Maleic Anhydride-β-Cyclodextrin Functionalized Magnetic Nanoparticles for the Removal of Uranium (VI) from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MAH-β-CD
2.3. Synthesis of IONPs@MAH-β-CD
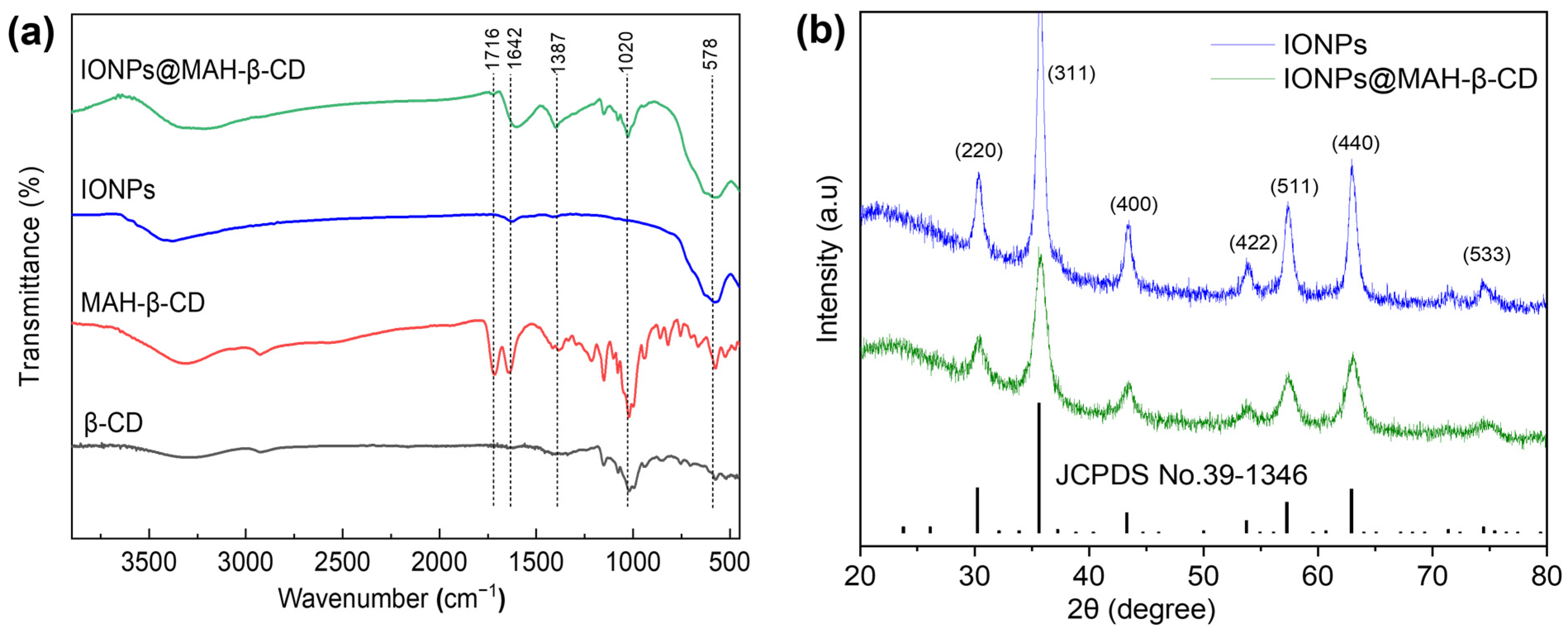
2.4. Characterization
2.5. Adsorption Experiment
3. Results and Discussion
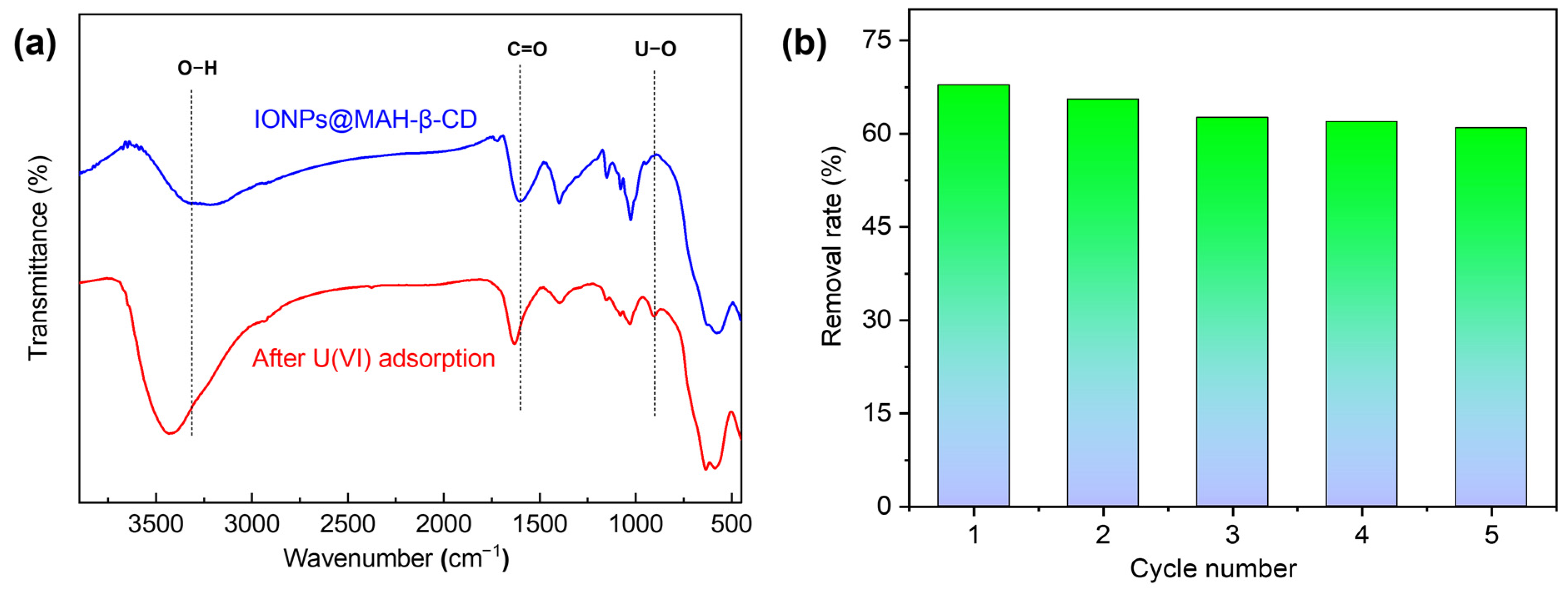
3.1. Characterization Results
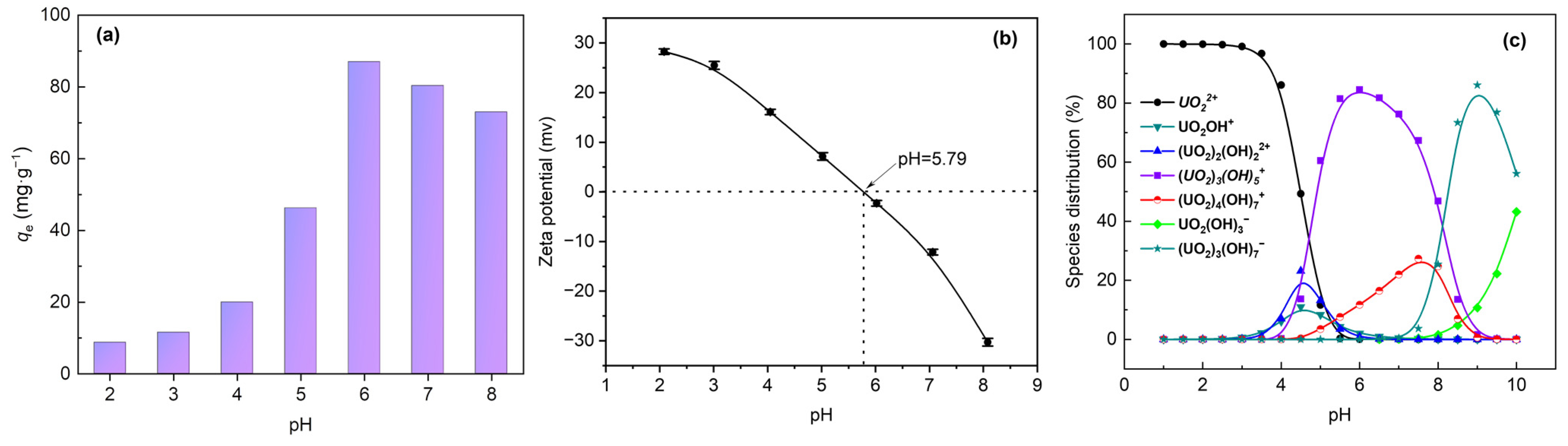
3.2. Effect of Initial pH
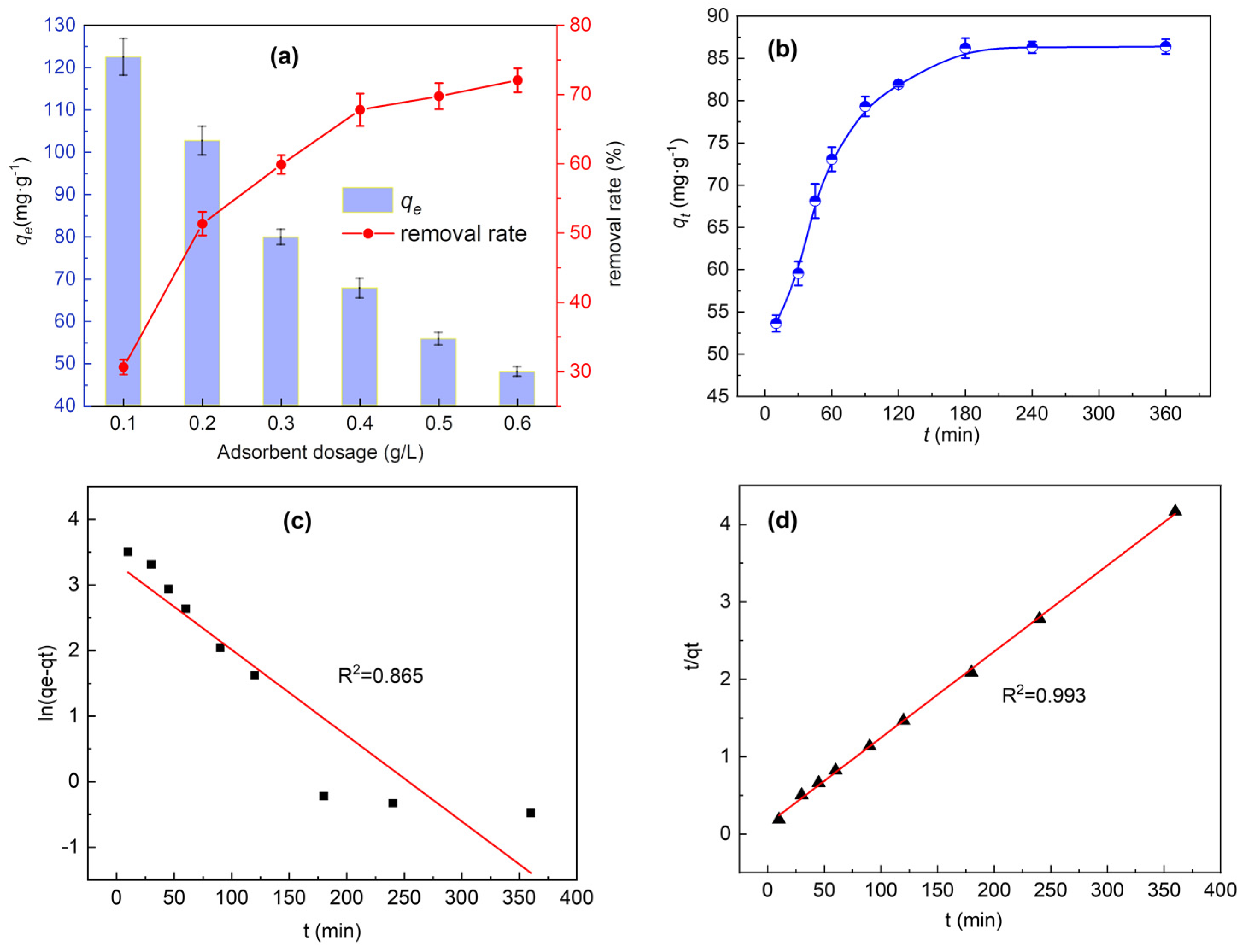
3.3. Effect of Adsorbent Dosage
3.4. Effect of Contact Time and Adsorption Kinetics
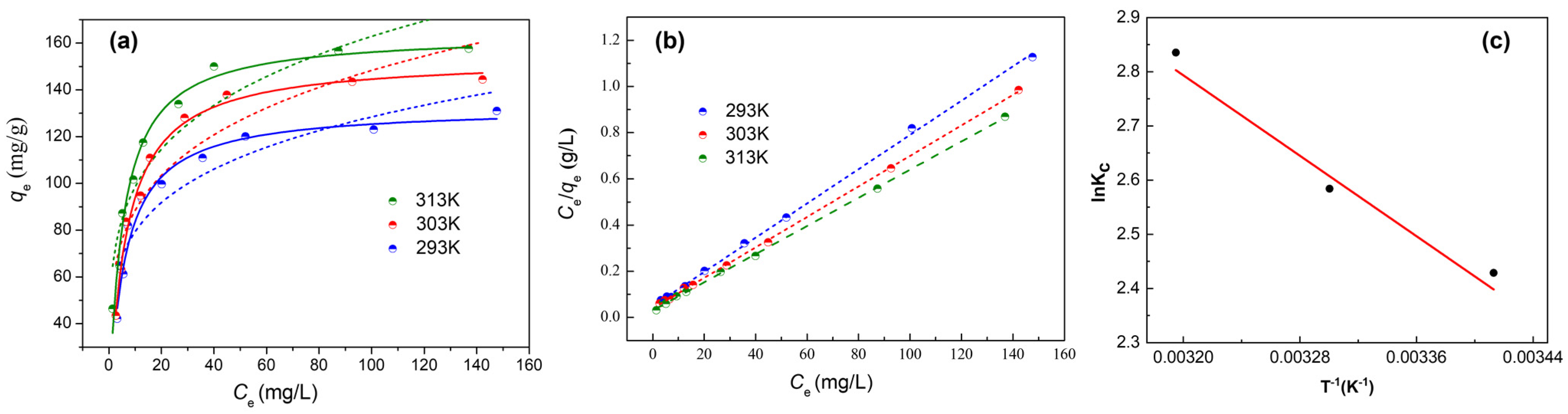
3.5. Adsorption Isotherms and Thermodynamics
3.6. Mechanism of Adsorption
3.7. Recyclability of IONPs@MAH-β-CD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsouris, C. Uranium extraction: Fuel from seawater. Nat. Energy 2017, 2, 17022. [Google Scholar] [CrossRef]
- Degueldre, C. Uranium as a renewable for nuclear energy. Prog. Nucl. Energ. 2017, 94, 174–186. [Google Scholar] [CrossRef]
- Cui, W.R.; Zhang, C.R.; Jiang, W.; Li, F.F.; Liang, R.P.; Liu, J.; Qiu, J.D. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Ruan, Y.; Zheng, Q.; Su, M.; Diao, Z.; Chen, D.; Hou, L.a.; Chang, X.; Shih, K. Uranium extraction using hydroxyapatite recovered from phosphorus containing wastewater. J. Hazard. Mater. 2020, 382, 120784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.; Zhang, Y.; Wang, D.; Zhou, H.; Li, J. Effective inspissation of uranium (VI) from radioactive wastewater using flow electrode capacitive deionization. Sep. Purif. Technol. 2022, 283, 120172. [Google Scholar] [CrossRef]
- Tang, N.; Liang, J.; Niu, C.; Wang, H.; Luo, Y.; Xing, W.; Ye, S.; Liang, C.; Guo, H.; Guo, J.; et al. Amidoxime-based materials for uranium recovery and removal. J. Mater. Chem. A 2020, 8, 7588–7625. [Google Scholar]
- Yuan, Y.; Yu, Q.; Yang, S.; Wen, J.; Guo, Z.; Wang, X.; Wang, N. Ultrafast recovery of uranium from seawater by bacillus velezensis strain UUS-1 with innate anti-biofouling activity. Adv. Sci. 2019, 6, 1900961. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Li, Q.; Li, Y.; Cao, L.; Li, W. Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater. Carbohydr. Polym. 2020, 245, 116627. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Y.; Jia, D.; Chen, W.; Cui, Z.; Wang, X. Layered silicate RUB-15 for efficient removal of UO2 2+ and heavy metal ions by ion-exchange. Environ. Sci. Nano. 2017, 4, 1851–1858. [Google Scholar] [CrossRef]
- Cui, W.R.; Li, F.F.; Xu, R.H.; Zhang, C.R.; Chen, X.R.; Yan, R.H.; Liang, R.P.; Qiu, J.D. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. Engl. 2020, 59, 17684–17690. [Google Scholar] [CrossRef]
- Zhong, L.; He, F.; Liu, Z.; Dong, B.; Ding, J. Adsorption of uranium (VI) ions from aqueous solution by acrylic and diaminomaleonitrile modified cellulose. Colloids Surf. A 2022, 641, 128565. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Zhuang, H.; Lu, B.; Sun, L.; Wang, G.; Qiu, J. Facile synthesis of low-cost MnPO4 with hollow grape-like clusters for rapid removal uranium from wastewater. J. Hazard. Mater. 2022, 434, 128894. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.M.; Lee, H.K.; Park, S.; Kim, T.J. Purification of uranium-contaminated radioactive water by adsorption: A review on adsorbent materials. Sep. Purif. Technol. 2021, 278, 119675. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, Z.; Pan, D.; Fan, F.; Tang, J.; Hu, Y.; Xu, Y.; Zhang, P.; Li, P.; Kong, X.Y.; et al. An efficient uranium adsorption magnetic platform based on amidoxime-functionalized flower-like Fe3O4@TiO2 core-shell microspheres. ACS Appl. Mater. Interfaces 2021, 13, 17931–17939. [Google Scholar] [CrossRef]
- Duan, S.; Xu, X.; Liu, X.; Wang, Y.; Hayat, T.; Alsaedi, A.; Meng, Y.; Li, J. Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic Fe3O4 nanoparticles. J. Colloid. Interf. Sci. 2018, 513, 92–103. [Google Scholar] [CrossRef]
- Ji, W.C.; Hu, P.; Wang, X.Y.; Saji, S.E.; Chang, T.; Zhu, X.Y.; Yang, F.F.; Cao, Q.G.; Dang, R.; Wang, K.S. One-step carbothermal synthesis of super nanoadsorbents for rapid and recyclable wastewater treatment. Crystals 2021, 11, 75. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid. Interfac. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Hazel, G.S.S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids Surf. A 2010, 367, 85–95. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.; Tay, A.S.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl-beta-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: Synthesis and adsorption studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef]
- Yang, S.; Zong, P.; Hu, J.; Sheng, G.; Wang, Q.; Wang, X. Fabrication of β-cyclodextrin conjugated magnetic HNT/iron oxide composite for high-efficient decontamination of U(VI). Chem. Eng. J. 2013, 214, 376–385. [Google Scholar] [CrossRef]
- Helal, A.S.; Mazario, E.; Mayoral, A.; Decorse, P.; Losno, R.; Lion, C.; Ammar, S.; Hémadi, M. Highly efficient and selective extraction of uranium from aqueous solution using a magnetic device: Succinyl-β-cyclodextrin-APTES@maghemite nanoparticles. Environ. Sci. Nano. 2018, 5, 158–168. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-capacity amidoxime-functionalized beta-cyclodextrin/graphene aerogel for selective uranium capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Wang, G.; Zhao, J.; Guo, Z.J.R.A. A novel bifunctional molecularly imprinted polymer for determination of Congo red in food. RSC Adv. 2015, 5, 22811–22817. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan–cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Frag. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Zhou, Q.; Shuang, C.; Zhou, W.; Wang, M. Effect of pore size distribution on tetracycline adsorption using magnetic hypercrosslinked resins. Micropor. Mesopor. Mat. 2014, 184, 105–111. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Rosenholm, J.M.; Gu, H.C. Synthesis and characterization of pore size-tunable magnetic mesoporous silica nanoparticles. J. Colloid Interf. Sci. 2011, 361, 16–24. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Samoilo, A.S.; Atuchin, V.V. Influence of alkyl substituents in 1,3-diethyl-2-thiobarbituric acid on the coordination environment in M(H2O)2 (1,3-diethyl-2-thiobarbiturate)2 M = Ca2+, Sr2+. J. Coord. Chem. 2016, 69, 957–965. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V.; Razumkova, I.A.; Atuchin, V.V. Crystal structure, vibrational, spectroscopic and thermochemical properties of double sulfate crystalline hydrate [CsEu(H2O)3(SO4)2]·H2O and its thermal dehydration product CsEu(SO4)2. Crystals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Luo, C.; Shea, J.J.; Huang, J. A carboxylate group-based organic anode for sustainable and stable sodium ion batteries. J. Power Sources 2020, 453, 227904. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.L.; Teng, B. Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohyd. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef]
- Chen, B.; Sun, J.; Fan, F.; Zhang, X.; Qin, Z.; Wang, P.; Li, Y.; Zhang, X.; Liu, F.; Liu, Y. Ferumoxytol of ultrahigh magnetization produced by hydrocooling and magnetically internal heating co-precipitation. Nanoscale 2018, 10, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Flores-Cano, D.A.; Caetano Passamani, E. Differentiating nanomaghemite and nanomagnetite and discussing their importance in arsenic and lead removal from contaminated effluents: A critical review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Bae, D.H.; Kwon, S.H.; Choi, H.J. Magnetite embedded mini-emulsion polymerized polystyrene particles and their magnetorheology. Macromol. Res. 2018, 26, 353–358. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Abbasi, A. Synthesis of Fe3O4@ZrO2 core–shell nanoparticles through new approach and its solar light photocatalyst application. J. Mater. Sci-Mater. El. 2017, 28, 4871–4878. [Google Scholar] [CrossRef]
- Li, N.; Gao, P.; Chen, H.; Li, F.; Wang, Z. Amidoxime modified Fe3O4@TiO2 particles for antibacterial and efficient uranium extraction from seawater. Chemosphere 2021, 287, 132137. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Krohling, A.; López, E.O.; Litterst, F.J.; Passamani, E. Superspinglass behavior of maghemite nanoparticles dispersed in mesoporous silica. J. Magn. Magn. Mater. 2019, 485, 142–150. [Google Scholar] [CrossRef]
- Lajevardi, A.; Sadr, M.H.; Yaraki, M.T.; Badiei, A.; Armaghan, M. A pH-responsive and magnetic Fe3O4@silica@MIL-100 (Fe)/β-CD nanocomposite as a drug nanocarrier: Loading and release study of cephalexin. New J. Chem. 2018, 42, 9690–9701. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, J.; Zhou, L.; Li, T.; Li, Z.; Liu, Z.; Huang, G.; Adesina, A.A. Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2016, 311, 2029–2037. [Google Scholar] [CrossRef]
- Li, W.; Han, X.; Wang, X.; Wang, Y.; Wang, W.; Xu, H.; Tan, T.; Wu, W.; Zhang, H. Recovery of uranyl from aqueous solutions using amidoximated polyacrylonitrile/exfoliated Na-montmorillonite composite. Chem. Eng. J. 2015, 279, 735–746. [Google Scholar] [CrossRef]
- Zong, P.; Xu, X.; Shao, M.; Cao, D.; Wang, S.; Zhang, H. Efficient elimination of uranium by carboxymethyl-β-cyclodextrin nanoparticles decorated iron oxides: Water chemistry influences and mechanism researches. Int. J. Hydrogen Energ. 2021, 46, 1370–1384. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Alsaedi, A.; Hayat, T.; Li, J. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J. 2019, 360, 941–950. [Google Scholar] [CrossRef]
- Ma, F.; Nian, J.; Bi, C.; Yang, M.; Zhang, C.; Liu, L.; Dong, H.; Zhu, M.; Dong, B. Preparation of carboxylated graphene oxide for enhanced adsorption of U(VI). J. Solid State Chem. 2019, 277, 9–16. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Shao, D.; Li, Y.; Xu, Z.; Cheng, C.; Asiri, A.M.; Marwani, H.M.; Hu, S. Adsorption of U(VI) on bentonite in simulation environmental conditions. J. Mol. Liq. 2017, 242, 678–684. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Wang, J.; Dong, L.; Zhang, W.; Lu, Z.; Liang, J.; Pan, D.; Fan, Q. Carboxyl groups on g-C3N4 for boosting the photocatalytic U(VI) reduction in the presence of carbonates. Chem. Eng. J. 2021, 414, 128810. [Google Scholar] [CrossRef]
- Diao, Z.; Pang, H.; Tang, H.; Wang, X.; Yu, S.; Song, G. Magnetic-dictyophora indusiata derived biochar composite for efficient removal of U(VI) and mechanism investigation. Chinese Sci. Bull. 2020, 65, 3954–3964. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, J.; Zhu, J.; Liu, Q.; Zhang, H.; Liu, J.; Chen, R.; Li, Y.; Wang, J. Cyclized polyacrylonitrile amidoxime with local conjugate domain for high-efficiency extraction of uranium from seawater. Appl. Catal. B-Environ 2022, 316, 121677. [Google Scholar] [CrossRef]
- Kavci, E.; Erkmen, J.; Bingöl, M.S. Removal of methylene blue dye from aqueous solution using citric acid modified apricot stone. Chem. Eng. Commun. 2021, 210, 1–16. [Google Scholar] [CrossRef]
- Han, H.; Cheng, C.; Hu, S.; Li, X.; Wang, W.; Xiao, C.; Xu, Z.; Shao, D. Facile synthesis of gelatin modified attapulgite for the uptake of uranium from aqueous solution. J. Mol. Liq. 2017, 234, 172–178. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Wang, Q.; Shao, D.; Wang, X. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys. Chem. Chem. Phys. 2015, 17, 398–406. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Zhang, Z. Chemical bonding between uranium and oxygen in U6+-containing compounds. J. Nucl. Mater. 2012, 420, 222–225. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, R.; Xu, G.; Guo, K.; Hu, C. Adsorptive removal of uranium (VI) from wastewater using a crosslinked amidoxime-functionalized β-cyclodextrin polymer. New J. Chem. 2022, 46, 20592–20601. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Y.; Liu, L.; Fang, Y.; Ma, F.; Zhang, C.; Dong, H. Efficient and magnetically recoverable U(VI) adsorbent: Fe3O4 loaded hypercrosslink copoly (styrene/maleic anhydride). Colloids Surf. A 2022, 632, 127644. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Vinnik, D.; Gavrilova, T.; Gudkova, S.; Isaenko, L.; Jiang, X.; Pokrovsky, L.; Prosvirin, I.; Mashkovtseva, L.; Lin, Z. Flux crystal growth and the electronic structure of BaFe12O19 hexaferrite. J. Phys. Chem. C 2016, 120, 5114–5123. [Google Scholar] [CrossRef]
- Pinaeva, L.; Prosvirin, I.; Chesalov, Y.; Atuchin, V. High-temperature abatement of N2O over FeOx/CeO2-Al2O3 catalysts: The effects of oxygen mobility. Catalysts 2022, 12, 938. [Google Scholar] [CrossRef]
- Li, B.; Ma, L.; Tian, Y.; Yang, X.; Li, J.; Bai, C.; Yang, X.; Zhang, S.; Li, S.; Jin, Y. A catechol-like phenolic ligand-functionalized hydrothermal carbon: One-pot synthesis, characterization and sorption behavior toward uranium. J. Hazard. Mater. 2014, 271, 41–49. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-ray photoelectron spectroscopic characterization of iron oxide nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]




| Adsorbent | T(K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | n | KF | R2 | ||
| IONPs@MAH-β-CD | 293 | 134.77 | 0.153 | 0.976 | 3.90 | 41.552 | 0.814 |
| 303 | 151.52 | 0.169 | 0.989 | 3.52 | 43.044 | 0.839 | |
| 313 | 163.93 | 0.202 | 0.978 | 3.70 | 50.253 | 0.881 | |
| Adsorbent | ΔG0 (KJ mol−1) | ΔH0 (KJ mol−1) | ΔS0 (J mol−1 K−1) | ||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| IONPs@MAH-β-CD | −5.84 | −6.57 | −7.29 | 15.43 | 72.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Yuan, Q.; Wang, Q.; Hu, C.; Guo, K.; Ouyang, J.; Chen, M. Maleic Anhydride-β-Cyclodextrin Functionalized Magnetic Nanoparticles for the Removal of Uranium (VI) from Wastewater. Crystals 2022, 12, 1731. https://doi.org/10.3390/cryst12121731
Zhong X, Yuan Q, Wang Q, Hu C, Guo K, Ouyang J, Chen M. Maleic Anhydride-β-Cyclodextrin Functionalized Magnetic Nanoparticles for the Removal of Uranium (VI) from Wastewater. Crystals. 2022; 12(12):1731. https://doi.org/10.3390/cryst12121731
Chicago/Turabian StyleZhong, Xing, Qiaozhulin Yuan, Qiang Wang, Caixia Hu, Kai Guo, Jinbo Ouyang, and Mingyang Chen. 2022. "Maleic Anhydride-β-Cyclodextrin Functionalized Magnetic Nanoparticles for the Removal of Uranium (VI) from Wastewater" Crystals 12, no. 12: 1731. https://doi.org/10.3390/cryst12121731




