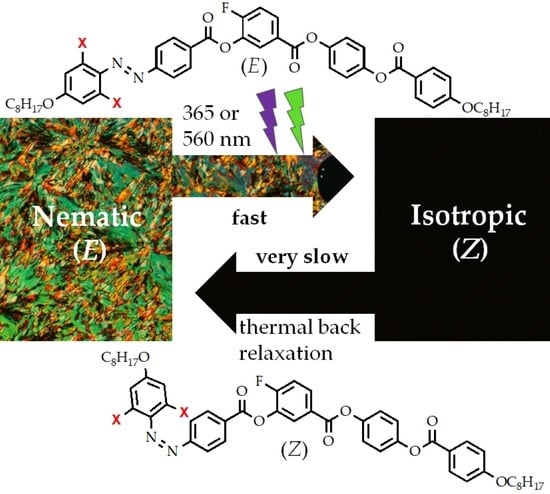
Photosensitive Bent-Core Liquid Crystals with Laterally Substituted Azobenzene Unit
Abstract
:1. Introduction
2. Materials and Methods
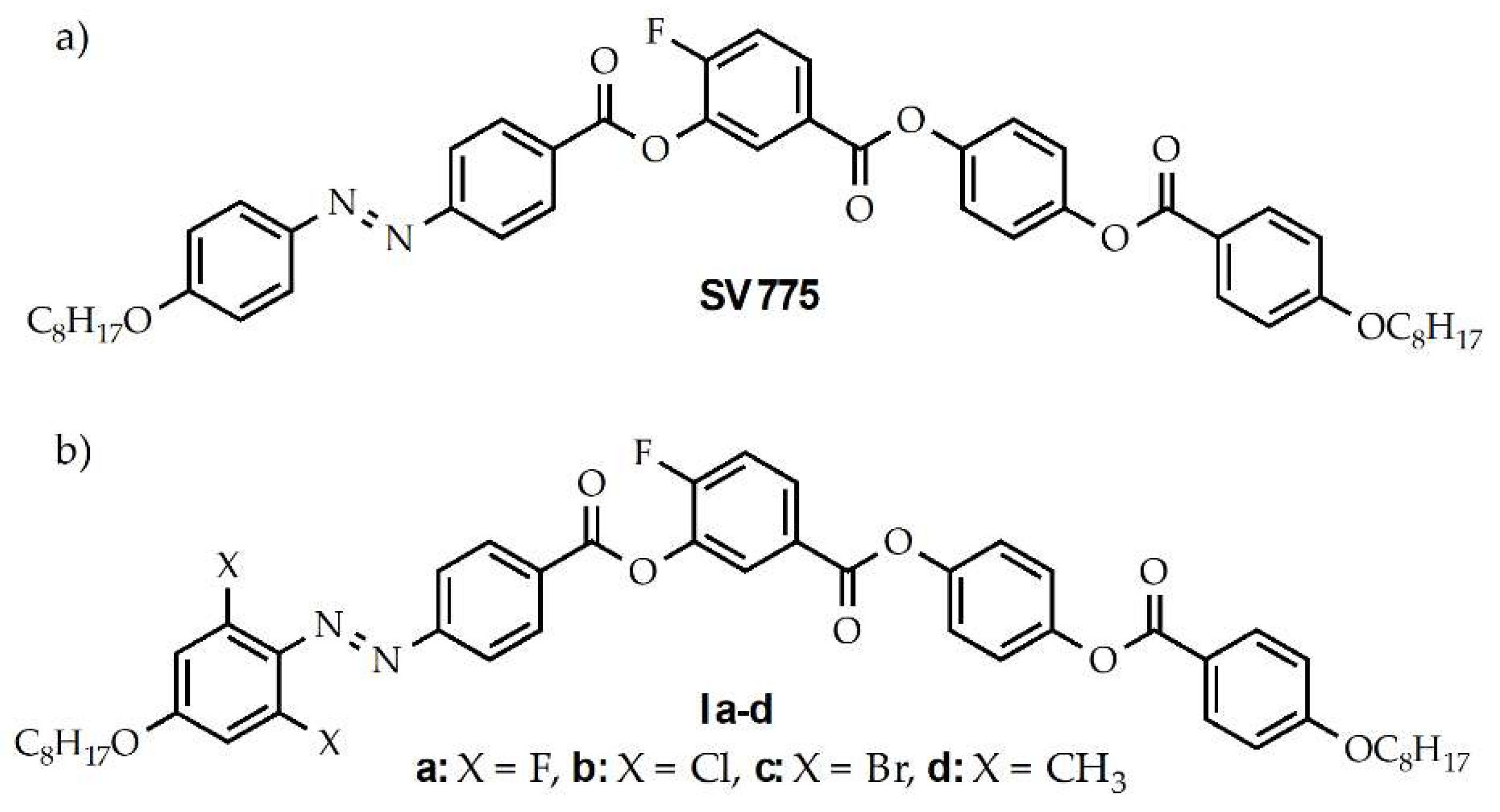
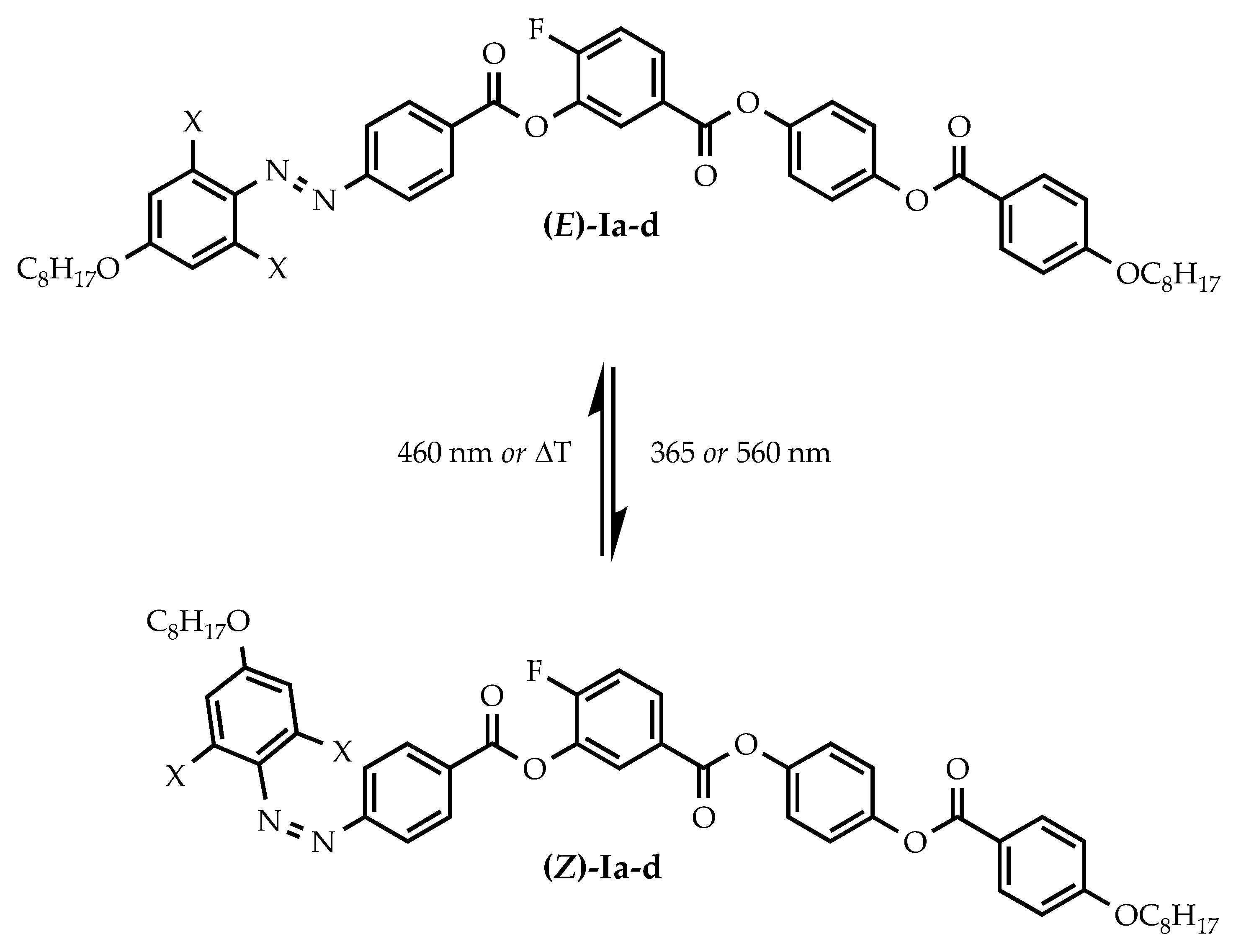
2.1. Design and Synthesis of Materials
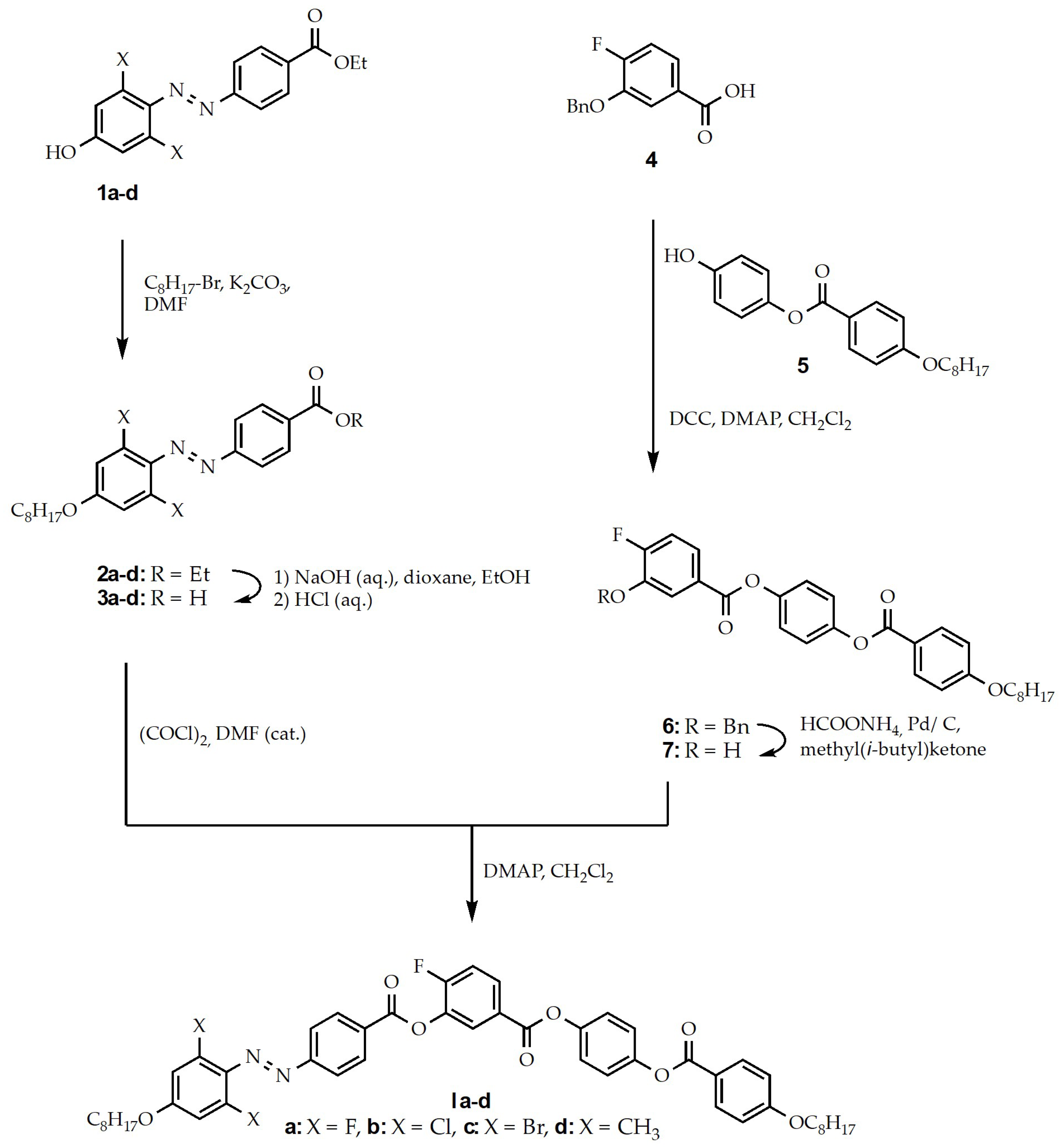
2.2. Synthesis and Characterization
2.3. Characterization of Mesomorphic and Photochemical Behavior
3. Results and Discussion
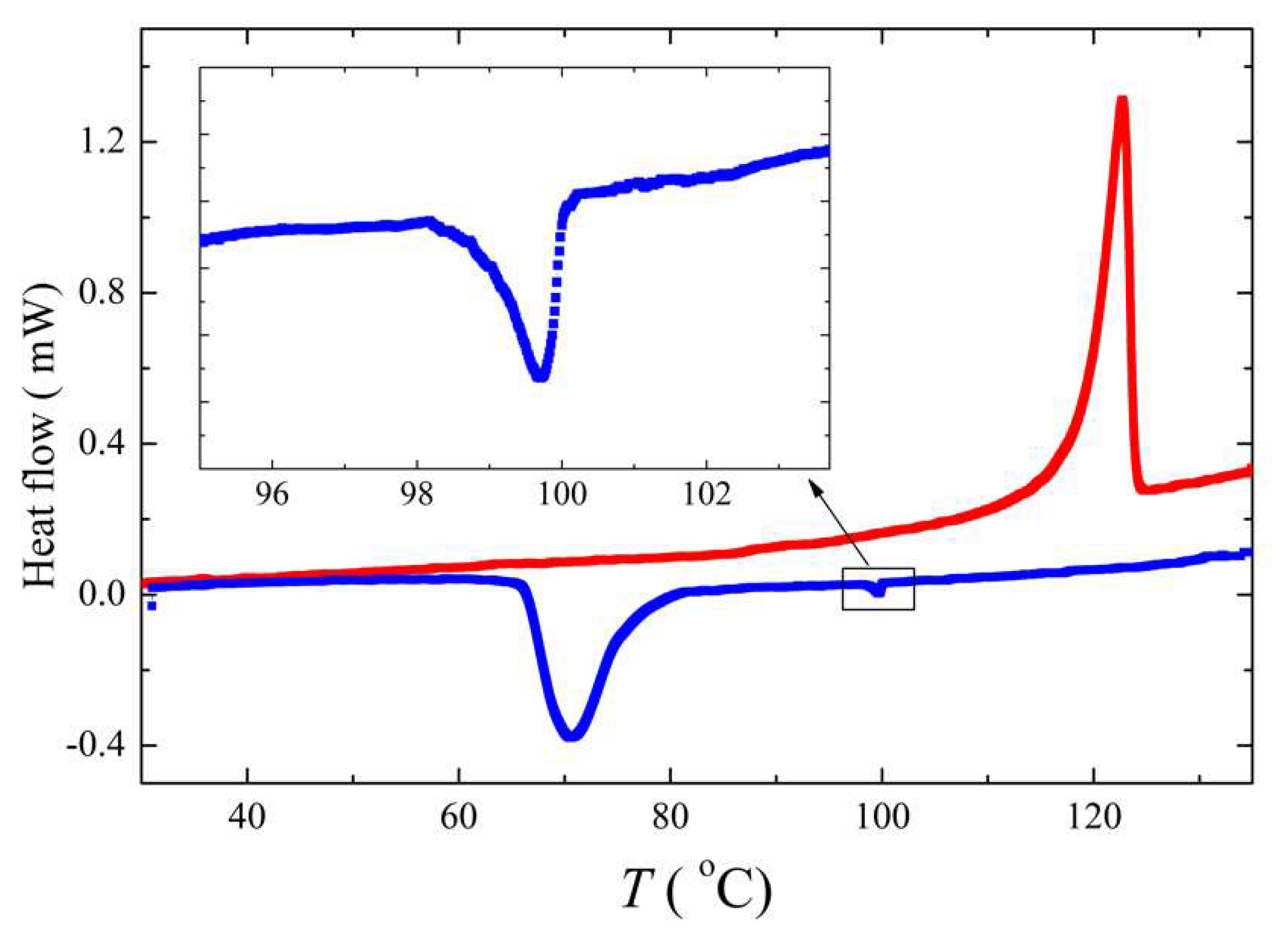
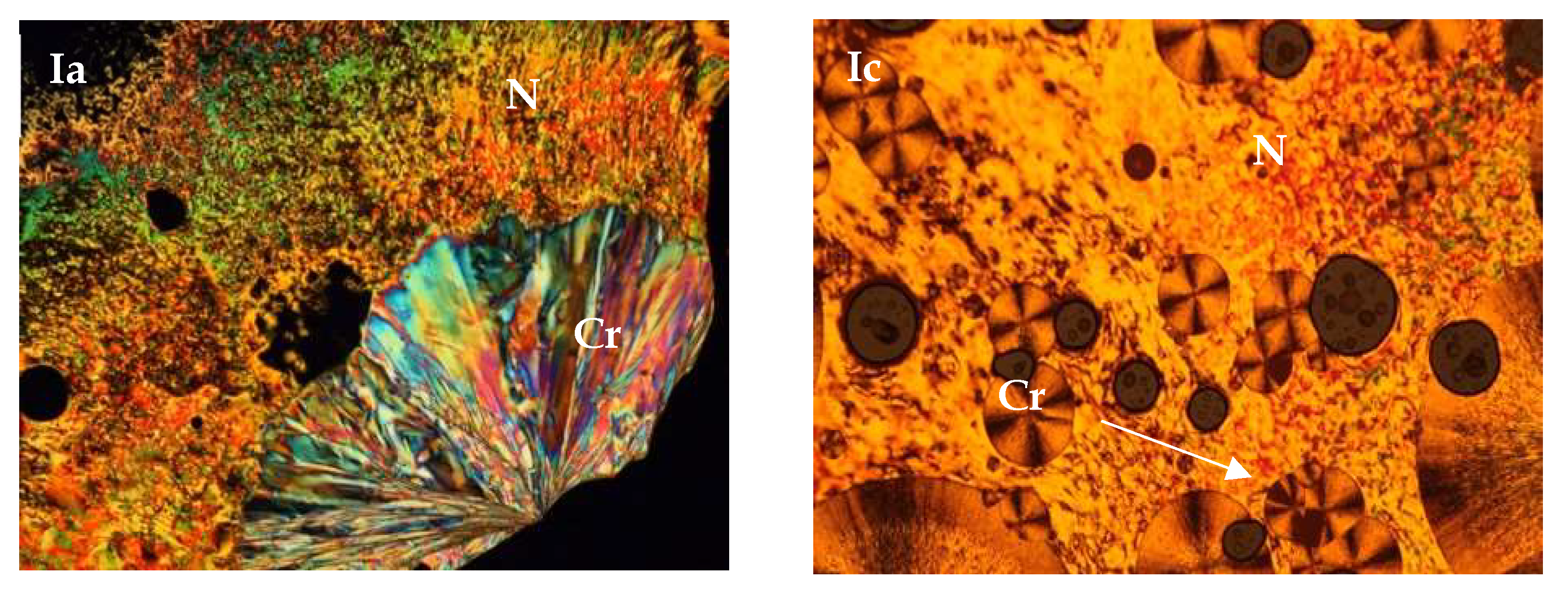
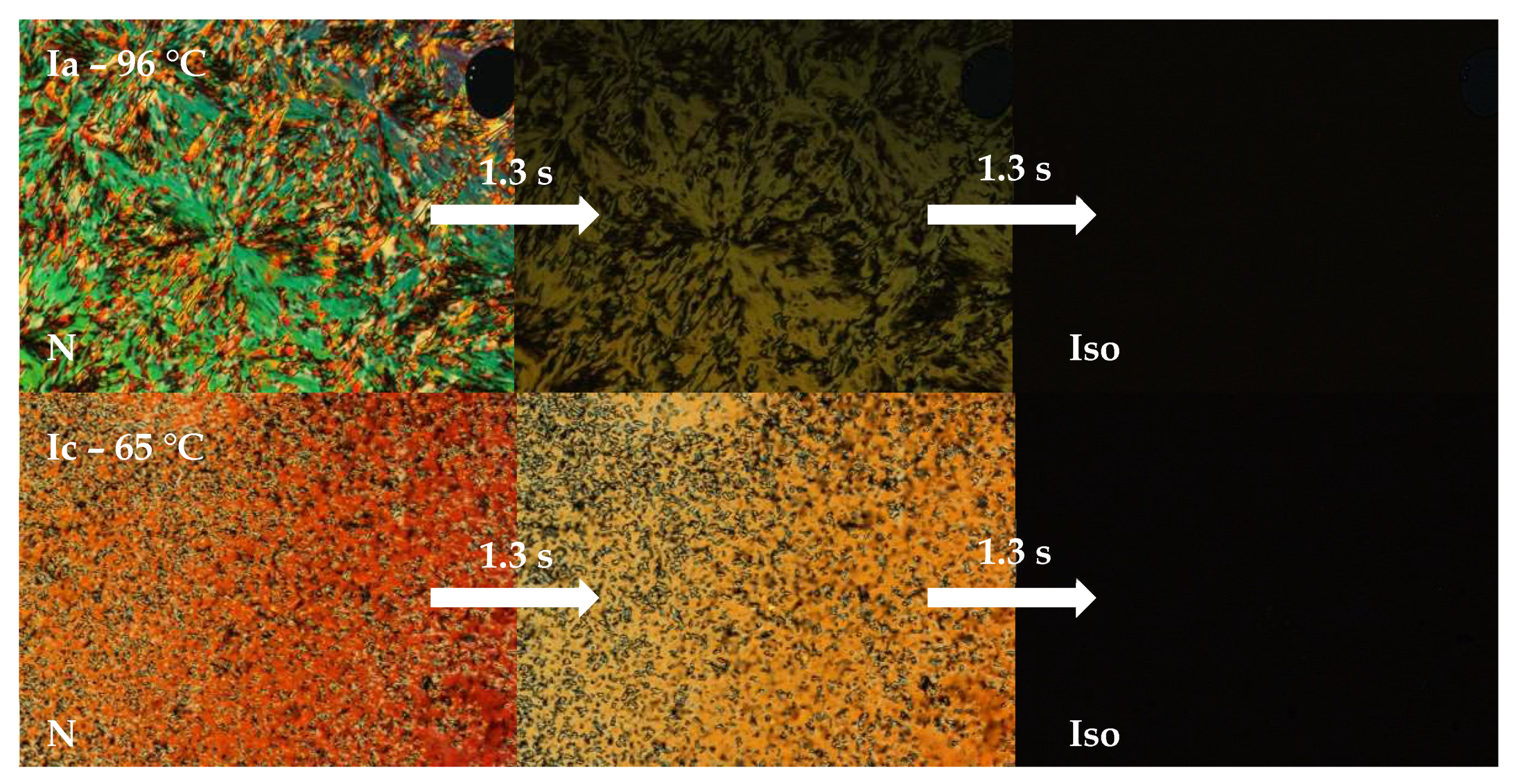
3.1. Mesomorphic Properties
3.2. Photosensitivity of the Materials
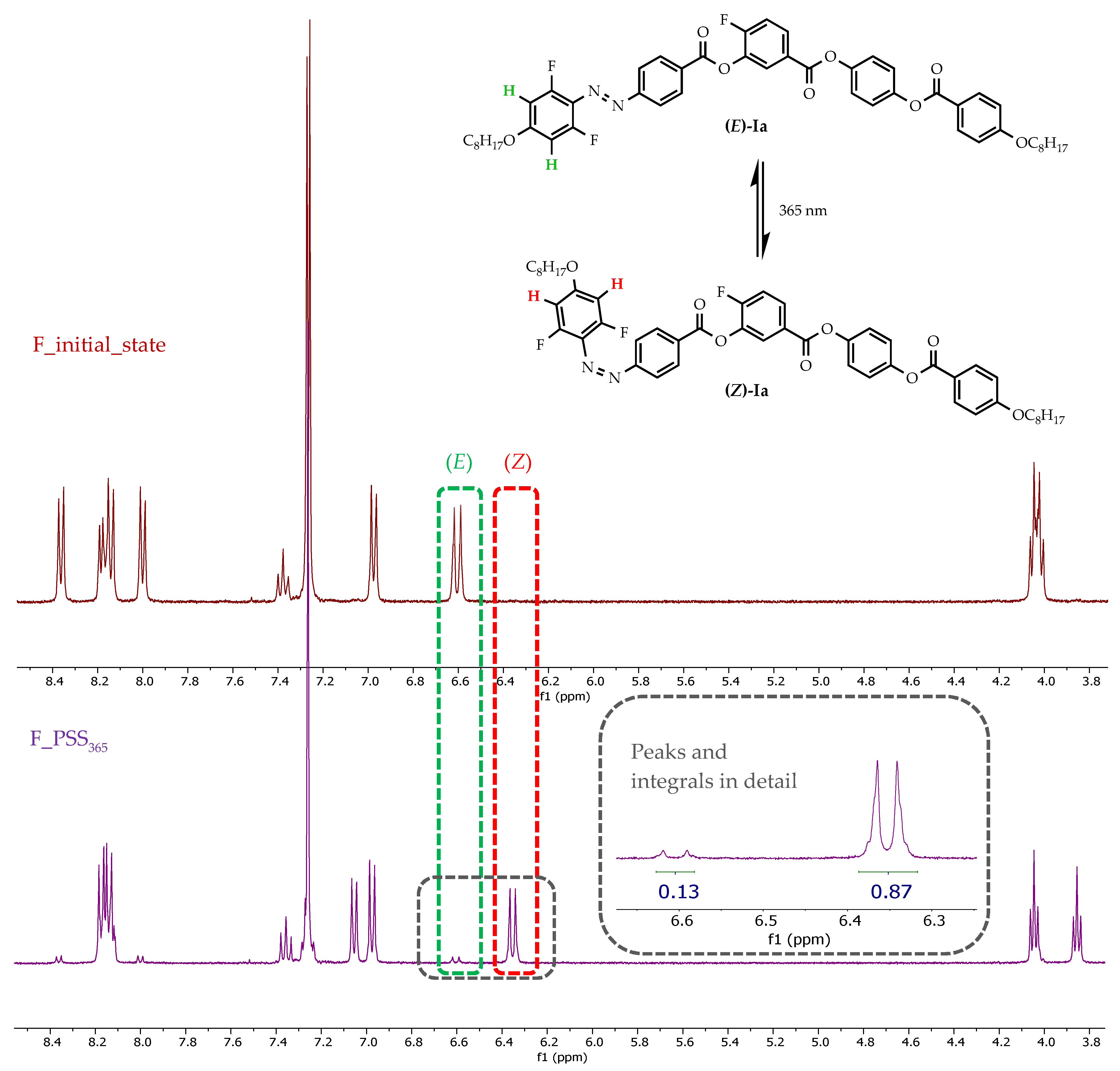
3.2.1. Photo-Response in Solution
3.2.2. Photo-Response in Mesophase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierste, C.; Gleeson, H.; Raynes, P.; Vill, V. (Eds.) Handbook of Liquid Crystals; Wiley-VCH Verlag: Weinheim, Germany, 2014. [Google Scholar]
- Jing, H.; Xu, M.; Xiang, Y.; Wang, E.; Liu, D.; Poryvai, A.; Kohout, M.; Éber, N.; Buka, Á. Light Tunable Gratings Based on Flexoelectric Effect in Photoresponsive Bent-Core Nematics. Adv. Opt. Mater. 2019, 7, 1801790. [Google Scholar] [CrossRef]
- Asquini, R.; d’Alessandro, A. Tunable Photonic Devices Based on Liquid Crystals and Composites; SPIE: San Diego, CA, USA, 2013; Volume 8828. [Google Scholar]
- Kawata, S.; Kawata, Y. Three-Dimensional Optical Data Storage Using Photochromic Materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Petsch, S.; Rix, R.; Khatri, B.; Schuhladen, S.; Müller, P.; Zentel, R.; Zappe, H. Smart artificial muscle actuators: Liquid crystal elastomers with integrated temperature feedback. Sens. Actuators A Phys. 2015, 231, 44–51. [Google Scholar] [CrossRef]
- Zeng, H.; Wani, O.M.; Wasylczyk, P.; Kaczmarek, R.; Priimagi, A. Self-Regulating Iris Based on Light-Actuated Liquid Crystal Elastomer. Adv. Mater. 2017, 29, 1701814. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M. Azobenzene-containing bent-core liquid crystals: An overview. Liq. Cryst. 2016, 43, 2208–2243. [Google Scholar] [CrossRef]
- Poryvai, A.; Bubnov, A.; Kohout, M. Chiral Photoresponsive Liquid Crystalline Materials Derived from Cyanoazobenzene Central Core: Effect of UV Light Illumination on Mesomorphic Behavior. Crystals 2020, 10, 1161. [Google Scholar] [CrossRef]
- Poryvai, A.; Bubnov, A.; Pociecha, D.; Svoboda, J.; Kohout, M. The effect of the length of terminal n-alkyl carboxylate chain on self-assembling and photosensitive properties of chiral lactic acid derivatives. J. Mol. Liq. 2019, 275, 829–838. [Google Scholar] [CrossRef]
- Šmahel, M.; Poryvai, A.; Xiang, Y.; Pociecha, D.; Troha, T.; Novotná, V.; Svoboda, J.; Kohout, M. Photosensitive bent-core nematic liquid crystals with various linking units in the side arms: Structure-properties relationships. J. Mol. Liq. 2020, 306, 112743. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Leistner, A.-L.; Kirchner, S.; Karcher, J.; Bantle, T.; Schulte, M.L.; Gödtel, P.; Fengler, C.; Pianowski, Z.L. Fluorinated Azobenzenes Switchable with Red Light. Chemistry 2021, 27, 8094–8099. [Google Scholar] [CrossRef]
- Cigl, M.; Bubnov, A.; Kašpar, M.; Hampl, F.; Hamplová, V.; Pacherová, O.; Svoboda, J. Photosensitive chiral self-assembling materials: Significant effects of small lateral substituents. J. Mater. Chem. C 2016, 4, 5326–5333. [Google Scholar] [CrossRef]
- Prasad, V.; Nagendrappa Gowdru, N.; Manjunath, M. Thermally stable azo-substituted bent-core nematogens: Observation of chiral domains in the nematic mesophases composed of smectic nano clusters. Liq. Cryst. 2018, 45, 666–679. [Google Scholar] [CrossRef]
- Hrozhyk, U.; Serak, S.; Tabiryan, N.; Bunning, T.J. Wide Temperature Range Azobenzene Nematic and Smectic LC Materials. Mol. Cryst. Liq. Cryst. 2006, 454, 235–245. [Google Scholar] [CrossRef]
- Nersesyan, V.; Dadalyan, T.; Beeckman, J.; Beunis, F.; Neyts, K.; Drampyan, R. Refractive Bessel lattice in azobenzene liquid crystal. J. Mod. Opt. 2018, 65, 2034–2043. [Google Scholar] [CrossRef]
- Serak, S.V.; Tabiryan, N.V.; Assanto, G. Nematicons in Azobenzene Liquid Crystals. Mol. Cryst. Liq. Cryst. 2012, 559, 202–213. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D. Non-symmetrical bent-shaped liquid crystals based on a laterally substituted naphthalene central core with four ester groups. Liq. Cryst. 2011, 38, 1099–1110. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D.; Glogarová, M.; Gorecka, E. A nematic-polar columnar phase sequence in new bent-shaped liquid crystals based on a 7-hydroxynaphthalene-2-carboxylic acid core. J. Mater. Chem. 2009, 19, 3153–3160. [Google Scholar] [CrossRef]
- Trišović, N.; Matović, L.; Tóth-Katona, T.; Saha, R.; Jákli, A. Mesomorphism of novel stilbene-based bent-core liquid crystals. Liq. Cryst. 2021, 48, 1054–1064. [Google Scholar] [CrossRef]
- Tschierske, C.; Photinos, D.J. Biaxial nematic phases. J. Mater. Chem. 2010, 20, 4263–4294. [Google Scholar] [CrossRef]
- Vanakaras, A.G.; Photinos, D.J. Thermotropic biaxial nematic liquid crystals: Spontaneous or field stabilized? J. Chem. Phys. 2008, 128, 154512. [Google Scholar] [CrossRef]
- Peroukidis, S.D.; Karahaliou, P.K.; Vanakaras, A.G.; Photinos, D.J. Biaxial nematics: Symmetries, order domains and field-induced phase transitions. Liq. Cryst. 2009, 36, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Madsen, L.A.; Dingemans, T.J.; Nakata, M.; Samulski, E.T. Thermotropic Biaxial Nematic Liquid Crystals. Phys. Rev. Lett. 2004, 92, 145505. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Primak, A.; Kumar, S. Biaxial Nematic Phase in Bent-Core Thermotropic Mesogens. Phys. Rev. Lett. 2004, 92, 145506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harden, J.; Mbanga, B.; Éber, N.; Fodor-Csorba, K.; Sprunt, S.; Gleeson, J.T.; Jákli, A. Giant Flexoelectricity of Bent-Core Nematic Liquid Crystals. Phys. Rev. Lett. 2006, 97, 157802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapanowski, A. Flexoelectric Effect in Biaxial Nematics. Acta Phys. Pol. A 2011, 122. [Google Scholar] [CrossRef]
- Jákli, A. Liquid crystals of the twenty-first century—Nematic phase of bent-core molecules. Liq. Cryst. Rev. 2013, 1, 65–82. [Google Scholar] [CrossRef]
- Jákli, A.; Lavrentovich, O.D.; Selinger, J.V. Physics of liquid crystals of bent-shaped molecules. Rev. Mod. Phys. 2018, 90, 045004. [Google Scholar] [CrossRef] [Green Version]
- Matharu, A.S.; Jeeva, S.; Ramanujam, P.S. Liquid crystals for holographic optical data storage. Chem. Soc. Rev. 2007, 36, 1868–1880. [Google Scholar] [CrossRef]
- Sobolewska, A.; Bartkiewicz, S.; Mysliwiec, J.; Singer, K.D. Holographic memory devices based on a single-component phototropic liquid crystal. J. Mater. Chem. C 2014, 2, 1409–1412. [Google Scholar] [CrossRef]
- Gibbons, W.M.; Kosa, T.; Palffy-Muhoray, P.; Shannon, P.J.; Sun, S.T. Continuous grey-scale image storage using optically aligned nematic liquid crystals. Nature 1995, 377, 43–46. [Google Scholar] [CrossRef]
- McPhail, D.; Gu, M. Use of polarization sensitivity for three-dimensional optical data storage in polymer dispersed liquid crystals under two-photon illumination. Appl. Phys. Lett. 2002, 81, 1160–1162. [Google Scholar] [CrossRef] [Green Version]
- Kohout, M.; Kozmík, V.; Slabochová, M.; Tůma, J.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core. Liq. Cryst. 2015, 42, 87–103. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef] [PubMed]
- Delden, R.A.v.; Mecca, T.; Rosini, C.; Feringa, B.L. A Chiroptical Molecular Switch with Distinct Chiral and Photochromic Entities and Its Application in Optical Switching of a Cholesteric Liquid Crystal. Chem. Eur. J. 2004, 10, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Moran, M.J.; Magrini, M.; Walba, D.M.; Aprahamian, I. Driving a Liquid Crystal Phase Transition Using a Photochromic Hydrazone. J. Am. Chem. Soc. 2018, 140, 13623–13627. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.; Balszuweit, J.; Saccone, M.; Wölper, C.; Doblas Jiménez, D.; Mezger, M.; Voskuhl, J.; Giese, M. Photo-switching and -cyclisation of hydrogen bonded liquid crystals based on resveratrol. Chem. Commun. 2020, 56, 1105–1108. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Ryabchun, A.; Cigl, M.; Hamplová, V.; Kašpar, M.; Hampl, F.; Shibaev, V. New azobenzene-based chiral-photochromic substances with thermally stable Z-isomers and their use for the induction of a cholesteric mesophase with a phototunable helix pitch. J. Mater. Chem. C 2014, 2, 8622–8629. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Cigl, M.; Hamplová, V.; Hampl, F.; Elyashevitch, G. Photochromic LC–polymer composites containing azobenzene chromophores with thermally stable Z-isomers. J. Mater. Chem. C 2014, 2, 4482–4489. [Google Scholar] [CrossRef]

| Material | m.p. ΔH | Cr | TCr ΔH | M2 | TTr | M1 | TTr ΔH | Iso |
|---|---|---|---|---|---|---|---|---|
| SV775 | 124 | • | 105 | SmX | 110 | N | 128 | • |
| Ia | 115 +49.8 | • | 92 −70.6 | - | - | N | 110 −1.2 | • |
| Ib | 100 +36.9 | • | 49 −15.6 | - | - | N | 73 −0.6 | • |
| Ic | 101 +34.1 | • | 59 −10.2 | - | - | N | 80 −0.58 | • |
| Id | 119 +46.4 | • | 75 −31.4 | - | - | N | 100 −0.25 | • |
| (E)/(Z) Ratios | ||||
|---|---|---|---|---|
| X | PSS365 | PSS460 | PSS560 | |
| Ia | F | 13/87 | 70/30 | 45/55 |
| Ib | Cl | 24/76 | 68/32 | 36/64 |
| Ic | Br | 52/48 | 84/16 | 64/36 |
| Id | CH3 | 36/64 | 80/20 | 70/30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jágerová, D.; Šmahel, M.; Poryvai, A.; Macháček, J.; Novotná, V.; Kohout, M. Photosensitive Bent-Core Liquid Crystals with Laterally Substituted Azobenzene Unit. Crystals 2021, 11, 1265. https://doi.org/10.3390/cryst11101265
Jágerová D, Šmahel M, Poryvai A, Macháček J, Novotná V, Kohout M. Photosensitive Bent-Core Liquid Crystals with Laterally Substituted Azobenzene Unit. Crystals. 2021; 11(10):1265. https://doi.org/10.3390/cryst11101265
Chicago/Turabian StyleJágerová, Diana, Michal Šmahel, Anna Poryvai, Jan Macháček, Vladimíra Novotná, and Michal Kohout. 2021. "Photosensitive Bent-Core Liquid Crystals with Laterally Substituted Azobenzene Unit" Crystals 11, no. 10: 1265. https://doi.org/10.3390/cryst11101265
APA StyleJágerová, D., Šmahel, M., Poryvai, A., Macháček, J., Novotná, V., & Kohout, M. (2021). Photosensitive Bent-Core Liquid Crystals with Laterally Substituted Azobenzene Unit. Crystals, 11(10), 1265. https://doi.org/10.3390/cryst11101265