Heterotridentate Organomonophosphines in Pt(κ3–X1P1X2)(Y) (X1,2 = N1,2 or S1,2), Pt(κ3–P1N1X1)(Y) (X1 = O, C, S or Se) Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL)—Structural Aspects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pt(κ3–P1N1N2)(Y) Derivatives
2.2. Pt(κ3–P1N1O1)(Y) Derivatives
2.3. Pt(κ3–P1N1C1)(Y) Derivatives
2.4. Pt(κ3–P1N1S1)(Y) Derivatives
2.5. Pt(κ3–P1N1Se1)(Y) Derivatives
2.6. Pt(κ3–N1P1N2)(Cl) and Pt(κ3–S1P1S2)(Cl) Derivatives
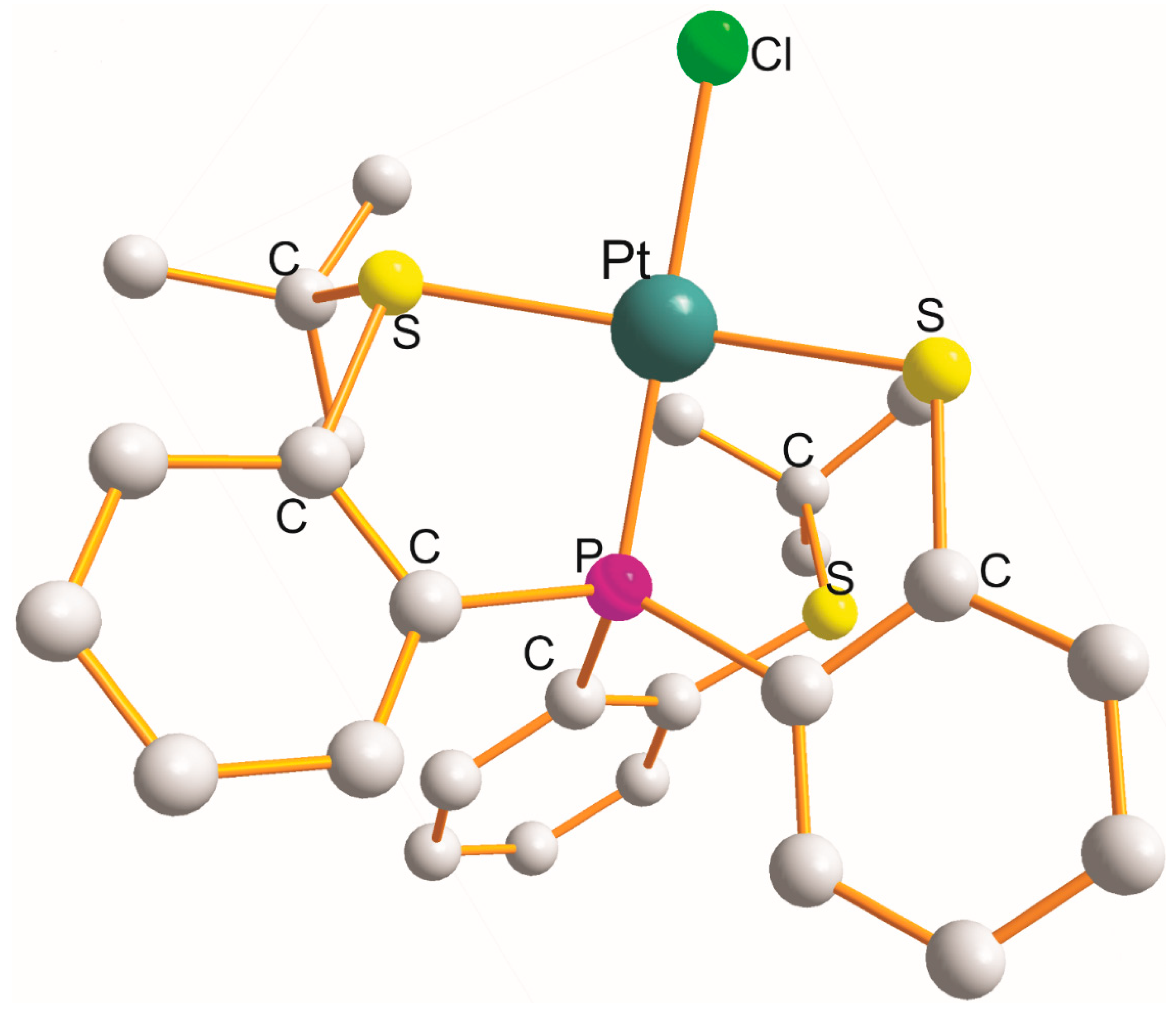
2.7. Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL) Derivatives
- 5 + 5—membered: P1C2P1C2N2 (1 example), P1C2N1NCO1 (1 example), P1C2N1NCC1 (1 example) and S1C2N1C2S2 (2 examples)
- 6 + 5—membered: P1C3N1C2N2 (6 examples), P1C3N1NCN2, (1 example), P1C3N1C2C1, (1 example) and P1C3N1NCS1 (1 example)
- 5 + 6—membered: P1C2N1C3O1 (3 examples), P1C2S1C2BCl1, (1 example) and P1C2Si1C3N1 (1 example)
- 6 + 6—membered: P1C3N1C3N2, P1C3N1C3O1, P1C3N1C3S1, P1C3N1C3Se1 and N1C2NP1NC2N2 (each 1 example)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| But2P1(CH2)(C5H3N1)(CH2)N2Et2 | (2-(di-t-butylphosphinomethyl)-6-diethyl-aminomethyl)pyridine) |
| ButS1(C6H4)P1(C6H4SBut)(C6H4)S2) | (2-((2-(t-butylsulfanyl)phenyl)(2-t-butyl-sulfanyl)phenyl)phosphino)benzenethiazato |
| cyh2P1(C6H4)Si1(CH3)(C7H6N1(Me2)) | ((2-(dicyclohexylphosphanyl)(2-(dimethyl-amino)methyl)phenyl)methylsilyl) |
| m | monoclinic |
| N1(C6H6)N(C6H10)NP1(Pri)(C6H6)N2 | (2-isopropyl-1,3-bis(2-pyridylmethyl)-octahydro-1H-1,3,2-benzodiazophosphore |
| or | orthorhombic |
| Ph2P1((C6H4CHN1NC(S1).NHMe) | (2-(diphenylphosphino)thiosemicarbazide |
| Ph2P1(C23H28S1)(B(Ph2)Cl1) | ((2,7-di-t-butyl-5-((chloro)(diphenyl)-25-boranyl)-9,9-dimethyl-9H-thioxantin-4-yl)(diphonylphosphine) |
| Ph2P1(C6H4N1)(C7H4ClO1)(C7H8N2) | (4-chloro-2-(((2-(diphenylphosphino)phenylimino)methylphenylate |
| Ph2P1(C6H4N1)(C8H7NOO1) | (2-(((2-(diphenylphosphino)phenylimino)methyl-4-methoxyphenylato) |
| Ph2P1(C7H5N1)(C2H2O)N2(C6H4OH) | (N-(2-(diphenylphosphinobenzylidene)-N-(2-hydroxyphenyl)glycinamidato) |
| Ph2P1(C7H5N1)(C2H2O)N2(C6H4OH) | (N2-(2-(diphenylphosphino)benzylidene)-N-(3-hydroxyphenyl)glycinamidato |
| Ph2P1(C7H5N1)(C2H2O)N2(C7H6OH) | (N-(2-(diphenylphosphino)benzylidene)-N-(2-hydroxymethylphenyl)glycine-amidato) |
| Ph2P1(C7H5N1)(C3H6)N2(C7H5O2) | (N-(2-((2-(diphenylphosphino)benzylidene)amino)propyl)-2-hydroxybenzamidato) |
| Ph2P1(C7H5N1)(C5H7O)N2(C10H10N2) | R7C-(N-(5,7-(dimethyl-1,8-naphtylridin-2-yl)-N2-(2-(diphenylphosphinyl)benzylidene) valinamidato) |
| Ph2P1(C7H5N1)(C6H4)N2(C10H9NO3) | (N2-benzyloxycarbinol)-N-(2-(((2-(diphenylphosphanyl)phenyl)methylidene) amino)phenyl)glycinamide) |
| Ph2P1(C7H5N1)(C7H8C1)(C7H8N2) | (2-(1b)-1-(((2-diphenylphosphinobenzylidene)amine) ethyl)phenyl) |
| Ph2P1(C7H5N1)(MeS1)(ButNH2) | (N-{N-[2-(diphenylphosphino)benzylidene)]-D/L-methionyl}-terc-butylamine |
| Ph2P1(C7H5N1)(NC5H4N2) | (2-(2-(diphenylphosphino)benzylidene)-1-(pyridine-2-yl)diazanido |
| Ph2P1(C7H5N1C3H6Se1(Ph) | (N-(2-(diphenylphosphino)benzylidene)-N-(3-(phenylseleno)propyl)amine |
| Ph2P1(C7H5N1O1) | (2-diphenylphosphino)-2-aminobenzaldehyde) |
| Ph2P1(C7H6N1)(C7H8N)(C7H8N2) | (N2-(2-(diphenylphosphino)benzyl)-N,N-bis(2-pyridyl-2-ethyl)amine) |
| Ph2P1(C7H6N1)(NC7H5O1)Ph2P2(C15H13N2O) | (N-(2-(diphenylphosphino)-1-phenylformyl)benzohydiazino)-N-(2-(diphenylphosphino)-1-phenylvinyl)benzohydeazone) |
| Ph2P1(C7H6N1 = NCC1C5H6) | (2-((2-(diphenylphosphino)-4-methylphenyl)diazinyl)-5-methylphenyl)pyridine |
| PriS1(C6H4)P1(C6H4SPri).(C6H4)S2) | (2-(((2-(isopropysulfanyl)phenyl)(2-isopropylsulfanyl)phenyl)phosphino)benzenethiazato) |
| py | pyridine |
| tr | triclinic |
References
- Holloway, C.E.; Melnik, M. Structural aspect of platinum coordination compounds: Part III—Monomeric square planar (PtA2XY and PtABXY) and trigonal bipyramidal PtII coordination compounds. Rev. Inorg. Chem. 2004, 24, 135–299. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Structural aspect of platinum coordination compounds: Part I—Monomeric Pt0, PtI and PtIIA4 derivatives. Rev. Inorg. Chem. 2002, 22, 163–284. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Structural Aspect of Platinum Coordination Compounds: Part II—Monomeric PtII Compounds with PtA3B and PtA2B2 Composition. Rev. Inorg. Chem. 2003, 23, 125–287. [Google Scholar] [CrossRef]
- Melnik, M.; Holloway, C.E. Stereochemistry of platinum coordination compounds. Coord. Chem. Rev. 2006, 250, 2261–2270. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Distortion isomers of cis-PtP2X2 and cis-PtP2XY derivatives—Structural aspects. Rev. Inorg. Chem. 2020, 40, 153–165. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Ligand isomerism in Pt(II) complexes—Structural aspects. Rev. Inorg. Chem. 2022, 42, 21–28. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Heterotridentate organodiphosphines in Pt(η3–P1X1P2)(Y) (X1 = B, S, or Si) and Pt(η3–P1P2Si1)(Y) derivatives-structural aspects. Rev. Inorg. Chem. 2021, 42, 21–28. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Organodiphosphines in Pt{η2-P(X)nP}Cl2 (n = 9–15, 17, 18) derivatives—Structural aspects. Rev. Inorg. Chem. 2021, 41, 41–48. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Heterotridentate organodiphosphines in Pt(η3–P1X1P2)(Y) derivatives-structural aspects. Rev. Inorg. Chem. 2021, 41, 41–48. [Google Scholar] [CrossRef]
- Vuzman, D.; Poverenov, E.; Shimon, L.J.W.; Diskin-Posner, Y.; Milstein, D. Cationic, Neutral and Anionic Platinum(II) Complexes Based on an Electron-Rich PNN Ligand. New Modes of Reactivity Based on Pincer Hemilability and Dearomatization. Organometallics 2008, 27, 2627–2634. [Google Scholar] [CrossRef]
- Durran, S.E.; Elsegood, M.R.J.; Hammond, S.R.; Smith, M.B. Flexible κ4-PNN′O-Tetradentate Ligands: Synthesis, Complexation and Structural Studies. Dalton Trans. 2010, 39, 7136–7146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durran, S.E.; Elsegood, M.R.J.; Hammond, S.R.; Smith, M.B. Coordination Studies of a New Unsymmetrical κ4-PNN‘N‘ ‘-Tetradentate Ligand: Stepwise Formation and Structural Characterization. Inorg. Chem. 2007, 46, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.A.; Clarke, M.L.; ZSlawin, A.M. A Supramolecular Approach to Chiral Ligand Modification: Coordination Chemistry of a Multifunctionalised Tridentate Amine-Phosphine Ligand. New J. Chem. 2008, 32, 689–693. [Google Scholar] [CrossRef]
- Elsegood, M.R.J.; Sanchez-Ballester, N.M.; Smith, M.B. New κ3-PNN′- and κ4-PNN′O-Polydentate Ligands: Synthesis, Coordination and Structural Studies. Inorg. Chim. Acta 2011, 379, 115–121. [Google Scholar] [CrossRef]
- Chang, M.; Horiki, H.; Nakajima, K.; Kobayashi, A.; Chang, H.-C.; Kato, M. Acid–Base Behavior of Substituted Hydrazone Complexes Controlled by the Coordination Geometry. Bull. Chem. Soc. Jpn. 2010, 83, 905–910. [Google Scholar] [CrossRef]
- Watkins, S.E.; Craig, D.C.; Colbran, S.B. Towards Co-Operative Reactivity in Conjoint Classical-Organometallic Heterometallic Complexes: The Co-Ordination Chemistry of Novel Ligands with Triphenylphosphine and Bis(Pyridylethyl)Amine or Triazacyclononane Domains. J. Chem. Soc. Dalton Trans. 2002, 12, 2423–2436. [Google Scholar] [CrossRef]
- Ahmad, M.; Perera, S.D.; Shaw, B.L.; Thornton-Pett, M. Uni-, Bi- and Ter-Dentate Complexes Formed from PPh2CH2C(R)NNHC(O)Ph (R = But or Ph) and Pd or Pt: Crystal Structures of [PdCl{PPh2CH2C(But)NNC(Ph)O}], [Pt{PPh2CHC(Ph)NNC(Ph)O}{PPh2CH2C(Ph)NNHC(O)Ph}] and [Pd{PPh2CHC(But)NHNC(O)Ph}2]. J. Chem. Soc. Dalton Trans. 2002, 9, 1954–1962. [Google Scholar] [CrossRef]
- Ní Dhubhghaill, O.M.; Lennon, J.; Drew, M.G.B. Palladium(Ii) and Platinum(Ii) Complexes with Tridentate Iminophosphine Ligands; Synthesis and Structural Studies. Dalton Trans. 2005, 19, 3213–3220. [Google Scholar] [CrossRef]
- Jircitano, A.J.; Mertes, K.B. Ligands with Dual Denticity: Crystal and Molecular Structure of Dichloro-Bis(o-Diphenylphosphino)-Benzaldehyde)Platinum(II). Inorg. Chim. Acta 1985, 103, L11–L13. [Google Scholar] [CrossRef]
- Kano, N.; Yamamura, M.; Meng, X.; Yasuzuka, T.; Kawashima, T. Different Coordination Modes of 2-(Diphenylphosphino)Azobenzenes in Complexation with Hard and Soft Metals. Dalton Trans. 2012, 41, 11491–11496. [Google Scholar] [CrossRef]
- Ramírez, P.; Contreras, R.; Valderrama, M.; Carmona, D.; Lahoz, F.J.; Balana, A.I. Cyclometallated Platinum(II) Complexes Containing the Chiral Ligand [2-(Diphenyl-Phosphanyl)-Benzylidene]-(1-Phenyl-Ethyl)-Amine: Synthesis and Molecular Structures of the Compounds [PtCl(Me){κ2-(R)-Ph2P(C6H4)CHNCH(Ph)Me-P,N}] and [Pt{κ3-(S)-Ph2P(C6H4)CHN. J. Organomet. Chem. 2008, 693, 349–356. [Google Scholar] [CrossRef]
- You, D.; Kang, S.O.; Ko, J.J.; Choi, M. Cycloplatinated complexes of thiosemicarbazones. Synthesis and crystal structure of [Ph2PC6H4CHNNC(S)NHCH3PtCl]. Bull. Korean Chem. Soc. 1997, 18, 305–310. [Google Scholar]
- Ankersmit, H.A.; Veldman, N.; Spek, A.L.; Vrieze, K.; van Koten, G. Methyl-, Acetyl- and Allyl-Palladium and -Platinum Complexes Containing Novel Terdentate PNS and NN’S Ligands. Inorg. Chim. Acta 1996, 252, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Durran, S.E.; Elsegood, M.R.J.; Smith, M.B. New Complexes of Functionalised Ligands Bearing P/N/Se or P2Se Donor Sets. New J. Chem. 2002, 26, 1402–1408. [Google Scholar] [CrossRef]
- Edwards, P.G.; Kariuki, B.; Newman, P.D. Coordination Behaviour in Transition Metal Complexes of Asymmetric NPN Ligands. Polyhedron 2011, 30, 935–941. [Google Scholar] [CrossRef]
- Takeda, N.; Tanaka, Y.; Oma, R.; Sakakibara, F.; Unno, M. Activation of C-S Bond by Group 10 Metal Complexes: Reaction of Phosphine Ligand Tethered with Three tert-Butylthiophenyl Groups with Group 10 Metal Compounds. Bull. Chem. Soc. Jpn. 2016, 89, 922–930. [Google Scholar] [CrossRef]
- Emslie, D.J.H.; Cowie, B.E.; Oakley, S.R.; Huk, N.L.; Jenkins, H.A.; Harrington, L.E.; Britten, J.F. A Study of M–X–BR3 (M = Pt, Pd or Rh; X = Cl or I) Interactions in Square Planar Ambiphilic Ligand Complexes: Structural, Spectroscopic, Electrochemical and Computational Comparisons with Borane-Free Analogues. Dalton Trans. 2012, 41, 3523–3535. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Ito, S.; Nomoto, H.; Saito, N.; Kirai, N.; Iwasawa, N. Fluorine-Controlled C–H Borylation of Arenes Catalyzed by a PSiN-Pincer Platinum Complex. Chem. Commun. 2015, 51, 17662–17665. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(i) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]




| Complex | Space gr. Cryst. cl. Z | a [Å] b [Å] c [Å] | α[°] β[°] γ[°] | Chromophore (Chelate Rings) Ʈ4 b | Pt-L c [Å] | L-Pt-L c [°] | Ref. REFCODE |
|---|---|---|---|---|---|---|---|
| A: Pt(κ3-P1N1N2)(Y) | |||||||
| [Pt{κ3-But2P(CH2)(C5H3N1). (CH2)N2Et2}(Cl)].C6H6 (at 120 K) | tr Pī 2 | 9.158(0) 10.963(0) 16.018(0) | 77.29(0) 76.97(0) 69.11(0) | PtP1N1N2Cl P1C2N1C2N2 0.044 | P1 2.236(1) N1 1.997(2) N2 2.149(2) Cl 2.296(2) | P1,N1 85.5 d N1,N2 83.4 d P1,N2 168.0 P1,Cl 98.5 N2,Cl 92.6 N1,Cl 176.0 | [10] WOGDAY |
| [Pt{κ3-Ph2P1(C7H5N1). (C2H2O)N2C6H4OH)}(CH3)]. (CHCl3) (at 150 K) | tr Pī 2 | 9.917(1) 11.944(2) 14.872(2) | 99.17(0) 103.82(0) 112.53(0) | PtP1N1N2C P1C3N1C2N2 0.032 | P1 2.179(1) N1 2.050(2) N2 2.089(2) H3C 2.045(2) | P1,N1 95.3 e N1,N2 80.5 d P1,N2 173.7 P1,C 89.0 N2,C 95.5 N1,C 174.7 | [11] GAJMOV |
| [Pt{κ3-Ph2P1(C7H5N1). (C2H2O)N2(C6H4OH)}(CH3)]. 1.5 toluene (at 150 K) | m P21/c 4 | 11.882(1) 14.184(1) 21.892(1) | 103.75(0) | PtP1N1N2C P1C3N1C2N2 0.027 | P1 2.184(2) N1 2.061(3) N2 2.075(3) H3C 2.051(2) | P1,N1 95.4 e N1,N2 81.0 d P1,N2 176.4 P1,C 90.5 N2,C 93.0 N1,C 174.0 | [11] GAJMUB |
| [Pt{κ3-Ph2P1(C7H5N1). (C3H6)N2(C7H5O2)}(CH3)]. 2 toluene (at 150 K) | m P21/c 4 | 14.859(0) 15.607(0) 16.287(0) | 95.88(0) | PtP1N1N2C P1C3N1C2N2 0.033 | P1 2.189(1) N1 2.077(2) N2 2.070(2) H3C 2.062(2) | P1,N1 95.2 e N1,N2 80.3 d P1,N2 175.4 P1,C 89.4 N2,C 94.9 N1,C 172.6 | [11] GAJNAI |
| [Pt{κ3-Ph2P1(C7H5N1)(C6H4)N2 (C10H9NO3)}(CH3)]Et2O (at 150 K) | m P21/c 4 | 10.992(0) 20.133(0) 16.933(0) | 101.72(0) | PtP1N1N2C P1C3N1C2N2 0.040 | P1 2.184(1) N1 2.087(2) N2 2.086(2) H3C 2.062(2) | P1,N1 92.6 e N1,N2 79.4 d P1,N2 171.8 P1,C 91.5 N2,C 96.6 N1,C 173.7 | [12] QICYAD |
| [Pt{κ3-Ph2P1(C7H5N1)(C5H7O) N2(C10H10N2)}(CH3)]H2O (at 93 K) | m P21/c 4 | 8.739 (1) 14.988(2) 25.469(2) | 94.23(0) | PtP1N1N2C P1C3N1C2N2 0.052 | P1 2.190(1) N1 2.061(2) N2 2.094(2) H3C 2.083(2) | P1,N1 89.4 e N1,N2 79.6 d P1,N2 169.0 P1,C 92.2 N2,C 93.7 N1,C 172.1 | [13] DIYYIU |
| [Pt{κ3-Ph2P1(C7H5N1)(C2H2O) N2(C6H4OH)}(CH3)]CHCl3 (at 150 K) | m P21/c 4 | 10.191(0) 16.863(1) 17.525(1) | 97.30(0) | PtP1N1N2C P1C3N1C2N2 0.030 | P1 2.183(1) N1 2.059(1) N2 2.061(1) H3C 2.055(1) | P1,N1 95.1 e N1,N2 81.0 d P1,N2 175.8 P1,C 91.2 N2,C 92.6 N1,C 173.4 | [14] CAJLAC |
| [Pt{κ3–Ph2P1(C7H6N1 = NCC1. C5H6)}(Cl)] (at 150 K) | tr Pī 2 | 7.431(2) 10.031(3) 14.797(5) | 101.10(0) 95.70(0) 98.76(0) | PtP1N1N2C P1C3N1NCN2 0.038 | P1 2.219(1) N1 2.164(2) N2 2.050(1) Cl 2.297(2) | P1,N1 95.8 e N1,N2 79.3 d P1,N2 173.0 P1,Cl 90.8 N2,Cl 94.2 N1,Cl 173.4 | [15] XUYWEU |
| [Pt{κ3-Ph2P1(C7H5N1)(C7H8N) (C7H8N2)}(Cl)]PF6 | m P21/c 4 | 18.910(3) 10.098(1) 19.429(3) | 118.93(1) | PtP1N1N2Cl P1C3N1CN2 0.020 | P1 2.234(1) N1 2.120(1) N2 2.104(1) Cl 2.284(1) | P1,N1 93.3 e N1,N2 85.6 d P1,N2 178.7 P1,Cl 91.8 N2,Cl 89.2 N1,Cl 174.0 | [16] IFUQEF |
| B: Pt(κ3–P1N1O1)(Y) | |||||||
| [Pt{κ3-Ph2P1(C8H6N1)(N. C7H5O1)}{κ1-Ph2P. (C15H13N2O)}].CH2Cl2 (at 200 K) | tr Pī 2 | 12.614(2) 13.671(2) 15.754(3) | 100.26(0) 99.33(0) 110.68(0) | PtP1N1O1P P1C2N1NCO1 0.067 | P1 2.233(2) N1 1.985(2) O1 2.050(2) LP 2.261(1) | P1,N1 83.6 d N1,O1 78.8 d P1,O1 162.4 P1,P 102.9 O1,P 94.7 N1,Cl 173.3 | [17] EFODAE |
| [Pt{κ3-Ph2P1(C6H4N1). (C7H4ClO1)}(P(p-tolyl3)]ClO4 (at 200 K) | m P21/c 4 | 12.614(14) 20.280(20) 16.972(17) | 98.96(1) | PtP1N1O1P P1C2N1C3O1 0.028 | P1 2.21(1) N1 2.05(2) O1 2.03(2) LP 2.269(1) | P1,N1 82.7 N1,O1 91.2 P1,O1 172.1 P1,P 99.6 O1,P 86.5 N1,P 177.7 | [18] KAVZOX |
| [Pt{κ3-Ph2P1(C6H4N1). (C8H7OO1)} (Cl)] | m P21/n 4 | 12.350(12) 12.138(14) 15.550(17) | 97.70(1) | PtP1N1O1Cl P1C2N1C3O1 0.007 | P1 2.195(1) N1 2.005(2) O1 2.080(2) Cl 2.303(1) | P1,N1 83.6 d N1,O1 92.3 e P1,O1 178.5 P1,Cl 93.5 O1,Cl 87.9 N1,Cl 178.9 | [18] KAVZAJ |
| [Pt{κ3-Ph2P1(C6H4N1). (C8H7OO1)}(I)](CH2Cl2) | m P21/c 4 | 10.446(11) 16.389(17) 16.507(0) | 100.241(1) | PtP1N1O1I P1C2N1C3O1 0.014 | P1 2.207(1) N1 2.011(2) O1 2.045(2) I 2.620(1) | P1,N1 84.8 d N1,O1 91.9 e P1,O1 176.6 P1,I 92.6 O1,I 89.2 N1,I 178.2 | [18] KAVZEN |
| [Pt{κ3-Ph2P1(C8H7N1O1)}(Cl)] | or Pna21 4 | 18.88(2) 13.10(1) 9.66(1) | PtP1N1O1Cl P1C3N1C3O1 0.027 | P1 2.206(1) N1 1.88(1) O1 2.14(1) Cl 2.386(4) | P1,N1 94.8(4) e N1,O1 93.3(4) e P1,O1 175.5 P1,Cl 89.1(2) O1,Cl 84.0(2) N1,Cl174.8 | [19] DERNIX | |
| C: Pt(κ3–P1N1C1)(Y) | |||||||
| [Pt{κ3-Ph2P1(C7H6N1 = NC. C1C5H6)}(Cl)] (at 120 K) | m P21/n 4 | 8.632(4) 17.191(8) 15.216(7) | 96.3(0) | PtP1N1C1Cl P1C2N1NCC1 0.060 | P1 2.291(2) N1 1.972(2) C1 2.023(2) Cl 2.309(1) | P1,N1 85.2 d N1,C1 78.7 d P1,C1 163.9 P1,Cl 99.9 C1,Cl 84.0 N1,Cl174.6 | [20] YEHMOP |
| [Pt{κ3-Ph2P1(C7H5N1). (C7H8C1)}(py)]BF4 (at 100 K) | m P21 4 | 9.356(0) 19.892(1) 15.084(1) | 90.76(0) | PtP1N1C1N P1C3N1C2C1 0.042 | P1 2.292(1) N1 2.000(2) C1 2.035(2) pyN 2.026(1) | P1,N1 92.1 e N1,C1 82.3 d P1,C1 174.3 P1,N 92.4 C1,N 93.6 N1,Cl170.7 | [21] NIVCAX |
| D: Pt(κ3–P1N1S1)(Y) | |||||||
| [Pt{κ3-Ph2P1(C6H4CHN1NC. (S1)NHMe}(Cl)] | m P21/c 4 | 14.695(6) 16.683(7) 19.297(9) | 102.83(6) | PtP1N1S1Cl P1C3N1NCS1 0.022 | P1 2.239(5) N1 2.03(2) S1 2.298(5) Cl 2.304(5) | P1,N1 95.8(4) e N1,S1 84.9(4) d P1,S1 177.8(2) P1,Cl 89.5(2) S1,Cl 89.8(2) N1,Cl174.4 (4) | [22] HAFMOQ |
| [Pt{κ3-Ph2P1(C7H5N1)(MeS1). (But.NH2)}(I)] | tr Pī 2 | 10.529(1) 11.558(1) 14.550(1) | 77.37(1) 84.45(1) 79.72(1) | PtP1N1S1I P1C3N1C3S1 0.023 | P1 2.240(2) N1 2.056(6) S1 2.363(2) I 2.580(1) | P1,N1 89.1(1) e N1,S1 93.2(1) e P1,S1 176.2(2) P1,I 93.6(2) S1,I 84.2(2) N1,I 175.4(2) | [23] ROBHOP |
| E: Pt(κ3–P1N1Se1)(Cl) | |||||||
| [Pt{κ3-Ph2P1(C7H5N1). (C3H6Se1)(Ph)}(Cl)]BF4 (at 150K) | m P21/c 4 | 9.869(0) 23.847(0) 11.740(0) | 99.65(0) | PtP1N1Se1Cl P1C3N1C3Se1 0.012 | P1 2.407(14) N1 2.028(4) Se1 2.489(1) Cl 2.308(1) | P1,N1 87.5(1) e N1,Se1 95.7(1) e P1,Se1 176.7(1) P1,Cl 93.0(1) Se1,Cl 83.7(1) N1,Cl 178.8(1) | [24] MULZIC |
| Complex | Space gr. Cryst. cl. Z | a [Å] b [Å] c [Å] | α [°] β [°] γ [°] | Chromophore (Chelate Rings) Ʈ4 b | Pt-L c [Å] | L-Pt-L c [°] | Ref. REFCODE |
|---|---|---|---|---|---|---|---|
| [Pt{κ3-N1(C6H6)N(C6H10)N.. P1(Pri) (C6H6)N2}(Cl)].H2O (at 150 K) | or P212121 6 | 14.373(0) 9.906(0) 17.590(0) | PtN1P1N2Cl N1C2NP1NC2N2 0.032 | N1 2.035 P1 2.187 N2 2.039 Cl 2.375 | N1,P1 91.1 e P1,N2 91.0 e N1,N2 175.4 N1,Cl 90.4 N2,Cl 91.0 P1,Cl 173.0 | [25] IRAWOO | |
| [Pt{κ3-PriS1(C6H4)P1. (C6H4SPri) (C6H4)S2}(Cl)] (at 123 K) | m P21/n 4 | 8.790(0) 18.706(1) 15.508(1) | 95.89(0) | PtS1P1S2Cl S1C2P1C2S2 0.055 | S1 2.289 P1 2.189 S2 2.292 Cl 2.374 | S1,P1 88.2 d P1,S2 87.7 d S1,S2 162.1 S1,Cl 93.0 S2,Cl 90.8 P1,Cl 178.3 | [26] EZORAO |
| [Pt{κ3-ButS1(C6H4)P1. (C6H4SBut)(C6H4)S2}(Cl)].0.5CHCl3 (at 123 K) | m P21/n 4 | 10.250(1) 18.715(2) 15.320(1) | 96.65(0) | PtS1P1S2Cl S1C2P1C2S2 0.063 | S1 2.287 P1 2.198 S2 2.297 Cl 2.360 | S1,P1 88.7 d P1,S2 88.8 d S1,S2 158.7 N1,Cl 92.8 N2,Cl 90.8 P1,Cl 178.6 | [26] EZOQIV |
| [Pt{κ3-Ph2P1(C23H28S1). (B)(Ph2)Cl1)}(Cl)].2CH2Cl2 (at 123 K) | or Pna21 4 | 21.373(0) 8.959(0) 25.330(3) | PtP1S1Cl1Cl P1C2S1C2BCl1 0.033 | P1 2.212 S1 2.243 Cl1 2.391 Cl2 2.321 | P1,S1 87.9 d S1,Cl1 87.1 e P1,Cl1 174.7 P1,Cl 93.4 Cl1,Cl 91.7 S1,Cl 173.3 | [27] DASMER | |
| [Pt{κ3-cyh2P1(C6H4)Si1. (CH3)(C7H6)N1(CH3)2)}. (OSO2CF3)] (at 123 K) | m P21/c 4 | 19.851(1) 20.837(3) 15.443(2) | 99.52(0) | PtP1Si1N1O P1C2Si1C3N1 0.033 | P1 2.228 Si1 2.260 N1 2.177 LO 2.353 | P1,Si1 85.8 d Si1,N1 82.7 e P1,N1 169.2 P1,O 95.0 N1,O 86.4 Si1,O 179.0 | [28] WUXFAI |
| Metallocyclic Rings | α- L-Pt-L [°] | β- L’-Pt-Y [°] | Ʈ4 |
|---|---|---|---|
| 5 + 5—membered | 163.0 | 176.2 | 0.058 |
| 6 + 5—membered | 172.9 | 173.6 | 0.037 |
| 5 + 6—membered | 174.2 | 177.5 | 0.023 |
| 6 + 6—membered | 176.5 | 175.6 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melník, M.; Mikuš, P. Heterotridentate Organomonophosphines in Pt(κ3–X1P1X2)(Y) (X1,2 = N1,2 or S1,2), Pt(κ3–P1N1X1)(Y) (X1 = O, C, S or Se) Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL)—Structural Aspects. Crystals 2022, 12, 1772. https://doi.org/10.3390/cryst12121772
Melník M, Mikuš P. Heterotridentate Organomonophosphines in Pt(κ3–X1P1X2)(Y) (X1,2 = N1,2 or S1,2), Pt(κ3–P1N1X1)(Y) (X1 = O, C, S or Se) Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL)—Structural Aspects. Crystals. 2022; 12(12):1772. https://doi.org/10.3390/cryst12121772
Chicago/Turabian StyleMelník, Milan, and Peter Mikuš. 2022. "Heterotridentate Organomonophosphines in Pt(κ3–X1P1X2)(Y) (X1,2 = N1,2 or S1,2), Pt(κ3–P1N1X1)(Y) (X1 = O, C, S or Se) Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL)—Structural Aspects" Crystals 12, no. 12: 1772. https://doi.org/10.3390/cryst12121772
APA StyleMelník, M., & Mikuš, P. (2022). Heterotridentate Organomonophosphines in Pt(κ3–X1P1X2)(Y) (X1,2 = N1,2 or S1,2), Pt(κ3–P1N1X1)(Y) (X1 = O, C, S or Se) Pt(κ3–P1S1Cl1)(Cl) and Pt(κ3–P1Si1N1)(OL)—Structural Aspects. Crystals, 12(12), 1772. https://doi.org/10.3390/cryst12121772







