Understanding the Antipathogenic Performance of Nanostructured and Conventional Copper Cold Spray Material Consolidations and Coated Surfaces
Abstract
1. Introductory Remarks
General Background
2. Materials and Methods
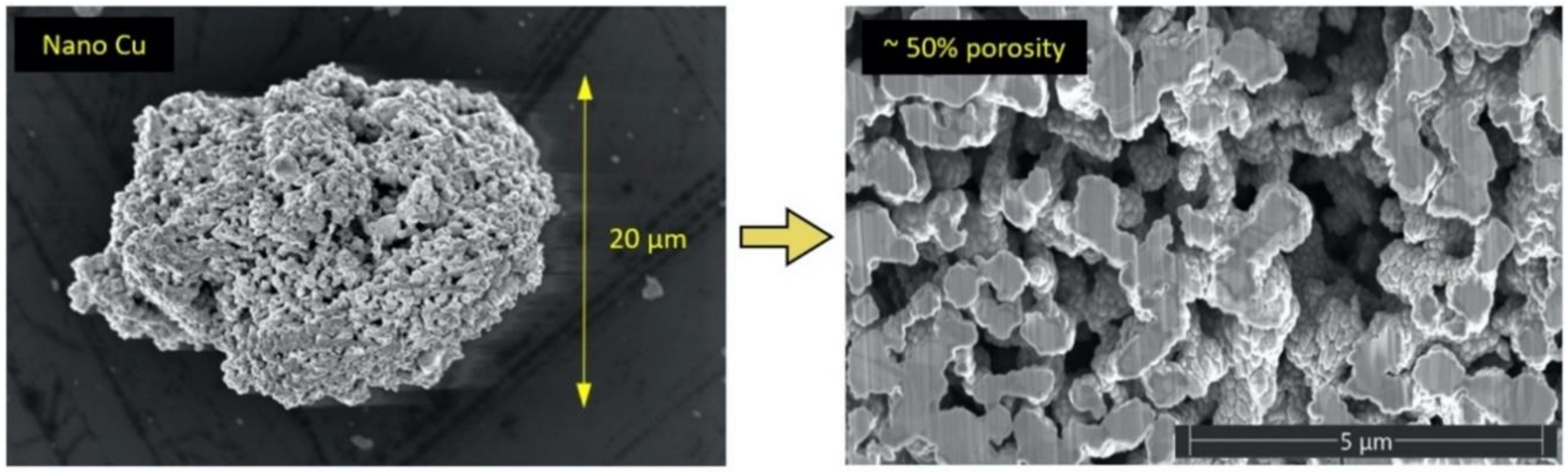
2.1. Feedstock Powders
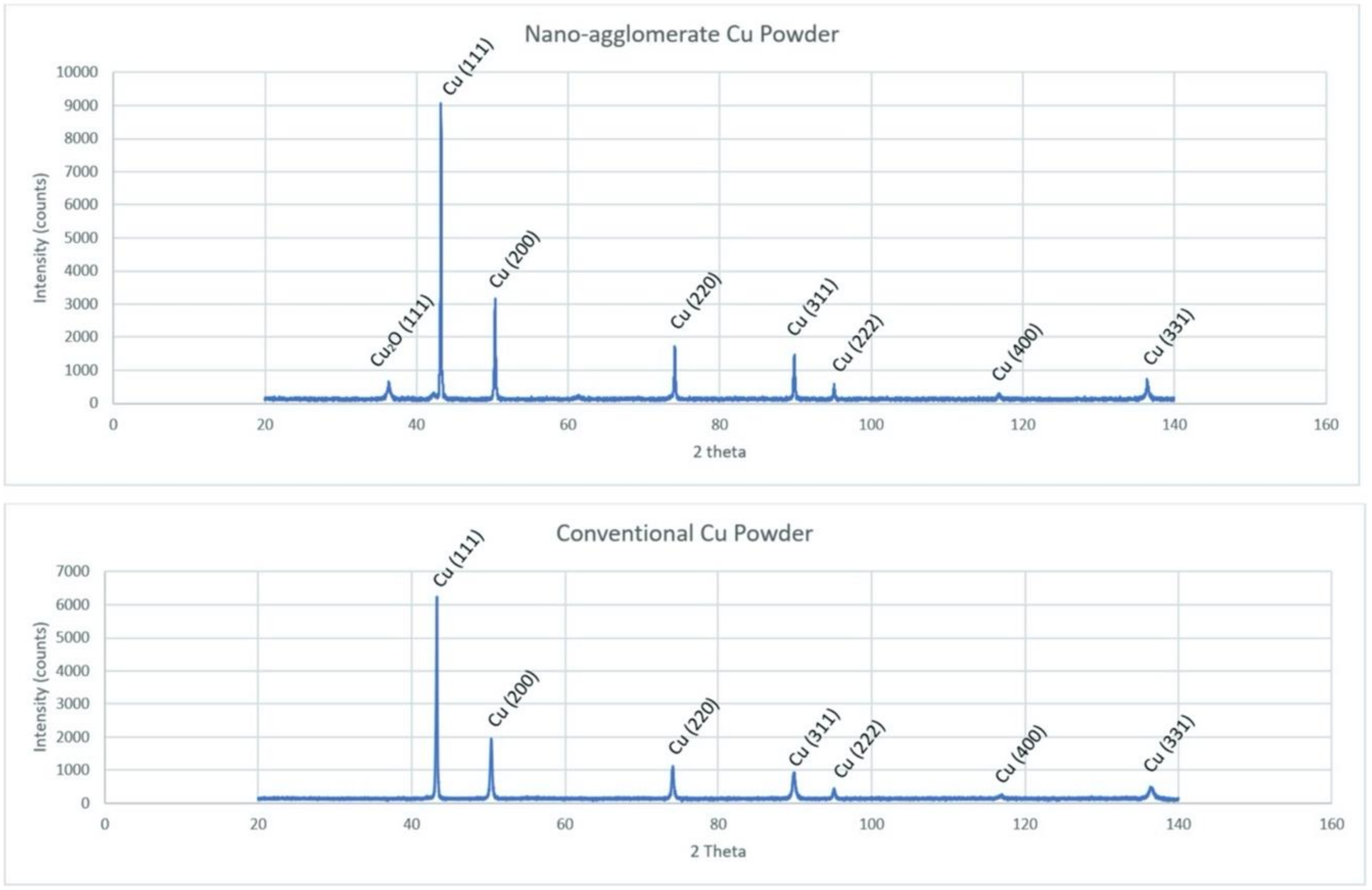
2.2. Powder X-ray Diffraction
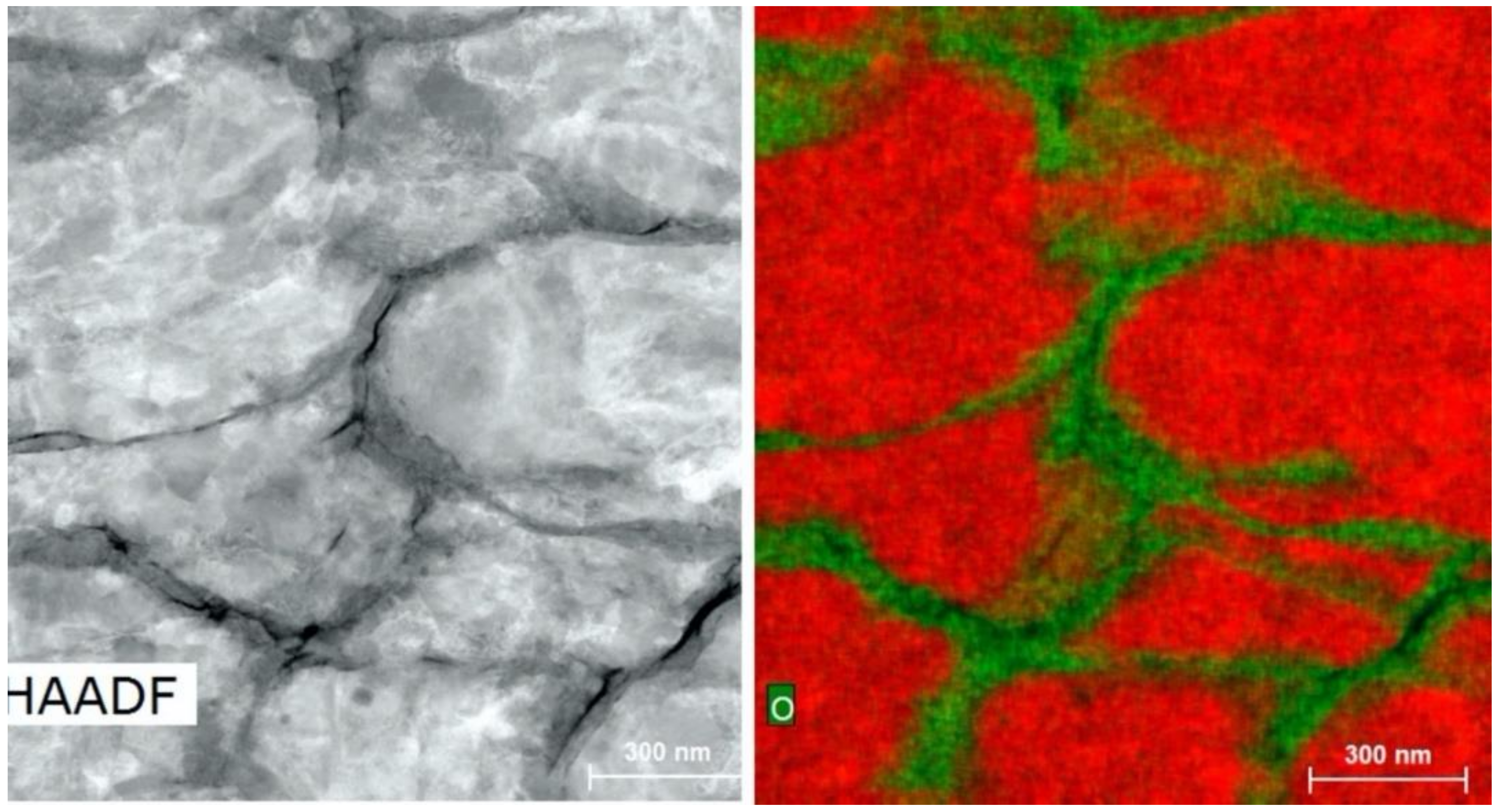
2.3. Microstructural Analysis
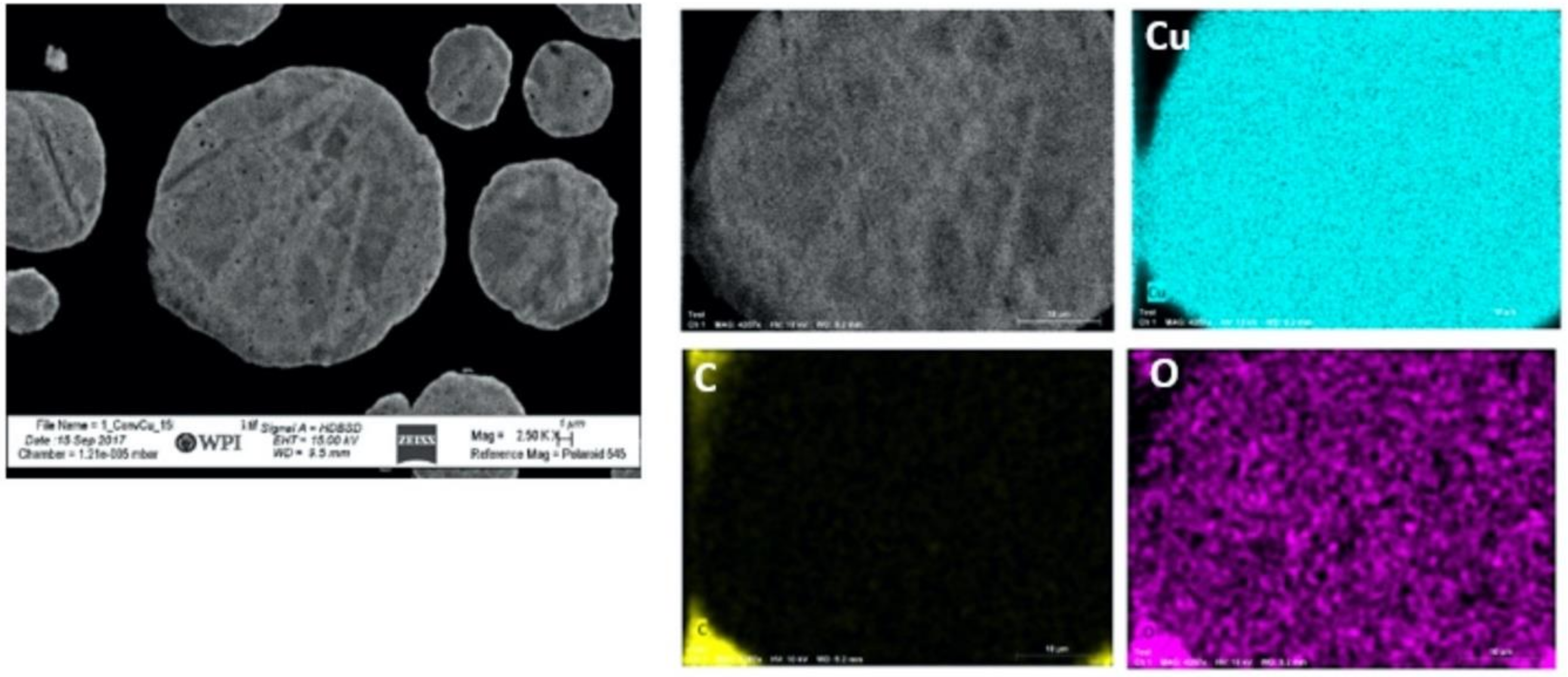
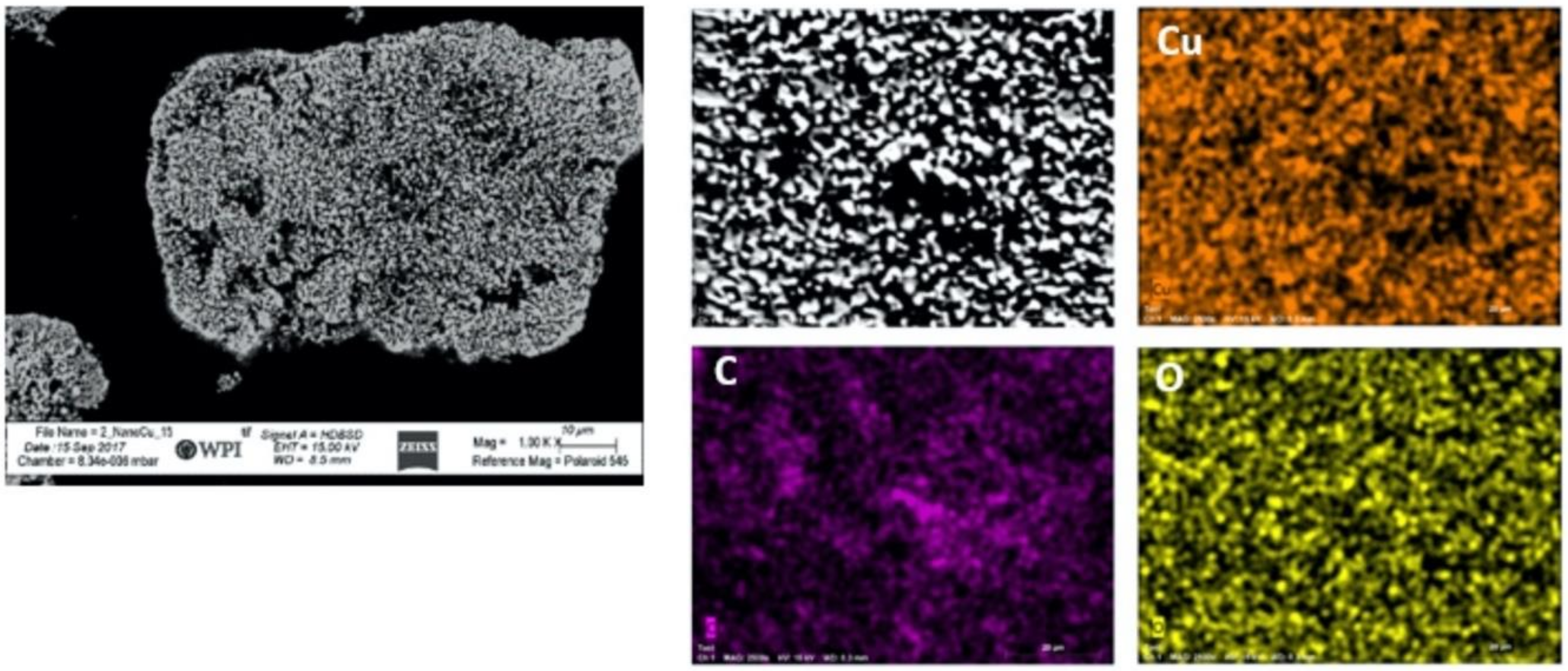
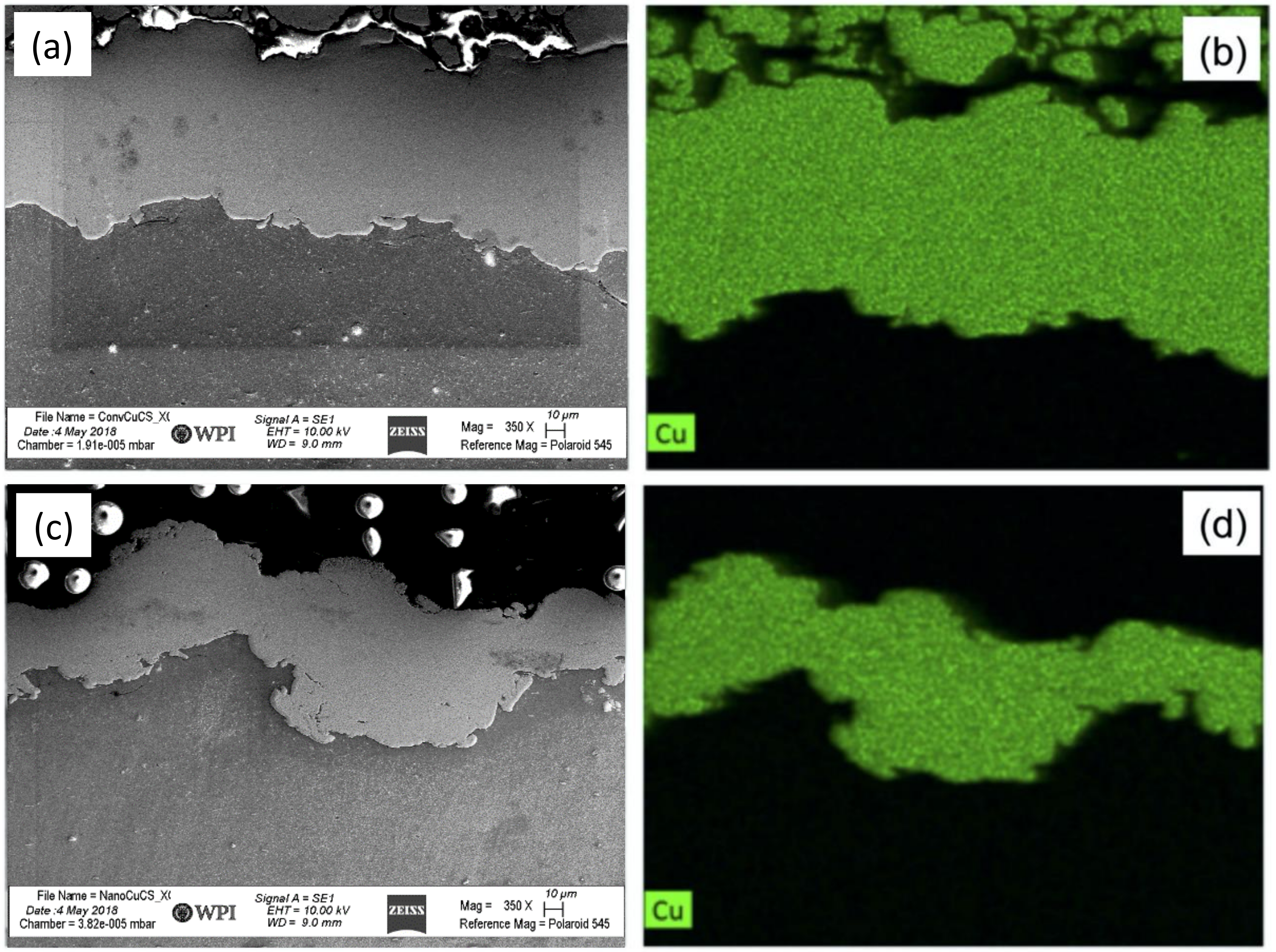
2.4. Energy-Dispersive X-ray Spectroscopy
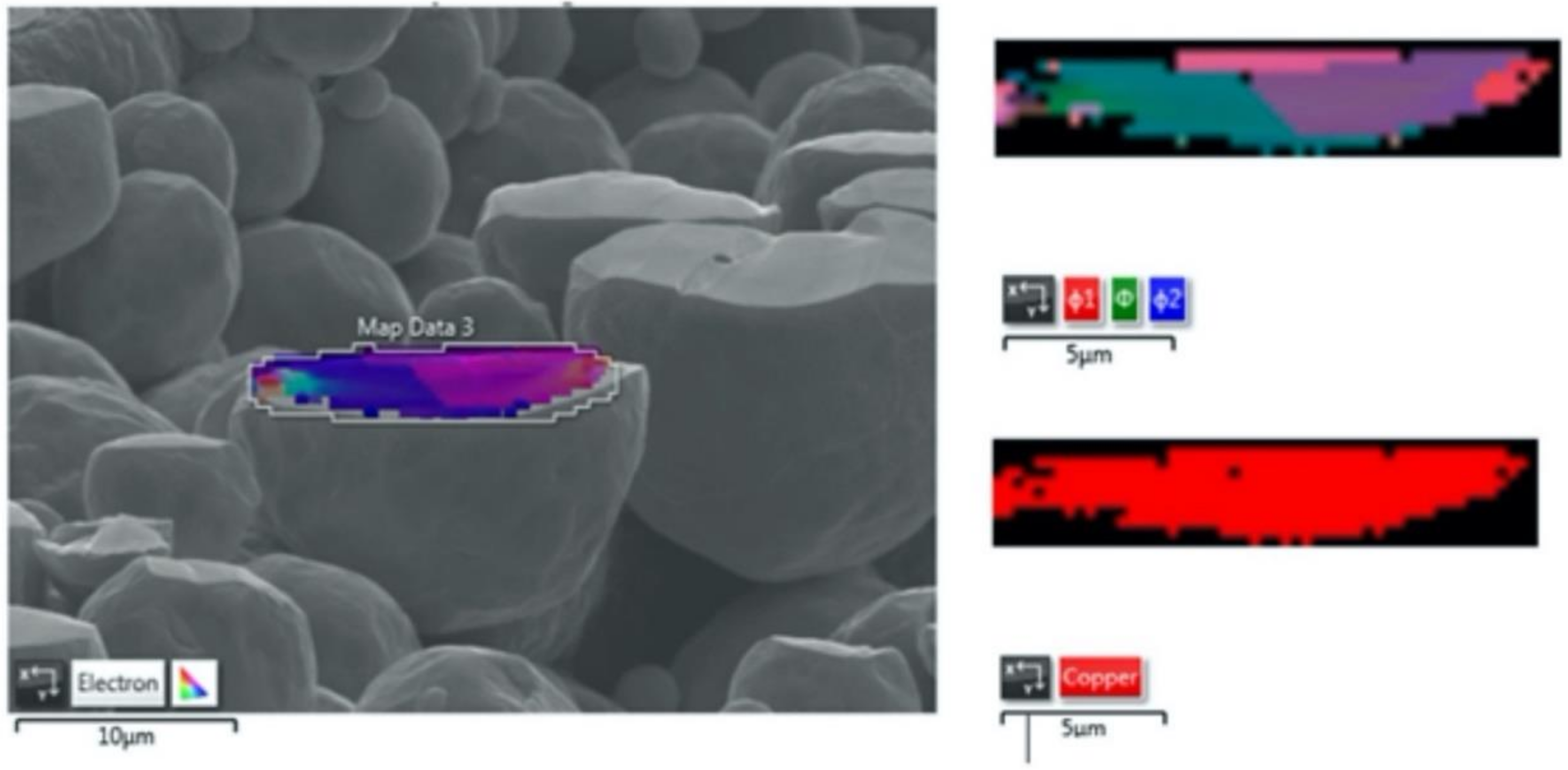
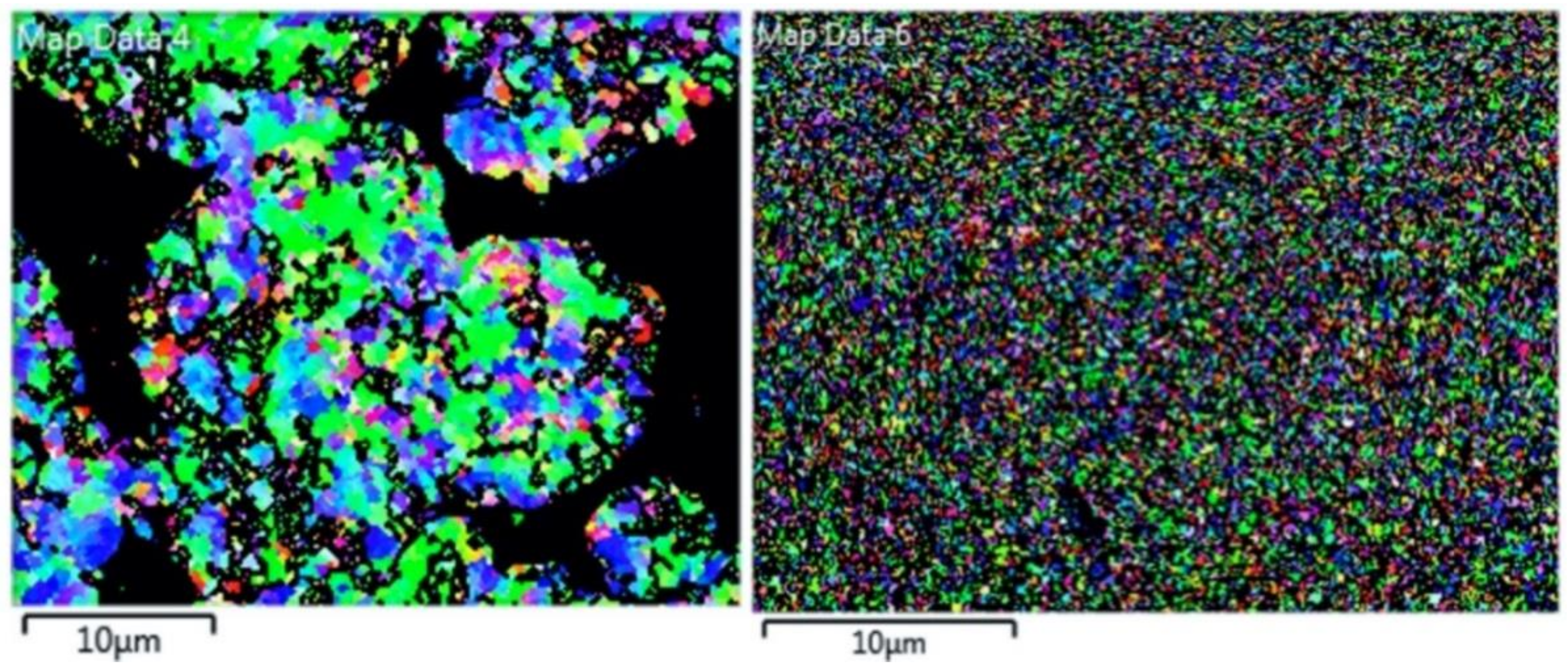
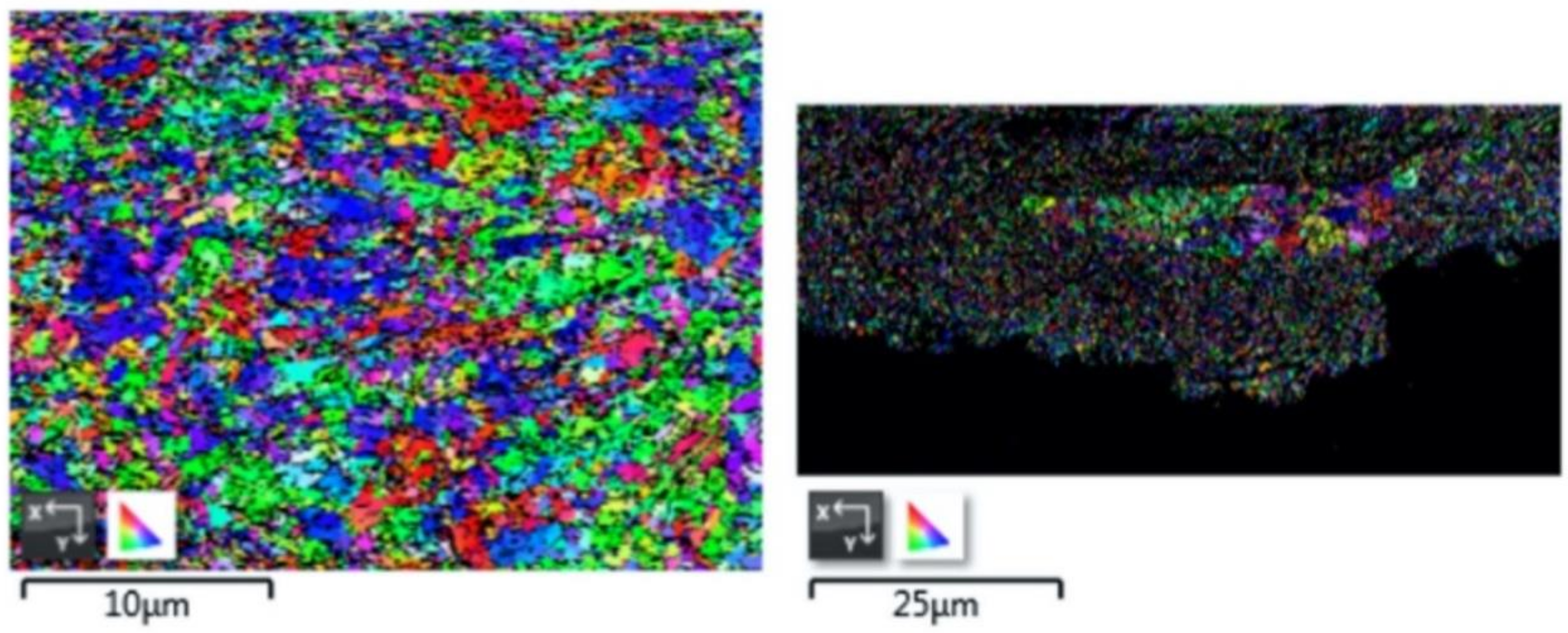
2.5. Electron Backscatter Diffraction
2.6. Nanoindentation Testing
2.7. High Strain-Rate Single-Particle Experiments
2.8. Additional Electron Microscopy
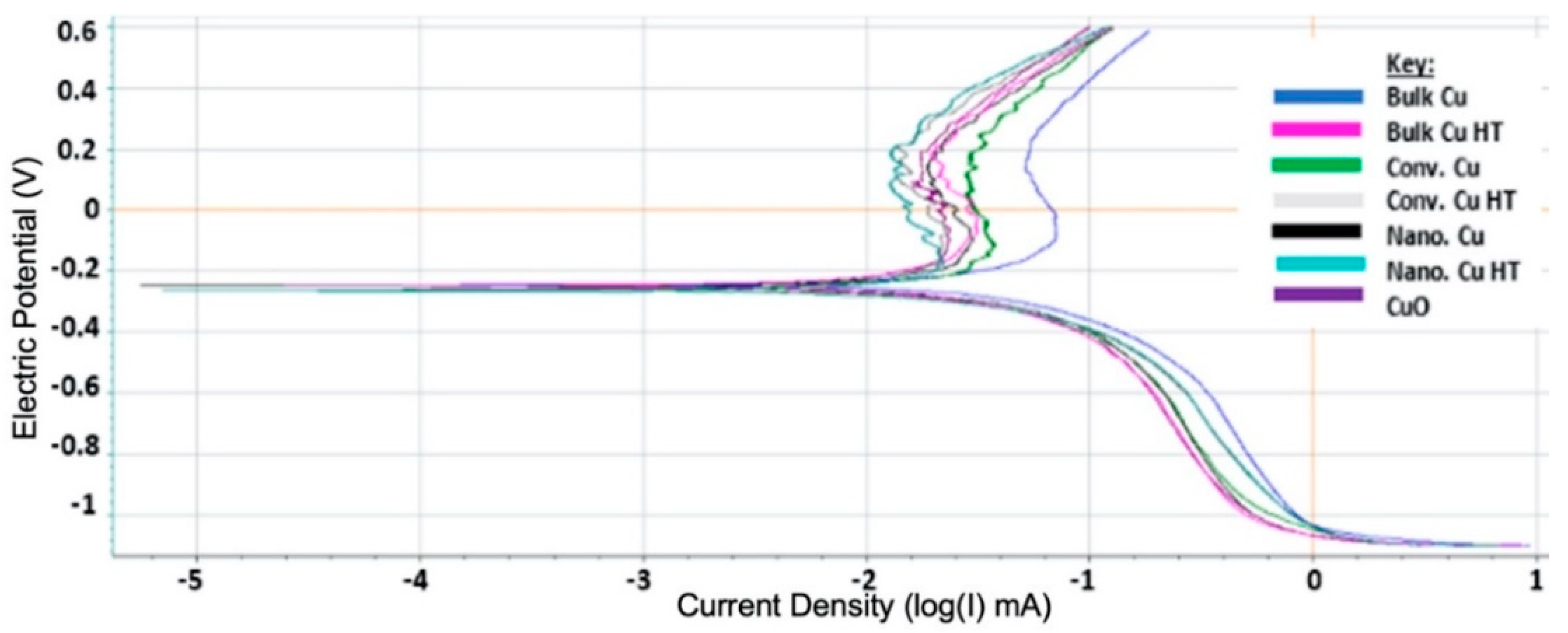
2.9. Electrochemical Analysis
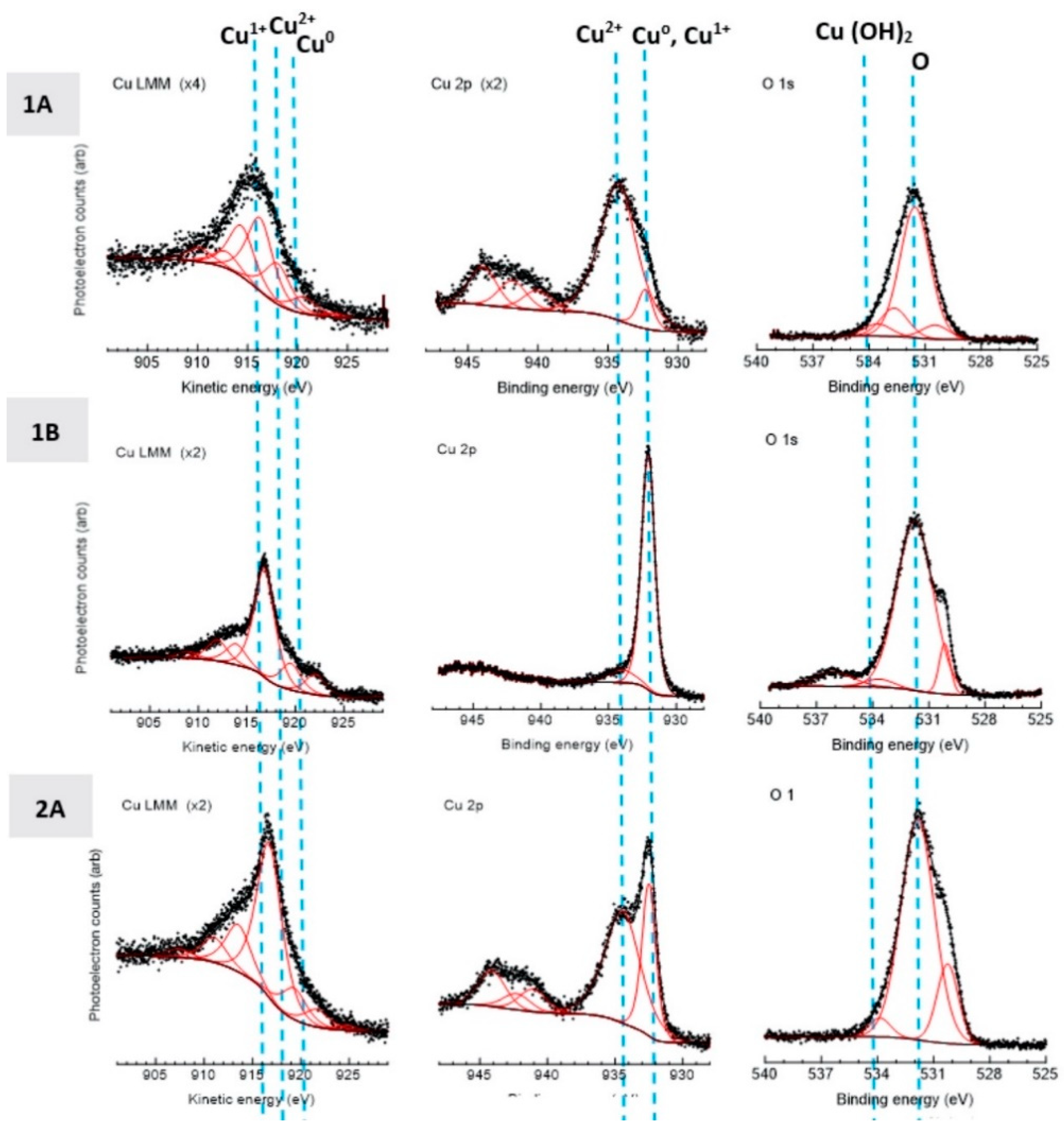
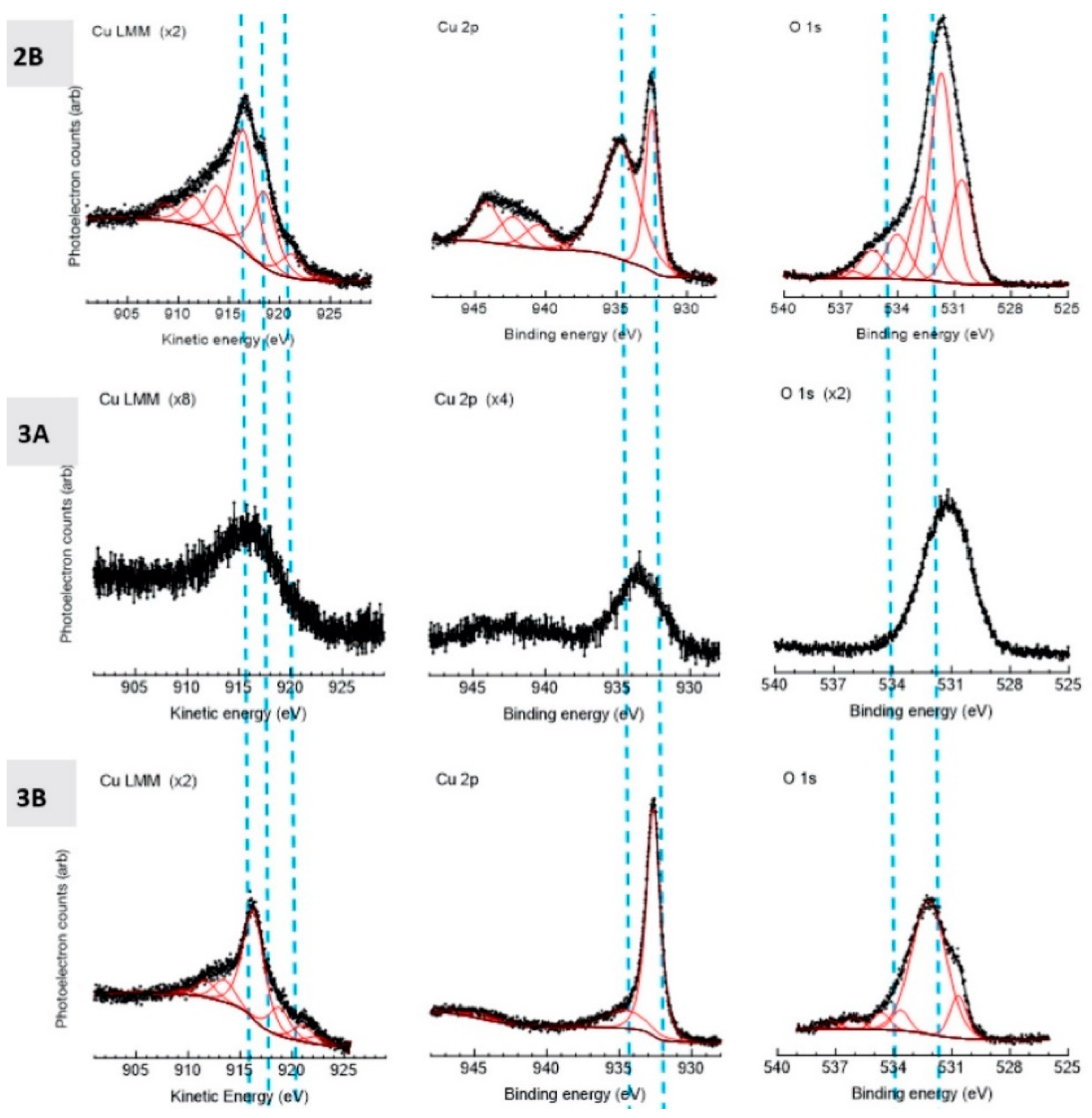
2.10. X-ray Photoelectron Spectroscopy
2.11. Cooling Rate Model Calculations
3. Results and Discussion
3.1. Feedstock Powder Properties
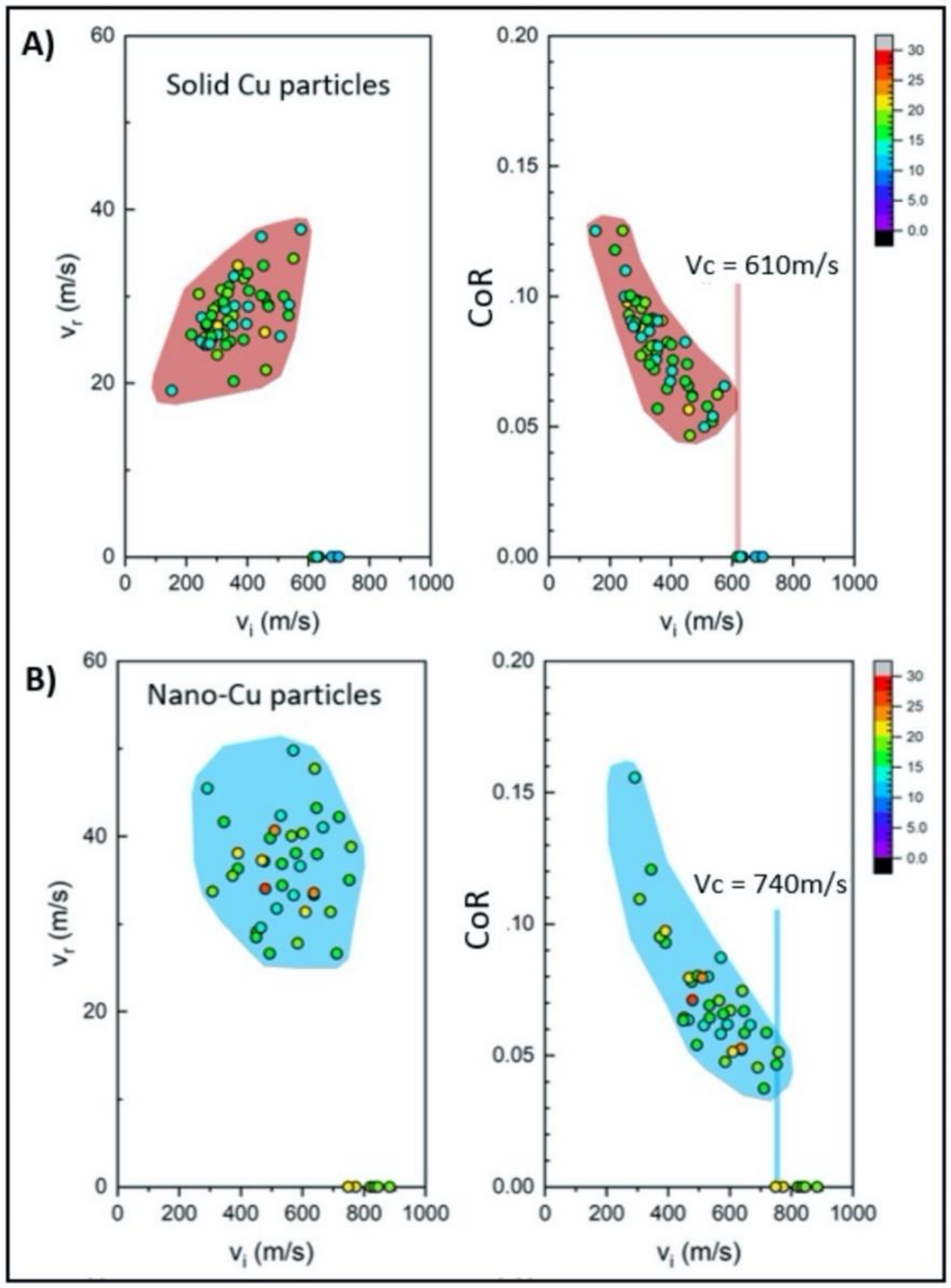
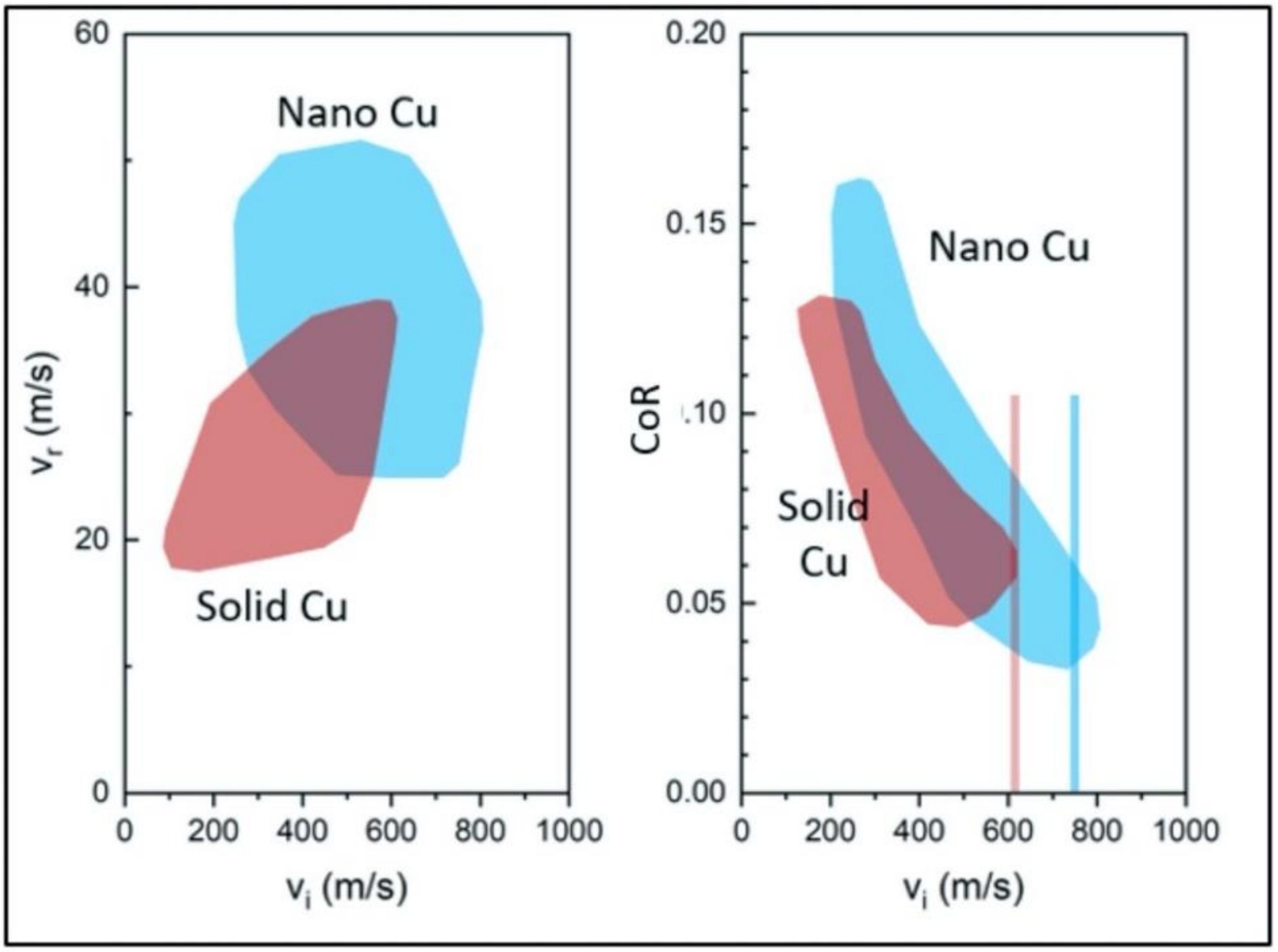
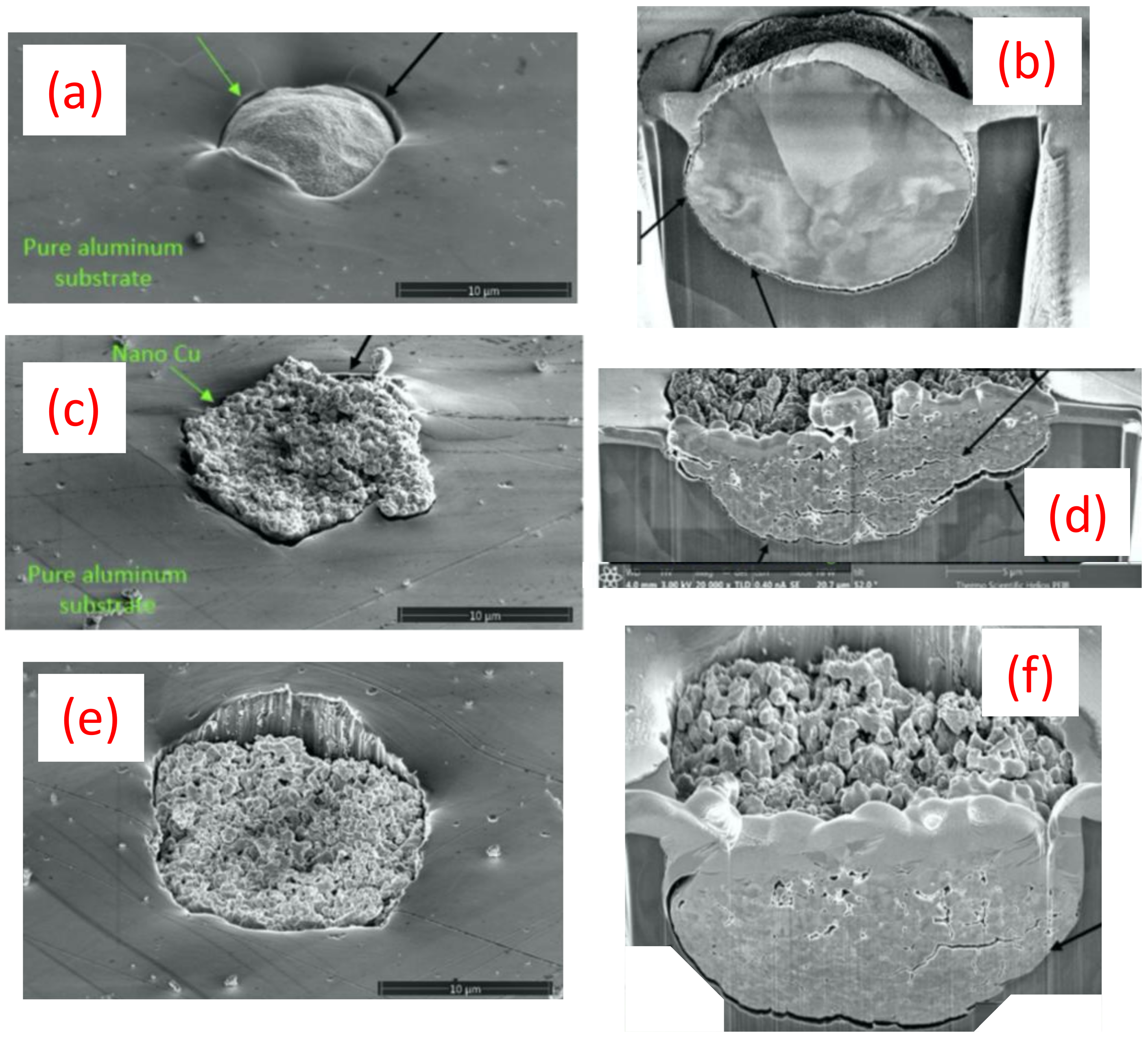
3.2. Laser-Induced Projectile Impact Behavior
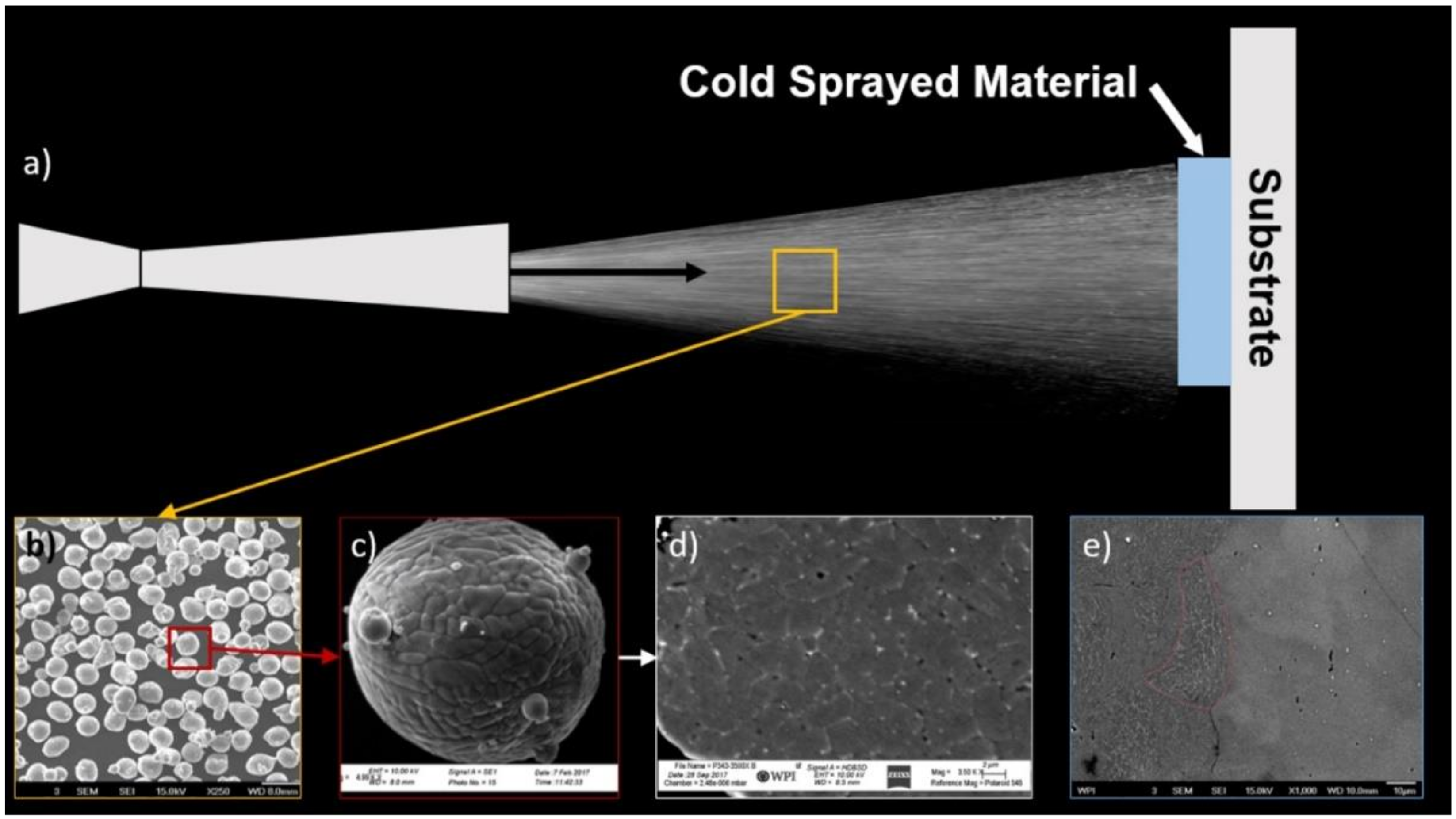
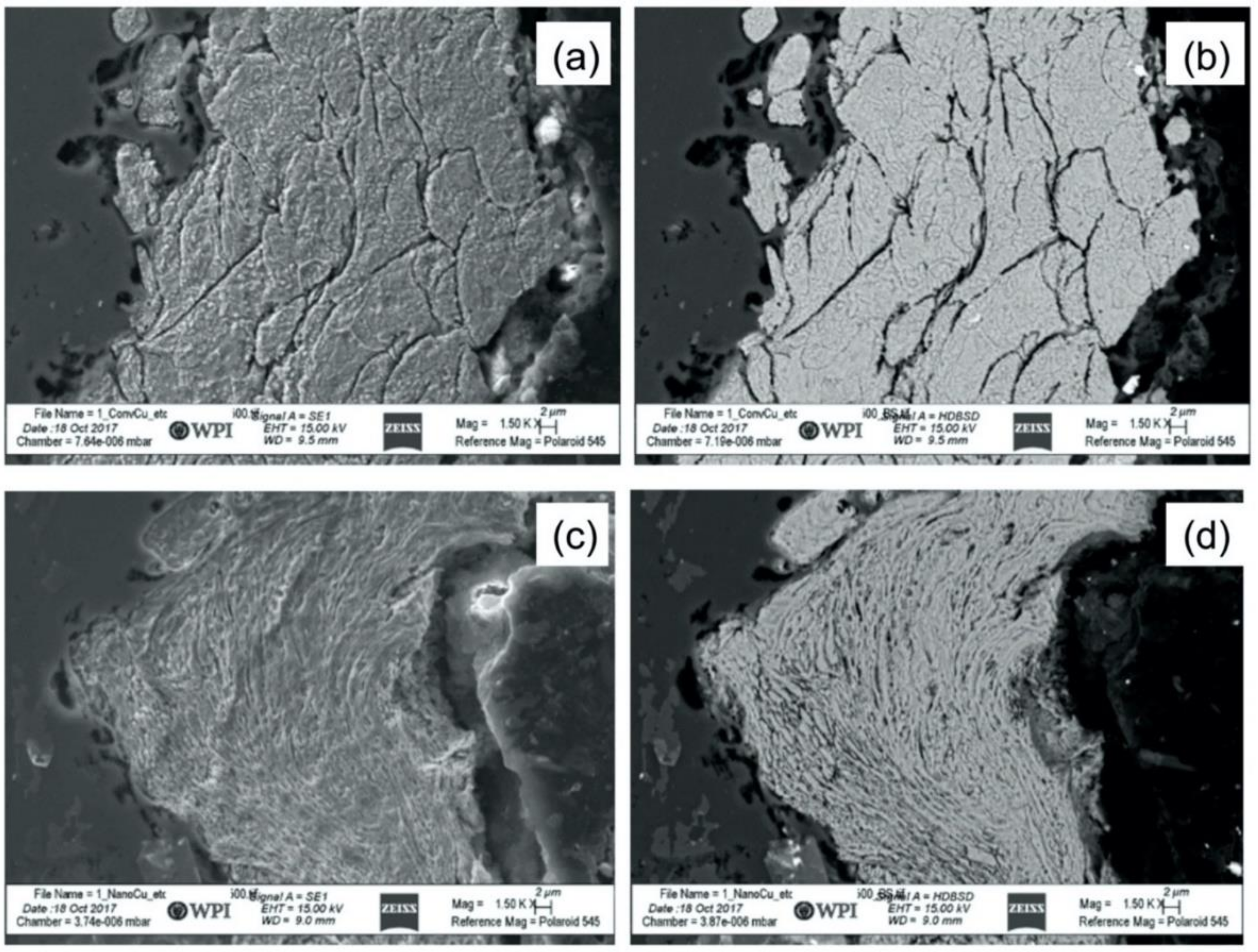
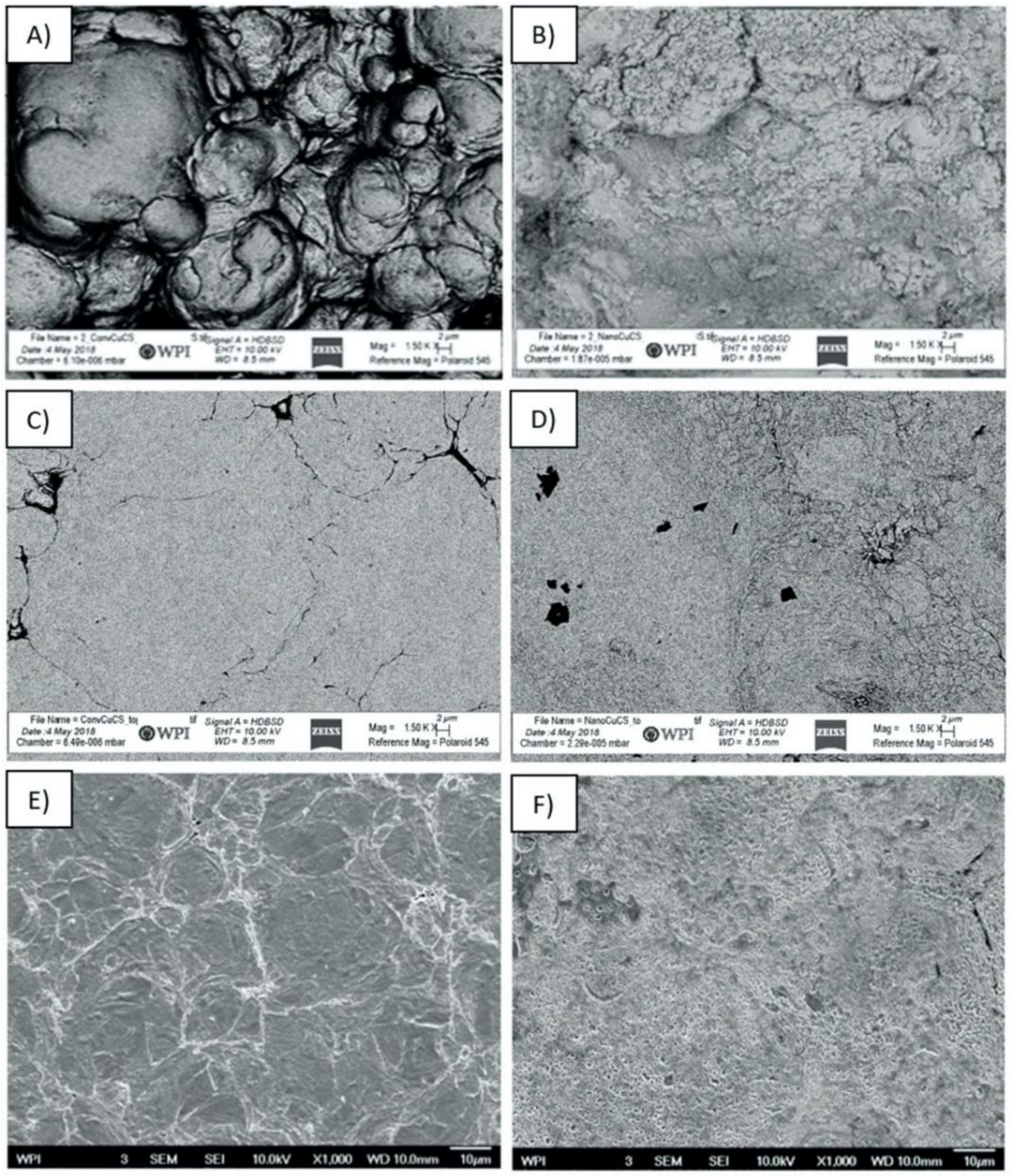
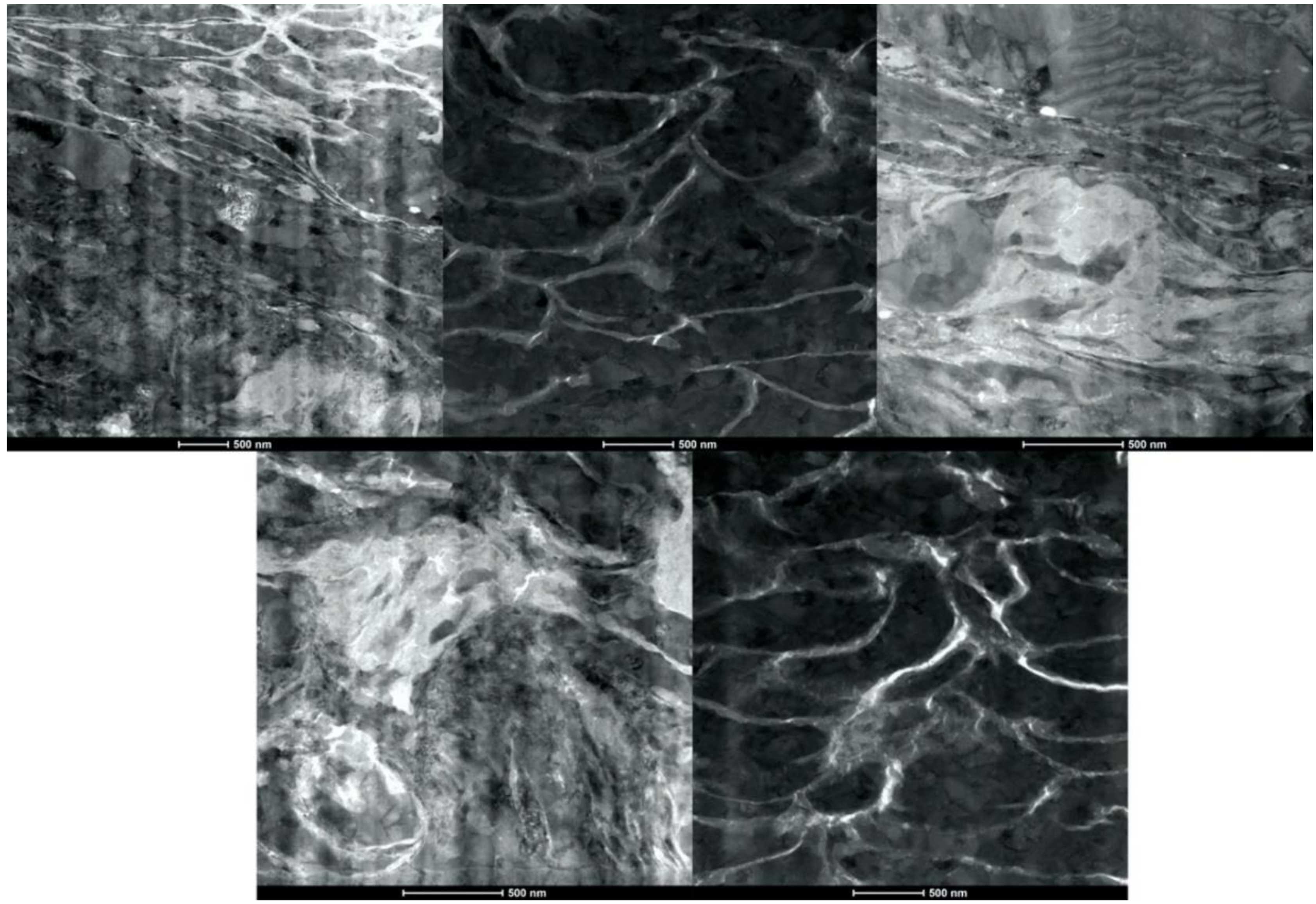
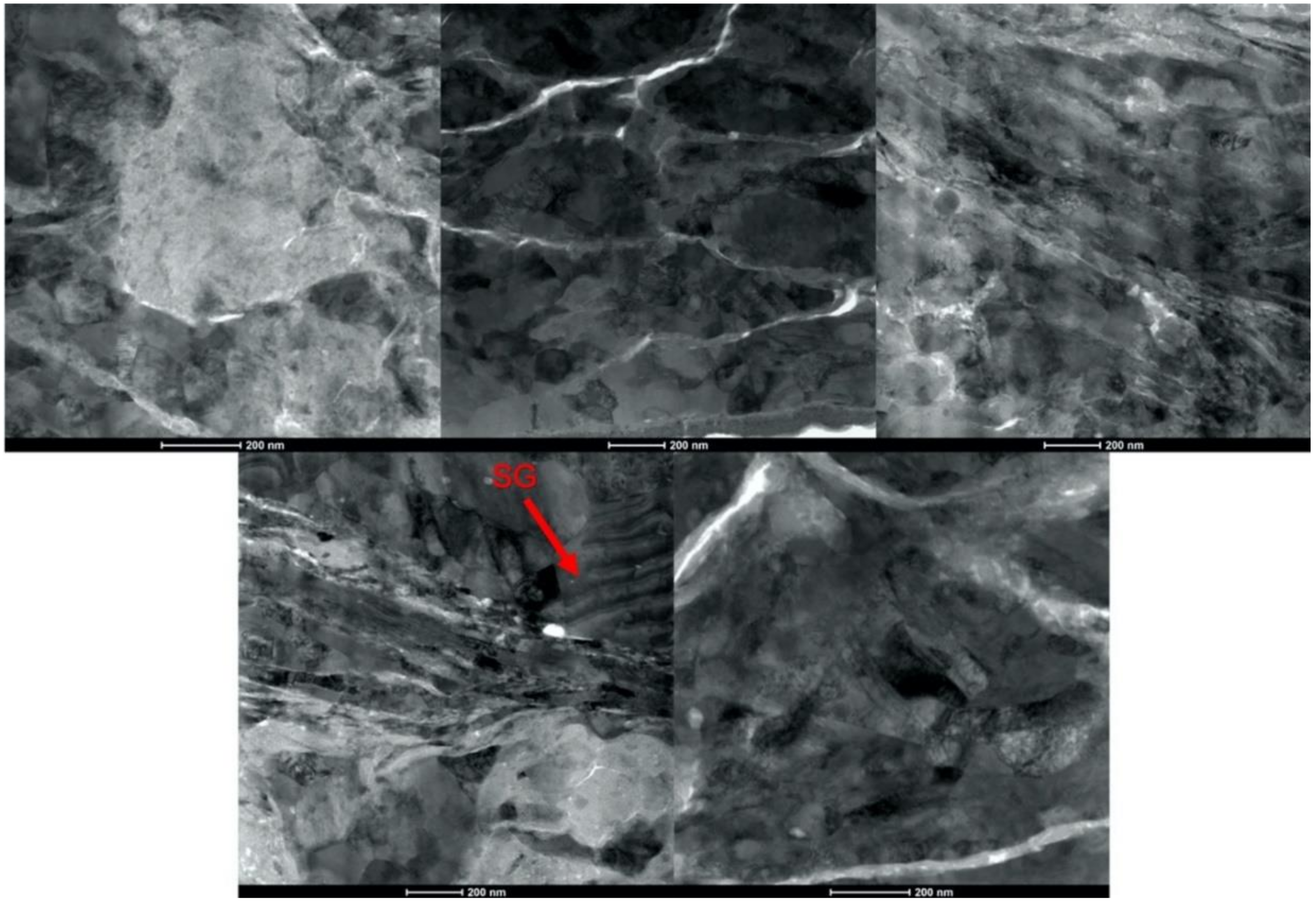
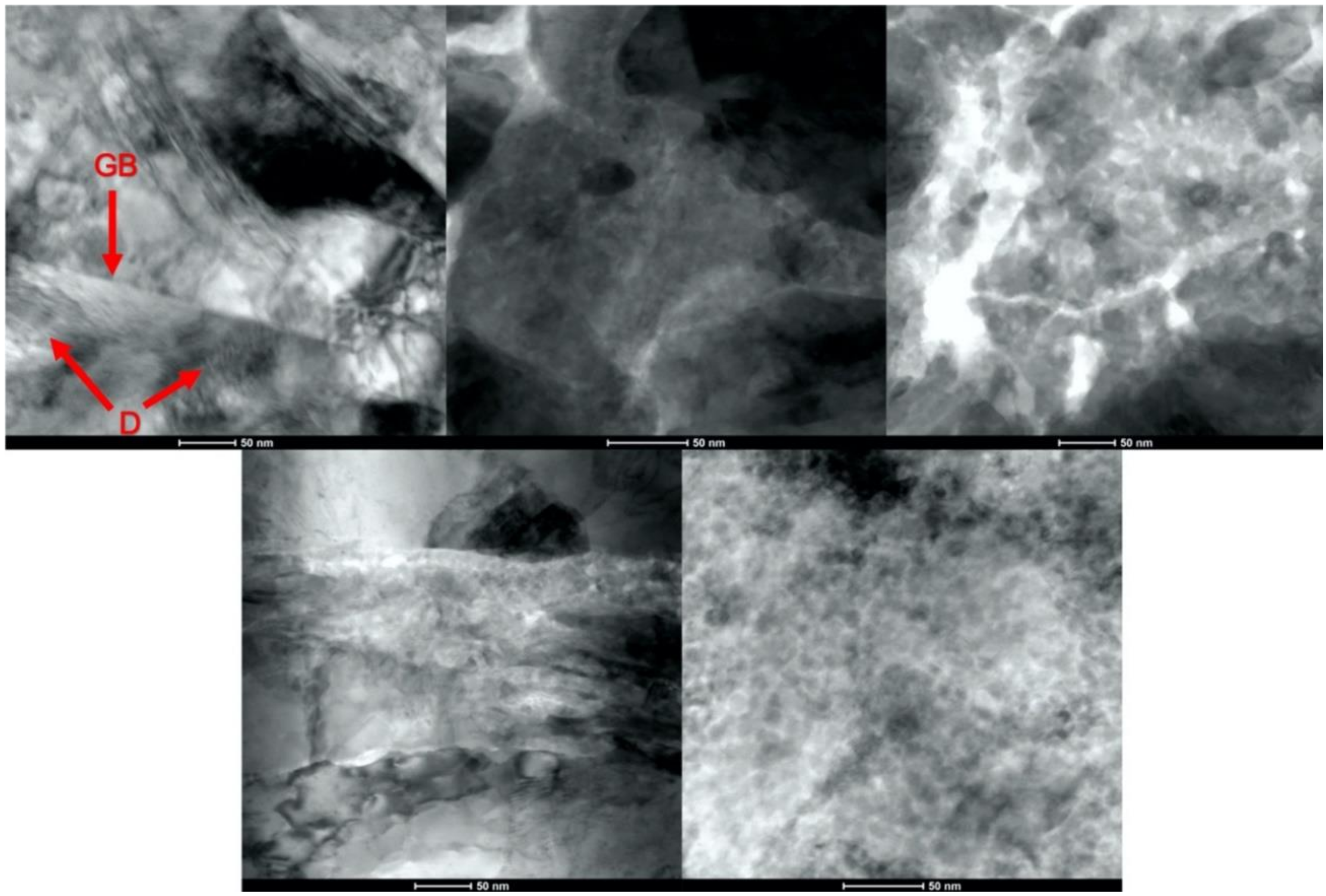
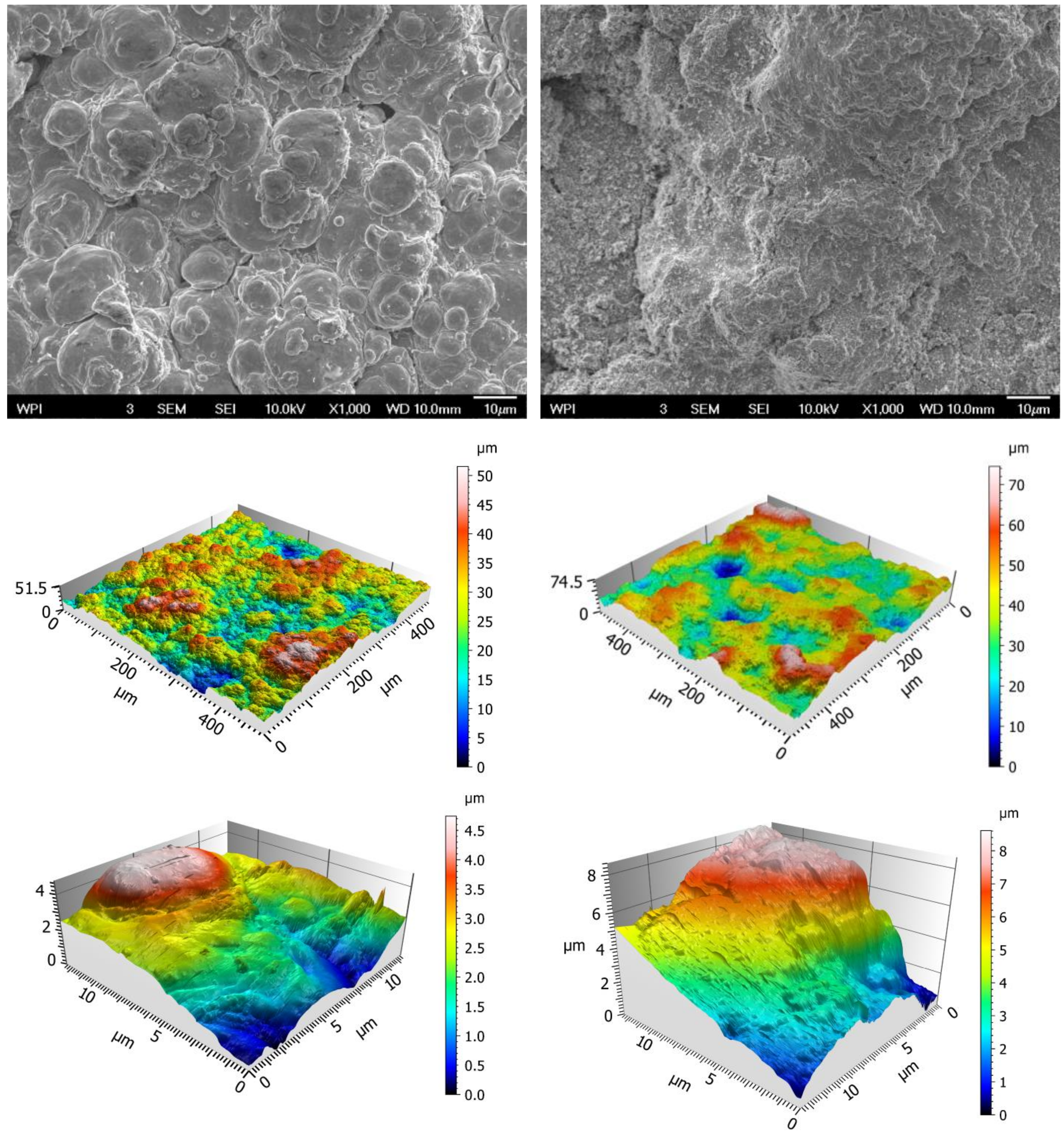
3.3. Characterization of Cold Sprayed Coatings
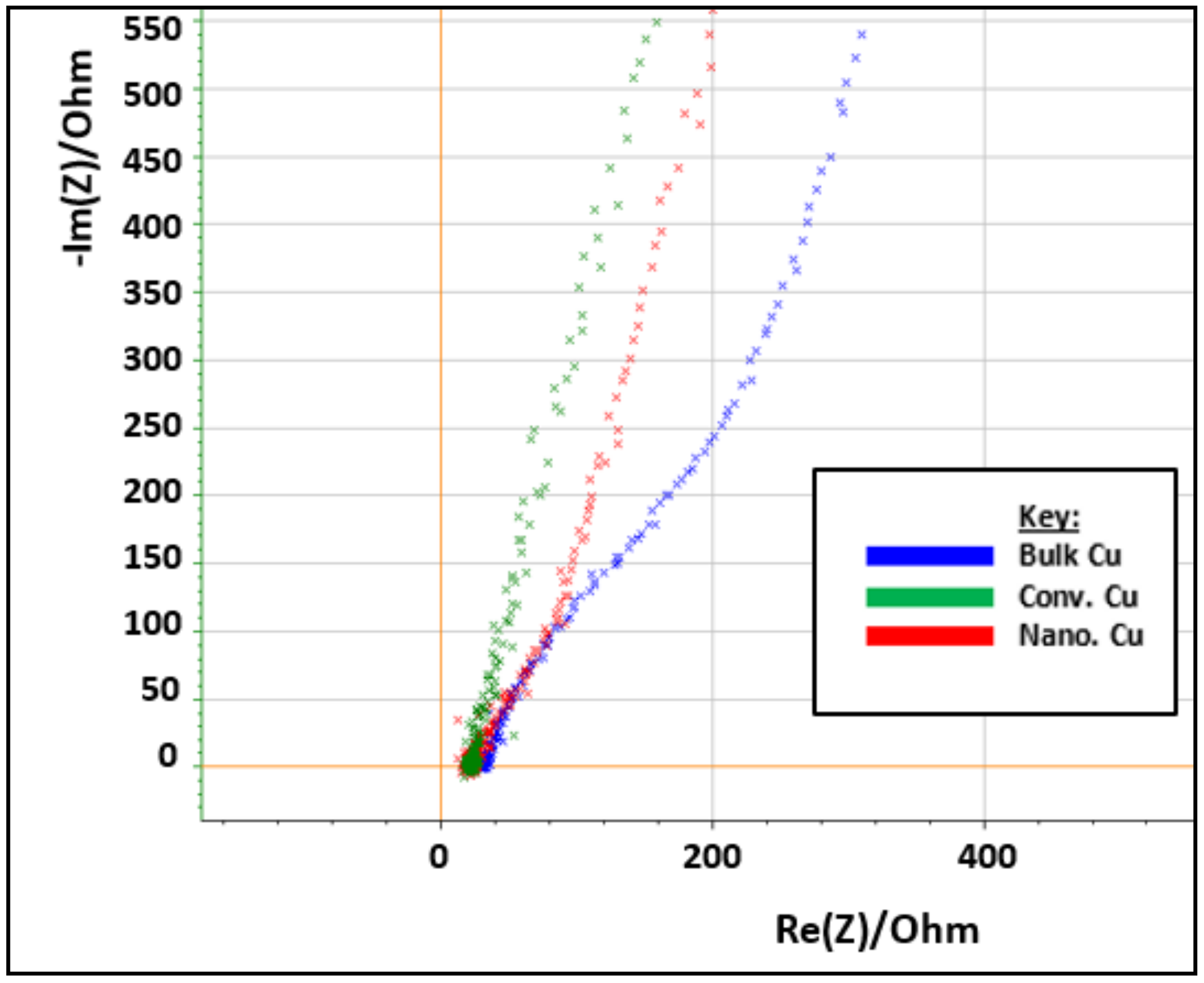
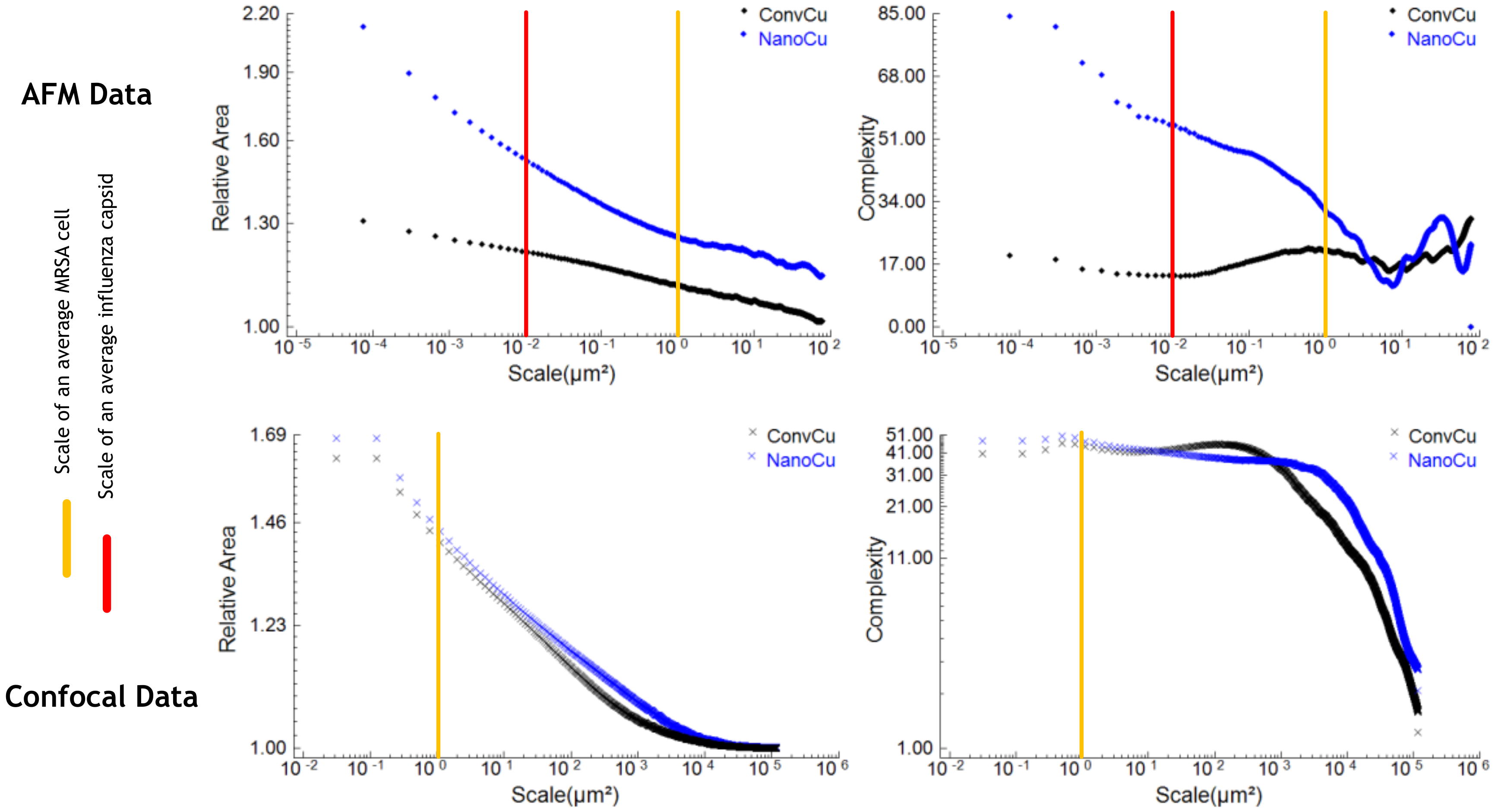
3.4. Electrochemical, Confocal and XPS Analysis
3.5. Relation to Contact Killing Mechanisms
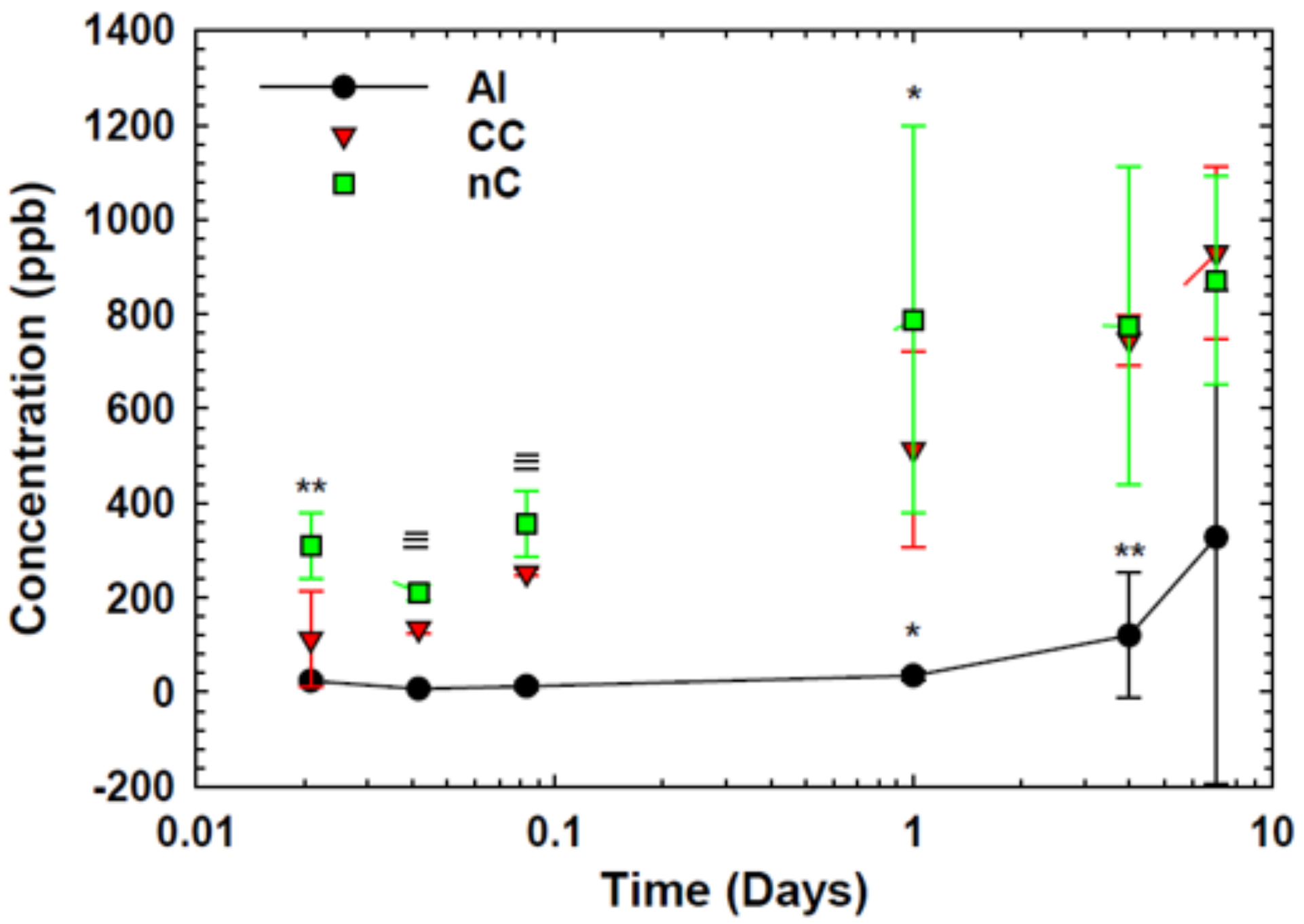
3.5.1. On Atomic Cu Ion Diffusion
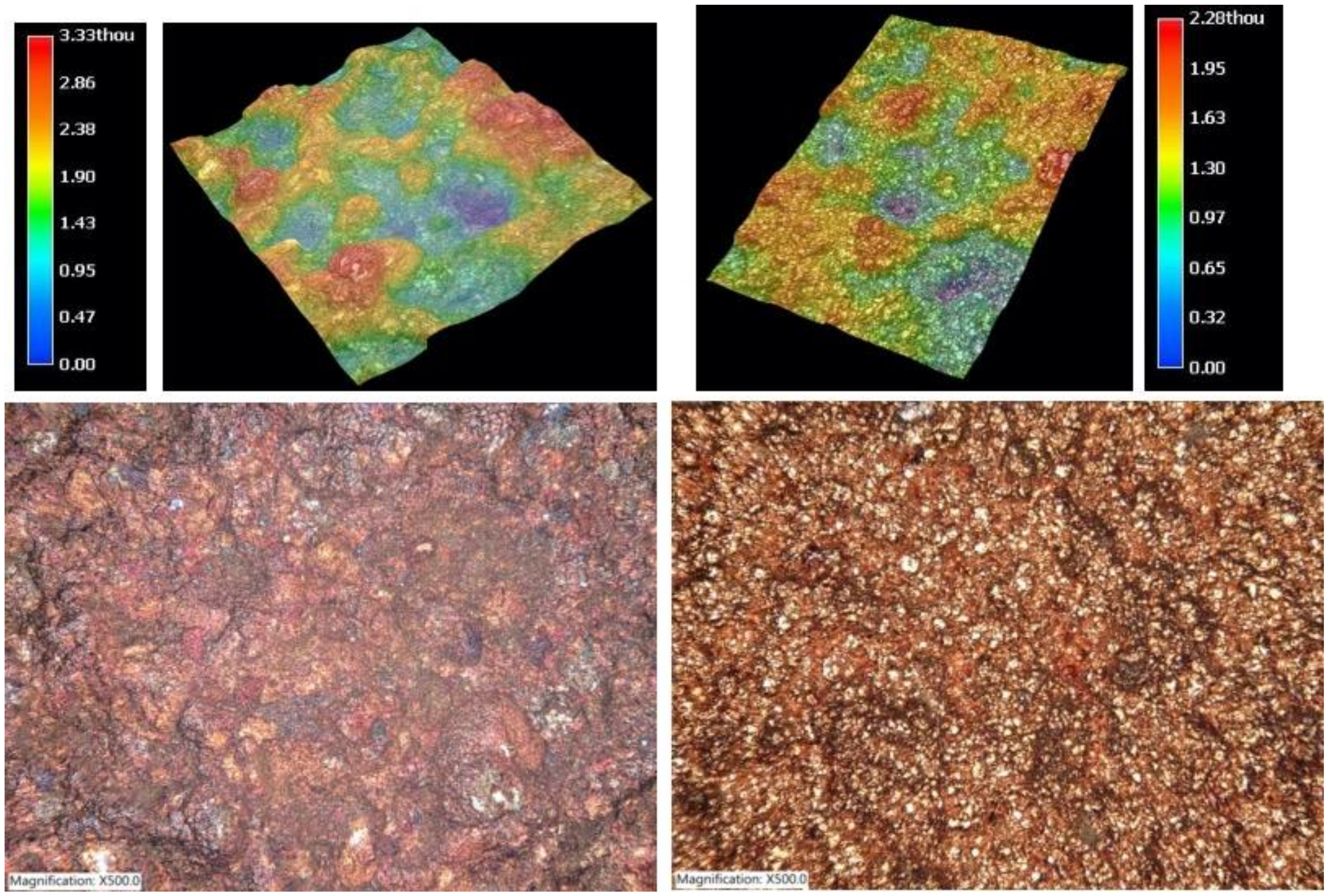
3.5.2. Surface Metrology and Roughness
3.6. On Copper and Covid-19
4. Concluding Remarks
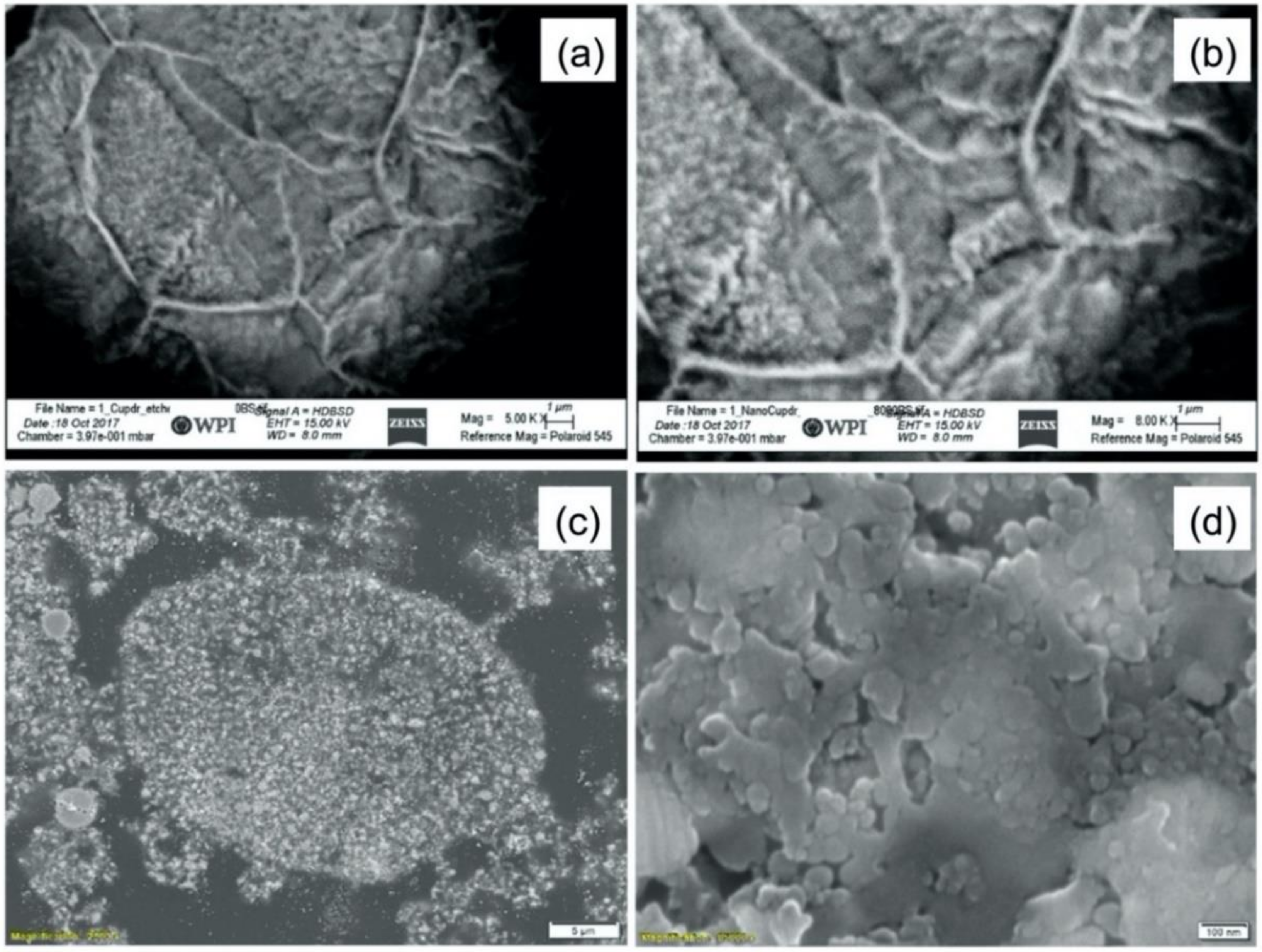
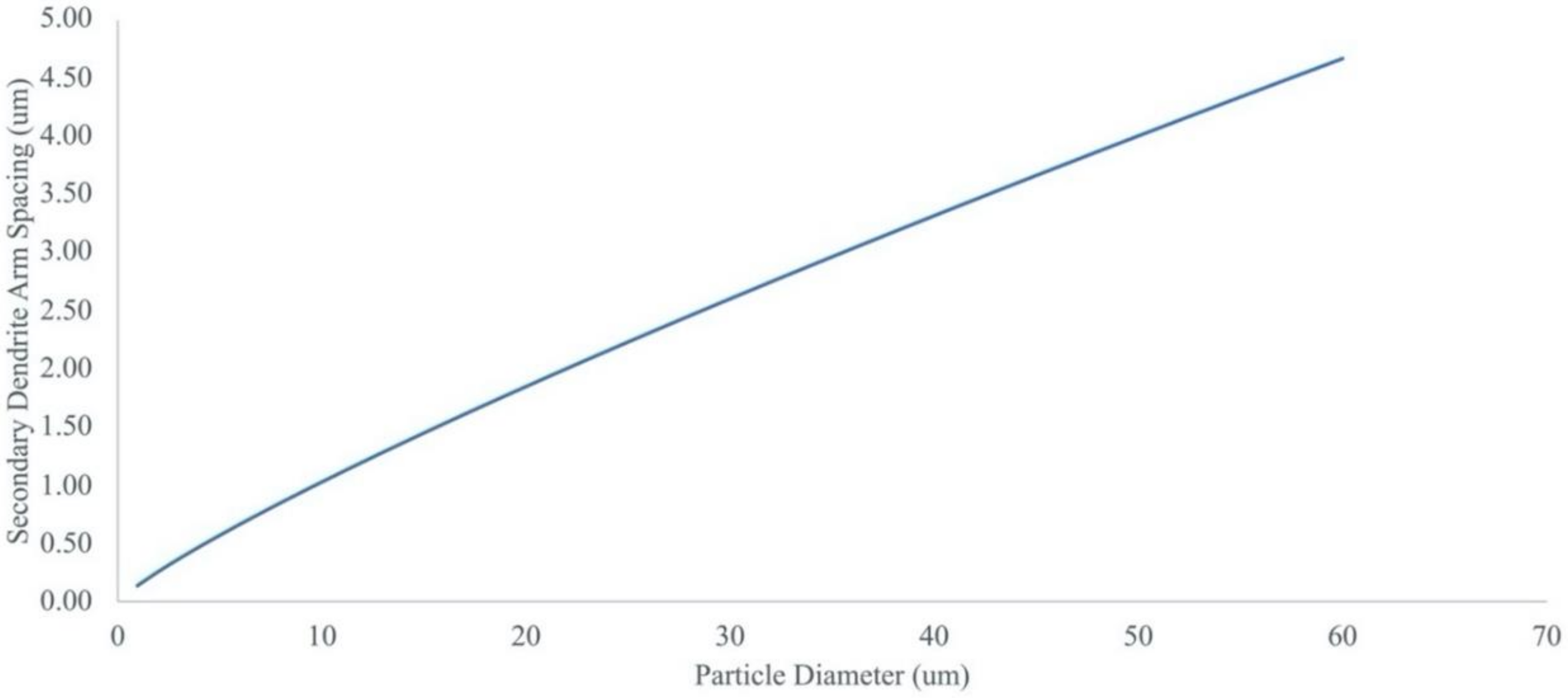
- The spray-dried Cu powder was shown to have finer grains and greater porosity than that of the GA Cu powder. The limited porosity of the GA Cu powder is attributable to trapped gas during solidification. The 50% porosity measured for the spray-dried Cu powder is directly attributable to the spray-drying manufacturing process itself. The measured microstructural feature sizes of the GA Cu powder were shown to agree well with theoretical predictions of the SDAS as a function of particle diameter and therefore particle cooling rate during the rapid solidification experienced in association with the GA process.
- PXRD revealed that Cu2O was present in the spray-dried Cu powder but not the GA Cu powder. EDS was performed in conjunction with PXRD. Future work should explore this difference between the two powders and the influence of the Cu2O oxide species upon the deposition of spray-dried Cu during cold spray processing. Prior work that utilized XPS had been reported regarding the surface species and composition for each of the coatings [24]. Future work could attempt to build upon the role of this Cu-oxide species upon contact killing/inactivation mechanisms and efficacy.
- The advanced LIPIT testing identified a critical impact velocity of 610 m/s for the GA Cu particles with a 15–20 μm diameter. As for the spray-dried Cu powder, particles with a diameter of 15–20 μm were found to have a critical impact velocity of 740 m/s. Although prior research has demonstrated increases in the degree of deformation experienced by conventionally manufactured Cu feedstock and impact velocity, a clear trend in densification as a function of impact velocity for the nanostructured powder was determined during the course of this work. Spray-dried Cu powder porosity was found to be 50% and was shown to reduce to 10% upon impact. Cross-sectional micrographs of single particles of spray-dried Cu powder deposited using LIPIT unveiled a porosity gradient where the region of the impacted powder closest to the substrate had the lowest retained porosity.
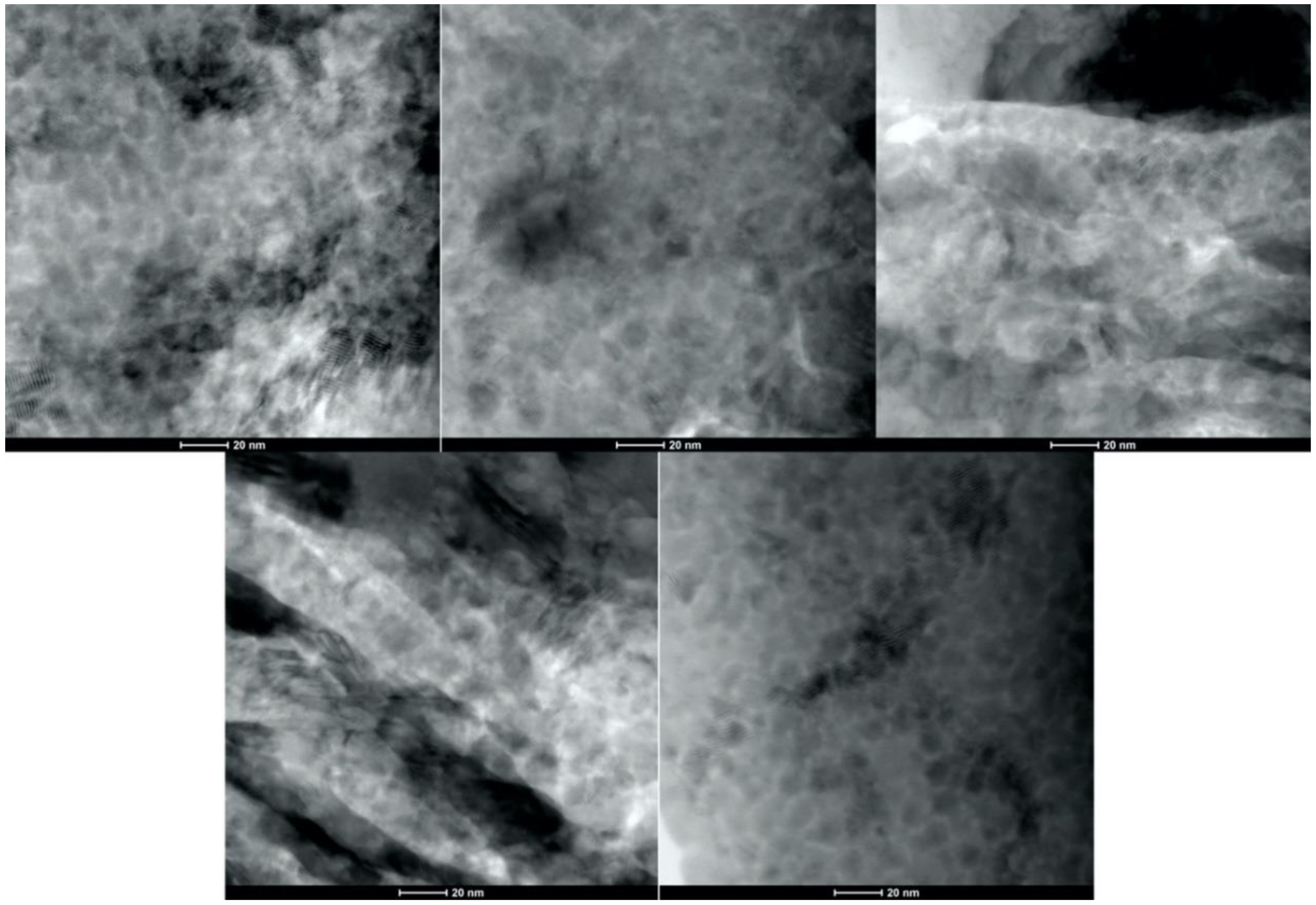
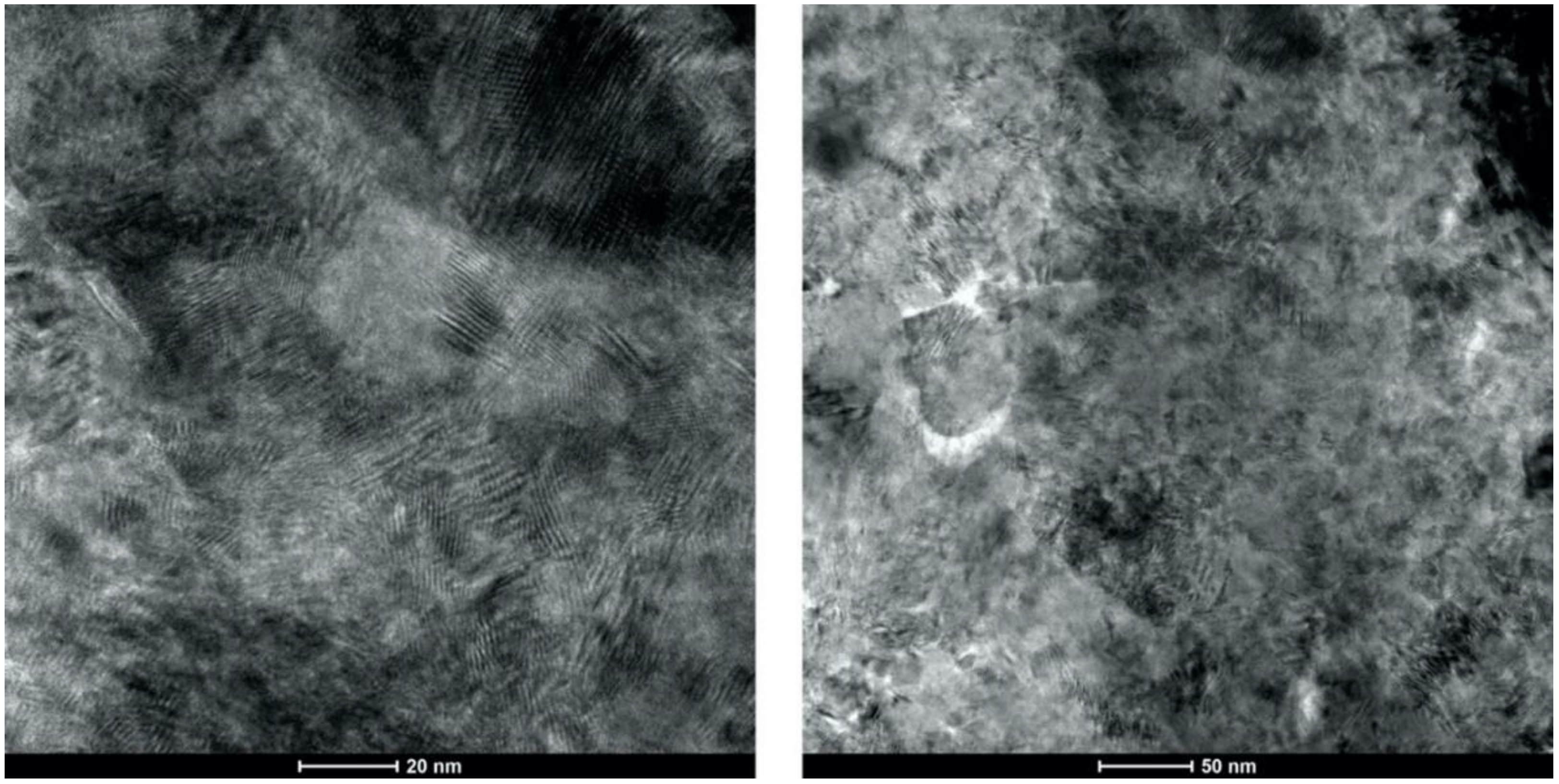
- Analysis of the cross-sections of the Cu cold spray coatings produced via conventionally GA Cu powder and spray-dried Cu powder supported the advanced LIPIT results when considering regions of microstructural variation within severely plastically deformed particles. Characterization of the conventional Cu cold spray coatings as well as the nanostructured Cu cold spray coatings was performed using EBSD, SEM, STEM and EDS. The three regions within a deformed powder particle can be classified as (1) dynamically recrystallized at the particle/substrate interface, (2) elongated and compressed in the middle of the particle and (3) retaining microstructural features at the top of the particle. However, these gradient regions undergo continued refinement as particles subsequently impact previously deposited particles. Therefore, regions (1) and (2) remain while region (3) becomes more comparable to that of (1) as particle/particle interfaces emerge.
- Microstructural analysis of the cross-sectional orientation as well as the orthogonal orientation of the Cu coatings demonstrated the fact that the conventional Cu coating had a larger grain size than the nanostructured material. This was shown to be the case regardless of orientation relative to the spray direction. The grain size of the conventional coating in the cross-sectional orientation was found to be 0.57 μm, while the grain size in the orthogonal orientation relative to the spray direction was found to be 0.90 μm. STEM revealed nanoscale grains within the nanostructured coating that were less than the initial feature size of the spray-dried Cu powder. Twinning and dislocations could also be observed via STEM. The decrease in grain size within the nanostructured Cu coating illustrates the occurrence of grain refinement even when unconventional feedstock is used in cold spray processing.
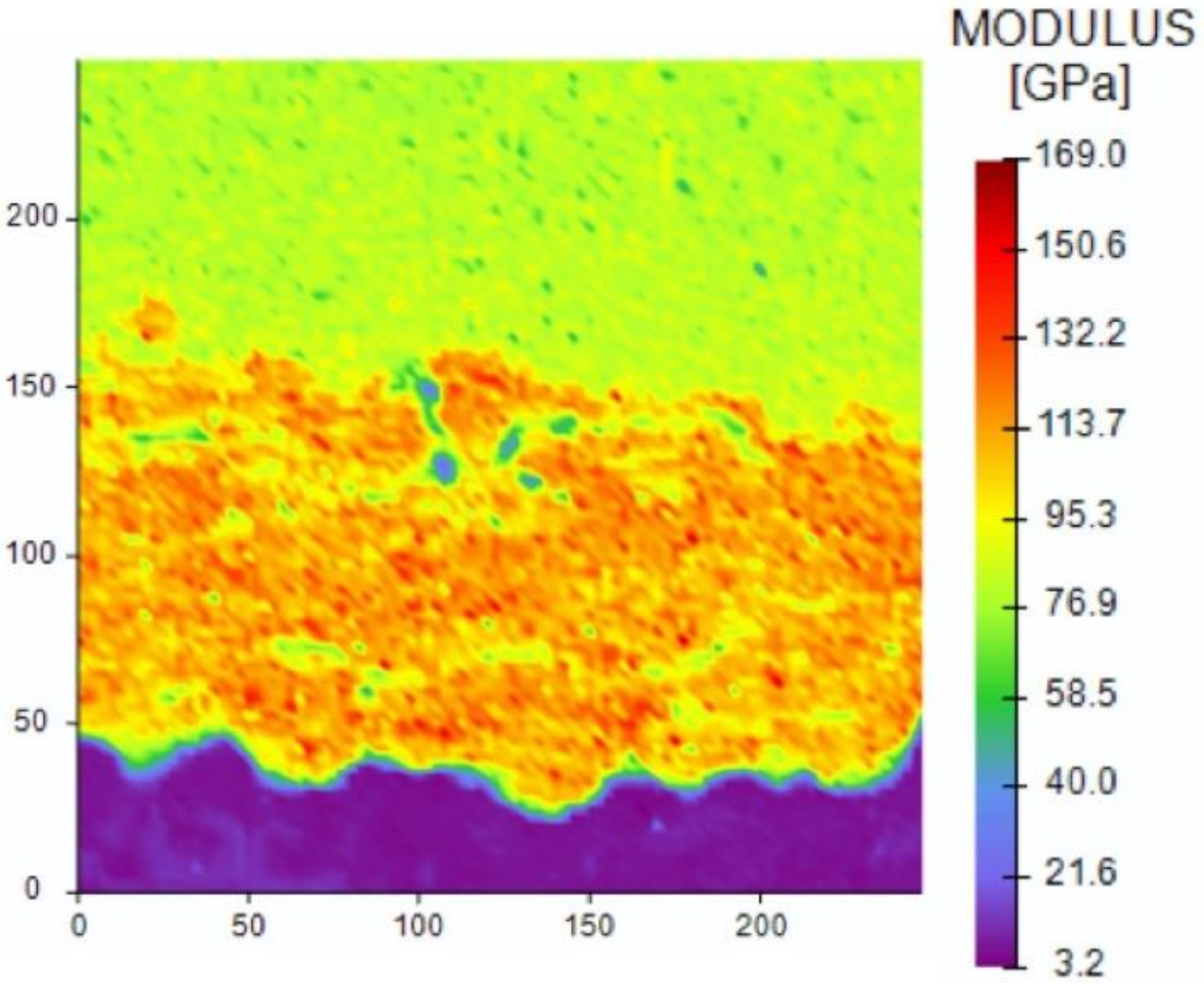
- Nanomechanical indentation testing measured the hardness of the two cold sprayed coatings in the orthogonal orientation. The hardness of the nanostructured Cu coating was found to be greater than the hardness of the conventional Cu coating. Specifically, the hardness of the nanostructured Cu coating in the orthogonal orientation was found to be 2.37 GPa, whereas the conventional Cu coating in the orthogonal orientation had a hardness of 2.01 GPa. These findings are consistent with the Hall–Petch relation. Future work relating the hardness values to the microstructural feature size via the Hall–Petch relation should be entertained in so far as derived feature sizes could be considered in the context of antipathogenic efficacy. For example, smaller feature sizes yield a greater concentration of grain boundaries within a given area. Since pathogens are killed/inactivated via a contact killing/inactivation mechanism, which is believed to be related to Cu ion diffusion, future research could consider the energetics of grain boundaries in relation to Cu ion diffusion and therefore pathogen kill/inactivation rate. Preliminary calculations found that grain-boundary mediated ion diffusion was an order of magnitude greater than the dislocation pipe diffusion contribution to the effective atomic diffusivity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhattiprolu, V.S.; Johnson, K.W.; Ozdemir, O.C.; Crawford, G.A. Influence of feedstock powder and cold spray processing parameters on microstructure and mechanical properties of Ti-6Al-4V cold spray depositions. Surf. Coat. Technol. 2018. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, Z.; Li, N.; Qiu, X.; Shen, Y.; Cui, X.; Du, H.; Wang, J.; Xiong, T. Influence of feedstock powder on microstructure and mechanical properties of Ta cold spray depositions. Surf. Coat. Technol. 2019. [Google Scholar] [CrossRef]
- Bhattiprolu, V.S.; Johnson, K.W.; Crawford, G.A. Influence of powder microstructure on the microstructural evolution of as-sprayed and heat treated cold-sprayed Ti-6Al-4V coatings. J. Therm. Spray Technol. 2019. [Google Scholar] [CrossRef]
- Bracker, G.P.; Hodges, E.; Scott, M.; Berdos, R.; Rigali, J.; Champagne, V.K.; Hyers, R.W. Electroplating powder for cold spray applications. In The Minerals, Metals and Materials Series; Springer: Berlin, Germany, 2020. [Google Scholar]
- Li, Y.J.; Luo, X.T.; Li, C.J. Dependency of deposition behavior, microstructure and properties of cold sprayed Cu on morphology and porosity of the powder. Surf. Coat. Technol. 2017. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Vuoristo, P. Effect of powder type and composition on structure and mechanical properties of Cu+Al2O3 coatings prepared by using low-pressure cold spray process. J. Therm. Spray Technol. 2010. [Google Scholar] [CrossRef]
- Huang, R.; Ma, W.; Fukanuma, H. Development of ultra-strong adhesive strength coatings using cold spray. Surf. Coat. Technol. 2014. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Champagne, V.K.; Nelson, K.A.; Schuh, C.A. Adiabatic shear instability is not necessary for adhesion in cold spray. Acta Mater. 2018. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Klassen, T.; Kreye, H. Comment on ‘Adiabatic shear instability is not necessary for adhesion in cold spray’. Scr. Mater. 2019, 162, 512–514. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Champagne, V.K.; Nelson, K.A.; Schuh, C.A. Response to Comment on “Adiabatic shear instability is not necessary for adhesion in cold spray”. Scr. Mater. 2019, 162, 515–519. [Google Scholar] [CrossRef]
- Raletz, F.; Vardelle, M.; Ezo’o, G. Critical particle velocity under cold spray conditions. Surf. Coat. Technol. 2006. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. Melt-driven erosion in microparticle impact. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Wang, H.T.; Zhang, Q.; Yang, G.J.; Li, W.Y.; Liao, H.L. Influence of spray materials and their surface oxidation on the critical velocity in cold spraying. J. Therm. Spray. Technol. 2010, 19, 95–101. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M. A hybrid approach to determine critical and erosion velocities in the cold spray process. Appl. Surf. Sci. 2013. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Coleman, A.; Murray, K.; Kearns, M.; Vuoristo, P. High pressure cold sprayed (HPCS) and low pressure cold sprayed (LPCS) coatings prepared from OFHC Cu feedstock: Overview from powder characteristics to coating properties. J. Therm. Spray Technol. 2012, 21, 1065–1075. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, D.D.; Huang, C.J.; Yin, S.; Yu, M.; Wang, F.F.; Liao, H.L. Modelling of impact behaviour of cold spray particles: Review. Surf. Eng. 2014. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, C.; Li, C.J.; Liao, H. Modeling aspects of high velocity impact of particles in cold spraying by explicit finite element analysis. J. Therm. Spray Technol. 2009. [Google Scholar] [CrossRef]
- Sundberg, K. Application of Materials Characterization, Efficacy Testing, and Modeling Methods on Copper Cold Spray Coatings for Optimized Antimicrobial Properties; Worcester Polytechnic Institute: Worcester, MA, USA, 2019. [Google Scholar]
- Imbriglio, S.I.; Hassani-Gangaraj, M.; Veysset, D.; Aghasibeig, M.; Gauvin, R.; Nelson, K.A.; Schuh, C.A.; Chromik, R.R. Adhesion strength of titanium particles to alumina substrates: A combined cold spray and LIPIT study. Surf. Coat. Technol. 2019. [Google Scholar] [CrossRef]
- Zou, Y.; Qin, W.; Irissou, E.; Legoux, J.G.; Yue, S.; Szpunar, J.A. Dynamic recrystallization in the particle/particle interfacial region of cold-sprayed nickel coating: Electron backscatter diffraction characterization. Scr. Mater. 2009. [Google Scholar] [CrossRef]
- Cunningham, R.; Narra, S.P.; Ozturk, T.; Beuth, J.; Rollett, A.D. Evaluating the effect of processing parameters on porosity in electron beam melted Ti-6Al-4V via synchrotron X-ray microtomography. JOM 2016. [Google Scholar] [CrossRef]
- Markusson, L. Powder Characterization for Additive Manufactering Processes; Lulea University of Technology: Lulea, Sweeden, 2017. [Google Scholar]
- Champagne, V.; Sundberg, K.; Helfritch, D. Kinetically deposited copper antimicrobial surfaces. Coatings 2019, 9, 257. [Google Scholar] [CrossRef]
- Sundberg, K.; Champagne, V.; McNally, B.; Helfritch, D.; Sisson, R. Effectiveness of nanomaterial copper cold spray surfaces on inactivation of influenza A virus. J. Biotechnol. Biomater. 2015, 22, 16753–16763. [Google Scholar]
- Sundberg, K.; Wang, Y.; Mishra, B.; Carl, A.; Grimm, R.; Te, A.; Lozeau, L.; Sousa, B.; Sisson, R.; Cote, D. The effect of corrosion on conventional and nanomaterial copper cold spray surfaces for antimicrobial applications. Biomed. J. Sci. Tech. Res. 2019. [Google Scholar] [CrossRef]
- Sousa, B.; Sundberg, K.; Massar, C.; Champagne, V.; Cote, D. Spherical nanomechanical characterization of novel nanocrystalline Cu cold spray manufactured materials. In Proceedings of the APS March Meeting 2019, Boston, MA, USA, 4–8 March 2019. [Google Scholar]
- Sundberg, K.; Gleason, M.; Haddad, B.; Champagne, V.K.; Brown, C.; Sisson, R.D.; Cote, D. The effect of nano-scale surface roughness on copper cold spray inactivation of influenza A virus. Int. J. Nanotechnol. Med. Eng. 2019, 4, 33–40. [Google Scholar]
- Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015. [CrossRef]
- Ventola, C.L. The antibiotic resistance: Part 1: Causes and threats. P&T 2015, 40, 277–283. [Google Scholar]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Heal. 2014, 2, 145. [Google Scholar] [CrossRef]
- Gross, M. Antibiotics in crisis. Curr. Biol. 2013. [Google Scholar] [CrossRef]
- Piddock, L.J.V. The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Sikder, P.; Grice, C.R.; Lin, B.; Goel, V.K.; Bhaduri, S.B. Single-phase, antibacterial trimagnesium phosphate hydrate coatings on polyetheretherketone (PEEK) implants by rapid microwave irradiation technique. ACS Biomater. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evidence Based Complement. Altern. Med. 2015, 16. [Google Scholar] [CrossRef]
- Gansukh, E.; Anthonydhason, V.; Jung, S.; Kim, D.H.; Muthu, M.; Gopal, J.; Chun, S. Nanotherapeutic anti-influenza solutions: Current knowledge and future challenges. J. Clust. Sci. 2018, 29, 933–941. [Google Scholar] [CrossRef]
- Gould, I.M.; Bal, A.M. New antibiotic agents in the pipeline and how hey can help overcome microbial resistance. Virulence 2013. [Google Scholar] [CrossRef]
- Golkar, Z.; Bagasra, O.; Gene Pace, D. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Gagne, E. Regulation of microRNA-199 Upon Pseudomonas Aeruginosa Infection in Zebrafifish; University of Maine: Orono, ME, USA, 2016. [Google Scholar]
- Mohammed, M.M.M.; Chun, D.M. Electrochemical performance of few-layer graphene nano-flake supercapacitors prepared by the vacuum kinetic spray method. Coatings 2018, 8, 302. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004. [Google Scholar] [CrossRef]
- Leitner, A.; Maier-Kiener, V.; Kiener, D. Essential refinements of spherical nanoindentation protocols for the reliable determination of mechanical flow curves. Mater. Des. 2018, 146, 69–80. [Google Scholar] [CrossRef]
- Chen, Q.; Alizadeh, A.; Xie, W.; Wang, X.; Champagne, V.; Gouldstone, A.; Lee, J.H.; Müftü, S. High-strain-rate material behavior and adiabatic material instability in impact of micron-scale Al-6061 particles. J. Therm. Spray Technol. 2018. [Google Scholar] [CrossRef]
- Xie, W.; Alizadeh-Dehkharghani, A.; Chen, Q.; Champagne, V.K.; Wang, X.; Nardi, A.T.; Kooi, S.; Müftü, S.; Lee, J.-H. Dynamics and extreme plasticity of metallic microparticles in supersonic collisions. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Carl, A.D.; Kalan, R.E.; Obayemi, J.D.; Kana, M.G.Z.; Soboyejo, W.O.; Grimm, R.L. Synthesis and characterization of alkylamine-functionalized Si(111) for perovskite adhesion with minimal interfacial oxidation or electronic defects. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef]
- Walton, J.; Wincott, P.; Fairley, N.; Carrick, A. Peak Fitting with CasaXPS: A Casa Pocket Book; Accolyte Science: Knutsford, UK, 2010. [Google Scholar]
- Belsito, D. Application of Computational Thermodynamic and Solidification Kinetics to Cold Sprayable Powder Alloy Design; Worcester Polytechnic Institute: Worcester, MA, USA, 2014. [Google Scholar]
- Davis, J.R. Solidifcation Structures of Copper Alloy Ingots. In Copper and Copper Alloys; ASM International: Materials Park, OH, USA, 2001; pp. 360–368. [Google Scholar]
- Chen, K.C.; Wu, W.W.; Liao, C.N.; Chen, L.J.; Tu, K.N. Observation of atomic diffusion at twin-modified grain boundaries in copper. Science 2008. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.Z.; Zhao, Y.H.; Srinivasan, S.G.; Zhu, Y.T.; Valiev, R.Z.; Gunderov, D.V. Deformation twinning in nanocrystalline copper at room temperature and low strain rate. Appl. Phys. Lett. 2004. [Google Scholar] [CrossRef]
- Meyers, M.A.; Andrade, U.R.; Chokshi, A.H. The effect of grain size on the high-strain, high-strain-rate behavior of copper. Metall. Mater. Trans. A 1995. [Google Scholar] [CrossRef]
- Poirier, D.; Legoux, J.G.; Vo, P.; Blais, B.; Giallonardo, J.D.; Keech, P.G. Powder development and qualification for high-performance cold spray copper coatings on steel substrates. J. Therm. Spray Technol. 2019. [Google Scholar] [CrossRef]
- Kang, N.; Coddet, P.; Liao, H.; Coddet, C. Cold gas dynamic spraying of a novel micro-alloyed copper: Microstructure, mechanical properties. J. Alloys Compd. 2016. [Google Scholar] [CrossRef]
- King, P.C.; Zahiri, S.H.; Jahedi, M. Microstructural refinement within a cold-sprayed copper particle. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009. [Google Scholar] [CrossRef]
- King, P.C.; Bae, G.; Zahiri, S.H.; Jahedi, M.; Lee, C. An experimental and finite element study of cold spray copper impact onto two aluminum substrates. J. Therm. Spray Technol. 2010, 19, 620–634. [Google Scholar] [CrossRef]
- King, P.C.; Zahiri, S.H.; Jahedi, M. Focused ion beam micro-dissection of cold-sprayed particles. Acta Mater. 2008. [Google Scholar] [CrossRef]
- King, P.C.; Jahedi, M. Relationship between particle size and deformation in the cold spray process. Appl. Surf. Sci. 2010. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.; Guo, C.; Gong, M.; Yang, K. Mechanical property improvement induced by nanoscaled deformation twins in cold-sprayed Cu coatings. Mater. Sci. Eng. A 2018. [Google Scholar] [CrossRef]
- Borchers, C.; Gärtner, F.; Stoltenhoff, T.; Assadi, H.; Kreye, H. Microstructural and macroscopic properties of cold sprayed copper coatings. J. Appl. Phys. 2003. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, S.M.; Moridi, A.; Guagliano, M. Critical review of corrosion protection by cold spray coatings. Surf. Eng. 2015, 31, 11. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Kang, K.; Yoon, S.; Ji, Y.; Lee, C. Oxidation dependency of critical velocity for aluminum feedstock deposition in kinetic spraying process. Mater. Sci. Eng. A 2008. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013. [Google Scholar] [CrossRef]
- Palmer, D.A. Solubility measurements of crystalline Cu2O in aqueous solution as a function of temperature and pH. J. Solution Chem. 2011. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013. [Google Scholar] [CrossRef]
- Mathews, S.; Kumar, R.; Solioz, M. Copper reduction and contact killing of bacteria by iron surfaces. Appl. Environ. Microbiol. 2015. [Google Scholar] [CrossRef]
- Rahmati, S.; Zúñiga, A.; Jodoin, B.; Veiga, R.G.A. Deformation of copper particles upon impact: A molecular dynamics study of cold spray. Comput. Mater. Sci. 2020. [Google Scholar] [CrossRef]
- Suresh, S.; Lee, S.W.; Aindow, M.; Brody, H.D.; Champagne, V.K.; Dongare, A.M. Unraveling the mesoscale evolution of microstructure during supersonic impact of aluminum powder particles. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Li, C.J.; Li, W.Y.; Liao, H. Examination of the critical velocity for deposition of particles in cold spraying. J. Therm. Spray Technol. 2006, 15, 212–222. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. In-situ observations of single micro-particle impact bonding. Scr. Mater. 2018. [Google Scholar] [CrossRef]
- Viscusi, A.; Astarita, A.; Gatta, R.D.; Rubino, F. A perspective review on the bonding mechanisms in cold gas dynamic spray. Surf. Eng. 2019, 35, 743–771. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J. Microstructure of kinetic spray coatings: A review. J. Therm. Spray Technol. 2015, 24, 592–610. [Google Scholar] [CrossRef]
- Grujicic, M.; Saylor, J.R.; Beasley, D.E.; DeRosset, W.S.; Helfritch, D. Computational analysis of the interfacial bonding between feed-powder particles and the substrate in the cold-gas dynamic-spray process. Appl. Surf. Sci. 2003. [Google Scholar] [CrossRef]
- Klinkov, S.V.; Kosarev, V.F.; Rein, M. Cold spray deposition: Significance of particle impact phenomena. Aerosp. Sci. Technol. 2005. [Google Scholar] [CrossRef]
- Lee, J.H.; Loya, P.E.; Lou, J.; Thomas, E.L. Dynamic mechanical behavior of multilayer graphene via supersonic projectile penetration. Science 2014. [Google Scholar] [CrossRef]
- Villafuerte, J. Modern Cold Spray: Materials, Process, and Applications; Springer: Berlin, Germany, 2015; ISBN 9783319167725. [Google Scholar]
- Fukumoto, M.; Terada, H.; Mashiko, M.; Sato, K.; Yamada, M.; Yamaguchi, E. Deposition of copper fine particle by cold spray process. Mater. Trans. 2009. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, K.S.; Kim, H.J.; Park, C.H.; Lee, K.A. Microstructure and room temperature compressive deformation behavior of cold-sprayed high-strength Cu bulk material. J. Therm. Spray Technol. 2017. [Google Scholar] [CrossRef]
- Yin, S.; Suo, X.; Su, J.; Guo, Z.; Liao, H.; Wang, X. Effects of substrate hardness and spray angle on the deposition behavior of cold-sprayed Ti particles. J. Therm. Spray Technol. 2014, 23, 76–83. [Google Scholar] [CrossRef]
- Huang, K.; Logé, R.E. A review of dynamic recrystallization phenomena in metallic materials. Mater. Des. 2016, 111, 548–574. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Planche, M.P.; Deng, S.; Huang, R.; Ren, Z.; Liao, H. Strengthened peening effect on metallurgical bonding formation in cold spray additive manufacturing. J. Therm. Spray Technol. 2019. [Google Scholar] [CrossRef]
- Wang, Q.; Birbilis, N.; Zhang, M.X. Interfacial structure between particles in an aluminum deposit produced by cold spray. Mater. Lett. 2011. [Google Scholar] [CrossRef]
- Balani, K.; Agarwal, A.; Seal, S.; Karthikeyan, J. Transmission electron microscopy of cold sprayed 1100 aluminum coating. Scr. Mater. 2005. [Google Scholar] [CrossRef]
- Hou, X.D.; Bushby, A.J.; Jennett, N.M. Study of the interaction between the indentation size effect and Hall-Petch effect with spherical indenters on annealed polycrystalline copper. J. Phys. D. Appl. Phys. 2008. [Google Scholar] [CrossRef]
- Hansen, N. Hall-Petch relation and boundary strengthening. Scr. Mater. 2004. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Schuh, C.A. The Hall-Petch breakdown in nanocrystalline metals: A crossover to glass-like deformation. Acta Mater. 2007. [Google Scholar] [CrossRef]
- Aristia, G.; Hoa, L.Q.; Bäßler, R. Corrosion of carbon steel in artificial geothermal brine: Influence of carbon dioxide at 70 °C and 150 °C. Materials 2019, 12, 3801. [Google Scholar] [CrossRef]
- Koivuluoto Heli, H.; Bolelli, G.; Lusvarghi, L.; Casadei, F.; Vuoristo, P. Corrosion resistance of cold-sprayed Ta coatings in very aggressive conditions. Surf. Coat. Technol. 2010. [Google Scholar] [CrossRef]
- Champagne, V.K.; Helfritch, D.J. A demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 1980; ISBN 3175723993. [Google Scholar]
- Electrochemical Impedance: Analysis and Interpretation; American Society for Testing Materials: West Conshohocken, PA, USA, 1993; ISBN 0803118619.
- Michels, H.T.; Wilks, S.A.; Noyce, J.O.; Keevil, C.W. Copper alloys for human infectious disease control. Mater. Sci. Technol. 2005, 1, 12. [Google Scholar]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayam, M.; Nakayam, T.; Sugamat, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper(i) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar] [CrossRef]
- Klinger, L.; Rabkin, E. Diffusion along the grain boundaries in crystals with dislocations. Interface Sci. 1998. [Google Scholar] [CrossRef]
- Legros, M.; Dehm, G.; Arzt, E.; Balk, T.J. Observation of giant diffusivity along dislocation cores. Science 2008. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Sheinerman, A.G. Grain-boundary dislocations and enhanced diffusion in nanocrystalline bulk materials and films. Philos. Mag. 2003. [Google Scholar] [CrossRef]
- Lu, K.; Huo, C.F.; He, Y.; Yin, J.; Liu, J.; Peng, Q.; Guo, W.P.; Yang, Y.; Li, Y.W.; Wen, X.D. Grain boundary plays a key role in carbon diffusion in carbon irons revealed by a reaxff study. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Parmar, V.; Changela, K.; Srinivas, B.; Sankar, M.M.; Mohanty, S.; Panigrahi, S.K.; Hariharan, K.; Kalyanasundaram, D. Relationship between dislocation density and antibacterial activity of cryo-rolled and cold-rolled copper. Materials 2019, 12, 200. [Google Scholar] [CrossRef]
- Da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M.; Caires, C.S.A.; Lima, A.R.; Silva, C.M.; Oliveira, S.L.; Caires, A.R.L.; et al. Corrosion resistance and antibacterial properties of copper coating deposited by cold gas spray. Surf. Coat. Technol. 2019. [Google Scholar] [CrossRef]
- Landau, P.; Osovski, S.; Venkert, A.; Gärtnerová, V.; Rittel, D. The genesis of adiabatic shear bands. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- De Orio, R.L. Electromigration Modeling and Simulation; Vienna University of Technology: Vienna, Austria, 2010. [Google Scholar]
- Chen, J.; Lu, L.; Lu, K. Hardness and strain rate sensitivity of nanocrystalline Cu. Scr. Mater. 2006. [Google Scholar] [CrossRef]
- Choi, S.; Christiansen, C.; Cao, L.; Zhang, J.; Filippi, R.; Shen, T.; Yeap, K.B.; Ogden, S.; Zhang, H.; Fu, B.; et al. Effect of metal line width on electromigration of BEOL Cu interconnects. In Proceedings of the IEEE International Reliability Physics Symposium, Burlingame, CA, USA, 11–15 March 2018. [Google Scholar]
- Hutchings, I.M. The contributions of David Tabor to the science of indentation hardness. J. Mater. Res. 2009. [Google Scholar] [CrossRef]
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical micro/mesoporous copper structure with enhanced antimicrobial property via laser surface texturing. Adv. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Michels, H.T.; Michels, C.A. Can copper help fight COVID-19? Adv. Mater. Process. 2020, 1–4. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, B.C.; Sundberg, K.L.; Gleason, M.A.; Cote, D.L. Understanding the Antipathogenic Performance of Nanostructured and Conventional Copper Cold Spray Material Consolidations and Coated Surfaces. Crystals 2020, 10, 504. https://doi.org/10.3390/cryst10060504
Sousa BC, Sundberg KL, Gleason MA, Cote DL. Understanding the Antipathogenic Performance of Nanostructured and Conventional Copper Cold Spray Material Consolidations and Coated Surfaces. Crystals. 2020; 10(6):504. https://doi.org/10.3390/cryst10060504
Chicago/Turabian StyleSousa, Bryer C., Kristin L. Sundberg, Matthew A. Gleason, and Danielle L. Cote. 2020. "Understanding the Antipathogenic Performance of Nanostructured and Conventional Copper Cold Spray Material Consolidations and Coated Surfaces" Crystals 10, no. 6: 504. https://doi.org/10.3390/cryst10060504
APA StyleSousa, B. C., Sundberg, K. L., Gleason, M. A., & Cote, D. L. (2020). Understanding the Antipathogenic Performance of Nanostructured and Conventional Copper Cold Spray Material Consolidations and Coated Surfaces. Crystals, 10(6), 504. https://doi.org/10.3390/cryst10060504






