Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil
Abstract
:1. Introduction
2. Results
2.1. Hydrotreatment Reactions: Catalytic Performance
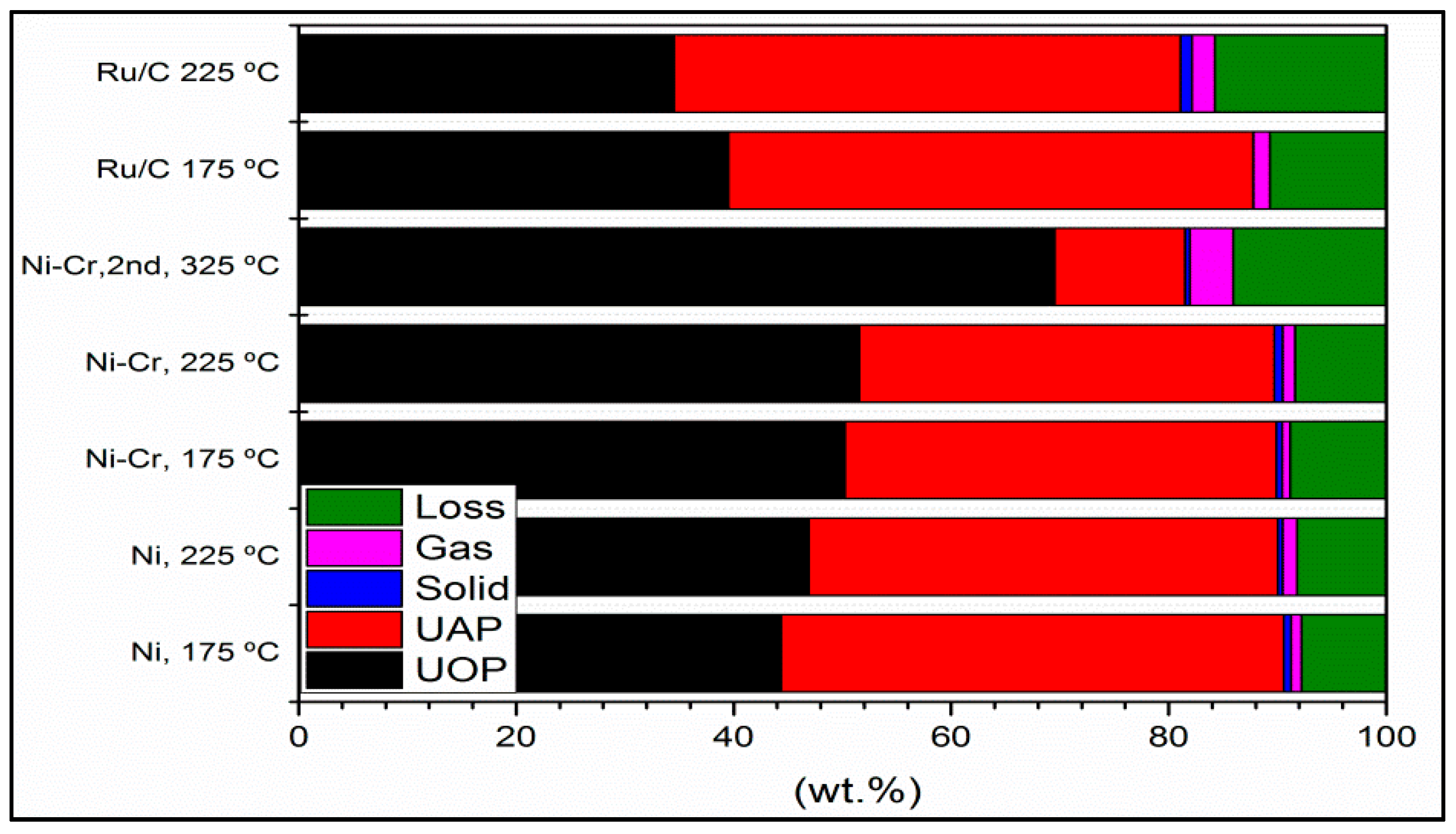
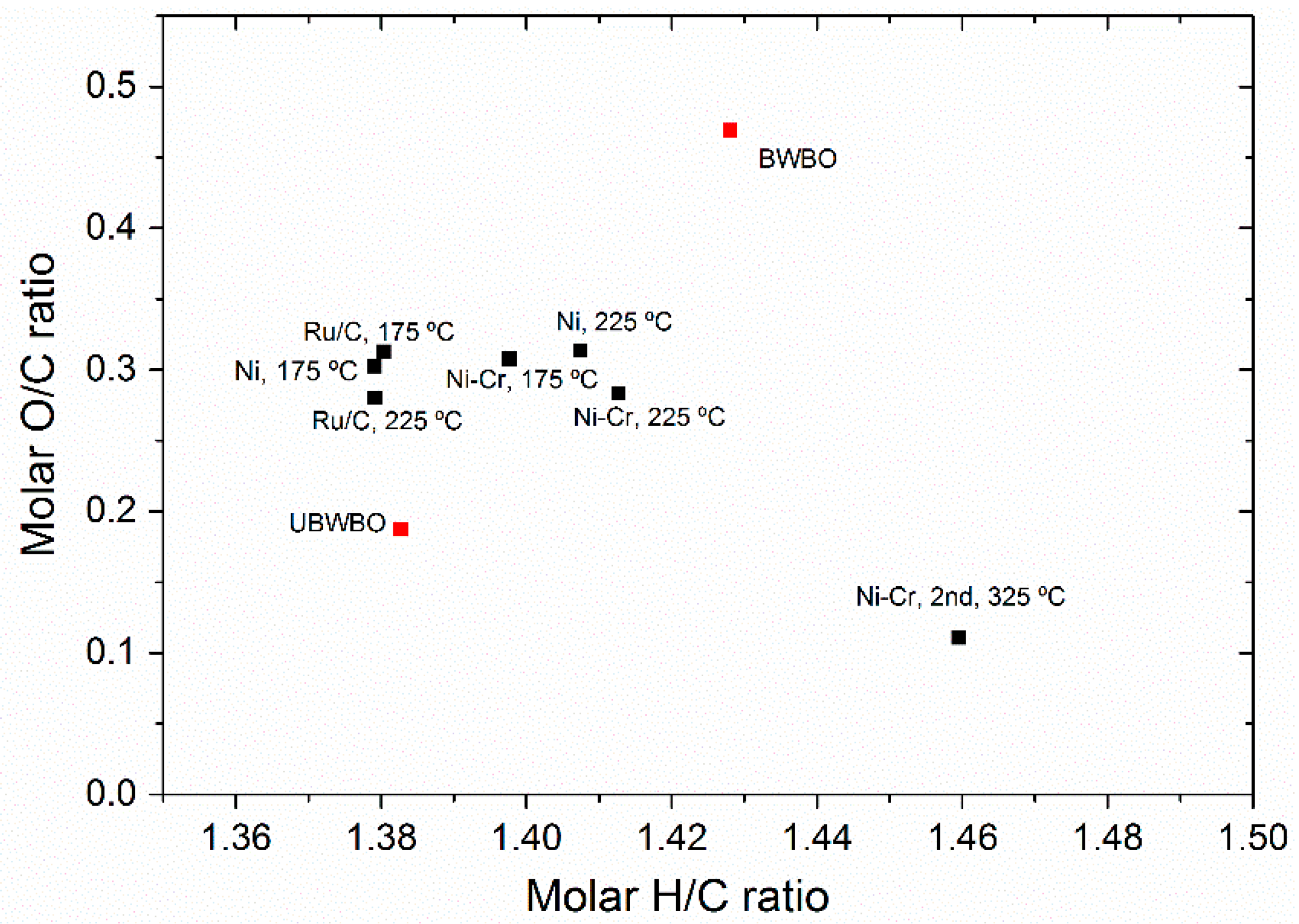
2.1.1. Products Distribution, Elemental Analysis, and Physicochemical Properties
2.1.2. Detailed Chemical Composition of Liquid Products and Feedstocks by 1H-NMR and GC-MS/FID
2.1.3. Characterization of Upgraded Liquid Products and Feedstocks by GC-MS/FID
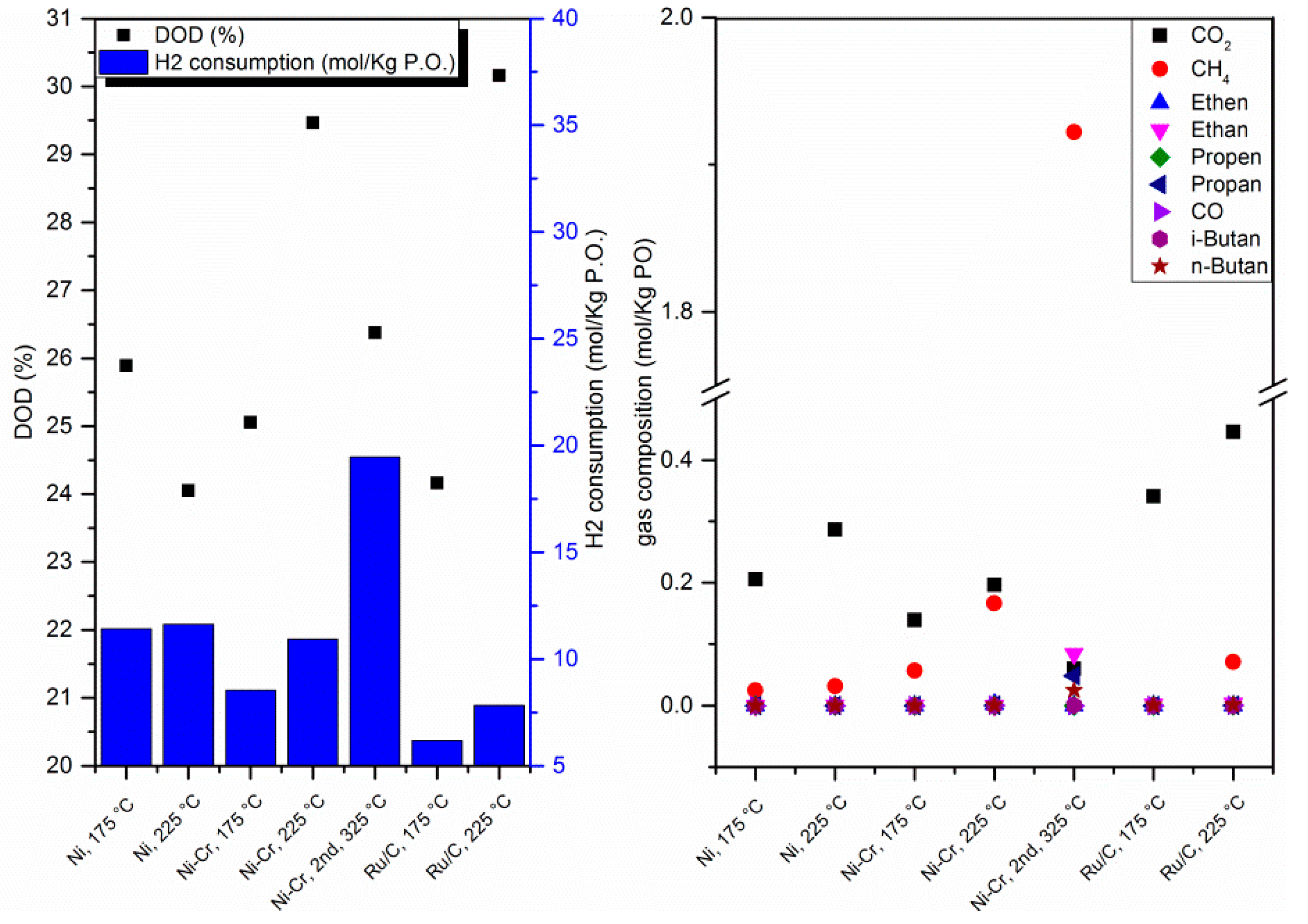
2.1.4. Degree of Deoxygenation (DOD), H2 Consumption, and Gaseous Products of Upgrading Reactions
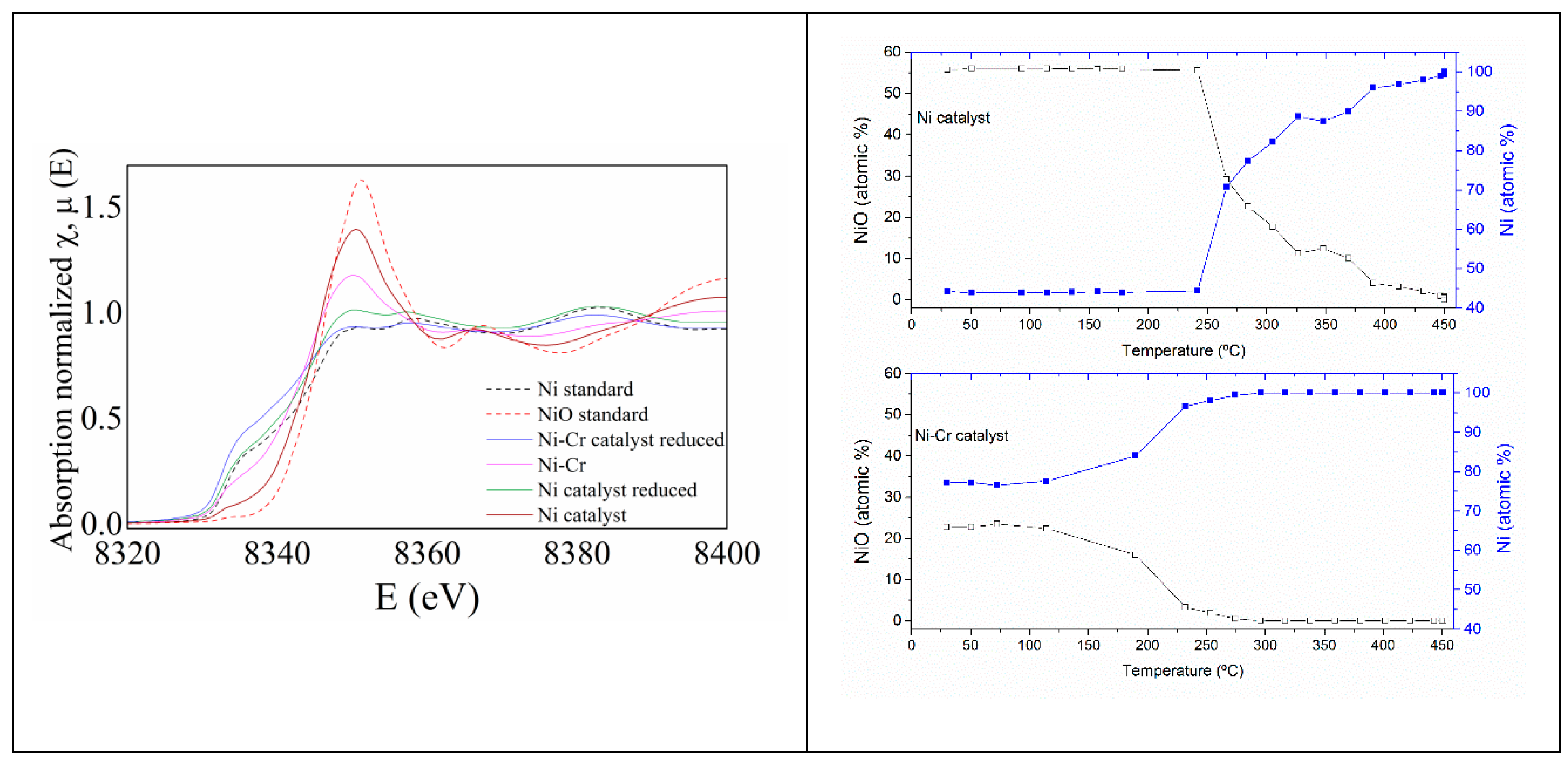
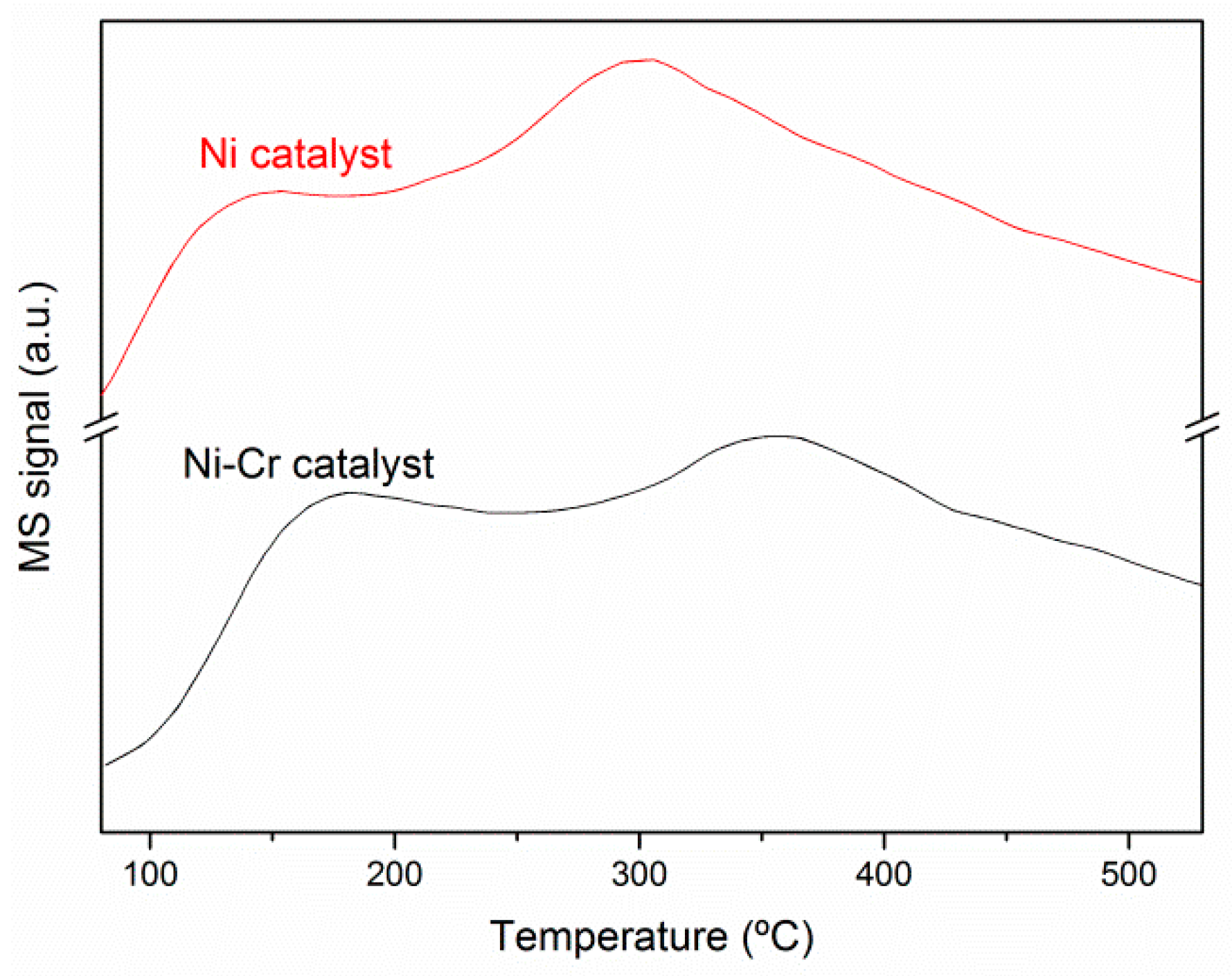
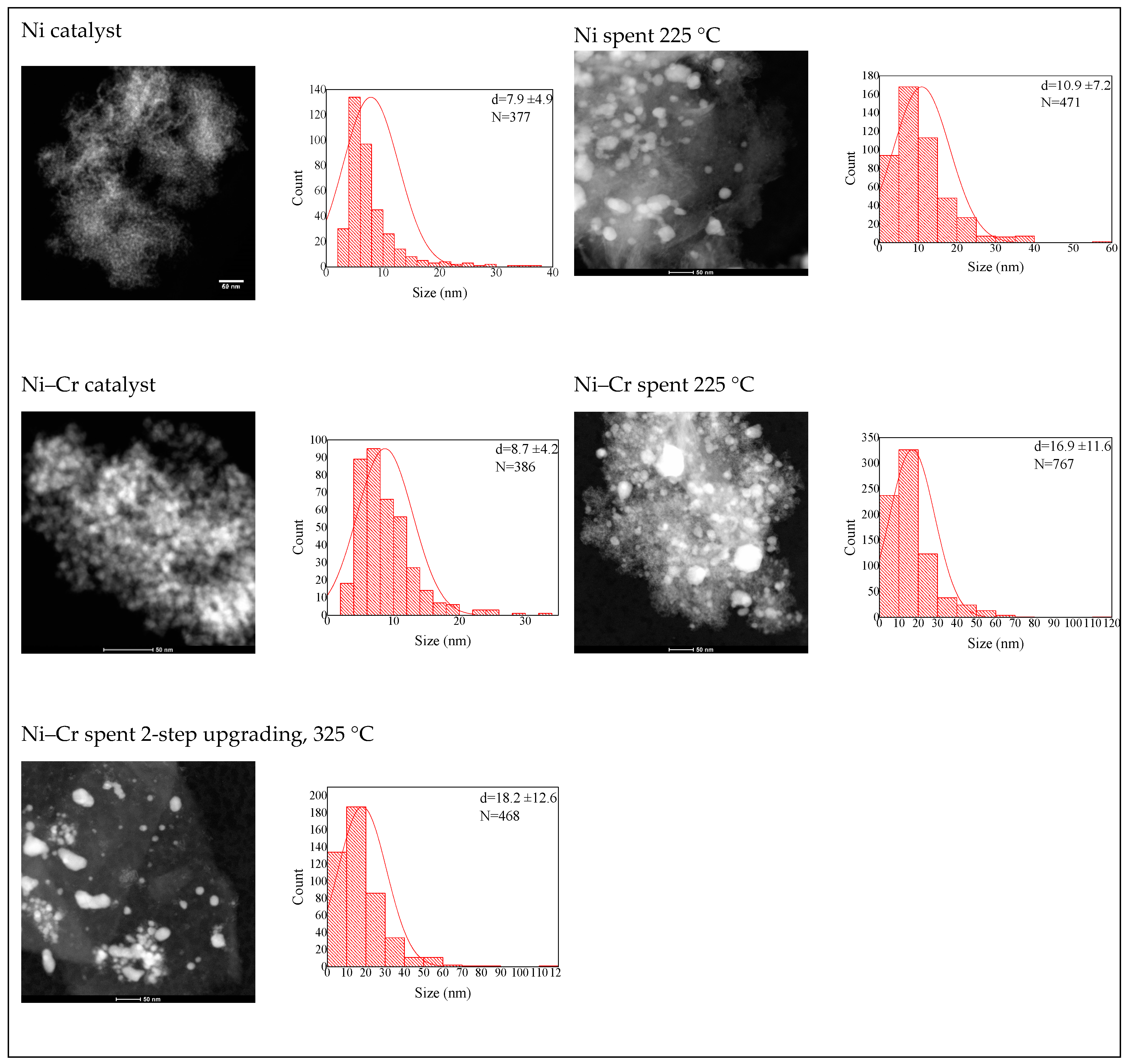
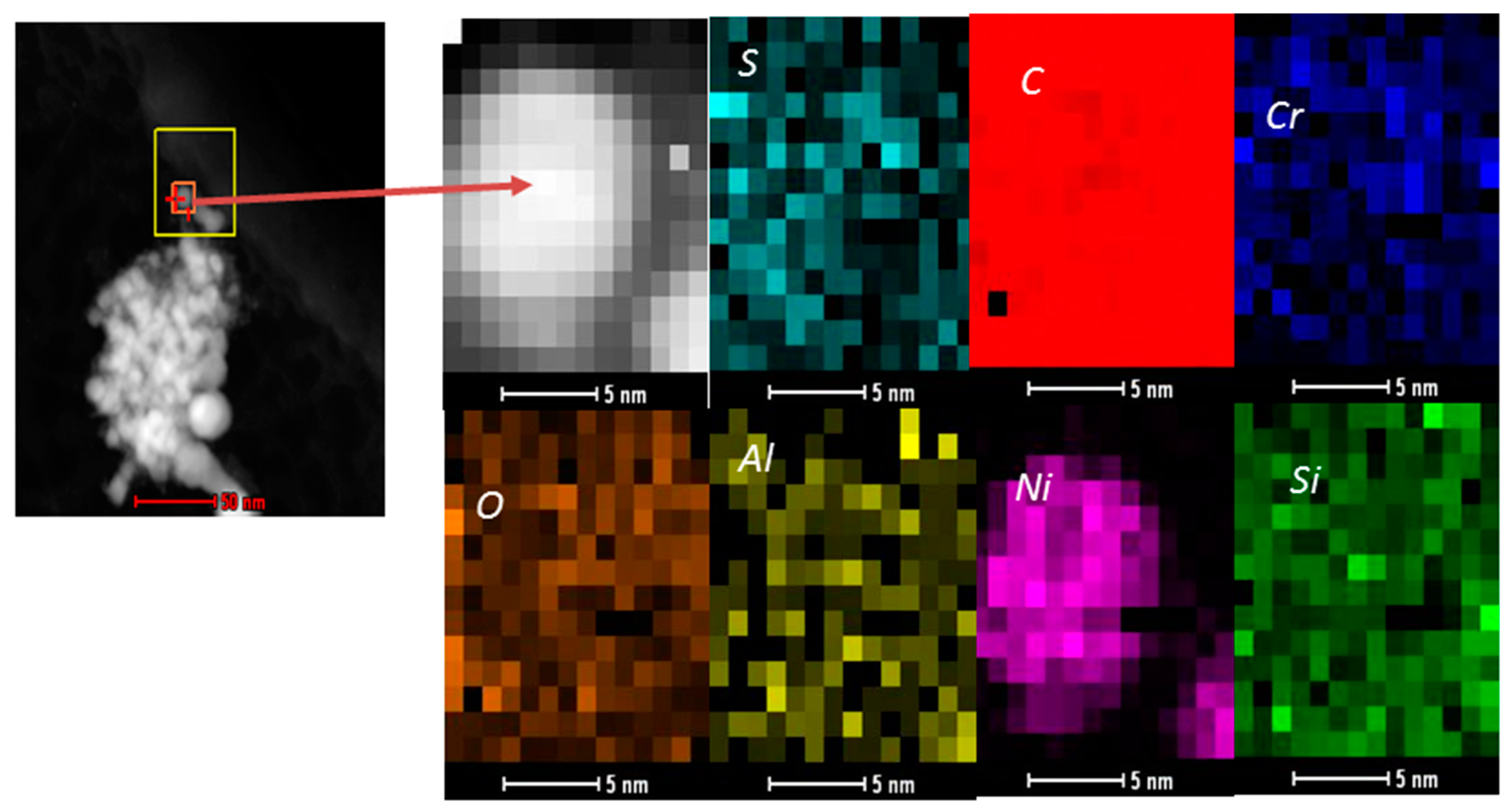
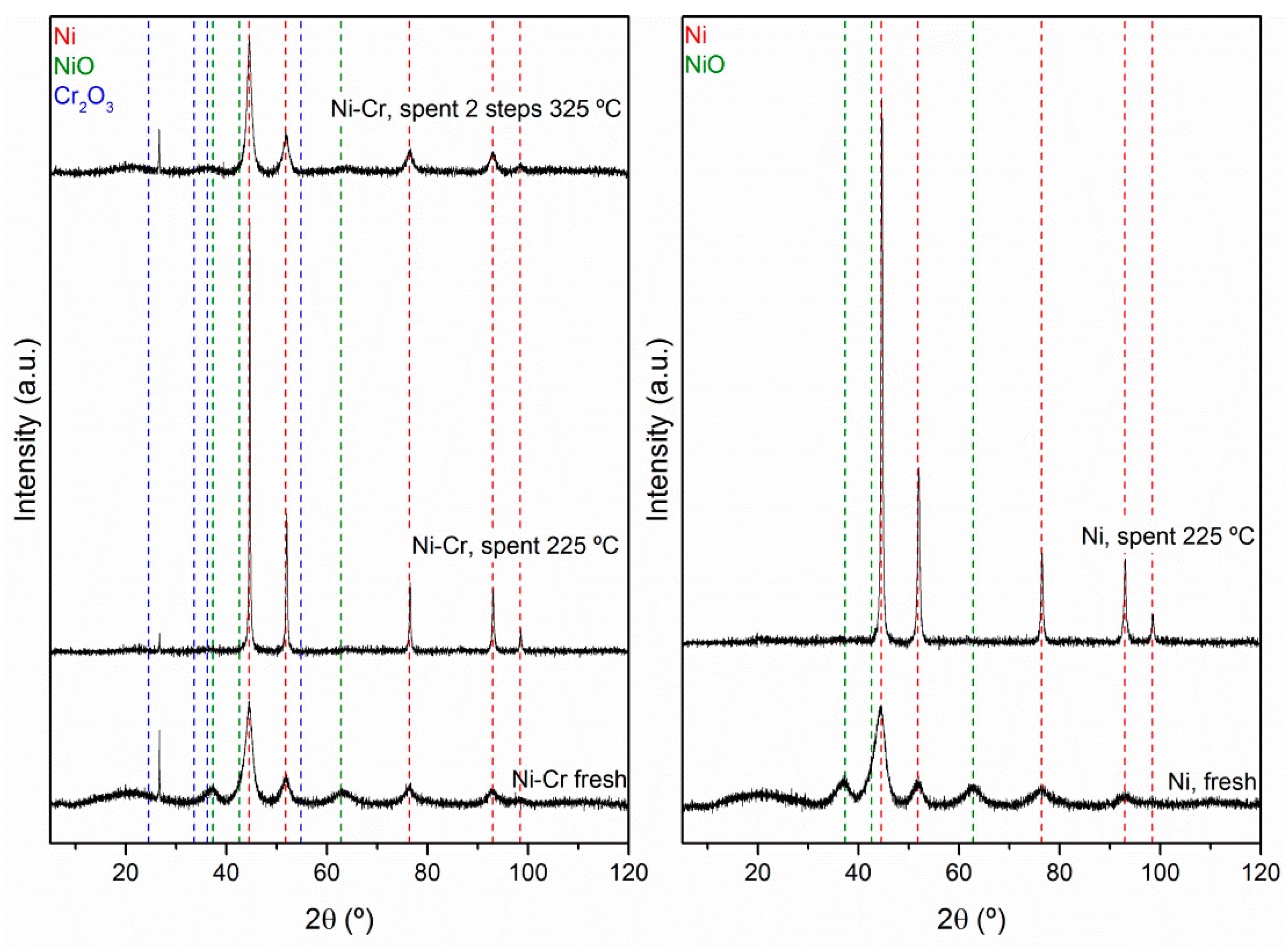
2.2. Catalysts Characterization
Correlation of Catalytic Performance vs. Properties of Ni-Based Catalysts
3. Materials and Methods
3.1. Fast Pyrolysis Bio-Oils
3.2. Catalysts
3.3. Methodology
3.3.1. Reaction Conditions
3.3.2. Liquid Products and Feedstock Characterization
3.3.3. Gas Product Characterization
3.3.4. Characterization of Ni-Based Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BWBO | Beech wood fast pyrolysis bio-oil |
| UBWBO | Upgraded beech wood bio-oil |
| LP | Light Phase |
| HP | Heavy Phase |
| UAP | Upgraded aqueous phase |
| UOP | Upgraded oil phase |
| LPBWBO | Light phase of beech wood fast pyrolysis bio-oil |
| HPBWBO | Heavy phase of beech wood fast pyrolysis bio-oil |
| UAPNi, 225 °C | Upgraded aqueous phase with Ni catalyst at 225 °C |
| UOPNi, 225 °C | Upgraded oil phase with Ni catalyst at 225 °C |
| UAPNi–Cr, 225 °C | Upgraded aqueous phase with Ni–Cr catalyst at 225 °C |
| UOPNi–Cr, 225 °C | Upgraded oil phase with Ni–Cr catalyst at 225 °C |
| UAP2nd, Ni–Cr, 325 °C | 2-step upgraded aqueous phase with Ni–Cr catalyst at 325 °C |
| UOP2nd, Ni–Cr, 325 °C | 2-step upgraded oil phase with Ni–Cr catalyst at 325 °C |
References
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Bozell, J.J.; Holladay, J.E.; Johnson, D.; White, J.F. Top Value-Added Chemicals from Biomass Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Energy: Washington, DC, USA, 2007; Volume II.
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 126–1140. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Melián-Cabrera, I.V.; Heeres, H.J. Catalytic hydrotreatment of fast pyrolysis oil using bimetallic Ni-Cu catalysts on various supports. Appl. Catal. A Gen. 2012, 449, 121–130. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Dabros, T.M.H.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.-D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- Boscagli, C.; Yang, C.; Welle, A.; Wang, W.; Behrens, S.; Raffelt, K.; Grunwaldt, J.D. Effect of pyrolysis oil components on the activity and selectivity of nickel-based catalysts during hydrotreatment. Appl. Catal. A Gen. 2017, 544, 161–172. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Reolon, M.G.; Zimmermann, M.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. Synthesis and Regeneration of Nickel-Based Catalysts for Hydrodeoxygenation of Beech Wood Fast Pyrolysis Bio-Oil. Catalysts 2018, 8, 449. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]
- French, R.J.; Stunkel, J.; Baldwin, R.M. Mild hydrotreating of bio-oil: Effect of reaction severity and fate of oxygenated species. Energy Fuels 2011, 25, 3266–3274. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Gunawan, R.; Hu, X.; De Miguel Mercader, F.; Westerhof, R.; Chaitwat, W.; Hasan, M.M.; Mourant, D.; Li, C.Z. Effects of temperature on the hydrotreatment behaviour of pyrolysis bio-oil and coke formation in a continuous hydrotreatment reactor. Fuel Process. Technol. 2016, 148, 175–183. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Nickel sulfide, nickel phosphide and nickel carbide catalysts for bio-hydrotreated fuel production. Energy Convers. Manag. 2017, 151, 324–333. [Google Scholar] [CrossRef]
- Boscagli, C.; Raffelt, K.; Grunwaldt, J.D. Reactivity of platform molecules in pyrolysis oil and in water during hydrotreatment over nickel and ruthenium catalysts. Biomass Bioenergy 2017, 106, 63–73. [Google Scholar] [CrossRef]
- Wildschut, J.; Iqbal, M.; Mahfud, F.H.; Cabrera, I.M.; Venderbosch, R.H.; Heeres, H.J. Insights in the hydrotreatment of fast pyrolysis oil using a ruthenium on carbon catalyst. Energy Environ. Sci. 2010, 3, 962. [Google Scholar] [CrossRef]
- De Wild, P.J.; Huijgen, W.J.J.; Kloekhorst, A.; Chowdari, R.K.; Heeres, H.J. Bioresource Technology Biobased alkylphenols from lignins via a two-step pyrolysis – Hydrodeoxygenation approach. Bioresour. Technol. 2017, 229, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ardiyani, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117–118, 105–117. [Google Scholar] [CrossRef]
- Mercader, F.D.M.; Koehorst, P.J.J.; Heeres, H.J.; Kersten, S.R.A.; Hogendoorn, J.A. Competition Between Hydrotreating and Polymerization Reactions During Pyrolysis Oil Hydrodeoxygenation. Aiche J. 2011, 55, 3160–3170. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R. Catalytic hydroprocessing of chemical models for bio-oil. Energy Fuels 2009, 23, 631–637. [Google Scholar] [CrossRef]
- Li, X.; Gunawan, R.; Wang, Y.; Chaiwat, W.; Hu, X.; Gholizadeh, M.; Mourant, D.; Bromly, J.; Li, C.-Z. Upgrading of bio-oil into advanced biofuels and chemicals. Part III. Changes in aromatic structure and coke forming propensity during the catalytic hydrotreatment of a fast pyrolysis bio-oil with Pd/C catalyst. Fuel 2014, 116, 642–649. [Google Scholar] [CrossRef]
- Chaiwat, W.; Gunawan, R.; Gholizadeh, M.; Li, X.; Lievens, C.; Hu, X.; Wang, Y.; Mourant, D.; Rossiter, A.; Bromly, J.; et al. Upgrading of bio-oil into advanced biofuels and chemicals. Part II. Importance of holdup of heavy species during the hydrotreatment of bio-oil in a continuous packed-bed catalytic reactor. Fuel 2013, 112, 302–310. [Google Scholar] [CrossRef]
- Gunawan, R.; Li, X.; Lievens, C.; Gholizadeh, M.; Chaiwat, W.; Hu, X.; Mourant, D.; Bromly, J.; Li, C.Z. Upgrading of bio-oil into advanced biofuels and chemicals. Part I. Transformation of GC-detectable light species during the hydrotreatment of bio-oil using Pd/C catalyst. Fuel 2013, 111, 709–717. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.K.; Jae, J.; Park, S.H.; Jung, S.C.; Park, Y.K. Catalytic Hydrodeoxygenation of Bio-oil Model Compounds over Pt/HY Catalyst. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Vispute, T.P.; Huber, G.W. Hydrodeoxygenation of the aqueous fraction of bio-oil with Ru/C and Pt/C catalysts. Appl. Catal. B Environ. 2015, 165, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Shen, D.; Sepúlveda-Escribano, A.; Gu, S.; Ramirez Reina, T. Catalytic upgrading of biomass model compounds: Novel approaches and lessons learnt from traditional hydrodeoxygenation—A review. ChemCatChem 2019, 11, 924–960. [Google Scholar] [CrossRef]
- Oh, S.; Choi, H.S.; Choi, I.-G.; Choi, J.W. Evaluation of hydrodeoxygenation reactivity of pyrolysis bio-oil with various Ni-based catalysts for improvement of fuel properties. RSC Adv. 2017, 7, 15116–15126. [Google Scholar] [CrossRef] [Green Version]
- Boscagli, C.; Raffelt, K.; Zevaco, T.A.; Olbrich, W.; Otto, T.N.; Sauer, J.; Grunwaldt, J.D. Mild hydrotreatment of the light fraction of fast-pyrolysis oil produced from straw over nickel-based catalysts. Biomass Bioenergy 2015, 83, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Furimsky, E. Deactivation of hydroprocessing catalysts. Catal. Today 2002, 52, 381–495. [Google Scholar] [CrossRef]
- Dongil, A.B.; Ghampson, I.T.; García, R.; Fierro, J.L.G.; Escalona, N. Hydrodeoxygenation of guaiacol over Ni/carbon catalysts: Effect of the support and Ni loading. RSC Adv. 2016, 6, 2611–2623. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of pinyon-juniper catalytic pyrolysis oil using red mud-supported nickel catalysts. Appl. Catal. B Environ. 2018, 236, 1–12. [Google Scholar] [CrossRef]
- Sápi, A.; Rémiás, R.; Kónya, Z.; Kukovecz, Á.; Kordás, K.; Kiricsi, I. Synthesis and characterization of nickel catalysts supported on different carbon materials. React. Kinet. Catal. Lett. 2009, 96, 379–389. [Google Scholar] [CrossRef]
- Boscagli, C.; Tomasi Morgano, M.; Raffelt, K.; Leibold, H.; Grunwaldt, J.-D. Influence of feedstock, catalyst, pyrolysis and hydrotreatment temperature on the composition of upgraded oils from intermediate pyrolysis. Biomass Bioenergy 2018, 116, 236–248. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Bykova, M.V.; Khromova, S.A.; Yin, W.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Ni-Based Catalysts for the Hydrotreatment of Fast Pyrolysis Oil. Energy Fuels 2016, 30, 1544–1554. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Nguyen, L.H.; Nguyen, D.A. Hydrodeoxygenation of Bio-Oil on Bimetallic Catalysts: From Model Compound to Real Feed. J. Sustain. Bioenergy Syst. 2015, 5, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Olarte, M.V.; Zacher, A.H.; Padmaperuma, A.B.; Burton, S.D.; Job, H.M.; Lemmon, T.L.; Swita, M.S.; Rotness, L.J.; Neuenschwander, G.N.; Frye, J.G.; et al. Stabilization of Softwood-Derived Pyrolysis Oils for Continuous Bio-oil Hydroprocessing. Top. Catal. 2016, 59, 55–64. [Google Scholar] [CrossRef]
- Gómez-Cazalilla, M.; Infantes-Molina, A.; Mérida-Robles, J.; Rodríguez-Castellón, E.; Jiménez-López, A. Chromium species as captors of sulfur molecules on nickel-based hydrotreating catalysts. Energy Fuels 2009, 23, 101–110. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Yin, W.; Venderbosch, R.H.; Alekseeva, M.V.; Figueirêdo, M.B.; Heeres, H.; Khromova, S.A.; Yakovlev, V.A.; Cannilla, C.; Bonura, G.; Frusteri, F.; et al. Hydrotreatment of the carbohydrate-rich fraction of pyrolysis liquids using bimetallic Ni based catalyst: Catalyst activity and product property relations. Fuel Process. Technol. 2018, 169, 258–268. [Google Scholar] [CrossRef]
- Huazhang, L. Ammonia Synthesis Catalysts: Innovation and Practice; World Scientific: Singapore, 2013; ISBN 978-981-4355-78-0. [Google Scholar]
- Thi, Q.; Bui, P.; Kim, Y.; Pil, S.; Han, J.; Chul, H.; Woo, S.; Won, C. Steam reforming of simulated biogas over plate Ni Cr catalysts: Influence of pre-oxidation on catalytic activity. Appl. Catal. B Environ. 2015, 166–167, 335–344. [Google Scholar]
- Deutschmann, O.; Knözinger, H.; Kochloefl, K.; Turek, T. Heterogeneous Catalysis and Solid Catalysts, 3. Industrial Applications. Ullmann’s Encycl. Ind. Chem. 2011. [Google Scholar] [CrossRef]
- French, R.J.; Stunkel, J.; Black, S.; Myers, M.; Yung, M.M.; Iisa, K. Evaluate impact of catalyst type on oil yield and hydrogen consumption from mild hydrotreating. Energy Fuels 2014, 28, 3086–3095. [Google Scholar] [CrossRef]
- Rover, M.R.; Hall, P.H.; Johnston, P.A.; Smith, R.G.; Brown, R.C. Stabilization of bio-oils using low temperature, low pressure hydrogenation. Fuel 2015, 153, 224–230. [Google Scholar] [CrossRef]
- Pucher, H.; Schwaiger, N.; Feiner, R.; Ellmaier, L.; Pucher, P.; Chernev, B.S.; Siebenhofer, M. Biofuels from liquid phase pyrolysis oil: A two-step hydrodeoxygenation (HDO) process. Green Chem. 2015, 17, 1291–1298. [Google Scholar] [CrossRef]
- Mercader, F.D.M. Pyrolysis oil Upgrading for Co-Processing in Standard Refinery Units. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2010. [Google Scholar]
- Venderbosch, R.; Heeres, H. Stabilisation of Biomass derived Pyrolysis Oils by Catalytic Hydrotreatment. Biocoup.Com. 2010, 85, 1–28. [Google Scholar]
- Schmitt, C.C.; Raffelt, K.; Zimina, A.; Krause, B.; Otto, T.; Rapp, M.; Grunwaldt, J.D.; Dahmen, N. Hydrotreatment of Fast Pyrolysis Bio-oil Fractions Over Nickel-Based Catalyst. Top. Catal. 2018. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Ardiyanti, A.; Venderbosch, R.H.; Heeres, H.J. Characterization of hydrotreated fast pyrolysis liquids. Energy Fuels 2010, 24, 5264–5272. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Garcia-Perez, M. Quantification of Bio-Oil Functional Groups and Evidences of the Presence of Pyrolytic Humins. Energy Fuels 2016, 30, 6505–6524. [Google Scholar] [CrossRef]
- Wan, H.; Chaudhari, R.V.; Subramaniam, B. Aqueous phase hydrogenation of acetic acid and its promotional effect on p-cresol hydrodeoxygenation. Energy Fuels 2013, 27, 487–493. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Yang, G.-X.; Jiang, H.; Liu, W.-J.; Ding, H.-S. Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 2013, 3, 1120. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Fujita, N. Hydrogenation of aliphatic carboxylic acids to corresponding aldehydes over Cr2O3-based catalysts. Appl. Catal. A Gen. 2004, 276, 179–185. [Google Scholar] [CrossRef]
- Martínez, J.D.; Veses, A.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Puy, N.; Artigues, A.; Bartrolí, J.; García, T. Co-pyrolysis of biomass with waste tyres: Upgrading of liquid bio-fuel. Fuel Process. Technol. 2014, 119, 263–271. [Google Scholar] [CrossRef]
- Elliott, D.C. Biofuel from fast pyrolysis and catalytic hydrodeoxygenation. Curr. Opin. Chem. Eng. 2015, 9, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.C.; Moreira, R.; Cruz, R.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.; Dahmen, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Dongil, A.B.; Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Fierro, J.L.G.; Escalona, N. Effect of Cu loading on Ni/carbon nanotubes catalyst for hydrodeoxygenation of guaiacol. Rsc Adv. 2016, 3, 26658–26667. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzin, D. Hydrodeoxygenation of Lignin-Derived Phenols: From Fundamental Studies towards Industrial Applications. Catalysts 2017, 7, 265. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin Pyrolysis Components and Upgrading-Technology Review. Bioenergy Res. 2013, 6, 1183–1204. [Google Scholar] [CrossRef]
- Rencoret, J.; Del Río, J.C.; Nierop, K.G.J.; Gutiérrez, A.; Ralph, J. Rapid Py-GC/MS assessment of the structural alterations of lignins in genetically modified plants. J. Anal. Appl. Pyrolysis 2016, 121, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Haibing, G.; Hexing, L.; Yeping, X.; Minghui, W. Liquid phase glucose hydrogenation over Cr-promoted Ru-B amorphous alloy catalysts. Mater. Lett. 2002, 57, 392–398. [Google Scholar]
- Li, H.; Li, H.; Wang, M. Glucose hydrogenation over promoted Co-B amorphous alloy catalysts. Appl. Catal. A Gen. 2001, 207, 129–137. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Deng, J.F. Glucose hydrogenation over Ni-B/SiO2 amorphous alloy catalyst and the promoting effect of metal dopants. Catal. Today 2002, 74, 53–63. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Zacker, A.H. Catalytic Hydroprocessing of Biomass Fast Pyrolysis Bio-oil to Produce Hydrocarbon Products. Environ. Prog. Sustain. Energy 2009, 28, 441–449. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Li, X.; Cheng, H.; Liang, G.; He, L.; Lin, W.; Yu, Y.; Zhao, F. Effect of Phosphine Doping and the Surface Metal State of Ni on the Catalytic Performance of Ni/Al2O3 Catalyst. Catalysts 2015, 5, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Jensen, A.D. Screening of catalysts for hydrodeoxygenation of phenol as a model compound for bio-oil. ACS Catal. 2013, 3, 1774–1785. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Ang, M.L.; Mo, L.; Li, Z.; Oemar, U.; Kawi, S. Highly active and coke resistant Ni/SiO2 catalysts for oxidative reforming of model biogas: Effect of low ceria loading. J. CO2 Util. 2017, 19, 284–295. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Shpeizer, B.G.; Sagar, G.V.; Chary, K.V.R.; Clearfield, A. Hydrogenation of Naphthalene over NiO/SiO2-Al2O3 catalysts: Structure-activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; de la Peña O’Shea, V.A.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Effect of metal-support interaction on the selective hydrodeoxygenation of anisole to aromatics over Ni-based catalysts. Appl. Catal. B Environ. 2014, 145, 91–100. [Google Scholar] [CrossRef]
- Gervasini, A. Microcalorimetric Study of the Acidity and Basicity. J. Phys. Chem. 1990, 94, 6371–6379. [Google Scholar]
- Aksel, S.; Eder, D. Catalytic effect of metal oxides on the oxidation resistance in carbon nanotube-inorganic hybrids. J. Mater. Chem. 2010, 20, 9149–9154. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Chen, B.; Wang, W. Synergetic Catalysis of Nickel Oxides with Oxygen Vacancies and Nickel Phosphide for the Highly Efficient Hydrodeoxygenation of Phenolic Compounds. ChemCatChem 2018, 10, 2612–2619. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Wu, T.; Zhao, M.; Yan, W.; Zhang, Y.; Li, H.; Wang, Y.; Xiao, T.; Zhao, Y. Synergistic Effect of Oxygen Vacancies and Ni Species on Tuning Selectivity of Ni/ZrO2 Catalyst for Hydrogenation of Maleic Anhydride into Succinic Anhydride and γ-Butyrolacetone. Nanomaterials 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Jensen, A.D. Influence on nickel particle size on the hydrodeoxygenation of phenol over Ni/SiO2. Catal. Today 2016, 259, 277–284. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. High-loaded nickel–silica catalysts for hydrogenation, prepared by sol–gel. Appl. Catal. A Gen. 2003, 245, 277–288. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Boscagli, C.; Rapp, M.; Raffelt, R.; Dahmen, N. Characterization of light and heavy phase of pyrolysis-oil from distinct biomass for further upgrading reactions. Proc. Eur. Biomass Conf. Exhib. 2017, 12–15. [Google Scholar]
- Zimina, A.; Dardenne, K.; Denecke, M.A.; Doronkin, D.E.; Huttel, E.; Lichtenberg, H.; Mangold, S.; Pruessmann, T.; Rothe, J.; Spangenberg, T.; et al. CAT-ACT—A new highly versatile x-ray spectroscopy beamline for catalysis and radionuclide science at the KIT synchrotron light facility ANKA. Rev. Sci. Instrum. 2017, 88, 113113. [Google Scholar] [CrossRef]
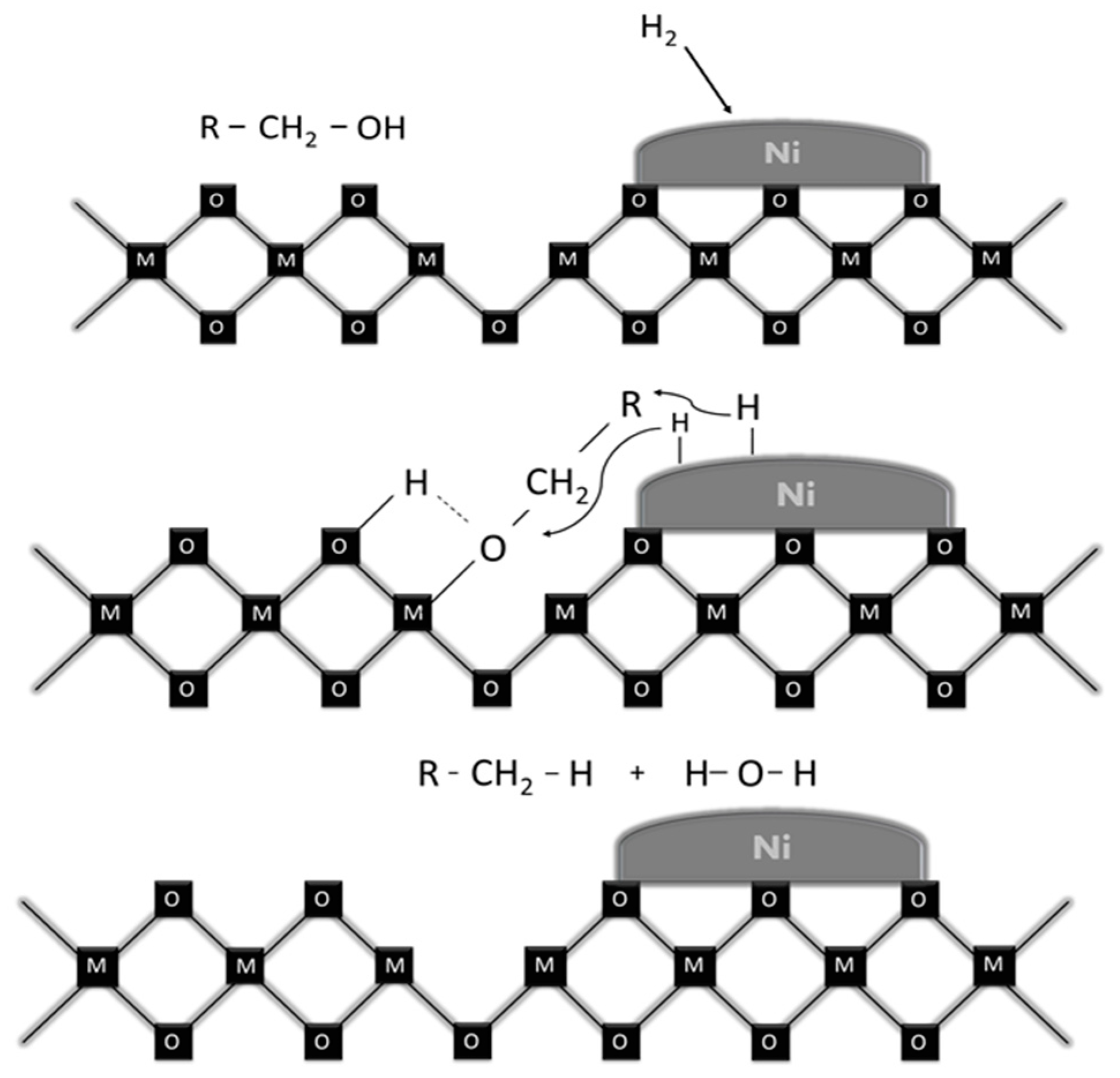
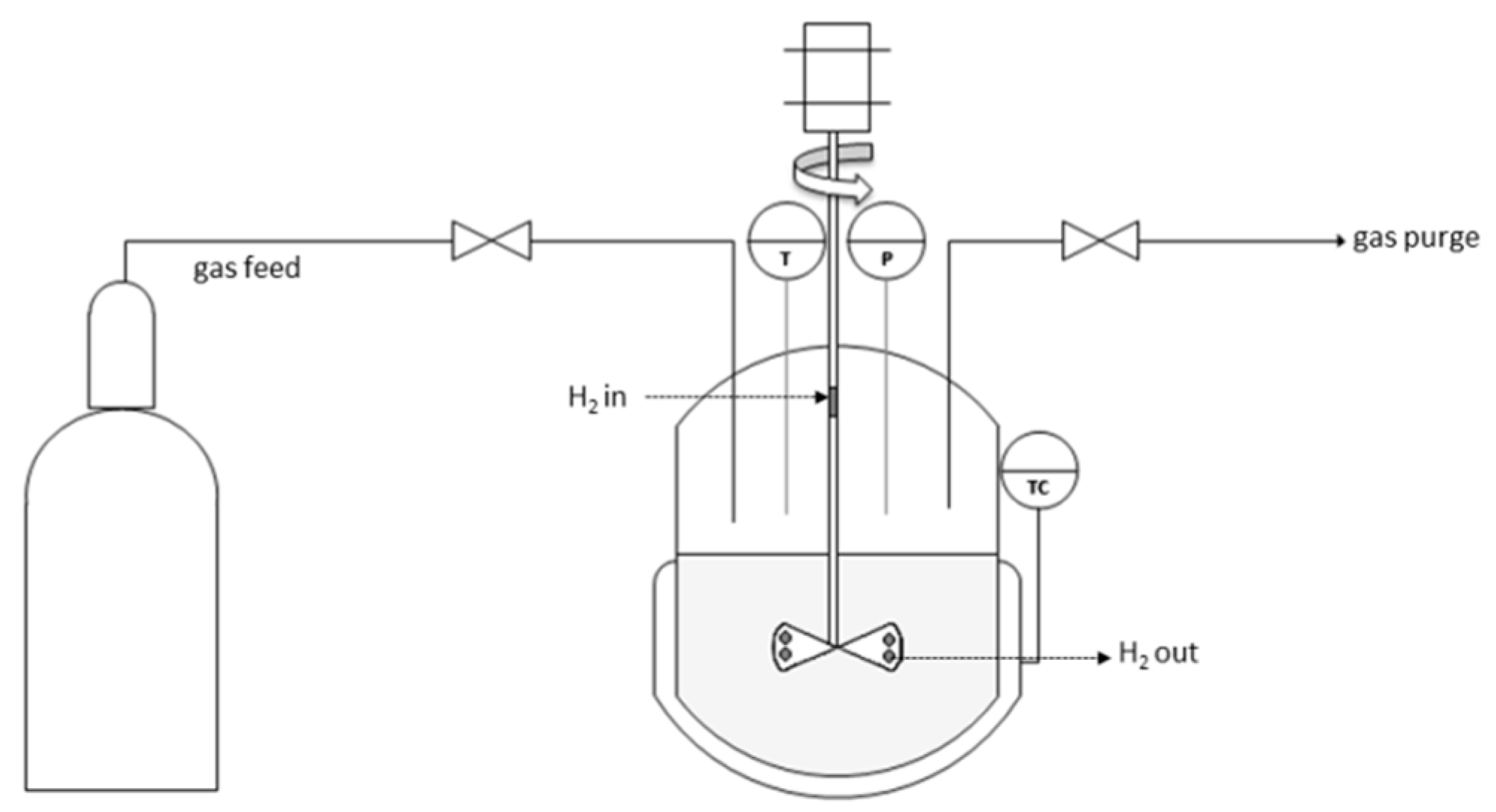
| Ni, 175 °C 1-step UR | Ni, 225 °C 1-step UR | Ni–Cr, 175 °C 1-step UR | Ni–Cr, 225 °C 1-step UR | Ru/C, 175 °C 1-step UR | Ru/C, 225 °C 1-step UR | Ni–Cr, 325 °C 2-step UR 1 | BWBO 2 | UBWBO 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upgraded oils and feedstocks - wet basis (wb) and dry basis (bd) | ||||||||||||||||||
| wb | bd | Wb | bd | wb | bd | wb | bd | wb | bd | wb | bd | wb | bd | wb | db | wb | db | |
| C (wt.%) | 57.9 | 65.9 | 58.3 | 65.1 | 58.5 | 65.5 | 60.1 | 66.8 | 57.6 | 65.2 | 59.9 | 67.0 | 76.8 | 78.6 | 46.1 | 59.9 | 69.1 | 72.9 |
| H (wt.%) | 8.0 | 7.6 | 8.0 | 7.6 | 8.0 | 7.6 | 8.2 | 7.9 | 7.9 | 7.5 | 8.1 | 7.7 | 9.6 | 9.6 | 7.8 | 6.8 | 8.5 | 8.4 |
| O (wt.%) | 34.1 | 26.5 | 33.7 | 27.2 | 33.5 | 26.8 | 31.7 | 25.3 | 34.4 | 27.2 | 31.8 | 25.0 | 13.4 | 11.6 | 46.0 | 33.0 | 21.8 | 18.2 |
| N (wt.%) | <0.1 | - | <1.0 | - | <1.0 | - | <1.0 | - | <0.2 | - | <0.2 | - | <0.2 | <0.2 | <0.2 | <0.2 | 0.3 | 0.3 |
| S (wt.%) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | 0.011 | 0.015 | 0.225 | 0.264 | |||||||
| pH value | 3.6 | - | 4.1 | - | 3.2 | - | 3.3 | - | 3.3 | - | 3.6 | - | 4.9 | - | 3LP; 2.7HP | - | 3.0 | - |
| H2O (wt.%) | 12.1 | - | 10.5 | - | 10.7 | - | 10.1 | - | 11.7 | - | 10.6 | - | 2.3 | - | 23.0 | - | 5.1 | - |
| HHV 4 (MJ/Kg) | 26.3 | - | 26.3 | - | 26.2 | - | 27.3 | - | 25.5 | - | 26.7 | - | 36.9 | - | 19.41 | - | 30.93 | - |
| Integration Range (ppm) 1 | Ni, 1-step 175 °C mmol⋅g−1 | Ni, 1-step 225 °C mmol⋅g−1 | Ni–Cr 1-step 175 °C mmol⋅g−1 | Ni–Cr 1-step 225 °C mmol⋅g−1 | Ni–Cr 2-step 325 °C mmol⋅g−1 | Ru/C 1-step 175 °C mmol⋅g−1 | Ru/C 1-step 225 °C mmol⋅g−1 | UBWBO mmol⋅g−1 | BWBO mmol⋅g−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UOP | UAP | UOP | UAP | UOP | UAP | UOP | UAP | UOP | UAP | UOP | UAP | UOP | UAP | UOP | HP | LP | |
| 0.5–1.5 | 16.66 | 7.05 | 17.39 | 7.47 | 16.79 | 6.54 | 21.55 | 5.85 | 41.80 | 0.75 | 13.40 | 5.74 | 16.30 | 4.90 | 22.0 | 8.20 | 3.0 |
| 1.5–3.0 | 21.56 | 9.45 | 23.22 | 9.94 | 21.38 | 6.92 | 25.94 | 6.09 | 36.50 | 4.30 | 20.30 | 8.30 | 21.90 | 4.27 | 39.20 | 17.80 | 11.0 |
| 3.0–4.3 | 13.96 | 20.76 | 13.66 | 19.81 | 16.45 | 18.18 | 15.55 | 14.00 | 5.90 | 0.50 | 14.40 | 16.10 | 13.30 | 11.4 | 11.50 | 13.20 | 13.90 |
| 4.3–6.0 | 22.11 | 64.00 | 21.76 | 68.99 | 27.74 | 60.26 | 22.78 | 53.62 | 6.30 | 111.7 | 29.90 | 64.30 | 24.80 | 77.5 | 13.50 | 28.50 | 50.20 |
| 6.0–8.5 | 6.07 | 0.67 | 6.04 | 0.79 | 5.21 | 0.82 | 6.01 | 0.50 | 5.80 | 0.10 | 5.90 | 0.55 | 4.80 | 0.40 | 7.50 | 8.14 | 3.20 |
| 9.5–10.1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.01 | 0.06 |
| Compounds | BWBO 1 | UBWBO | Ni, 225 °C, 1-step UR | Ni–Cr, 225 °C, 1-step UR | Ni–Cr, 325 °C, 2-step UR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wb 2 (wt.%) | db 3 (wt.%) | wb 2 (wt.%) | db 3 (wt.%) | UOP wb 2 (wt.%) | UOP db 3 (wt.%) | UAP wb 2 (wt.%) | UAP db 3 (wt.%) | UOP wb 2 (wt.%) | UOP db 3 (wt.%) | UAP wb 2 (wt.%) | UAP db 3 (wt.%) | UOP wb 2 (wt.%) | UOP db 3 (wt.%) | UAP wb 2 (wt.%) | UAP db 3 (wt.%) | |
| Nonaromatic compounds | 17.41 | 24.74 | 13.69 | 14.61 | 15.00 | 16.91 | 28.70 | 55.68 | 13.15 | 14.64 | 20.72 | 41.57 | 5.13 | 5.28 | 2.96 | 80.99 |
| Acids | 5.56 | 7.87 | 9.71 | 10.37 | 8.64 | 9.73 | 10.02 | 19.45 | 7.96 | 8.86 | 4.55 | 9.14 | - | - | 2.73 | 74.72 |
| Nonaromatic Esters | 0.33 | 0.47 | 0.08 | 0.08 | 0.59 | 0.66 | 0.79 | 1.53 | 0.27 | 0.31 | 0.58 | 1.16 | - | - | - | - |
| Nonaromatic Alcohols | 1.96 | 2.89 | 0.18 | 0.19 | 4.16 | 4.69 | 17.51 | 33.97 | 4.34 | 4.83 | 15.54 | 31.17 | 0.46 | 0.47 | 0.11 | 3.12 |
| Nonaromatic Aldehydes | 3.49 | 4.99 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Nonaromatic Ketones | 6.05 | 8.51 | 3.72 | 3.98 | 1.57 | 1.77 | 0.38 | 0.73 | 0.58 | 0.65 | 0.05 | 0.10 | 1.84 | 1.89 | 0.12 | 3.15 |
| Hydrocarbons | - | - | - | - | 0.04 | 0.05 | - | - | - | - | - | - | 2.84 | 2.92 | - | - |
| Heterocyclic compounds | 2.39 | 3.30 | 0.70 | 0.75 | 2.41 | 2.72 | 1.88 | 3.66 | 2.52 | 2.80 | 1.85 | 3.71 | 0.34 | 0.35 | 0.16 | 4.44 |
| Furans | 2.19 | 3.04 | 0.70 | 0.75 | 2.23 | 2.51 | 1.76 | 3.42 | 2.27 | 2.53 | 1.70 | 3.41 | 0.34 | 0.35 | 0.16 | 4.44 |
| Pyrans | 0.19 | 0.27 | - | - | 0.18 | 0.20 | 0.12 | 0.24 | 0.25 | 0.27 | 0.15 | 0.29 | - | - | - | - |
| Aromatic Compounds | 4.46 | 5.813 | 4.47 | 4.77 | 7.27 | 8.2 | 1.54 | 2.98 | 6.25 | 6.95 | 0.93 | 1.87 | 1.94 | 1.99 | - | 0.09 |
| Benzenes | 0.05 | 0.05 | 0.07 | 0.08 | 0.06 | 0.06 | - | - | 0.10 | 0.11 | - | - | 0.60 | 0.62 | - | - |
| Aromatic Alcohols | - | - | - | - | - | - | 0.05 | 0.11 | - | - | - | - | - | - | - | - |
| Aromatic Aldehydes | 0.03 | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Aromatic Ketones | 0.01 | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Aromatic Esters | - | - | - | - | - | - | 0.05 | 0.10 | 0.08 | 0.09 | - | - | - | - | - | - |
| Lignin Derived Phenols | 0.5 | 0.64 | 0.86 | 0.91 | 0.70 | 0.79 | 0.06 | 0.11 | 0.71 | 0.79 | 0.03 | 0.07 | 0.68 | 0.70 | - | - |
| Guaiacols | 3.68 | 4.81 | 3.54 | 3.78 | 5.53 | 6.23 | 1.37 | 2.67 | 5.21 | 5.80 | 0.90 | 1.80 | 0.66 | 0.68 | - | - |
| Syringols | 0.19 | 0.25 | - | - | 0.23 | 0.26 | - | - | 0.15 | 0.17 | - | - | - | - | - | - |
| Carbohydrate | 6.79 | 9.96 | - | - | - | - | 1.31 | 2.54 | - | - | 0.19 | 0.39 | - | - | - | - |
| Sugars | 6.79 | 9.96 | - | - | - | - | 1.31 | 2.54 | - | - | 0.19 | 0.39 | - | - | ||
| Other organic compounds 4 | 0.68 | 0.03 | 0.07 | 0.07 | 0.45 | 0.51 | 0.65 | 1.26 | 0.20 | 0.22 | 0.56 | 1.11 | 0.20 | 0.21 | 0.01 | 0.16 |
| Total (wt.%) 5 | 34.61LP 28.14HP | 53.66LP 32.83HP | 18.93 | 20.21 | 34.08 | 66.12 | 25.14 | 28.33 | 24.25 | 48.66 | 22.12 | 24.62 | 3.14 | 85.68 | 7.61 | 7.84 |
| Sample | Low Temperature NH3 Desorption (mol.g−1) | High Temperature NH3 Desorption (mol.g−1) | Density of Acid Site (μmol/m2) 1 |
|---|---|---|---|
| Ni | 3.32 × 10−4 | 1.00 × 10−3 | 7.01 |
| Ni–Cr | 1.92 × 10−4 | 4.65 × 10−4 | 6.99 |
| Element | C | O | Al | Si | Ni | Cr | S |
|---|---|---|---|---|---|---|---|
| at.% | 88.46 | 5.49 | 0.37 | 1.10 | 2.63 | 0.60 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriel Schmitt, C.; Zimina, A.; Fam, Y.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil. Catalysts 2019, 9, 784. https://doi.org/10.3390/catal9090784
Carriel Schmitt C, Zimina A, Fam Y, Raffelt K, Grunwaldt J-D, Dahmen N. Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil. Catalysts. 2019; 9(9):784. https://doi.org/10.3390/catal9090784
Chicago/Turabian StyleCarriel Schmitt, Caroline, Anna Zimina, Yakub Fam, Klaus Raffelt, Jan-Dierk Grunwaldt, and Nicolaus Dahmen. 2019. "Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil" Catalysts 9, no. 9: 784. https://doi.org/10.3390/catal9090784
APA StyleCarriel Schmitt, C., Zimina, A., Fam, Y., Raffelt, K., Grunwaldt, J.-D., & Dahmen, N. (2019). Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil. Catalysts, 9(9), 784. https://doi.org/10.3390/catal9090784







