholar] [CrossRef]
- Barcan, V.; Sylina, A. The appraisal of snow sampling for environmental pollution valuation. Water. Air. Soil Pollut. 1996, 89, 49–65. [Google Scholar] [CrossRef]
- Yakovlev, E.; Druzhinina, A.; Druzhinin, S.; Zykov, S.; Ivanchenko, N. Assessment of physical and chemical properties, health risk of trace metals and quality indices of surface waters of the rivers and lakes of the Kola Peninsula (Murmansk Region, North-West Russia). Environ. Geochem. Health 2021, 1–30. [Google Scholar] [CrossRef]
- Barcan, V.; Kovnatsky, E. Soil Surface Geochemical Anomaly Around the Copper-Nickel Metallurgical Smelter. Water. Air. Soil Pollut. 1998, 103, 197–218. [Google Scholar] [CrossRef]
- Koptsik, G.N.; Koptsik, S.V.; Smirnova, I.E.; Sinichkina, M.A. Remediation of Technogenic Barren Soils in the Kola Subarctic: Current State and Long-Term Dynamics. Eurasian Soil Sci. 2021, 54, 619–630. [Google Scholar] [CrossRef]
- Nikolaev, A.I.; Samburov, G.O.; Kalashnikova, G.O. Method for the Extraction of Silver from Pyrometallurgical Waste. RU Patent Application No. 2021124566A, 16 February 2022. [Google Scholar]
- Merlino, S.; Pasero, M.; Khomyakov, A.P. The crystal structure of lintisite, Na3LiTi2[Si2O6]2O2·2H2O, a new titanosilicate from Lovozero (USSR). Z. Krist. 1990, 193, 137–148. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Kalashnikova, G.O.; Shefer, K.I.; Mel’gunova, E.A.; Panchenko, V.N.; Nikolaev, A.I.; Gil, A. Effect of the acid activation on a layered titanosilicate AM-4: The fine-tuning of structural and physicochemical properties. Appl. Clay Sci. 2020, 186, 105445. [Google Scholar] [CrossRef]
- Gerasimova, L.G.; Shchukina, E.S.; Maslova, M.V.; Gladkikh, S.N.; Garaeva, G.R.; Kolobkova, V.M. Synthesis of rutile titanium dioxide from Russian raw materials. Polym. Sci. Ser. D 2017, 10, 23–27. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T. Raman spectroscopy of some complex arsenate minerals—implications for soil remediation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2797–2804. [Google Scholar] [CrossRef]
- Filippi, M. Oxidation of the arsenic-rich concentrate at the Přebuz abandoned mine (Erzgebirge Mts., CZ): Mineralogical evolution. Sci. Total Environ. 2004, 322, 271–282. [Google Scholar] [CrossRef]
- Filippi, M.; Doušová, B.; Machovič, V. Mineralogical speciation of arsenic in soils above the Mokrsko-west gold deposit, Czech Republic. Geoderma 2007, 139, 154–170. [Google Scholar] [CrossRef]
- Yakovenchuk, V.; Pakhomovsky, Y.; Panikorovskii, T.; Zolotarev, A.; Mikhailova, J.; Bocharov, V.; Krivovichev, S.; Ivanyuk, G. Chirvinskyite, (Na,Ca)13(Fe,Mn,□)2(Ti,Nb)2(Zr,Ti)3-(Si2O7)4(OH,O,F)12, a New Mineral with a Modular Wallpaper Structure, from the Khibiny Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Pakhomovsky, Y.A.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, J.A.; Krivovichev, S.V.; Bocharov, V.N.; Kalashnikov, A.O. Selivanovaite, NaTi3(Ti,Na,Fe,Mn)4[(Si2O7)2O4(OH,H2O)4]·nH2O, a new rock-forming mineral from the eudialyte-rich malignite of the Lovozero alkaline massif (Kola Peninsula, Russia). Eur. J. Mineral. 2018, 30, 525–535. [Google Scholar] [CrossRef]
- Krivovichev, S. Topology of Microporous Structures. Rev. Mineral. Geochem. 2005, 57, 17–68. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Yakovenchuk, V.N.; Yanicheva, N.Y.; Pakhomovsky, Y.A.; Shilovskikh, V.V.; Bocharov, V.N.; Krivovichev, S.V. Crystal chemistry of ivanyukite-group minerals, A3 − x H1 + x [Ti4O4 (SiO4)3](H2O)n (A = Na, K, Cu), (n = 6–9, x = 0–2): Crystal structures, ion-exchange, chemical evolution. Mineral. Mag. 2021, 85, 607–619. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in cation adsorption and cation exchange—A review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Rumsey, M.S.; Mills, S.J.; Spratt, J. Natropharmacoalumite, NaAl4[(OH)4(AsO4)3]·4H2O, a new mineral of the pharmacosiderite supergroup and the renaming of aluminopharmacosiderite to pharmacoalumite. Mineral. Mag. 2010, 74, 929–936. [Google Scholar] [CrossRef]
- Bedlivy, D.; Mereiter, K. Preisingerite, Bi3O(OH)(AsO4)2, a new species from San Juan Province, Argentina: Its description and crystal structure. Am. Mineral. 1982, 67, 833–840. [Google Scholar]
- Ridkosil, T.; Srein, V.; Fabry, J.; Hybler, J.; Maximov, B.A. Mrazekite, Bi2Cu3(OH)2O2(PO4)2. Can. Mineral. 1992, 30, 215–224. [Google Scholar]
- Siidra, O.I.; Zinyakhina, D.O.; Zadoya, A.I.; Krivovichev, S.V.; Turner, R.W. Synthesis and Modular Structural Architectures of Mineralogically Inspired Novel Complex Pb Oxyhalides. Inorg. Chem. 2013, 52, 12799–12805. [Google Scholar] [CrossRef]
- Voropanova, L.A.; Gagieva, Z.A.; Pukhova, V.P.; Vil’ner, N.A. Method of Extracting Lead Ions Pb2+ from Acidic Solutions. RU Patent Application No. 2393244C1, 27 June 2010. [Google Scholar]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, C.; Cui, W.; He, F.; Zhang, J.; Zhang, T.C.; He, C. Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem. Eng. J. 2020, 391, 123581. [Google Scholar] [CrossRef]
- Pavlovic, I.; Perez, M.; Barriga, C.; Ulibarri, M. Adsorption of Cu2+, Cd2+ and Pb2+ ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2,3-dimercaptosuccinate. Appl. Clay Sci. 2009, 43, 125–129. [Google Scholar] [CrossRef]

| Temperature/K | 293 (2) |
|---|---|
| Crystal system | cubic |
| Space group | P-43m |
| a = b = c/Å | 7.8037 (7) |
| α = β = γ/° | 90 |
| Volume/Å3 | 475.23 (13) |
| Z | 1 |
| ρcalcg/cm3 | 2.956 |
| μ/mm−1 | 13.003 |
| F (000) | 388.0 |
| Crystal size/mm3 | 0.12 × 0.12 × 0.12 |
| Radiation | Mo Kα (λ = 0.71073) |
| 2θ range for data collection/° | 7.384 to 52.998 |
| Index ranges | −6 ≤ h ≤ 9, −9 ≤ k ≤ 4, −9 ≤ l ≤ 9 |
| Reflections collected | 612 |
| Independent reflections | 200 [Rint = 0.0486, Rsigma = 0.0310] |
| Data/restraints/parameters | 200/0/23 |
| Goodness-of-fit on F2 | 1.116 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0497, wR2 = 0.1186 |
| Final R indexes [all data] | R1 = 0.0644, wR2 = 0.1257 |
| Largest diff. peak/hole/e Å−3 | 0.71/−0.64 |
| Flack parameter | 0.008(19) |
| Constituent | Pb-Exchanged SIV | Pb-Exchanged Natural Ivanyukite | |||
|---|---|---|---|---|---|
| SiO2 | 18.11 | 19.08 | 19.40 | 18.23 | 19.23 |
| TiO2 | 30.74 | 30.01 | 31.64 | 31.65 | 32.50 |
| Al2O3 | 0.45 | 0.23 | |||
| FeO | 0.22 | 0.31 | |||
| Nb2O5 | 1.64 | 1.68 | |||
| K2O | 0.44 | 0.32 | 1.85 | ||
| PbO | 38.60 | 38.40 | 36.03 | 37.59 | 34.87 |
| H2O * | 9.70 | 10.00 | 11.00 | 10.10 | 10.10 |
| Total | 98.04 | 97.81 | 100.15 | 99.43 | 98.69 |
| Si4+ | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Ti4+ | 3.83 | 3.55 | 3.68 | 3.92 | 3.81 |
| Al3+ | 0.09 | 0.04 | |||
| Fe2+ | 0.03 | 0.04 | |||
| Nb5+ | 0.12 | 0.12 | |||
| Sum O | 3.92 | 3.55 | 3.72 | 4.07 | 3.97 |
| K+ | 0.09 | 0.06 | 0.36 | ||
| Pb2+ | 1.72 | 1.63 | 1.50 | 1.67 | 1.46 |
| Sum A | 1.81 | 1.69 | 1.86 | 1.67 | 1.46 |
| OH− | 10.72 | 10.49 | 11.35 | 11.09 | 10.51 |
| C(Pb), g/L | q, mg/g | A, % | |
|---|---|---|---|
| Initial solution 1 | 0.39 | ||
| SIV | 0.0089 | 38.11 | 97.72 |
| SL3 | 0.31 | 8 | 20.51 |
| AM-4 | 0.0008 | 38.92 | 99.79 |
| Initial solution 2 | 0.88 | ||
| SIV | 0.004 | 87.6 | 99.55 |
| SL3 | 0.80 | 8 | 9.09 |
| AM-4 | 0.0009 | 87.91 | 99.90 |
| Initial solution 3 | 1.79 | ||
| SIV | 0.0026 | 178.74 | 99.85 |
| AM-4 | 0.0009 | 178.91 | 99.95 |
| Initial solution 4 | 1.78 | ||
| SIV | 0.97 | 403 | 45.28 |
| AM-4 | 1.73 | 26 | 2.92 |
| Initial solution 5 * | 0.16 | ||
| SIV | 0.016 | 14.4 | 90 |
| AM-4 | 0.017 | 14.3 | 89 |
| Initial solution 6 * | 23.70 | ||
| SIV | 0.11 | 2.4 | 99.5 |
| AM-4 | <0.03 | 2.4 | 99.9 |
| Initial solution 7 * | 56.23 | ||
| SIV | 55.04 | 0.1 | 2.1 |
| AM-4 | 55.68 | 0.1 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samburov, G.O.; Kalashnikova, G.O.; Panikorovskii, T.L.; Bocharov, V.N.; Kasikov, A.; Selivanova, E.; Bazai, A.V.; Bernadskaya, D.; Yakovenchuk, V.N.; Krivovichev, S.V. A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations. Crystals 2022, 12, 311. https://doi.org/10.3390/cryst12030311
Samburov GO, Kalashnikova GO, Panikorovskii TL, Bocharov VN, Kasikov A, Selivanova E, Bazai AV, Bernadskaya D, Yakovenchuk VN, Krivovichev SV. A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations. Crystals. 2022; 12(3):311. https://doi.org/10.3390/cryst12030311
Chicago/Turabian StyleSamburov, Gleb O., Galina O. Kalashnikova, Taras L. Panikorovskii, Vladimir N. Bocharov, Aleksandr Kasikov, Ekaterina Selivanova, Ayya V. Bazai, Daria Bernadskaya, Viktor N. Yakovenchuk, and Sergey V. Krivovichev. 2022. "A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations" Crystals 12, no. 3: 311. https://doi.org/10.3390/cryst12030311
APA StyleSamburov, G. O., Kalashnikova, G. O., Panikorovskii, T. L., Bocharov, V. N., Kasikov, A., Selivanova, E., Bazai, A. V., Bernadskaya, D., Yakovenchuk, V. N., & Krivovichev, S. V. (2022). A Synthetic Analog of the Mineral Ivanyukite: Sorption Behavior to Lead Cations. Crystals, 12(3), 311. https://doi.org/10.3390/cryst12030311









