Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of PNC
2.2. Preparation of Nano-Chlorophyll
2.3. Preparation of ZnO nanoparticles
2.4. DSSC Assembly
2.5. Morphology and Particle Size Analyses
2.6. Photovoltaic Measurements
2.7. Working Principles of DSSC
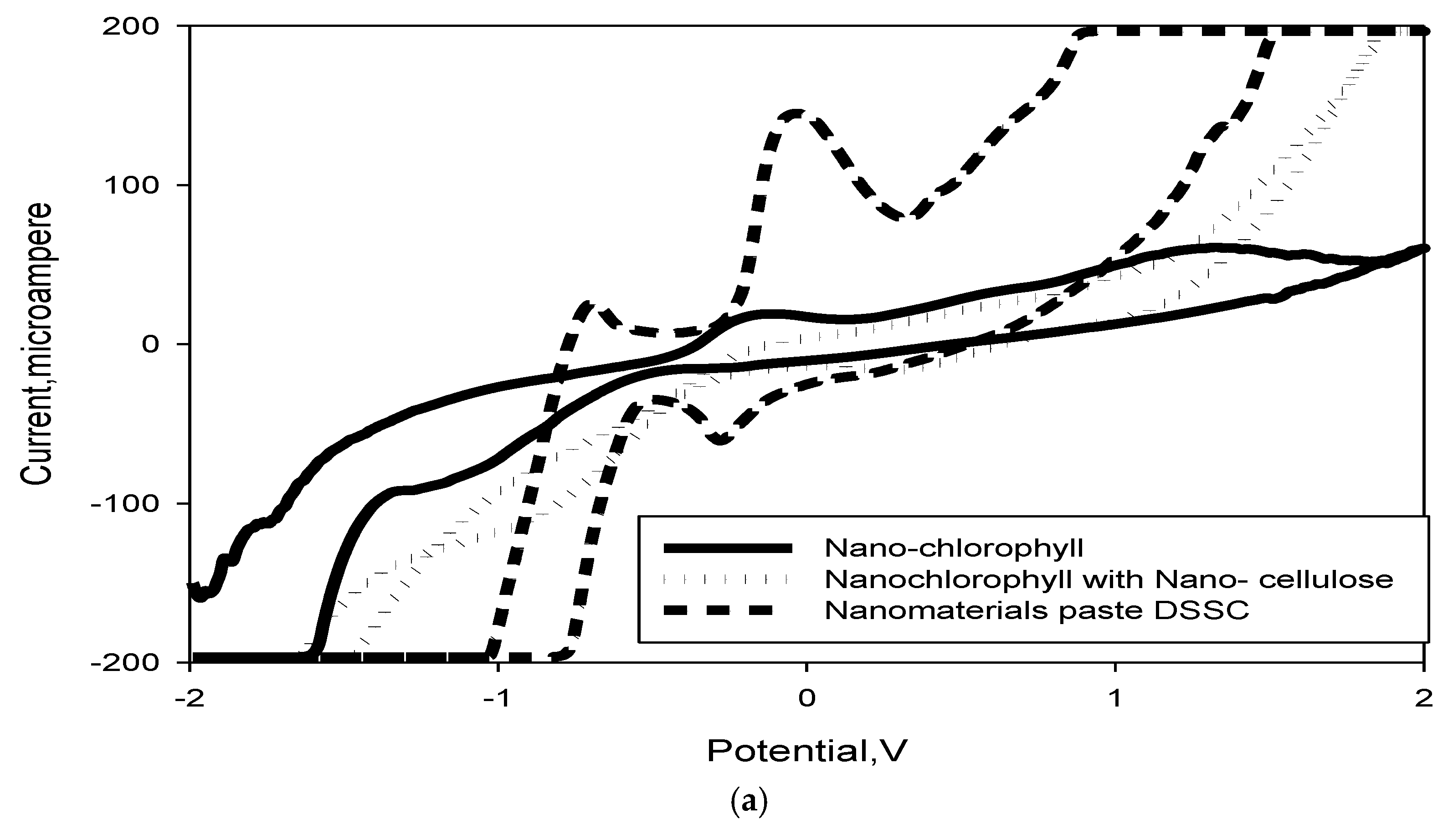
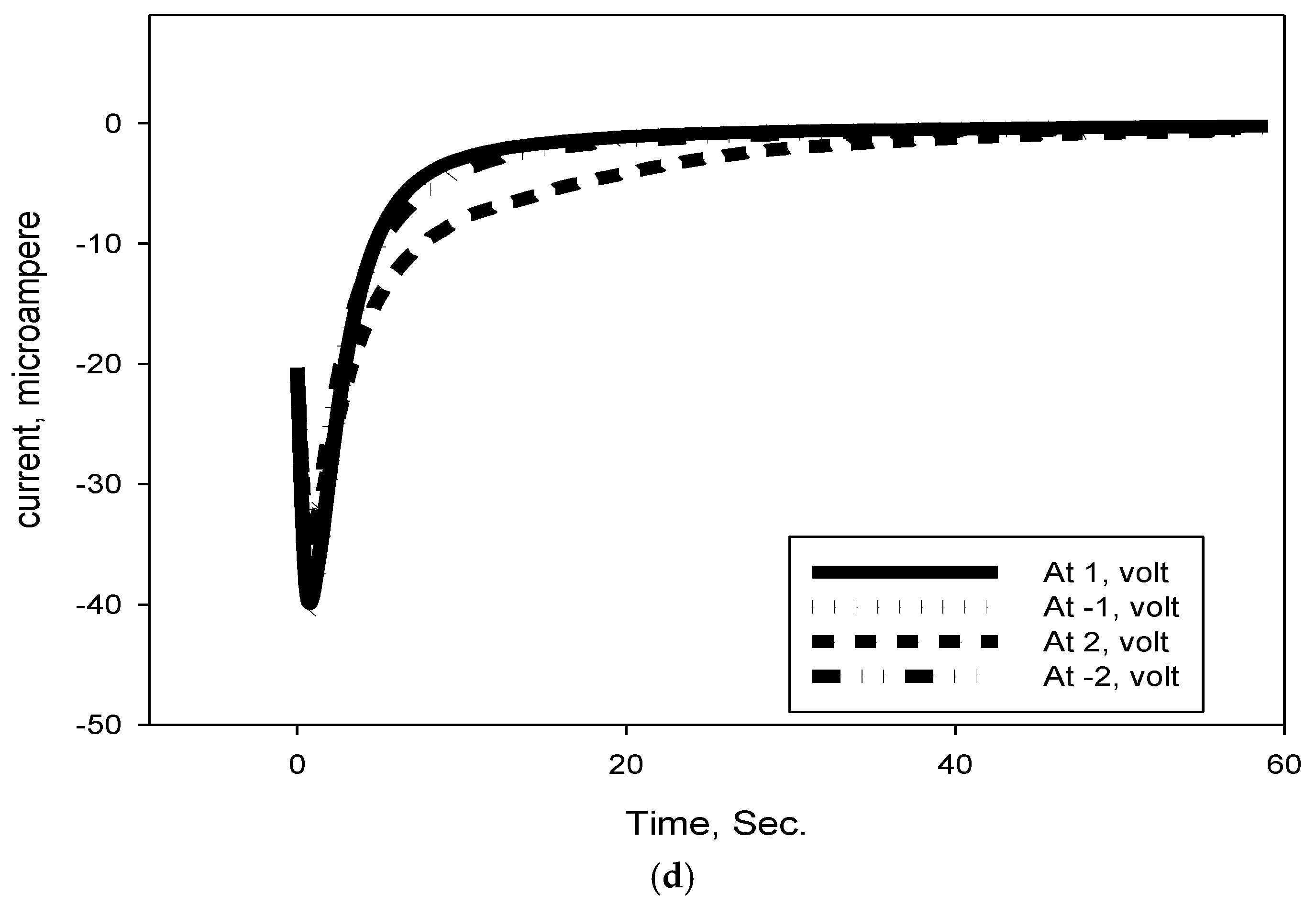
2.8. Cyclic Voltammetry Measurements
3. Results and Discussion
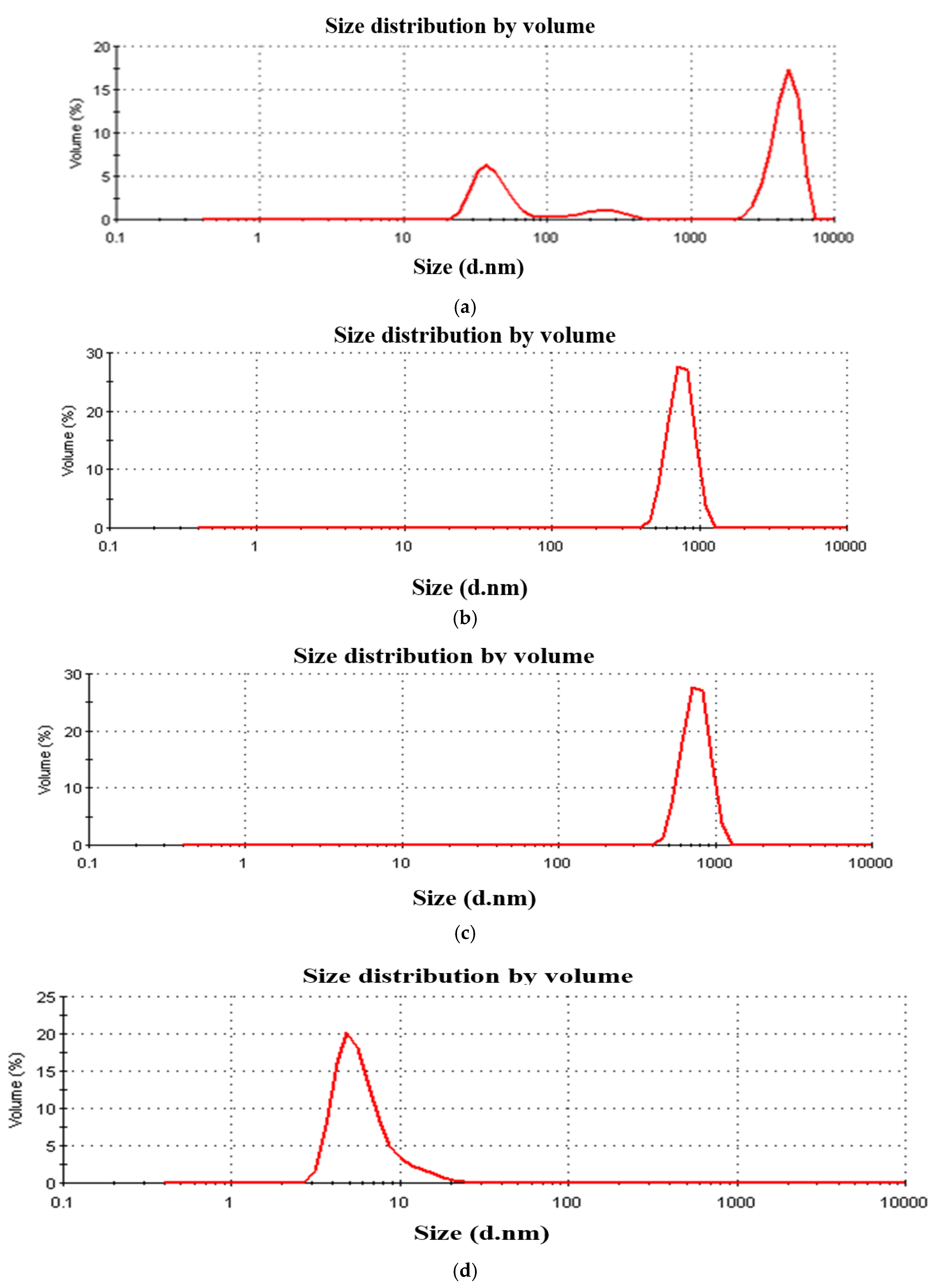
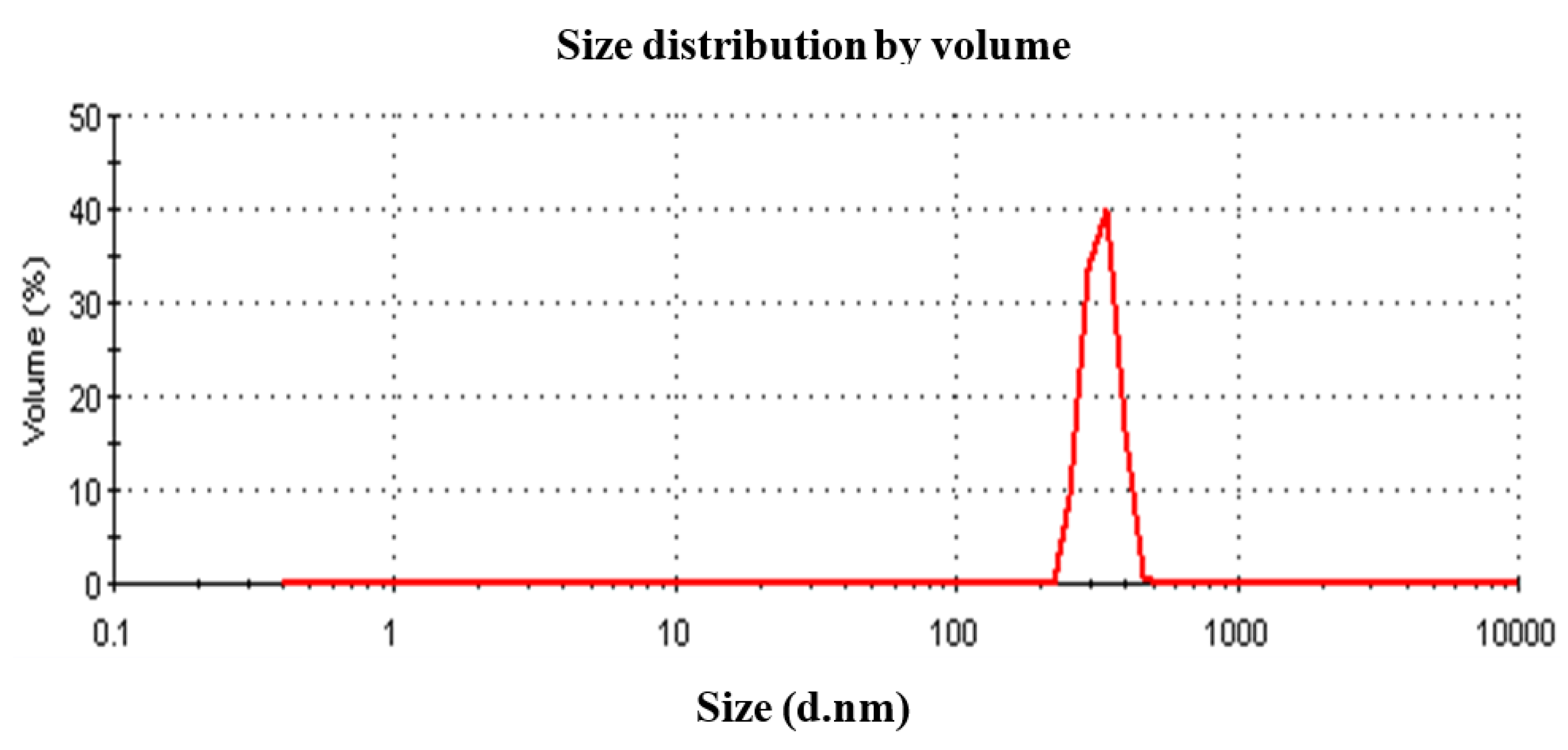
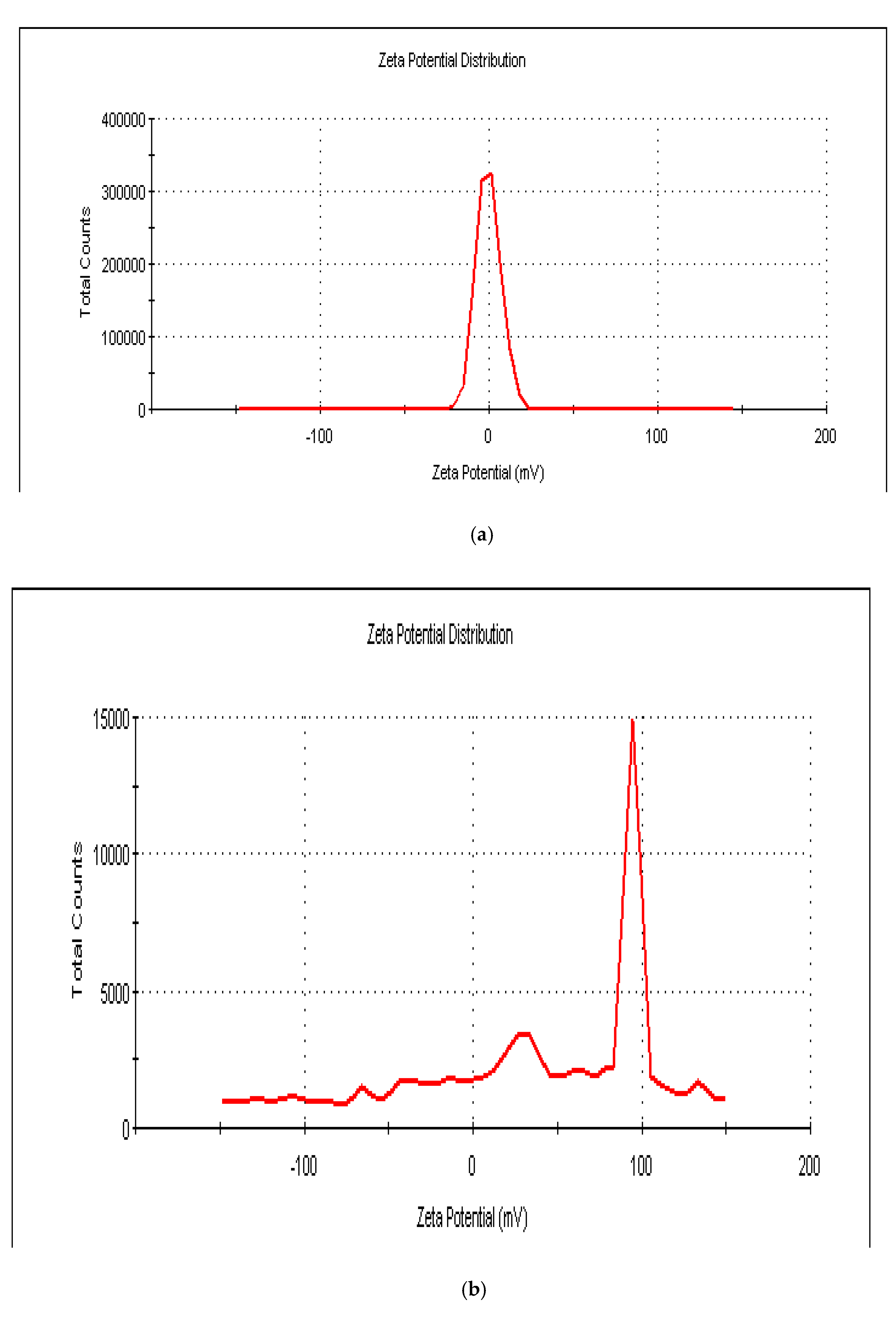
3.1. DLS Particle Size
3.2. Chemical Bonding
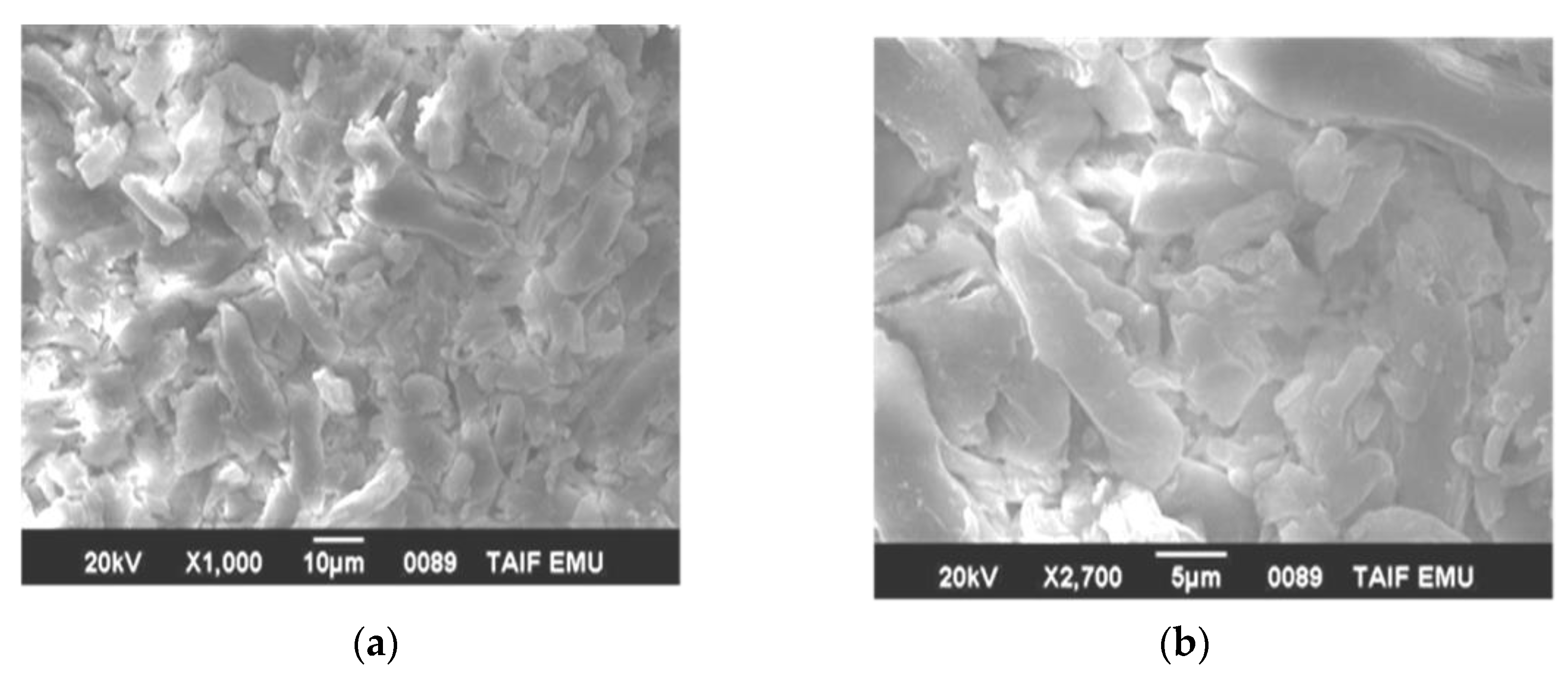
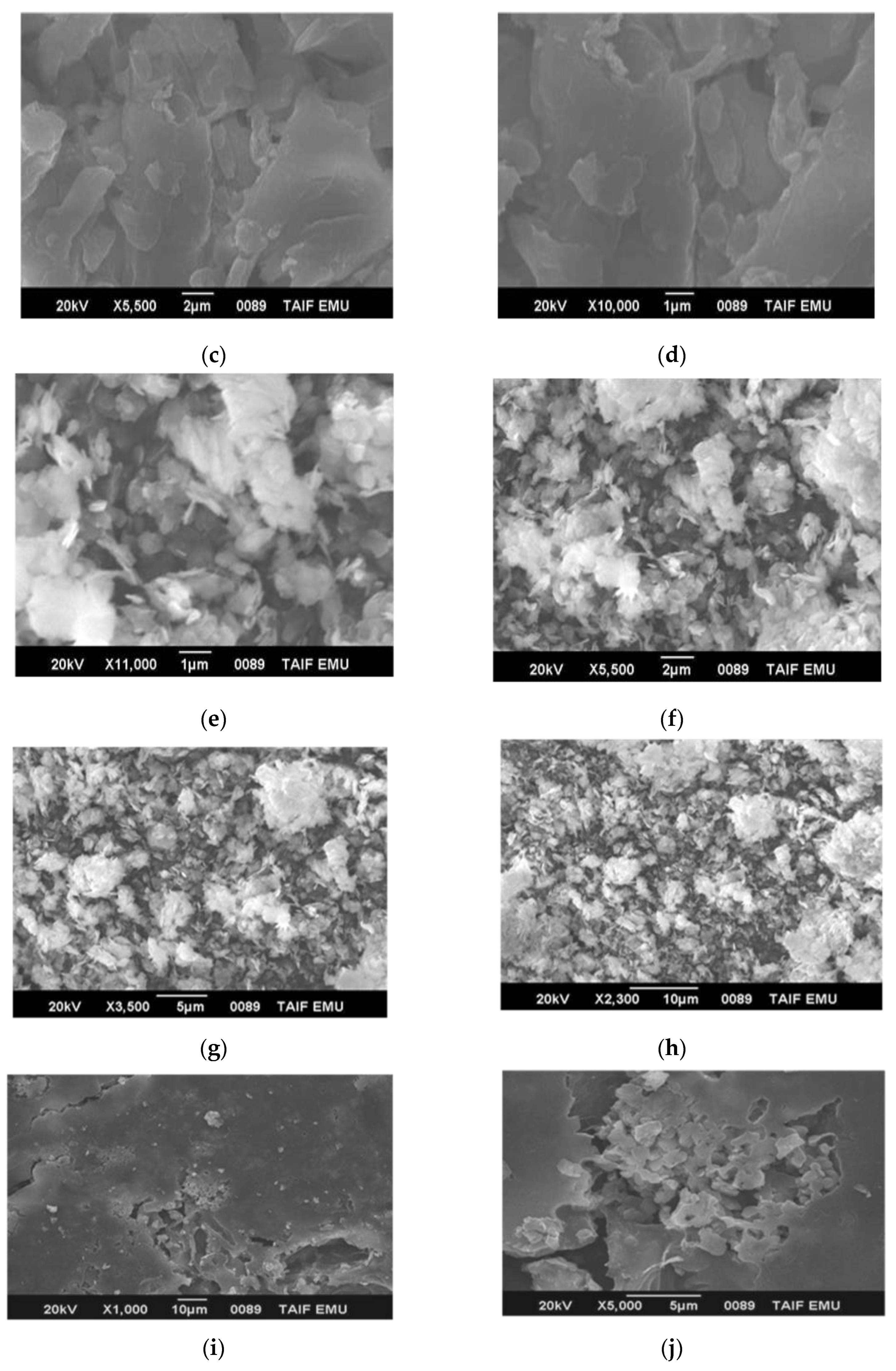
3.3. Microstructure
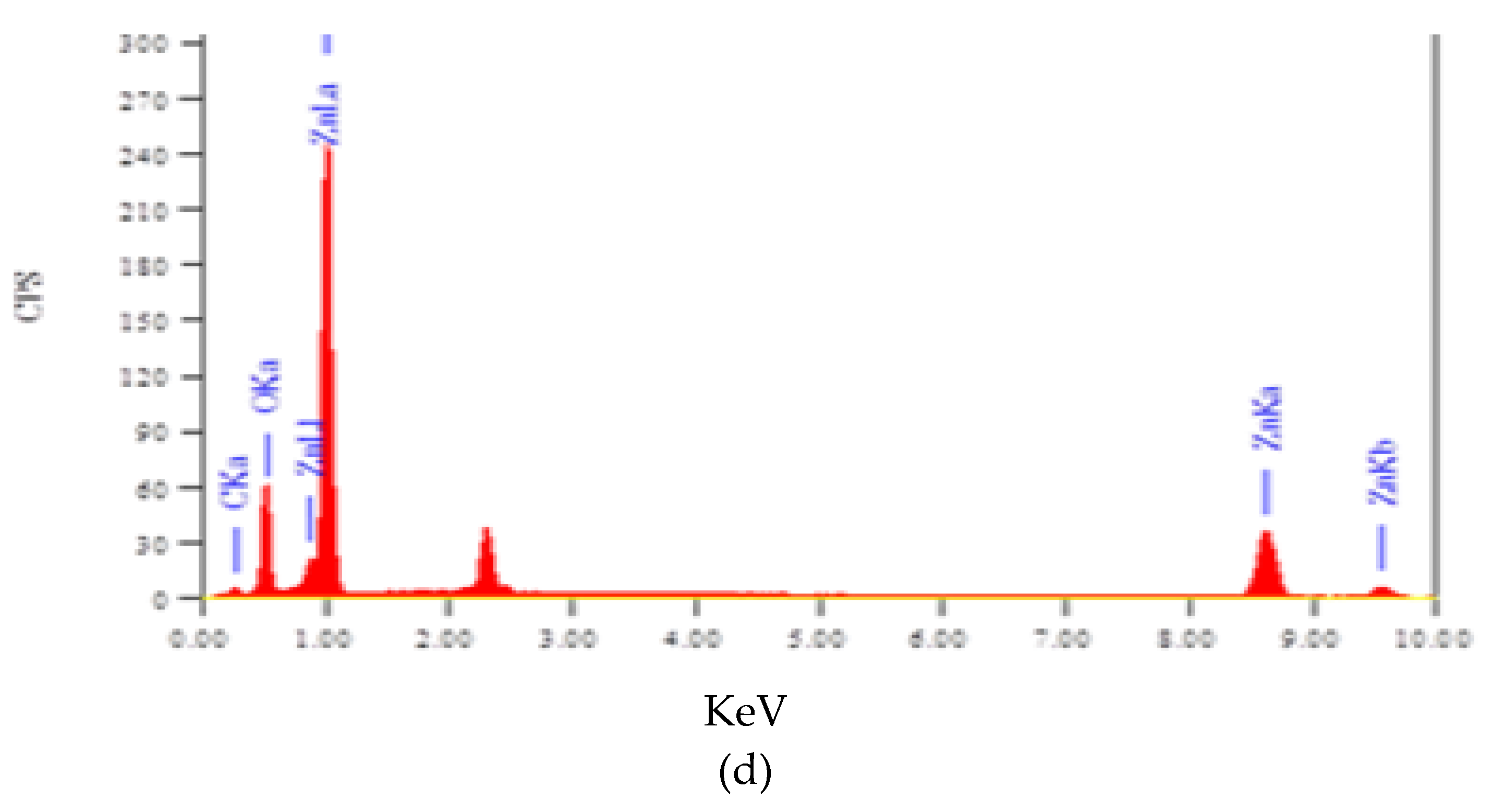
3.4. Elemental Composition
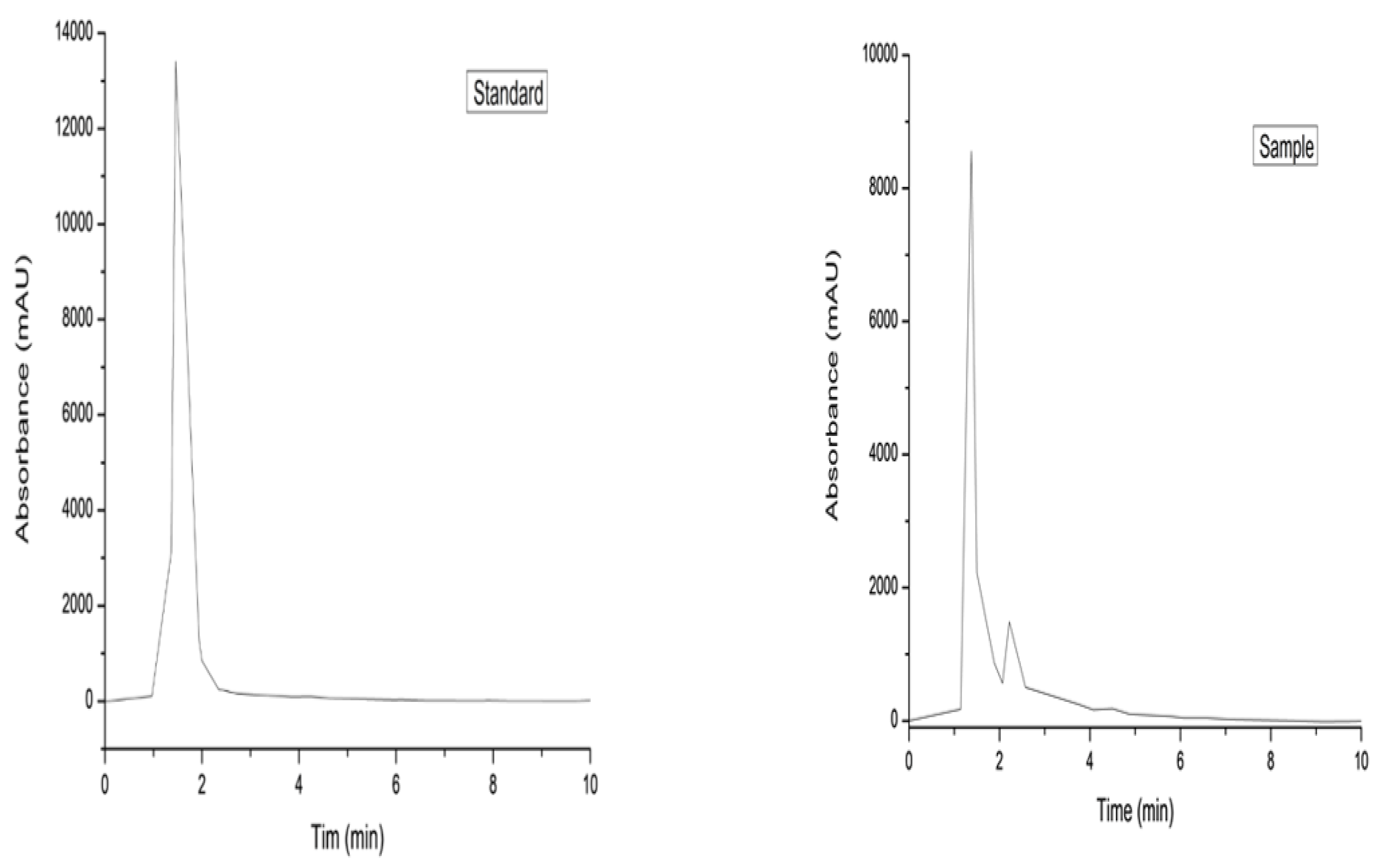
3.5. Dye Purification, Conductivity, Concentration, and Structure
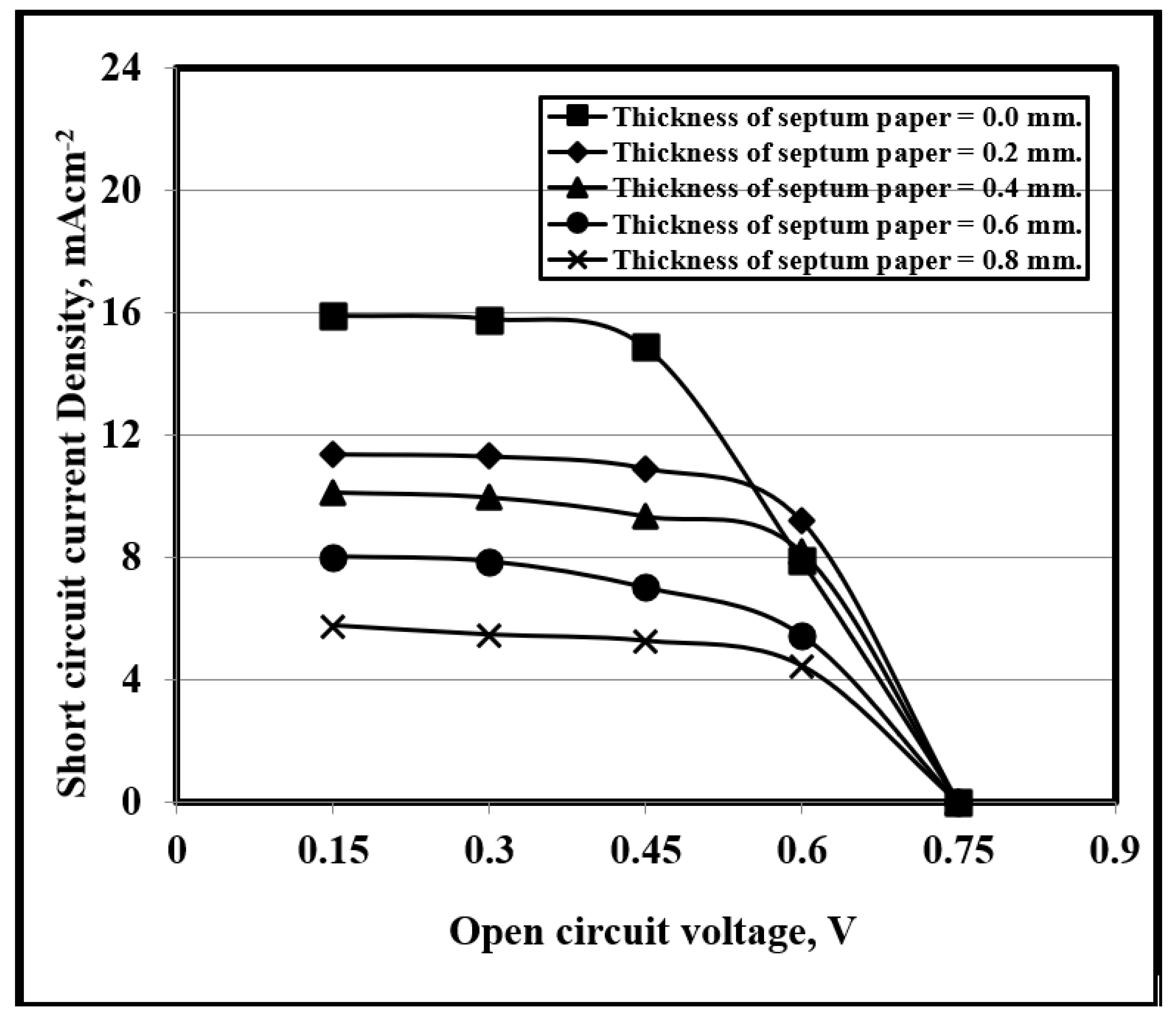
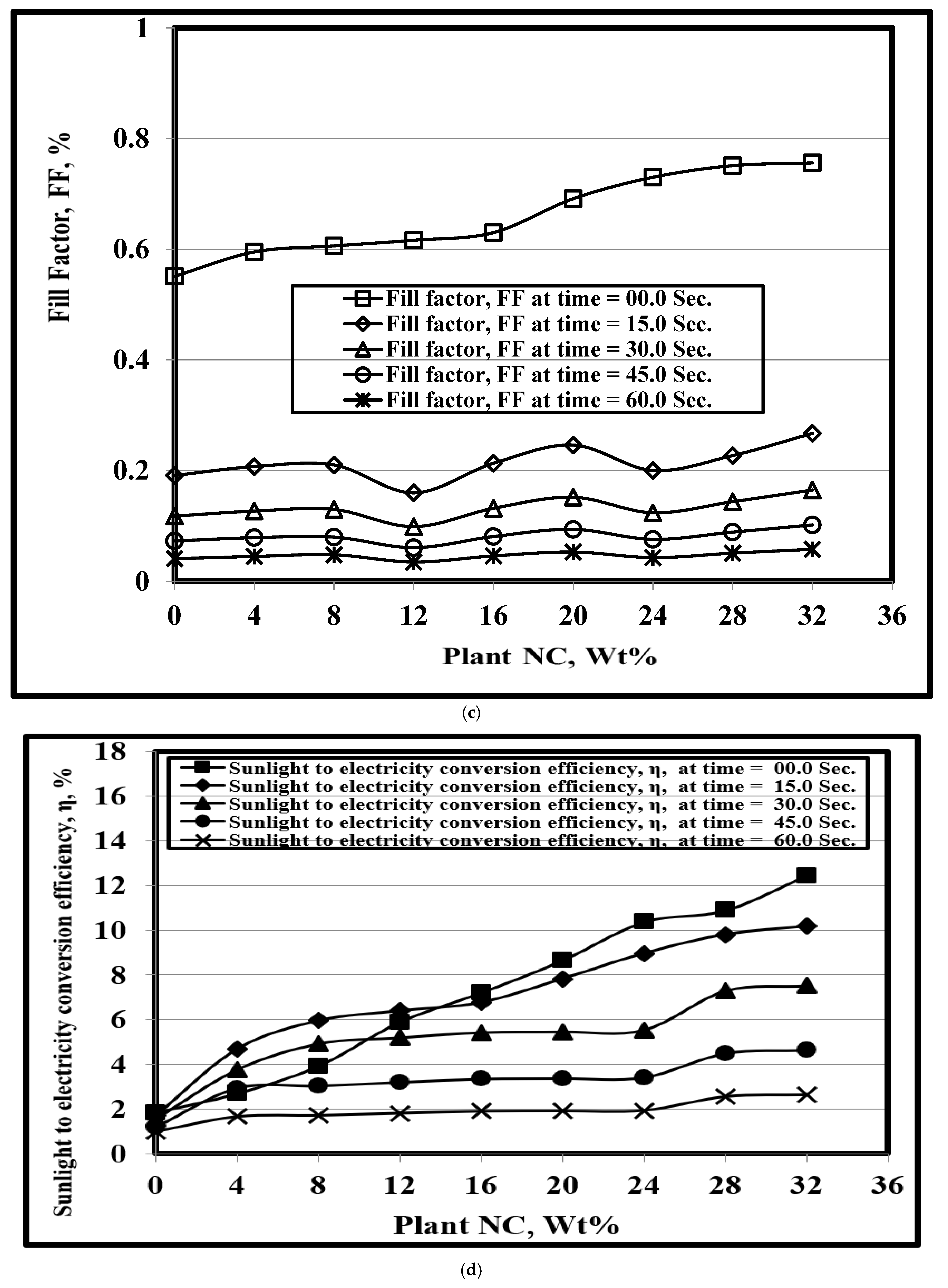
3.6. Effect of PNC Content on the PV Properties
3.7. DSSC PV Performance and Stability
3.8. DSSC Stability and Voltage Decay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Pye, S.; Li, P.H.; Keppo, I.; O’Gallachoir, B. Technology interdependency in the United Kingdom’s low carbon energy transition. Energy Strategy Rev. 2019, 24, 314–330. [Google Scholar] [CrossRef]
- Ortner, A.; Totschnig, G. The future relevance of electricity balancing markets in Europe—A 2030 case study. Energy Strategy Rev. 2019, 24, 111–120. [Google Scholar] [CrossRef]
- Solarin, S.A. Will energy strategies to reduce oil imports by countries dependent on foreign oil be effective? Evidence from residual augmented least squares and cross-sectionally augmented panel unit root tests. Energy Strategy Rev. 2019, 24, 268–278. [Google Scholar] [CrossRef]
- Sonter, L.J.; Dade, M.C.; Watson, J.E.M.; Valenta, R.K. Renewable energy production will exacerbate mining threats to biodiversity. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A. Recent Advances in Renewable Energy and Clean Energy. Energies 2022, 15, 3204. [Google Scholar] [CrossRef]
- Smith, O.; Cattell, O.; Farcot, E.; O’Dea, R.D.; Hopcraft, K.I. The effect of renewable energy incorporation on power grid stability and resilience. Sci. Adv. 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; El-kharouf, A.; Soyhan, H.S. For Sustainable Development: Future Trends in Renewable Energy and Enabling Technologies. Renew. Energy Resour. Chall. Appl. 2020, 1–10. [Google Scholar] [CrossRef]
- Arndt, C.; Arent, D.; Hartley, F.; Merven, B.; Mondal, A.H. Annual review of resource economics faster than you think: Renewable energy and developing countries. Annu. Rev. Resour. Econ. 2019, 11, 149–168. [Google Scholar] [CrossRef]
- Saba, C.S.; Ngepah, N. Convergence in renewable energy sources and the dynamics of their determinants: An insight from a club clustering algorithm. Energy Rep. 2022, 8, 3483–3506. [Google Scholar] [CrossRef]
- Kinolikar, A.; Garg, S.; Pundir, S.; Shakya, M.K. Power generation using advanced solar panel. Int. J. Eng. Res. Technol. 2014, 3, 1695–1697. [Google Scholar]
- Laha, S.K.; Sadhu, P.K.; Ganguly, A.; Naskar, A.K. Convergence tuning of solar power for enhanced energy harvesting. Lat. Am. Appl. Res. Int. J. 2018, 48, 113–118. [Google Scholar] [CrossRef]
- Angowski, M.; Kijek, T.; Lipowski, M.; Bondos, I. Factors Affecting the Adoption of Photovoltaic Systems in Rural Areas of Poland. Energies 2021, 14, 5272. [Google Scholar] [CrossRef]
- Ata, N.K.; Dolmatov, I.A. Which factors infuence the decisions of renewable energy investors? Empirical evidence from OECD and BRICS countries. Environ. Sci. Pollut. Res. 2022, 1–24. [Google Scholar] [CrossRef]
- Alam, S.S.; Masukujjaman, M.; Lin, C.Y.; Omar, N.A.; Na, M.; Othman, A.S. Factors affecting photo voltaic solar energy usage intention in rural households in bangladesh: A structural equation modelling approach. Environ. Clim. Technol. 2022, 26, 276–293. [Google Scholar] [CrossRef]
- Dincer, F.; Meral, M.E. Critical Factors that Affecting Efficiency of Solar Cells. Smart Grid Renew. Energy 2010, 1, 47–50. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Balzani, V. Solar Electricity and Solar Fuels: Status and perspectives in the Context of the energy transition. Chem. Eur. J. 2016, 22, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Patni, N.; Pillai, S.G.; Sharma, P. Effect of using betalain, anthocyanin and chlorophyll dyes together as a sensitizer on enhancing the efficiency of dye-sensitized solar cell. Int. J. Energy. Res. 2020, 44, 10846–10859. [Google Scholar] [CrossRef]
- Dinesh, V.P.; Kumar, R.S.; Sukhananazerin, A.; Sneha, J.M.; Kumar, P.M.; Biji, P. Novel stainless steel based, eco-friendly dye-sensitized solar cells using electrospun porous ZnO nanofibers. Nano-Struct. Nano-Objects 2019, 19, 100311. [Google Scholar] [CrossRef]
- Pratiwi, D.D.; Nurosyid, F.; Kusumandari; Supriyanto, A.; Suryana, R. Effect of Ni Doping into Chlorophyll Dye on the Efficiency of Dye-Sensitized Solar Cells (DSSC); AIP Publishing: Long Island, NY, USA, 2018; pp. 267–276. [Google Scholar]
- Kohn, P.S.; Großerhode, C.; Storck, J.L.; Grötsch, G.; Cornelißen, A.; Cornelißen, C.; Pfeiffer, A.S.; Ehrmann, A. Commercially available teas as possible dyes for dye-sensitized solar cells. Optik 2019, 202, 178–182. [Google Scholar] [CrossRef]
- Junger, I.J.; Wehlage, D.; Böttjer, R.; Grothe, T.; Juhász, L.; Grassmann, C.; Blachowicz, T.; Ehrmann, A. Dye-Sensitized Solar Cells with Electrospun Nanofiber Mat-Based Counter Electrodes. Materials 2018, 11, 1604. [Google Scholar] [CrossRef]
- Khammee, P.; Unpaprom, Y.; Subhasaen, U.; Ramaraj, R. Potential evaluation of yellow cotton (cochlospermum regium) pigments for dye sensitized solar cells application. Glob. J. Sci. Eng. 2020, 2, 16–21. [Google Scholar] [CrossRef]
- Amin, M.T.; Abo-Dief, H.M.; Abu-Ali, O.A.; Mohamed, A.T. Effect of cabbage color on the improvement of dye-sensitized solar cell (DSSC) Parameters using nanostructured zinc oxide. In Proceedings of the #VSESA Conference, Jeddah, KSA, 1–2 December 2020; pp. 1–10. [Google Scholar]
- Atli, A.; Atilgan, A.; Altinkaya, C.; Oze, K.; Yildiz, A. St. Lucie cherry, yellow jasmine, and madder berries as novel natural sensitizers for dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 3914–3922. [Google Scholar] [CrossRef]
- Junger, I.J.; Udomrungkhajorncha, S.; Grimmelsmann, N.; Blachowicz, T.; Ehrmann, A. Effect of caffeine copigmentation of anthocyanin dyes on dssc efficiency. Materials 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bashar, H.; Bhuiyan, M.; Hossain, M.; Kabir, F.; Rahaman, S.; Manir, M.; Ikegami, T. Study on combination of natural red and green dyes to improve the power conversion efficiency of dye sensitized solar cells. Optik 2019, 185, 620–625. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Rouhani, S.; Gharanjig, K. Extraction and application of natural pigments for fabrication of green dye-sensitized solar cells. Opto-Electronics Rev. 2018, 26, 165–171. [Google Scholar] [CrossRef]
- Franchi, D.; Calamante, M.; Coppola, C.; Alessandro Mordini, A.; Reginato, G.; Adalgisa Sinicropi, A.; Lorenzo Zani, L. Synthesis and characterization of new organic dyes containing the Indigo core. Molecules 2020, 25, 3377. [Google Scholar] [CrossRef]
- Navar, A.O.; Hernández, I.A.; Luke, T.L.; Zarazúa, I.; Arellano, V.R.; Guerrero, J.P.; Soto, N.O. Photoconversion efficiency of Titania solar cells co-sensitized with natural pigments from cochineal, papaya peel and microalga Scenedesmus obliquus. J. Photochem. Photobiol. A Chem. 2020, 388, 1–10. [Google Scholar]
- Velasco, P.H.; Atilano, I.M.; Delgado, M.R.; Delgado, J.M.R.; Moreno, D.L.; Alanís, F.G.Á.; Villarreal-Chiu, J.F. Photoelectric evaluation of dye-sensitized solar cells based on prodigiosin pigment derived from Serratia marcescens 11E. Dye. Pigment. 2020, 177, 108278. [Google Scholar] [CrossRef]
- Arifin, Z.; Soeparman, S.; Widhiyanuriyawan, D.; Suyitno, S.; Setyaji, A.T. Improving stability of chlorophyll as natural dye for dye-sensitized solar cells. J. Teknol. 2017, 80, 27–33. [Google Scholar] [CrossRef]
- Chatterjee, S.; Karki, I.B. Effect of Photoanodes on the Performance of Dye-Sensitized Solar Cells. J. Inst. Eng. 2020, 15, 62–68. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Das, P.P.; Roy, A.; Das, S.; Devi, P.S. Enhanced stability of Zn2SnO4 with N719, N3 and eosin Y dye molecules for DSSC application. Phys. Chem. Chem. Phys. 2016, 18, 1429–1438. [Google Scholar]
- Lee, K.E.; Gomez, M.; Elouatik, S.; Demopoulos, G. Further understanding of the adsorption mechanism of N719 sensitizer on anatase TiO2 Films for DSSC application. Langmuir 2010, 26, 9575–9583. [Google Scholar] [CrossRef]
- Calamante, M.; Mordini, A.; Franchi, D.; Reginato, G.; Zani, L.; Peruzzini, M.; Taddei, M.; de Biani, F.F.; Basosi, R.; Sinicropi, A.; et al. Two New Dyes with Carboxypyridinium Regioisomers as Anchoring Groups for Dye-Sensitized Solar Cells. Synlett 2015, 26, 2389–2394. [Google Scholar] [CrossRef]
- Reginato, G.; Calamante, M.; Zani, L.; Mordini, A.; Franchi, D. Design and synthesis of organic sensitizers with enhanced anchoring stability in dye-sensitized solar cells. Pure Appl. Chem. 2017, 90, 363–376. [Google Scholar] [CrossRef]
- Pham, H.D.; Do, T.T.; Kim, J.; Charbonneau, C.; Manzhos, S.; Feron, K.; Tsoi, W.C.; Durrant, J.R.; Jain, S.M.; Sonar, P. Molecular engineering using an anthanthrone dye for low-cost hole transport materials: A strategy for dopant-free, high-efficiency, and stable perovskite solar cells. Adv. Energy Mater. 2018, 8, 1703007. [Google Scholar] [CrossRef]
- Pham, H.D.; Do, T.T.; Kim, J.; Charbonneau, C.; Manzhos, S.; Feron, K.; Tsoi, W.C.; Durrant, J.R.; Jain, S.M.; Sonar, P. One step facile synthesis of a novel anthanthrone dye-based, dopant-free hole transporting material for efficient and stable perovskite solar cells. J. Mater. Chem. 2018, 6, 3699–3708. [Google Scholar] [CrossRef]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol-Gel Sci. Technol. 2013, 66, 212–219. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef]
- Siddick, S.Z.; Lai, C.W.; Juan, J.C. An investigation of the dye-sensitized solar cell performance using graphene-titania (TrGO) photoanode with conventional dye and natural green chlorophyll dye. Mater. Sci. Semicond. Process. 2018, 74, 267–276. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.-F.; Dall’Agnese, C.; Wei, Y.; Chen, G.; Tamiaki, H.; Sanehira, Y.; Sasaki, S.-I. P-type P3HT interfacial layer induced performance improvement in chlorophyll-based solid-state solar cells. J. Photochem. Photobiol. A Chem. 2018, 371, 349–354. [Google Scholar] [CrossRef]
- Almaz, W.; Muhamad, D.; Daud, Z.; Salleh, H. The sensitization effect of anthocyanin and chlorophyll dyes on optical and photovoltaic properties of zinc oxide based dye-sensitized solar cells. Optik 2020, 207, 458–464. [Google Scholar]
- Wang, S.; Duan, S.; Wang, Y.; Sun, C.; Wang, X.-F.; Sasaki, S.-I. Chlorophyll-based organic solar cells with improved power conversion efficiency. J. Energy Chem. 2019, 38, 88–93. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, W.; Ogasawara, S.; Wang, X.F.; Tamiaki, H. Fabrication and performance of all-solid-state dye-sensitized solar cells using synthetic carboxylated and pyridylated chlorophyll derivatives. J. Photochem. Photobiol. A Chem. 2018, 353, 625–630. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Al-Mashaan, A.B.S.A.; Abdullah, M.F. Performance of the dye-sensitized solar cells fabricated using natural dyes from Ixora coccinea flowers and Cymbopogon schoenanthus leaves as sensitizers. Int. J. Energy Res. 2019, 43, 7229–7239. [Google Scholar]
- Delgado, R.L.; Plascencia, M.T.; Ramos, M.E.Á.; Ayón, A. Increasing the solar cell efficiency using glass balls. Version Rec. Sci. Direct 2019, 1–15. [Google Scholar]
- Li, M.; Li, N.; Chen, G.; Sasaki, S.-I.; Miyasaka, T.; Tamiaki, H.; Dall’Agnese, C.; Wang, X.-F. Perovskite solar cells based on chlorophyll hole transporters: Dependence of aggregation and photovoltaic performance on aliphatic chains at C17-propionate residue. Dye. Pigment. 2019, 162, 763–770. [Google Scholar] [CrossRef]
- Ridwan, M.A.; Noor, E.; Rusli, M.S.; Akhiruddin, A. Fabrication of dye-sensitized solar cell using chlorophylls pigment from sargassum. In Proceedings of the 1st International Conference on Tropical Studies and Its Application (ICTROPS), Samarinda, Indonesia, 9 November 2017; pp. 1–8. [Google Scholar]
- Wang, X.; Liu, C.; Shi, Z.; Pan, M.; Yu, D. Protein-encapsulated chlorophyll a molecules for biological solar cells. Mater. Des. 2020, 195, 108983. [Google Scholar] [CrossRef]
- Tamiaki, H.; Hagio, N.; Tsuzuki, S.; Cui, Y.; Zouta, T.; Wang, X.-F.; Kinoshita, Y. Synthesis of carboxylated chlorophyll derivatives and their activities in dye-sensitized solar cells. Tetrahedron 2018, 74, 4078–4085. [Google Scholar] [CrossRef]
- Wang, X.; Pan, M.; Shi, Z.; Yu, D.; Huang, F. Chlorophyll derivative-sensitized tio2 electron transport layer for record efficiency of Cs2AgBiBr6 double Perovskite solar cells. ACS Appl. Bio Mater. 2021, 4, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ezike, S.C.; Hyelnasinyi, C.N.; Salawu, M.A.; Wansah, J.F.; Ossai, A.N.; Agu, N.N. Synergestic effect of chlorophyll and anthocyanin Co-sensitizers in TiO2-based dye-sensitized solar cells. Surf. Interfaces 2020, 22, 100882. [Google Scholar] [CrossRef]
- Zhao, W.; Agnese, C.D.; Duan, S.; Sanehira, Y.; Wei, Y.; Tamiaki, H.; Tamiaki, S.; Wang, X.F. Trilayer chlorophyll-based cascade biosolar cells. ACS Energy Lett. 2019, 4, 384–389. [Google Scholar] [CrossRef]
- Lasrado, D.; Ahankari, S.; Kar, K. Nanocellulose-based polymer composites for energy applications—A review. J. Appl. Polym. Sci. 2020, 137, 48959. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; Abo-Dief, H.M.; Mohamed, A.T. Polymer/steel tribological characteristics and corrosion and under external voltages. J. Appl. Mech. Eng. 2021, 6, 1–9. [Google Scholar]
- Poskela, A.; Miettunen, K.E.; Borghei, M.; Vapaavuori, J.; Greca, L.G.; Lehtonen, J.; Solin, K.; Ago, M.; Lund, P.D.; Rojas, O.J. Nanocellulose and Nanochitin Cryogels Improve the Efficiency of Dye Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 10257–10265. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Arazi, R.; Mirjalili, B.B.F.; Azad, S. Synthesis and application of Fe3O4 @nanocellulose/TiCl as a nanofiller for high performance of quasisolid-based dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 4483–4494. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Polysaccharide-based polymer electrolytes for future renewable energy sources. In Sustainable Materials for Next Generation Energy Devices, Challenges and Opportunities; Elsevier: Amsterdam, The Netherlands, 2021; pp. 283–316. [Google Scholar]
- Bita, B.; Iftimie, S.; Radu, A.; Antohe, V.A.; Coman, D.; Miron, C.; Staicu, D.; Dan, L.; Ion, L.; Antohe, S. On the Electrical and Photo-Electrical Behavior of the Photovoltaic Cells Based on Polymeric and Chlorophyll-a Thin Films; The Romanian Academy: Bucharest, Romania, 2019; Volume 20, pp. 51–57. [Google Scholar]
- Hsu, H.H.; Zhong, W. Nanocellulose-Based Conductive Membranes for Free-Standing Supercapacitors: A Review. Membranes 2019, 9, 74. [Google Scholar] [CrossRef]
- Raga, S.R.; Santiago, F.F. Temperature effects in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 2328–2336. [Google Scholar] [CrossRef]
- Radziemska, E.K. The effect of temperature on the power drop in crystalline solar cells. Renew. Energy 2003, 28, 1–12. [Google Scholar] [CrossRef]
- Savadogo, M.; Soro, B.; Konate, R.; Sourabié, I.; Zoungrana, M.; Zerbo, I.; Dieudonné Joseph Bathiebo, D.J. Temperature Effect on Light Concentration Silicon Solar Cell’s Operating Point and Conversion Efficiency. Smart Grid Renew. Energy 2020, 11, 61–72. [Google Scholar] [CrossRef]
- Shreedhana, K.; Ilavarasi, R. Fabrication of nanocrystalline cellulose from banana peel obtained from unripe plantain bananas. In Proceedings of the International Web Conference on Advanced Material Science and Nanotechnology (NANOMAT-2020), Nandgaon Khandeshwar, India, 20–21 June 2020. [Google Scholar]
- Delgadoa, R.L.; Plascenciac, M.T.; Ramosb, M.E.A.; Ayóna, A. Silicon Solar Cell Efficiency Improvement Employing Photoluminescent Properties of Chlorophyll-A; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar]
- Szutt, A.; Śródka, A.D. Chlorophyll content in senescent pelargonium graveolens leaves. Proc. ECOpole 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Knapp, M.; Jo, M.; Dill, Z.; Bridwell-Rabb, J. Rieske Oxygenase Catalyzed C–H Bond Functionalization Reactions in Chlorophyll b Biosynthesis. ACS Central Sci. 2022, 8, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Ashtaputre, S.S.; Deshpande, A.; Marathe, S.; Wankhede, M.E.; Chimanpure, J.; Pasricha, R.; Urban, J.; Haram, S.K.; Gosavi, S.W.; Kulkarni, S.K. Synthesis and analysis of ZnO and CdSe nanoparticles. Pramana 2005, 65, 615–620. [Google Scholar] [CrossRef]
- Bhakat, C.; Singh, P.P. Zinc oxide nanorods: Synthesis and its applications in solar cell. Int. J. Mod. Eng. Res. 2012, 2, 2452–2454. [Google Scholar]
- Sekine, N.; Chou, C.-H.; Kwan, W.L.; Yang, Y. ZnO nano-ridge structure and its application in inverted polymer solar cell. Org. Electron. 2009, 10, 1473–1477. [Google Scholar] [CrossRef]
- Noh, Y.-J.; Na, S.-I.; Kim, S.-S. Inverted polymer solar cells including ZnO electron transport layer fabricated by facile spray pyrolysis. Sol. Energy Mater. Sol. Cells 2013, 117, 139–144. [Google Scholar] [CrossRef]
- Moyassari, E.; Thomas Roth, T.; Kücher, S.; Chang, C.C.; Hou, S.C.; Spingler, F.B.; Jossen, A. The role of silicon in silicon-graphite composite electrodes regarding specific capacity, cycle stability, and expansion. J. Electrochem. Soc. 2022, 169, 010504. [Google Scholar] [CrossRef]
- Gouyon, J.; D’Orlyé, F.; Griveau, S.; Bedioui, F.; Varenne, A. Characterization of home-made graphite/PDMS microband electrodes for amperometric detection in an original reusable glass-NOA®-PDMS electrophoretic microdevice. Electrochim. Acta 2019, 329, 135164. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Luo, J.; Sun, J.; Gao, L. A bifunctional TiO2 sol for convenient low-temperature fabrication of dye-sensitized solar cells. Mater. Lett. 2011, 67, 60–63. [Google Scholar] [CrossRef]
- Chiappone, A.; Bella, F.; Nair, J.R.; Meligrana, G. Structure–performance correlation of nanocellulose-based polymer electrolytes for efficient quasi-solid DSSCs. ChemElectroChem 2014, 1, 1350–1358. [Google Scholar] [CrossRef]
- Shafiee, A.; Salleh, M.M.; Yahaya, M. Determination of HOMO and LUMO of [6,6]-Phenyl C61-butyric Ac id3-ethylthiophene Ester and Poly (3-octyl-thiophene-2, 5-diyl) through Voltametry Characterization. Sains Malays. 2011, 40, 173–176. [Google Scholar]
- De Lile, J.R.; Kang, S.G.; Son, Y.A.; Lee, S.G. Do homo−lumo energy levels and band gaps provide sufficient understanding of dye-sensitizer activity trends for water purification? ACS Omega 2020, 5, 15052–15062. [Google Scholar] [CrossRef]
- Shabir, G.; Arooj, S.; Javed, A.H.; Saeed, A.; Shahzad, N.; Iqbal, N.; Jabeen, E. The development of highly fluorescent hemicyanine and dicyanoisophorone dyes for applications in dye-sensitized solar cells. J. Fluoresc. 2022, 32, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Djurovich, P.I.; Mayo, E.I.; Forrest, S.R.; Thompson, M. Measurement of the lowest unoccupied molecular orbital energies of molecular organic semiconductors. Org. Electron. 2009, 10, 515–520. [Google Scholar] [CrossRef]
- Al-Ibrahim, M.; Roth, H.K.; Schroedner, M.; Alexander Konkin, A.; Zhokhavet, U.; Gobsch, G.; Scharff, P.; Sensfus, S. The influence of the optoelectronic properties of poly(3-alkylthiophenes) on The device parametersin flexible polymer solar cells. Org. Electron. 2005, 6, 65–77. [Google Scholar] [CrossRef]
- Lin, L.Y.; Tsai, C.H.; Wong, K.T.; Huang, T.W.; Hsieh, L.; Liu, S.H.; Lin, H.W. Organic dyes containing coplanar diphenyl-substituted dithienosilole core for efficient dye-sensitized solar cells. J. Org. Chem. 2010, 75, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-P.; Lu, H.-P.; Chiu, C.-L.; Lee, C.-W.; Chuang, S.-H.; Mai, C.-L.; Yen, W.-N.; Hsu, S.-J.; Diau, E.W.-G.; Yeh, C.-Y. Synthesis and characterization of porphyrin sensitizers with various electron-donating substituents for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2009, 20, 1127–1134. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Datta, S.; Forrest, S.R.; Djurovich, P.; Polikarpov, E.; Thompson, M.E. Relationship between the ionization and oxidation potentials of molecular organic semiconductors. Org. Electron. 2005, 6, 11–20. [Google Scholar] [CrossRef]
- Bredas, J.L.; Silbey, R.; Boudreux, D.S.; Chance, R.R. Chain-length dependence of electronic and electrochemical properties of conjugated systems: Polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, M.; Mujumdar, A.S.; Zhong, O.; Wang, Z. Effects of ultrasonic pretreatments on quality, energy consumption and sterilization of barley grass in freeze drying. Ultrason. Sonochemistry 2018, 40, 333–340. [Google Scholar] [CrossRef]
- Yang, A.; Liao, Y.; An, M.; Cao, Y.; Yang, Z.; Ren, H.; Su, H.; Zou, Q.; Chen, L. Effect of ultrasonic pretreatment on flocculation filtration of low-rank coal slurry. Molecules 2022, 27, 6460. [Google Scholar] [CrossRef] [PubMed]
- Sompech, S.; Srion, A.; Nuntiya, A. The Effect of Ultrasonic Treatment on the Particle Size and Specific Surface Area of LaCoO3. Procedia Eng. 2012, 32, 1012–1018. [Google Scholar] [CrossRef]
- Aydar, A.Y.; Aydın, T.; Yılmaz, T.; Kothakota, A.; Terezia, S.C.; Leontin, C.F.; Pandiselvam, R. Investigation on the influence of ultrasonic pretreatment on color, quality and antioxidant attributes of microwave dried Inula viscosa (L.). Ultrason. Sonochemistry 2022, 90, 106184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Ma, D.; Ahmed, S.; Qin, W.; Liu, Y. Effects of ultrasonication duration and graphene oxide and nano-zinc oxide contents on the properties of polyvinyl alcohol nanocomposites. Ultrason. Sonochemistry 2019, 59, 104731. [Google Scholar] [CrossRef]
- Barabaszová, K.C.; Holešová, S.; Šulcová, K.; Hundáková, M.; Thomasová, B. Effects of ultrasound on zinc oxide/vermiculite/chlorhexidine nanocomposite preparation and their antibacterial activity. Nanomaterials 2019, 9, 1309. [Google Scholar] [CrossRef]
- Anžlovar, A.; Orel, Z.C.; Kogej, K.; Igon, M. Polyol-Mediated Synthesis of Zinc Oxide Nanorods and Nanocomposites with Poly(methyl methacrylate). J. Nanomater. 2012, 2012, 760872. [Google Scholar] [CrossRef]
- Rammah, Y.S.; El-Agawany, F.I.; Mahmoud, K.A.; El-Mallawany, R.; Ilik, E.; Kilic, G. FTIR, UV–Vis–NIR spectroscopy, and gamma rays shielding competence of novel ZnO-doped vanadium borophosphate glasses. J. Mater. Sci. Mater. Electron. 2020, 31, 9099–9113. [Google Scholar] [CrossRef]
- Alomairy, S.; Al-Buriahi, M.S.; Abdel Wahab, E.A.; Sriwunkum, C.; Shaaban, K. Synthesis, FTIR, and neutron/charged particle transmission properties of Pb3O4–SiO2–ZnO–WO3 glass system. Ceram. Int. 2021, 47, 17322–17330. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C. Production of nanocellulose from waste cellulose. Chem. Eng. Trans. 2019, 73, 1–6. [Google Scholar]
- Ogawa, Y.; Putaux, J.L. Transmission Electron Microscopy of Cellulose. Part 2: Technical and Practical Aspects. Cellulose; Springer: New York, NY, USA, 2019; Volume 26, pp. 17–34. [Google Scholar]
- Javed, A.H.; Shahzad, N.; Khan, M.A.; Ayub, M.; Iqbal, N.; Hassan, M.; Hussain, N.; Rameel, M.I.; Shahzad, M.I. Effect of ZnO nanostructures on the performance of dye sensitized solar cells. Sol. Energy 2021, 230, 492–500. [Google Scholar] [CrossRef]
- Muryani, B.Y.; Sarifah, N.; Kusumawardani, D.R.; Nurosyid, F. Effect concentration of dye solution binahong leaves to the efficiency of dye-sensitized solar cell (DSSC). In Proceedings of the International Conference on Science and Applied Science (ICSAS), Surakarta, Indonesia, 19 July 2019; pp. 020122-1–020122-4. [Google Scholar]
- Woodward, R.B.; Ayer, W.A.; Beaton, J.M.; Bickelhaupt, F.; Bonnett, R.; Buchschacher, P.; Closs, G.L.; Dutler, H.; Hannah, J.; Hauck, F.P.; et al. The total synthesis of chlorophyll a. Tetrahedron 1990, 46, 7599–7659. [Google Scholar] [CrossRef]
- Wu, N.; Wu, Y.; Walter, D.; Shen, H.; Duong, T.; Grant, D.; Barugkin, C.; Fu, X.; Peng, J.; White, T.; et al. Identifying the Cause of Voltage and Fill Factor Losses in Perovskite Solar Cells by Using Luminescence Measurements. Energy Technol. 2017, 5, 1827–1835. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, T.; Kang, M.G.; Song, H.-E.; Kang, Y.; Lee, H.-S.; Kim, D.; Park, S.; Lee, S.H. An Analysis of Fill Factor Loss Depending on the Temperature for the Industrial Silicon Solar Cells. Energies 2020, 13, 2931. [Google Scholar] [CrossRef]
- Amadi, L.; Jenny, S.S.; Ahmed, A.; Brown, N.; Yadav, S.; Brown, D.; Ghann, W.; Gayrama, A.; Mintesinot Jiru, M.; Uddin, J. Creation of Natural Dye Sensitized Solar Cell by Using Nanostructured Titanium Oxide. Nanosci. Nanoeng. 2015, 3, 25–32. [Google Scholar] [CrossRef]
- Thube, S.G.; Nikale, V.M.; Patil, M.A. Measurement of solar constant using simple route. JETIR 2021, 8, 1362–1366. [Google Scholar]
- Hendry, E.; Koeberg, M.; O’Regan, B.; Bonn, M. Local Field Effects on Electron Transport in Nanostructured TiO2 Revealed by Terahertz Spectroscopy. Nano Lett. 2006, 6, 755–759. [Google Scholar] [CrossRef]
- Rojas, J.; Lopez, A.; Guisao, S.; Ortiz, C. Evaluation of several microcrystalline celluloses obtained from agricultural by-products. J. Adv. Pharm. Technol. Res. 2011, 2, 144. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.H.; Kumar, V.; Banker, G.S. Comparative evaluations of powder and mechanical properties of low crystallinity celluloses, microcrystalline celluloses, and powdered celluloses. Int. J. Pharm. 2002, 232, 69–80. [Google Scholar] [CrossRef] [PubMed]









| Variable | Structure |
|---|---|
| Type | Chlorophyll A |
| Molecular Formula | C55H72O5N4Mg |
| C3 Group | −CH=CH2 |
| C7 Group | −CH3 |
| C8 Group | −CH2CH3 |
| C17 Group | −CH2CH2COO-Phytyl |
| C17-C18 Bond | Single |
| Occurrence | Universal |
| Filter Paper Thickness (X = mm) | JSC mAcm−2 | Voc mV | Jm mAcm−2 | Vm mV | Vm*Jm | (Voc X Jc)/A = PCell W/cm2 | PAM1.5 W/m2 | FF % | η % | ηx/η |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 16.60 | 0.96 | 14.67 | 0.525 | 7.702 | 12.45 | 1000.0 1366.0 | 0.76 | 12.45 9.154 | 100 |
| 2.0 | 11.393 | 0.75 | 10.051 | 0.525 | 5.277 | 9.74 | 1000.0 1366.0 | 0.617 | 9.74 7.16 | 78.22 |
| 4.0 | 10.283 | 0.75 | 8.965 8.674 | 0.525 0.525 | 4.707 4.554 | 9.71 | 1000.0 1366.0 | 0.611 0.591 | 9.71 7.14 | 77.92 |
| 6.0 | 8.141 | 0.75 | 7.439 6.232 | 0.525 0.525 | 3.905 3.272 | 6.93 | 1000.0 1366.0 | 0.639 | 6.93 5.11 | 55.66 |
| 8.0 | 6.998 | 0.75 | 5.945 | 0.525 | 3.121 | 5.95 | 1000.0 1366.0 | 0.600 | 5.95 4.38 | 47.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.K.; Abo-Dief, H.M.; Alothman, Z.A.; Mohamed, A.T.; Pramanik, T.; Alotaibi, S.H. Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance. Crystals 2022, 12, 1771. https://doi.org/10.3390/cryst12121771
Alanazi AK, Abo-Dief HM, Alothman ZA, Mohamed AT, Pramanik T, Alotaibi SH. Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance. Crystals. 2022; 12(12):1771. https://doi.org/10.3390/cryst12121771
Chicago/Turabian StyleAlanazi, Abdullah K., Hala M. Abo-Dief, Zeid A. Alothman, Ashraf T. Mohamed, Tanay Pramanik, and Saad H. Alotaibi. 2022. "Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance" Crystals 12, no. 12: 1771. https://doi.org/10.3390/cryst12121771
APA StyleAlanazi, A. K., Abo-Dief, H. M., Alothman, Z. A., Mohamed, A. T., Pramanik, T., & Alotaibi, S. H. (2022). Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance. Crystals, 12(12), 1771. https://doi.org/10.3390/cryst12121771







