Multicolor Photochromism of Two-Component Diarylethene Crystals Containing Oxidized and Unoxidized Benzothiophene Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of 2a
3. Results and Discussion
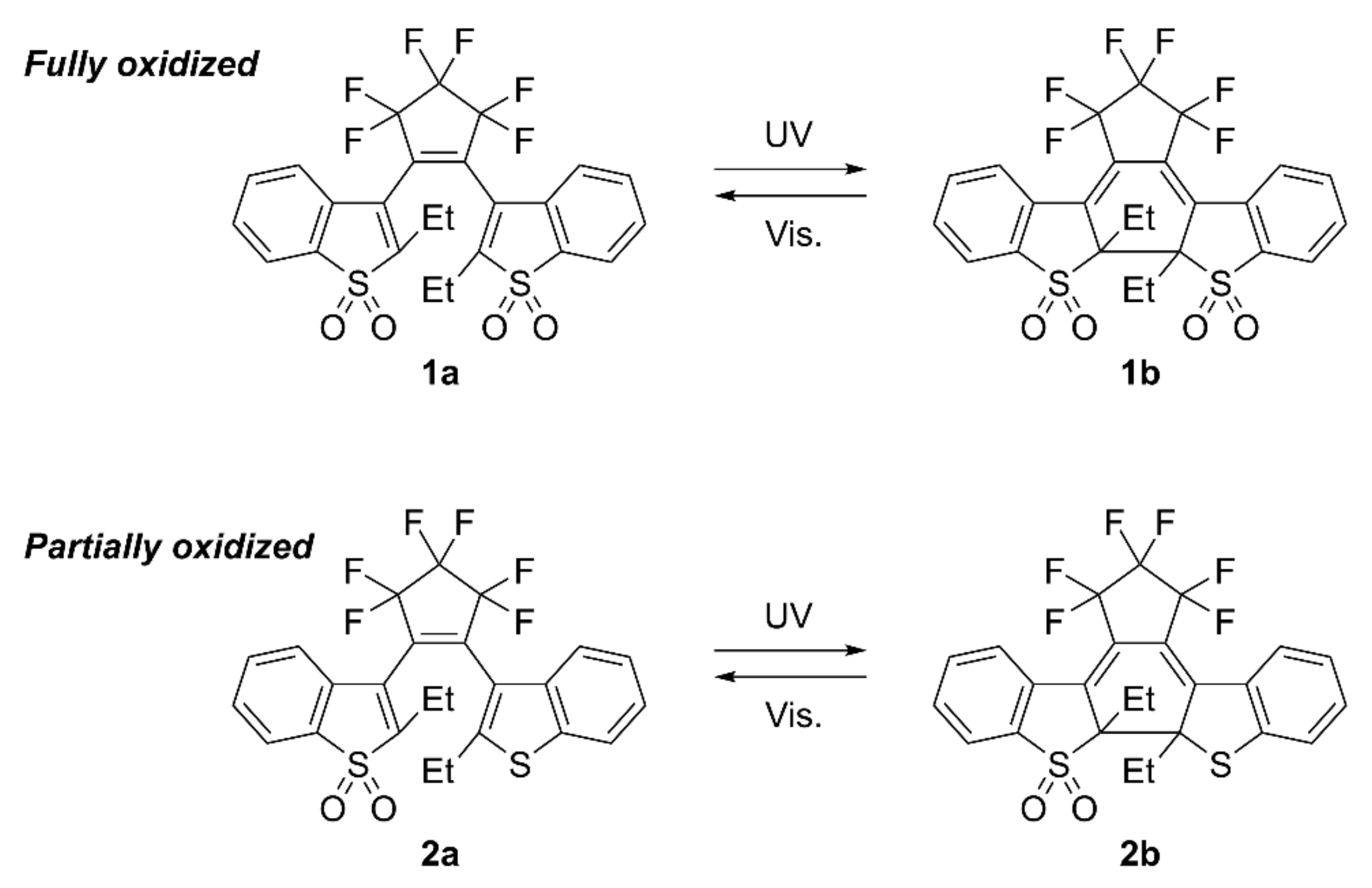
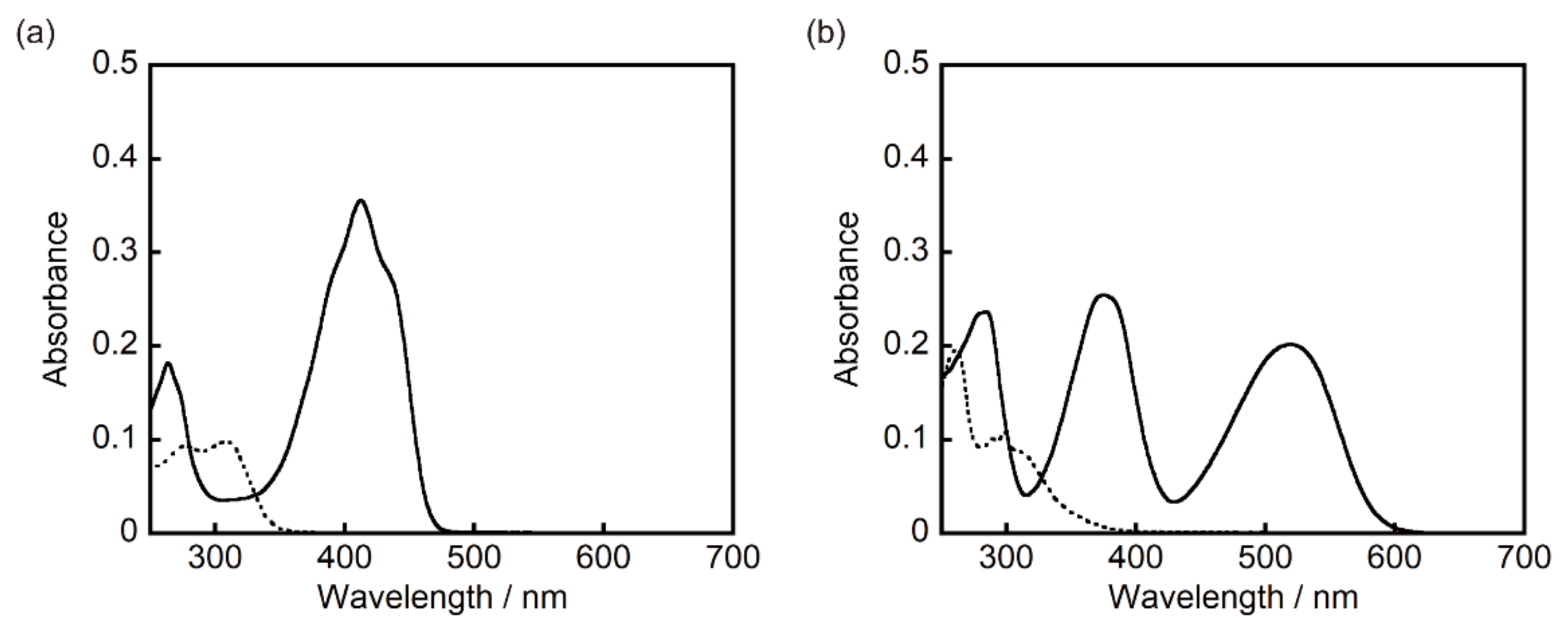
3.1. Photochromism of 1 and 2 in Solution
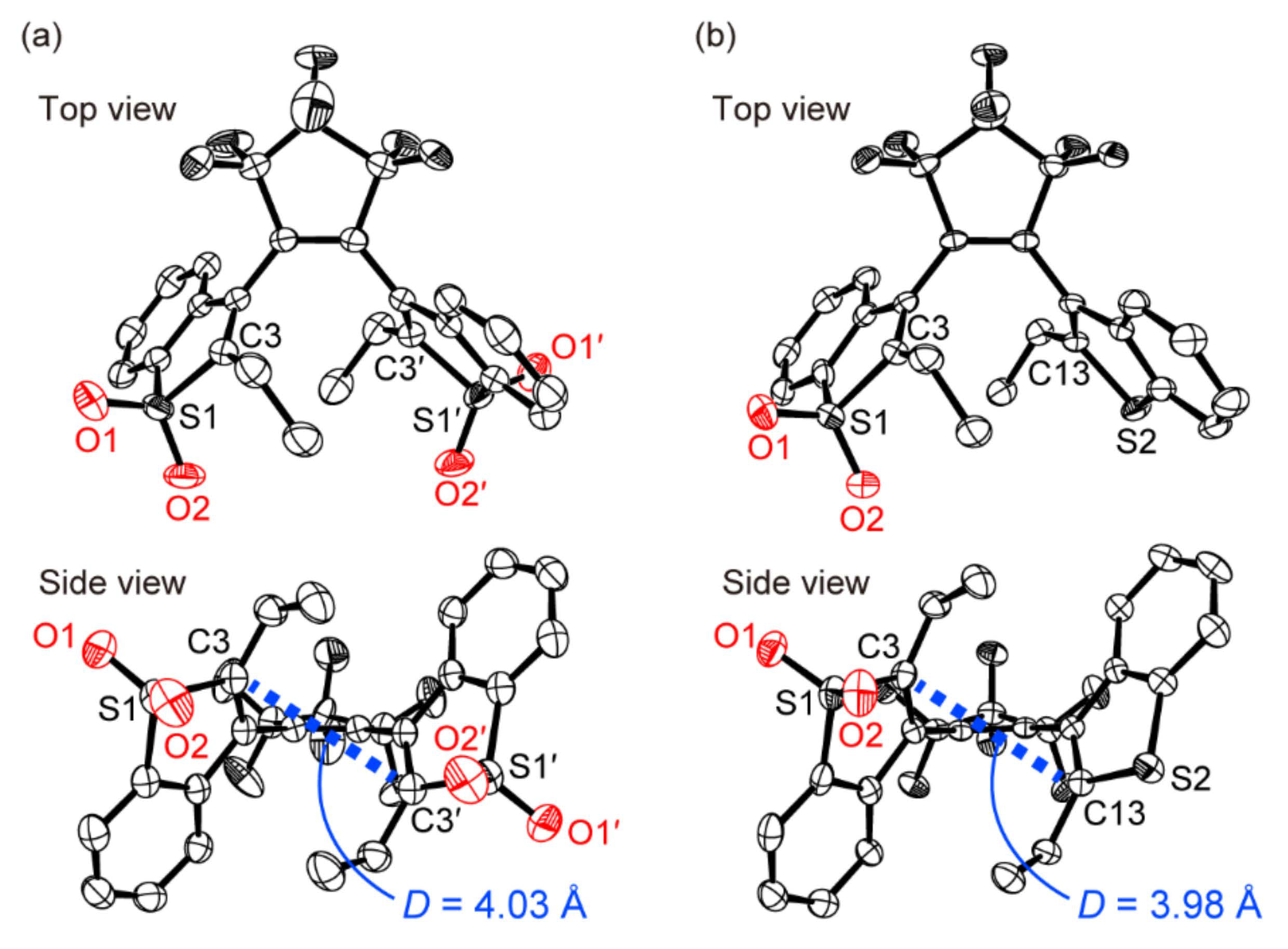
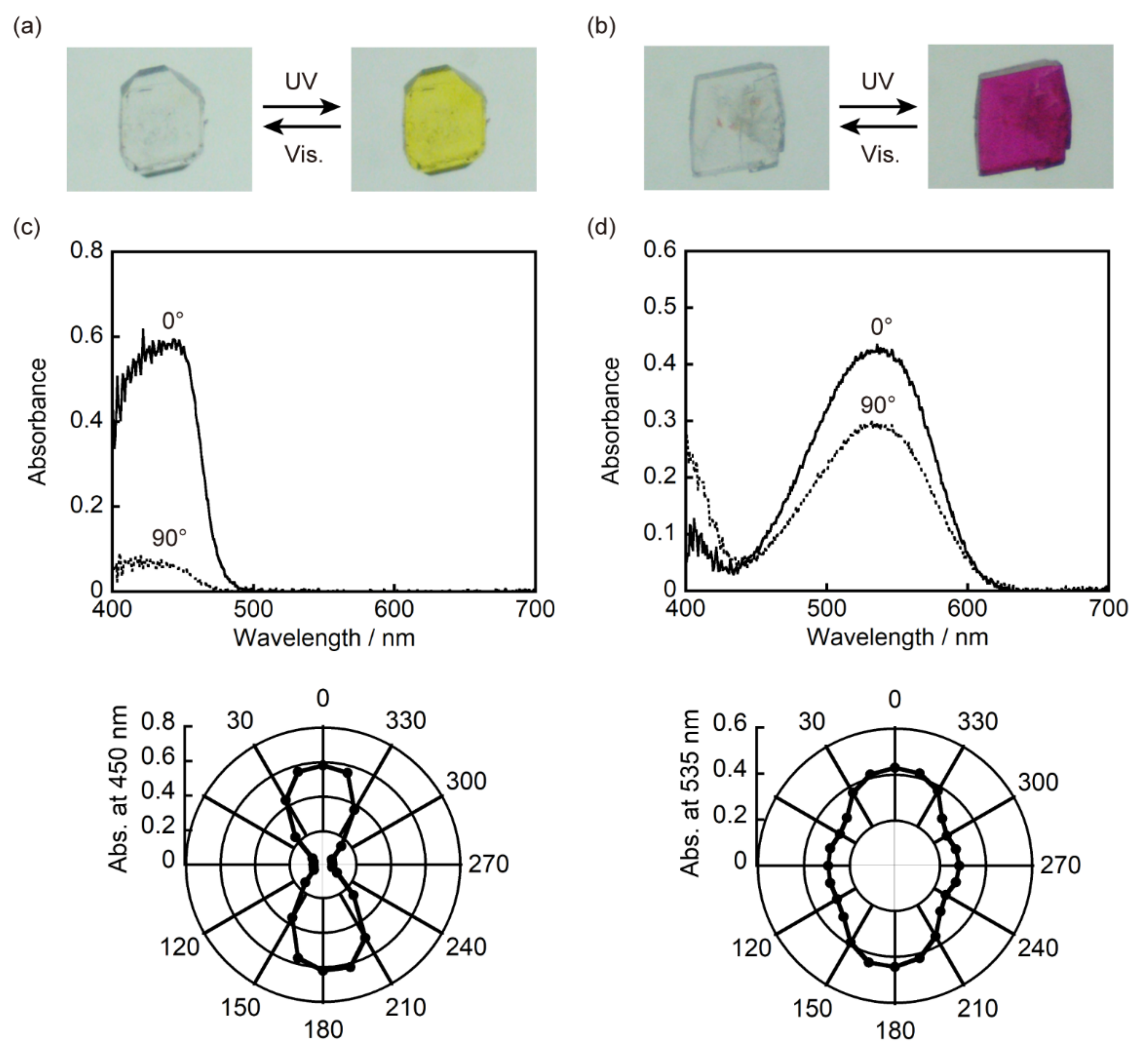
3.2. Single-Component Crystals of 1a and 2a
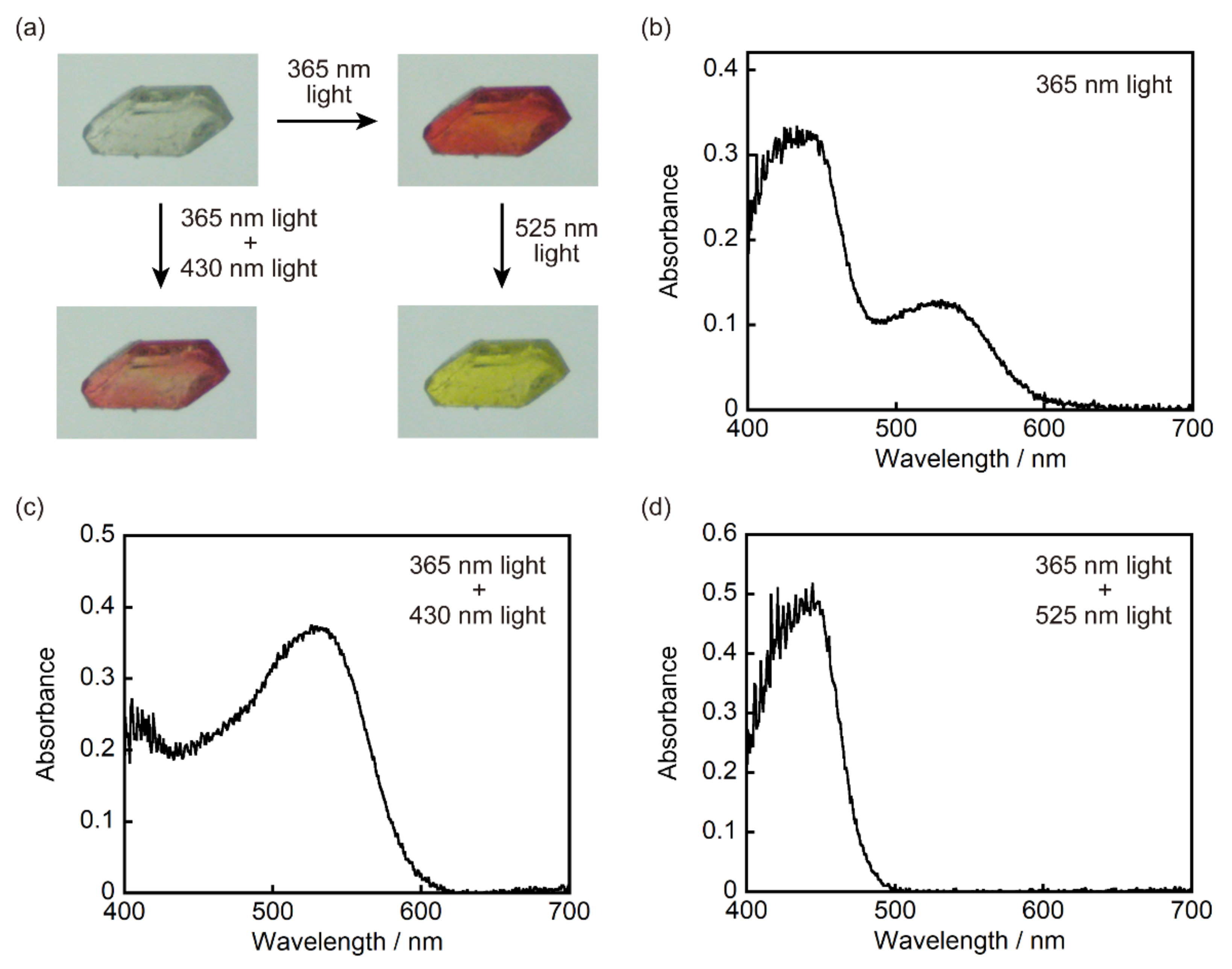
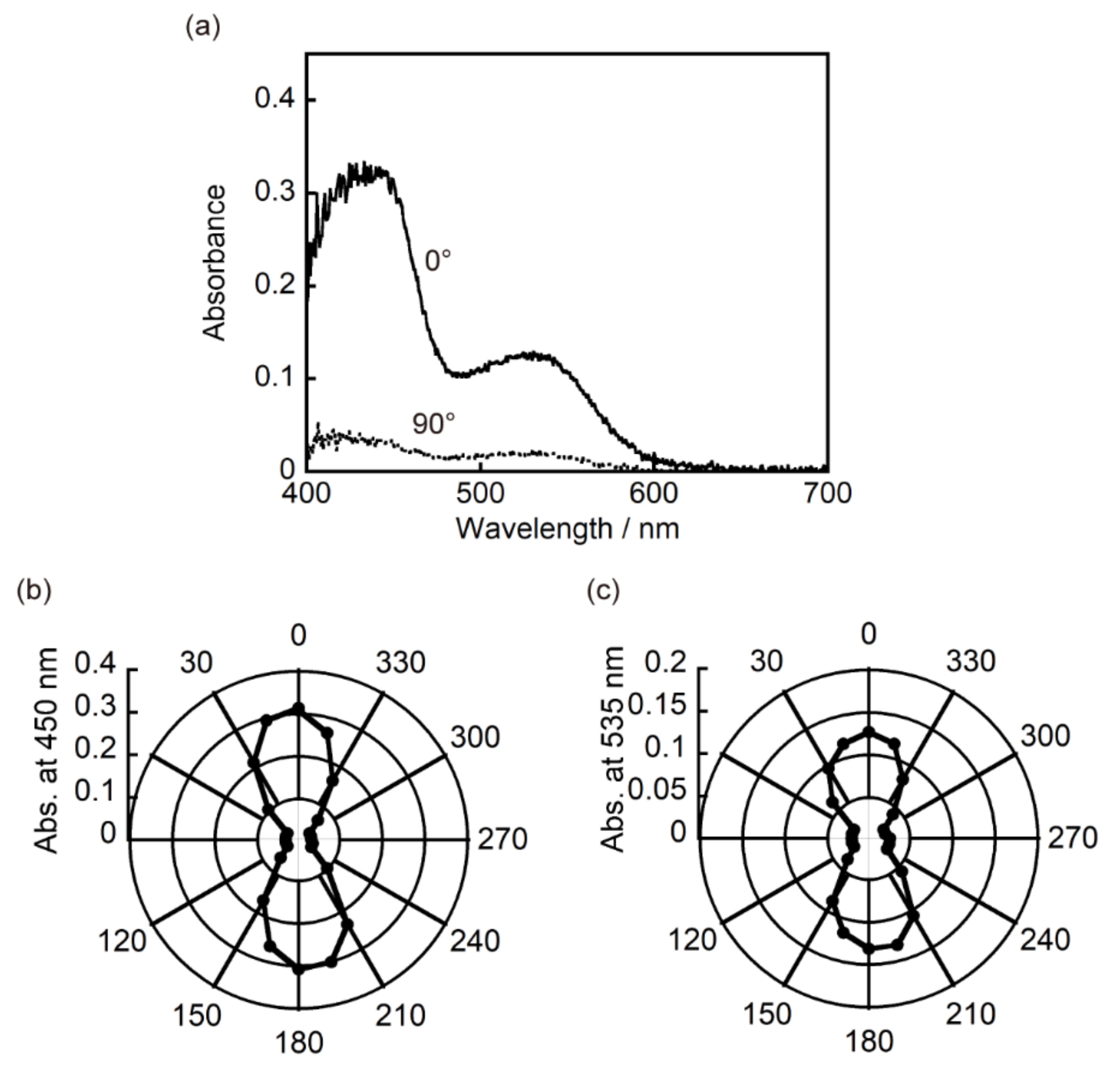
3.3. Two-Component Mixed Crystals Containing 1a and 2a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dürr, H.; Bouas-Laurent, H. Photochromism: Molecules and Systems; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Tian, H.; Zhang, J. Photochromic Materials: Preparation, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef] [PubMed]
- Irie, M. Diarylethene Molecular Photoswitches: Concepts and Fundamentals; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Kobatake, S.; Irie, M. Single-crystalline photochromism of diarylethenes. Bull. Chem. Soc. Jpn. 2004, 77, 195–210. [Google Scholar] [CrossRef]
- Morimoto, M.; Irie, M. Photochromism of diarylethene single crystals: Crystal structures and photochromic performance. Chem. Commun. 2005, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Jean-Ruel, H.; Cooney, R.R.; Gao, M.; Lu, C.; Kochman, M.A.; Morrison, C.A.; Miller, R.J.D. Femtosecond Dynamics of the Ring Closing Process of Diarylethene: A Case Study of Electrocyclic Reactions in Photochromic Single Crystals. J. Phys. Chem. A 2011, 115, 13158–13168. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, S.; Takami, S.; Muto, H.; Ishikawa, T.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Irie, M. A diarylethene cocrystal that converts light into mechanical work. J. Am. Chem. Soc. 2010, 132, 14172–14178. [Google Scholar] [CrossRef]
- Terao, F.; Morimoto, M.; Irie, M. Light-Driven Molecular-Crystal Actuators: Rapid and Reversible Bending of Rodlike Mixed Crystals of Diarylethene Derivatives. Angew. Chem. Int. Ed. 2012, 51, 901–904. [Google Scholar] [CrossRef]
- Kitagawa, D.; Nishi, H.; Kobatake, S. Photoinduced Twisting of a Photochromic Diarylethene Crystal. Angew. Chem. Int. Ed. 2013, 52, 9320–9322. [Google Scholar] [CrossRef]
- Kitagawa, D.; Tsujioka, H.; Tong, F.; Dong, X.N.; Bardeen, C.J.; Kobatake, S. Control of Photomechanical Crystal Twisting by Illumination Direction. J. Am. Chem. Soc. 2018, 140, 4208–4212. [Google Scholar] [CrossRef]
- Dong, X.M.; Tong, F.; Hanson, K.M.; Al-Kaysi, R.O.; Kitagawa, D.; Kobatake, S.; Bardeen, C.J. Hybrid Organic Inorganic Photon-Powered Actuators Based on Aligned Diarylethene Nanocrystals. Chem. Mater. 2019, 31, 1016–1022. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Lusi, M. A rough guide to molecular solid solutions: Design, synthesis and characterization of mixed crystals. CrystEngComm 2018, 20, 7042–7052. [Google Scholar] [CrossRef]
- Lusi, M. Engineering Crystal Properties through Solid Solutions. Cryst. Growth Des. 2018, 18, 3704–3712. [Google Scholar] [CrossRef]
- Kitaigorodsky, A.I. Mixed Crystals; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Schur, E.; Nauha, E.; Lusi, M.; Bernstein, J. Kitaigorodsky Revisited: Polymorphism and Mixed Crystals of Acridine/Phenazine. Chem. Eur. J. 2015, 21, 1735–1742. [Google Scholar] [CrossRef]
- Morimoto, M.; Kobatake, S.; Irie, M. Multicolor Photochromism of Two- and Three-Component Diarylethene Crystals. J. Am. Chem. Soc. 2003, 125, 11080–11087. [Google Scholar] [CrossRef]
- Kuroki, L.; Takami, S.; Shibata, K.; Irie, M. Photochromism of single crystals composed of dioxazolylethene and dithiazolylethene. Chem. Commun. 2005, 6005–6007. [Google Scholar] [CrossRef]
- Takami, S.; Kuroki, L.; Irie, M. Photochromism of Mixed Crystals Containing Bisthienyl-, Bisthiazolyl-, and Bisoxazolylethene Derivatives. J. Am. Chem. Soc. 2007, 129, 7319–7326. [Google Scholar] [CrossRef]
- Ohshima, S.; Morimoto, M.; Irie, M. Light-driven bending of diarylethene mixed crystals. Chem. Sci. 2015, 6, 5746–5752. [Google Scholar] [CrossRef]
- Hanazawa, M.; Sumiya, R.; Horikawa, Y.; Irie, M. Thermally irreversible photochromic systems. Reversible photocyclization of 1,2-bis (2-methylbenzo[b]thiophen-3-yl)perfluorocyclocoalkene derivatives. J. Chem. Soc. Chem. Commun. 1992, 206–207. [Google Scholar] [CrossRef]
- Sumi, T.; Takagi, Y.; Yagi, A.; Morimoto, M.; Irie, M. Photoirradiation wavelength dependence of cycloreversion quantum yields of diarylethenes. Chem. Commun. 2014, 50, 3928–3930. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Yang, S.I.; Ahn, K.-H.; Kim, E. Highly fluorescent photochromic diarylethene in the closed-ring form. Chem. Commun. 2005, 2503–2505. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Park, D.G.; Kim, E.; Ahn, K.-H.; Yang, S.I. Fatigue-resistant photochromic dithienylethenes by controlling the oxidation state. Chem. Commun. 2006, 1881–1883. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Park, D.G.; Lee, I.S.; Yang, S.I.; Ahn, K.-H. Highly fluorescent photochromic diarylethene with an excellent fatigue property. J. Mater. Chem. 2009, 19, 97–103. [Google Scholar] [CrossRef]
- Uno, K.; Niikura, H.; Morimoto, M.; Ishibashi, Y.; Miyasaka, H.; Irie, M. In Situ Preparation of Highly Fluorescent Dyes upon Photoirradiation. J. Am. Chem. Soc. 2011, 133, 13558–13564. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Morimoto, M. Photoswitchable Turn-on Mode Fluorescent Diarylethenes: Strategies for Controlling the Switching Response. Bull. Chem. Soc. Jpn. 2018, 91, 237–250. [Google Scholar] [CrossRef]
- Morimoto, M.; Kashihara, R.; Mutoh, K.; Kobayashi, Y.; Abe, J.; Sotome, H.; Ito, S.; Miyasaka, H.; Irie, M. Turn-on mode fluorescence photoswitching of diarylethene single crystals. CrystEngComm 2016, 18, 7241–7248. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Yamaguchi, T.; Irie, M. Photochromism of bis(2-alkyl-1-benzothiophen-3-yl)perfluorocyclopentene derivatives. J. Photochem. Photobiol. A 2006, 178, 162–169. [Google Scholar] [CrossRef]
- Kobatake, S.; Uchida, K.; Tsuchida, E.; Irie, M. Single-crystalline photochromism of diarylethenes: Reactivity-structure relationship. Chem. Commun. 2002, 2804–2805. [Google Scholar] [CrossRef]
- Kobatake, S.; Yamada, T.; Uchida, K.; Kato, N.; Irie, M. Photochromism of 1,2-Bis(2,5-dimethyl-3-thienyl)perfluorocyclopentene in a Single Crystalline Phase. J. Am. Chem. Soc. 1999, 121, 2380–2386. [Google Scholar] [CrossRef]

| 1a | 2a | 1a·2a | |
|---|---|---|---|
| Crystallization solvent | Acetone | Diethyl ether | Acetone |
| Formula | C25H18F6O4S2 | C25H18F6O2S2 | C25H18F6O3.57S2 |
| Formula weight | 560.51 | 528.51 | 553.60 |
| T/K | 223 (2) | 100 (2) | 223 (2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P21/c | C2/c |
| a/Å | 15.0346 (11) | 13.1649 (8) | 15.0533 (5) |
| b/Å | 15.1774 (11) | 10.5410 (6) | 15.1878 (5) |
| c/Å | 10.7072 (7) | 16.5297 (10) | 10.6375 (3) |
| β/° | 107.706 (2) | 99.859 (2) | 107.8093 (13) |
| V/Å3 | 2327.5 (3) | 2260.0 (2) | 2315.47 (13) |
| Z | 4 | 4 | 4 |
| R1 (I > 2σ(I)) | 0.0328 | 0.0571 | 0.0529 |
| wR2 (all data) | 0.0906 | 0.1265 | 0.1420 |
| CCDC No. | 2217447 | 2217448 | 2217449 |
| Entry | Feed Ratio in Solution (1a:2a) | Composition Ratio in Crystal (1a:2a) |
|---|---|---|
| 1 | 90:10 | 97:3 |
| 2 | 70:30 | 85:15 |
| 3 | 50:50 | 53:47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, R.; Nagakawa, Y.; Morimoto, M. Multicolor Photochromism of Two-Component Diarylethene Crystals Containing Oxidized and Unoxidized Benzothiophene Groups. Crystals 2022, 12, 1730. https://doi.org/10.3390/cryst12121730
Nishimura R, Nagakawa Y, Morimoto M. Multicolor Photochromism of Two-Component Diarylethene Crystals Containing Oxidized and Unoxidized Benzothiophene Groups. Crystals. 2022; 12(12):1730. https://doi.org/10.3390/cryst12121730
Chicago/Turabian StyleNishimura, Ryo, Yurika Nagakawa, and Masakazu Morimoto. 2022. "Multicolor Photochromism of Two-Component Diarylethene Crystals Containing Oxidized and Unoxidized Benzothiophene Groups" Crystals 12, no. 12: 1730. https://doi.org/10.3390/cryst12121730
APA StyleNishimura, R., Nagakawa, Y., & Morimoto, M. (2022). Multicolor Photochromism of Two-Component Diarylethene Crystals Containing Oxidized and Unoxidized Benzothiophene Groups. Crystals, 12(12), 1730. https://doi.org/10.3390/cryst12121730






