Abstract
In the construction of heterobimetallic coordination polymers based on dithiocarbamato–carboxylate (DTCC ligands), platinum as a thiophilic metal center can be replaced by the cheaper nickel or palladium. The compounds Zn[Pd(HL)2] and Zn2[M(L)2] (M = Ni, Pd; L = {SSC-N(CH2COO)2}3−) were prepared in a sequential approach starting from K3(L). The products were characterized by IR and NMR spectroscopy, thermal analyses, and single-crystal X-ray diffraction. The products decompose under nitrogen between 300 and 400 °C. Zn[Pd(HL)2] · 6H2O forms polymeric chains in the solid state, and the Zn2[M(L)2] · 14H2O (M = Ni, Pd) exhibit two-dimensional polymeric structures, each being isotypic with the respective Zn/Pt analogs. While the carboxylate groups in all these products are coordinated to zinc in a κO-monodentate mode, a structural variant of Zn2[Ni(L)2] having κO:κO′-briding carboxylate groups was also obtained. Exchange of the metal sites in the two Ni/Zn compounds was not observed, and these compounds are therefore diamagnetic.
1. Introduction
Coordination polymers are potentially able to form three-dimensional porous structures, so-called metal-organic frameworks (MOFs). In the recent decade, compounds containing more than one different metal have attracted significant research interest, as they can exhibit completely different properties than their homometallic analogs, offering new reaction patterns and novel fields of application [1,2,3,4]. An efficient strategy for the construction of heterobimetallic coordination polymers is a sequential approach, using a metal complex with free donor groups in the backbone of the ligands, being able to coordinate to a second metal (“metalloligand” or “metallolinker”) [1]. While many ligand systems that are potentially suitable for this approach are difficult to synthesize and therefore expensive, dithiocarbamate-functionalized carboxylates (DTCC) are very easily accessible from commercially available amino carboxylic acids and can coordinate selectively to a soft (thiophilic) and hard (oxyphilic) metal center at the same time (Scheme 1) [5]. In a recent contribution, we demonstrated that polymeric structures can be constructed using platinum(II) and zinc for this purpose [6]. Thereby, DTCC ligands derived from sarcosine and L-proline result in the formation of zigzag-chain or helical structures, respectively, while the iminodiacetic-acid derived DTCC ligand {SSC-N(CH2COO)2}3− (L) forms a two-dimensional array with platinum(II) and zinc ions. The latter compound has been found to be significantly more thermally stable than the one-dimensional coordination polymers and decompose at 398 °C under nitrogen [6]. Since platinum is a less abundant and expensive metal, which detracts from the attractivity of the resulting materials for applications, we were interested in the question if comparable coordination polymers can be obtained using the cheaper metals palladium or nickel. Several nickel(II) and palladium(II) dithiocarbamates are described in the literature, and their molecular structures are closely related to those of the corresponding platinum(II) complexes. In November 2019, the Cambridge Structural Database (CSD) contained ca. 170 entries on homoleptic Ni, Pd, and Pt bis(dithiocarbamate) complexes, in which the metal is always coordinated by two chelating sulfur ligands in a square-planar fashion [7]. As previously reported by Leka et al., the potential metalloligands [M(H2L)2] (M = Ni, Pd) are readily available from (NH4)3(L) and an appropriate metal salt in aqueous solution, followed by protonation of the carboxylate groups with hydrochloric acid [8]. In the present study, we have investigated if these compounds can be used for the straightforward synthesis of Zn/Ni or Zn/Pd heterobimetallic structures. We report here four new heterobimetallic coordination polymers, including their spectroscopic characterization (IR, NMR), thermal and magnetic properties, and crystal structures.
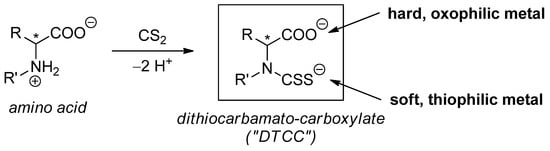
Scheme 1.
General synthesis of dithiocarbamato-carboxylate (DTCC) ligands from amino carboxylic acids and their potential ability to coordinate hard and soft metal centers selectively (R, R′ = alkyl residues; * = potential chirality center).
2. Materials and Methods
General: All operations were performed under atmospheric conditions without exclusion of air. All starting materials and solvents were obtained from commercial suppliers (e.g., Sigma-Aldrich) and used without further purification. The potassium salt K3(L) (1) was prepared as described previously [6]. NMR spectra were recorded on a Bruker AVIII 400 machine (5 mm BBO; 1H: 400.1 MHz, 13C: 100.6 MHz) at 295(2) K. Chemical shifts are referenced internally to the corresponding solvent signals. IR spectra were measured on a Bruker Vertex V70 FTIR spectrometer equipped with a diamond ATR unit, and elemental analyses (C, H, N, S) were performed using a VARIO EL cube. The single-crystal X-ray intensity data were collected on a STOE IPDS 2T diffractometer equipped with a 34cm image-plate detector, using Mo-Kα radiation, at T = 133(2) K. The crystal structures were solved with SHELXT-2018/3 [9] and refined by full matrix least-squares methods on F2 using SHELXL-2018/3 [10], using the Olex 1.2 environment [11]. Numerical absorption correction was applied to the intensity data [12]. Simultaneous TG/DTA investigations were carried out on a Netzsch STA 449F5 thermo balance. Ca. 2 mg of each sample were placed in an Al2O3 crucible, applying a gas flow of 50 mL/min N2 and a heating rate of 10 K/min during measurement.
Synthesis of [M(H2L)2] (2; M = Pd, Ni): Following previously reported procedures [6,8], a solution of K3(L) (1; 12.9 g, 40 mmol) in 250 mL water was reacted with solid PdCl2 (3.55 g, 20 mmol) or with a solution of NiCl2 · 6H2O (4.75 g, 20 mmol) in 50 mL water, followed by addition of hydrochloric acid (1.0 mol/L; 80 mL, 80 mmol). The so-obtained products are microcrystalline, yellow (2-Pd) or green (3-Ni) powders, which are sparingly soluble in alcohols and water, and moderately soluble in DMSO. 2-Pd [8]: Yield: 9.31 g (89%). Dec. 248 °C. 1H NMR (DMSO-D6): δ 4.45 (s; CH2) ppm; COOH not observed. 13C NMR (DMSO-D6): 51.5 (CH2), 168.5 (COOH), 213.4 (CSS) ppm. IR: ν 3445(m br), 3380(m br), 2981(w), 2940(w), 2909(w), 1706(s), 1628(m), 1483(s), 1430(w), 1412(m), 1375(m), 1348(m br), 1319(s), 1293(m), 1270(s), 1219(s), 1164(s), 1044(w br), 1018(w), 997(m), 951(m), 937(m), 907(s), 778(m), 715(s), 636(s), 622(sh), 603(w), 582(w), 545(sh), 512(s), 476(s), 407(w), 389(m), 363(w), 345(s), 290(m), 259(m), 229(m), 205(sh) cm−1. 2-Ni [8]: Yield: 7.70 g (81%). Dec. 217 °C. 1H NMR (DMSO-D6): δ 4.35 (s; CH2) ppm; COOH not observed. 13C NMR (DMSO-D6): 51.3 (CH2), 168.0 (COOH), 208.9 (CSS) ppm. IR: ν 3087(m br), 2980(w), 2873(w br), 1731(sh), 1720(s), 1686(s), 1478(s), 1448(w), 1432(m), 1415(w), 1402(s), 1393(sh), 1344(m), 1327(m), 1282(m), 1238(s), 1219(s), 1177(s), 1023(m), 1006(m), 954(m), 939(w), 904(m), 873(m), 810(sh), 789(s), 724(m), 679(m), 617(s), 592(s), 581(m), 562(m), 541(m), 517(s), 504(sh), 486(sh), 437(m), 399(s), 392(s), 380(m), 368(m), 447(s), 291(sh), 280(w), 268(s), 258(sh), 243(m), 222(w), 204(w) cm−1.
Synthesis of Zn[Pd(HL)2] (3-Pd): Similar as previously described for the related 3-Pt [6], a suspension of [Pd(H2L)2] (2-Pd; 0.52 mg, 1.0 mmol) and zinc acetate–dihydrate (0.22 g, 1.0 mmol) in 100 mL water and 100 mL ethanol were stirred at 80 °C for 3 h. The resulting mixture was filtered while hot, and the ethanol was allowed to evaporate slowly from the filtrate upon standing for several days at ambient temperature. The so-formed crystals of 3-Pd · 6H2O were suitable for single-crystal X-ray structure determination, but decomposed to 2-Pd and 4-Pd (as identified by IR and NMR spectroscopy) upon attempted isolation.
Attempted synthesis of Zn[Ni(HL)2] (3-Ni): Similar as described for 3-Pd, a suspension of [Ni(H2L)2] (2-Pd; 0.52 mg, 1.0 mmol) and zinc acetate–dihydrate (0.22 g, 1.0 mmol) in 100 mL water and 100 mL ethanol were stirred at 80 °C for 3 h. The resulting mixture was filtered while hot, and slow evaporation of the filtrate at ambient temperature afforded an oily, pale blue residue, which solidified to an undefined, almost colorless solid upon addition of a few drops ethanol.
Synthesis of Zn2[Pd(L)2] (4-Pd): Similar as previously described for the related 4-Pt [6], a mixture of [Pd(H2L)2] (2-Pd; 0.48 mg, 1.0 mmol) and zinc acetate–dihydrate (0.88 g, 4.0 mmol) in 80 mL water was stirred at ambient temperature until the precursor complex had completely dissolved (30–60 min). The solution was filtered if necessary, then carefully layered with 80 mL acetone and allowed to stand for a few days. Subsequently, the precipitated yellow, needle-like crystals of 4-Pd · 14H2O were isolated by vacuum filtration, washed with ethanol and acetone and dried at 40 °C overnight. The so-obtained microcrystalline powders are slightly soluble in water and insoluble in organic solvents. Yield of 4-Pd · 12H2O: 0.76 g (88%). Dec. 377 °C. Anal. calcd. for C10H32N2O20PdS4Zn2 (M = 865.80 g mol−1): C 13.87, H 3.72, N 3.24, S 14.81%; found: C 13.84, H 3.79, N 3.23, S 14.74%. 1H NMR (D2O): δ 4.29 (s; CH2) ppm. 13C NMR (D2O): 53.6 (CH2), 173.7 (COO), 211.0 (CSS) ppm. IR: ν 3596(w br), 3515(w br), 3287(w br), 2978(w), 1588(s), 1496(s), 1425(m), 1395(sh), 1387(s), 1309(s), 1292(sh), 1240(sh), 1228(s), 1175(m), 1028(m), 1001(w), 953(m), 910(w), 735(s), 716(sh), 693(sh), 621(s), 613(sh), 607(sh), 573(sh), 557(s), 535(sh), 401(w br), 354(sh), 344(s), 291(w), 266(w), 256(w) cm−1.
Synthesis of Zn2[Ni(L)2] (4-Ni and 5-Ni): Compound 4-Ni was prepared similarly as described for 4-Pd, using [Ni(H2L)2] (2-Ni; 0.48 g, 1.0 mmol). Green, needle-like crystals of 4-Ni · 14H2O were obtained from a dark green solution, that turned into green, microcrystalline powder upon isolation. Yield of 4-Ni · 2H2O: 0.52 g (81%). Dec. 387 °C. Anal. calcd. for C10H12N2NiO10S4Zn2 (M = 637.92 g mol−1): C 18.83, H 1.90, N 4.39, S 20.10%; found: C 18.85, H 1.90, N 4.41, S 20.14%. 1H NMR (D2O): δ 4.17 (s; CH2) ppm. 13C NMR (D2O): 53.4 (CH2), 173.6 (COO), 207.2 (CSS) ppm. IR: ν 2929(w), 1652(sh), 1575(s), 1483(s), 1428(m), 1387(s), 1306(s), 1281(sh), 1226(s), 1168(m), 1028(m), 1007(m), 953(m), 917(m), 747(m), 730(sh), 714(sh), 648(sh), 622(s), 613(sh), 603(w), 596(sh), 573(sh), 546(m), 388(s), 369(sh), 298(s), 285(sh), 267(sh), 238(w), 223(sh), 205(sh) cm−1. When the reaction solution was stirred at 80 °C for 2 h, the initial dark green color changed to pale brownish-green. After cooling to ambient temperature, the precipitated yellow-green solid was isolated by vacuum filtration, washed with ethanol and acetone and dried at 40 °C overnight. Yield of 5-Ni · 2H2O: 0.26 g (41%). Dec. 307 °C. Anal. calcd. for C10H12N2NiO10S4Zn2 (M = 637.92 g mol−1): C 18.83, H 1.90, N 4.39, S 20.10%; found: C 18.83, H 1.92, N 4.38, S 20.12%. 1H NMR (D2O): δ 4.19 (s; CH2) ppm. 13C NMR (D2O): 53.4 (CH2), 173.6 (COO), 207.2 (CSS) ppm. IR: ν 3363(m br), 3223(w br), 2935(w), 2901(w), 1642(m), 1579(s), 1552(sh), 1471(m), 1448(s), 1424(s), 1402(m), 1380(s), 1324(m), 1300(s), 1293(sh), 1217(s), 1191(m), 1023(s), 960(m), 939(w), 912(m), 742(w), 709(s), 668(s), 640(m), 623(sh), 613(w), 602(sh), 593(sh), 580(m), 562(w), 553(w), 490(w br), 411(sh), 398(m), 379(m), 369(sh), 339(w), 305(sh), 281(m), 253(s), 236(s), 210(m), 202(sh) cm−1. Single crystals of 4-Ni · 2.5acetone · 8H2O suitable for X-ray structure determination were formed at ambient temperature when the aqueous filtrate was carefully layered with 80 mL acetone.
3. Results
3.1. Precoursor Complexes
Heterobimetallic coordination polymers with DTCC ligands having Ni or Pd as a thiophilic metal center were prepared in a two-step synthesis starting from the potassium salt of the appropriate DTCC ligand, K3(L) (1; L = {SSC-N(CH2COO)2}3−). Aqueous solutions of 1 react cleanly with PdCl2 or NiCl2 · 6H2O under selective complexation of the dithiocarbamate moiety of the ligand. The resulting complexes are most conveniently isolated in their poorly soluble, protonated forms, [M(H2L)2] (2; M = Ni, Pd; Scheme 2). The isolated yields of 89% (2-Pd) and 81% (2-Ni) were similar to those reported by Leka et al., using the ammonium salt (NH4)3(L) as a precursor [8]. In this previous contribution, the products were characterized by IR spectroscopy and thermal analyses, but for better comparison with our target products, we provide our own analysis data together with NMR data of 2-Pd and 2-Ni. According to Leka et al., the complexes decompose under air at ca. 250 °C (2-Pd) and 230 °C (2-Ni), respectively, under elimination of CO2 [8]. In our hands, similar values at 248 °C (2-Pd) and 217 °C (2-Ni) were obtained under an inert atmosphere of nitrogen (Table 1), which also indicate that 2-Pd exhibits a comparable thermal stability to the related 2-Pt (Dec. 241 °C, CO2 loss) [6], while 2-Ni is slightly less thermally stable. While palladium(II) and platinum(II) usually adopt a square-planar coordination in which the metal center is diamagnetic [13,14], nickel(II) is also able to realize tetrahedral or octahedral coordinations, in which it is paramagnetic to the extent of two unpaired electrons each [14,15,16]. However, determination of the magnetic susceptibility of 2-Ni confirmed that this compound is certainly diamagnetic; therefore suggesting that the compound is structurally related to 2-Pd and 2-Pt, comprising a square-planar coordinated metal center. In the IR spectra, strong bands around 1700 cm−1 are diagnostical for the protonated carboxylate groups. The νas(M-S) bands of the M(dithiocarbamate)2 core between 300 and 400 cm−1 were assigned by comparison with the literature data [17], which was in agreement with the assignment done by Leka et al. for 2-Ni, but different for 2-Pd [8]. The 1H and 13C NMR in DMSO-D6 are also very similar for all three compounds, containing one set of signals of the ligand L. The observation of sharp signals in the NMR spectra of 2-Ni with similar chemical shifts as for 2-Pd and 2-Pt also confirmed the diamagnetic nature of this compound. However, one exception is the 13C signal of the CSS group, which was observed at 209.0 ppm for 2-Ni. Compound 2-Pd gave a CSS signal that was significantly downfield-shifted at 213.4 ppm, which is much closer to the value observed for 2-Pt (214.4 pm) [6]. The COOH chemical shift is virtually identical at ca. 168 ppm for all three compounds.
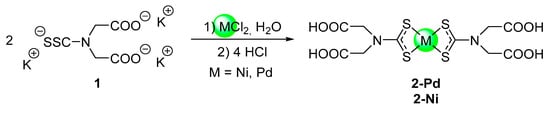
Scheme 2.
Preparation of the complexes [M(H2L)2] (2; M = Ni, Pd) from the potassium DTCC salt 1.

Table 1.
Selected characterization data for [M(H2L)2] (2), Zn2[M(L)2] (4 and 5; M = Ni, Pd, Pt).
3.2. Heterobimetallic Coordination Polymers
The complexes 2-Pd and 2-Ni showed a similar reactivity with zinc acetate as reported previously for 2-Pt [6] and dissolve readily in aqueous solutions of the zinc salt. However, the isolation of partially metallated products of the type Zn[M(HL)2] (3) failed in both cases. For palladium, single crystals of 3-Pd · 6H2O were obtained after treatment with a stoichiometric amount of Zn(OAc)2, which decomposed to 2-Pd and the fully metallated form Zn2[Pd(L)2] (4-Pd; vide infra) upon attempted isolation. Therefore, we surmise that the palladium system behaves somewhat similar to the platinum system regarding stepwise metalation of the COOH groups, but the intermediate 3-Pd is less stable against disproportionation than observed for 3-Pt (Scheme 3). Compound 2-Ni showed a less clear behavior when treated with one equiv. Zn(OAc)2 under comparable conditions, resulting in the formation of an undefined, light blue product mixture.
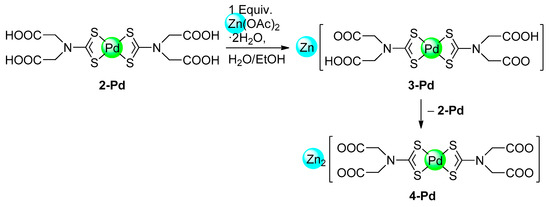
Scheme 3.
Formation and assumed disproportionation of Zn[Pd(HL)2] (3-Pd).
Single-crystal X-ray structure determination of 3-Pd · 6H2O revealed that this compound is isotypic with 3-Pt · 6H2O [6], existing as a one-dimensional polymer in the solid state (Figure 1). The Pd and Zn atoms are situated on crystallographic centers of inversion and adopt a typical square planar or octahedral coordination, respectively. The Pd-S bond lengths are similar at 231.6(1) and 232.4(1) pm, which is within the typical range observed for palladium(II) bis(dithiocarbamate) complexes [7]. The Zn atom is coordinated by two monodentate carboxylate groups in a trans arrangement (O-Zn-O = 180°), and coordinatively saturated by four H2O ligands. Similar as in the related 3-Pt · 6H2O, the resulting Zn(H2O)4 fragment shows rotational disorder around the (CO)O-Zn-O(CO) vector [6]. The Zn-O(carboxylate) bond length is 212.3(3) pm and, therefore, virtually identical with that in 3-Pt · 6H2O (212.0(2) pm). The polymeric chain structure is supported by intramolecular O-H···O bonds between H2O ligands and carboxylate C=O moieties, and the chains are cross-linked by additional O-H···O bonds involving COOH groups and H2O ligands. Numerous zinc bis(carboxylates) have been reported in which the coordinative environment of the Zn atom is similar as in 3-Pd · 6H2O, e.g., with CSD [7] refcode RAYRAM (Zn-O 217.8(1) pm) [18] and UNEQUB (Zn-O 206.5(5) pm) [19].
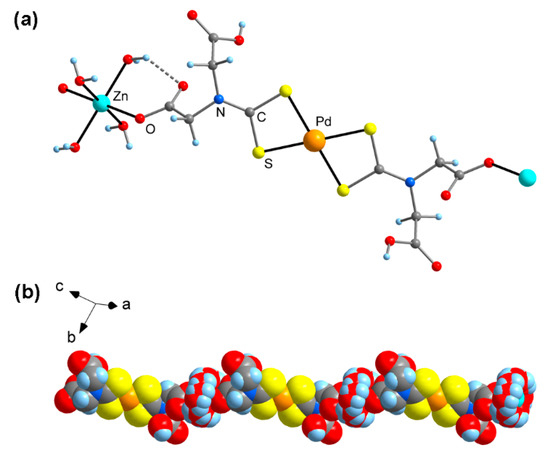
Figure 1.
Molecular structure of Zn[Pd(HL)2] (3-Pd) · 6H2O in the crystal (non-coordinated crystal water omitted for clarity). (a) Ball-and-stick model of one formula unit; (b) Representation of the one-dimensional polymeric structure (space-filling model, showing rotational disorder of the Zn(H2O)4 units).
Both 2-Pd and 2-Ni reacted cleanly with excess zinc acetate at ambient temperature (Scheme 4). The so-obtained aqueous solutions afforded crystals of Zn2[M(L)2] (4; M = Ni, Pd) · 14H2O, which showed partial loss of crystal water upon isolation. Accordingly, yellow Zn2[Pd(L)2] (4-Pd) · 12H2O and green Zn2[Ni(L)2] (4-Ni) · 2H2O were obtained in good isolated yields. However, running the reaction of 2-Ni at elevated temperature led to color change of the solution from intense green to pale brownish-green (cf. Figure S1 in Supplementary Information, SI), and a yellow-green powder (5-Ni) could be isolated. This product gave identical elemental analyses results as 4-Ni, and, therefore, it could be assumed that its composition was also Zn2Ni(L)2 · 2H2O. All three products 4-Pd, 4-Ni, and 5-Ni are sparingly soluble in water and insoluble in the usual set of organic solvents. We forewent characterization of the products by powder X-ray diffraction in order to prove their phase purity, as the partial crystal water loss and potential presence of more than one crystalline polymorphic form did not allow for a reasonable comparison with the single-crystal data. These problems were outlined in detail previously for a series of related Zn/Pt coordination polymers [6]. Upon heating the products under nitrogen, the residual water was completely released around 110 °C for 4-Pd and 4-Ni, and at 127 °C for 5-Ni (cf. Figure S6 in the SI). Thermal decomposition was detected at 377 °C (4-Pd) and 348 °C (4-Ni), which is lower than for the related 4-Pt (398 °C [6]; cf. Table 1). However, the decomposition temperature of 5-Ni is considerably increased to 387 °C as compared to 4-Ni. Magnetic measurements revealed that both nickel compounds 4-Ni and 5-Ni are diamagnetic, and, therefore, an exchange of the Ni and Zn sites could be ruled out for 5-Ni. Coordination of Ni2+ by carboxylic groups would most likely lead to an octahedral coordination and, therefore, to a paramagnetic nature of the compound.
Scheme 4.
Preparation of the heterobimetallic compounds Zn2[M(L)2] (4; M = Ni, Pd) from the protonated metalloligands 2.
Similar to what was reported for the platinum compound [6], the deprotonation of the carboxylic acid groups in 2-Pd and 2-Ni can be easily monitored by IR spectroscopy, as the νC-O bands are significantly shifted from ca. 1700 cm−1 to smaller wavenumbers (cf. Figure S3 in the SI). The spectra of 4-Pd · 12H2O and 4-Ni ·2 H2O are almost identical with that of 4-Pt · 10H2O and each display two strong bands between 1600 and 1480 cm−1 [6]. IR spectroscopy of 5-Ni · 2H2O revealed an additional νC-O band at 1642 cm−1, which is in agreement with the more complex coordination pattern of the carboxylate groups in this compound (vide infra). In all four products, the position of the νas(M-S) band between 300 and 400 cm−1 is comparable with those in the respective precursor compounds 2 (cf. Table 1). The 1H and 13C NMR in D2O are again very similar for all compounds, containing one set of signals of the ligand L (cf. Figures S4 and S5 in the SI). The NMR spectra of 4-Ni · 2H2O and 5-Ni · 2H2O showed sharp signals in the typical chemical shift ranges (e.g., for CH2, δH 4.3–4.5 ppm and δC 51–54 ppm), thus confirming the diamagnetic nature of these products. The 13C chemical shifts of the CSS group in the two nickel compounds are identical at 207.2 pm and, therefore, significantly upfield-shifted as compared to 4-Pd (211.0 ppm) and 4-Pt (211.6 pm) [6]. The COO chemical shift is virtually identical at ca. 174 ppm for all compounds, which is ca. 6 ppm downfield-shifted as compared to the COOH signals in compounds 2.
The crystal structures of 4-Pd ·14H2O and 4-Ni · 14H2O are isotypic with that of 4-Pt · 14H2O [6]. The Pd or Ni center is situated on a crystallographic center of inversion and is coordinated by two chelating dithiocarbamate groups in a square-planar fashion (Figure 2a). The Pd-S bond lengths in 4-Pd · 14H2O are virtually identical with those in 3-Pd · 6H2O (Table 2). Similarly, the Ni-S bonds in 4-Ni · 14H2O do not differ significantly in length, and are in good agreement with the values observed for other nickel bis(dithiocarbamate) complexes [7]. The Zn atoms display an octahedral coordination by two monodentate carboxylate groups and four H2O ligands. Thereby, different from 3-Pd · 6H2O, the carboxalate moieties are in a cis arrangement with virtually identical O-Zn-O coordination angles at ca. 97°. The two Zn-OOC bonds are slightly different in length, and the respective values of ca. 204 and 207 pm are virtually identical within the series 4-Ni/4-Pd/4-Pt. The cis-carboxylate coordination is relatively rare and has been observed in only few other zinc bis(carboxylates), e.g., with CSD [7] refcode GUQVAR (Zn-O 206.8(2) pm, O-Zn-O 91.0(1) pm) [20] and XOKKEQ (Zn-O 206.3(1) pm, O-Zn-O 92.06(6) pm) [21]. This connectivity between Zn2+ ions and [M(L)2]4− metalloligands results in the formation of puckered, two-dimensional arrays with significant porosity (Figure 2b). The vacancies are filled with non-coordinated water of crystallization and have a size of approx. 0.5 × 0.7 nm (M···H2C and Zn-OH2···H2O-Zn separations, respectively). The layers are linked in the third dimension by Zn-OH2···H2O-Zn hydrogen bonds.
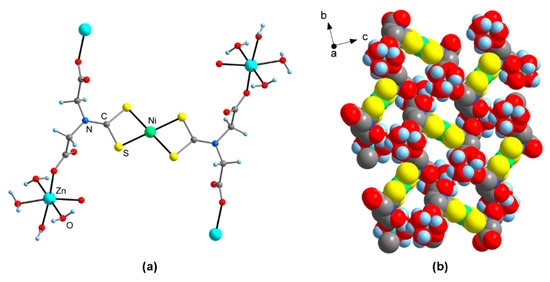
Figure 2.
Molecular structure of Zn2[M(H2L)2] (4; M = Ni, Pd) · 14H2O in the crystal, as exemplified at 4-Ni · 14H2O (non-coordinated crystal water omitted for clarity). (a) Ball-and-stick model of one formula unit; (b) Representation of the two-dimensional polymeric structure (space-filling model).

Table 2.
Selected interatomic distances (pm) and angles (deg.) in heterobimetallic coordination polymers of the type Zn2[M(L)2] (M = Ni, Pd, Pt).
The crystal structure of 5-Ni · 2.5acetone · 8.5H2O comprises two symmetry-independent, centrosymmetric [Ni(L)2]4− ions, and two different Zn2+ sites. Five out of the 8.5 equiv. H2O of crystallization are zinc-coordinated. The coordination of the Ni center is virtually identical as in 4-Ni · 14H2O, while the environment of the Zn atoms is considerably different. While in 4-Ni · 14H2O, all carboxylate groups are coordinated to Zn in a monodentate mode, this applies to only one of the two carboxylate groups of each L3− ligand in solvated 5-Ni (Figure 3a). The other COO− moiety adopts a syn,syn-arranged κO:κO′-bridging coordination to two symmetry-equivalent Zn atoms, resulting in the formation of centrosymmetric zinc-carboxylate dimeric units. This connectivity is supported by a µ-bridging H2O ligand between the two Zn atoms. Another carboxylate moiety of an adjacent [Ni(L)2]4− metalloligand is coordinated to each Zn atom in a monodentate mode, and this ligating moiety is further fixed by an O-H···O bond with the µ-H2O ligand. For each Zn atom, octahedral coordination is completed by two additional, terminal H2O ligands. In total, the Zn atoms in acetone/H2O-solvated 5-Ni are coordinated by three carboxylate O atoms and three H2O ligands in a meridional arrangement. In the resulting cage-like [Zn2(carboxylate-κO:κO′)2(carboxylate-κO)2(µ-H2O)] structure, the Zn-O bonds to the terminal carboxylate groups are similar in lengths as in compounds 4 · 14H2O, while the bridging COO moieties are significantly elongated to 214.2(4)–214.8(4) pm. Several hydrated zinc bis(carboxylates), which are structurally related to 5-Ni, have been reported. In these compounds, the Zn-O bonds to bridging carboxylate groups are often shorter than those to terminal carboxylate groups, e.g., with CSD [7] refcode KEGHUA (Zn-O(bridge) 208.4(2)–208.7(1) pm, Zn-O(terminal) 213.8(1)–214.7(2) pm) [22] and LUPCOQ (Zn-O(bridge) 208.7(2)–210.4(2) pm, Zn-O(terminal) 219.2(2) pm) [23]. Similar to that for 4-Ni, the polymeric structure of 5-Ni extends in two dimensions (Figure 3b). The solvent-filled voids have a size of ca. 0.8 × 1.0 nm (Ni···Ni and OH2···OOC distances, respectively) and are, therefore, larger than in 4-Ni.
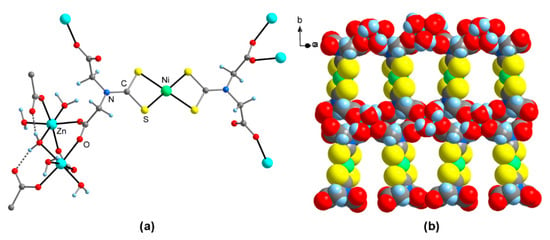
Figure 3.
Molecular structure of Zn2[Ni(H2L)2] (5-Ni) · 2.5acetone · 8.5H2O in the crystal (non-coordinated solvent of crystallization omitted for clarity). (a) Balls-and-sticks model of one formula unit; (b) Representation of the two-dimensional polymeric structure (space-filling model).
4. Discussion and Conclusions
Heterobimetallic coordination polymers with the dithiocarbamato-carboxylate (DTCC) ligand {SSC-N(CH2COO)2}3− (= L) are deliberately available in a sequential synthetic approach. In this study, we have demonstrated that platinum(II) as a thiophilic metal center can be easily replaced by the cheaper palladium or nickel in their divalent oxidation states. Therefore, the metalloligands [M(H2L)2] (2; M = Pd, Ni) react cleanly with aqueous solutions of excess Zn(OAc)2 to afford Zn2[M(L)2] (4) in good yields. The products are structurally virtually identical with the Pt-analog compound, but exhibit slightly lower thermal stabilities. However, the relatively high decomposition temperature of 5-Ni indicated that the thermal properties depend not only on the thiophilic metal, but also on the bonding situation of the carboxylate groups. Generally, the results are particularly surprising in view of the richer redox and coordination chemistry of nickel as compared to palladium and platinum, which makes it difficult to substitute the heavier group 10 metals by their lightest homolog in many cases. For instance, Ni2+ can switch quite easily between different coordination polyhedra and is able to adopt an octahedral coordination similar as Zn2+ does. Moreover, Ni2+ is much more oxophilic than divalent Pd and Pt, and consequently, it can be expected that coordination by the carboxylate groups instead of the dithiocarbamate moiety should be also possible. It is, therefore, a particularly interesting finding that an exchange of the metal sites has not been observed for the Ni/Zn system, that is, even nickel is complexed highly selective by the CSS− group. However, the outcome of the reactions with zinc acetate was found to be not entirely independent from the choice of the thiophilic metal. Firstly, the partially protonated species Zn[M(HL)2] (3) could not be prepared in a straightforward manner as it has been done for M = Pt, as the stability towards disproportionation into 2 and 4 seems to decrease in going from Pt to Pd to Ni. Secondly, the reaction of 2-Ni with excess zinc acetate afforded a new structural variant of Zn2[Ni(L)2] (5-Ni) when the reaction solution was heated; comparable products were not obtained with Pd or Pt. In both cases, it is improbable that these differences arise from electronic differences between the thiophilic metals, as the metal bis(dithiocarbamate) core and the carboxylate groups are well-separated by aliphatic CH2 spacers. More likely, the thiophilic metal center may exert some influence on the solubility of different complex species, and, therefore, on which product crystallizes first. The different colors of the reaction mixtures of 4-Ni (formed at room temperature) and 5-Ni (formed at 80 °C) effectively illustrated that in solution not only are solvent-separated Zn2+ ions and [M(L)2]4− metalloligands present, but different aggregates can form under different conditions. The formation of such aggregates seems to be irreversible to some extent, since the pale brownish-green solution of 5-Ni did not change back to the intense green color of 4-Ni upon cooling to ambient temperature. In future research, we will investigate the impact of the oxophilic metal ions on the formation, structures and properties of heterobimetallic coordination polymers with DTCC ligands.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/6/505/s1. Photographic images of reaction solutions and single crystals, details on single-crystal X-ray structural analyses.
Author Contributions
Conceptualization, P.L.; methodology, P.L.; validation, P.L. and F.O.; formal analysis, P.L. and F.O.; investigation, P.L. and J.W.; data curation, P.L., F.O. and J.W.; writing—original draft preparation, P.L.; writing—review and editing, P.L. and F.O.; visualization, P.L.; supervision, P.L.; project administration, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
General financial support by the Otto von Guericke University is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, S.R.; Du, D.Y.; Tan, K.; Qin, J.S.; Dong, H.Q.; Li, S.L.; He, W.W.; Lan, Y.Q.; Shen, P.; Su, Z.M. Self-assembly versus stepwise synthesis: Heterometal-organic frameworks based on metalloligands with tunable luminescence properties. Chem. Eur. J. 2013, 19, 11279–11286. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-López, L.; Valverde-Muñoz, F.J.; Seredyuk, M.; Muñoz, M.C.; Haukka, M.; Real, J.A. Guest Induced Strong Cooperative One- and Two-Step Spin Transitions in Highly Porous Iron(II) Hofmann-Type Metal-Organic Frameworks. Inorg. Chem. 2017, 56, 7038–7047. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.Z.; Xu, H.; Cheng, P.; Zhao, B. 3d-4f Heterometal-Organic Frameworks for Efficient Capture and Conversion of CO2. Cryst. Growth Des. 2017, 17, 3128–3133. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Chen, J.; Zhang, X.; Wang, L.; Xu, N.; Yang, G.; Cheng, P. A multi-responsive luminescent sensor for organic small-molecule pollutants and metal ions based on a 4d-4f metal-organic framework. Dalton Trans. 2017, 46, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, G. Transition Metal Dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2005; Volume 53, ISBN 0471463701. [Google Scholar]
- Liebing, P.; Witzorke, J.; Oehler, F.; Schmeide, M. Dithiocarbamatocarboxylate (DTCC) Ligands—Building Blocks for Hard/Soft-Heterobimetallic Coordination Polymers. Inorg. Chem. 2020, 59, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- Leka, Z.B.; Leovac, V.M.; Lukić, S.; Sabo, T.J.; Trifunović, S.R.; Szécsényi, K.M. Synthesis and physico-chemical characterization of new dithiocarabamato ligand and its complexes with copper(II), nickel(II) and palladium(II). J. Therm. Anal. Calorim. 2006, 83, 687–691. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Stoe & Cie, X-Area, X-Step and X-Shape; Stoe & Cie: Darmstadt, Germany, 2002.
- Kamimura, H.; Koide, S.; Sekiyama, H.; Sugano, S. Magnetic Properties of the Pd and Pt Group Transition Metal Complexes. J. Phys. Soc. Jpn. 1960, 15, 1264–1272. [Google Scholar] [CrossRef]
- Banks, C.V.; Haar, R.W.V.; Wal, R.P.V. Magnetic Studies of Nickel(II) and Palladium(II) Complexes with Some vic-Dioximes. J. Am. Chem. Soc. 1955, 77, 324–325. [Google Scholar] [CrossRef]
- Russell, C.D.; Cooper, G.R.; Vosburgh, W.C. Complex Ions. V. The Magnetic Moments of Some Complex Ions of Nickel and Copper. J. Am. Chem. Soc. 1943, 65, 1301–1306. [Google Scholar] [CrossRef]
- Willis, J.B.; Mellor, D.P. The Magnetic Susceptibility of Some Nickel Complexes in Solution. J. Am. Chem. Soc. 1947, 69, 1237–1240. [Google Scholar] [CrossRef]
- Ojima, I.; Onishi, T.; Iwamoto, T.; Inamoto, N.; Tamaru, K. A new route to metal chelates of dimethyldithiocarbamate and their far infrared spectra. Inorg. Nucl. Chem. Lett. 1970, 6, 65–69. [Google Scholar] [CrossRef]
- Tepavitcharova, S.; Rabadjieva, D.; Havlíček, D.; Němec, I.; Vojtíšek, P.; Plocek, J.; Koleva, Z. Crystallization and characterization of the compounds Gly·MSO 4·mH2O (M = Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+; M = 0, 3, 5, 6). J. Mol. Struct. 2012, 1018, 113–121. [Google Scholar] [CrossRef]
- Marandi, F.; Hashemi, L.; Morsali, A.; Krautscheid, H. Sonochemical synthesis and characterization of three nano zinc(II) coordination polymers; Precursors for preparation of zinc(II) oxide nanoparticles. Ultrason. Sonochem. 2016, 32, 86–94. [Google Scholar] [CrossRef]
- Okamura, T.A.; Furuya, R.; Onitsuka, K. Regulation of the hydrolytic activity of Mg2+-dependent phosphatase models by intramolecular NH···O hydrogen bonds. J. Am. Chem. Soc. 2014, 136, 14639–14641. [Google Scholar] [CrossRef]
- Larionov, S.V.; Rakhmanova, M.I.; Glinskaya, L.A.; Naumov, D.Y.; Vinogradov, A.S.; Karpov, V.M.; Platonov, V.E.; Fadeeva, V.P. Zinc(II) Complexes with Tetrafluoroterephthalic and Octafluorobiphenyl-4,4′-dicarboxylic Acid Anions and 1,10-Phenanthroline. Russ. J. Gen. Chem. 2019, 89, 261–265. [Google Scholar] [CrossRef]
- Brown, D.A.; Fitzpatrick, N.J.; Muller-Bunz, H.; Ryan, Á.T. Di-, tri-, and tetranuclear zinc hydroxamate complexes as structural models for the inhibition of zinc hydrolases by hydroxamic acids. Inorg. Chem. 2006, 45, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-P.; Chen, J.; Mu, Y.-H.; Du, M. Exceptional sensitivity to the synthetic approach and halogen substituent for Zn(ii) coordination assemblies with 5-halonicotinic acids. Dalton Trans. 2015, 44, 11109–11118. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
