Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation
Abstract
1. Introduction
2. Methodology
2.1. Starting Materials
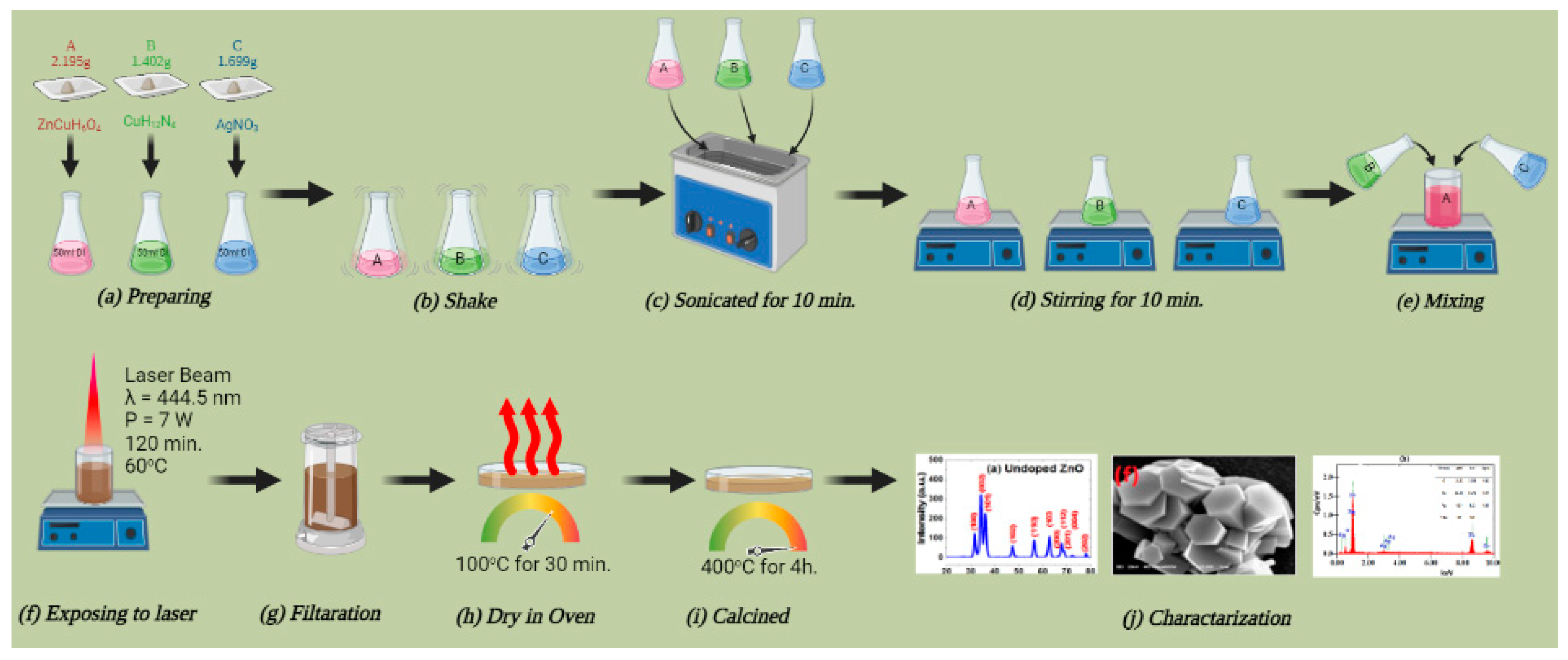
2.2. Catalyst Preparation
2.3. Synthesis ZnO Submicron Powder
2.4. Synthesis Ag-Doped ZnO Submicronflowers
2.5. Characterization
2.6. Photocatalyst Experiment
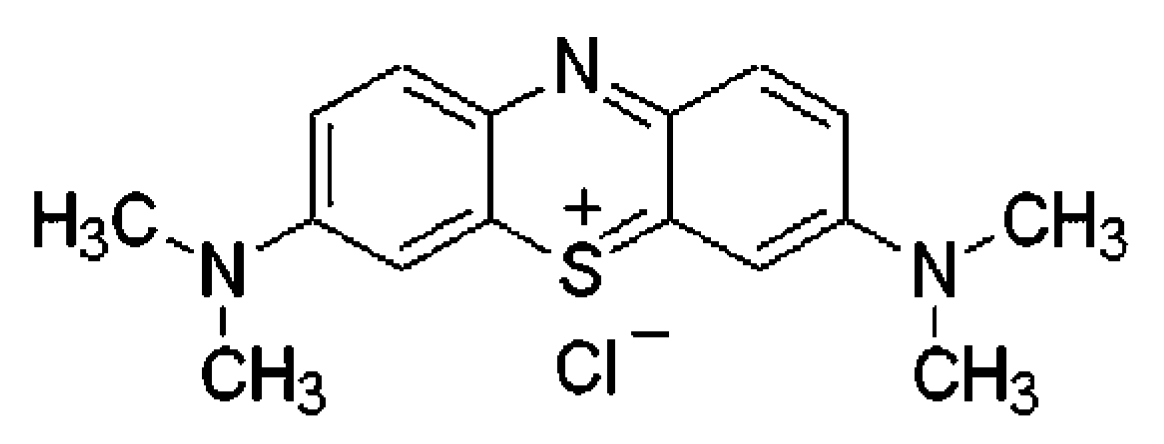
2.6.1. Preparation of Methyl Blue (MB) Solution
2.6.2. Photocatalytic Degradation of MB
3. Results and Discussion
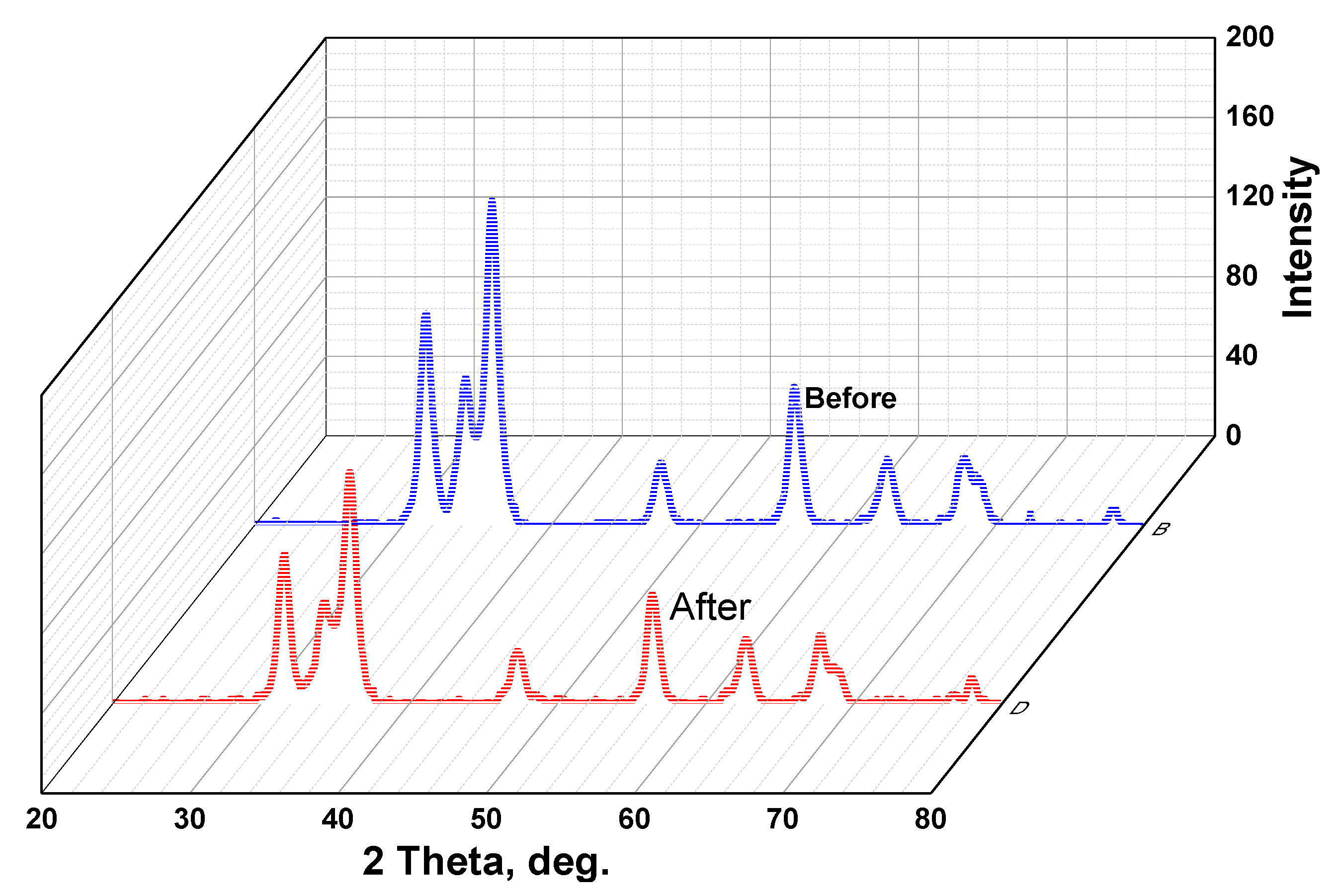
3.1. X-ray Diffraction (XRD)
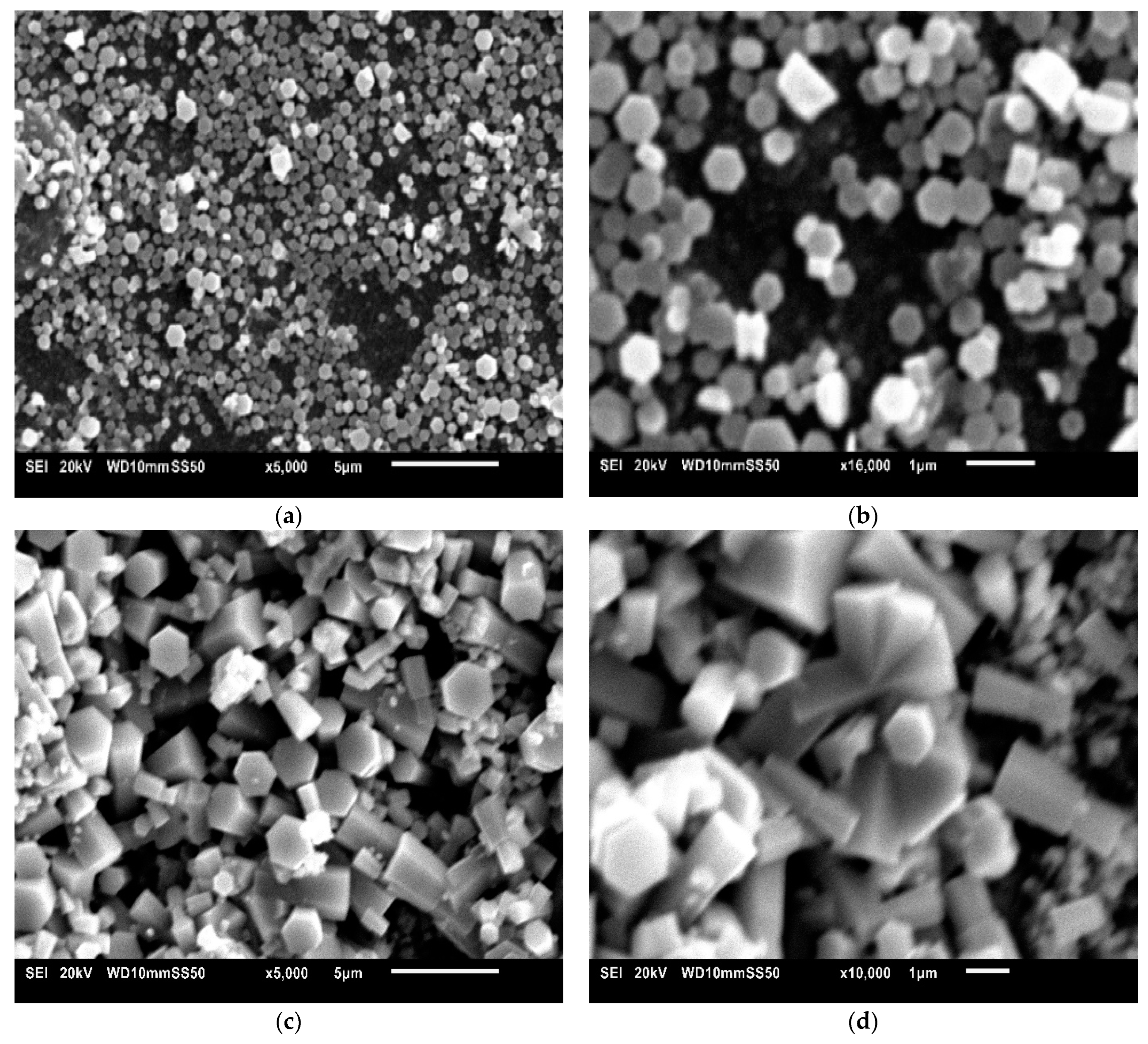
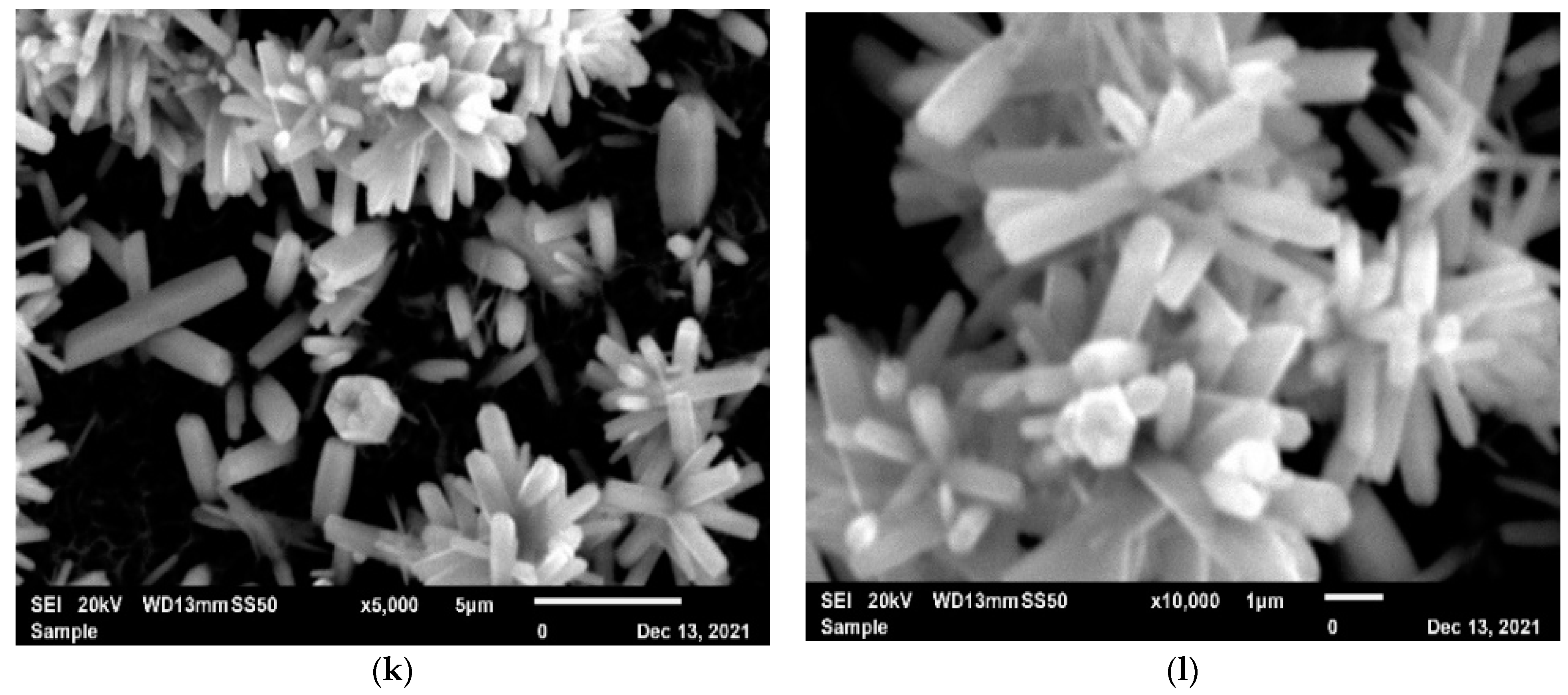
3.2. Scanning Electron Microscope (SEM)
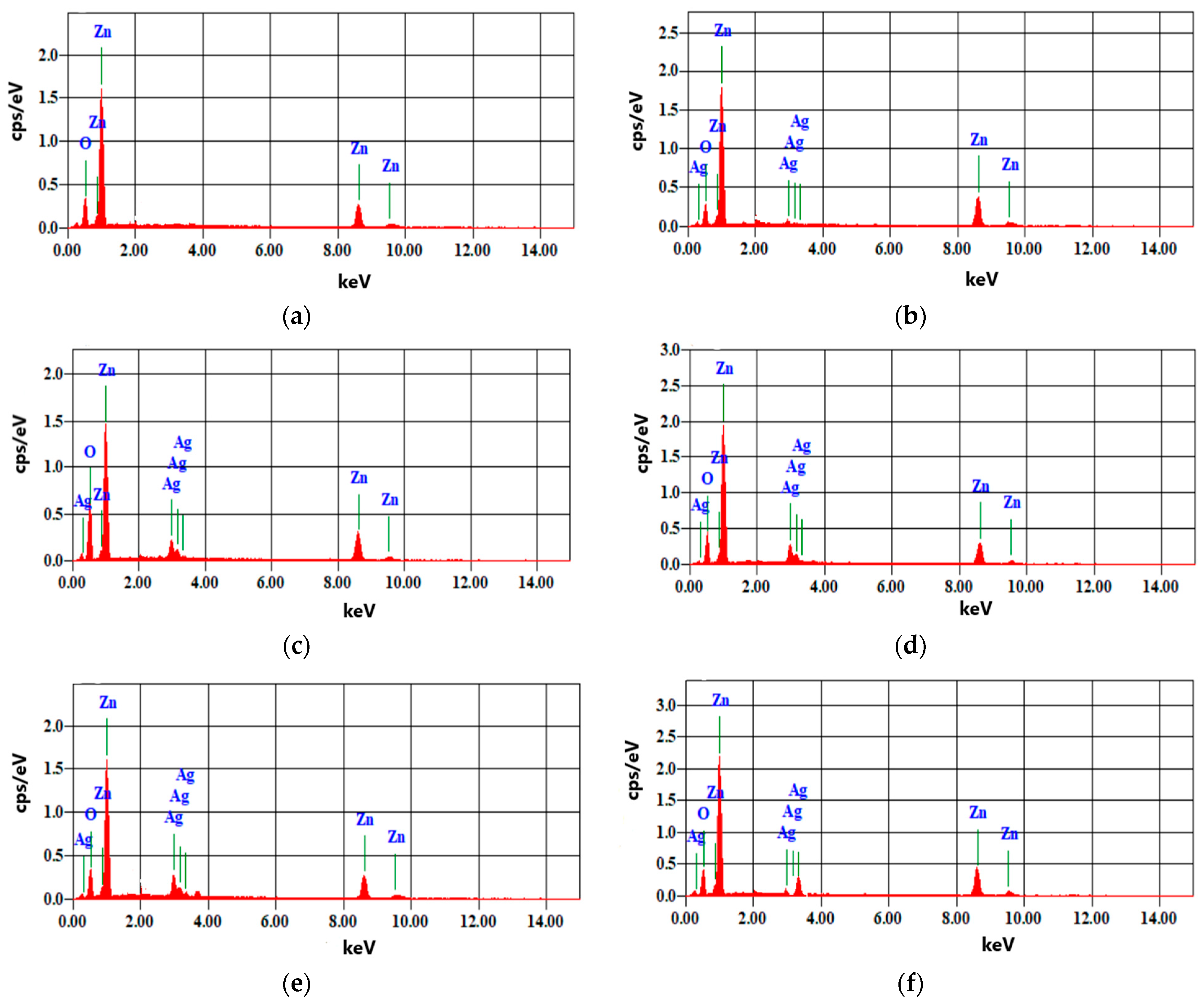
3.3. Energy Dispersive X-ray Diffractive (EDX)
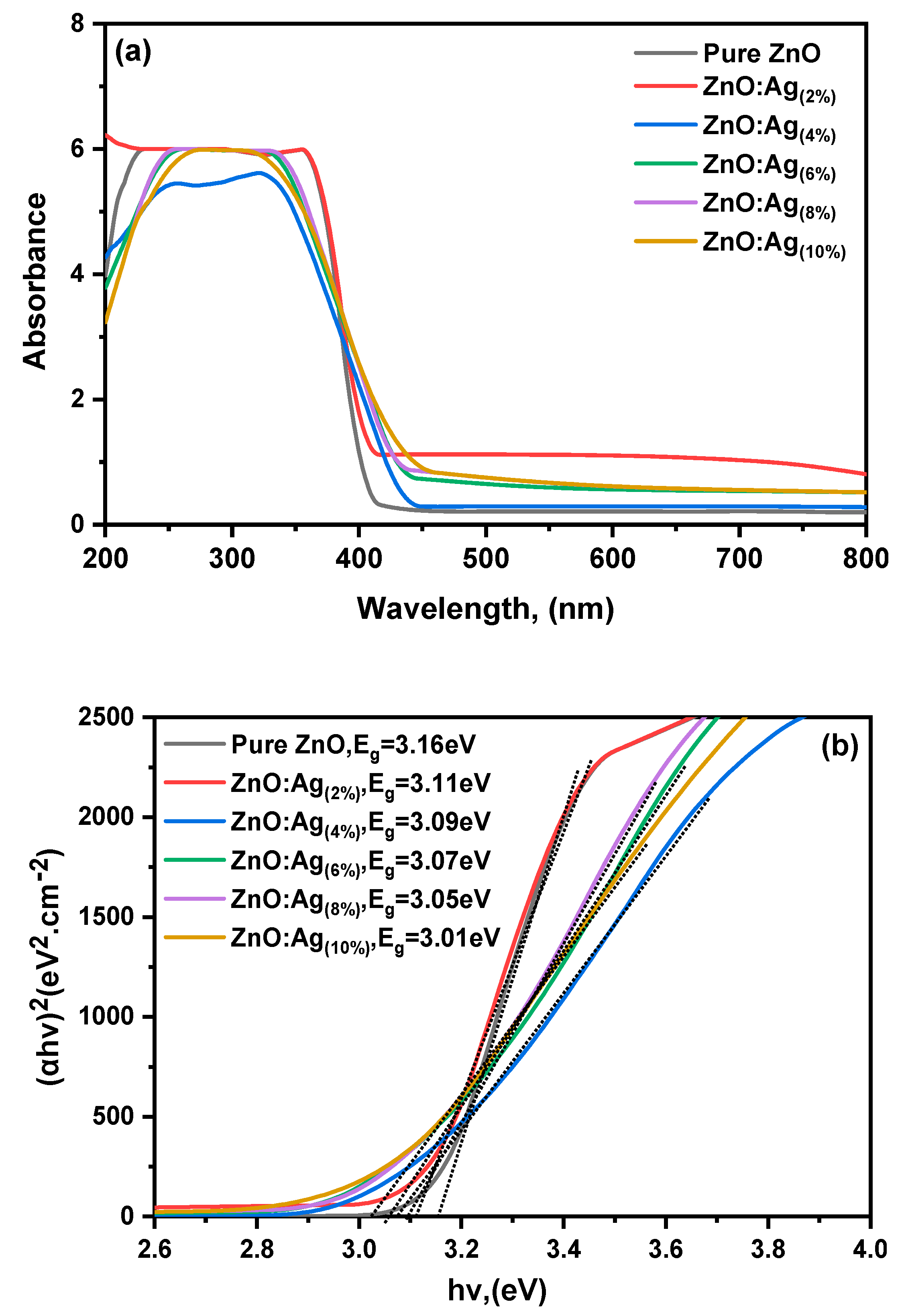
3.4. UV-Vis Absorption
3.5. FTIR
3.6. Photocatalyst Study
3.6.1. Photocatalytic Degradation under Blue Laser Irradiation/UV-Vis Studies
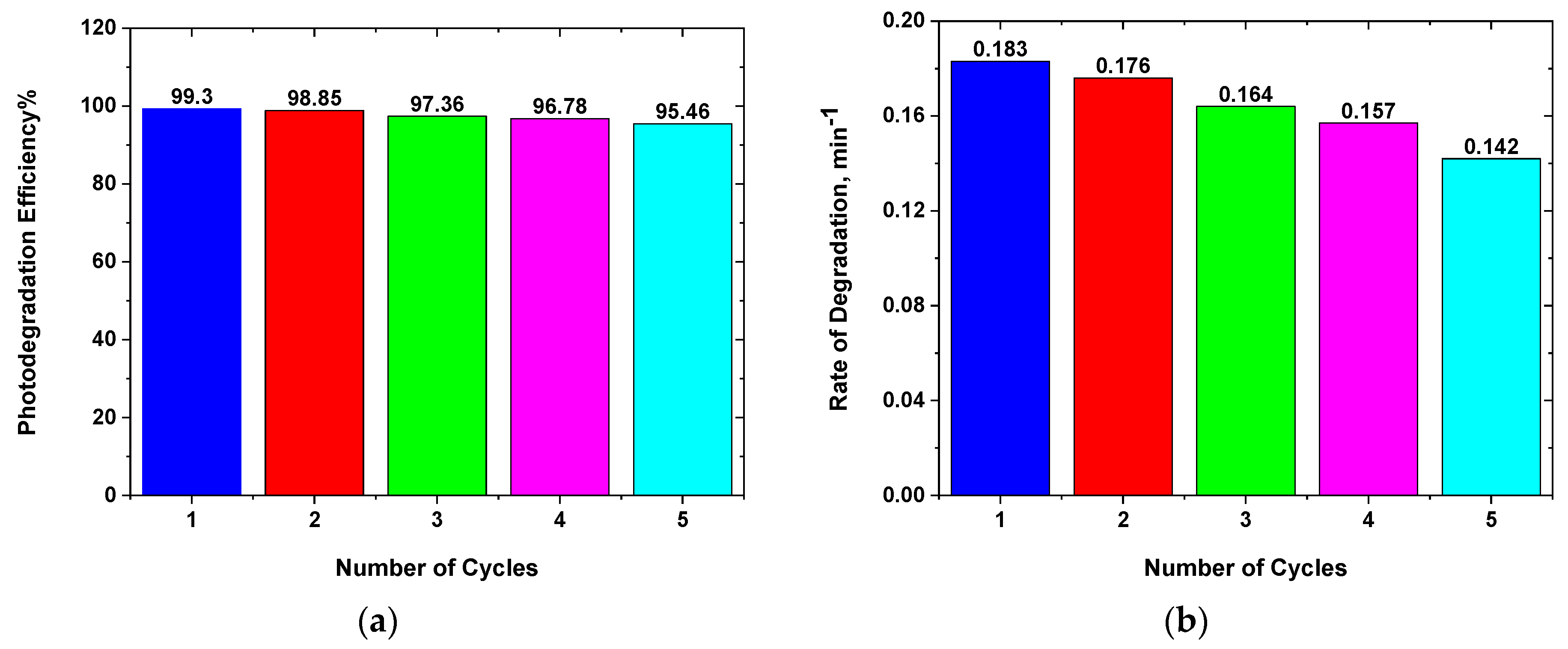
3.6.2. Photocatalytic Stability
3.6.3. The Mechanism of Photocatalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.U.; Ahn, H.J.; et al. Emerging trends in the remediation of persistent organic pollutants using nanomaterials and related processes: A review. Nanomaterials 2022, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Koop, S.H.A.; van Leeuwen, C. The challenges of water, waste and climate change in cities. Environ. Dev. Sustain. 2017, 19, 385–418. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.X.; Lin, K.L.; Zheng, Z.S.; Xiao, F.B.; Li, S.X. Sulfanilic acid-modified P25 TiO2 nanoparticles with improved photocatalytic degradation on Congo red under visible light. Dye. Pigment. 2012, 92, 1278–1284. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, S.; Yáñez, J.; Mansilla, H.D.; Riaz, U.; Chauhan, N.P.S. Conducting polymers/zinc oxide-based photocatalysts for environmental remediation: A review. Environ. Chem. Lett. 2022, 20, 2063–2083. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Qin, L.; Mawignon, F.J.; Hussain, M.; Ange, N.K.; Lu, S.; Hafezi, M.; Dong, G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials 2021, 14, 4083. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-dimensional ZnO hierarchical nanostructures: Solution phase synthesis and applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly efficient visible light active doped ZnO photocatalysts for the treatment of wastewater contaminated with dyes and pathogens of emerging concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Lemos, S.C.S.; Rezende, T.K.D.L.; Assis, M.; Romeiro, F.D.C.; Peixoto, D.A.; Gomes, E.D.O.; Jacobsen, G.M.; Teodoro, M.D.; Gracia, L.; Ferrari, J.L.; et al. Efficient Ni and Fe doping process in ZnO with enhanced photocatalytic activity: A theoretical and experimental investigation. Mater. Res. Bull. 2022, 152, 111849. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Hellín, P.; Flores, P.; Navarro, S. Heterogeneous photocatalytic oxidation of cyprodinil and fludioxonil in leaching water under solar irradiation. Chemosphere 2011, 85, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Xu, H.; Zhang, J.; Guo, J.; Cao, B. Direct hydrothermal growth of ZnO nanosheets on electrode for ethanol sensing. Sens. Actuators B Chem. 2014, 201, 444–451. [Google Scholar] [CrossRef]
- Luo, J.; Ma, S.Y.; Sun, A.M.; Cheng, L.; Yang, G.J.; Wang, T.; Gz, D.J. Ethanol sensing enhancement by optimizing ZnO nanostructure: From 1D nanorods to 3D nanoflower. Mater. Lett. 2014, 137, 17–20. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G.; Mi, J.; Wu, Z. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Avciata, O.; Benli, Y.; Gorduk, S.; Koyun, O. Ag doped TiO2 nanoparticles prepared by hydrothermal method and coating of the nanoparticles on the ceramic pellets for photocatalytic study: Surface properties and photoactivity. J. Eng. Technol. Appl. Sci. 2016, 1, 34–50. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Z.; Chen, D.; Liu, F.; Yang, Z.; Li, X.; Yu, H.; Liu, H.; Zhou, W. Laser synthesis and microfabrication of micro/nanostructured materials toward energy conversion and storage. Nano-Micro Lett. 2021, 13, 49. [Google Scholar] [CrossRef]
- Palneedi, H.; Park, J.H.; Maurya, D.; Peddigari, M.; Hwang, G.T.; Annapureddy, V.; Kim, J.W.; Choi, J.J.; Hahn, B.D.; Priya, S.; et al. Laser irradiation of metal oxide films and nanostructures: Applications and advances. Adv. Mater. 2018, 30, 1705148. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Ahmed, N.M.; Lahewil, A.S.Z.; Omar, A.F. Micro spot ZnO nanotubes using laser assisted chemical bath deposition: A low-cost approach to UV photodetector fabrication. Sens. Actuators A Phys. 2022, 338, 113485. [Google Scholar] [CrossRef]
- Palneedi, H.; Park, J.H.; Maurya, D.; Peddigari, M.; Hwang, G.-T.; Annapureddy, V.; Kim, J.-W.; Choi, J.-J.; Hahn, B.-D.; Priya, S.; et al. Laser processing of metal oxides: Laser irradiation of metal oxide films and nanostructures: Applications and advances. Adv. Mater. 2018, 30, 1870094. [Google Scholar] [CrossRef]
- Vulfrano González-Fernández, J.; David Pinzón-Moreno, D.; Neciosup-Puican, A.A.; Verónica Carranza-Oropeza, M. Green method, optical and structural characterization of zno nanoparticles synthesized using leaves extract of m. Oleifera. J. Renew. Mater. 2022, 10, 833. [Google Scholar]
- Primo, J.d.O.; Horsth, D.F.; Correa, J.d.S.; Das, A.; Bittencourt, C.; Umek, P.; Buzanich, A.G.; Radtke, M.; Yusenko, K.V.; Zanette, C.; et al. Synthesis and Characterization of Ag/ZnO Nanoparticles for Bacteria Disinfection in Water. Nanomaterials 2022, 12, 1764. [Google Scholar] [CrossRef]
- Primc, G.; Brenčič, K.; Mozetič, M.; Gorjanc, M.; de Giacomo, A. Recent advances in the plasma-assisted synthesis of zinc oxide nanoparticles. Nanomaterials 2021, 11, 1191. [Google Scholar] [CrossRef]
- Wagner, E.; Maudez, W.; Bagdzevicius, S.; Sandu, S.C.; Benvenuti, G. Chemical beam vapour deposition technique with Sybilla equipment: Review of main results in its 20-year anniversary. Oxide-Based Mater. Devices XII 2021, 11687, 135–154. [Google Scholar]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. II. Adiabatic principle of X-ray diffraction analysis of mixtures. J. Appl. Crystallogr. 1974, 7, 526–531. [Google Scholar] [CrossRef]
- Pakma, O.; Özaydın, C.; Özden, Ş.; Kariper, I.A.; Güllü, Ö. Synthesis and characterization of vanadium oxide thin films on different substrates. J. Mater. Sci. Mater. Electron. 2017, 28, 10909–10913. [Google Scholar] [CrossRef]
- Bharathi, V.; Sivakumar, M.; Udayabhaskar, R.; Takebe, H.; Karthikeyan, B. Optical, structural, enhanced local vibrational and fluorescence properties in K-doped ZnO nanostructures. Appl. Phys. A 2014, 116, 395–401. [Google Scholar] [CrossRef]
- Modwi, A.; Ali, M.K.M.; Taha, K.K.; Ibrahem, M.A.; El-Khair, H.M.; Eisa, M.H.; Elamin, M.R.; AAldaghri, O.; Alhathlool, R.; Ibnaouf, K.H. Structural and optical characteristic of chalcone doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 2791–2796. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Popović, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M.G.; Jain, R.K. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. 2011, 123, 11619–11622. [Google Scholar] [CrossRef]
- Stetsenko, M.; Rudenko, S.P.; Maksimenko, L.S.; Serdega, B.K.; Pluchery, O.; Snegir, S.V. Optical properties of gold nanoparticle assemblies on a glass surface. Nanoscale Res. Lett. 2017, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel green route of Synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci. Part A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Dai, H. Probing dissolved CO2 (aq) in aqueous solutions for CO2 electroreduction and storage. Sci. Adv. 2022, 8, eabo0399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wen, J.Z.; Zhao, P.; Anderson, W.A. Synthesis of vertically-aligned zinc oxide nanowires and their application as a photocatalyst. Nanomaterials 2017, 7, 9. [Google Scholar] [CrossRef]
- Alrebdi, T.A.; Rezk, R.A.; Alghamdi, S.M.; Ahmed, H.A.; Alkallas, F.H.; Pashameah, R.A.; Mostafa, A.M.; Mwafy, E.A. Photocatalytic Performance Improvement by Doping Ag on ZnO/MWCNTs Nanocomposite Prepared with Pulsed Laser Ablation Method Based Photocatalysts Degrading Rhodamine B Organic Pollutant Dye. Membranes 2022, 12, 877. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef]
- Han, X.G.; He, H.Z.; Kuang, Q.; Zhou, X.; Zhang, X.H.; Xu, T.; Xie, Z.X.; Zheng, L.S. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C 2009, 113, 584–589. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Mosquera, E.; Rojas-Michea, C.; Morel, M.; Gracia, F.; Fuenzalida, V.; Zárate, R.A. Zinc oxide nanoparticles with incorporated silver: Structural, morphological, optical and vibrational properties. Appl. Surf. Sci. 2015, 347, 561–568. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Q.; Liu, J.; Cui, N.; Guan, G.; Huang, W. Electron promoted ZnO for catalytic synthesis of higher alcohols from syngas. Green Energy Environ. 2022, 7, 1390–1400. [Google Scholar] [CrossRef]
- Sun, M.; Kong, W.; Zhao, Y.; Liu, X.; Xuan, J.; Liu, Y.; Jia, F.; Yin, G.; Wang, J.; Zhang, J. Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials 2019, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Das, T.; Baruah, S. Improvement of photocatalytic activity of Zinc Oxide nanoparticles using Zinc Sulphide Shell. ADBU J. Eng. Technol. 2016, 4, 137–141. [Google Scholar]
- Alahmadi, N. Recent Progress in Photocatalytic Removal of Environmental Pollution Hazards in Water Using Nanostructured Materials. Separations 2022, 9, 264. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Bakhtiari, M.; Hayati, B.; Shekarchi, A.A.; Bagheri, A.; Rahimi, S. Environmentally friendly ultrasound-assisted synthesis of magnetic zeolitic imidazolate framework-Graphene oxide nanocomposites and pollutant removal from water. J. Mol. Liq. 2019, 282, 115–130. [Google Scholar] [CrossRef]
- Mohajershojaei, K.; Mahmoodi, N.M.; Khosravi, A. Immobilization of laccase enzyme onto titania nanoparticle and decolorization of dyes from single and binary systems. Biotechnol. Bioprocess Eng. 2015, 20, 109–116. [Google Scholar] [CrossRef]









| NO | (h, k, l) | (deg) | (deg) | I | D (Å) | a = b (Å) | c (Å) | c/a | L (Å) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnO | (100) | 31.63 | 2.82 | 0.86 | 83 | 1.67 | 3.26 | 5.65 | 5.65 | 0.48 | 8.68 | 2.82 | 0.36 | 2.04 |
| (002) | 34.26 | 2.61 | 0.88 | 218 | 1.64 | 3.02 | 5.23 | 5.23 | −7.02 | 0.55 | 2.61 | 0.36 | 1.88 | |
| (101) | 36.04 | 2.48 | 0.99 | 153 | 1.46 | 2.88 | 4.98 | 4.98 | −11.47 | −4.25 | 2.49 | 0.36 | 1.79 | |
| ZnO:Ag 2% | (100) | 31.62 | 2.82 | 0.67 | 47 | 2.14 | 3.26 | 5.65 | 5.65 | 0.50 | 8.70 | 2.82 | 0.36 | 2.04 |
| (002) | 34.22 | 2.61 | 0.82 | 118 | 1.76 | 3.02 | 5.23 | 5.23 | −6.91 | 0.67 | 2.61 | 0.36 | 1.89 | |
| (101) | 36.08 | 2.48 | 0.75 | 89 | 1.93 | 2.87 | 4.97 | 4.97 | −11.56 | −4.35 | 2.48 | 0.36 | 1.79 | |
| ZnO:Ag 4% | (100) | 31.62 | 2.82 | 0.77 | 112 | 1.87 | 3.26 | 5.65 | 5.65 | 0.51 | 8.71 | 2.82 | 0.36 | 2.04 |
| (002) | 34.21 | 2.61 | 0.81 | 194 | 1.78 | 3.02 | 5.24 | 5.24 | −6.89 | 0.69 | 2.62 | 0.36 | 1.89 | |
| (101) | 36.06 | 2.48 | 0.84 | 191 | 1.73 | 2.87 | 4.97 | 4.97 | −11.53 | −4.32 | 2.48 | 0.36 | 1.88 | |
| ZnO:Ag 6% | (100) | 31.61 | 2.82 | 0.69 | 158 | 2.07 | 3.26 | 5.65 | 5.65 | 0.54 | 8.74 | 2.82 | 0.36 | 2.04 |
| (002) | 34.22 | 2.61 | 0.76 | 205 | 1.88 | 3.02 | 5.23 | 5.23 | −6.92 | 0.66 | 2.61 | 0.36 | 1.89 | |
| (101) | 36.06 | 2.48 | 0.78 | 254 | 1.85 | 2.87 | 4.98 | 4.97 | −11.53 | −4.31 | 2.48 | 0.36 | 1.79 | |
| ZnO:Ag 8% | (100) | 31.59 | 2.82 | 0.73 | 179 | 1.97 | 3.26 | 5.66 | 5.66 | 0.59 | 8.79 | 2.83 | 0.36 | 2.04 |
| (002) | 34.21 | 2.61 | 0.82 | 204 | 1.76 | 3.02 | 5.24 | 5.23 | −6.90 | 0.68 | 2.61 | 0.36 | 1.89 | |
| (101) | 36.06 | 2.48 | 0.77 | 311 | 1.89 | 2.87 | 4.98 | 4.97 | −11.52 | −4.31 | 2.48 | 0.36 | 1.79 | |
| ZnO:Ag 10% | (100) | 31.59 | 2.82 | 0.91 | 73 | 1.57 | 3.26 | 5.66 | 5.66 | 0.59 | 8.79 | 2.83 | 0.36 | 2.04 |
| (002) | 34.31 | 2.61 | 1.18 | 51 | 1.23 | 3.01 | 5.22 | 5.22 | −7.17 | 0.39 | 2.61 | 0.36 | 1.88 | |
| (101) | 36.00 | 2.49 | 0.98 | 112 | 1.48 | 2.87 | 4.98 | 4.98 | −11.37 | −4.15 | 2.49 | 0.36 | 1.80 |
| Samples | Pure ZnO | ZnO:Ag(2%) | ZnO:Ag(4%) | ZnO:Ag(6%) | ZnO:Ag(8%) | ZnO:Ag(10%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | wt% | At% | wt% | At% | wt% | At% | wt% | At% | wt% | At% | wt% | At% |
| O | 19.70 | 50.06 | 10.31 | 32.36 | 14.51 | 42.03 | 24.20 | 58.84 | 19.32 | 52.02 | 15.53 | 45.5 |
| Zn | 80.30 | 49.94 | 85.56 | 65.72 | 76.03 | 53.90 | 58.97 | 35.09 | 60.63 | 39.97 | 62.98 | 45.16 |
| Ag | 0 | 0 | 4.13 | 1.92 | 9.46 | 4.07 | 16.83 | 6.07 | 20.06 | 8.01 | 21.49 | 9.34 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Synthesis Method | Catalyst Type | Catalyst Amount | Dye Concentration | Light Source | %Deg | Time (min) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Co-precipitation | ZnO:Ag(0.05) | 10 mg/100 mL | MB (20 ppm) | visible | 98 | 0.017 | 120 | [40] |
| Co-precipitation | ZnO:Ag(0.10) | 15 mg/25 mL | MB (25 ppm) | visible | 98 | 120 | [41] | |
| Hydrothermal | ZnO:Ag(0.05) | 50 mg/100 mL | MB (10 ppm) | visible | 92.9 | 210 | [42] | |
| Co-precipitation | ZnO:Ag(0.02) | 10 mg/50mL | MB (20 ppm) | visible | 96 | 80 | [43] | |
| LACBS | ZnO | 10 mg/50 mL | MB (20 ppm) | Blue | 68.5 | 0.045 | 25 | This work |
| LACBS | ZnO:Ag(2%) | 10 mg/50 mL | MB (20 ppm) | Blue | 68.5 | 0.052 | 25 | This work |
| LACBS | ZnO:Ag(4%) | 10 mg/50 mL | MB (20 ppm) | Blue | 83.5 | 0.097 | 25 | This work |
| LACBS | ZnO:Ag(6%) | 10 mg/50 mL | MB (20 ppm) | Blue | 92.9 | 0.106 | 25 | This work |
| LACBS | ZnO:Ag(8%) | 10 mg/50 mL | MB (20 ppm) | Blue | 94.1 | 0.098 | 25 | This work |
| LACBS | ZnO:Ag(10%) | 10 mg/50 mL | MB (20 ppm) | Blue | 99.3 | 0.183 | 25 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyoud, S.H.; Yahia, I.S.; Shahwan, M.; Zyoud, A.H.; Zahran, H.Y.; Abdel-wahab, M.S.; Daher, M.G.; Nasor, M.; Makhadmeh, G.N.; Hassan, N.; et al. Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation. Crystals 2023, 13, 229. https://doi.org/10.3390/cryst13020229
Zyoud SH, Yahia IS, Shahwan M, Zyoud AH, Zahran HY, Abdel-wahab MS, Daher MG, Nasor M, Makhadmeh GN, Hassan N, et al. Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation. Crystals. 2023; 13(2):229. https://doi.org/10.3390/cryst13020229
Chicago/Turabian StyleZyoud, Samer H., Ibrahim S. Yahia, Moyad Shahwan, Ahed H. Zyoud, Heba Y. Zahran, Mohamed Sh. Abdel-wahab, Malek G. Daher, Mohamed Nasor, Ghaseb N. Makhadmeh, Nageeb Hassan, and et al. 2023. "Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation" Crystals 13, no. 2: 229. https://doi.org/10.3390/cryst13020229
APA StyleZyoud, S. H., Yahia, I. S., Shahwan, M., Zyoud, A. H., Zahran, H. Y., Abdel-wahab, M. S., Daher, M. G., Nasor, M., Makhadmeh, G. N., Hassan, N., Ashames, A., & Qamhieh, N. (2023). Fast and Excellent Enhanced Photocatalytic Degradation of Methylene Blue Using Silver-Doped Zinc Oxide Submicron Structures under Blue Laser Irradiation. Crystals, 13(2), 229. https://doi.org/10.3390/cryst13020229







