Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties
Abstract
1. Introduction
2. Cocrystals for the Pharmaceutical Formulation Development
- Formation of the supersaturated metastable state.
- Maintaining the above condition.
- (1)
- The solubility of the crystalline (stable) state is low.
- (2)
- The metastable form (amorphous) has a maximum solubility but rapidly decreases to the poorly soluble form (crystalline form).
3. Coformer Screening and Selection Techniques
3.1. Hypothetical/Theoretical Methods
3.1.1. Hydrogen Bonding
- “All good proton donors and acceptors are used in hydrogen bonding”
- “Six-membered-ring intramolecular hydrogen bonds form in preference to intermolecular hydrogen bond”
- “The remaining best proton donors and acceptors, after intramolecular hydrogen-bond formation, form intermolecular hydrogen bonds with one another”
3.1.2. Supramolecular Synthon Approach
- “Identification of functional groups in the API and coformer molecules”.
- “Assessment of homo interactions in the individual pure molecules of the target compounds”.
- “Identification of the possible functional groups which may compete with the homo interactions of pure compounds”.
- “Assessment of the likelihood of hetero interactions”.
- “Selection of coformers based on the assessment of homo/ hetero interactions”.
3.1.3. ΔpKa Rule of Three
3.1.4. Hansen Solubility Parameter
- (δd) = Dispersion.
- (δp) = Polarity.
- (δh) = Hydrogen bonding.
3.2. In-Silico Techniques
3.2.1. Cambridge Structural Database (CSD)
3.2.2. Conductor-Like Screening Model for Real Solvents (COSMO-RS)
3.2.3. Molecular Electrostatic Potential Surface (MEPS)
3.2.4. Lattice Energy Assessment
- Elatt = Lattice energy.
- Uinter = intermolecular lattice energy.
- ΔEintra = conformational intramolecular energy.
3.3. Pick and Trial Approaches
3.4. Thermal Screening Techniques
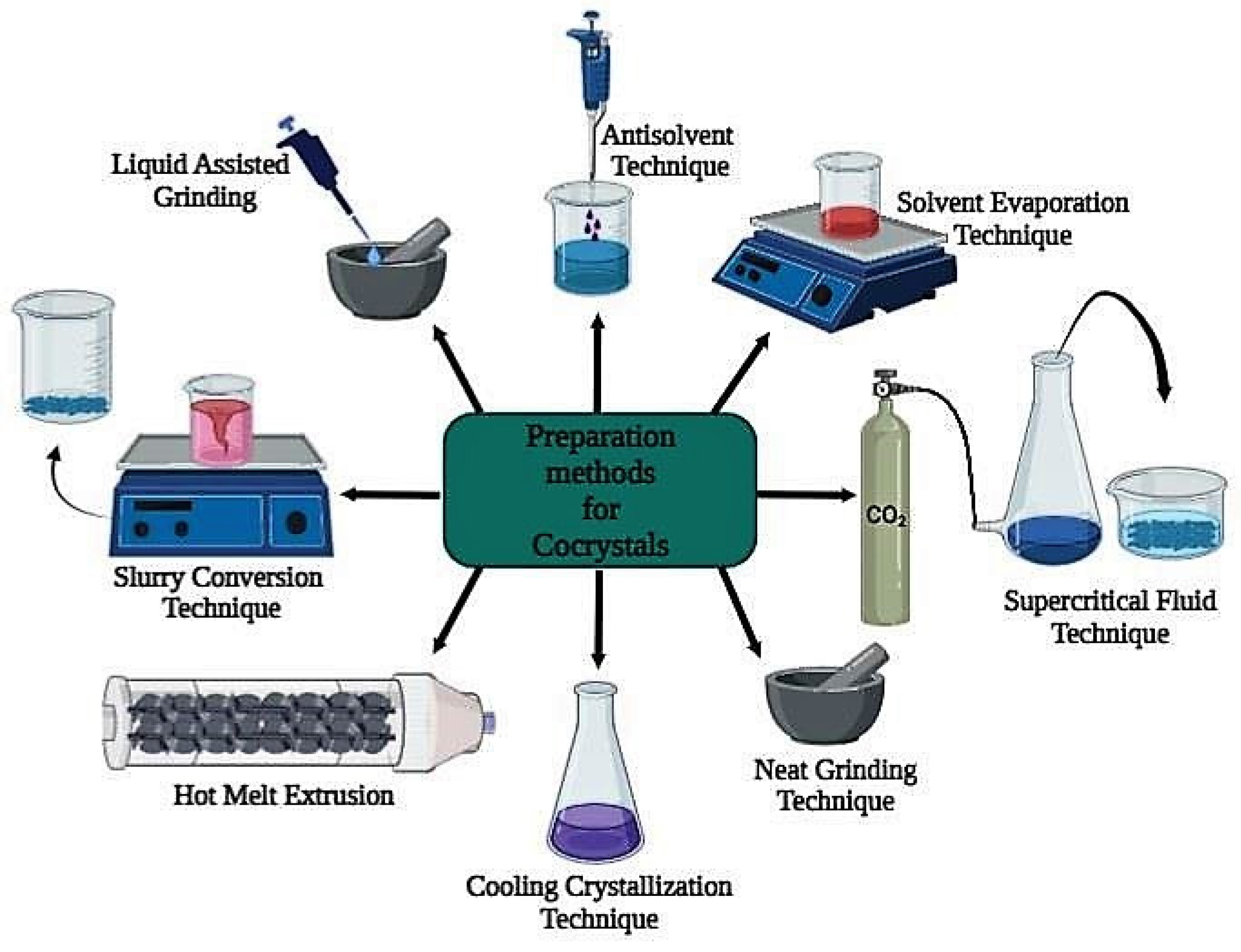
4. Methods for Preparing Cocrystals
4.1. Green Synthesis Techniques or Non-Solvent Techniques
4.2. Solvent-Based Techniques
4.3. Supercritical Fluid Approach
5. Characterization of Cocrystals
6. Anti-Cancer Drug Cocrystals
Drug–Drug Cocrystals
| Anti-Cancer Drugs | Coformers Used | Preparation Techniques | Enhanced Parameters from Pure Drug | Reference |
|---|---|---|---|---|
| 4,4′-ethylenebispyridine | Flavonoids | Solvent Evaporation method | Improved dissolution behaviour, and anti-tumor activity | [146] |
| 5-Fluorouracil | Ferulic acid | Solvent assisted co-grinding | Improved solubility and permeability | [147] |
| 5-Fluorouracil | Nicotinamide and Iso-Nicotinamide | Solvent-free grinding technique | Improved solubility and stability | [148] |
| 5-Fluorouracil | Proline | Solvent evaporation technique, and Liquid assisted grinding | Improved solubility, permeability | [131] |
| 5-Fluorouracil | Succinic acid, Malic acid Cinnamic acid, and Benzoic acid | Neat grinding and slow solvent evaporation | Improved anticancer activity | [149] |
| 5-Fluorouracil | Urea, Thiourea, Pyrazinamide | Supercritical solvent technique | Improved solubility | [150] |
| 5-Fluorouracil | Urea, Thiourea and Acetanilide | Solid-state grinding method | Improved solubility | [151] |
| 5-Fluorouracil | Gentisic acid, 3,4-Dihydroxybenzoic, 4-Aminopyridine | Solvent-assisted grinding and solution crystallization | Improved permeability and antitumor efficacy | [152] |
| 6-Mercaptopurine monohydrate | 2,4-Dihydroxybenzoic acid and Hydroxybenzoic acid | Slurry reactive crystallization | Improved solubility and stability | [153] |
| 6-Mercaptopurine monohydrate | Zinc Tri-fluoro methane sulfonate | Solvent evaporation | Improved solubility and dissolution | [154] |
| Artemisinin | Acetylene dicarboxylic acid | Green synthesis technique | Improved solubility and dissolution | [155] |
| Axitinib | Glutaric acid | Solvent evaporation technique | Improved solubility and stability | [156] |
| Axitinib | Fumaric acid, Cinnamic acid | Liquid assisted grinding and slurry methods | Improved solubility, stability, and oral bioavailability | [157] |
| Baicalein | Nicotinamide | Slurry method | Improved solubility, dissolution, and bioavailability | [158] |
| Betulinic acid | Ascorbic acid | Slow solvent evaporation | Improved bioavailability, solubility | [159] |
| Ceritinib | Nicotinamide, Quercitin | Neat grinding | Improved solubility and stability | [160] |
| Coumarin | Thiourea | Neat grinding method | Improved antioxidant activity and solubility | [161] |
| Curcumin | N-acetylcysteine | Anti-Solvent gas Supercritical fluid technique | Improved oral bioavailability | [162] |
| Curcumin | Ascorbic acid | Solvent evaporation method | Improved solubility, stability, and biological activities | [163] |
| Curcumin | Trimesic acid | Solid-state grinding, slow cooling crystallization, Evaporative crystallization | Enhanced dissolution | [164] |
| Curcumin | N-acetylcysteine | Supercritical fluid technology | Improved solubility and dissolution | [165] |
| Cytarabine | Nicotinamide | Slow solvent evaporation method | Improved solubility | [166] |
| Dabrafenib | Fumaric acid, Succinic acid and Adipic acid | Solvent drop grinding method | Improved solubility and dissolution | [167] |
| Dasatinib | N-methyl-4-hydroxybenzoate, Nicotinamide, Ethyl gallate, Methyl gallate, Propyl gallate, Vanillin | Solvent evaporation technique | Improved solubility, stability, and oral bioavailability | [168] |
| Docetaxel | Nicotinamide | Slow solvent evaporation technique | Improved solubility, dissolution and penetration | [169] |
| Doxorubicin | Maraviroc | Slow solvent evaporation method | Improved solubility | [166] |
| Emodin | Nicotinamide | Solvent evaporation | Improved solubility, oral absorption, and bioavailability | [170] |
| Gefitinib | Isonicotinamide and Vanillin | Solvent evaporation method | Improved intrinsic dissolution, bioavailability, and cell inhibition response | [171] |
| Ibrutinib | Hydroxy benzoic acid, Succinic acid, Hydroxy naphthoic acid | Slurry method, ultrasonic method | Improved solubility, stability | [65] |
| Imatinib | Syringic acid | Slurry method | Improved solubility, dissolution, and permeation | [172] |
| Lapachone | Resorcinol | Mechanical grinding | Improved solubility and oral bioavailability | [173] |
| Lenalidomide | Urea and 3,5-dihydroxybenzoic acid | Solvent evaporation technique | Improved solubility | [174] |
| Lenalidomide | Gallic acid | Liquid assisted grinding | Improved solubility, stability, and dissolution | [175] |
| Lenvatinib | Sulfamerazine | Slow solvent evaporation method | Improved Solubility and stability | [176] |
| Lenvatinib | Salicylic acid | Slow solvent evaporation method | Improved Solubility and stability | [176] |
| Lenvatinib | Sulfamerazine and salicylic acid | Slurry reactive crystallization | Improved solubility, stability, and dissolution | [176] |
| Luteolin | Caffeine | Liquid assisted grinding and rapid solvent removal method | Improved solubility, thermal stability, and bioavailability | [177] |
| Megestrol acetate | Saccharin | Slow solvent evaporation technique | Improved dissolution | [178] |
| Nandrolone | Salicylic acid | Grinding method | Improved Anticancer activity | [130] |
| Nandrolone | 3-amino-1,2,4-triazole | Solution reflux method | Improved Anticancer activity | [130] |
| Palbociclib | Resorcinol | Solvent evaporation technique | Improved solubility, bioavailability, and biosafety | [128] |
| Palbociclib | Orcinol | Solvent evaporation technique | Improved solubility and dissolution | [128] |
| Pterostilbene | Picolinic acid | Liquid assisted grinding | Improved oral bioavailability | [179] |
| Regorafenib | Malonic acid, Glutaric acid and Pimelic acid | Liquid assisted grinding | Improved solubility and dissolution | [180] |
| Resveratrol | Nicotinamide | Supercritical CO2 by the gas antisolvent (GAS) technique | Improved dissolution and bioavailability | [181] |
| Tegafur | Syringic acid | Liquid assisted grinding and solvent evaporation | Prolonged half-life and increased bioavailability | [133] |
| Temozolomide | Succinic acid, Oxalic acid | Solvent evaporation technique | Improved solid-state stability, bioavailability, dissolution | [182] |
| Zoledronic acid | Tartaric acid and Nicotinamide | Slurry method, solvent evaporation, and dry grinding | Improved solubility | [183] |
| Drug Combinations | Therapeutic Category | Preparation Methods | Observations | Reference |
|---|---|---|---|---|
| Dihydromyricetin with Pentoxifylline | Anticancer and Anti ischemic | Slurry method | Improved solubility and synergistic anticancer effect | [143] |
| Lobaplatin with Quercetin, Myricetin, Fisetin, Naringenin, and Luteolin | Anticancer and Flavonoids | Slow solvent evaporation method | Improved dissolution, stability, and antitumor effect | [140] |
| Oxaliplatin with Baicalein | Anticancer and Flavonoids | Solvent evaporation method | Reduced release rate | [144] |
| Oxaliplatin with Naringenin | Anticancer and Flavonoids | Solvent evaporation method | Delayed hydrolysis | [144] |
| Temozolomide with Hesperetin | Anticancer and Flavonoids | Liquid assisted grinding | Improved stability and extended dissolution | [145] |
| Theophylline with Quercetin | Anticancer drugs | Liquid assisted grinding | Improved thermal properties, stability, solubility, and anticancer effect | [184] |
| 5-fluorouracil with Sorafenib | Anticancer drugs | Slurry method | Improved solubility, stability, and antitumor effect | [185] |
| 5-fluorouracil with Regorafenib | Anticancer drugs | Hetero seeding method | Improved solubility, stability, and antitumor effect | [185] |
| Propylthiouracil with kaempferol | Anti-thyroid and Anticancer drugs | Solvent evaporation | Improved bioavailability | [186] |
| Erlotinib with Furosemide | Anticancer and Diuretic drugs | Liquid assisted grinding | Improved solubility, thermal stability, and dissolution | [187] |
| Gefitinib with Mefenamic | Anticancer and NSAIDs | Liquid assisted grinding | Improved solubility | [187] |
| Oxaliplatin with Baicalein | Anticancer and Flavonoids | Solution crystallization and liquid assisted grinding | Improved physicochemical properties | [144] |
| Oxaliplatin with Naringenin | Anticancer and Flavonoids | Solution crystallization | Improved physicochemical properties | [144] |
| 5-fluorouracil with kaempferol | Anticancer and Flavonoids | Slurry method | Improved stability and dissolution | [188] |
| Berberine chloride with Myricetin and Dihydromyricetin | Anticancer and Flavonoids | Green synthesis | Enhanced solubility and synergistic anticancer effect | [136] |
| Luteolin with Isoniazid | Anticancer and Antituberculosis | Liquid assisted grinding and rapid solvent removal method | Improved solubility, thermal stability, and bioavailability | [177] |
| Doxorubicin HCl with Quercetin hydrate and Naringin | Anticancer and Flavonoids | Slow solvent evaporation technique | Improved solubility | [189] |
| 5-Fluorouracil with Aspirin | Anticancer and NSAIDs | Solid-state grinding method | Improved solubility | [151] |
| Emodin with Berberine Chloride | Anticancer drugs | Slurry method | Improved sustained-release hence absorption | [190] |
| Imatinib mesylate with 5-fluorouracil | Anticancer drugs | Solvent evaporation technique | Improved solubility and stability | [191] |
| Dacarbazine with Quercetin | Anticancer drugs | Solvent drop grinding | Improved solubility and dissolution | [192] |
| Temozolomide with Hesperitin | Anticancer and Flavonoids | Liquid assisted grinding | Improved solubility, stability, and dissolution | [145] |
7. Prospects of Cocrystals in the Future
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novais, M.V.M.; Gomes, E.R.; Miranda, M.C.; Silva, J.O.; Gomes, D.A.; Braga, F.C.; Pádua, R.M.; Oliveira, M.C. Liposomes Co-Encapsulating Doxorubicin and Glucoevatromonoside Derivative Induce Synergic Cytotoxic Response against Breast Cancer Cell Lines. Biomed. Pharmacother. 2021, 136, 111123. [Google Scholar] [CrossRef]
- Layek, B.; Gidwani, B.; Tiwari, S.; Joshi, V.; Jain, V.; Vyas, A. Recent Advances in Lipid-Based Nanodrug Delivery Systems in Cancer Therapy. Curr. Pharm. Des. 2020, 26, 3218–3233. [Google Scholar] [CrossRef] [PubMed]
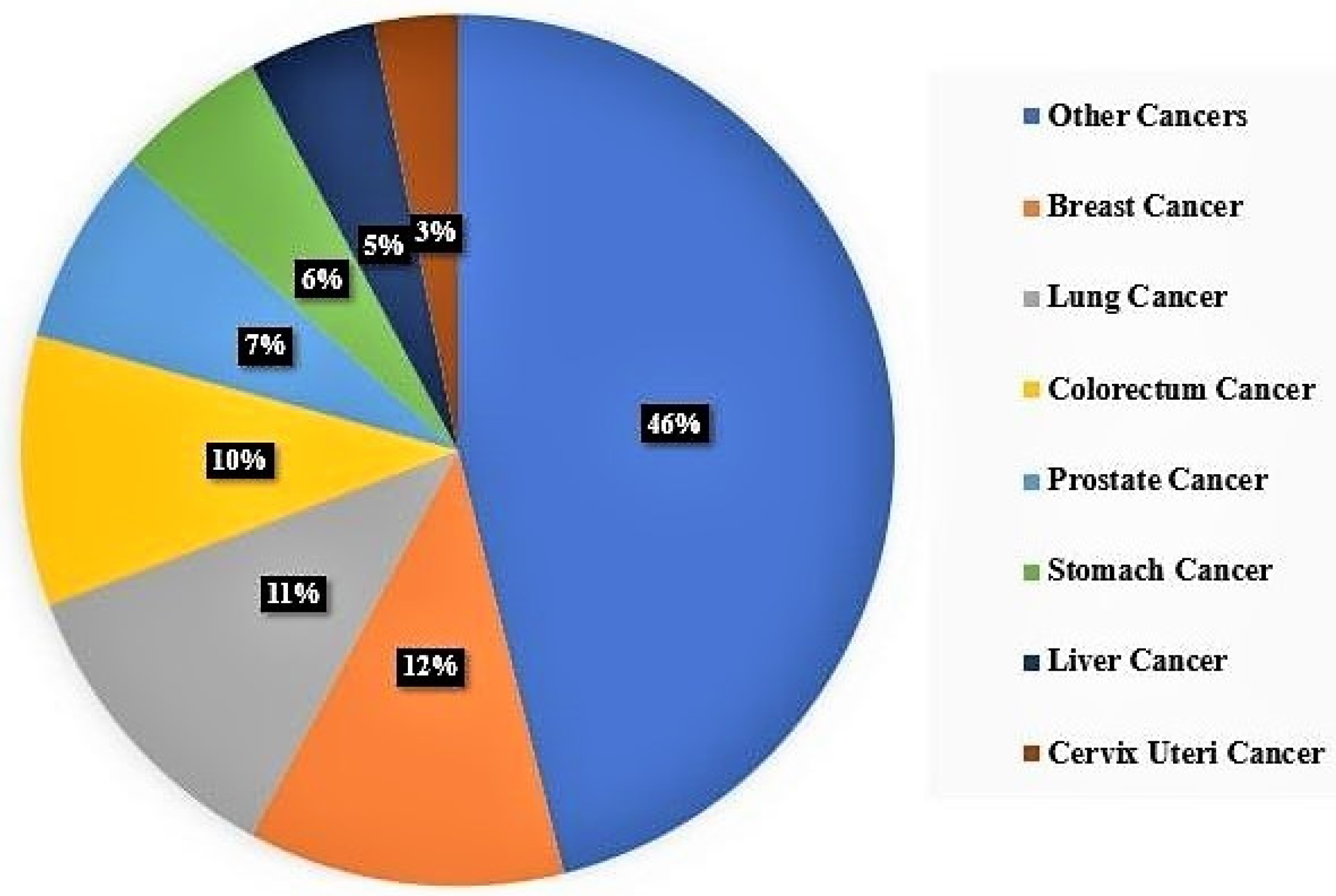
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; van Delft, F.L. Chapter 1 Introduction to Antibody–Drug Conjugates. In Cytotoxic Payloads for Antibody–Drug Conjugates; Royal Society of Chemistry: London, UK, 2021; pp. 1–31. [Google Scholar] [CrossRef]
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2000. [Google Scholar]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Sawicki, E.; Schellens, J.H.M.; Beijnen, J.H.; Nuijen, B. Inventory of Oral Anticancer Agents: Pharmaceutical Formulation Aspects with Focus on the Solid Dispersion Technique. Cancer Treat. Rev. 2016, 50, 247–263. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Sarwar, H.S.; Naveed, S.; Salman, O.; Bukhari, N.I.; Hussain, I.; Webster, T.J.; Shahnaz, G. Advancements in the Oral Delivery of Docetaxel: Challenges, Current State-of-the-Art and Future Trends. Int. J. Nanomed. 2018, 13, 3145–3161. [Google Scholar] [CrossRef]
- Han, W.; Xie, B.; Li, Y.; Shi, L.; Wan, J.; Chen, X.; Wang, H. Orally Deliverable Nanotherapeutics for the Synergistic Treatment of Colitis-Associated Colorectal Cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef]
- Gala, U.H.; Miller, D.A.; Williams, R.O. Harnessing the Therapeutic Potential of Anticancer Drugs through Amorphous Solid Dispersions. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188319. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C.; Anand, I. Biopharmaceutics Classification System. Syst. Rev. Pharm. 2010, 1, 62. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical Solids as a Platform to Improve Solubility and Bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Suzuki, M.; Takebe, G.; Takagi, T.; Tsukada, H. Characterization of Novel Paclitaxel Nanoparticles Prepared by Laser Irradiation. Chem. Pharm. Bull. 2022, 70, 269–276. [Google Scholar] [CrossRef]
- Parvataneni, D.M.; Devraj, R.; Mangamoori, L.N. Micelles Entrapped Microparticles Technology: A Novel Approach to Resolve Dissolution and Bioavailability Problems of Poorly Water Soluble Drugs. J. Microencapsul. 2020, 37, 254–269. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-Based Advanced Materials for Docetaxel and Paclitaxel Delivery: Recent Advances and Future Directions in Cancer Theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Du, S.; Lin, Y.; Lu, B.; Yang, C.; Wang, J.; Zeng, Y. Structural and Computational Insights into the Enhanced Solubility of Dipfluzine by Complexation: Salt and Salt-Cocrystal. New J. Chem. 2018, 42, 15068–15078. [Google Scholar] [CrossRef]
- Al-Obaidi, H.; Granger, A.; Hibbard, T.; Opesanwo, S. Pulmonary Drug Delivery of Antimicrobials and Anticancer Drugs Using Solid Dispersions. Pharmaceutics 2021, 13, 1056. [Google Scholar] [CrossRef] [PubMed]
- Khatun, B.; Baishya, P.; Ramteke, A.; Maji, T.K. Study of the Complexation of Structurally Modified Curcumin with Hydroxypropyl Beta Cyclodextrin and Its Effect on Anticancer Activity. New J. Chem. 2020, 44, 4887–4897. [Google Scholar] [CrossRef]
- Lerata, M.S.; D’Souza, S.; Sibuyi, N.R.S.; Dube, A.; Meyer, M.; Samaai, T.; Antunes, E.M.; Beukes, D.R. Encapsulation of Variabilin in Stearic Acid Solid Lipid Nanoparticles Enhances Its Anticancer Activity in Vitro. Molecules 2020, 25, 830. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Alshahrouri, B.; Yang, F.; Schwing, Q.; Dürig, T.; Fassihi, R. Hot-Melt Extrusion Based Sustained Release Ibrutinib Delivery System: An Inhibitor of Bruton’s Tyrosine Kinase (BTK). Int. J. Pharm. 2021, 607, 120981. [Google Scholar] [CrossRef] [PubMed]
- Caruana, R.; Montalbano, F.; Zizzo, M.G.; Puleio, R.; Caldara, G.; Cicero, L.; Cassata, G.; Licciardi, M. Enhanced Anticancer Effect of Quercetin Microparticles Formulation Obtained by Spray Drying. Int. J. Food Sci. Technol. 2022, 57, 2739–2746. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Alam, M.; Harshita; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-Lipid Hybrid Nanoparticles: A Next-Generation Nanocarrier for Targeted Treatment of Solid Tumors. Curr. Pharm. Des. 2020, 26, 1206–1215. [Google Scholar] [CrossRef]
- Abuzar, S.M.; Hyun, S.-M.; Kim, J.-H.; Park, H.J.; Kim, M.-S.; Park, J.-S.; Hwang, S.-J. Enhancing the Solubility and Bioavailability of Poorly Water-Soluble Drugs Using Supercritical Antisolvent (SAS) Process. Int. J. Pharm. 2018, 538, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Lee, B.-J.; Tran, T.T.D. Strategies and Formulations of Freeze-Dried Tablets for Controlled Drug Delivery. Int. J. Pharm. 2021, 597, 120373. [Google Scholar] [CrossRef]
- Khanfar, M.; Al-Remawi, M.; Al-Akayleh, F.; Hmouze, S. Preparation and Evaluation of Co-Amorphous Formulations of Telmisartan—Amino Acids as a Potential Method for Solubility and Dissolution Enhancement. AAPS PharmSciTech 2021, 22, 112. [Google Scholar] [CrossRef]
- Ngilirabanga, J.B.; Samsodien, H. Pharmaceutical Co-Crystal: An Alternative Strategy for Enhanced Physicochemical Properties and Drug Synergy. Nano Sel. 2021, 2, 512–526. [Google Scholar] [CrossRef]
- Bharate, S.S. Recent Developments in Pharmaceutical Salts: FDA Approvals from 2015 to 2019. Drug Discov. Today 2021, 26, 384–398. [Google Scholar] [CrossRef]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef]
- Feng, Y.; Meng, Y.; Tan, F.; Lv, L.; Li, Z.; Wang, Y.; Yang, Y.; Gong, W.; Yang, M. Effect of Surfactants and Polymers on the Dissolution Behavior of Supersaturable Tecovirimat-4-Hydroxybenzoic Acid Cocrystals. Pharmaceutics 2021, 13, 1772. [Google Scholar] [CrossRef] [PubMed]
- Buddhadev, S.S.; Garala, K.C. Pharmaceutical Cocrystals—A Review. Proceedings 2021, 62, 14. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.-R.; Mei, X. Enhancing the Stability of Active Pharmaceutical Ingredients by the Cocrystal Strategy. CrystEngComm 2022, 24, 2002–2022. [Google Scholar] [CrossRef]
- Yousef, M.A.E.; Vangala, V.R. Pharmaceutical Cocrystals: Molecules, Crystals, Formulations, Medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Cocrystals: Along the Path to Improved Medicines. Chem. Commun. 2015, 52, 640–655. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Kumar, A.; Nanda, A. In-Silico Methods of Cocrystal Screening: A Review on Tools for Rational Design of Pharmaceutical Cocrystals. J. Drug Deliv. Sci. Technol. 2021, 63, 102527. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical Cocrystals: Regulatory and Strategic Aspects, Design and Development. Adv. Pharm. Bull. 2016, 6, 479–494. [Google Scholar] [CrossRef]
- Chivate, A.; Garkal, A.; Dhas, N.; Mehta, T. Hot-Melt Extrusion: An Emerging Technique for Solubility Enhancement of Poorly Water-Soluble Drugs. PDA J. Pharm. Sci. Technol. 2021, 75, 357–373. [Google Scholar] [CrossRef]
- Shoaib, A.; Mangla, B.; Javed, S.; Sultan, M.H.; Alqahtani, S.S.; Shakeel, F. Vicissitudes of Liquid Crystals for Solubility Enhancement of Poorly Soluble Drugs. J. Mol. Liq. 2021, 321, 114924. [Google Scholar] [CrossRef]
- Bavishi, D.D.; Borkhataria, C.H. Spring and Parachute: How Cocrystals Enhance Solubility. Prog. Cryst. Growth Charact. Mater. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Y.; Zhang, A.; Wang, X.; Zhao, Z.; Zhang, Y.; Yin, T.; Gou, J.; Wang, Y.; He, H.; et al. Rod-Shaped Nintedanib Nanocrystals Improved Oral Bioavailability through Multiple Intestinal Absorption Pathways. Eur. J. Pharm. Sci. 2022, 168, 106047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Zhang, H.-M.; Cui, B.-J.; Hao, C.-Y.; Zhu, H.-Y.; Guan, J.; Wang, D.; Jin, Y.; Feng, B.; Cai, J.-H.; et al. “Felodipine-Indomethacin” Co-Amorphous Supersaturating Drug Delivery Systems: “Spring-Parachute” Process, Stability, in Vivo Bioavailability, and Underlying Molecular Mechanisms. Eur. J. Pharm. Biopharm. 2021, 166, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Goto, S.; Chatani, H.; Otsuka, Y.; Shimada, Y.; Terada, H.; Inoo, K. The Function of Oxybuprocaine: A Parachute Effect That Sustains the Supersaturated State of Anhydrous Piroxicam Crystals. RSC Adv. 2020, 10, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Parwati, R.D. Challenges and Progress in Nonsteroidal Anti-Inflammatory Drugs Co-Crystal Development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Yang, X.; Wang, Y.; Wang, Q.; Hu, R.; Anwaier, G.; Di, C.; Qi, R.; Huang, Y. HPMC Improves Protective Effects of Naringenin and Isonicotinamide Co-Crystals against Abdominal Aortic Aneurysm. Cardiovasc. Drugs Ther. 2021, 1–11. [Google Scholar] [CrossRef]
- Ilie, A.-R.; Griffin, B.T.; Vertzoni, M.; Kuentz, M.; Kolakovic, R.; Prudic-Paus, A.; Malash, A.; Bohets, H.; Herman, J.; Holm, R. Exploring Precipitation Inhibitors to Improve in Vivo Absorption of Cinnarizine from Supersaturated Lipid-Based Drug Delivery Systems. Eur. J. Pharm. Sci. 2021, 159, 105691. [Google Scholar] [CrossRef]
- Malamatari, M.; Ross, S.A.; Douroumis, D.; Velaga, S.P. Experimental Cocrystal Screening and Solution Based Scale-up Cocrystallization Methods. Adv. Drug Deliv. Rev. 2017, 117, 162–177. [Google Scholar] [CrossRef]
- Salem, A.; Nagy, S.; Pál, S.; Széchenyi, A. Reliability of the Hansen Solubility Parameters as Co-Crystal Formation Prediction Tool. Int. J. Pharm. 2019, 558, 319–327. [Google Scholar] [CrossRef]
- Gunawardana, C.A.; Aakeröy, C.B. Co-Crystal Synthesis: Fact, Fancy, and Great Expectations. Chem. Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef]
- Nugrahani, I.; Jessica, M.A. Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review. Molecules 2021, 26, 3279. [Google Scholar] [CrossRef] [PubMed]
- Jagia, M.; Kale, D.P.; Bansal, A.K.; Patel, S. Novel Co-Crystals and Eutectics of Febuxostat: Characterization, Mechanism of Formation, and Improved Dissolution. AAPS PharmSciTech 2021, 23, 43. [Google Scholar] [CrossRef]
- Sarkar, N.; Sinha, A.S.; Aakeröy, C.B. Systematic Investigation of Hydrogen-Bond Propensities for Informing Co-Crystal Design and Assembly. CrystEngComm 2019, 21, 6048–6055. [Google Scholar] [CrossRef]
- Sarathi, P.; Padhi, S. Insight of the Various in Silico Screening Techniques Developed for Assortment of Cocrystal Formers and Their Thermodynamic Characterization. Drug Dev. Ind. Pharm. 2022, 47, 1523–1534. [Google Scholar] [CrossRef]
- MacEachern, L.; Kermanshahi-pour, A.; Mirmehrabi, M. Supercritical Carbon Dioxide for Pharmaceutical Co-Crystal Production. Cryst. Growth Des. 2020, 20, 6226–6244. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M. Selection of Effective Cocrystals Former for Dissolution Rate Improvement of Active Pharmaceutical Ingredients Based on Lipoaffinity Index. Eur. J. Pharm. Sci. 2017, 107, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C. Hydrogen Bonds as Design Elements in Organic Chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Nikam, V.J.; Patil, S.B. Pharmaceutical Cocrystals of Nebivolol Hydrochloride with Enhanced Solubility. J. Cryst. Growth 2020, 534, 125488. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, C.; Sharma, N.; Kumar, J. COCRYSTALS: AN ALTERNATIVE APPROACH TO MODIFY PHYSICOCHEMICAL PROPERTIES OF DRUGS. Res. J. Pharm. Biol. Chem. Sci. 2015, 2015, 427–436. [Google Scholar]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Rajendrakumar, S.; Surampudi Venkata Sai Durga, A.; Balasubramanian, S. Strategic Synthon Approach in Obtaining Cocrystals and Cocrystal Polymorphs of a High-Z′ System Deferiprone—An Anti-Thalassemia Drug. Acta Cryst. B 2021, 77, 946–964. [Google Scholar] [CrossRef]
- Shi, X.; Wang, C.; Chen, Q.; Shen, S.; Song, S.; Zhou, X. Improving Physicochemical Properties of Ibrutinib with Cocrystal Strategy Based on Structures and Natures of the Carboxylic Acid Co-Formers. J. Drug Deliv. Sci. Technol. 2021, 63, 102554. [Google Scholar] [CrossRef]
- dos Santos, J.A.B.; Chaves Júnior, J.V.; de Araújo Batista, R.S.; de Sousa, D.P.; Ferreira, G.L.R.; de Lima Neto, S.A.; de Santana Oliveira, A.; de Souza, F.S.; Aragão, C.F.S. Preparation, Physicochemical Characterization and Solubility Evaluation of Pharmaceutical Cocrystals of Cinnamic Acid. J. Therm. Anal. Calorim. 2021, 145, 379–390. [Google Scholar] [CrossRef]
- Ghosh, T.; Juturu, T.; Nagar, S.N.; Kamath, S. Cocrystals of Modafinil-Nicotinic Acid: A Novel Cocrystal for Enhanced Bioavailability. Proceedings 2021, 62, 12. [Google Scholar] [CrossRef]
- Salem, A.; Hagymási, A.; Vörös-Horváth, B.; Šafarik, T.; Balić, T.; Szabó, P.; Gősi, F.; Nagy, S.; Pál, S.; Kunsági-Máté, S.; et al. Solvent Dependent 4-Aminosalicylic Acid-Sulfamethazine Co-Crystal Polymorph Control. Eur. J. Pharm. Sci. 2021, 156, 105599. [Google Scholar] [CrossRef]
- Devogelaer, J.-J.; Brugman, S.J.T.; Meekes, H.; Tinnemans, P.; Vlieg, E.; de Gelder, R. Cocrystal Design by Network-Based Link Prediction. CrystEngComm 2019, 21, 6875–6885. [Google Scholar] [CrossRef]
- Devogelaer, J.-J.; Meekes, H.; Tinnemans, P.; Vlieg, E.; Gelder, R. de Co-Crystal Prediction by Artificial Neural Networks**. Angew. Chem. Int. Ed. 2020, 59, 21711–21718. [Google Scholar] [CrossRef]
- Heng, T.; Yang, D.; Wang, R.; Zhang, L.; Lu, Y.; Du, G. Progress in Research on Artificial Intelligence Applied to Polymorphism and Cocrystal Prediction. ACS Omega 2021, 6, 15543–15550. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Zhu, B.; Mei, X.; Luo, X. Machine-Learning-Guided Cocrystal Prediction Based on Large Data Base. Cryst. Growth Des. 2020, 20, 6610–6621. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y.; Zhang, X.; Zhou, X. Novel Pharmaceutical Cocrystal of Lenalidomide with Nicotinamide: Structural Design, Evaluation, and Thermal Phase Transition Study. Int. J. Pharm. 2022, 613, 121394. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Q.; Ji, W.; An, Q.; Song, J.; Xing, C.; Yang, D.; Zhang, L.; Lu, Y.; Du, G. Cocrystals of Praziquantel with Phenolic Acids: Discovery, Characterization, and Evaluation. Molecules 2022, 27, 2022. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Churakov, A.V.; Proshin, A.N.; Dai, X.-L.; Lu, T.; Perlovich, G.L. Cocrystals of a 1,2,4-Thiadiazole-Based Potent Neuroprotector with Gallic Acid: Solubility, Thermodynamic Stability Relationships and Formation Pathways. Phys. Chem. Chem. Phys. 2018, 20, 14469–14481. [Google Scholar] [CrossRef] [PubMed]
- Savla, P.P. The Selection and Screening of Conformers. Bombay Technol. 2021, 68, 1–8. [Google Scholar] [CrossRef]
- Mswahili, M.E.; Lee, M.-J.; Martin, G.L.; Kim, J.; Kim, P.; Choi, G.J.; Jeong, Y.-S. Cocrystal Prediction Using Machine Learning Models and Descriptors. Appl. Sci. 2021, 11, 1323. [Google Scholar] [CrossRef]
- Khan, H.W.; Elgharbawy, A.A.M.; Bustam, A.; Moniruzzaman, M. Design and Selection of Ionic Liquids Via COSMO for Pharmaceuticals and Medicine. In Application of Ionic Liquids in Drug Delivery; Goto, M., Moniruzzaman, M., Eds.; Springer: Singapore, 2021; pp. 137–164. ISBN 9789811643651. [Google Scholar]
- Vologzhanina, A.V. Intermolecular Interactions in Functional Crystalline Materials: From Data to Knowledge. Crystals 2019, 9, 478. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, B.; Yao, Q.; Hou, B.; Zhou, L.; Xie, C.; Gong, J.; Hao, H.; Chen, W. Evaluation on Cocrystal Screening Methods and Synthesis of Multicomponent Crystals: A Case Study. Cryst. Growth Des. 2021, 21, 4531–4546. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Y.; Shi, P.; Du, S.; Wu, S.; Gong, J. Similar but Not the Same: Difference in the Ability to Form Cocrystals between Nimesulide and the Pyridine Analogues. Cryst. Growth Des. 2021, 21, 287–296. [Google Scholar] [CrossRef]
- Barbas, R.; Kumar, V.; Vallcorba, O.; Prohens, R.; Frontera, A. Sildenafil–Resorcinol Cocrystal: XRPD Structure and DFT Calculations. Crystals 2020, 10, 1126. [Google Scholar] [CrossRef]
- Yang, D.; Cao, J.; Heng, T.; Xing, C.; Yang, S.; Zhang, L.; Lu, Y.; Du, G. Theoretical Calculation and Structural Analysis of the Cocrystals of Three Flavonols with Praziquantel. Cryst. Growth Des. 2021, 21, 2292–2300. [Google Scholar] [CrossRef]
- Khalaji, M.; Potrzebowski, M.J.; Dudek, M.K. Virtual Cocrystal Screening Methods as Tools to Understand the Formation of Pharmaceutical Cocrystals—A Case Study of Linezolid, a Wide-Range Antibacterial Drug. Cryst. Growth Des. 2021, 21, 2301–2314. [Google Scholar] [CrossRef]
- Sun, G.; Liu, X.; Abramov, Y.A.; Nilsson Lill, S.O.; Chang, C.; Burger, V.; Broo, A. Current State-of-the-Art In-House and Cloud-Based Applications of Virtual Polymorph Screening of Pharmaceutical Compounds: A Challenging Case of AZD1305. Cryst. Growth Des. 2021, 21, 1972–1983. [Google Scholar] [CrossRef]
- Dal Magro, C.; dos Santos, A.E.; Ribas, M.M.; Aguiar, G.P.S.; Volfe, C.R.B.; Lopes, M.L.L.C.; Siebel, A.M.; Müller, L.G.; Bortoluzzi, A.J.; Lanza, M.; et al. Production of Curcumin-Resveratrol Cocrystal Using Cocrystallization with Supercritical Solvent. J. Supercrit. Fluids 2021, 171, 105190. [Google Scholar] [CrossRef]
- Stanton, S.A.; Du, J.J.; Lai, F.; Stanton, G.; Hawkins, B.A.; Ong, J.A.; Groundwater, P.W.; Platts, J.A.; Hibbs, D.E. Understanding Hygroscopicity of Theophylline via a Novel Cocrystal Polymorph: A Charge Density Study. J. Phys. Chem. A 2021, 125, 9736–9756. [Google Scholar] [CrossRef]
- Haskins, M.M.; Zaworotko, M.J. Screening and Preparation of Cocrystals: A Comparative Study of Mechanochemistry vs Slurry Methods. Cryst. Growth Des. 2021, 21, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Earle, R.R.; Radha, G.V. Engineering Cocrystals of Paliperidone with Enhanced Solubility and Dissolution Characteristics. Arh. Farm. 2021, 71, 393–409. [Google Scholar] [CrossRef]
- O’Malley, C.; McArdle, P.; Erxleben, A. Formation of Salts and Molecular Ionic Cocrystals of Fluoroquinolones and α,ω-Dicarboxylic Acids. Cryst. Growth Des. 2022, 22, 3060–3071. [Google Scholar] [CrossRef]
- Ferreira, P.O.; de Moura, A.; de Almeida, A.C.; dos Santos, É.C.; Kogawa, A.C.; Caires, F.J. Mechanochemical Synthesis, Thermoanalytical Study and Characterization of New Multicomponent Solid Forms of Norfloxacin with Saccharin. J. Therm. Anal. Calorim. 2022, 147, 1985–1997. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Savjani, J.K.; Savjani, K.T.; Shah, H. Improved Pharmaceutical Properties of Ritonavir through Co-Crystallization Approach with Liquid-Assisted Grinding Method. Drug Dev. Ind. Pharm. 2022, 47, 1633–1642. [Google Scholar] [CrossRef]
- Pawar, N.; Saha, A.; Nandan, N.; Parambil, J.V. Solution Cocrystallization: A Scalable Approach for Cocrystal Production. Crystals 2021, 11, 303. [Google Scholar] [CrossRef]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced Methodologies for Cocrystal Synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Murthy, S.; Korpale, S.; Yadav, A.; Sajane, S.; Sakhare, S.; Doijad, R. Cocrystals of Itraconazole with Amino Acids: Screening, Synthesis, Solid State Characterization, in Vitro Drug Release and Antifungal Activity. J. Drug Deliv. Sci. Technol. 2015, 28, 46–55. [Google Scholar] [CrossRef]
- Saganowska, P.; Wesolowski, M. DSC as a Screening Tool for Rapid Co-Crystal Detection in Binary Mixtures of Benzodiazepines with Co-Formers. J. Therm. Anal. Calorim. 2018, 133, 785–795. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Nanda, A. Hot Stage Microscopy and Its Applications in Pharmaceutical Characterization. Appl. Microsc. 2020, 50, 12. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Yoon, W.; Yun, J.; Ban, E.; Yun, H.; Kim, A. Emodin-Nicotinamide (1:2) Cocrystal Identified by Thermal Screening to Improve Emodin Solubility. Int. J. Pharm. 2019, 557, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Zhou, Z.; Chan, H.M.; Sung, H.H.-Y.; Tong, H.H.Y.; Zheng, Y. Identification of New Cocrystal Systems with Stoichiometric Diversity of Salicylic Acid Using Thermal Methods. Pharm. Res. 2016, 33, 1030–1039. [Google Scholar] [CrossRef]
- Pal, S.; Roopa, B.N.; Abu, K.; Manjunath, S.G.; Nambiar, S. Thermal Studies of Furosemide–Caffeine Binary System That Forms a Cocrystal. J. Therm. Anal. Calorim. 2014, 115, 2261–2268. [Google Scholar] [CrossRef]
- Borkar, D.; Puranik, P.; Trivedi, H. Experimental Design Approach for Development of Cocrystals and Immediate Release Cocrystal Tablet of Atorvastatin Calcium for Enhancement of Solubility and Dissolution. J. Res. Pharm. 2020, 24, 720–737. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef]
- Hossain Mithu, M.S.; Ross, S.A.; Hurt, A.P.; Douroumis, D. Effect of Mechanochemical Grinding Conditions on the Formation of Pharmaceutical Cocrystals and Co-Amorphous Solid Forms of Ketoconazole—Dicarboxylic Acid. J. Drug Deliv. Sci. Technol. 2021, 63, 102508. [Google Scholar] [CrossRef]
- Myz, S.A.; Mikhailenko, M.A.; Mikhailovskaya, A.V.; Bulina, N.V.; Gerasimov, K.B.; Politov, A.A.; Kuznetsova, S.A.; Shakhtshneider, T.P. Cocrystals of Betulin with Adipic Acid: Preparation and Thermal Behavior. J. Therm. Anal. Calorim. 2021, 147, 8235–8242. [Google Scholar] [CrossRef]
- Medina, C.; Daurio, D.; Nagapudi, K.; Alvarez-Nunez, F. Manufacture of Pharmaceutical Co-crystals Using Twin Screw Extrusion: A Solvent-less and Scalable Process. J. Pharm. Sci. 2010, 99, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Gajda, M.; Nartowski, K.P.; Pluta, J.; Karolewicz, B. Continuous, One-Step Synthesis of Pharmaceutical Cocrystals via Hot Melt Extrusion from Neat to Matrix-Assisted Processing—State of the Art. Int. J. Pharm. 2019, 558, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, Y.; Setyawan, D. Siswandono Formation of Ketoprofen-Malonic Acid Cocrystal by Solvent Evaporation Method. Indones. J. Chem. 2017, 17, 161–166. [Google Scholar] [CrossRef][Green Version]
- Przybyłek, M.; Ziółkowska, D.; Kobierski, M.; Mroczyńska, K.; Cysewski, P. Utilization of Oriented Crystal Growth for Screening of Aromatic Carboxylic Acids Cocrystallization with Urea. J. Cryst. Growth 2016, 433, 128–138. [Google Scholar] [CrossRef]
- Urano, M.; Kitahara, M.; Kishi, K.; Goto, E.; Tagami, T.; Fukami, T.; Ozeki, T. Physical Characteristics of Cilostazol–Hydroxybenzoic Acid Cocrystals Prepared Using a Spray Drying Method. Crystals 2020, 10, 313. [Google Scholar] [CrossRef]
- Yang, J.; Hong, B.; Wang, N.; Li, X.; Huang, X.; Bao, Y.; Xie, C.; Hao, H. Thermodynamics and Molecular Mechanism of the Formation of the Cocrystals of P-Hydroxybenzoic Acid and Glutaric Acid. CrystEngComm 2019, 21, 6374–6381. [Google Scholar] [CrossRef]
- Vaksler, Y.A.; Benedis, D.; Dyshin, A.A.; Oparin, R.D.; Correia, N.T.; Capet, F.; Shishkina, S.V.; Kiselev, M.G.; Idrissi, A. Spectroscopic Characterization of Single Co-Crystal of Mefenamic Acid and Nicotinamide Using Supercritical CO2. J. Mol. Liq. 2021, 334, 116117. [Google Scholar] [CrossRef]
- Gaddam, M.; Ravouru, N. A Crystal Engineering Design to Enhance the Solubility, Dissolution, Stability and Micrometric Properties of Omeprazole via Co-Crystallization Techniques. Res. J. Pharm. Technol. 2021, 14, 356–362. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Banerjee, N.; Chaudhari, P. A Critical Review on the Particle Generation and Other Applications of Rapid Expansion of Supercritical Solution. Int. J. Pharm. 2021, 608, 121089. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Deng, S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics 2021, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Development and Evaluation of Nano-Formulations for Immediate Release Oral Dosage Forms of Poorly Soluble Drugs. Ph.D. Thesis, Freie Universitaet Berlin, Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Sellers, M.S.; Lísal, M.; Brennan, J.K. Free-Energy Calculations Using Classical Molecular Simulation: Application to the Determination of the Melting Point and Chemical Potential of a Flexible RDX Model. Phys. Chem. Chem. Phys. 2016, 18, 7841–7850. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Maggi, L.; Mustarelli, P.; Sakaj, M.; Friuli, V.; Ferrara, C.; Berbenni, V.; Girella, A.; Milanese, C.; Marini, A. Enhancing the Pharmaceutical Behavior of Nateglinide by Cocrystallization: Physicochemical Assessment of Cocrystal Formation and Informed Use of Differential Scanning Calorimetry for Its Quantitative Characterization. J. Pharm. Sci. 2019, 108, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Hong, M.; Yi, D.; Qi, M.; Ren, G. Structure Properties of Scoparone: Polymorphs and Cocrystals. J. Mol. Struct. 2019, 1191, 323–336. [Google Scholar] [CrossRef]
- Budziak-Wieczorek, I.; Maciołek, U. Synthesis and Characterization of a (−)-Epicatechin and Barbituric Acid Cocrystal: Single-Crystal X-Ray Diffraction and Vibrational Spectroscopic Studies. ACS Omega 2021, 6, 8199–8209. [Google Scholar] [CrossRef]
- Cerreia Vioglio, P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical Aspects of Salt and Cocrystal Forms of APIs and Characterization Challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- Wei, H.; Gao, N.; Dang, L. Solubility and Thermodynamic Properties of Sulfamethazine–Saccharin Cocrystal in Pure and Binary (Acetonitrile + 2-Propanol) Solvents. Trans. Tianjin Univ. 2021, 27, 460–472. [Google Scholar] [CrossRef]
- Arabiani, M.R.; Lodagekar, A.; Yadav, B.; Chavan, R.B.; Shastri, N.R.; Purohit, P.Y.; Shelat, P.; Dave, D. Mechanochemical Synthesis of Brexpiprazole Cocrystals to Improve Its Pharmaceutical Attributes. CrystEngComm 2019, 21, 800–806. [Google Scholar] [CrossRef]
- Waterman, K.C.; Waterman, A.K.; Botoy, T.M.; Li, J.; Qiu, F.; Hawley, M. Stability Screening of Pharmaceutical Cocrystals. Pharm. Dev. Technol. 2021, 26, 1130–1135. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to Cancer Chemotherapy: Failure in Drug Response from ADME to P-Gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.C.P.; de O. Pepino, R.; de Melo, C.C.; Tenorio, J.C.; Ellena, J. Controlled Synthesis of New 5-Fluorocytosine Cocrystals Based on the pKa Rule. Cryst. Growth Des. 2014, 14, 4383–4393. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Li, M.; Wang, J.-R.; Mei, X. Modulating the Dissolution and Mechanical Properties of Resveratrol by Cocrystallization. Cryst. Growth Des. 2017, 17, 3989–3996. [Google Scholar] [CrossRef]
- Duan, C.; Liu, W.; Tao, Y.; Liang, F.; Chen, Y.; Xiao, X.; Zhang, G.; Chen, Y.; Hao, C. Two Novel Palbociclib-Resorcinol and Palbociclib-Orcinol Cocrystals with Enhanced Solubility and Dissolution Rate. Pharmaceutics 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of Quercetin with Improved Solubility and Oral Bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef]
- Iqbal, U.; Choudhary, M.I.; Yousuf, S. Synthesis of Co-Crystals of Anti-Cancer Nandrolone as a Potential Leads towards Treatment of Cancer. J. Mol. Struct. 2021, 1224, 128981. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Liu, L.; Bu, F.-Z.; Li, Y.-T.; Yan, C.-W.; Wu, Z.-Y. A Novice Cocrystal Nanomicelle Formulation of 5-Fluorouracil with Proline: The Design, Self-Assembly and in Vitro/Vivo Biopharmaceutical Characteristics. Int. J. Pharm. 2022, 617, 121635. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.-R.; Zhang, Q.; Li, M.; Guo, C.; Ren, G.; Mei, X. Stable Cocrystals and Salts of the Antineoplastic Drug Apatinib with Improved Solubility in Aqueous Solution. Cryst. Growth Des. 2018, 18, 4701–4714. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Yu, M.-C.; Wang, L.-Y.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. A Supramolecular Adduct of Tegafur and Syringic Acid: The First Tegafur-Nutraceutical Cocrystal with Perfected in Vitro and in Vivo Characteristics as Well as Synergized Anticancer Activities. New J. Chem. 2020, 44, 15994–16005. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Zhang, Q.; Peng, B.; Zhu, B.; Wang, J.; Liu, L.; Mei, X. Fine-Tuning the Colors of Natural Pigment Emodin with Superior Stability through Cocrystal Engineering. Cryst. Growth Des. 2018, 18, 6123–6132. [Google Scholar] [CrossRef]
- Shiraki, K.; Takata, N.; Takano, R.; Hayashi, Y.; Terada, K. Dissolution Improvement and the Mechanism of the Improvement from Cocrystallization of Poorly Water-Soluble Compounds. Pharm. Res. 2008, 25, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ramaiah, T.; Zhang, M.; Zhang, Y.; Huang, Y.; Lou, B. Two Cocrystals of Berberine Chloride with Myricetin and Dihydromyricetin: Crystal Structures, Characterization, and Antitumor Activities. Cryst. Growth Des. 2020, 20, 157–166. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef] [PubMed]
- Xuan, B.; Wong, S.N.; Zhang, Y.; Weng, J.; Tong, H.H.Y.; Wang, C.; Sun, C.C.; Chow, S.F. Extended Release of Highly Water Soluble Isoniazid Attained through Cocrystallization with Curcumin. Cryst. Growth Des. 2020, 20, 1951–1960. [Google Scholar] [CrossRef]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug–Drug Cocrystals: Opportunities and Challenges. Asian J. Pharm. Sci. 2021, 16, 307–317. [Google Scholar] [CrossRef]
- Yin, H.-M.; Xie, J.-Y.; Jiang, J.-Y.; Hong, M.; Zhu, B.; Ren, G.-B.; Qi, M.-H. Strategy to Tune the Performance of Two Drug Components: Drug–Drug Cocrystals of Lobaplatin with Flavonoids. Cryst. Growth Des. 2022, 22, 2602–2610. [Google Scholar] [CrossRef]
- da Silva, C.C.P.; de Melo, C.C.; Souza, M.S.; Diniz, L.F.; Carneiro, R.L.; Ellena, J. 5-Fluorocytosine/5-Fluorouracil Drug–Drug Cocrystal: A New Development Route Based on Mechanochemical Synthesis. J. Pharm. Innov. 2019, 14, 50–56. [Google Scholar] [CrossRef]
- Thorat, S.H.; Sahu, S.K.; Patwadkar, M.V.; Badiger, M.V.; Gonnade, R.G. Drug–Drug Molecular Salt Hydrate of an Anticancer Drug Gefitinib and a Loop Diuretic Drug Furosemide: An Alternative for Multidrug Treatment. J. Pharm. Sci. 2015, 104, 4207–4216. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhang, M.; Zhang, Y.; Lou, B. A Drug–Drug Cocrystal of Dihydromyricetin and Pentoxifylline. J. Pharm. Sci. 2022, 111, 82–87. [Google Scholar] [CrossRef]
- Yin, H.-M.; Wu, N.; Zhou, B.-J.; Hong, M.-H.; Zhu, B.; Qi, M.-H.; Ren, G.-B. Slow-Release Drug–Drug Cocrystals of Oxaliplatin with Flavonoids: Delaying Hydrolysis and Reducing Toxicity. Cryst. Growth Des. 2021, 21, 75–85. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.-L.; Lu, T.-B.; Chen, J.-M. Temozolomide–Hesperetin Drug–Drug Cocrystal with Optimized Performance in Stability, Dissolution, and Tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Yin, H.-M.; Zhou, B.; Hong, M.; Zhu, B.; Qi, M.-H.; Ren, G.-B. Cocrystals of Flavonoids with 4,4′-Ethylenebispyridine: Crystal Structures Analysis, Dissolution Behavior, and Anti-Tumor Activity. J. Mol. Struct. 2022, 1252, 132150. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Niu, Y.-Y.; Wang, L.-Y.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Supramolecular Self-Assembly and Perfected in Vitro/Vivo Property of 5-Fluorouracil and Ferulic Acid on the Strength of Double Optimized Strategy: The First 5-Fluorouracial-Phenolic Acid Nutraceutical Cocrystal with Synergistic Antitumor Efficacy. Analyst 2021, 146, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Heinen, T.; Hoelscher, S.; Vasylyeva, V. Structural Study of Anhydrous and Hydrated 5-Fluorouracil Co-Crystals with Nicotinamide and Isonicotinamide. Z. Krist. Cryst. Mater. 2022, 237, 109–116. [Google Scholar] [CrossRef]
- Jubeen, F.; Liaqat, A.; Amjad, F.; Sultan, M.; Iqbal, S.Z.; Sajid, I.; Khan Niazi, M.B.; Sher, F. Synthesis of 5-Fluorouracil Cocrystals with Novel Organic Acids as Coformers and Anticancer Evaluation against HCT-116 Colorectal Cell Lines. Cryst. Growth Des. 2020, 20, 2406–2414. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.R.; Türk, M.; Pando, C. Cocrystallization of the Anticancer Drug 5-Fluorouracil and Coformers Urea, Thiourea or Pyrazinamide Using Supercritical CO2 as an Antisolvent (SAS) and as a Solvent (CSS). J. Supercrit. Fluids 2020, 160, 104813. [Google Scholar] [CrossRef]
- Jubeen, F.; Liaqat, A.; Sultan, M.; Zafar Iqbal, S.; Sajid, I.; Sher, F. Green Synthesis and Biological Evaluation of Novel 5-Fluorouracil Derivatives as Potent Anticancer Agents. Saudi Pharm. J. 2019, 27, 1164–1173. [Google Scholar] [CrossRef]
- Gautam, M.K.; Besan, M.; Pandit, D.; Mandal, S.; Chadha, R. Cocrystal of 5-Fluorouracil: Characterization and Evaluation of Biopharmaceutical Parameters. AAPS PharmSciTech 2019, 20, 149. [Google Scholar] [CrossRef]
- Xu, L.-L.; Chen, J.-M.; Yan, Y.; Lu, T.-B. Improving the Solubility of 6-Mercaptopurine via Cocrystals and Salts. Cryst. Growth Des. 2012, 12, 6004–6011. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J.-M.; Xu, Y.-B.; Lu, T.-B. Enhancing the Solubility of 6-Mercaptopurine by Formation of Ionic Cocrystal with Zinc Trifluoromethanesulfonate: Single-Crystal-to-Single-Crystal Transformation. Cryst. Growth Des. 2014, 14, 5019–5025. [Google Scholar] [CrossRef]
- Makadia, J.; Madu, S.J.; Arroo, R.; Seaton, C.C.; Li, M. Artemisinin–Acetylenedicarboxylic Acid Cocrystal: Screening, Structure Determination, and Physicochemical Property Characterisation. CrystEngComm 2022, 24, 1056–1067. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Dai, X.-L.; Chen, J.-M.; Lu, T.-B. Two Anhydrous Forms and One Monohydrate of a Cocrystal of Axitinib and Glutaric Acid: Characterization, Property Evaluation and Phase Transition Study. CrystEngComm 2022, 24, 2138–2148. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Dai, X.-L.; Wang, J.; Wu, C.; Lu, T.-B.; Chen, J.-M. Cocrystallization of Axitinib with Carboxylic Acids: Preparation, Crystal Structures and Dissolution Behavior. CrystEngComm 2021, 23, 5504–5515. [Google Scholar] [CrossRef]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Zhang, B.; Qi, D.; Guo, P.; Li, N.; Liu, Z. A Nano-Cocrystal Strategy to Improve the Dissolution Rate and Oral Bioavailability of Baicalein. Asian J. Pharm. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nicolov, M.; Ghiulai, R.M.; Voicu, M.; Mioc, M.; Duse, A.O.; Roman, R.; Ambrus, R.; Zupko, I.; Moaca, E.A.; Coricovac, D.E.; et al. Cocrystal Formation of Betulinic Acid and Ascorbic Acid: Synthesis, Physico-Chemical Assessment, Antioxidant, and Antiproliferative Activity. Front. Chem. 2019, 7, 92. [Google Scholar] [CrossRef]
- Awasthi, A.; Dheeraj, H.D.; Birangal, S.; Pai, A.; Pai, G.; Sathyanarayana, M.B. Fabrication of ceritinib cocrystals with improved solubility: Preparation, solid-state characterization, solubility studies, and molecular docking studies. RJC 2021, 14, 905–913. [Google Scholar] [CrossRef]
- Shahbaz, M.; Khan, U.A.; Chaudhary, M.I.; Yousuf, S. A New Bioactive Cocrystal of Coumarin-3-Carboxylic Acid and Thiourea: Detailed Structural Features and Biological Activity Studies. Acta Cryst. C 2022, 78, 192–200. [Google Scholar] [CrossRef]
- Paulazzi, A.R.; Alves, B.O.; Zilli, G.A.L.; dos Santos, A.E.; Petry, F.; Soares, K.D.; Danielli, L.J.; Pedroso, J.; Apel, M.A.; Aguiar, G.P.S.; et al. Curcumin and N-Acetylcysteine Cocrystal Produced with Supercritical Solvent: Characterization, Solubility, and Preclinical Evaluation of Antinociceptive and Anti-Inflammatory Activities. Inflammopharmacology 2022, 30, 327–341. [Google Scholar] [CrossRef]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel Curcumin Ascorbic Acid Cocrystal for Improved Solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Sathisaran, I.; Devidas Bhatia, D.; Vishvanath Dalvi, S. New Curcumin-Trimesic Acid Cocrystal and Anti-Invasion Activity of Curcumin Multicomponent Solids against 3D Tumor Models. Int. J. Pharm. 2020, 587, 119667. [Google Scholar] [CrossRef]
- Ribas, M.M.; Sakata, G.S.B.; Santos, A.E.; Dal Magro, C.; Aguiar, G.P.S.; Lanza, M.; Oliveira, J.V. Curcumin Cocrystals Using Supercritical Fluid Technology. J. Supercrit. Fluids 2019, 152, 104564. [Google Scholar] [CrossRef]
- Dheeraj, H.M.; Awasthi, A.; Pai, A.; Birangal, S.; Vasanthraju, S.G.; Pai, G.; Sathyanarayana, M.B. Designing of stable cocrystals of cytarabine and doxorubicin hcl using suitable coformers. Lat. Am. J. Pharm. 2021, 40, 39–48. [Google Scholar]
- Rai, S.K.; Gunnam, A.; Mannava, M.K.C.; Nangia, A.K. Improving the Dissolution Rate of the Anticancer Drug Dabrafenib. Cryst. Growth Des. 2020, 20, 1035–1046. [Google Scholar] [CrossRef]
- Chiodo, T.; Hafner, A.; Hintermann, T.; Salvador, B.; Szelagiewicz, M.; Blatter, F.; Siebenhaar, B.; Vossen, M. Multicomponent Crystals Comprising Dasatinib and Selected Cocrystal Formers. International Patent Application No. PCT/IB2013/054832, 17 May 2017. [Google Scholar]
- Fan, X.; Chen, J.; Shen, Q. Docetaxel–Nicotinamide Complex-Loaded Nanostructured Lipid Carriers for Transdermal Delivery. Int. J. Pharm. 2013, 458, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; An, S.H.; Park, B.; Park, M.; Yoon, N.-E.; Jung, B.H.; Kim, A. Improved Solubility and Oral Absorption of Emodin-Nicotinamide Cocrystal Over Emodin with PVP as a Solubility Enhancer and Crystallization Inhibitor. J. Pharm. Sci. 2020, 109, 3660–3667. [Google Scholar] [CrossRef]
- Pandit, D.; Chadha, R.; Laha, B.; Gautam, M.K.; Karan, M.; Mandal, S.K. Novel Pharmaceutical Cocrystals of Gefitinib: A Credible Upswing in Strategic Research to Ameliorate Its Biopharmaceutical Challenges. Cryst. Growth Des. 2022, 22, 2218–2229. [Google Scholar] [CrossRef]
- Reggane, M.; Wiest, J.; Saedtler, M.; Harlacher, C.; Gutmann, M.; Zottnick, S.H.; Piechon, P.; Dix, I.; Müller-Buschbaum, K.; Holzgrabe, U.; et al. Bioinspired Co-Crystals of Imatinib Providing Enhanced Kinetic Solubility. Eur. J. Pharm. Biopharm. 2018, 128, 290–299. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Chen, Y.; Chen, Z.; Chen, H.; Pui, Y.; Qian, F. Oral Bioavailability Enhancement of β-Lapachone, a Poorly Soluble Fast Crystallizer, by Cocrystal, Amorphous Solid Dispersion, and Crystalline Solid Dispersion. Eur. J. Pharm. Biopharm. 2018, 124, 73–81. [Google Scholar] [CrossRef]
- Song, J.-X.; Yan, Y.; Yao, J.; Chen, J.-M.; Lu, T.-B. Improving the Solubility of Lenalidomide via Cocrystals. Cryst. Growth Des. 2014, 14, 3069–3077. [Google Scholar] [CrossRef]
- Song, J.-X.; Chen, J.-M.; Lu, T.-B. Lenalidomide–Gallic Acid Cocrystals with Constant High Solubility. Cryst. Growth Des. 2015, 15, 4869–4875. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Ji, W.; Qi, M.-H.; Ren, G. Cocrystals of Lenvatinib with Sulfamerazine and Salicylic Acid: Crystal Structure, Equilibrium Solubility, Stability Study, and Anti-Hepatoma Activity. Cryst. Growth Des. 2021, 21, 3714–3727. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, S.; Zhou, J.; Chen, J.; Tian, L.; Gao, W.; Zhang, Y.; Ma, A.; Li, L.; Zhou, Z. Luteolin Cocrystals: Characterization, Evaluation of Solubility, Oral Bioavailability and Theoretical Calculation. J. Drug Deliv. Sci. Technol. 2019, 50, 248–254. [Google Scholar] [CrossRef]
- Hisada, N.; Takano, R.; Takata, N.; Shiraki, K.; Ueto, T.; Tanida, S.; Kataoka, M.; Yamashita, S. Characterizing the Dissolution Profiles of Supersaturable Salts, Cocrystals, and Solvates to Enhance in Vivo Oral Absorption. Eur. J. Pharm. Biopharm. 2016, 103, 192–199. [Google Scholar] [CrossRef]
- Bofill, L.; Barbas, R.; de Sande, D.; Font-Bardia, M.; Ràfols, C.; Albertí, J.; Prohens, R. A Novel, Extremely Bioavailable Cocrystal of Pterostilbene. Cryst. Growth Des. 2021, 21, 2315–2323. [Google Scholar] [CrossRef]
- Jia, J.-L.; Dai, X.-L.; Che, H.-J.; Li, M.-T.; Zhuang, X.-M.; Lu, T.-B.; Chen, J.-M. Cocrystals of Regorafenib with Dicarboxylic Acids: Synthesis, Characterization and Property Evaluation. CrystEngComm 2021, 23, 653–662. [Google Scholar] [CrossRef]
- Pessoa, A.S.; Aguiar, G.P.S.; Vladimir Oliveira, J.; Bortoluzzi, A.J.; Paulino, A.; Lanza, M. Precipitation of Resveratrol-Isoniazid and Resveratrol-Nicotinamide Cocrystals by Gas Antisolvent. J. Supercrit. Fluids 2019, 145, 93–102. [Google Scholar] [CrossRef]
- Babu, N.J.; Sanphui, P.; Nangia, A. Crystal Engineering of Stable Temozolomide Cocrystals. Chem. Asian J. 2012, 7, 2274–2285. [Google Scholar] [CrossRef]
- Varma, A.; Laxmi, P.; Pai, A.; Pai, G.; Sg, V.; Badamane Sathyanarayana, M. Designing of Stable Co-Crystals of Zoledronic Acid Using Suitable Coformers. Chem. Pharm. Bull. 2019, 67, 816–823. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Xu, X.; Xu, X.; Wang, Q.; Li, D.; Zhang, H. Drug–Drug Cocrystals of Theophylline with Quercetin. J. Drug Deliv. Sci. Technol. 2022, 70, 103228. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Wan, S.; Liu, L.; Sun, C.C.; Qian, F. An Elusive Drug–Drug Cocrystal Prepared Using a Heteroseeding Strategy. Cryst. Growth Des. 2021, 21, 5659–5668. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, W.; Zhou, L.; Hao, H.; Bao, Y.; Yin, Q.; Xie, C. Growth Mechanism of the Spherulitic Propylthiouracil–Kaempferol Cocrystal: New Perspectives into Surface Nucleation. CrystEngComm 2021, 23, 2367–2375. [Google Scholar] [CrossRef]
- George, C.P.; Thorat, S.H.; Shaligram, P.S.; Suresha, P.R.; Gonnade, R.G. Drug–Drug Cocrystals of Anticancer Drugs Erlotinib–Furosemide and Gefitinib–Mefenamic Acid for Alternative Multi-Drug Treatment. CrystEngComm 2020, 22, 6137–6151. [Google Scholar] [CrossRef]
- Lv, W.-T.; Liu, X.-X.; Dai, X.-L.; Long, X.-T.; Chen, J.-M. A 5-Fluorouracil–Kaempferol Drug–Drug Cocrystal: A Ternary Phase Diagram, Characterization and Property Evaluation. CrystEngComm 2020, 22, 8127–8135. [Google Scholar] [CrossRef]
- Laxmi, P.; Varma, A.; Pai, A.; Sathyanarayana, M.B. Experimental Data of Fabricated Co-Crystals of Doxorubicin HCl with Flavonoids. Indian J. Pharm. Educ. Res. 2019, 53, s225–s230. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Huang, Y.; Zhang, M.; Lou, B. Preparation, Crystal Structures, and Oral Bioavailability of Two Cocrystals of Emodin with Berberine Chloride. Cryst. Growth Des. 2018, 18, 7481–7488. [Google Scholar] [CrossRef]
- Veverka, M.; Šimon, P.; Gallovič, J.; Jorík, V.; Veverková, E.; Dubaj, T. Imatinib Mesylate Cocrystals: Synthesis, Screening, and Preliminary Characterization. Mon. Chem 2012, 143, 1405–1415. [Google Scholar] [CrossRef]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Danihelová, M.; Šimon, P. Cocrystals of Quercetin: Synthesis, Characterization, and Screening of Biological Activity. Mon. Chem 2015, 146, 99–109. [Google Scholar] [CrossRef]
- Albrecht, W.; Geier, J.; RABE, S.; Palacios, D.P. Co-Crystals of Ibrutinib with Carboxylic Acids. U.S. Patent 20180072737A1, 13 August 2019. [Google Scholar]
- Ross, S.A.; Lamprou, D.A.; Douroumis, D. Engineering and Manufacturing of Pharmaceutical Co-Crystals: A Review on Solvent-Free Manufacturing Technologies. Chem. Commun. 2016, 52, 8772–8786. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Du, W.; Liu, X.; Zhang, X.; Dong, H.; Hu, W. Cocrystals Strategy towards Materials for Near-Infrared Photothermal Conversion and Imaging. Angew. Chem. Int. Ed. 2018, 57, 3963–3967. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, D.D.; Rathnanand, M. Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties. Crystals 2022, 12, 1337. https://doi.org/10.3390/cryst12101337
Kara DD, Rathnanand M. Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties. Crystals. 2022; 12(10):1337. https://doi.org/10.3390/cryst12101337
Chicago/Turabian StyleKara, Divya Dhatri, and Mahalaxmi Rathnanand. 2022. "Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties" Crystals 12, no. 10: 1337. https://doi.org/10.3390/cryst12101337
APA StyleKara, D. D., & Rathnanand, M. (2022). Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties. Crystals, 12(10), 1337. https://doi.org/10.3390/cryst12101337






