Controllable Fabrication of Organic Cocrystals with Interior Hollow Structure Based on Donor-Acceptor Charge Transfer Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses of (E)-4-(2(anthracen-9-yl)vinyl)pyridine (APE)
2.2. Preparation of APE-TCNB Single-Crystals for Structure Determination
2.3. Preparation of APE-TCNB Hollow Microcrystals Preparation
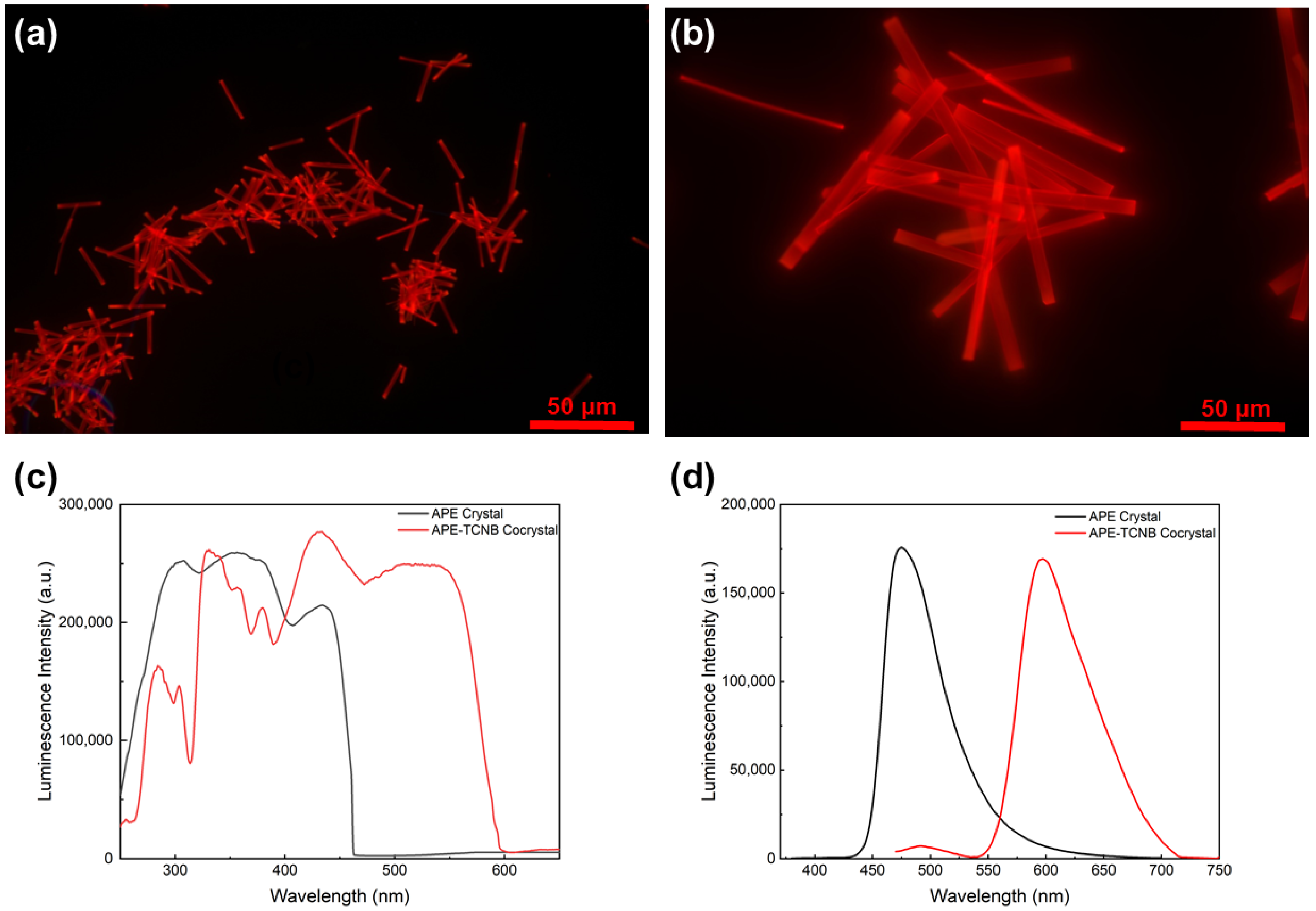
2.4. Measurements and Characterization
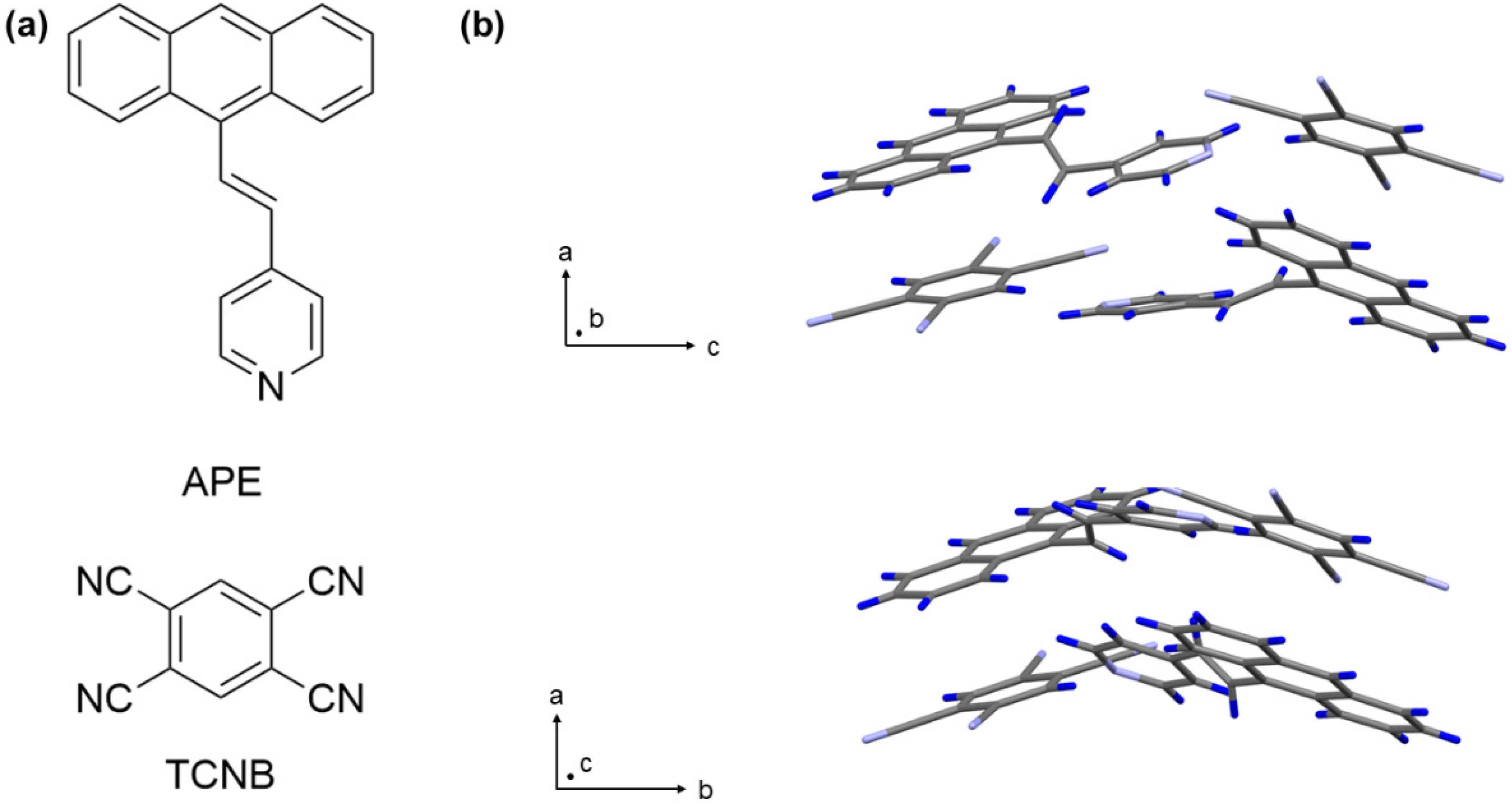
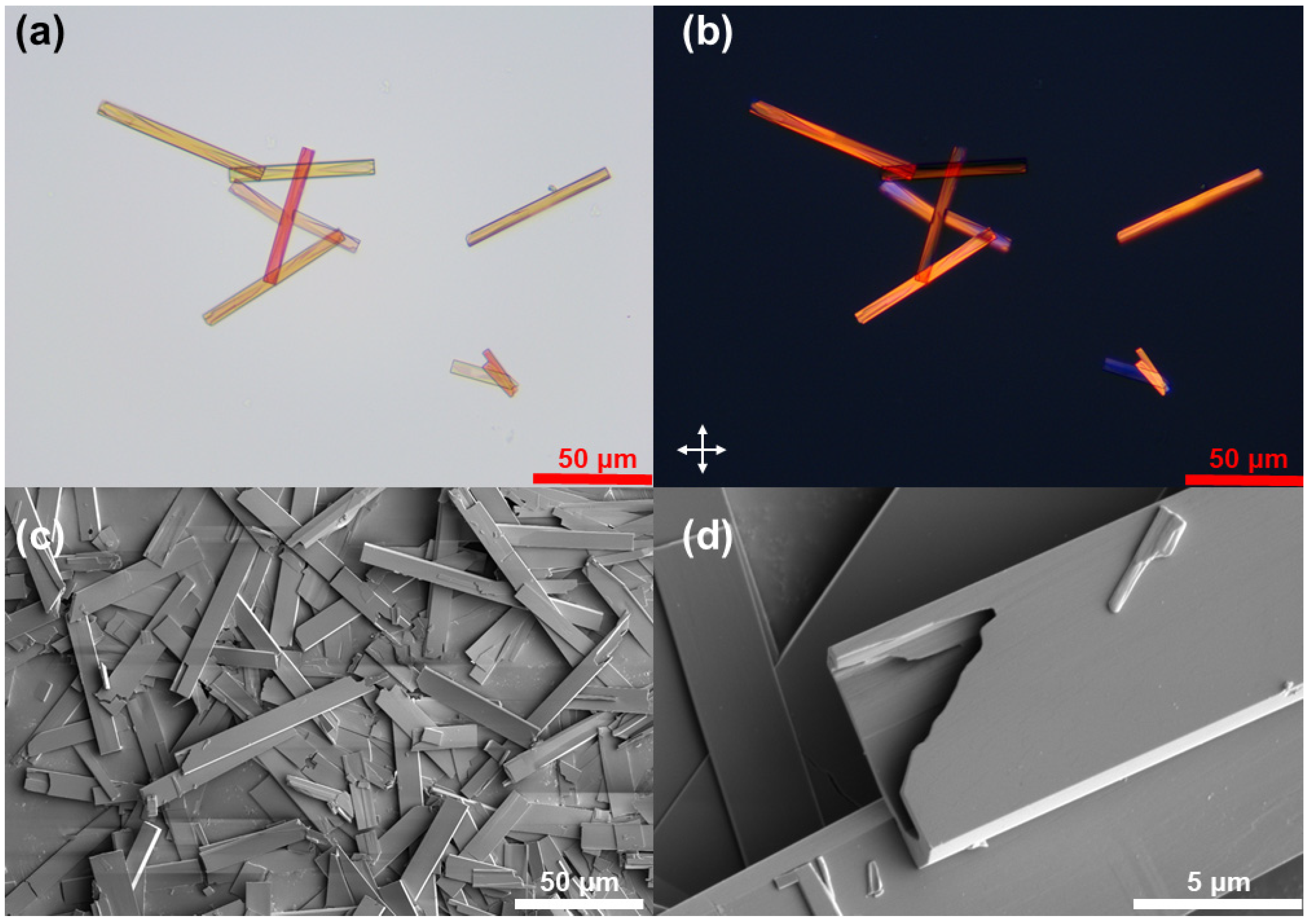
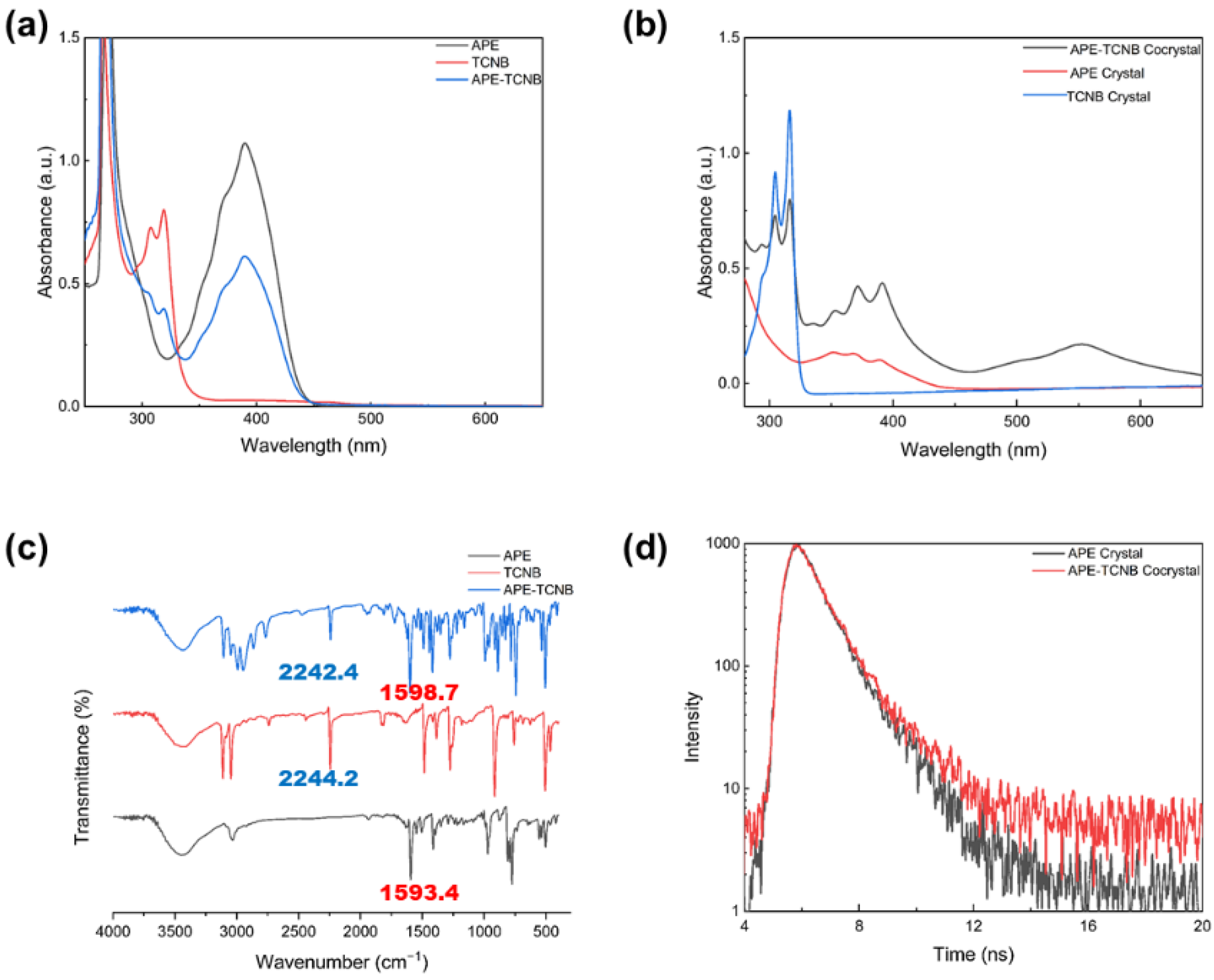
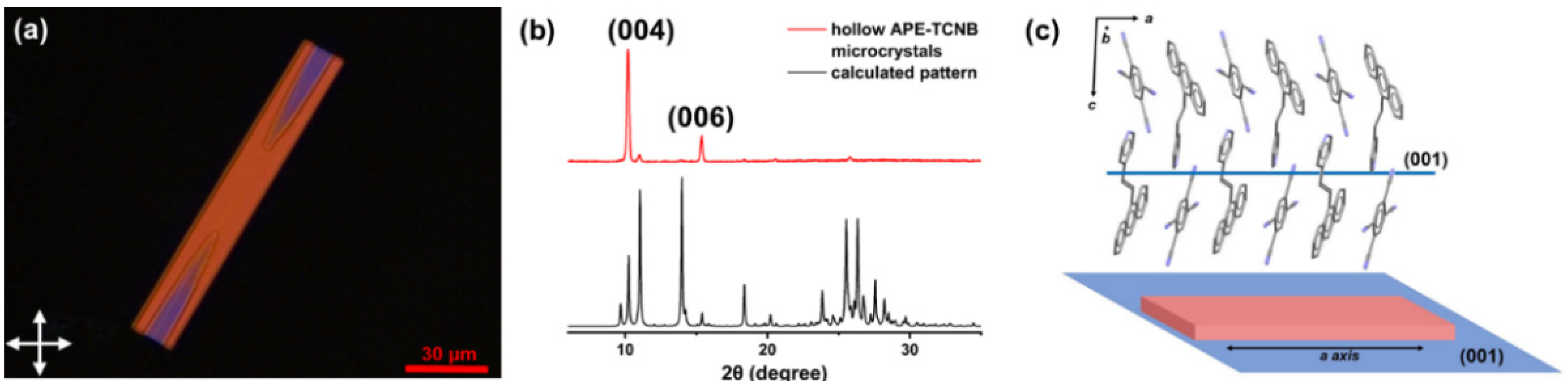
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Accession Codes
References
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhen, Y.; Dong, H.; Hu, W. Crystal engineering of organic optoelectronic materials. Chem 2019, 5, 2814–2853. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, Z.; Wu, Y.; Tong, F.; Chen, K.; Liu, G.; Zhang, L.; Wang, Z.; Qu, D.-H.; Tian, H. Vibratile dihydrophenazines with controllable luminescence enabled by precise regulation of π-conjugated wings. CCS Chem. 2022, 4, 2344–2353. [Google Scholar] [CrossRef]
- Naumov, P.; Chizhik, S.; Panda, M.K.; Nath, N.K.; Boldyreva, E. Mechanically responsive molecular crystals. Chem. Rev. 2015, 115, 12440–12490. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Qu, D.-H. Engineering shapes and sizes of molecular crystals to achieve versatile photomechanical behaviors. Langmuir 2022, 38, 4793–4801. [Google Scholar] [CrossRef]
- Kitagawa, D.; Bardeen, C.J.; Kobatake, S. Symmetry breaking and photomechanical behavior of photochromic organic crystals. Symmetry 2020, 12, 1478. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Pan, J.; Feng, T.; Wu, D.; Zhang, X.; Yang, B.; Zhang, X.; Jie, J. Graphene-quantum-dots-induced centimeter-sized growth of monolayer organic crystals for high-performance transistors. Adv. Mater. 2020, 32, 2003315. [Google Scholar] [CrossRef]
- Karunatilaka, C.; Bučar, D.K.; Ditzler, L.R.; Friščić, T.; Swenson, D.C.; MacGillivray, L.R.; Tivanski, A.V. Softening and hardening of macro-and nano-sized organic cocrystals in a single-crystal transformation. Angew. Chem. Int. Ed. 2011, 50, 8642–8646. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Punin, Y.O.; Gujral, A.; Kahr, B. Growth actuated bending and twisting of single crystals. Angew. Chem. Int. Ed. 2014, 53, 672–699. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Zapien, J.A.; Ismathullakhan, S.; Kang, Z.; Jie, J.; Zhang, X.; Chang, J.C.; Lee, C.-S.; Lee, S.-T. Polyhedral organic microcrystals: From cubes to rhombic dodecahedra. Angew. Chem. Int. Ed. 2009, 48, 9121–9123. [Google Scholar] [CrossRef]
- Lei, Y.; Liao, Q.; Fu, H.; Yao, J. Phase- and shape-controlled synthesis of single crystalline perylene nanosheets and its optical properties. J. Phys. Chem. C 2009, 113, 10038–10043. [Google Scholar] [CrossRef]
- Lovette, M.A.; Browning, A.R.; Griffin, D.W.; Sizemore, J.P.; Snyder, R.C.; Doherty, M.F. Crystal shape engineering. Ind. Eng. Chem. Res. 2008, 47, 9812–9833. [Google Scholar] [CrossRef] [Green Version]
- Shtukenberg, A.G.; Ward, M.D.; Kahr, B. Crystal growth with macromolecular additives. Chem. Rev. 2017, 117, 14042–14090. [Google Scholar] [CrossRef] [PubMed]
- Darkins, R.; McPherson, I.J.; Ford, I.J.; Duffy, D.M.; Unwin, P.R. Critical step length as an indicator of surface supersaturation during crystal growth from solution. Cryst. Growth Des. 2022, 22, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Nakabayashi, S.; Yoshikawa, H.Y. Control of organic crystal shape by femtosecond laser ablation. Cryst. Growth Des. 2018, 18, 4829–4833. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Sonigara, K.K.; Beuvier, T.; Gibaud, A.; Soni, S.S. Iodine induced 1-D lamellar self assembly in organic ionic crystals for solid state dye sensitized solar cells. Nanoscale 2017, 9, 15949–15957. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, R.; Fu, X.; Fu, H.; Shi, Q.; Zhen, Y.; Dong, H.; Hu, W. Revealing the charge-transfer interactions in self-assembled organic cocrystals: Two-dimensional photonic applications. Angew. Chem. Int. Ed. 2015, 54, 6785–6789. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, D.; Yu, T.; Zhang, F.; Xie, S.; Zhang, L.; Deng, Y.; Wan, Y.; Tu, B.; Zhao, D. Mesoporous carbon single-crystals from organic-organic self-assembly. J. Am. Chem. Soc. 2007, 129, 7746–7747. [Google Scholar] [CrossRef]
- Tong, F.; Xu, W.; Al-Haidar, M.; Kitagawa, D.; Al-Kaysi, R.O.; Bardeen, C.J. Photomechanically induced magnetic field response by controlling molecular orientation in 9-methylanthracene microcrystals. Angew. Chem. Int. Ed. 2018, 57, 7080–7084. [Google Scholar] [CrossRef]
- Tong, F.; Kitagawa, D.; Bushnak, I.; Al-Kaysi, R.O.; Bardeen, C.J. Light-powered autonomous flagella-like motion of molecular crystal microwires. Angew. Chem. Int. Ed. 2021, 60, 2414–2423. [Google Scholar] [CrossRef]
- Tong, F.; Al-Haidar, M.; Zhu, L.; Al-Kaysi, R.O.; Bardeen, C.J. Photoinduced peeling of molecular crystals. Chem. Commun. 2019, 55, 3709–3712. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaysi, R.O.; Tong, F.; Al-Haidar, M.; Zhu, L.; Bardeen, C.J. Highly branched photomechanical crystals. Chem. Commun. 2017, 53, 2622–2625. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-S.; Guo, Y.-G.; Liang, H.-P.; Wan, L.-J.; Jiang, L. Three-dimensional self-organization of supramolecular self-assembled porphyrin hollow hexagonal nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-Y.; Tong, F.; Xu, H.; Wang, M.-Q.; Tian, H.; Qu, D.-H. Engineering photomechanical molecular crystals to achieve extraordinary expansion based on solid-state [2 + 2] photocycloaddition. J. Am. Chem. Soc. 2022, 144, 6278–6290. [Google Scholar] [CrossRef]
- Nagai, A.; Nishimura, R.; Hattori, Y.; Hatano, E.; Fujimoto, A.; Morimoto, M.; Yasuda, N.; Kamada, K.; Sotome, H.; Miyasaka, H. Molecular crystalline capsules that release their contents by light. Chem. Sci. 2021, 12, 11585–11592. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M.; Kitagawa, D.; Kobatake, S. Effect of substrate surfaces for crystal growth of a photochromic diarylethene by sublimation. Cryst. Growth Des. 2022, 22, 5489–5496. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Sheng, P.; Zhao, G.; Zhu, D. Organic donor–acceptor complexes as novel organic semiconductors. Acc. Chem. Res. 2017, 50, 1654–1662. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal engineering: A collaborative strategy toward functional materials. Adv. Mater. 2019, 31, 1902328. [Google Scholar] [CrossRef]
- Bond, A.D. What is a co-crystal? CrystEngComm 2007, 9, 833–834. [Google Scholar] [CrossRef]
- Al-Kaysi, R.O.; Müller, A.M.; Frisbee, R.J.; Bardeen, C.J. Formation of cocrystal nanorods by solid-state reaction of tetracyanobenzene in 9-methylanthracene molecular crystal nanorods. Cryst. Growth Des. 2009, 9, 1780–1785. [Google Scholar] [CrossRef]
- Deibel, C.; Strobel, T.; Dyakonov, V. Role of the charge transfer state in organic donor–acceptor solar cells. Adv. Mater. 2010, 22, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Rogers, R.D.; Myerson, A. Cocrystal formation by ionic liquid-assisted grinding: Case study with cocrystals of caffeine. CrystEngComm 2018, 20, 3817–3821. [Google Scholar] [CrossRef] [Green Version]
- Stahly, G.P. A survey of cocrystals reported prior to 2000. Cryst. Growth Des. 2009, 9, 4212–4229. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, Z.; Chen, Z.; Zhang, Q. Organic cocrystals: Beyond electrical conductivities and field-effect transistors (FETs). Angew. Chem. Int. Ed. 2019, 58, 9696–9711. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef]
- Tong, F.; Li, W.; Li, Z.; Islam, I.; Al-Kaysi, R.O.; Bardeen, C.J. Molecular crystal microcapsules: Formation of sealed hollow chambers via surfactant-mediated growth. Angew. Chem. Int. Ed. 2020, 59, 23035–23039. [Google Scholar] [CrossRef]
- Bakshi, M.S. How surfactants control crystal growth of nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133. [Google Scholar] [CrossRef]
- Karanjkar, P.U.; Lee, J.W.; Morris, J.F. Surfactant effects on hydrate crystallization at the water-oil interface: Hollow-conical crystals. Cryst. Growth Des. 2012, 12, 3817–3824. [Google Scholar] [CrossRef]
- Zhou, S.W.; Tong, F.; Chen, M.; Gu, R.; Shi, C.Y.; Yu, C.Y.; Zhang, Q.; Qu, D.H. Self-evolution of high mechanical strength dry-network polythiourethane thermosets into neat macroscopic hollow structures. Angew. Chem. Int. Ed. 2022, 61, e202117195. [Google Scholar]
- Wang, Q.; Zhang, Q.; Zhang, Q.-W.; Li, X.; Zhao, C.-X.; Xu, T.-Y.; Qu, D.-H.; Tian, H. Color-tunable single-fluorophore supramolecular system with assembly-encoded emission. Nat. Commun. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, H.; Shibata, N. Growth mechanism of hollow ZnO crystals from ZnSe. J. Cryst. Growth 1974, 24, 357–361. [Google Scholar] [CrossRef]


| Temperature | 37 °C | 47 °C | 57 °C | |
|---|---|---|---|---|
| Surfactant | ||||
| SDS | Solid | Solid | Solid | |
| BS12 | Solid | Obvious hollow cavities | Obscure hollow structures | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, P.; Duan, Z.; Zhang, T.; Tong, F. Controllable Fabrication of Organic Cocrystals with Interior Hollow Structure Based on Donor-Acceptor Charge Transfer Molecules. Crystals 2022, 12, 1781. https://doi.org/10.3390/cryst12121781
Li Y, Wang P, Duan Z, Zhang T, Tong F. Controllable Fabrication of Organic Cocrystals with Interior Hollow Structure Based on Donor-Acceptor Charge Transfer Molecules. Crystals. 2022; 12(12):1781. https://doi.org/10.3390/cryst12121781
Chicago/Turabian StyleLi, Yuhao, Peiyao Wang, Zhongzhao Duan, Tianle Zhang, and Fei Tong. 2022. "Controllable Fabrication of Organic Cocrystals with Interior Hollow Structure Based on Donor-Acceptor Charge Transfer Molecules" Crystals 12, no. 12: 1781. https://doi.org/10.3390/cryst12121781
APA StyleLi, Y., Wang, P., Duan, Z., Zhang, T., & Tong, F. (2022). Controllable Fabrication of Organic Cocrystals with Interior Hollow Structure Based on Donor-Acceptor Charge Transfer Molecules. Crystals, 12(12), 1781. https://doi.org/10.3390/cryst12121781







