Morpholino-Substituted BODIPY Species: Synthesis, Structure and Electrochemical Studies
Abstract
1. Introduction
2. Experimental Part
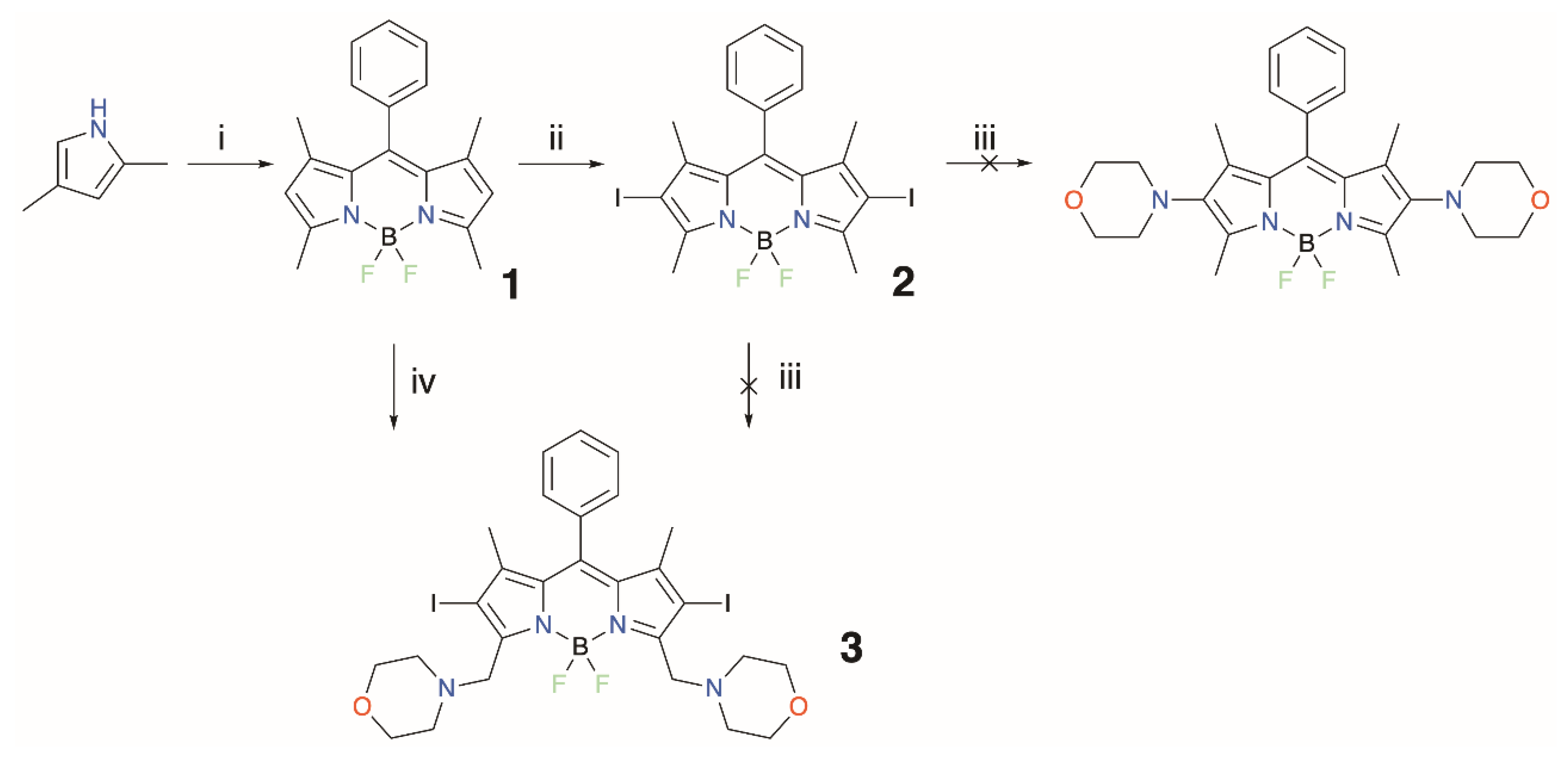
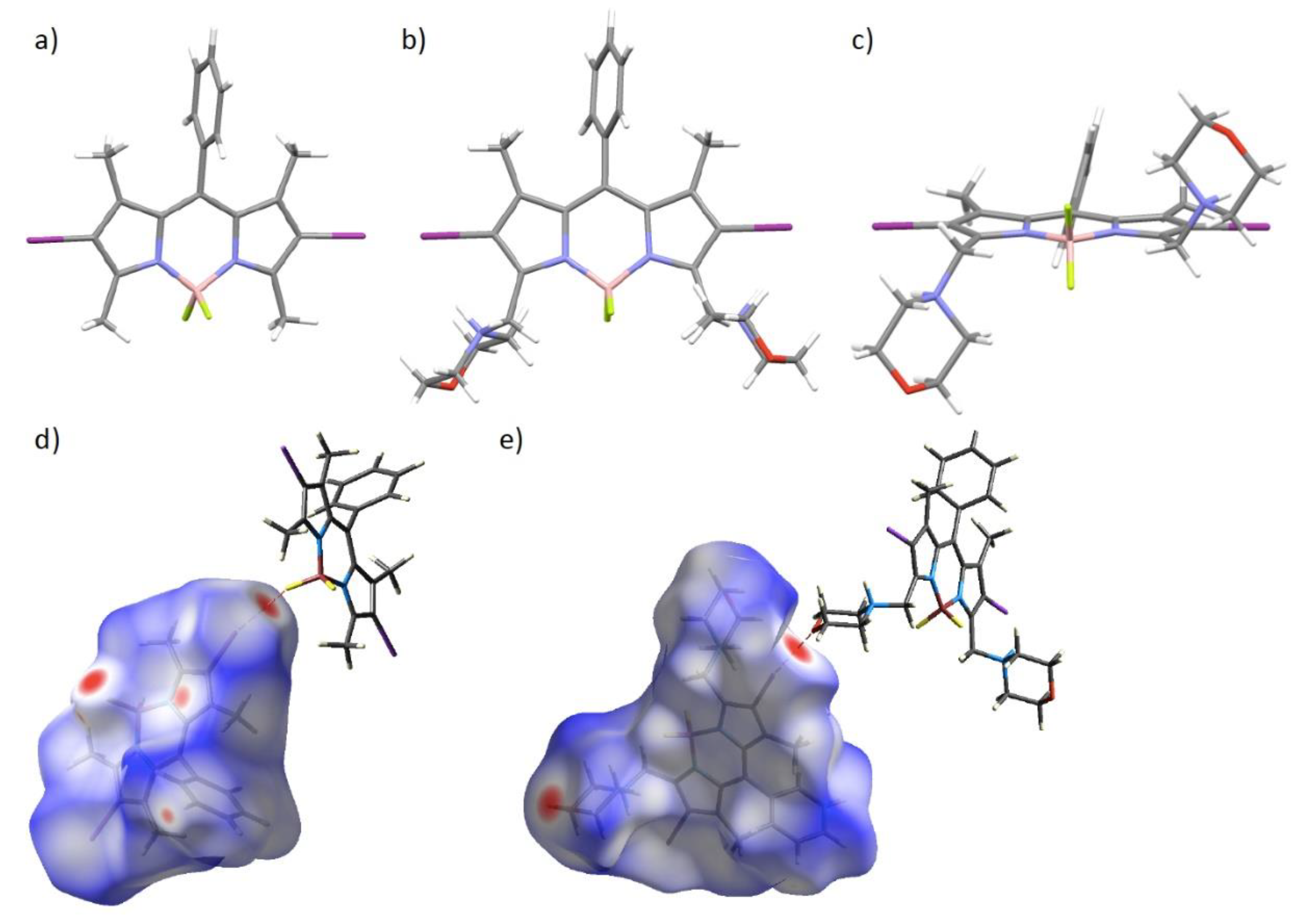
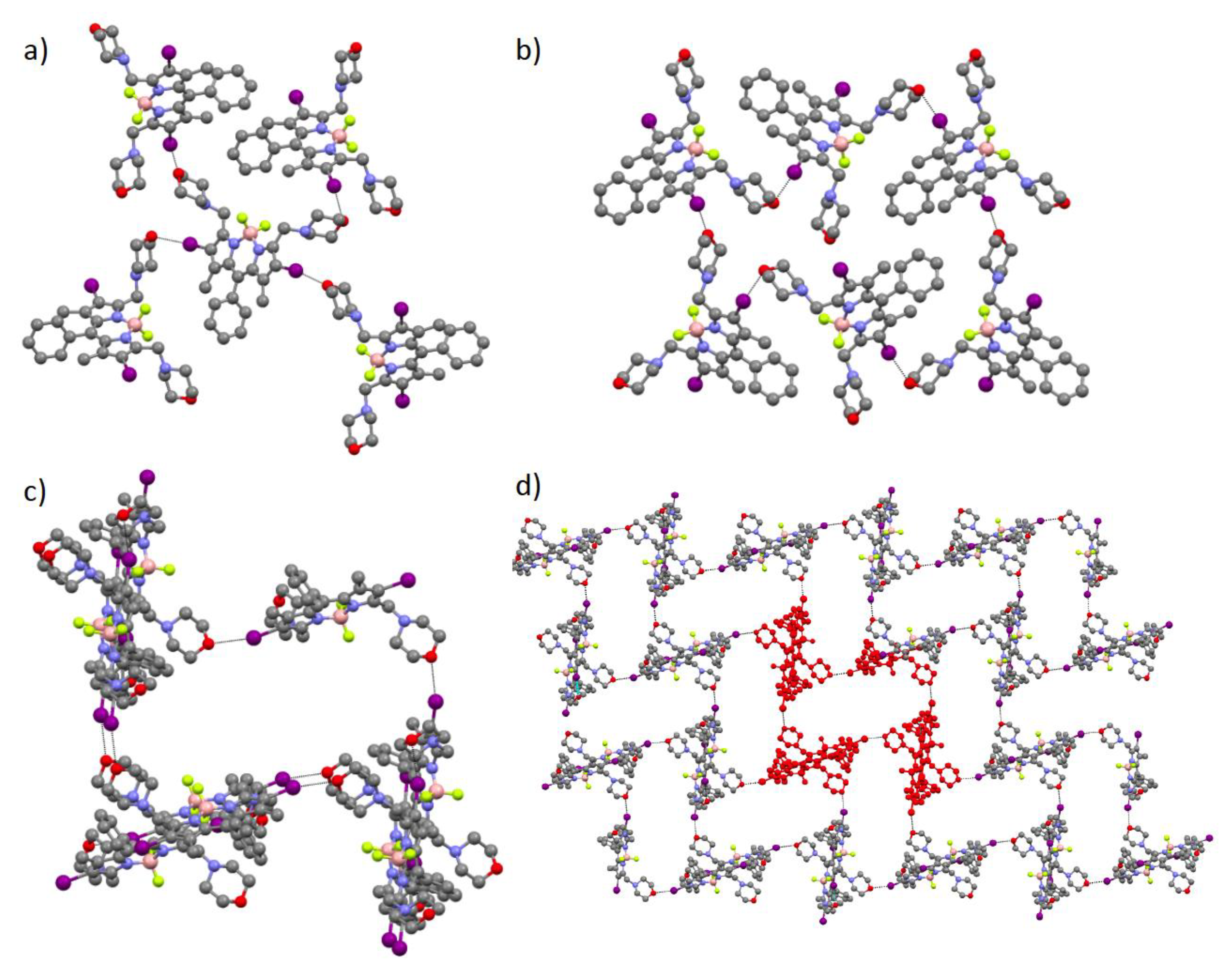
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.-W.; Cui, A.-J.; Cai, X.-X.; Wan, Y.; Chen, Q.; He, M.-Y.; Zhang, W. Regioselective 2,6-dihalogenation of BODIPYs in 1,1,1,3,3,3-hexafluoro-2-propanol and preparation of novel meso-alkyl polymeric BODIPY dyes. RSC Adv. 2013, 3, 9219–9222. [Google Scholar] [CrossRef]
- Wagner, R.W.; Lindsey, J.S. Boron-dipyrromethene dyes for incorporation in synthetic multi-pigment light-harvesting arrays. Pure Appl. Chem. 1996, 68, 1373–1380. [Google Scholar] [CrossRef]
- El-Khouly, M.E.; Fukuzumi, S.; D’Souza, F. Photosynthetic Antenna–Reaction Center Mimicry by Using Boron Dipyrromethene Sensitizers. ChemPhysChem 2014, 15, 30–47. [Google Scholar] [CrossRef]
- Kumaresan, D.; Thummel, R.P.; Bura, T.; Ulrich, G.; Ziessel, R. Color Tuning in New Metal-Free Organic Sensitizers (Bodipys) for Dye-Sensitized Solar Cells. Chem. Eur. J. 2009, 15, 6335–6339. [Google Scholar] [CrossRef]
- Coskun, A.; Deniz, E.; Akkaya, E.U. Effective PET and ICT Switching of Boradiazaindacene Emission: A Unimolecular, Emission-Mode, Molecular Half-Subtractor with Reconfigurable Logic Gates. Org. Lett. 2005, 7, 5187–5189. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, M.; Wei, Y.; Zhou, X.; Liu, S.; Zhang, S.; Zhang, B. Solvent effects on the triplet–triplet annihilation upconversion of diiodo-Bodipy and perylene. Phys. Chem. Chem. Phys. 2017, 19, 1516–1525. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Wu, S.; Wang, Z.; Wu, W.; Ma, J.; Guo, S.; Huang, L. Intramolecular RET Enhanced Visible Light-Absorbing Bodipy Organic Triplet Photosensitizers and Application in Photooxidation and Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2013, 135, 10566–10578. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Haefele, A. A General Synthetic Route to 3,5-Substituted Boron Dipyrromethenes: Applications and Properties. J. Org. Chem. 2012, 77, 4298–4311. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef]
- Ziessel, R.; Bonardi, L.; Retailleau, P.; Ulrich, G. Isocyanate-, Isothiocyanate-, Urea-, and Thiourea-Substituted Boron Dipyrromethene Dyes as Fluorescent Probes. J. Org. Chem. 2006, 71, 3093–3102. [Google Scholar] [CrossRef]
- Moon, S.Y.; Cha, N.R.; Kim, Y.H.; Chang, S.K. New Hg2+-selective chromo- and fluoroionophore based upon 8-hydroxyquinoline. J. Org. Chem. 2004, 69, 181–183. [Google Scholar] [CrossRef]
- Li, G.; Otsuka, Y.; Matsumiya, T.; Suzuki, T.; Li, J.; Takahashi, M.; Yamada, K. A Straightforward Substitution Strategy to Tune BODIPY Dyes Spanning the Near-Infrared Region via Suzuki–Miyaura Cross-Coupling. Materials 2018, 11, 1297. [Google Scholar] [CrossRef]
- Li, L.; Nguyen, B.; Burgess, K. Functionalization of the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core. Bioorg. Med. Chem. Lett. 2008, 18, 3112–3116. [Google Scholar] [CrossRef]
- Rohand, T.; Baruah, M.; Qin, W.; Boens, N.; Dehaen, W. Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem. Commun. 2006, 266–268. [Google Scholar] [CrossRef]
- Leen, V.; Braeken, E.; Luckermans, K.; Jackers, C.; Van der Auweraer, M.; Boens, N.; Dehaen, W. 2- and 3-Monohalogenated BODIPY Dyes and Their Functionalized Analogues: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2011, 4386–4396. [Google Scholar] [CrossRef]
- Lakshmi, V.; Rajeswara Rao, M.; Ravikanth, M. Halogenated boron-dipyrromethenes: Synthesis, properties and applications. Org. Biomol. Chem. 2015, 13, 2501–2517. [Google Scholar] [CrossRef]
- Rohand, T.; Qin, W.; Boens, N.; Dehaen, W. Palladium-Catalyzed Coupling Reactions for the Functionalization of BODIPY Dyes with Fluorescence Spanning the Visible Spectrum. Eur. J. Org. Chem. 2006, 4658–4663. [Google Scholar] [CrossRef]
- Leen, V.; Braeken, E.; Luckermans, K.; Jackers, C.; Van der Auweraer, M.; Boens, N.; Dehaen, W. A versatile, modular synthesis of monofunctionalized BODIPY dyes. Chem. Commun. 2009, 4515–4517. [Google Scholar] [CrossRef]
- Zhao, N.; Xuan, S.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Stepwise Polychlorination of 8-Chloro-BODIPY and Regioselective Functionalization of 2,3,5,6,8-Pentachloro-BODIPY. J. Org. Chem. 2015, 80, 8377–8383. [Google Scholar] [CrossRef] [PubMed]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Kubas, G.J.; Monzyk, B.; Crumblis, A.L. Tetrakis(Acetonitrile)Copper(I) Hexafluorophosphate. Inorg. Synth. 2007, 68. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A: Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, M.A.S.D. CrystalExplorer (Version 3.1); University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, M.A.S.D. CrystalExplorer (Version 17.5); University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Fu, G.-L.; Pan, H.; Zhao, Y.-H.; Zhao, C.-H. Solid-state emissive triarylborane-based BODIPY dyes: Photophysical properties and fluorescent sensing for fluoride and cyanide ions. Org. Biomol. Chem. 2011, 9, 8141–8146. [Google Scholar] [CrossRef]
- Hassanain, H.; Davies, E.S.; Lewis, W.; Kays, D.L.; Champness, N.R. Structural characterization and optical properties of two copper(I)-iodide BODIPY coordination polymers. CrystEngComm 2019, 21, 4551–4556. [Google Scholar] [CrossRef]
- Gorbe, M.; Costero, A.M.; Sancenón, F.; Martínez-Máñez, R.; Ballesteros-Cillero, R.; Ochando, L.E.; Chulvi, K.; Gotor, R.; Gil, S. Halogen-containing BODIPY derivatives for photodynamic therapy. Dye. Pigment. 2019, 160, 198–207. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Robins, L.T.; Zhou, Z.; Bobadova-Parvanova, P.; Cottam, M.; McCandless, G.T.; Fronczek, F.R.; Vicente, M.G.H. Spectroscopic, computational modeling and cytotoxicity of a series of meso-phenyl and meso-thienyl-BODIPYs. Bioorg. Med. Chem. 2013, 21, 5770–5781. [Google Scholar] [CrossRef]
- Swaminathan, S.; Fowley, C.; Raj Thapaliya, E.; McCaughan, B.; Tang, S.; Fraix, A.; Captain, B.; Sortino, S.; Callan, J.F.; Raymo, F.M. Supramolecular nanoreactors for intracellular singlet-oxygen sensitization. Nanoscale 2015, 7, 14071–14079. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yu, G.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef]
- LeBlanc, L.E.; Gibbs, J.H.; Vicente, M.G.H.; Fronczek, F.R. CCDC 972982: Experimental Crystal Structure Determination. 2013. [Google Scholar] [CrossRef]
- Sabatini, R.P.; Lindley, B.; McCormick, T.M.; Lazarides, T.; Brennessel, W.W.; McCamant, D.W.; Eisenberg, R. Efficient Bimolecular Mechanism of Photochemical Hydrogen Production Using Halogenated Boron-Dipyrromethene (Bodipy) Dyes and a Bis(dimethylglyoxime) Cobalt (III) Complex. J. Phys. Chem. B 2016, 120, 527–534. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Brammer, L.; Minguez Espallargas, G.; Libri, S. Combining metals with halogen bonds. CrystEngComm 2008, 10, 1712–1727. [Google Scholar] [CrossRef]
- Metrangolo, P.; Pilati, T.; Terraneo, G.; Biella, S.; Resnati, G. Anion coordination and anion-templated assembly under halogen bonding control. CrystEngComm 2009, 11, 1187–1196. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A.; Jaskólski, M. Crystal engineering with hydrogen bonds and halogen bonds. CrystEngComm 2005, 7, 355–358. [Google Scholar] [CrossRef]
- Caronna, T.; Liantonio, R.; Logothetis, T.A.; Metrangolo, P.; Pilati, T.; Resnati, G. Halogen Bonding and π···π Stacking Control Reactivity in the Solid State. J. Am. Chem. Soc. 2004, 126, 4500–4501. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Richards, V.J.; Gower, A.L.; Smith, J.E.H.B.; Davies, E.S.; Lahaye, D.; Slater, A.G.; Lewis, W.; Blake, A.J.; Champness, N.R.; Kays, D.L. Synthesis and characterisation of BODIPY radical anions. Chem. Commun. 2012, 48, 1751–1753. [Google Scholar] [CrossRef] [PubMed]

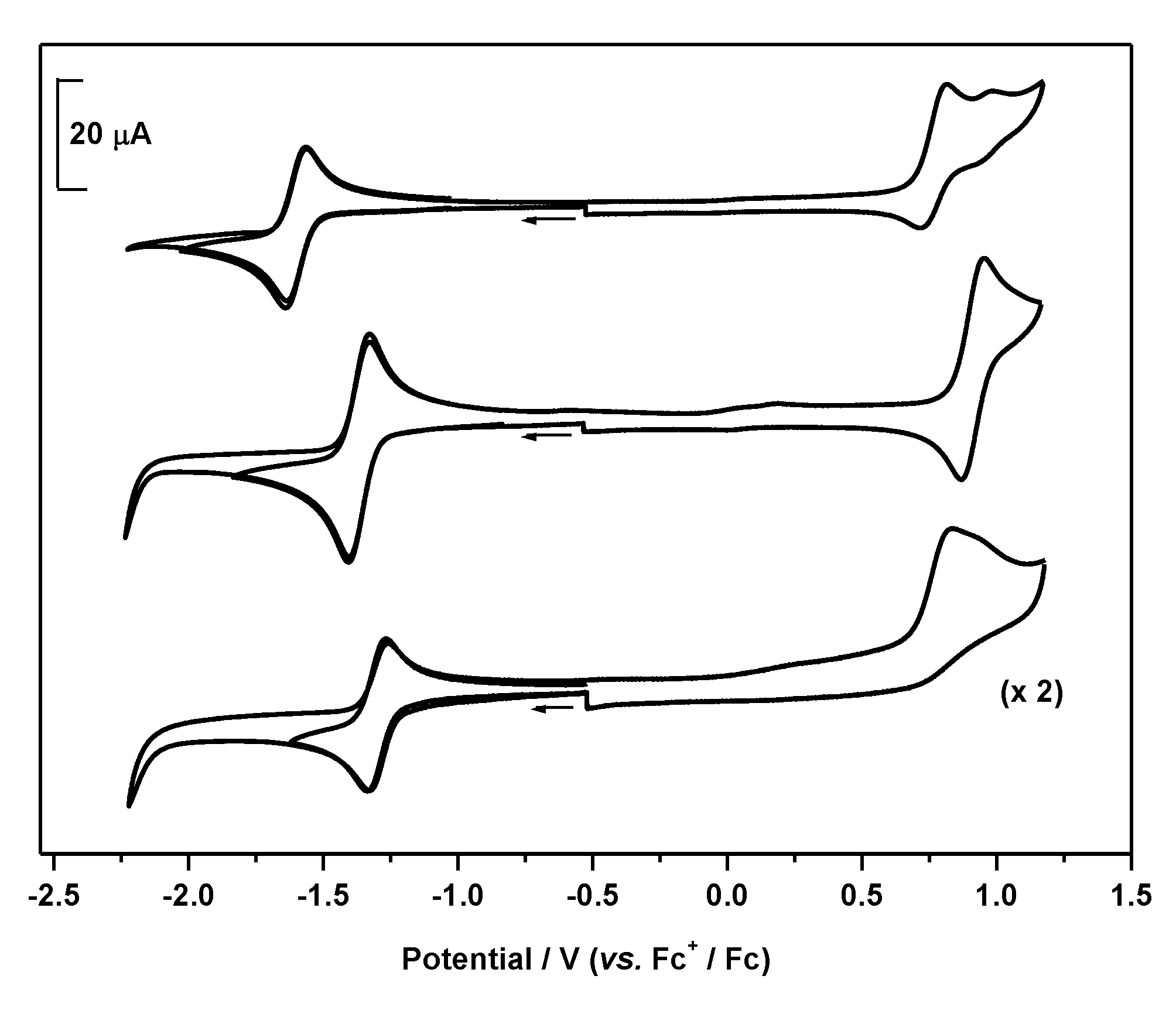
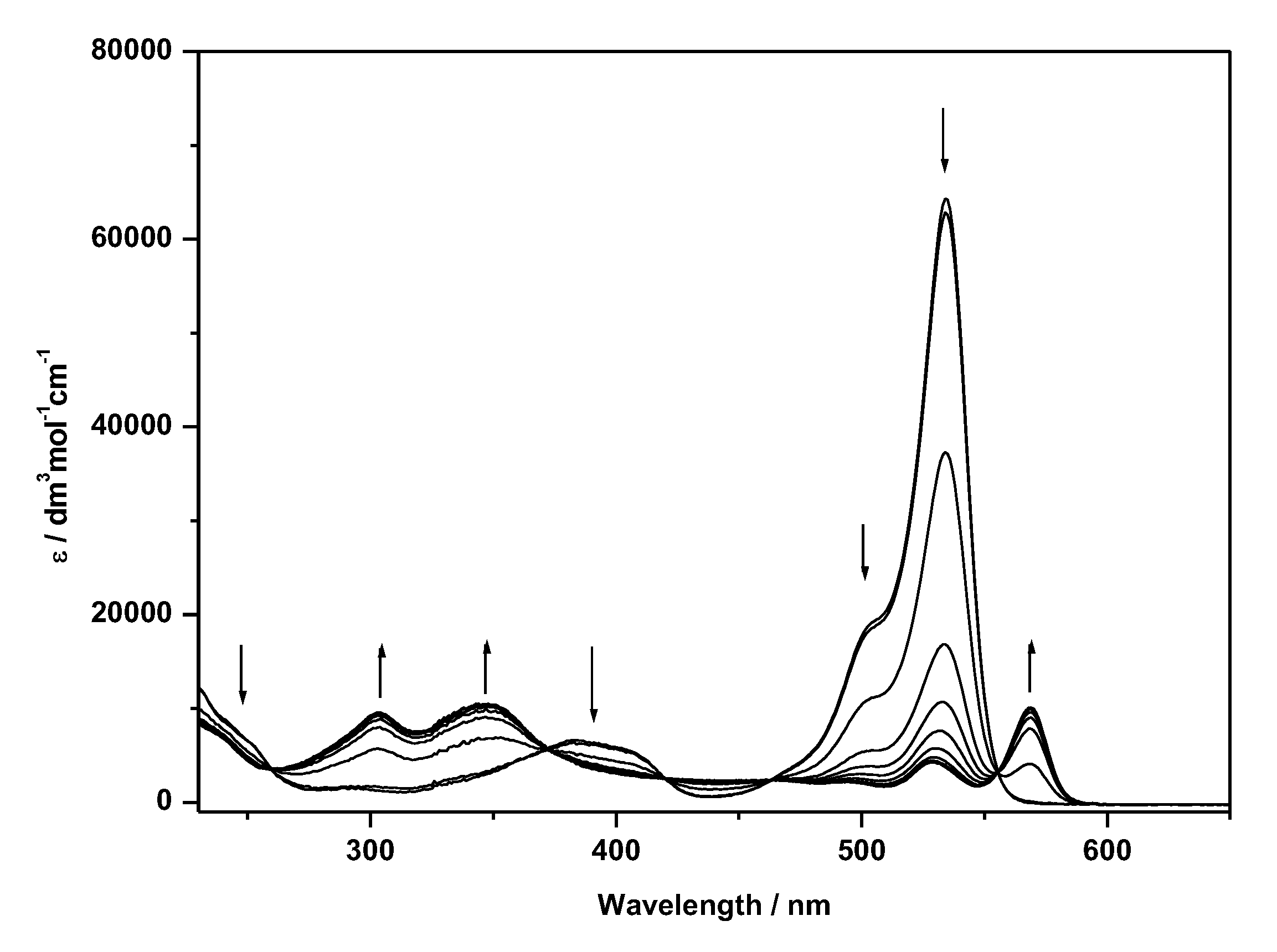
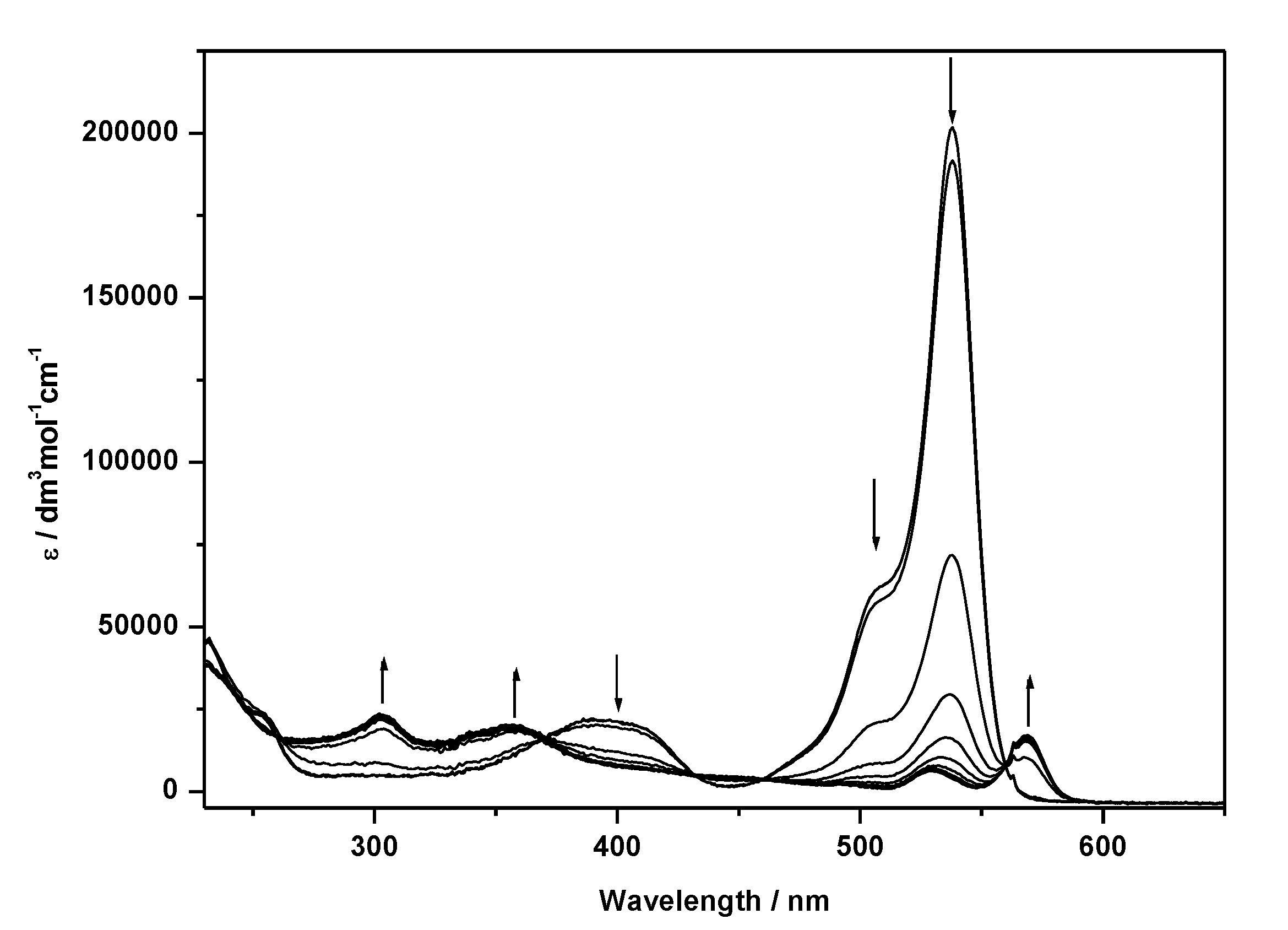
| Compound | E1/2/V (ΔE) | λabs/nm (ε × 10−4/dm3 mol−1 cm−1) b | λem/nm e | ΦF Quantum Yield e | |
|---|---|---|---|---|---|
| Neutral Species | Radical Anion | ||||
| 1 | −1.60 (0.08) c | 501 (1.1) | 287 (0.2), 340 (0.2), 473 (0.1), 528 (0.1), 571 (0.1) | 511 f | 0.63 f |
| 2 | −1.37 (0.08) c | 384 (0.7), 534 (6.4) | 304 (1.0), 343 (1.1), 493 (0.2), 528 (0.4), 569 (1.0) | 550 f | 0.05 f |
| 3 | −1.30 (0.07) d | 389 (2.2), 538 (20.2) | 303 (2.3), 357 (2.0), 529 (0.6), 568 (1.7) | 556 | 0.0006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanain, H.; Davies, E.S.; Lewis, W.; Kays, D.L.; Champness, N.R. Morpholino-Substituted BODIPY Species: Synthesis, Structure and Electrochemical Studies. Crystals 2020, 10, 36. https://doi.org/10.3390/cryst10010036
Hassanain H, Davies ES, Lewis W, Kays DL, Champness NR. Morpholino-Substituted BODIPY Species: Synthesis, Structure and Electrochemical Studies. Crystals. 2020; 10(1):36. https://doi.org/10.3390/cryst10010036
Chicago/Turabian StyleHassanain, Hawazen, E. Stephen Davies, William Lewis, Deborah L. Kays, and Neil R. Champness. 2020. "Morpholino-Substituted BODIPY Species: Synthesis, Structure and Electrochemical Studies" Crystals 10, no. 1: 36. https://doi.org/10.3390/cryst10010036
APA StyleHassanain, H., Davies, E. S., Lewis, W., Kays, D. L., & Champness, N. R. (2020). Morpholino-Substituted BODIPY Species: Synthesis, Structure and Electrochemical Studies. Crystals, 10(1), 36. https://doi.org/10.3390/cryst10010036






