Three New Lead Iodide Chain Compounds, APbI3, Templated by Molecular Cations
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis
2.3. Characterization
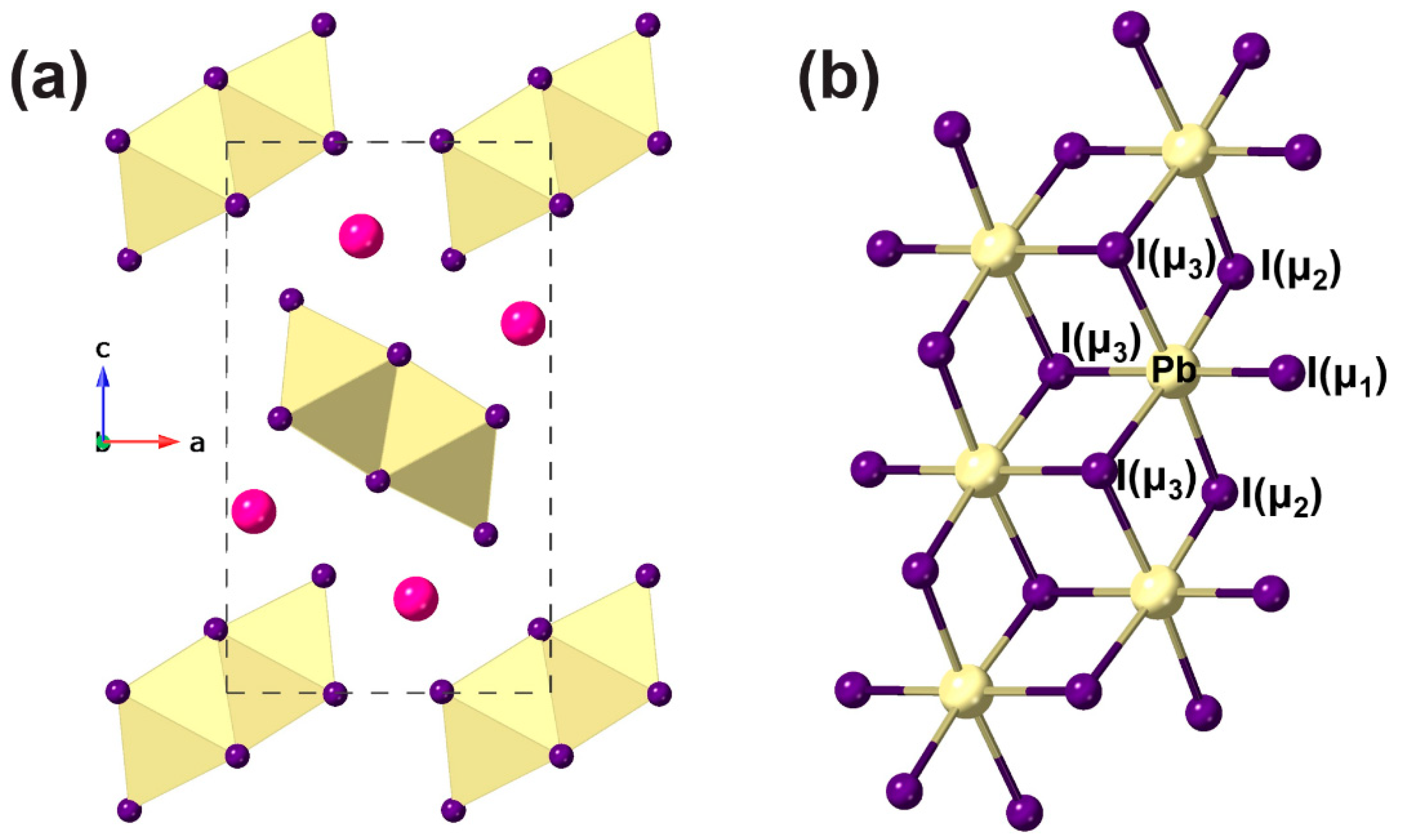
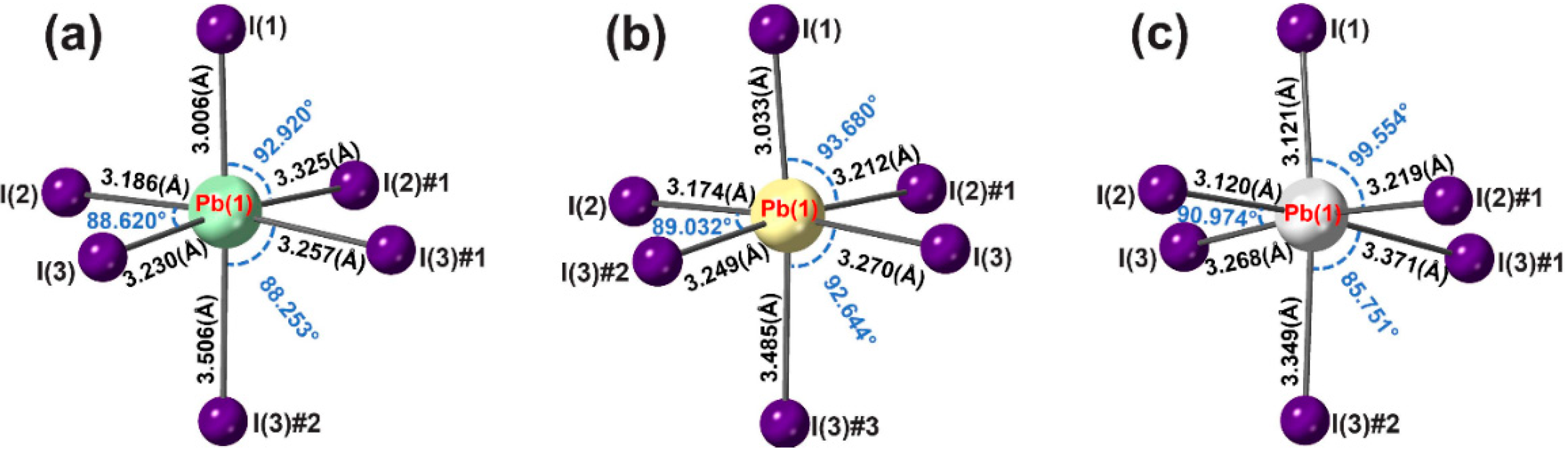
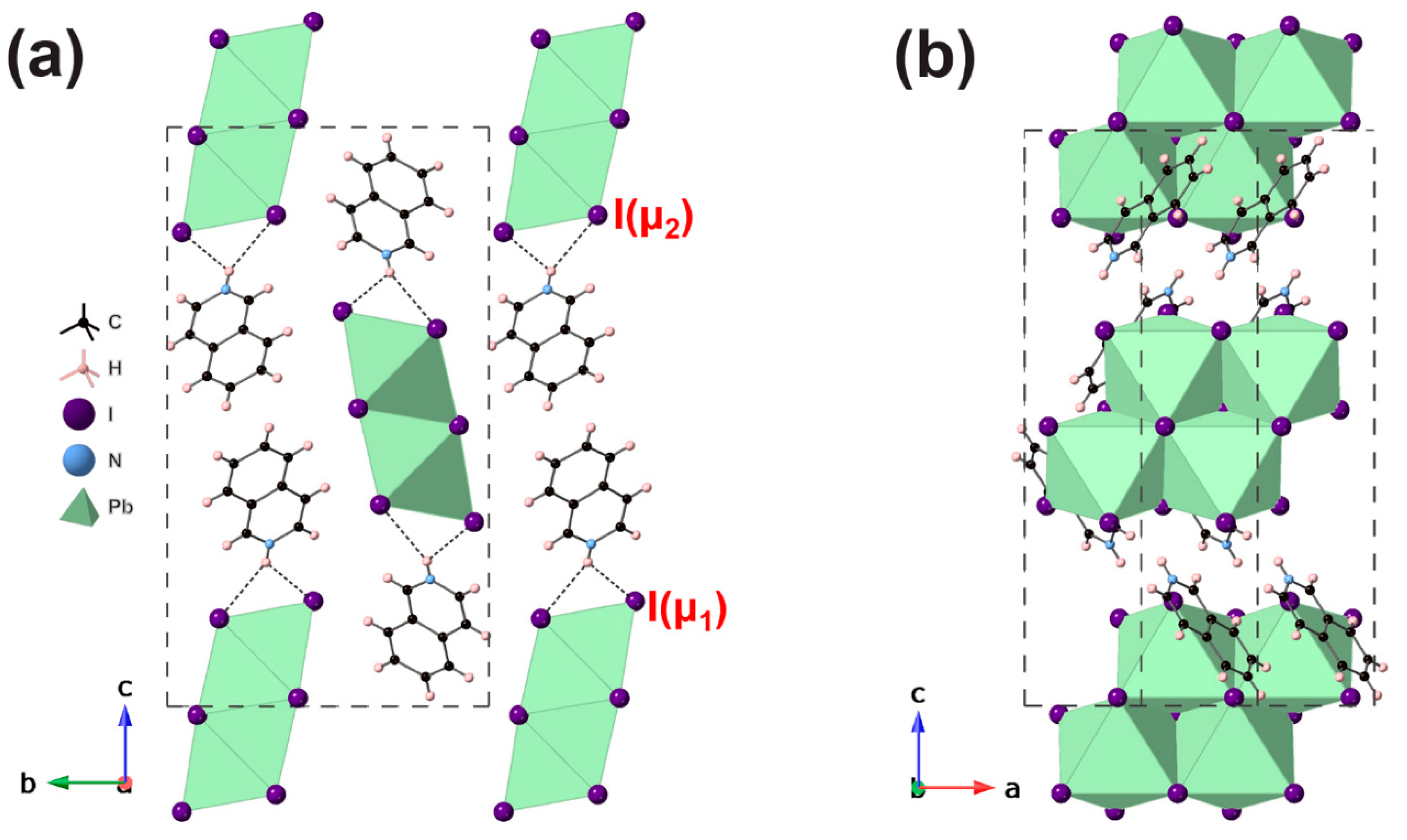
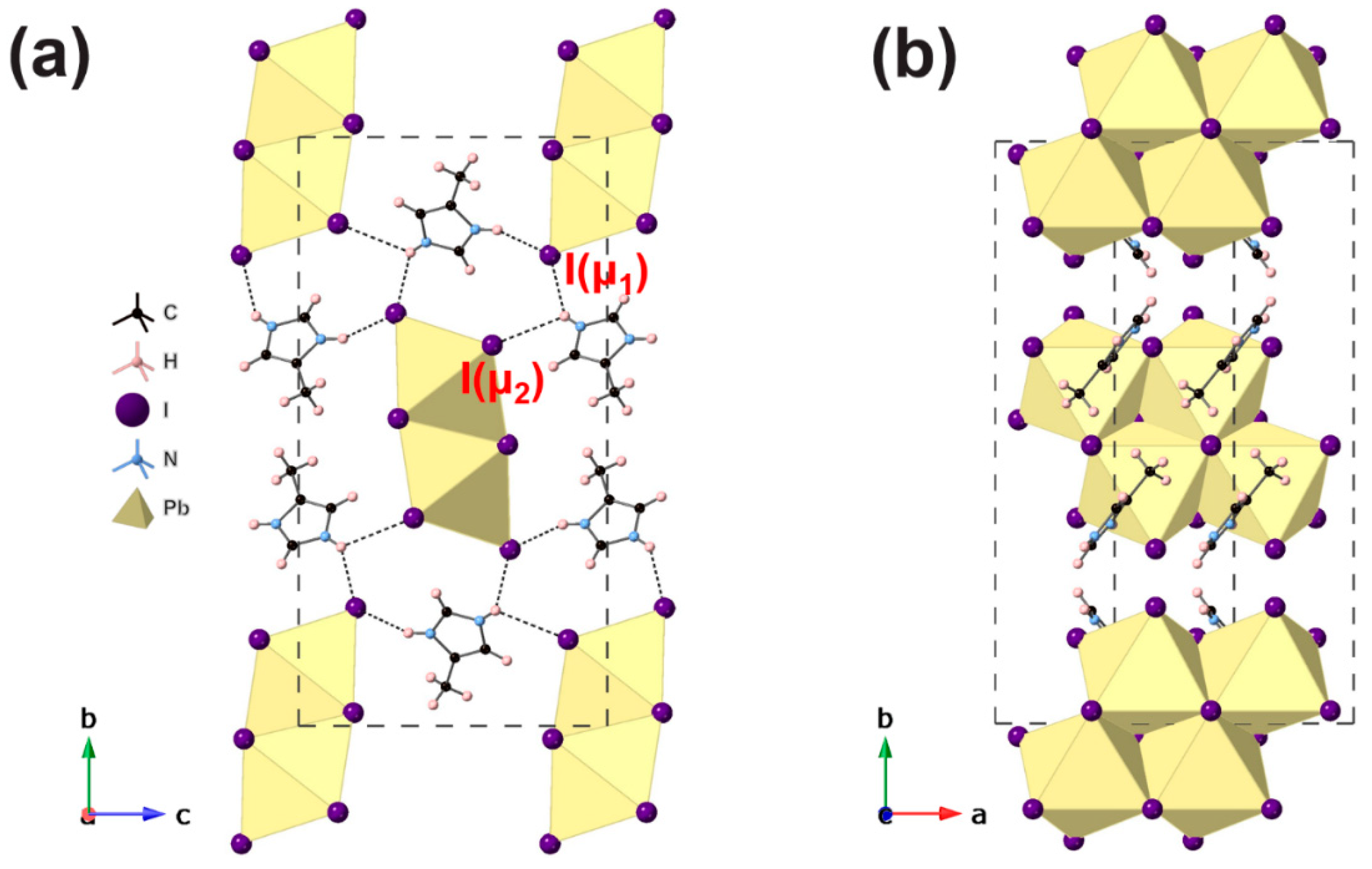
3. Results and Discussion
#1 x − 1, y, z; #2 x + 1, y, z ([4MiH]PbI3)
#1 x − 1, y, z; #2 x − 1/2, −y + 3/2, −z + 1 ([BzH]PbI3).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goesten, M.G.; Hoffmann, R. Mirrors of Bonding in Metal Halide Perovskites. J. Am. Chem. Soc. 2018, 140, 12996–13010. [Google Scholar] [CrossRef]
- Clulow, R.; Bradford, A.; Lee, S.; Lightfoot, P. Perovzalates: a family of perovskite-related oxalates. Dalton Trans. 2019, 14461–14466. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Clulow, R.; Bradford, A.J.; Lee, S.L.; Slawin, A.M.Z.; Lightfoot, P. A hybrid fluoride layered perovskite, (enH2)MnF4. Dalton Trans. 2019, 48, 4784–4787. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; McNulty, J.A.; Mica, N.A.; Samuel, I.D.W.; Slawin, A.M.Z.; Bühl, M.; Lightfoot, P. Structure-directing effects in (110)-layered hybrid perovskites containing two distinct organic moieties. Chem. Commun. 2019, 55, 9935–9938. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional ruddlesden-popper perovskite solar cells. Nature 2016, 536, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hoffman, J.; Ke, W.; Chen, M.; Tsai, H.; Nie, W.; Mohite, A.D.; Kepenekian, M.; Katan, C.; Even, J.; et al. Two-Dimensional Halide Perovskites Incorporating Straight Chain Symmetric Diammonium Ions, (NH3CmH2mNH3)(CH3NH3)n-1PbnI3n+1 (m = 4-9; n = 1-4). J. Am. Chem. Soc. 2018, 140, 12226–12238. [Google Scholar] [CrossRef] [PubMed]
- Gautier, R.; Massuyeau, F.; Galnon, G.; Paris, M. Lead Halide Post-Perovskite-Type Chains for High-Efficiency White-Light Emission. Adv. Mater. 2019, 31, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. [Google Scholar] [CrossRef]
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M.J.; et al. Thermal unequilibrium of strained black CsPbI3 thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef]
- Straus, D.B.; Guo, S.; Cava, R.J. Kinetically Stable Single Crystals of Perovskite-Phase CsPbI3. J. Am. Chem. Soc. 2019, 141, 11435–11439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jin, S.F.; Huang, S.; Liu, N.; Ma, J.Y.; Xue, D.J.; Han, Q.; Ding, J.; Ge, Q.Q.; Feng, Y.; et al. Thermodynamically Stable Orthorhombic γ-CsPbI3 Thin Films for High-Performance Photovoltaics. J. Am. Chem. Soc. 2018, 140, 11716–11725. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Wu, X.T.; Chen, L. Structural overview and structure-property relationships of iodoplumbate and iodobismuthate. Coord. Chem. Rev. 2009, 253, 2787–2804. [Google Scholar] [CrossRef]
- Trots, D.M.; Myagkota, S.V. High-temperature structural evolution of caesium and rubidium triiodoplumbates. J. Phys. Chem. Solids 2008, 69, 2520–2526. [Google Scholar] [CrossRef]
- Schelhas, L.T.; Li, Z.; Christians, J.A.; Goyal, A.; Kairys, P.; Harvey, S.P.; Kim, D.H.; Stone, K.H.; Luther, J.M.; Zhu, K.; et al. Insights into operational stability and processing of halide perovskite active layers. Energy Environ. Sci. 2019, 12, 1341–1348. [Google Scholar] [CrossRef]
- Rolies, M.M.; De Ranter, C.J. A new investigation of ammonium cadmium chloride. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 3057–3059. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Jung, M.H.; Rhim, S.H.; Moon, D. TiO2/RbPbI3 halide perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 44–54. [Google Scholar] [CrossRef]
- Papavassiliou, G.C.; Mousdis, G.A.; Raptopoulou, C.P.; Terzis, A. Preparation and Characterization of [C6H5CH2NH3]2PbI4, [C6H5CH2CH2SC(NH2)2]3PbI5 and [C10H7CH2NH3]PbI3 Organic-Inorganic Hybrid Compounds. Z. Naturforsch 1999, 54b, 1405–1409. [Google Scholar] [CrossRef]
- Lermer, C.; Harm, S.P.; Birkhold, S.T.; Jaser, J.A.; Kutz, C.M.; Mayer, P.; Schmidt-Mende, L.; Lotsch, B.V. Benzimidazolium Lead Halide Perovskites: Effects of Anion Substitution and Dimensionality on the Bandgap. Zeitschrift Fur Anorg. Und Allg. Chemie 2016, 642, 1369–1376. [Google Scholar] [CrossRef]
- Samet, A.; Triki, S.; Abid, Y. Resonantly Enhanced White-Light Emission Involving Energy and Charge Transfer in One-Dimensional Hybrid Material: (ABT)2[PbBr3]. J. Phys. Chem. C 2019, 123, 6213–6219. [Google Scholar] [CrossRef]
- Medhioub, O.; Barkaoui, H.; Samet, A.; Pillet, S.; Triki, S.; Abid, Y. Blue Emission from Charge-Transfer Excitons in Hybrid Organic–Inorganic Quantum Wires: (ABT)[PbCl3]. J. Phys. Chem. C 2019, 123, 26547–26553. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Rigaku. CrystalClear; Rigaku Corporation: Tokyo, Japan, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Palmer, D.C. CrystalMaker; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report No. 88-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller distortions, cation ordering and octahedral tilting in perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 10–20. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic Elongation: A Quantitative Measure of Distortion in Coordination Polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
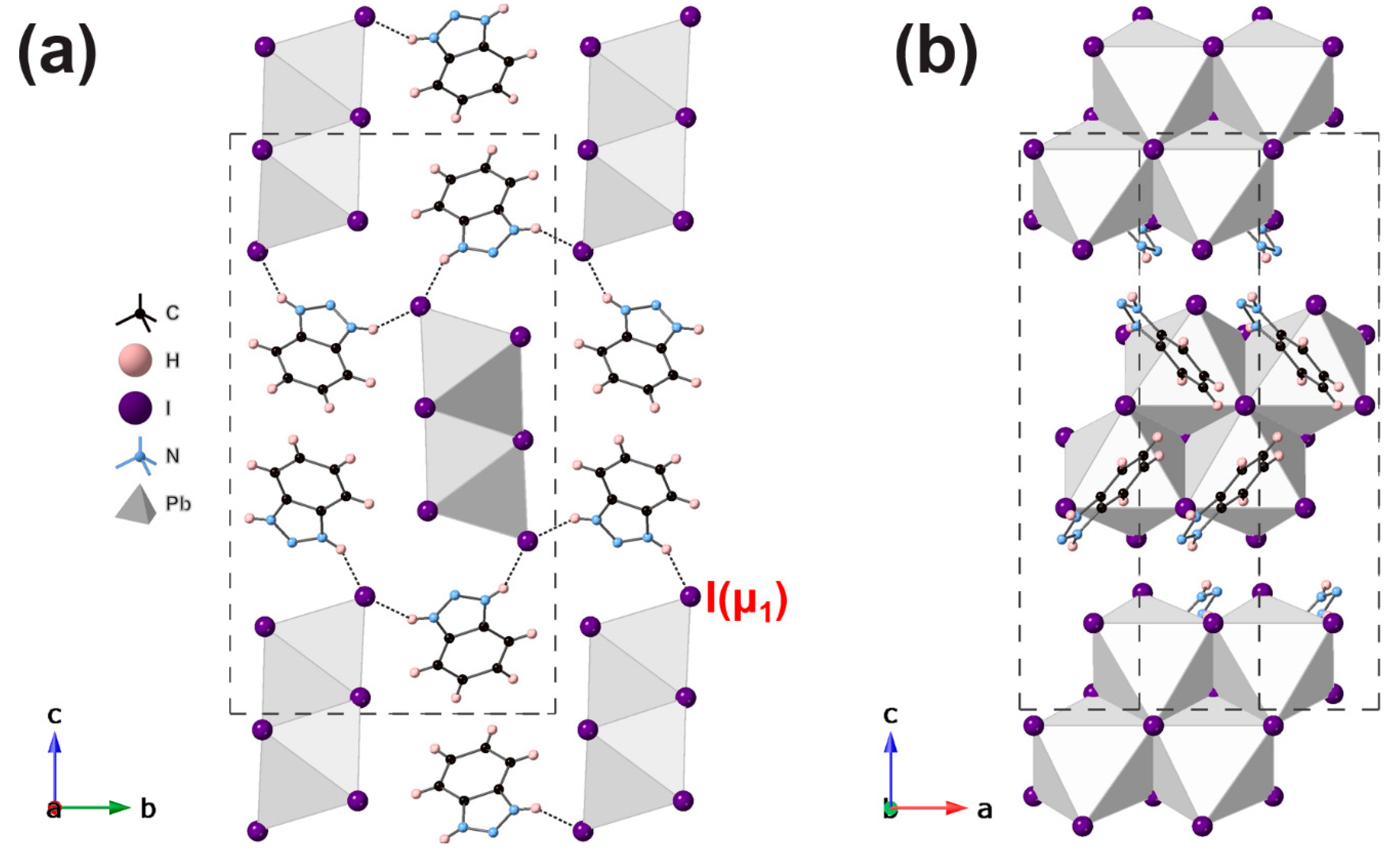
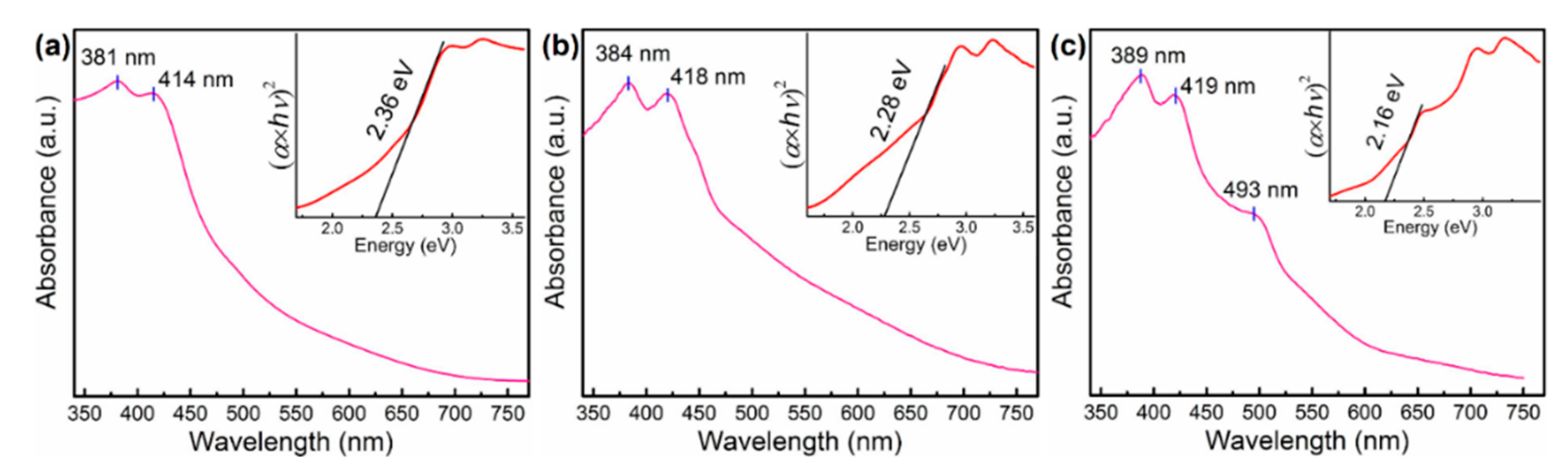
| Compound | [IqH]PbI3 | [4MiH]PbI3 | [BzH]PbI3 |
|---|---|---|---|
| Formula | C9NH8PbI3 | C4N2H7PbI3 | C6N3H6PbI3 |
| Formula Weight | 718.05 | 671.01 | 708.03 |
| Crystal System | Orthorhombic | Monoclinic | Orthorhombic |
| Space Group | P212121 | P21/c | P212121 |
| a/Å | 4.6946(2) | 4.6110(2) | 4.6102(2) |
| b/Å | 12.8898(9) | 22.2560(16) | 12.4712(9) |
| c/Å | 23.2093(16) | 11.7926(8) | 22.2047(16) |
| β/o | - | 98.801(9) | - |
| V/Å3 | 1404.45(15) | 1195.94(13) | 1276.65(14) |
| Z | 4 | 4 | 4 |
| MEASURED Ref | 11890 | 12122 | 13081 |
| Independent Ref | 2473 | 2717 | 2893 |
| [R(int) = 0.0399] | [R(int) = 0.0631] | [R(int) = 0.0556] | |
| GOOF | 1.067 | 0.952 | 0.749 |
| Final R Indices (I > 2σ(I)) | R1 = 0.0187 | R1 = 0.0272 | R1 = 0.0207 |
| wR2 = 0.0399 | wR2 = 0.0544 | wR2 = 0.0373 | |
| Flack Parameter | 0.357(5) | 0.006(5) |
| Compound | [IqH]PbI3 | [4MiH]PbI3 | [BzH]PbI3 | [C10H7CH2NH3]Pbl3 [19] | (C7H7N2)PbI3 [20] |
|---|---|---|---|---|---|
| Δd (× 10−4) | 21.30 | 17.32 | 9.34 | 18.68 | 8.34 |
| σ2 | 10.70 | 13.88 | 16.66 | 9.92 | 12.38 |
| Σν (Pb) | 1.82 | 1.86 | 1.79 | 1.80 | 1.78 |
| Σν (I1) | 0.54 | 0.50 | 0.40 | 0.54 | 0.42 |
| Σν (I2) | 0.56 | 0.66 | 0.70 | 0.48 | 0.64 |
| Σν (I3) | 0.71 | 0.70 | 0.68 | 0.78 | 0.72 |
| Equation (1) | |||||
| Equation (2) | |||||
| Equation (3) | |||||
| Compound | D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|---|
| [IqH]PbI3 | N(1)-H(1)...I(1) | 0.86 | 3.24 | 3.852(7) | 130.0 |
| N(1)-H(1)...I(2) | 0.86 | 2.94 | 3.626(7) | 138.3 | |
| [4MiH]PbI3 | N(1)-H(1)...I(2) | 0.86 | 3.04 | 3.612(7) | 126.3 |
| N(1)-H(1)...I(1)#4 | 0.86 | 3.07 | 3.812(6) | 145.2 | |
| N(2)-H(2)...I(1)#5 | 0.86 | 2.96 | 3.700(7) | 145.2 | |
| [BzH]PbI3 | N(1)-H(1)...I(1)#5 | 0.86 | 2.90 | 3.598(7) | 139.1 |
| N(3)-H(2)...I(1) | 0.86 | 2.79 | 3.490(7) | 139.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-Y.; Yang, L.-J.; Lightfoot, P. Three New Lead Iodide Chain Compounds, APbI3, Templated by Molecular Cations. Crystals 2019, 9, 616. https://doi.org/10.3390/cryst9120616
Guo Y-Y, Yang L-J, Lightfoot P. Three New Lead Iodide Chain Compounds, APbI3, Templated by Molecular Cations. Crystals. 2019; 9(12):616. https://doi.org/10.3390/cryst9120616
Chicago/Turabian StyleGuo, Yuan-Yuan, Lin-Jie Yang, and Philip Lightfoot. 2019. "Three New Lead Iodide Chain Compounds, APbI3, Templated by Molecular Cations" Crystals 9, no. 12: 616. https://doi.org/10.3390/cryst9120616
APA StyleGuo, Y.-Y., Yang, L.-J., & Lightfoot, P. (2019). Three New Lead Iodide Chain Compounds, APbI3, Templated by Molecular Cations. Crystals, 9(12), 616. https://doi.org/10.3390/cryst9120616






