Abstract
Potassium chloride crystals are good conductors in the UV to mid-infrared broadband and can be widely used as materials for high-power CO2 lasers. However, the most important use of potassium chloride crystals has been overlooked; their stable chemistry and wide electrochemical window allow them to form molten eutectic salts with other chlorides for the dry reprocessing of spent fuel. The aim of this work is to investigate the electrochemical properties of the actinide substitute cerium metal on liquid indium media in order to provide basic data for further realization of lanthanides (Ln)/actinides (An) separation. In this paper, we investigated the electrochemical behavior of 3LiCl-2KCl-CeCl3 melt at liquid metal media indium (In) electrode using various transient electrochemical analysis methods, such as cyclic voltammetry, square wave voltammetry and open circuit potential technique. The quasi-equilibrium potentials of Ce3+ at different temperatures on the liquid metal In electrode were determined. On the redox process, the reaction kinetics of the oxidation–reduction process in the 3LiCl-2KCl-CeCl3 system is studied. It was determined that the reduction of Ce3+ at the liquid metal In electrode is an irreversible single-step process with three electron transfers. The relationship between the diffusion coefficient and the reduction process of Ce3+ ions on liquid metal indium electrodes at different temperatures was determined. The expression for the diffusion coefficient and temperature of Ce3+ at the liquid metal indium electrode is: lnD = 1.43 − 7974.69/T.
1. Introduction
The continuous exploitation and use of fossil fuels have caused serious and difficult to treat environmental pollution problems. In recent years, efforts have been made to develop environmentally friendly and renewable clean energy sources to replace traditional fossil fuels, such as wind [1,2], solar-based (photovoltaics [3,4,5,6] or photocatalysis [7,8,9,10,11,12,13]), hydrogen-based (fuel cell [14,15] and electrocatalysis [16,17,18]), batteries [19,20], supercapacitors [21,22], thermoelectric [23,24,25,26,27] and nuclear energy [28,29]. More realistically, nuclear energy remains the most promising clean energy source for efficient energy supply and large-scale utilization [30,31]. Given the historical implementation of nuclear energy, we have full confidence in its use to replace organic fuels such as coal, oil and natural gas [32]. However, with the rapid development of nuclear energy, the radioactive materials in spent reactor fuel have increased, so the effective reprocessing of spent fuel has become the most difficult issue globally [33,34]. Therefore, finding and developing promising chloride and fluoride molten salt systems and determining the basic physicochemical properties of molten salt systems is one of the important ways to achieve breakthroughs [33,35]. It is widely believed that KCl crystals are good conductors in the broadband from ultraviolet to mid-infrared, and are broadly used for infrared spectroscopy and for manufacturing ultraviolet and infrared optical components [35]. At the same time, these crystals are also an important material for thin film substrates, especially for “film-substrate” separation applications, where these water-soluble substrates can be easily dissolved with water, leaving only the thin film [36]. More interestingly, KCl crystals have been found to be an extremely promising material for the formation of molten eutectic salt systems with other chlorides and for dry reprocessing of spent fuel [35,36,37].
Recently, molten salts have attracted considerable attention as the most promising novel technology among the many reaction media for separating Ln and An, and most importantly for their ability to efficiently process radioactive waste and spent nuclear fuel [38,39,40]. High-temperature thermochemical methods using molten salts and liquid metals in the reprocessing of spent nuclear reactor fuel are being investigated [41]. Researchers use liquid metals to selectively extract fissile elements dissolved in molten salts for the purpose of separation [42]. The chemical properties of the molten salt and the liquid metal determine the selectivity of the thermochemical separation process occurring between the interfaces of the two phases [41,43,44,45,46,47,48,49,50]. In addition, the nature and composition of the studied melt systems play a decisive role in the selective separation process of the fission products. Understanding the thermodynamic properties of fission elements in SNFs is essential for their applicability in practical separation processes [32,51,52,53]. The currently proposed method for the separation of fission elements at liquid electrodes is effective and novel, but previous work on the electrode separation of indium in liquid metal is scarce [54]. In this work, the electrochemical properties of the fissile element cerium (Ce) in spent fuel are emphasized. Ce is the most important fissile element in the lanthanide family and is chemically similar to An. Therefore, Ce has been widely used as a substitute for the behavior of the element An [55]. More importantly, despite its chemical similarity to many fissile elements, cerium metal is one of the most dangerous elements among fission products and, in addition, it is a neutron poison [56,57].
In order to improve the separation effect regarding An and Ln elements, some experts have conducted basic research on the application of Al, Bi and Cd cathodes; however, the high melting point of metal Al (660 °C) renders it difficult to realize the application of liquid cathodes [58,59,60,61]. Therefore, considering that indium metal is a liquid at low temperatures and located in the same main group as Al, it should have similar chemical properties and is expected to replace the Al electrode as a liquid metal cathode. The content of this chapter proposes to study the electrochemical behavior of Ce3+ on liquid metal indium in eutectic salt LiCl-KCl melt by using transient electrochemical methods such as cyclic voltammetry, square wave voltammetry and open circuit potential technique to provide basic parameters for the application of In as liquid metal in molten salt dry reprocessing process.
2. Experimental Section
A low eutectic mixture of LiCl-KCl (2:3 wt.%) was first dehydrated under vacuum drying oven at 473 K for more than 24 h to remove adsorbed water and then melted under a dry alumina crucible in a quartz glass capsule. Pre-electrolysis at 2.10 V for 4 h was used to remove impurities. CeCl3 (99.9 wt.%) after chlorination by CCl4 was used as a source of Ce3+ and added directly to the crucible containing the LiCl-KCl eutectic salt. All experiments were performed under argon atmosphere to prevent environmental O2 or H2O interference. The electrolytic cell is an alumina or glassy carbon crucible placed in a cylindrical quartz vessel with a placed eutectic salt. The experiments were performed under an inert argon atmosphere (99.999%). The cells are heated using a vertical heater connected to the programmable device to control the temperature of the heater to ±8 K. The operating temperature is measured using a NiCr thermocouple.
The experiments in this chapter employ a three-electrode system to study the electrochemical behavior of Ce3+ on indium cathode, the active liquid indium electrode is used as the working electrode, and the liquid In electrode is made by placing the liquid indium in a 15 mm × 20 mm alumina crucible with a mass of 5 g of indium. The specific electrochemical redox process is shown schematically in Figure 1. The reference electrode is composed of corundum tube containing silver wire (d = 1 mm, 99.99% pure silver) and AgCl (1 wt.%, molar fraction of 0.0039) of corundum tube, and the ratio of salt in the reference electrode was KCl-LiCl (3:2 wt.%). The silver wire was polished with SiC sandpaper and inserted into the melt to obtain a more accurate chemical reaction. CeCl3 with a concentration of about (2.80 wt.%) and In 4 g were simultaneously placed in a crucible containing KCl-LiCl eutectic salt and the furnace temperature was raised to 823 K. The auxiliary electrode was composed of a 3 mm glassy carbon rod (SU-2000). The electrode potentials during the experiments were used (vs. Ag/AgCl) as a reference.
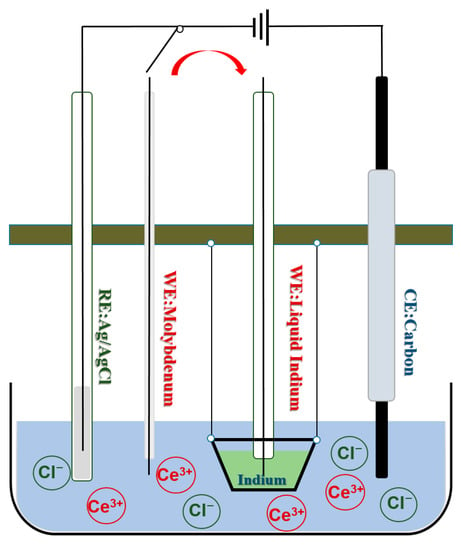
Figure 1.
Schematic diagram of the equilibrium potential determination of Ce3+ at liquid indium metal (In) electrode in the LiCl-KCl eutectic salt system.
Electrochemical measurements including CV, SWV, and OCP methods were used to study the electrochemical behavior of Ce on liquid metal indium electrodes. The electrochemical data obtained were measured and analyzed using a PGSTAT 302N electrochemical workstation (Autolab, Metrohm) and controlled by Nova 1.8 software package. The Ce-In alloy was obtained by constant potential electrolysis at the indium electrode at a selected applied potential. After the experiment, a small amount of sample was dissolved in nitric acid, and the cerium (uranium) concentration in the molten salt was determined by ICP-MS test. The indium containing the deposited cerium metal was washed and quantita-tively dissolved in nitric acid or perchloric acid. An ICP-MS test was subsequently con-ducted to obtain the alloy composition and determine the concentration of cerium on the metal indium.
3. Results and Discussion
The comparative cyclic voltammetry on the molybdenum electrode and the liquid indium metal electrode after the addition of Ce3+ to the molten eutectic LiCl-KCl melt at 723 K are shown in Figure 2, which clearly reflects the redox processes occurring at the different electrodes. Regarding the cyclic voltammetry of redox reactions occurring at the molybdenum electrode, the potential control ranges from −2.7 to 0 V, approximately at potentials of −2.6 V and −2.4 V representing the reduction and dissolution of lithium. When scanning in the positive direction, the Cl− undergoes oxidation to form Cl2 around +0.8 V. However, in this experiment, we chose to avoid scanning in the positive direction in order to prevent Cl2 from affecting the normal operation of the working electrode, so as not to produce a large amount of Cl2 on the surface of the working electrode. In addition to the precipitation of lithium metal in the black curve, a new pair of redox peaks appears in the curve, corresponding to the redox process of cerium, where Ce is also precipitated at a potential near −2.10 V and the oxidation peak potential is near −1.95 V, as shown in Figure 2. On the other hand, no other peak signals were observed before the precipitation of lithium metal, indicating that the redox process was a one-step reaction and that no co-deposition of Ce3+ and Li+ ions occurred. It can be seen that the peak shape corresponding to the redox of cerium metal on a molybdenum electrode is characterized by a typical electrochemical process of first generating solid-phase material on a heterogeneous inert electrode and then stripping and dissolving the deposited material into solution [61]. For the CV occurring at the indium metal electrode, redox peaks appear at −1.472 V and −1.183 V. This phenomenon is mainly attributed to the deposition of Ce3+ on the liquid metal indium electrode forming an In alloy and the oxidation of the alloy to Ce3+. In addition, the CV of Ce3+ at the liquid metal indium electrode observed only a pair of redox peaks, so the reduction of Ce3+ at the liquid metal indium electrode is a one-step reduction reaction with the number of transferred electrons (n) of 3. The calculated values of αn are listed in Table 1.
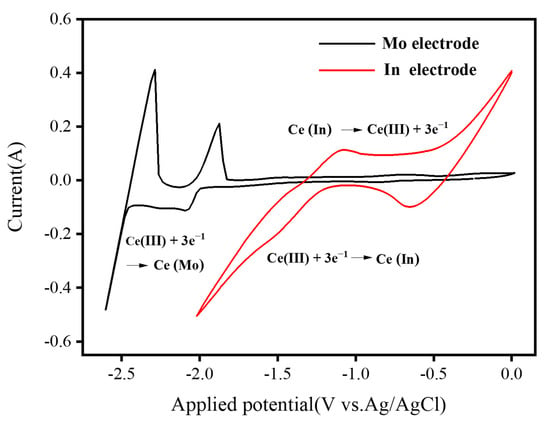
Figure 2.
Comparison between cyclic voltammetry of LiCl-KCl-CeCl3 melt at 723 K at molybdenum and liquid indium metal electrodes, respectively; sweep rate: 100 mV s−1.

Table 1.
Values of peak cathodic potential, half-front potential and αn of Ce3+ on liquid metal indium electrode in the molten salt system of LiCl-KCl-CeCl3 (3.0 wt.%) at 773 K.
The peak potential and peak current measured by CV versus sweep rate are important techniques to determine the reversibility of the reaction process, and systems in which the peak potential does not vary with the sweep rate usually exhibit reversibility. In this paper, the mechanism of the Ce3+/Ce reaction in an insoluble–soluble system is investigated by analyzing the CV at different potential scanning rates. The natural logarithmic function of the peak cathode potential and the scan rate showed regular changes, and the type of reaction control could be judged. The CV on the liquid metal indium electrode in LiCl-KCl-CeCl3 (3.0 wt.%) melt at different scan rates are shown in Figure 3a. We clearly find that the cyclic voltammetry in the figure gives mainly three pairs of redox peaks, where peaks M and M1 correspond to the redox potential of the In electrode. Peaks F and F1 correspond to the redox potential of Ce on the liquid metal In electrode. L and L1 correspond to the deposition and dissolution processes of Li on the In electrode. Here, we focus on the redox process of Ce on a liquid metal In electrode. As the scan rate increases from 50 mV·s−1 to 200 mV·s−1, the cathode peak potential changes significantly, with the potential moving from −1.472 V to a more negative −1.502 V. At the same time, the peak current shifts gradually in a more negative direction with the increase in scan rate. Figure 3b shows the curve of the cathode peak potential (EP,C) versus the logarithm of the sweep speed (lgv), from which it can be seen that the cathode peak potential and the sweep speed are linearly related, so it can be determined that the redox reaction of Ce3+ on the liquid metal indium electrode is irreversible. Moreover, both the results we obtained and those reported in the literature exhibit irreversible reactions at liquid metal gallium electrodes [62].
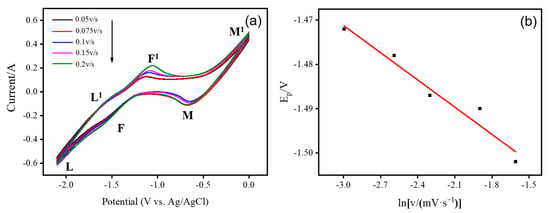
Figure 3.
(a) Cyclic voltammetry of LiCl-KCl-CeCl3 (3.0 wt.%) molten salt system at liquid metal indium electrode at 773 K at different sweep rates. Scan rate: 0.05–0.2 V-s−1, (b) Relationship between the peak cathode potential and the logarithm of the scan rate.
For irreversible reactions, the value of the electron transfer coefficient (α) can be calculated from Equation (1).
- where: EP,C—peak potential, V
- EP/2,C—half-peak potential, V
- n—number of electrons transferred during the reaction.
The transfer of Ce3+ ions in the melt to the indium electrode occurs solely via diffusion, and under the conditions where no other chemical reactions occur, it can be determined that the peak cathode current is proportional to the flux through the surface of the liquid metal electrode. In order to calculate the diffusion coefficient of Ce3+ at the liquid metal indium electrode in the molten system of LiCl-KCl, this chapter is calculated by derivation of equation.
- where: IP—peak current of cathode, A
- S—Surface area of liquid metal indium electrode, cm2
- F—Faraday constant, 96,485 C-mol−1
- c—Molar concentration of Ce3+ in LiCl-KCl melt, mol-cm−3
- R—Ideal gas constant, 8.314 J/(mol·K)
- T—Thermodynamic temperature of the reaction in the LiCl-KCl molten salt system, K
- D—Diffusion coefficient, cm2 s−1.
Figure 4a represents the relationship curves between the peak cathode current and the square root of the sweep rate at different temperatures. From Figure 4a, it can be seen that the peak current value of the cathode is linearly related to the square root of the sweep rate, and this result can indicate that the mass transfer during the reduction of Ce3+ on the surface of the liquid metal indium electrode to form the Ce-In alloy is controlled by diffusion. The obtained experimental numbers were substituted into Equation (2), and the diffusion coefficients of Ce3+ on the liquid metal indium electrode in LiCl-KCl melts at different temperatures were calculated and included in Table 2.
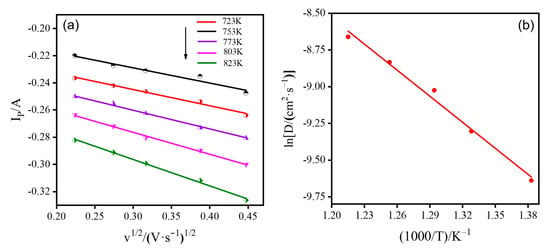
Figure 4.
(a) Relationship between the peak cathode current and the square root of the scan rate in the cyclic voltammetry of 3LiCl-2KCl-CeCl3 across different temperatures at the liquid metal indium electrode in LiCl-KCl melts. Scanning speed: 0.05–0.2 V-s−1, (b) Diffusion coefficient of Ce3+ on liquid metal indium electrode versus temperature in LiCl-KCl melt at different temperatures.

Table 2.
Diffusion coefficients of Ce3+ ions in LiCl-KCl melt at different temperatures at liquid metal indium electrodes.
The activation energy of Ce3+ diffusion in the LiCl-KCl melt into the liquid metal indium electrode is calculated according to the Arrhenius formula.
The relationship equation between diffusion coefficient and activation energy can be derived from Equation (3), and the corresponding results are obtained by fitting the calculated data using the software OriginPro 9.64, and the relationship curve between diffusion coefficient and temperature is depicted by Figure 4b.
According to Figure 4b, the expression of the diffusion coefficient of Ce3+ at the metal indium electrode versus temperature is:
The results show that the diffusion coefficient of Ce3+ in the LiCl-KCl melt at the liquid indium metal electrode increases with an increase in the experimental temperature within the temperature range of 723–823 K.
We also measured the equilibrium electrode potential of the Ce-In alloy using the OCP technique method. Figure 5a shows the equilibrium electrode potential versus time for a liquid indium working electrode (S = 0.38 cm2) in a molten eutectic salt 3LiCl-2KCl-CeCl3 (2.4 wt.%) melt after a short-time polarization in the temperature range of 723–823 K.
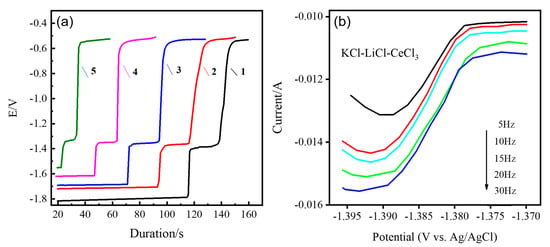
Figure 5.
(a) Potential energy (vs. Ag/AgCl) versus time in 3LiCl-2KCl-CeCl3 melts at different temperatures on liquid metal indium electrodes after short-time polarization under inert atmosphere conditions. Current—80 mA; Temperature: 1—723 K; 2—753 K; 3—773 K; 4—803 K; 5—823 K. (b) SWV curves of Ce3+ on liquid metal indium electrode.
The plateau that appears on the curve can be clearly seen in Figure 5a. The potential plateau corresponds to the composition of the electrode surface in a two-phase coexistence state, i.e., the quasi-equilibrium potential of the formed Ce-In alloy. Our clear hairline shows a plateau near −1.4 V. The diffusion of the deposited metal generates stable compounds as it reacts with the deposit, representing the formation of a Ce-In metal compound. In addition, the equilibrium potential of the solid Ce and liquid-metal-indium alloys shifts in a positive direction as the temperature increases. Finally, we find that the plateau is reproduced around −0.5 V, which is attributed to the residual potential of the indium electrode. Our results share similar characteristics with those reported in the literature for the generation of intermetallic compounds by Ce-Ga. The difference in potential is due to the difference in equilibrium potential generated by different intermetallic compounds [62].
The square wave voltammetry potential waveform is a superposition of a step wave reference potential and a bidirectional potential pulse. In addition, it constitutes the background current of the impurity redox reaction Faraday current, which is effectively deducted by differential subtraction. SWV is one of the most sensitive methods for transient electrochemical testing. With the property of eliminating capacitance and residual current, square wave voltammetry has been widely used in studies exploring the electrochemical behavior of lanthanides and actinides. In this paper, the SWV of Ce3+ in LiCl-KCl melt over liquid metal indium electrode were tested using different frequencies. We also confirmed the electrochemical process of intermetallic compound formation by the co-reduction of Ce3+ ions in the LiCl-KCl-CeCl3 molten salt system containing liquid metal indium electrodes from electrochemical methods such as CV and OCP. Figure 5b shows the SWV of Ce3+ at the liquid indium electrode at different frequencies (5 to 30 Hz). We found only one reduction peak in the range of −1.37 V to −1.4 V, which further indicates that the reduction of Ce3+ to αIn6Ce is a one-step process of transferring three electrons. Meanwhile, the reduction peak potential shifts from −1.389 V to −1.394 V as the frequency increases from 5 Hz to 30 Hz, indicating that the reduction of Ce3+ in this system is irreversible.
4. Conclusions
In this paper, the electrochemical behavior of cerium on liquid cathode indium in a molten eutectic salt 3LiCl-2KCl-CeCl3 system was studied, and the redox processes on liquid metal indium electrode were investigated using CV, SWV and OCP techniques, respectively. The quasi-equilibrium potentials of Ce3+ at different temperatures on the liquid metal In electrode were determined. The results show that the reduction of Ce3+ at the liquid In metal electrode in the 3LiCl-2KCl-CeCl3 system at a temperature of 773 K occurs at about −1.4 V and is a one-step reduction process with three electron transfers. The electrochemical reduction of Ce3+ at the liquid indium metal electrode is irreversible and controlled by mass transfer. In addition, the diffusion coefficients at 723–823 K were calculated as 0.6512 × 10−5, 0.9105 × 10−5, 1.2032 × 10−5, 1.4517 × 10−5, and 1.7326 × 10−5 cm2 s−1, respectively. The diffusion coefficient and temperature of Ce3+ at the liquid metal In electrode are: lnD = 1.43 − 7974.69/T.
Author Contributions
L.Q. and W.H. conceived the idea and supervised the project. M.X. Investigation, Writing—original draft, Formal analysis. M.X. and Y.D. Investigation. Y.Z. and Y.J. Software with the help of H.L., X.L. and L.Q. wrote the manuscript with input from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 12274061, 52072059 and 11774044), Foundation of Sichuan Excellent Young Talents (2021JDJQ0015) and Fundamental Research Funds for the Central Universities (ZYGX2020J023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The supporting data are available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Veers, P.; Dykes, K.; Lantz, E.; Barth, S.; Bottasso, C.L.; Carlson, O.; Clifton, A.; Green, J.; Green, P.; Holttinen, H.; et al. Grand challenges in the science of wind energy. Science 2019, 366, 6464. [Google Scholar] [CrossRef]
- Wiser, R.; Rand, J.; Seel, J.; Beiter, P.; Baker, E.; Lantz, E.; Gilman, P. Expert elicitation survey predicts 37% to 49% declines in wind energy costs by 2050. Nat. Energy 2021, 6, 555–565. [Google Scholar] [CrossRef]
- Mavlonov, A.; Razykov, T.; Raziq, F.; Gan, J.T.; Chantana, J.; Kawano, Y.; Nishimura, T.; Wei, H.M.; Zakutayev, A.; Minemoto, T.; et al. A review of Sb2Se3 photovoltaic absorber materials and thin-film solar cells. Sol. Energy 2020, 201, 227–246. [Google Scholar] [CrossRef]
- Zhao, M.; Su, J.W.; Zhao, Y.; Luo, P.; Wang, F.K.; Han, W.; Li, Y.; Qiao, L.; Zhai, T.Y. Sodium-Mediated Epitaxial Growth of 2D Ultrathin Sb2Se3 Flakes for Broadband Photodetection. Adv. Funct. Mater. 2021, 30, 1909849. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Qiao, L.; Xiao, H.Y.; Meyer, H.M.; Sun, J.N.; Rouleau, C.M.; Puretzky, A.A.; Geohegan, D.B.; Ivanov, I.N.; Yoon, M.; Weber, W.J.; et al. Nature of the band gap and origin of the electro-/photo-activity of Co3O4. J. Mater. Chem. C 2013, 1, 4628–4633. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Mane, S.K.B.; Faheem, M.B.; Ali, A.; Zhao, Y.; Han, S.B.; Cai, C.; Li, W.; et al. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B Environ. 2020, 270, 118867. [Google Scholar] [CrossRef]
- Raziq, F.; Humayun, M.; Ali, A.; Wang, T.T.; Khan, A.; Fu, Q.Y.; Luo, W.; Zeng, H.P.; Zheng, Z.P.; Khan, B.; et al. Synthesis of S-Doped porous g-C3N4 by using ionic liquids and subsequently coupled with Au-TiO2 for exceptional cocatalyst-free visible-light catalytic activities. Appl. Catal. B Environ. 2018, 237, 1082–1090. [Google Scholar] [CrossRef]
- Raziq, F.; He, J.X.; Gan, J.T.; Humayun, M.; Faheem, M.B.; Iqbal, A.; Hayat, A.; Fazal, S.; Yi, J.B.; Zhao, Y.; et al. Promoting visible-light photocatalytic activities for carbon nitride based 0D/2D/2D hybrid system: Beyond the conventional 4-electron mechanism. Appl. Catal. B Environ. 2020, 270, 118870. [Google Scholar] [CrossRef]
- Raziq, F.; Khan, K.; Ali, S.; Xu, H.; Ali, I.; Zada, A.; Ismail, P.M.; Ali, A.; Khan, H.; Wu, X.Q.; et al. Accelerating CO2 reduction on novel double perovskite oxide with sulfur, carbon incorporation: Synergistic electronic and chemical engineering. Chem. Eng. J. 2022, 446, 137161. [Google Scholar] [CrossRef]
- Ali, S.; Ali, S.; Ismail, P.M.; Shen, H.H.; Zada, A.; Ali, A.; Ahmad, I.; Shah, R.; Khan, I.; Chen, J.S.; et al. Synthesis and bader analyzed cobalt-phthalocyanine modified solar UV-blind beta-Ga2O3 quadrilateral nanorods photocatalysts for wide-visible-light driven H2 evolution. Appl. Catal. B Environ. 2022, 307, 121149. [Google Scholar] [CrossRef]
- Ali, S.; Yasin, G.; Iqbal, R.; Huang, X.; Su, J.; Ibraheem, S.; Zhang, Z.; Wu, X.Q.; Wahid, F.; Ismail, P.M.; et al. Porous aza-doped graphene-analogous 2D material a unique catalyst for CO2 conversion to formic-acid by hydrogenation and electroreduction approaches. Mol. Catal. 2022, 524, 112285. [Google Scholar] [CrossRef]
- Raziq, F.; Aligayev, A.; Shen, H.H.; Ali, S.; Shah, R.; Ali, S.; Bakhtiar, S.H.; Ali, A.; Zarshad, N.; Zada, A.; et al. Exceptional Photocatalytic Activities of rGO Modified (B,N) Co-Doped WO3, Coupled with CdSe QDs for One Photon Z-Scheme System: A Joint Experimental and DFT Study. Adv. Sci. 2022, 9, 2102530. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.C.; Kee, R.J.; Zhu, H.Y.; Karakaya, C.; Chen, Y.C.; Braun, R.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Spendelow, J.S.; Choe, Y.K.; Fujimoto, C.; Kim, Y.S. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 2016, 1, 16120. [Google Scholar] [CrossRef]
- Wu, X.Q.; He, J.X.; Zhang, M.; Liu, Z.R.; Zhang, S.; Zhao, Y.; Li, T.; Zhang, F.P.; Peng, Z.; Cheng, N.Y.; et al. Binary Pd/amorphous-SrRuO3 hybrid film for high stability and fast activity recovery ethanol oxidation electrocatalysis. Nano Energy 2020, 67, 104247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ding, X.; Qiao, L. Recent advances in the electrochemistry of layered post-transition metal chalcogenide nanomaterials for hydrogen evolution reaction. J. Energy Chem. 2021, 60, 451–479. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ilyas, N.; Yang, L.X.; Ali, S.; Zada, A.; Bakhtiar, S.H.; Wang, Y.; Shen, H.H.; Qiao, L. FeNi@CNS nanocomposite as an efficient electrochemical catalyst for N-2 -to-NH3 conversion under ambient conditions. J. Mater. Sci. Technol. 2022, 103, 59–66. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.P.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Fan, E.S.; Li, L.; Wang, Z.P.; Lin, J.; Huang, Y.X.; Yao, Y.; Chen, R.J.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.; He, Y.H.; Wu, X.Q.; Ge, R.X.; Zu, X.T.; Li, S.A.; Qiao, L. High-performance asymmetric supercapacitors realized by copper cobalt sulfide crumpled nanoflower and N, F co-doped hierarchical nanoporous carbon polyhedron. J. Power Sources 2020, 456, 228023. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Ding, X.; Liu, Z.R.; Tian, H.; Shen, H.H.; Zu, X.T.; Li, S.A.; Qiao, L. One-step colloid fabrication of nickel phosphides nanoplate/nickel foam hybrid electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 373, 1132–1143. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Qiao, L. Layered LaCuOSe: A Promising Anisotropic Thermoelectric Material. Phys. Rev. Appl. 2020, 13, 024038. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Gong, H.F.; Liu, Z.J.; Zu, X.T.; Qiao, L. Optimizing the thermoelectric transport properties of Bi2O2Se monolayer via biaxial strain. Phys. Chem. Chem. Phys. 2019, 21, 15097–15105. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, N.; Xiao, H.Y.; Zhang, H.B.; Liu, Z.J.; Zu, X.T.; Qiao, L. Improved thermoelectric performance of bilayer Bi2O2Se by the band convergence approach. J. Mater. Chem. C 2019, 7, 11029–11039. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.L.; Xiao, H.Y.; Gao, Z.B.; Liu, Z.J.; Zu, X.T.; Li, S.A.; Qiao, L. Band degeneracy enhanced thermoelectric performance in layered oxyselenides by first-principles calculations. NPJ Comput. Mater. 2021, 7, 18. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, N.; Zhang, S.; Hu, J.T.; Xiao, H.Y.; Gong, H.F.; Liu, Z.J.; Qiao, L.; Zu, X.T. A review of the properties, synthesis and applications of lanthanum copper oxychalcogenides. J. Phys. D Appl. Phys. 2022, 55, 273002. [Google Scholar] [CrossRef]
- Salvatores, M. Nuclear fuel cycle strategies including partitioning and transmutation. Nucl. Eng. Des. 2005, 235, 805–816. [Google Scholar] [CrossRef]
- Salvatores, M.; Palmiotti, G. Radioactive waste partitioning and transmutation within advanced fuel cycles achievements and challenges. Prog. Part. Nucl. Phys. 2011, 66, 144–166. [Google Scholar] [CrossRef]
- Cometto, M.; Wydler, P.; Chawla, R. Management of actinide waste inventories in nuclear phase-out scenarios. Ann. Nucl. Energy 2008, 35, 1447–1460. [Google Scholar] [CrossRef]
- Laidler, J.J.; Battles, J.E.; Miller, W.E.; Ackerman, J.P.; Carls, E.L. Development of pyro-processing technology. Prog. Nucl. Energy 1997, 31, 131–140. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.L.; Liu, K.; Tang, S.L.; Shi, W.Q. Electrochemical Extraction of Cerium by Forming Ce-Zn Alloys in LiCl-KCl Eutectic on W and Liquid. J. Electrochem. Soc. 2015, 165, E179–E184. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X. Nuclear Energy Development in China: A Study of Opportunities and Challenges. Energy 2010, 35, 4282–4288. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Buddenberg, T.; Wu, S.; Sigurbjornsson, O.; Tran, K.C.; Kakaras, E. The Challenge of Energy Storage in Europe: Focus on Power to Fuel. J. Energy Resour. Technol. 2016, 138, 04200201–04200210. [Google Scholar] [CrossRef]
- Armington, A.F.; Posen, H.; Lipson, H. Lipson Strengthening of halides for infrared windows. J. Electron. Mater. 1973, 2, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xia, J.; Li, X.Z.; Ru, F.; Chen, X.; Hua, Z.; Shao, R.W.; Wang, X.C.; Zhang, W.J.; Lee, C.S.; et al. Vapor phase epitaxy of PbS single-crystal films on water-soluble substrates and application to photodetectors. Nano Res. 2022, 15, 5402–5409. [Google Scholar] [CrossRef]
- Satoh, T.; Iwai, T.; Arai, Y. Electrolysis of Burnup-Simulated Uranium Nitride Fuels in LiCl-KCl Eutectic Melts. J. Nucl. Sci. Technol. 2009, 46, 557–563. [Google Scholar] [CrossRef][Green Version]
- Masset, P.; Konings, R.J.M.; Malmbeck, R.; Serp, J.; Glatz, J.P. Thermochemical properties of lanthanides (Ln = La, Nd) and actinides (An = U, Np, Pu, Am) in the molten LiCl-KCl eutectic. J. Nucl. Mater. 2005, 344, 173–179. [Google Scholar] [CrossRef]
- Bechtel, T.B.; Storvick, T.S. Activity coefficients of actinide and rare-earth chlorides in molten LiCl/KCl eutectic salt. Ind. Eng. Chem. Res. 1999, 38, 1723–1728. [Google Scholar] [CrossRef]
- Zhu, T.J.; Huang, W.; Gong, Y. Electrochemical Separation of Uranium from Lanthanide (La, Eu, Gd) Fluorides in Molten LiCl-KCl. Sep. Purif. Technol. 2020, 235, 116227. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Osipenko, A.; Maershin, A. Thermodynamics and separation factor of uranium from lanthanum in liquid eutectic gallium-indium alloy/molten salt system. Electrochim. Acta 2014, 145, 81–85. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kwon, K.C.; Park, B.H.; Seo, C.S. A Kinetic Study of the Oxidation of Uranium Dioxide. React. Kinet. Mech. Catal. 2006, 89, 269–275. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Volkovich, V.A. Thermodynamics of La and U and the separation factor of U/La in fused Me (Ga-40wt% In)/3LiCl-2KCl system. J. Nucl. Mater. 2017, 495, 285–290. [Google Scholar] [CrossRef]
- Smolenski, V.; Novoselova, A.; Osipenko, A.; Kormilitsyn, M. The influence of electrode material nature on the mechanism of cathodic reduction of ytterbium (III) ions in fused NaCl–KCl–CsCl eutectic. J. Electroanal. Chem. 2009, 633, 291–296. [Google Scholar] [CrossRef]
- Melchakov, S.Y.; Maltsev, D.S.; Volkovich, V.A.; Yamshchikov, L.F.; Osipenko, A.G. Separation of Uranium and Lanthanides in a Fused Salt - Liquid Gallium Based Alloy System. ECS Trans. 2014, 64, 369–375. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, Y.D.; Han, W.; Zhang, M.L.; Yuan, L.Y.; Lin, R.S.; Ye, G.A.; He, H.; Chai, Z.F.; Shi, W.Q. Electrochemical separation of Th from ThO2 and Eu2O3 assisted by AlCl3 in molten LiCl–KCl. Electrochim. Acta 2013, 114, 180–188. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kumar, J.R.; Kim, J.S.; Park, H.K.; Yoon, H.S. Liquid-liquid extraction/separation of platinum (IV) and rhodium (III) from acidic chloride solutions using tri-iso-octylamine. J. Hazard. Mater. 2009, 168, 424–429. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Hayashi, H.; Minato, K.; Gaune-Escard, M. Electrochemical transient techniques for determination of uranium and rare-earth metal separation coefficients in molten salts. Electrochim. Acta 2006, 51, 2463–2470. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.J.; Eun, H.C.; Kim, G.Y.; Lee, S. Uranium recovery with zinc distillation from a liquid zinc cathode for pyroprocessing. J. Radioanal. Nucl. 2018, 361, 649–654. [Google Scholar] [CrossRef]
- Fukasawa, K.; Uehara, A.; Nagai, T.; Sato, N.; Fujii, T.; Yamana, H. Thermodynamic properties of trivalent lanthanide and actinide ions in molten mixtures of LiCl and KCl. J. Nucl. Mater. 2012, 424, 17–22. [Google Scholar] [CrossRef]
- Xu, M.H.; Smolenski, V.; Liu, Q.; Novoselova, A.; Jiang, K.W.; Yu, J.; Liu, J.Y.; Chen, R.R.; Zhang, H.S.; Zhang, M.L.; et al. Thermodynamics, Solubility and the Separation of Uranium from Cerium in Molten In/3LiCl-2KCl System. J. Electrochem. Soc. 2021, 167, 136506. [Google Scholar] [CrossRef]
- Novoselova, A.; Smolenski, V. Solubility, Activity coefficients and the separation factor of U/Pr couple in Ga-In alloys of different compositions in fused LiCl-KCl-CsCl eutectic. J. Electrochem. Soc. 2020, 167, 126518. [Google Scholar] [CrossRef]
- Toda, T.; Maruyama, T.; Moritani, K.J.; Moriyama, H.; Hayashi, H. Thermodynamic Properties of Lanthanides and Actinides for Reductive Extraction of Minor Actinides. J. Nucl. Sci. Technol. 2009, 46, 18–25. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles. RSC Adv. 2013, 4, 4663–4671. [Google Scholar] [CrossRef]
- Kim, S.H.; Paek, S.; Kim, T.J.; Park, D.Y.; Ahn, D.H. Electrode reactions of Ce3+/Ce couple in LiCl–KCl solutions containing CeCl3 at solid W and liquid Cd electrodes. Electrochim. Acta 2012, 85, 35–40. [Google Scholar] [CrossRef][Green Version]
- Xu, M.H.; Smolenski, V.; Liu, Q.; Novoselova, A.; Jiang, K.W.; Yu, J.; Liu, J.Y.; Chen, R.R.; Zhang, H.S.; Zhang, M.L.; et al. Thermodynamic properties of stable states cerium compounds in fused 3LiCl-2KCl eutectic. J. Chem. 2021, 152, 106260. [Google Scholar] [CrossRef]
- Kato, T.; Inoue, T.; Iwai, T.; Arai, Y. Separation behaviors of actinides from rare-earths in molten salt electrorefining using saturated liquid cadmium cathode. J. Nucl. Mater. 2006, 357, 105–114. [Google Scholar] [CrossRef]
- Li, S.X.; Herrmann, S.D.; Simpson, M. Electrochemical analysis of actinides and rare earth constituents in liquid cadmium cathode product from spent fuel electrorefining. Nucl. Technol. 2010, 171, 292–299. [Google Scholar] [CrossRef]
- Castrillejo, Y.; Bermejo, M.R.; Arocas, P.D.; Martinez, A.M.; Barrado, E. The electrochemical behaviour of the Pr(III)/Pr redox system at Bi and Cd liquid electrodes in molten eutectic LiCl–KCl. J. Chem. 2005, 579, 343–358. [Google Scholar] [CrossRef]
- Castrillejo, Y.; Bermejo, M.R.; Martinez, A.M.; Barrado, E.; Arocas, P.D. Application of electrochemical techniques in pyrochemical processes – Electrochemical behaviour of rare earths at W, Cd, Bi and Al electrodes. J. Nucl. Mater. 2007, 360, 32–42. [Google Scholar] [CrossRef]
- Serp, J.; Lefebvre, P.; Malmbeck, R.; Rebizant, J.; Vallet, P.; Glatz, J.P. Separation of plutonium from lanthanum by electrolysis in LiCl-KCl onto molten bismuth electrode. J. Nucl. Mater. 2005, 340, 266–270. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.L.; Chai, Z.F.; Shi, W.Q. Evaluation of the Electroextractions of Ce and Nd from LiCl-KCl Molten Salt Using Liquid Ga Electrode. J. Electrochem. Soc. 2017, 164, D169–D178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).