(Z)-2-(2-(Anthracen-9-ylmethylene)hydrazineyl)pyrimidine-rhodium(III): Crystal Structure and DNA Binding Property
Abstract
1. Introduction
2. Results and Discussion
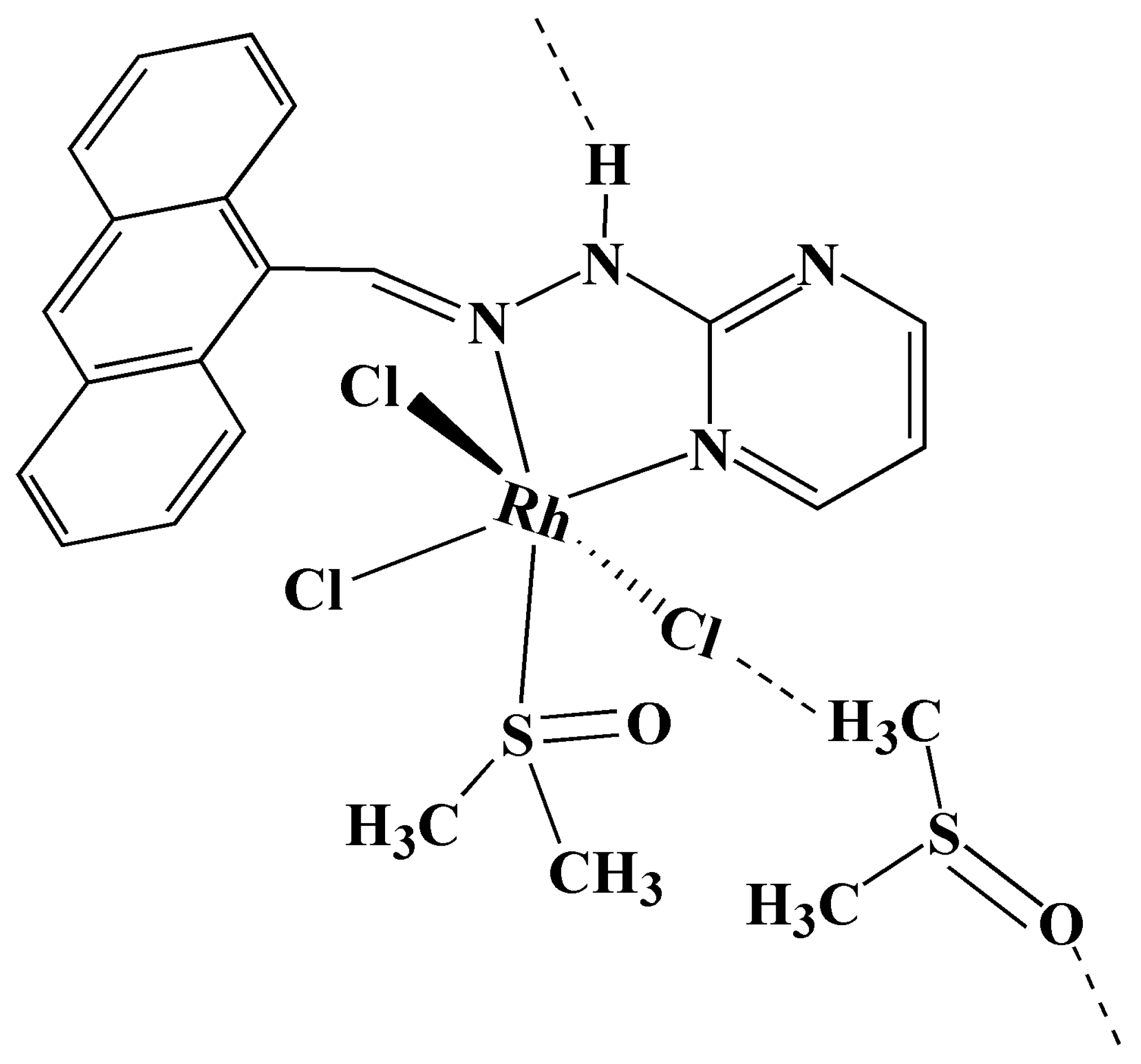
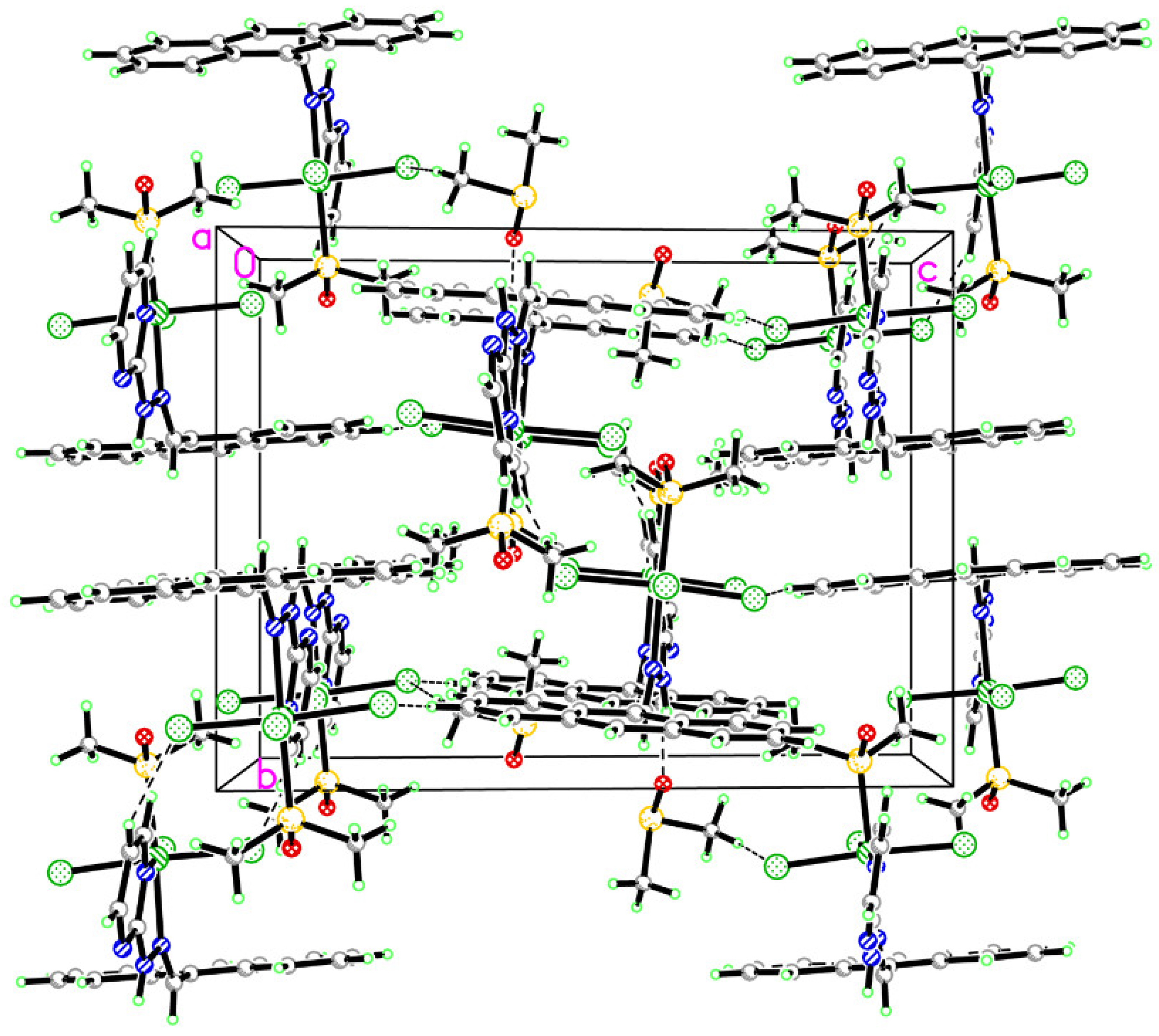
2.1. Crystal Structure Refinement and Analysis
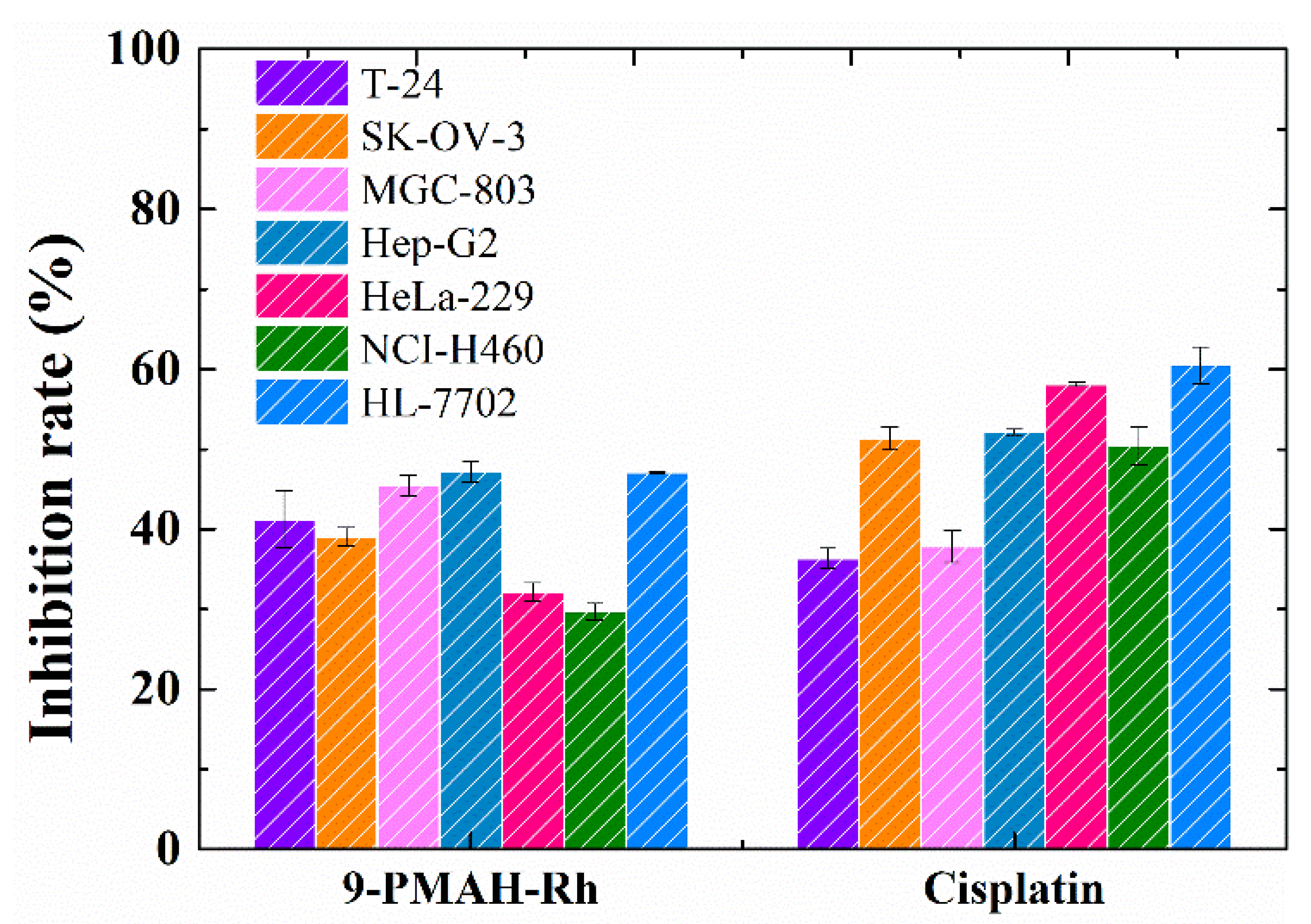
2.2. In Vitro Cytotoxicity Activities
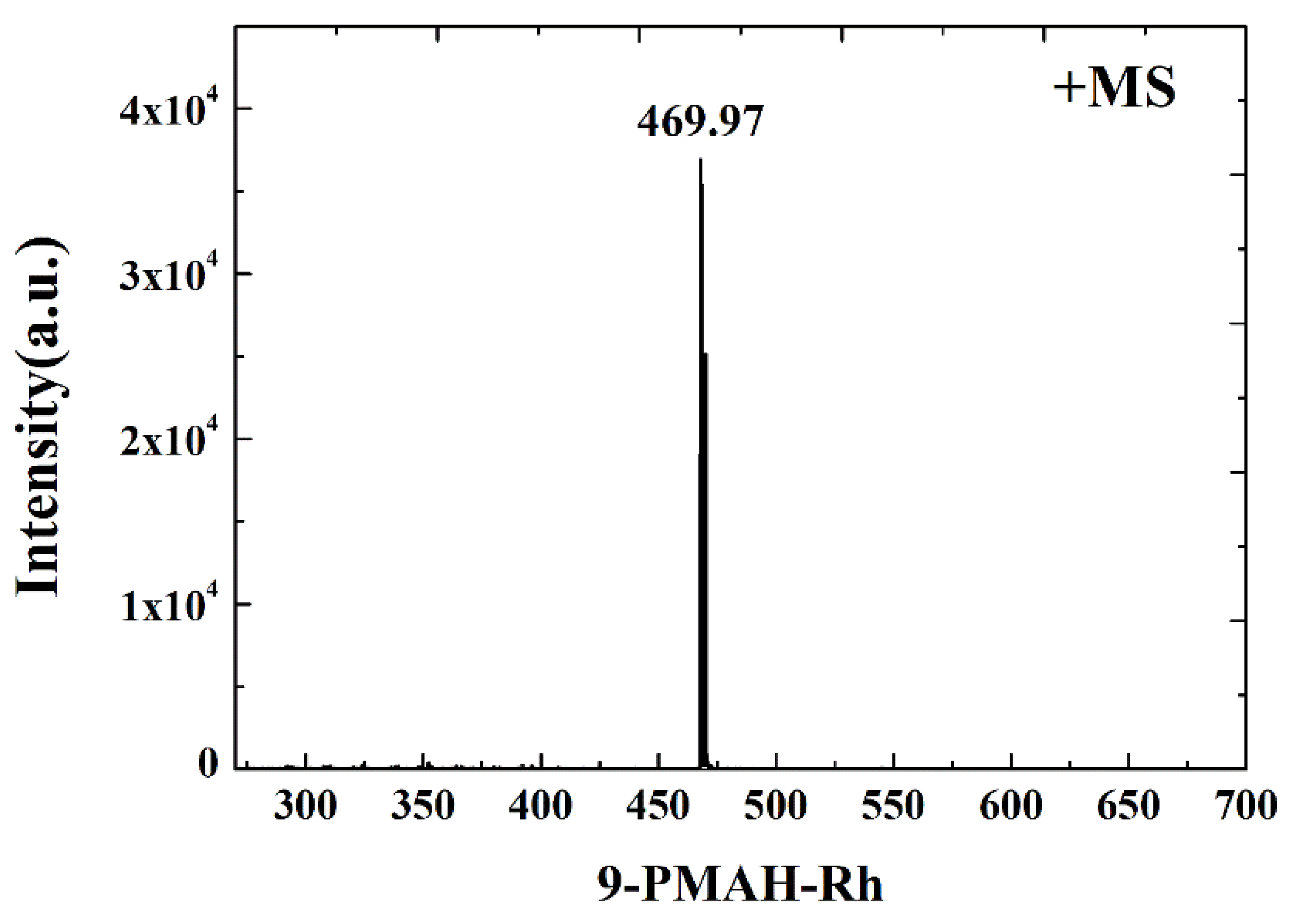
2.3. The Stability of the Complex in Solution
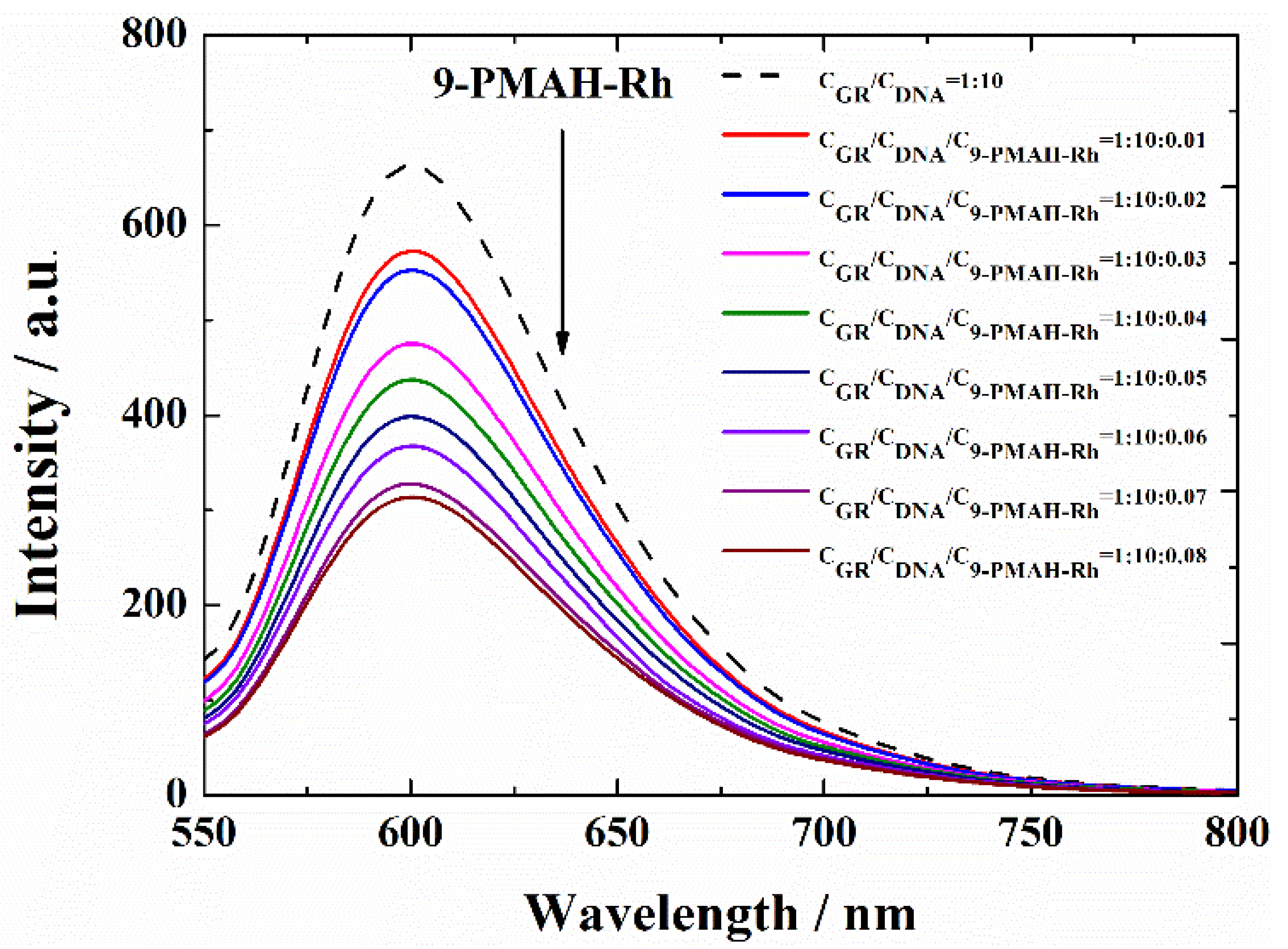
2.4. GR-Competitive Binding Studies
3. Experimental Section
3.1. Synthesis and Crystallization of 9-PMAH-Rh(III)
3.2. X-ray Crystallography
3.3. Biological Evaluation
3.3.1. Cell Culture
3.3.2. Cytotoxicity Analysis against Tumor Cell Lines
3.4. The Solution Chemistry Study of the Complex Examined by Spectroscopic Analysis
3.5. DNA Binding Study by Fluorescent Spectral Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panaretakis, T.; Pokrovskaja, K.; Shoshan, M.C.; Grandér, D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J. Biol. Chem. 2002, 277, 44317–44326. [Google Scholar] [CrossRef] [PubMed]
- Shenkenberg, T.D.; Von Hoff, D.D. Mitoxantrone: A new anticancer drug with significant clinical activity. Ann. Intern. Med 1986, 105, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, D.; Bourassa, P.; Bérubé, G.; Tajmir-Riahi, H.A.J. Review on the binding of anticancer drug doxorubicin with DNA and tRNA: Structural models and antitumor activity. Photochem. Photobiol. B. 2016, 158, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Coltman, C.A., Jr.; Osborne, C.K. Bisantrene, biological and clinical effects. Cancer Treat. Rev. 1984, 11, 285–288. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Liu, Y.-C.; Wang, H.-L.; Qin, J.-L.; Cheng, F.-J.; Tang, S.-F.; Liang, H. Synthesis and antitumor mechanisms of a copper(ii) complex of anthracene-9-imidazoline hydrazone (9-AIH). Metallomics 2015, 7, 1124–1136. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef]
- Zhong, Y.; Jia, C.; Zhang, X.; Liao, X.; Yang, B.; Cong, Y.; Pu, S.; Gao, C. Targeting drug delivery system for platinum (IV)-Based antitumor complexes. Eur. J. Med. Chem. 2020, 194, 112229. [Google Scholar] [CrossRef]
- Štarha, P. Multinuclear biologically active Ru, Rh, Os and Ir arene complexes. Coord. Chem. Rev. 2021, 431, 213690. [Google Scholar] [CrossRef]
- Bear, J.L.; Gray, H.B., Jr.; Rainen, L.; Chang, I.M.; Howard, R.; Serio, G.; Kimball, A.P. Interaction of Rhodium (II) Carboxylates with Molecules of Biologic. Cancer Chemother. Rep. 1975, 59, 611–620. [Google Scholar]
- Howard, R.A.; Kimball, A.P.; Bear, J.L. Mechanism of action of tetra-µ-carboxylatodirhodium (II) in L1210 tumor suspension culture. Cancer Res. 1979, 39, 2568–2573. [Google Scholar]
- Peng, Y.B.; Tao, C.; Tan, C.P.; Zhao, P. Mitochondrial targeted rhodium (III) complexes: Synthesis, characterized and antitumor mechanism investigation. J. Inorg. Biochem. 2021, 218, 111400. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. iotbx. cif: A comprehensive CIF toolbox. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Redetermination of cyclo-trimethylenetrinitramine. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, L.; Kaminsky, W.; Poluri, K.M.; Kollipara, M.R.J. Versatile coordination modes of benzothiazole hydrazone derivatives towards Ru(II), Rh(III) and Ir(III) complexes and their reactivity studies with azides and activated alkynes. Organomet. Chem. 2019, 891, 54–63. [Google Scholar] [CrossRef]
- Palepu, N.R.; Premkumar, J.R.; Verma, A.K.; Bhattacharjee, K.; Joshi, S.R.; Forbes, S.; Mozharivskyj, Y.K.; Rao, M. Antibacterial, in vitro antitumor activity and structural studies of rhodium and iridium complexes featuring the two positional isomers of pyridine carbaldehyde picolinic hydrazone ligand. Arab. J. Chem. 2018, 11, 714–728. [Google Scholar] [CrossRef]
- Pal, P.; Das, K.; Hossain, A.; Frontera, A.; Mukhopadhyay, S. Supramolecular and theoretical perspectives of 2,2′:6′,2′′-terpyridine based Ni(II) and Cu(II) complexes: On the importance of C–H⋯Cl and π⋯π interactions. New J. Chem. 2020, 44, 7310–7318. [Google Scholar] [CrossRef]
- Pramanik, S.; Pathak, S.; Frontera, A.; Mukhopadhyay, S. Syntheses, crystal structures and supramolecular assemblies of two Cu(II) complexes based on a new heterocyclic ligand: Insights into C–H⋯Cl and π⋯π interactions. Cryst. Eng. Comm. 2022, 24, 1598–1611. [Google Scholar] [CrossRef]
- Geldmacher, Y.; Kitanovic, I.; Alborzinia, H.; Bergerhoff, K.; Rubbiani, R.; Wefelmeier, P.; Prokop, A.; Gust, R.; Ott, I.; Wölfl, S.; et al. Cellular Selectivity and Biological Impact of Cytotoxic Rhodium (III) and Iridium (III) Complexes Containing Methyl-Substituted Phenanthroline Ligands. Chem. Med. Chem. 2011, 6, 429–439. [Google Scholar] [CrossRef]
- Khan, T.M.; Gul, N.S.; Lu, X.; Kumar, R.; Choudhary, M.I.; Liang, H.; Chen, Z.-F. Rhodium (III) complexes with isoquinoline derivatives as potential anticancer agents: In vitro and in vivo activity studies. Dalton Trans. 2019, 48, 11469–11479. [Google Scholar] [CrossRef]
- Skarka, A.; Škarydová, L.; Štambergová, H.; Wsól, V. Anthracyclines and their metabolism in human liver microsomes and the participation of the new microsomal carbonyl reductase. Chem-Biol. Interact. 2011, 191, 66–74. [Google Scholar] [CrossRef]
- Gothe, Y.; Marzo, T.; Messori, L.; Metzler-Nolte, N. Iridium(I) Compounds as Prospective Anticancer Agents: Solution Chemistry, Antiproliferative Profiles and Protein Interactions for a Series of Iridium(I) N-Heterocyclic Carbene Complexes. Chem. Eur. J. 2016, 22, 12487–12494. [Google Scholar] [CrossRef] [PubMed]
- Bachur, N.R. Encyclopedia of Cancer, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 57–61. [Google Scholar]
- Zhang, G.; Guo, J.; Zhao, N.; Wang, J. Study of interaction between kaempferol–Eu3+ complex and DNA with the use of the Neutral Red dye as a fluorescence probe. Sensor Actuat. B Chem. 2010, 144, 239–246. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; pp. 278–280. [Google Scholar]
- Liu, R.-X.; Wu, Y.-S.; Liu, Y.-C.; Luo, R.-Y.; Yang, L.-D.; Tang, M.-T.; Chen, Z.-F.; Liang, H. New anthrahydrazone derivatives and their cisplatin-like complexes: Synthesis, antitumor activity and structure–activity relationship. New J. Chem. 2019, 43, 18685–18694. [Google Scholar] [CrossRef]
- The MTT Assay Protocol for Tumor Cell Proliferation Is Available on the Web Site. Available online: https://www.thermofisher.cn/cn/zh/home/references/protocols/cell-and-tissue-analysis/protocols/vybrant-mtt-cell-proliferation-assay-protocol.html (accessed on 15 October 2022).


| Empirical Formula | C23H26Cl3N4O2RhS2 |
|---|---|
| Formula weight | 663.86 |
| Temperature/K | 296.15 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å, b/Å, c/Å | 11.573(5), 13.309(5), 17.577(7) |
| α/°, β/°, γ/° | 90.00, 94.955(6), 90.00 |
| Volume/Å3 | 2697.2(18) |
| Z | 4 |
| ρcalcg/cm3 | 1.635 |
| μ/mm−1 | 1.114 |
| F(000) | 1344.0 |
| Crystal size/mm3 | 0.41 × 0.23 × 0.12 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 3.84 to 52.74 |
| Index ranges | −14 ≤ h ≤ 14, −16 ≤ k ≤ 16, −20 ≤ l ≤ 21 |
| Reflections collected | 29946 |
| Independent reflections | 5478 [Rint = 0.0310, Rsigma = 0.0225] |
| Data/restraints/parameters | 5478/0/323 |
| Goodness-of-fit on F2 | 1.082 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0285, wR2 = 0.0684 |
| Final R indexes [all data] | R1 = 0.0363, wR2 = 0.0729 |
| Largest diff. peak/hole / e Å−3 | 0.77/−0.33 |
| Selected bond (Å) | |||
| Rh1-Cl1 | 2.3437(12) | Rh1-S1 | 2.2869(11) |
| Rh1-Cl2 | 2.3437(12) | Rh1-N1 | 2.118(2) |
| Rh1-Cl3 | 2.3320(11) | Rh1-N3 | 2.063(2) |
| Selected angles (°) | |||
| Cl2-Rh1-Cl1 | 93.45(3) | S1-Rh1-Cl1 | 91.07(3) |
| Cl3-Rh1-Cl1 | 174.79(3) | S1-Rh1-Cl2 | 84.14(3) |
| Cl3-Rh1-Cl2 | 90.62(3) | S1-Rh1-Cl3 | 92.58(3) |
| N1-Rh1-Cl1 | 90.22(6) | N1-Rh1-Cl3 | 85.95(6) |
| N1-Rh1-Cl2 | 98.47(6) | N1-Rh1-S1 | 177.01(6) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N2—H2···O2 i | 0.86 | 1.88 | 2.733(3) | 174 |
| C18—H18···Cl1 ii | 0.93 | 2.78 | 3.600(4) | 148 |
| C23—H23C···Cl3 iii | 0.96 | 2.83 | 3.713(4) | 154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Tang, Z.; Liu, R.; Liu, Y.; Liang, H.; Liu, Y. (Z)-2-(2-(Anthracen-9-ylmethylene)hydrazineyl)pyrimidine-rhodium(III): Crystal Structure and DNA Binding Property. Crystals 2022, 12, 1664. https://doi.org/10.3390/cryst12111664
Deng Q, Tang Z, Liu R, Liu Y, Liang H, Liu Y. (Z)-2-(2-(Anthracen-9-ylmethylene)hydrazineyl)pyrimidine-rhodium(III): Crystal Structure and DNA Binding Property. Crystals. 2022; 12(11):1664. https://doi.org/10.3390/cryst12111664
Chicago/Turabian StyleDeng, Qianjun, Zitian Tang, Ruixue Liu, Yuan Liu, Huaxuan Liang, and Yancheng Liu. 2022. "(Z)-2-(2-(Anthracen-9-ylmethylene)hydrazineyl)pyrimidine-rhodium(III): Crystal Structure and DNA Binding Property" Crystals 12, no. 11: 1664. https://doi.org/10.3390/cryst12111664
APA StyleDeng, Q., Tang, Z., Liu, R., Liu, Y., Liang, H., & Liu, Y. (2022). (Z)-2-(2-(Anthracen-9-ylmethylene)hydrazineyl)pyrimidine-rhodium(III): Crystal Structure and DNA Binding Property. Crystals, 12(11), 1664. https://doi.org/10.3390/cryst12111664








