Coordination Ability of 10-EtC(NHPr)=HN-7,8-C2B9H11 in the Reactions with Nickel(II) Phosphine Complexes
Abstract
1. Introduction
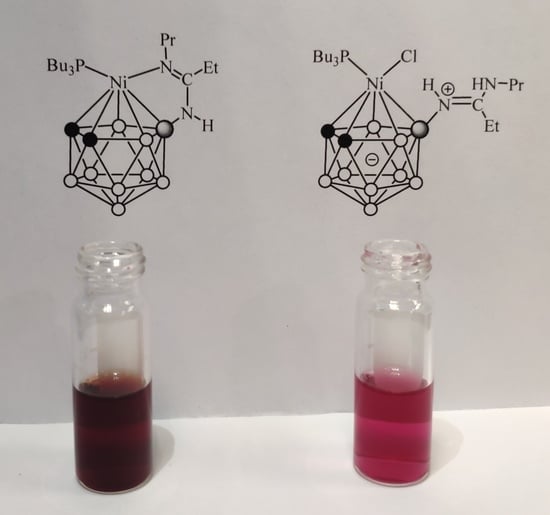
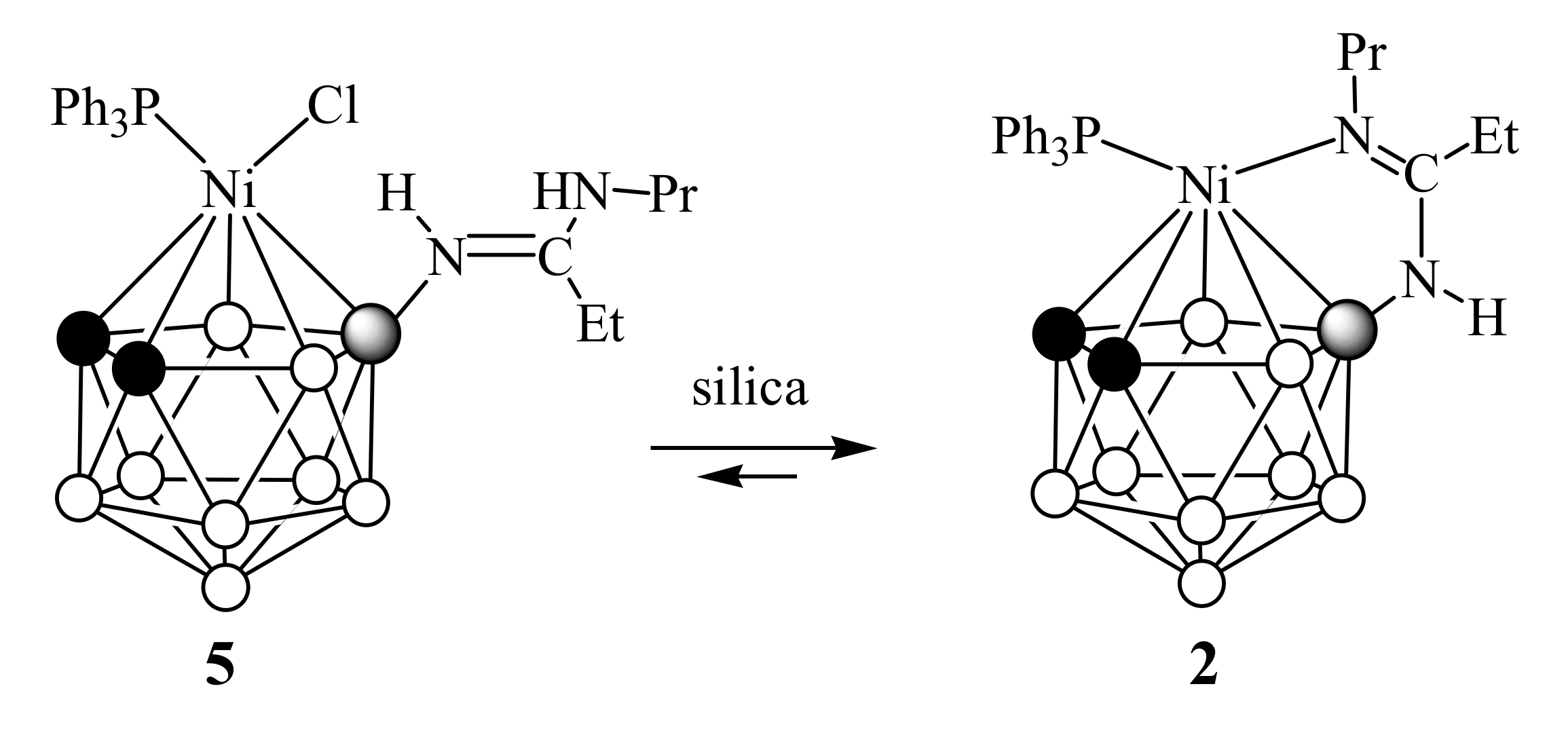
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Reagents and Methods
4.2. Synthesis of [3-Ph3P-3-(8-PrN=C(Et)NH)-closo-3,1,2-NiC2B9H10] (2)
4.3. Synthesis of [3-PhMe2P-3-(8-PrN=C(Et)NH)-closo-3,1,2-NiC2B9H10] (3)
4.4. Synthesis of [3-Bu3P-3-(8-PrN=C(Et)NH)-closo-3,1,2-NiC2B9H10] (4)
4.5. General Procedure for the Synthesis of [3-Cl-3-R’R2P-8-PrN=C(Et)NH-closo-3,1,2-NiC2B9H10] (5–7)
4.6. Single Crystal X-ray Diffraction Study
4.7. Quantum Chemical Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimes, R.N. Transitional metal metallacarbaboranes. In Comprehensive Organometallic Chemistry II; Elsevier: Oxford, UK, 1995; Volume 1, pp. 373–430. [Google Scholar]
- Grimes, R.N. Metallacarboranes in the new millennium. Coord. Chem. Rev. 2000, 200, 773–811. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Maguire, J.A. Metallacarboranes of d- and f-block metals. In Comprehensive Organometallic Chemistry III; Elsevier: Oxford, UK, 2007; Volume 3, pp. 175–264. [Google Scholar]
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992, 92, 209–223. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Yulin, Z.; Carpenter, K.; Maguire, J.A.; Hosmane, N.S. Synthesis and catalytic activities of Group 4 metal complexes derived from C(cage)-appended cyclohexyloxocarborane trianion. J. Organomet. Chem. 2005, 690, 2802–2808. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Lo Pei Sia, S.; Kooli, F.; Carpenter, K.; Kemp, R.A. Another example of carborane based trianionic ligand: Syntheses and catalytic activities of cyclohexylamino tailed ortho-carboranyl zirconium and titanium dicarbollides. J. Organomet. Chem. 2005, 690, 6284–6291. [Google Scholar] [CrossRef]
- Shen, H.; Chan, H.-S.; Xie, Z. Guanylation of amines catalyzed by a half-sandwich titanacarborane amide complex. Organometallics 2006, 25, 5515–5517. [Google Scholar] [CrossRef]
- Gao, M.; Tang, Y.; Xie, M.; Qian, C.; Xie, Z. Synthesis, structure, and olefin polymerization behavior of constrained-geometry Group 4 metallacarboranes incorporating imido-dicarbollyl ligands. Organometallics 2006, 25, 2578–2584. [Google Scholar] [CrossRef]
- Shen, H.; Xie, Z. Titanacarborane Amide catalyzed transamination of guanidines. Organometallics 2008, 27, 2685–2687. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Hosmane, N.S. Carborane-based transitional metal complexes and their catalytic application for olefin polymerization: Current and future perspectives. J. Organomet. Chem. 2013, 747, 25–29. [Google Scholar] [CrossRef]
- Sivaev, I.B. Ferrocene and transition metal bis(dicarbollides) as platform for design of rotatory molecular switches. Molecules 2017, 22, 2201. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. bis(dicarbollide) complexes of transition metals as a platform for molecular switches. Study of complexation of 8,8’-bis(methylsulfanyl) derivatives of cobalt and iron bis(dicarbollides). Molecules 2020, 25, 5745. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chan, H.-S.; Xie, Z. Synthesis, structure, and reactivity of [σ:η1:η5-(OCH2)(Me2NCH2)C2B9H9]Ti(NR2) (R = Me, Et). Organometallics 2007, 26, 2694–2704. [Google Scholar] [CrossRef]
- Lee, J.-D.; Lee, Y.-J.; Son, K.-C.; Han, W.-S.; Cheong, M.; Ko, J.; Kang, S.O. Synthesis, characterization, and reactivity of new types of constrained geometry Group 4 metal complexes derived from picolyl-substituted dicarbollide ligand systems. J. Organomet. Chem. 2007, 692, 5403–5413. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Nakamura, H. Two possible ways to combine boron and gadolinium for Gd-quided BNCT. A concept. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 910–917. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.-H.; Kim, S.-J.; Ko, J.; Kim, S.H.; Cho, S.; Lee, C.-H.; Kang, S.O. Preparation and reactions of a half-sandwich dicarbollyl nickel(II) complexes containing a dimethylamino pendent group. Organometallics 2001, 20, 4483–4491. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.-H.; Ko, J.; Kim, S.H.; Cho, S.; Lee, C.-H.; Kang, S.O. Half-sandwich iron(II) and ruthenium(II) complexes with the dicarbollylamino ligand system. Organometallics 2001, 20, 4632–4640. [Google Scholar] [CrossRef]
- Kim, D.-H.; Won, J.H.; Kim, S.-J.; Ko, J.; Kim, S.H.; Cho, S.; Kang, S.O. Dicarbollide analogues of the constrained-geometry polymerization catalyst. Organometallics 2001, 20, 4298–4300. [Google Scholar] [CrossRef]
- Lee, J.-D.; Lee, Y.-J.; Son, K.-C.; Cheong, M.; Ko, J.; Kang, S.O. New types of constrained geometry Group 4 metal complexes derived from the aminomethyldicarbollyl ligand system: Synthesis and structural characterization of mono-dicarbollylamino and bis-dicarbollylamino Group 4 metal complexes. Organometallics 2007, 26, 3374–3384. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Godovikov, I.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl amidines. J. Organomet. Chem. 2020, 909, 121111. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Bregadze, V.I. Nucleophilic addition reactions to the ethylnitrilium derivative of nido-carborane 10-EtC≡N-7,8-C2B9H11. New J. Chem. 2018, 42, 17958–17967. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10-NCCH2CH2OCH2CH2C≡N- 7,8-C2B9H11: Synthesis and reactions with various nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Clavier, H.; Nolan, S.P. Percent buried volume for phosphine and N-heterocyclic carbene ligands: Steric properties in organometallic chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef]
- Hunter, A.D.; Williams, T.R.; Zarzyczny, B.M.; Bottesch, H.W.; Dolan, S.A.; McDowell, K.A.; Thomas, D.N.; Mahler, C.H. Correlations among 31P NMR coordination chemical shifts, Ru–P bond distances, and enthalpies of reaction in Cp′Ru(PR3)2Cl complexes (Cp′ = η5-C5H5, η5-C5Me5; PR3 = PMe3, PPhMe2, PPh2Me, PPh3, PEt3, PnBu3). Organometallics 2016, 35, 2701–2706. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Jin, G.-X. Synthesis, reactivity, and structural transformation of mono- and binuclear carboranylamidinate-based 3d metal complexes and metallacarborane derivatives. Organometallics 2012, 31, 1767–1774. [Google Scholar] [CrossRef]
- Erdman, A.A.; Zubreichuk, Z.P.; Knizhnikov, V.A.; Maier, A.A.; Aleksandrov, G.G.; Nefedov, S.E.; Eremenko, I.L. Synthesis and the structure of the triphenylphosphine complex of o-nickelacarborane, 3,3-(PPh3)2-3,1,2-NiC2B9H11. Russ. Chem. Bull. 2001, 50, 2248–2250. [Google Scholar] [CrossRef]
- Standley, E.A.; Smith, S.J.; Müller, P.; Jamison, T.F. A broadly applicable strategy for entry into homogeneous nickel(0) catalysts from air-stable nickel(II) complexes. Organometallics 2014, 33, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Burlington, MA, USA, 2009. [Google Scholar]
- Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B-H···π Interactions between the B-H-B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 4436–4443. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]-. Some evidences of BH···X hydride-halogen bonds in 9- XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849-850, 315–323. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Tafur, S.; Masunov, A.E. Applicability of hybrid density functional theory methods to calculation of molecular hyperpolarizability. J. Chem. Phys. 2008, 129, 044109. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Masunov, A.E.; Antipin, M.Y. Conformational dependence of the first molecular hyperpolarizability in the computational design of nonlinear optical materials for optical switching. Mendeleev. Commun. 2008, 18, 265–267. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Lyssenko, K.A. Analysis of supramolecular architectures: Beyond molecular packing diagrams. Mendeleev. Commun. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Lyssenko, K.A.; Ananyev, I.V.; Kozeev, A.M.; Sheremetev, A.B. Role of weak intermolecular interactions in the crystal structure of tetrakis-furazano[3,4-c:3′,4′-g:3″,4″-k:3"’,4"’-o][1,2,5,6,9,10,13,14]octaazacyclohexadecine and its solvates. Cryst. Growth Des. 2014, 14, 4439–4449. [Google Scholar] [CrossRef]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The influence of halogen type on structural features of compounds containing α-halo-α,α-dinitroethyl moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]

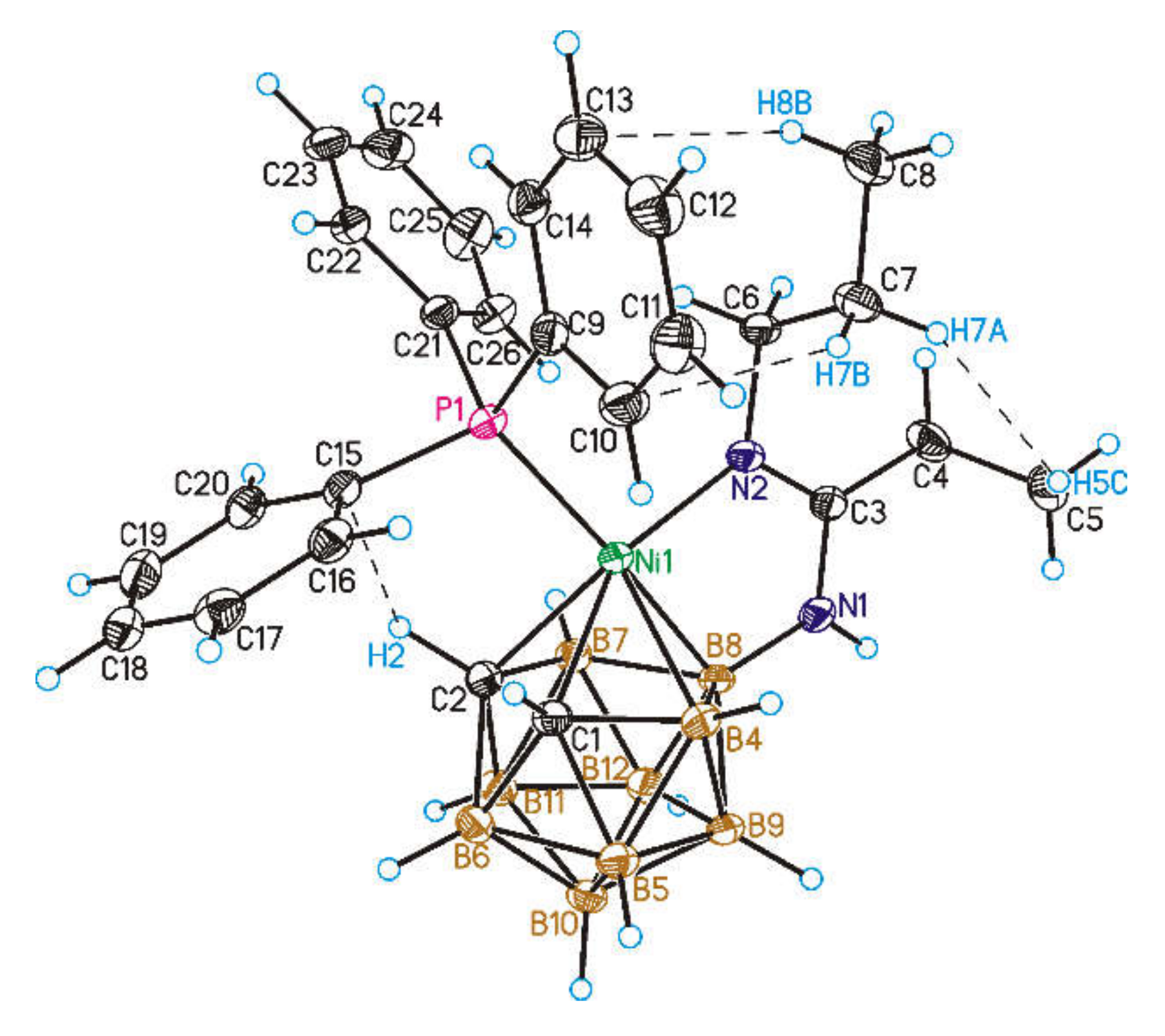


| Structure Parameters | Compound 2 (X-ray) | Compound 2 * | Compound 2 (calc) * |
|---|---|---|---|
| Ni-C2B3(centroid) | 1.526 (2) | 1.533 (2) | 1.545 |
| C2-Ni1-P1-C9 | 143.7 (2) | 128.3 (2) | 125.5 |
| C2-Ni1-P1-C15 | 26.3 (2) | 8.3 (2) | 8.0 |
| C2-Ni1-P1-C21 | −93.3 (2) | −108.9 (2) | −110.5 |
| B8-N1-C3-C4 | −179.0 (2) | −174.1 (2) | 172.0 |
| N1-C3-C4-C5 | −63.2 (2) | 100.0 (2) | −62.1 |
| Ni1-N2-C6-C7 | 104.7 (2) | 85.1 (2) | 95.9 |
| H2…C15 | 2.66 | 2.54 | 2.45 (−2.5) |
| H7B…C10 | 2.76 | 2.75 | 2.74 (−1.1) |
| H8B…C13 | 3.04 | - | 2.98 (−0.7) |
| H5C…H7A | 2.37 | 2.28 (H5C…H6B) | 2.30 (−1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Coordination Ability of 10-EtC(NHPr)=HN-7,8-C2B9H11 in the Reactions with Nickel(II) Phosphine Complexes. Crystals 2021, 11, 306. https://doi.org/10.3390/cryst11030306
Stogniy MY, Erokhina SA, Suponitsky KY, Sivaev IB, Bregadze VI. Coordination Ability of 10-EtC(NHPr)=HN-7,8-C2B9H11 in the Reactions with Nickel(II) Phosphine Complexes. Crystals. 2021; 11(3):306. https://doi.org/10.3390/cryst11030306
Chicago/Turabian StyleStogniy, Marina Yu., Svetlana A. Erokhina, Kyrill Yu. Suponitsky, Igor B. Sivaev, and Vladimir I. Bregadze. 2021. "Coordination Ability of 10-EtC(NHPr)=HN-7,8-C2B9H11 in the Reactions with Nickel(II) Phosphine Complexes" Crystals 11, no. 3: 306. https://doi.org/10.3390/cryst11030306
APA StyleStogniy, M. Y., Erokhina, S. A., Suponitsky, K. Y., Sivaev, I. B., & Bregadze, V. I. (2021). Coordination Ability of 10-EtC(NHPr)=HN-7,8-C2B9H11 in the Reactions with Nickel(II) Phosphine Complexes. Crystals, 11(3), 306. https://doi.org/10.3390/cryst11030306