A Promising Crystalline KCl: Electrolyte Material for Studying the Electrochemical Properties of Cerium on Liquid Indium Electrodes
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
- where: EP,C—peak potential, V
- EP/2,C—half-peak potential, V
- n—number of electrons transferred during the reaction.
- where: IP—peak current of cathode, A
- S—Surface area of liquid metal indium electrode, cm2
- F—Faraday constant, 96,485 C-mol−1
- c—Molar concentration of Ce3+ in LiCl-KCl melt, mol-cm−3
- R—Ideal gas constant, 8.314 J/(mol·K)
- T—Thermodynamic temperature of the reaction in the LiCl-KCl molten salt system, K
- D—Diffusion coefficient, cm2 s−1.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veers, P.; Dykes, K.; Lantz, E.; Barth, S.; Bottasso, C.L.; Carlson, O.; Clifton, A.; Green, J.; Green, P.; Holttinen, H.; et al. Grand challenges in the science of wind energy. Science 2019, 366, 6464. [Google Scholar] [CrossRef]
- Wiser, R.; Rand, J.; Seel, J.; Beiter, P.; Baker, E.; Lantz, E.; Gilman, P. Expert elicitation survey predicts 37% to 49% declines in wind energy costs by 2050. Nat. Energy 2021, 6, 555–565. [Google Scholar] [CrossRef]
- Mavlonov, A.; Razykov, T.; Raziq, F.; Gan, J.T.; Chantana, J.; Kawano, Y.; Nishimura, T.; Wei, H.M.; Zakutayev, A.; Minemoto, T.; et al. A review of Sb2Se3 photovoltaic absorber materials and thin-film solar cells. Sol. Energy 2020, 201, 227–246. [Google Scholar] [CrossRef]
- Zhao, M.; Su, J.W.; Zhao, Y.; Luo, P.; Wang, F.K.; Han, W.; Li, Y.; Qiao, L.; Zhai, T.Y. Sodium-Mediated Epitaxial Growth of 2D Ultrathin Sb2Se3 Flakes for Broadband Photodetection. Adv. Funct. Mater. 2021, 30, 1909849. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Qiao, L.; Xiao, H.Y.; Meyer, H.M.; Sun, J.N.; Rouleau, C.M.; Puretzky, A.A.; Geohegan, D.B.; Ivanov, I.N.; Yoon, M.; Weber, W.J.; et al. Nature of the band gap and origin of the electro-/photo-activity of Co3O4. J. Mater. Chem. C 2013, 1, 4628–4633. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Mane, S.K.B.; Faheem, M.B.; Ali, A.; Zhao, Y.; Han, S.B.; Cai, C.; Li, W.; et al. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B Environ. 2020, 270, 118867. [Google Scholar] [CrossRef]
- Raziq, F.; Humayun, M.; Ali, A.; Wang, T.T.; Khan, A.; Fu, Q.Y.; Luo, W.; Zeng, H.P.; Zheng, Z.P.; Khan, B.; et al. Synthesis of S-Doped porous g-C3N4 by using ionic liquids and subsequently coupled with Au-TiO2 for exceptional cocatalyst-free visible-light catalytic activities. Appl. Catal. B Environ. 2018, 237, 1082–1090. [Google Scholar] [CrossRef]
- Raziq, F.; He, J.X.; Gan, J.T.; Humayun, M.; Faheem, M.B.; Iqbal, A.; Hayat, A.; Fazal, S.; Yi, J.B.; Zhao, Y.; et al. Promoting visible-light photocatalytic activities for carbon nitride based 0D/2D/2D hybrid system: Beyond the conventional 4-electron mechanism. Appl. Catal. B Environ. 2020, 270, 118870. [Google Scholar] [CrossRef]
- Raziq, F.; Khan, K.; Ali, S.; Xu, H.; Ali, I.; Zada, A.; Ismail, P.M.; Ali, A.; Khan, H.; Wu, X.Q.; et al. Accelerating CO2 reduction on novel double perovskite oxide with sulfur, carbon incorporation: Synergistic electronic and chemical engineering. Chem. Eng. J. 2022, 446, 137161. [Google Scholar] [CrossRef]
- Ali, S.; Ali, S.; Ismail, P.M.; Shen, H.H.; Zada, A.; Ali, A.; Ahmad, I.; Shah, R.; Khan, I.; Chen, J.S.; et al. Synthesis and bader analyzed cobalt-phthalocyanine modified solar UV-blind beta-Ga2O3 quadrilateral nanorods photocatalysts for wide-visible-light driven H2 evolution. Appl. Catal. B Environ. 2022, 307, 121149. [Google Scholar] [CrossRef]
- Ali, S.; Yasin, G.; Iqbal, R.; Huang, X.; Su, J.; Ibraheem, S.; Zhang, Z.; Wu, X.Q.; Wahid, F.; Ismail, P.M.; et al. Porous aza-doped graphene-analogous 2D material a unique catalyst for CO2 conversion to formic-acid by hydrogenation and electroreduction approaches. Mol. Catal. 2022, 524, 112285. [Google Scholar] [CrossRef]
- Raziq, F.; Aligayev, A.; Shen, H.H.; Ali, S.; Shah, R.; Ali, S.; Bakhtiar, S.H.; Ali, A.; Zarshad, N.; Zada, A.; et al. Exceptional Photocatalytic Activities of rGO Modified (B,N) Co-Doped WO3, Coupled with CdSe QDs for One Photon Z-Scheme System: A Joint Experimental and DFT Study. Adv. Sci. 2022, 9, 2102530. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.C.; Kee, R.J.; Zhu, H.Y.; Karakaya, C.; Chen, Y.C.; Braun, R.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Spendelow, J.S.; Choe, Y.K.; Fujimoto, C.; Kim, Y.S. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 2016, 1, 16120. [Google Scholar] [CrossRef]
- Wu, X.Q.; He, J.X.; Zhang, M.; Liu, Z.R.; Zhang, S.; Zhao, Y.; Li, T.; Zhang, F.P.; Peng, Z.; Cheng, N.Y.; et al. Binary Pd/amorphous-SrRuO3 hybrid film for high stability and fast activity recovery ethanol oxidation electrocatalysis. Nano Energy 2020, 67, 104247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ding, X.; Qiao, L. Recent advances in the electrochemistry of layered post-transition metal chalcogenide nanomaterials for hydrogen evolution reaction. J. Energy Chem. 2021, 60, 451–479. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ilyas, N.; Yang, L.X.; Ali, S.; Zada, A.; Bakhtiar, S.H.; Wang, Y.; Shen, H.H.; Qiao, L. FeNi@CNS nanocomposite as an efficient electrochemical catalyst for N-2 -to-NH3 conversion under ambient conditions. J. Mater. Sci. Technol. 2022, 103, 59–66. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.P.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Fan, E.S.; Li, L.; Wang, Z.P.; Lin, J.; Huang, Y.X.; Yao, Y.; Chen, R.J.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.; He, Y.H.; Wu, X.Q.; Ge, R.X.; Zu, X.T.; Li, S.A.; Qiao, L. High-performance asymmetric supercapacitors realized by copper cobalt sulfide crumpled nanoflower and N, F co-doped hierarchical nanoporous carbon polyhedron. J. Power Sources 2020, 456, 228023. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Ding, X.; Liu, Z.R.; Tian, H.; Shen, H.H.; Zu, X.T.; Li, S.A.; Qiao, L. One-step colloid fabrication of nickel phosphides nanoplate/nickel foam hybrid electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 373, 1132–1143. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Qiao, L. Layered LaCuOSe: A Promising Anisotropic Thermoelectric Material. Phys. Rev. Appl. 2020, 13, 024038. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Gong, H.F.; Liu, Z.J.; Zu, X.T.; Qiao, L. Optimizing the thermoelectric transport properties of Bi2O2Se monolayer via biaxial strain. Phys. Chem. Chem. Phys. 2019, 21, 15097–15105. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, N.; Xiao, H.Y.; Zhang, H.B.; Liu, Z.J.; Zu, X.T.; Qiao, L. Improved thermoelectric performance of bilayer Bi2O2Se by the band convergence approach. J. Mater. Chem. C 2019, 7, 11029–11039. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Gao, Z.B.; Liu, Z.J.; Zu, X.T.; Li, S.A.; Qiao, L. Band degeneracy enhanced thermoelectric performance in layered oxyselenides by first-principles calculations. NPJ Comput. Mater. 2021, 7, 18. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, N.; Zhang, S.; Hu, J.T.; Xiao, H.Y.; Gong, H.F.; Liu, Z.J.; Qiao, L.; Zu, X.T. A review of the properties, synthesis and applications of lanthanum copper oxychalcogenides. J. Phys. D Appl. Phys. 2022, 55, 273002. [Google Scholar] [CrossRef]
- Salvatores, M. Nuclear fuel cycle strategies including partitioning and transmutation. Nucl. Eng. Des. 2005, 235, 805–816. [Google Scholar] [CrossRef]
- Salvatores, M.; Palmiotti, G. Radioactive waste partitioning and transmutation within advanced fuel cycles achievements and challenges. Prog. Part. Nucl. Phys. 2011, 66, 144–166. [Google Scholar] [CrossRef]
- Cometto, M.; Wydler, P.; Chawla, R. Management of actinide waste inventories in nuclear phase-out scenarios. Ann. Nucl. Energy 2008, 35, 1447–1460. [Google Scholar] [CrossRef]
- Laidler, J.J.; Battles, J.E.; Miller, W.E.; Ackerman, J.P.; Carls, E.L. Development of pyro-processing technology. Prog. Nucl. Energy 1997, 31, 131–140. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.L.; Liu, K.; Tang, S.L.; Shi, W.Q. Electrochemical Extraction of Cerium by Forming Ce-Zn Alloys in LiCl-KCl Eutectic on W and Liquid. J. Electrochem. Soc. 2015, 165, E179–E184. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X. Nuclear Energy Development in China: A Study of Opportunities and Challenges. Energy 2010, 35, 4282–4288. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Buddenberg, T.; Wu, S.; Sigurbjornsson, O.; Tran, K.C.; Kakaras, E. The Challenge of Energy Storage in Europe: Focus on Power to Fuel. J. Energy Resour. Technol. 2016, 138, 04200201–04200210. [Google Scholar] [CrossRef]
- Armington, A.F.; Posen, H.; Lipson, H. Lipson Strengthening of halides for infrared windows. J. Electron. Mater. 1973, 2, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xia, J.; Li, X.Z.; Ru, F.; Chen, X.; Hua, Z.; Shao, R.W.; Wang, X.C.; Zhang, W.J.; Lee, C.S.; et al. Vapor phase epitaxy of PbS single-crystal films on water-soluble substrates and application to photodetectors. Nano Res. 2022, 15, 5402–5409. [Google Scholar] [CrossRef]
- Satoh, T.; Iwai, T.; Arai, Y. Electrolysis of Burnup-Simulated Uranium Nitride Fuels in LiCl-KCl Eutectic Melts. J. Nucl. Sci. Technol. 2009, 46, 557–563. [Google Scholar] [CrossRef][Green Version]
- Masset, P.; Konings, R.J.M.; Malmbeck, R.; Serp, J.; Glatz, J.P. Thermochemical properties of lanthanides (Ln = La, Nd) and actinides (An = U, Np, Pu, Am) in the molten LiCl-KCl eutectic. J. Nucl. Mater. 2005, 344, 173–179. [Google Scholar] [CrossRef]
- Bechtel, T.B.; Storvick, T.S. Activity coefficients of actinide and rare-earth chlorides in molten LiCl/KCl eutectic salt. Ind. Eng. Chem. Res. 1999, 38, 1723–1728. [Google Scholar] [CrossRef]
- Zhu, T.J.; Huang, W.; Gong, Y. Electrochemical Separation of Uranium from Lanthanide (La, Eu, Gd) Fluorides in Molten LiCl-KCl. Sep. Purif. Technol. 2020, 235, 116227. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Osipenko, A.; Maershin, A. Thermodynamics and separation factor of uranium from lanthanum in liquid eutectic gallium-indium alloy/molten salt system. Electrochim. Acta 2014, 145, 81–85. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kwon, K.C.; Park, B.H.; Seo, C.S. A Kinetic Study of the Oxidation of Uranium Dioxide. React. Kinet. Mech. Catal. 2006, 89, 269–275. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Volkovich, V.A. Thermodynamics of La and U and the separation factor of U/La in fused Me (Ga-40wt% In)/3LiCl-2KCl system. J. Nucl. Mater. 2017, 495, 285–290. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Osipenko, A.; Kormilitsyn, M. The influence of electrode material nature on the mechanism of cathodic reduction of ytterbium (III) ions in fused NaCl–KCl–CsCl eutectic. J. Electroanal. Chem. 2009, 633, 291–296. [Google Scholar] [CrossRef]
- Melchakov, S.Y.; Maltsev, D.S.; Volkovich, V.A.; Yamshchikov, L.F.; Osipenko, A.G. Separation of Uranium and Lanthanides in a Fused Salt - Liquid Gallium Based Alloy System. ECS Trans. 2014, 64, 369–375. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, Y.D.; Han, W.; Zhang, M.L.; Yuan, L.Y.; Lin, R.S.; Ye, G.A.; He, H.; Chai, Z.F.; Shi, W.Q. Electrochemical separation of Th from ThO2 and Eu2O3 assisted by AlCl3 in molten LiCl–KCl. Electrochim. Acta 2013, 114, 180–188. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kumar, J.R.; Kim, J.S.; Park, H.K.; Yoon, H.S. Liquid-liquid extraction/separation of platinum (IV) and rhodium (III) from acidic chloride solutions using tri-iso-octylamine. J. Hazard. Mater. 2009, 168, 424–429. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Hayashi, H.; Minato, K.; Gaune-Escard, M. Electrochemical transient techniques for determination of uranium and rare-earth metal separation coefficients in molten salts. Electrochim. Acta 2006, 51, 2463–2470. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.J.; Eun, H.C.; Kim, G.Y.; Lee, S. Uranium recovery with zinc distillation from a liquid zinc cathode for pyroprocessing. J. Radioanal. Nucl. 2018, 361, 649–654. [Google Scholar] [CrossRef]
- Fukasawa, K.; Uehara, A.; Nagai, T.; Sato, N.; Fujii, T.; Yamana, H. Thermodynamic properties of trivalent lanthanide and actinide ions in molten mixtures of LiCl and KCl. J. Nucl. Mater. 2012, 424, 17–22. [Google Scholar] [CrossRef]
- Xu, M.H.; Smolenski, V.; Liu, Q.; Novoselova, A.; Jiang, K.W.; Yu, J.; Liu, J.Y.; Chen, R.R.; Zhang, H.S.; Zhang, M.L.; et al. Thermodynamics, Solubility and the Separation of Uranium from Cerium in Molten In/3LiCl-2KCl System. J. Electrochem. Soc. 2021, 167, 136506. [Google Scholar] [CrossRef]
- Novoselova, A.; Smolenski, V. Solubility, Activity coefficients and the separation factor of U/Pr couple in Ga-In alloys of different compositions in fused LiCl-KCl-CsCl eutectic. J. Electrochem. Soc. 2020, 167, 126518. [Google Scholar] [CrossRef]
- Toda, T.; Maruyama, T.; Moritani, K.J.; Moriyama, H.; Hayashi, H. Thermodynamic Properties of Lanthanides and Actinides for Reductive Extraction of Minor Actinides. J. Nucl. Sci. Technol. 2009, 46, 18–25. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles. RSC Adv. 2013, 4, 4663–4671. [Google Scholar] [CrossRef]
- Kim, S.H.; Paek, S.; Kim, T.J.; Park, D.Y.; Ahn, D.H. Electrode reactions of Ce3+/Ce couple in LiCl–KCl solutions containing CeCl3 at solid W and liquid Cd electrodes. Electrochim. Acta 2012, 85, 35–40. [Google Scholar] [CrossRef][Green Version]
- Xu, M.H.; Smolenski, V.; Liu, Q.; Novoselova, A.; Jiang, K.W.; Yu, J.; Liu, J.Y.; Chen, R.R.; Zhang, H.S.; Zhang, M.L.; et al. Thermodynamic properties of stable states cerium compounds in fused 3LiCl-2KCl eutectic. J. Chem. 2021, 152, 106260. [Google Scholar] [CrossRef]
- Kato, T.; Inoue, T.; Iwai, T.; Arai, Y. Separation behaviors of actinides from rare-earths in molten salt electrorefining using saturated liquid cadmium cathode. J. Nucl. Mater. 2006, 357, 105–114. [Google Scholar] [CrossRef]
- Li, S.X.; Herrmann, S.D.; Simpson, M. Electrochemical analysis of actinides and rare earth constituents in liquid cadmium cathode product from spent fuel electrorefining. Nucl. Technol. 2010, 171, 292–299. [Google Scholar] [CrossRef]
- Castrillejo, Y.; Bermejo, M.R.; Arocas, P.D.; Martinez, A.M.; Barrado, E. The electrochemical behaviour of the Pr(III)/Pr redox system at Bi and Cd liquid electrodes in molten eutectic LiCl–KCl. J. Chem. 2005, 579, 343–358. [Google Scholar] [CrossRef]
- Castrillejo, Y.; Bermejo, M.R.; Martinez, A.M.; Barrado, E.; Arocas, P.D. Application of electrochemical techniques in pyrochemical processes – Electrochemical behaviour of rare earths at W, Cd, Bi and Al electrodes. J. Nucl. Mater. 2007, 360, 32–42. [Google Scholar] [CrossRef]
- Serp, J.; Lefebvre, P.; Malmbeck, R.; Rebizant, J.; Vallet, P.; Glatz, J.P. Separation of plutonium from lanthanum by electrolysis in LiCl-KCl onto molten bismuth electrode. J. Nucl. Mater. 2005, 340, 266–270. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.L.; Chai, Z.F.; Shi, W.Q. Evaluation of the Electroextractions of Ce and Nd from LiCl-KCl Molten Salt Using Liquid Ga Electrode. J. Electrochem. Soc. 2017, 164, D169–D178. [Google Scholar] [CrossRef]
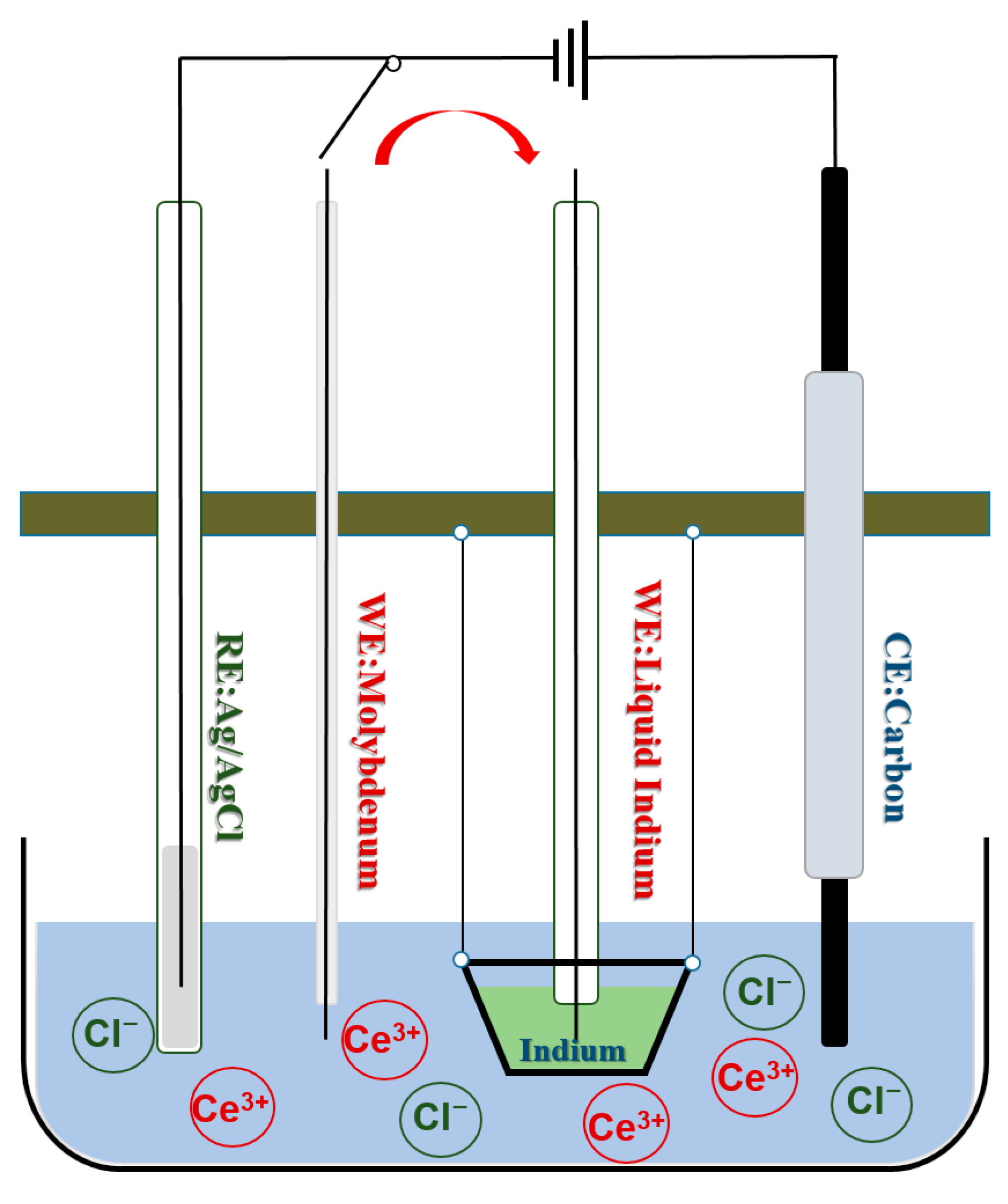
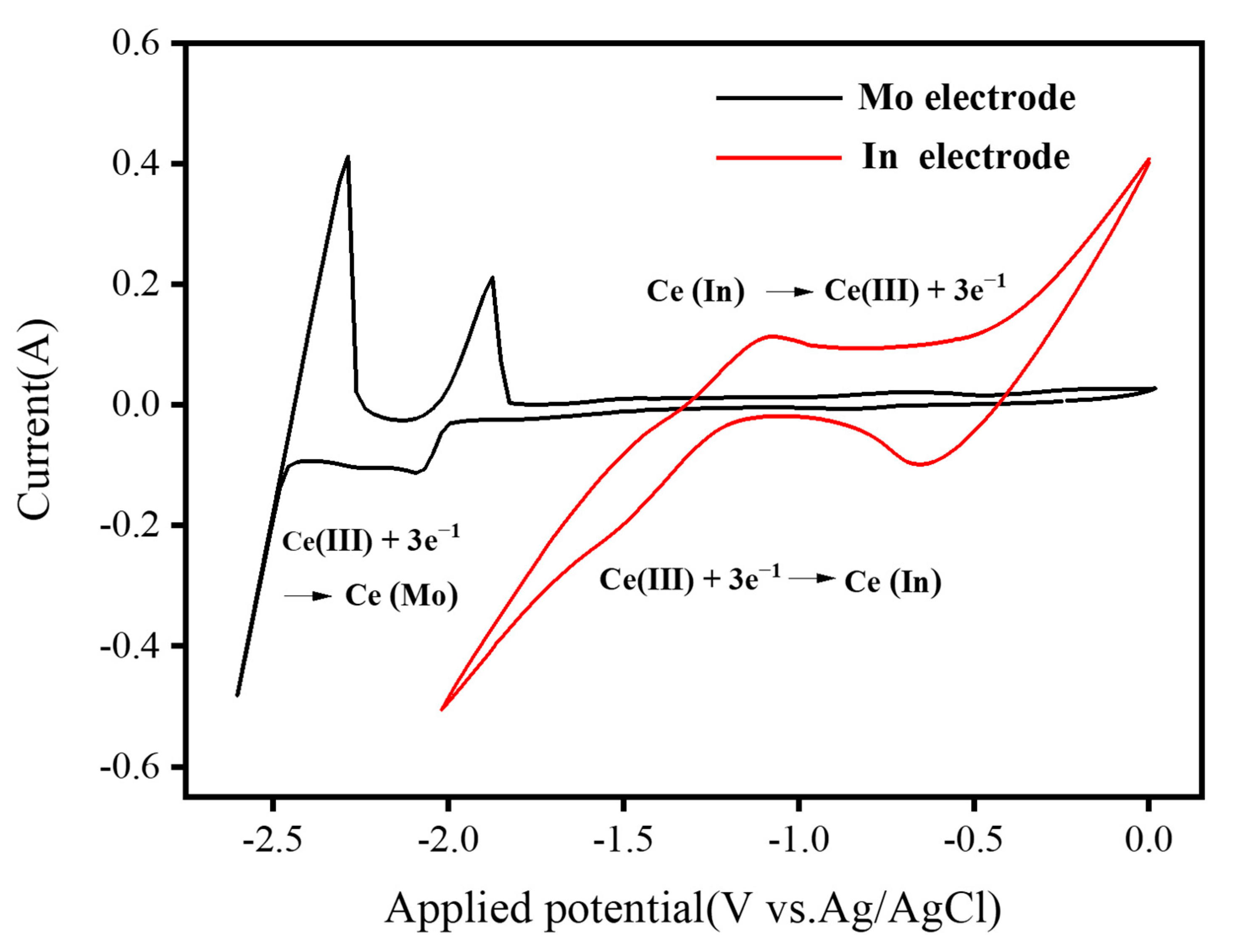



| v/(mV·s−1) | EP,C/V | EP/2,C/V | EP/2,C/V − EP,C/V | α |
|---|---|---|---|---|
| 50 | −1.472 | −1.342 | 0.130 | 0.953 |
| 75 | −1.480 | −1.347 | 0.133 | 0.931 |
| 100 | −1.487 | −1.351 | 0.136 | 0.912 |
| 150 | −1.492 | −1.353 | 0.139 | 0.892 |
| 200 | −1.503 | −1.360 | 0.143 | 0.867 |
| T(K) | 723 | 753 | 773 | 803 | 823 |
| D × 10−5/cm2 s−1 | 0.6512 | 0.9105 | 1.2032 | 1.4517 | 1.7326 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhao, Y.; Ji, Y.; Dong, Y.; Leng, H.; Li, X.; He, W.; Qiao, L. A Promising Crystalline KCl: Electrolyte Material for Studying the Electrochemical Properties of Cerium on Liquid Indium Electrodes. Crystals 2022, 12, 1509. https://doi.org/10.3390/cryst12111509
Xu M, Zhao Y, Ji Y, Dong Y, Leng H, Li X, He W, Qiao L. A Promising Crystalline KCl: Electrolyte Material for Studying the Electrochemical Properties of Cerium on Liquid Indium Electrodes. Crystals. 2022; 12(11):1509. https://doi.org/10.3390/cryst12111509
Chicago/Turabian StyleXu, Minghui, Yan Zhao, Yuanpeng Ji, Yunfa Dong, Huaqian Leng, Xiaowei Li, Weidong He, and Liang Qiao. 2022. "A Promising Crystalline KCl: Electrolyte Material for Studying the Electrochemical Properties of Cerium on Liquid Indium Electrodes" Crystals 12, no. 11: 1509. https://doi.org/10.3390/cryst12111509
APA StyleXu, M., Zhao, Y., Ji, Y., Dong, Y., Leng, H., Li, X., He, W., & Qiao, L. (2022). A Promising Crystalline KCl: Electrolyte Material for Studying the Electrochemical Properties of Cerium on Liquid Indium Electrodes. Crystals, 12(11), 1509. https://doi.org/10.3390/cryst12111509








