Optimizing the Aromatic Product Distribution from Catalytic Fast Pyrolysis of Biomass Using Hydrothermally Synthesized Ga-MFI Zeolites
Abstract
1. Introduction
2. Results and Discussion
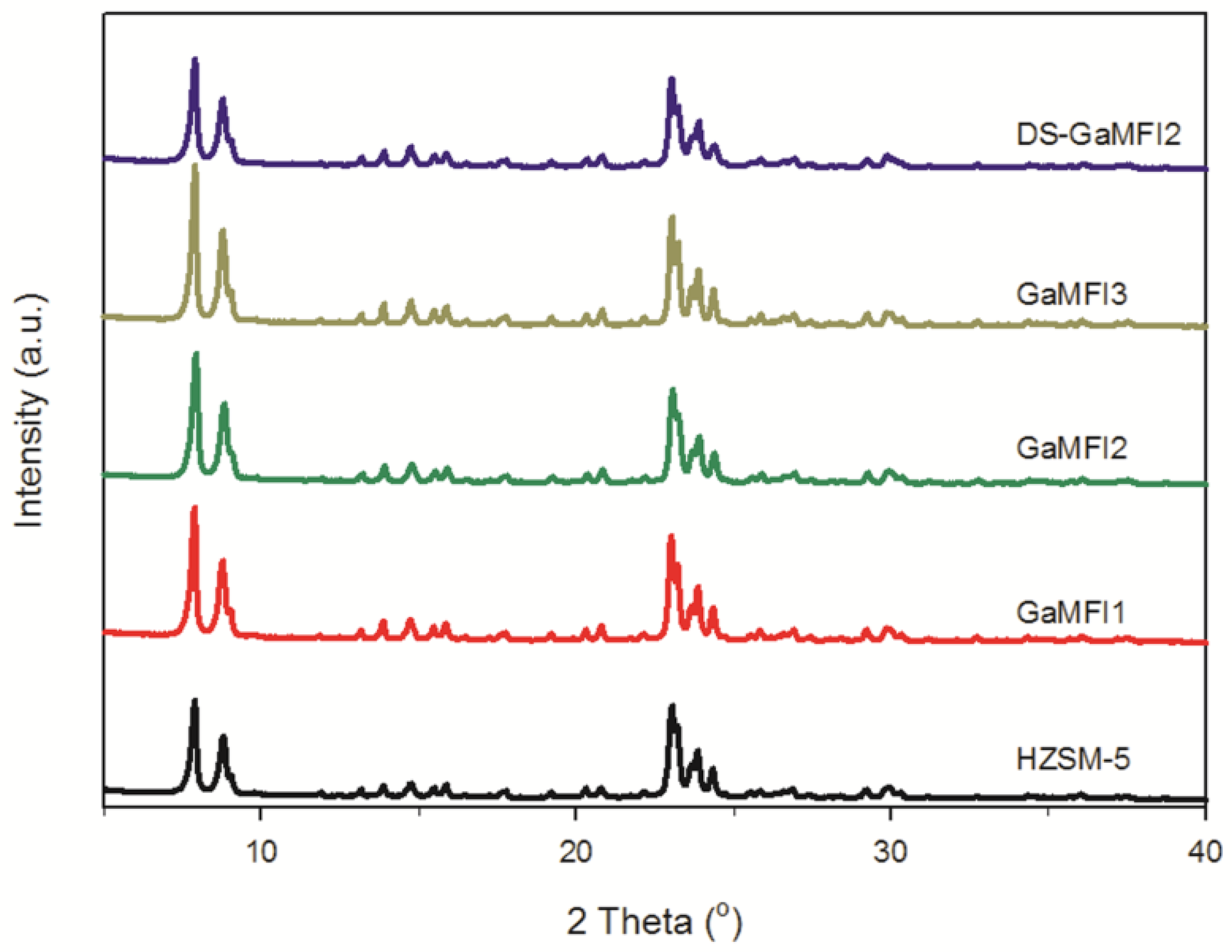
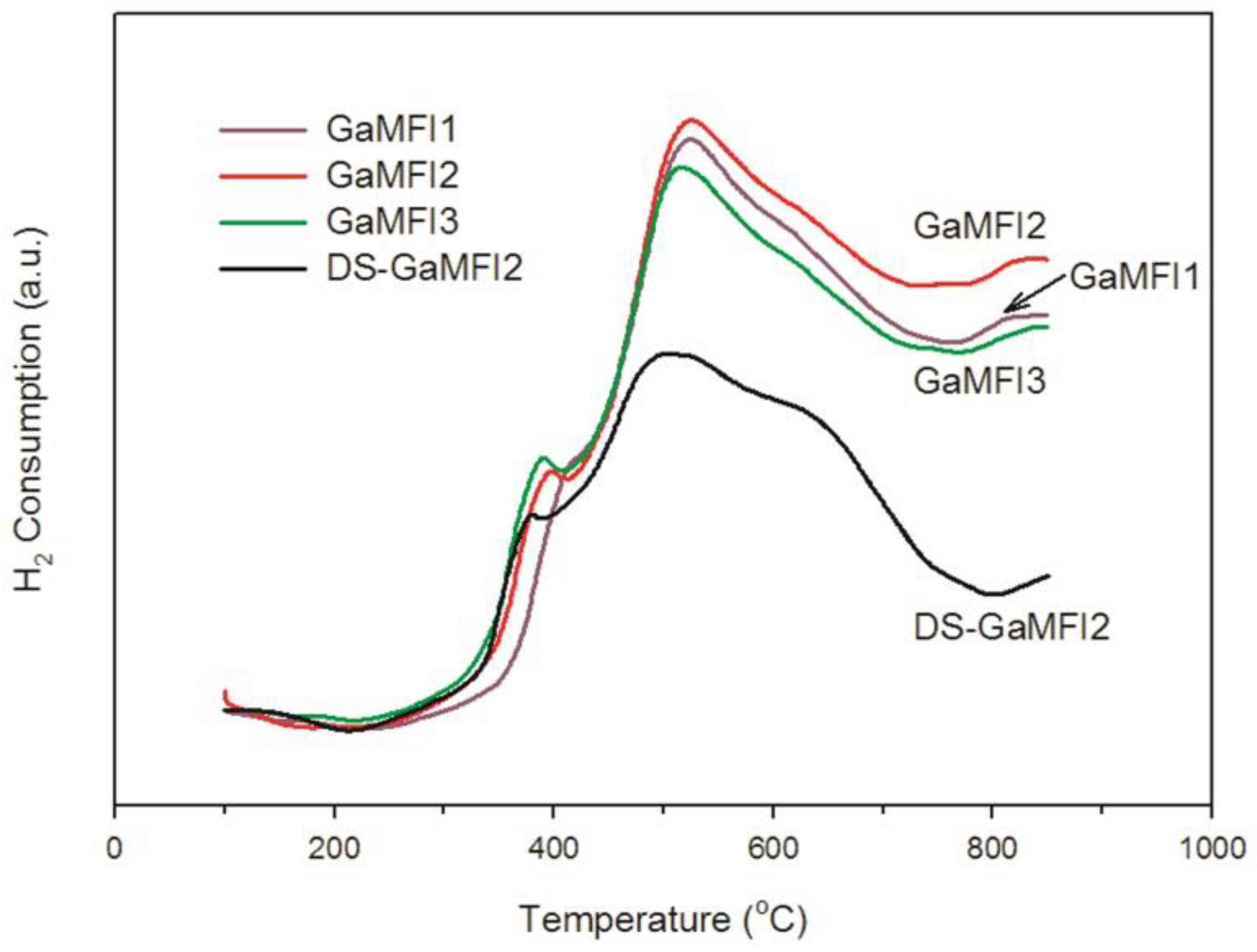
2.1. Zeolite Characterizations
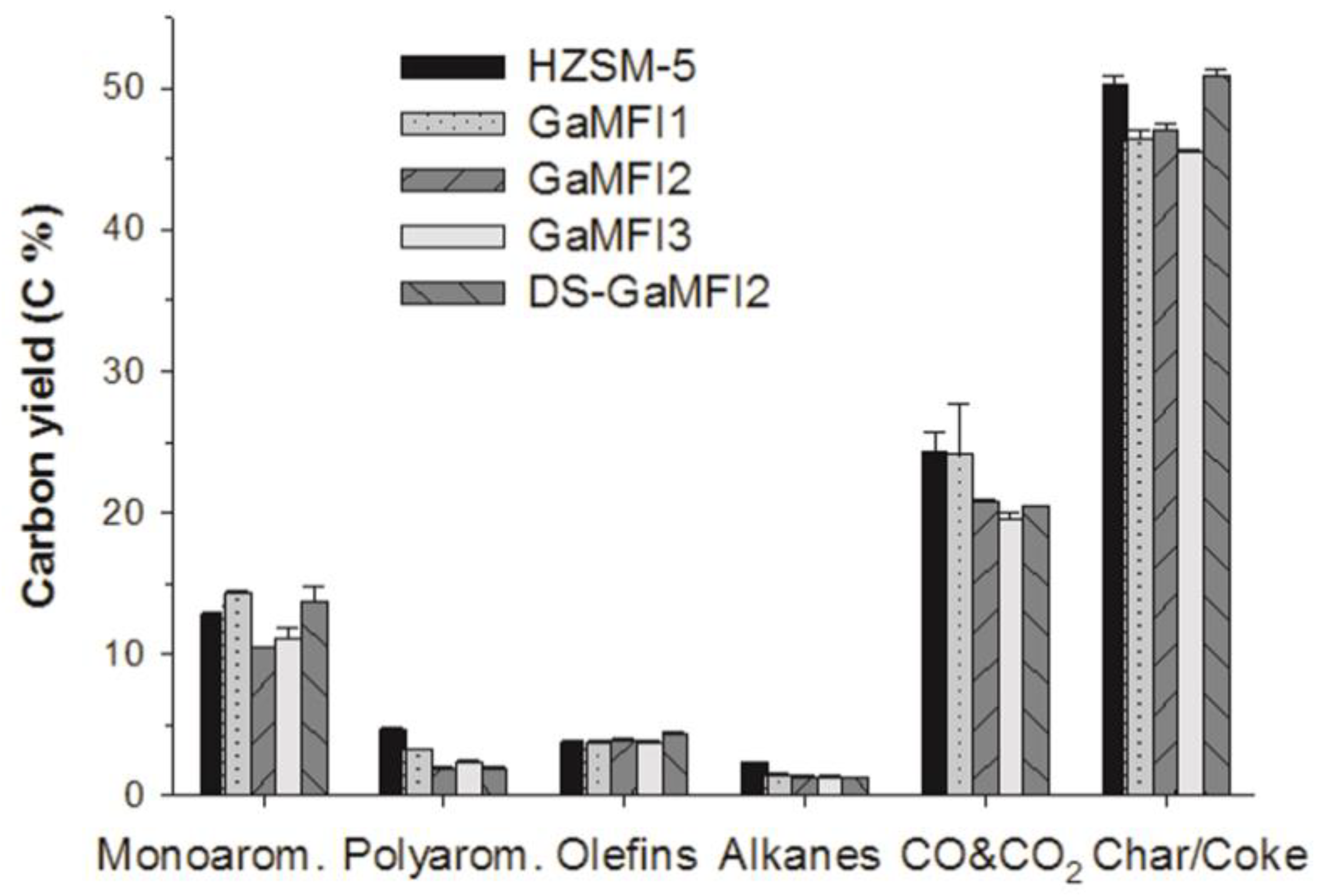
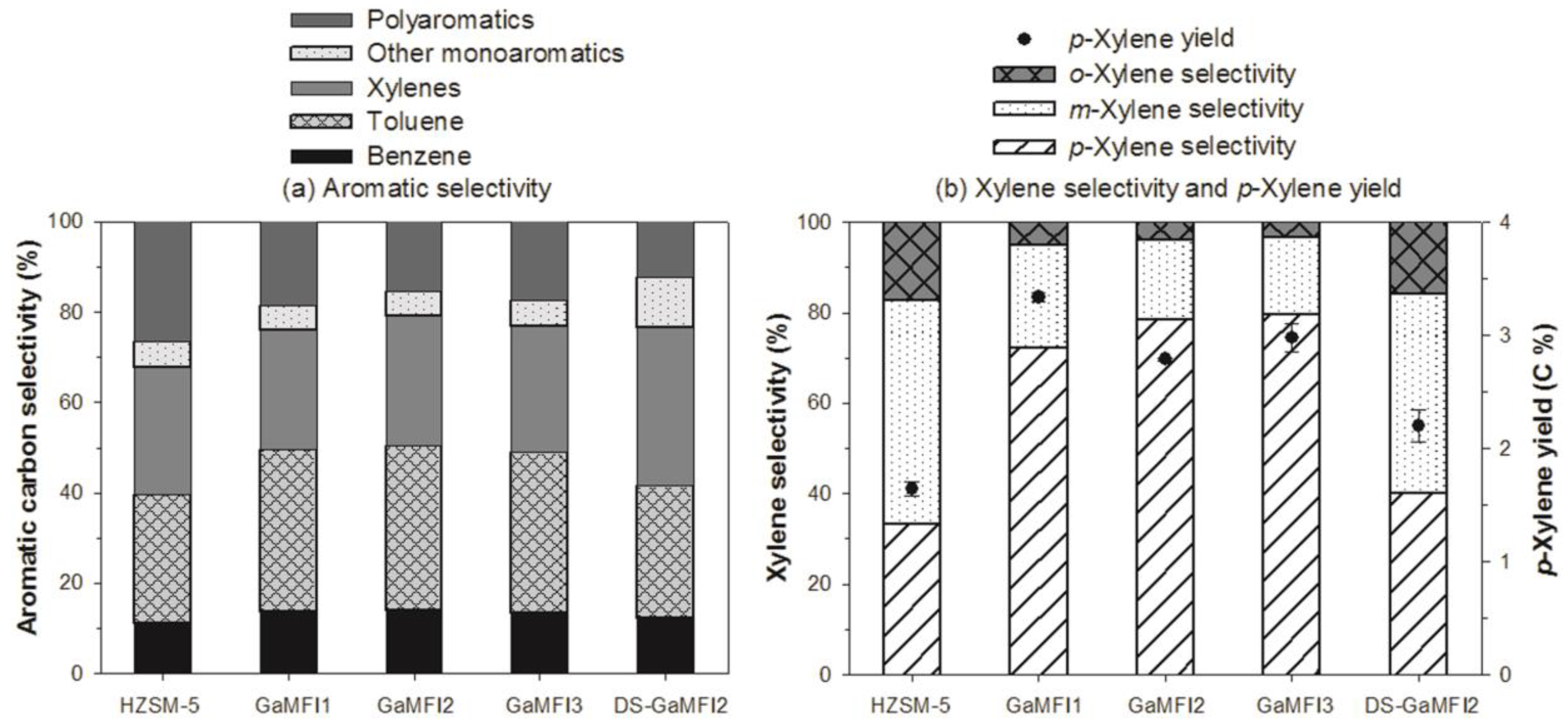
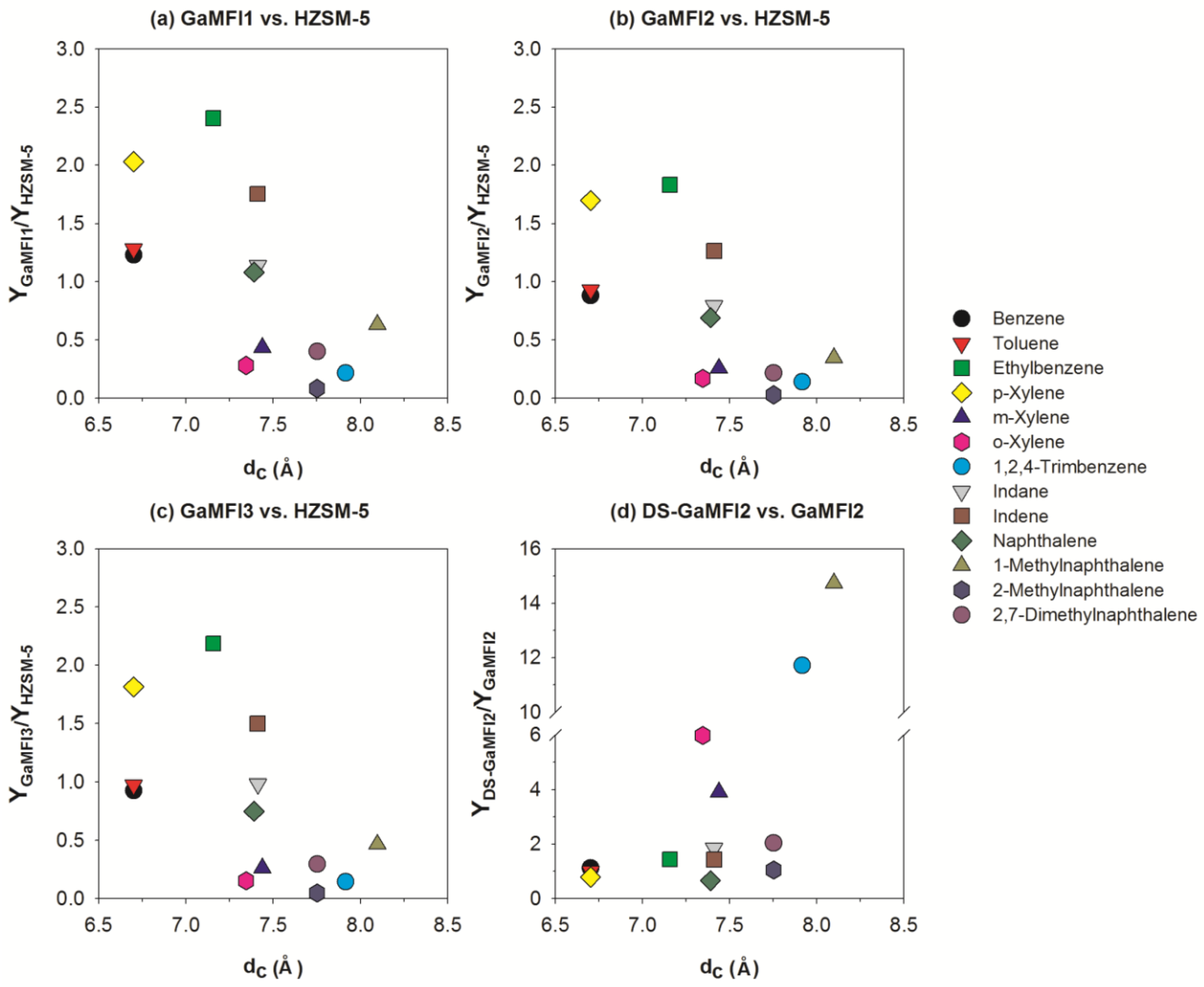
2.2. CFP of Beech Wood with HZSM-5 and the Ga-Containing MFI Zeolites
3. Materials and Methods
3.1. Materials
3.2. Zeolite Synthesis
3.3. Zeolite Characterizaitions
3.4. Catalytic Fast Pyrolysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Lei, H.W.; Chen, S.L.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eranen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Alejandro Martín, S.; Cerda-Barrera, C.; Montecinos, A. Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite. Catalysts 2017, 7, 356. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A 2012, 447–448, 115–123. [Google Scholar] [CrossRef]
- Foster, A.J.; Jae, J.; Cheng, Y.T.; Huber, G.W.; Lobo, R.F. Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl. Catal. A 2012, 423, 154–161. [Google Scholar] [CrossRef]
- Carlson, T.R.; Jae, J.; Lin, Y.C.; Tompsett, G.A.; Huber, G.W. Catalytic fast pyrolysis of glucose with HZSM-5: The combined homogeneous and heterogeneous reactions. J. Catal. 2010, 270, 110–124. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, Y.; Zuo, J.; Ma, F.; Yang, X.; Zhang, X.; Wang, Y. Improving the conversion of biomass in catalytic fast pyrolysis via white-rot fungal pretreatment. Bioresour. Technol. 2013, 134, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Gupta, R.B.; Tu, M.; Taylor, S. Production of hydrocarbon fuels from biomass using catalytic pyrolysis under helium and hydrogen environments. Bioresour. Technol. 2011, 102, 6742–6749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.; Li, X.; Wang, W.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Maximizing carbon efficiency of petrochemical production from catalytic co-pyrolysis of biomass and plastics using gallium-containing MFI zeolites. Appl. Catal. B Environ. 2015, 172–173, 154–164. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Wang, Z.; Gilbert, C.J.; Fan, W.; Huber, G.W. Production of p-xylene from biomass by catalytic fast pyrolysis using ZSM-5 catalysts with reduced pore openings. Angew. Chem. 2012, 51, 11097–11100. [Google Scholar] [CrossRef]
- Li, J.; Li, X.Y.; Zhou, G.Q.; Wang, W.; Wang, C.W.; Komarneni, S.; Wang, Y.J. Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl. Catal. A 2014, 470, 115–122. [Google Scholar] [CrossRef]
- Mei, C.; Wen, P.; Liu, Z.; Liu, H.; Wang, Y.; Yang, W.; Xie, Z.; Hua, W.; Gao, Z. Selective production of propylene from methanol: Mesoporosity development in high silica HZSM-5. J. Catal. 2008, 258, 243–249. [Google Scholar] [CrossRef]
- Khan, W.; Jia, X.; Wu, Z.; Choi, J.; Yip, A. Incorporating Hierarchy into Conventional Zeolites for Catalytic Biomass Conversions: A Review. Catalysts 2019, 9, 127–149. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Effect of desilication of H-ZSM-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Lai, P.C.; Chen, C.H.; Hsu, H.Y.; Lee, C.H.; Lin, Y.C. Methanol aromatization over Ga-doped desilicated HZSM-5. RSC Adv. 2016, 6, 67361–67371. [Google Scholar] [CrossRef]
- Sadowska, K.; Góra-Marek, K.; Drozdek, M.; Kuśtrowski, P.; Datka, J.; Martinez Triguero, J.; Rey, F. Desilication of highly siliceous zeolite ZSM-5 with NaOH and NaOH/tetrabutylamine hydroxide. Microporous Mesoporous Mater. 2013, 168, 195–205. [Google Scholar] [CrossRef]
- Vicente, J.; Gayubo, A.G.; Ereña, J.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Improving the DME steam reforming catalyst by alkaline treatment of the HZSM-5 zeolite. Appl. Catal. B Environ. 2013, 130–131, 73–83. [Google Scholar] [CrossRef]
- McGlone, J.; Priecel, P.; Da Vià, L.; Majdal, L.; Lopez-Sanchez, J. Desilicated ZSM-5 Zeolites for the Production of Renewable p-Xylene via Diels–Alder Cycloaddition of Dimethylfuran and Ethylene. Catalysts 2018, 8, 253. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Al-Khattaf, S. Physicochemical properties and catalytic performance of galloaluminosilicate in aromatization of lower alkanes: A comparative study with Ga/HZSM-5. J. Porous Mater. 2011, 19, 943–960. [Google Scholar] [CrossRef]
- Fricke, R.; Kosslick, H.; Lischke, G.; Richter, M. Incorporation of Gallium into Zeolites: Syntheses, Properties and Catalytic Application. Chem. Rev. 2010, 100, 2303–2405. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Huang, Y.; To, A.T.; Sooknoi, T.; Resasco, D.E. Hydrodeoxygenation of m-cresol over gallium-modified beta zeolite catalysts. J. Catal. 2012, 290, 90–100. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.H.; Jung, J.; Jeon, J.K.; Ko, C.H.; Jeong, K.E.; Park, Y.K. Catalytic pyrolysis of mandarin residue from the mandarin juice processing industry. Bioresour. Technol. 2013, 136, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Kinage, A.K.; Sivadinarayana, C.; Devadas, P.; Sansare, S.D.; Guisnet, M. H-gallosilicate (MFI) propane aromatization catalyst: Influence of Si/Ga ratio on acidity, activity and deactivation due to coking. J. Catal. 1996, 158, 34–50. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Holm, M.S.; Svelle, S.; Joensen, F.; Beato, P.; Christensen, C.H.; Bordiga, S.; Bjørgen, M. Assessing the acid properties of desilicated ZSM-5 by FTIR using CO and 2,4,6-trimethylpyridine (collidine) as molecular probes. Appl. Catal. A 2009, 356, 23–30. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Ogunronbi, K.; Al-Khattaf, S. Synthesis of stable H-galloaluminosilicate MFI with hierarchical pore architecture by surfactant-mediated base hydrolysis, and their application in propane aromatization. J. Mol. Catal. A Chem. 2012, 360, 1–15. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T.; Resasco, D.E. Catalytic deoxygenation of benzaldehyde over gallium-modified ZSM-5 zeolite. J. Catal. 2009, 268, 68–78. [Google Scholar] [CrossRef]
- Nowak, I. Effect of H2–O2 pre-treatments on the state of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. Appl. Catal. A 2003, 251, 107–120. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Bilbao, J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. I. Alcohols and phenols. Ind. Eng. Chem. Rev. 2004, 43, 2610–2618. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Olazar, M.; Bilbao, J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. H. Aldehydes, ketones, and acids. Ind. Eng. Chem. Rev. 2004, 43, 2619–2626. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. Origin of carbon in aromatic and olefin products derived from HZSM-5 catalyzed co-pyrolysis of cellulose and plastics via isotopic labeling. Appl. Catal. B Environ. 2015, 162, 338–345. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Abelló, S.; Bonilla, A.; Groen, J.C. Tailored Mesoporosity Development in Zeolite Crystals by Partial Detemplation and Desilication. Adv. Funct. Mater. 2009, 19, 164–172. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal. J. Catal. 2010, 271, 88–98. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Panjala, D.; Banerjee, S. Aromatization of propene and n-butene over H-galloaluminosilicate (ZSM-5 type) zeolite. Appl. Catal. A 2002, 231, 243–251. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Nayak, V.S.; Choudhary, T.V. Single-Component Sorption/Diffusion of Cyclic Compounds from Their Bulk Liquid Phase in H-ZSM-5 Zeolite. Ind. Eng. Chem. Rev. 1997, 36, 1812–1818. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Jeon, J.K.; Kim, J.; Ryoo, R.; Jeong, K.E.; Park, Y.K. Highly valuable chemicals production from catalytic upgrading of radiata pine sawdust-derived pyrolytic vapors over mesoporous MFI zeolites. Appl. Catal. B Environ. 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Jae, J.; Shi, J.; Fan, W.; Huber, G.W. Production of renewable aromatic compounds by catalytic fast pyrolysis of lignocellulosic biomass with bifunctional Ga/ZSM-5 catalysts. Angew. Chem. 2012, 51, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, K.H.; Nam, C.M.; Lee, J.S. Formation of Hierarchical Pore Structures in Zn/ZSM-5 to Improve the Catalyst Stability in the Aromatization of Branched Olefins. ChemCatChem 2012, 4, 1143–1153. [Google Scholar] [CrossRef]
- Ohayon, D.; Le van Mao, R.; Ciaravino, D.; Hazel, H.; Cochennec, A.; Rolland, N. Methods for pore size engineering in ZSM-5 zeolite. Appl. Catal. A 2001, 217, 241–251. [Google Scholar] [CrossRef]
- Torri, C.; Reinikainen, M.; Lindfors, C.; Fabbri, D.; Oasmaa, A.; Kuoppala, E. Investigation on catalytic pyrolysis of pine sawdust: Catalyst screening by Py-GC-MIP-AED. J. Anal. Appl. Pyrolysis 2010, 88, 7–13. [Google Scholar] [CrossRef]
| Catalyst | Ga2O3 a (%) | Ga2O3/Al2O3 a | SiO2/(Al2O3 + Ga2O3) a | SBET b (m2/g) | Vtotal (cm3/g) | Vmicro c (cm3/g) | Vmeso d (cm3/g) | ABrönsted e (μmol/g) | ALewis e (μmol/g) |
|---|---|---|---|---|---|---|---|---|---|
| HZSM-5 | - | - | 25.5 | 423.7 | 0.211 | 0.158 | 0.053 | 340.2 | 75.6 |
| GaMFI1 | 4.72 | 1.2 | 33.0 | 412.0 | 0.233 | 0.165 | 0.068 | 198.5 | 85.3 |
| GaMFI2 | 5.09 | 1.7 | 36.0 | 394.3 | 0.204 | 0.148 | 0.056 | 201.4 | 76.8 |
| GaMFI3 | 5.56 | 2.3 | 36.6 | 407.5 | 0.229 | 0.164 | 0.065 | 160.4 | 85.2 |
| DS-GaMFI2 | 5.74 | 1.7 | 31.5 | 404.0 | 0.254 | 0.139 | 0.115 | 129.5 | 95.2 |
| Carbon Yield (C%) | HZSM-5 | GaMFI1 | GaMFI2 | GaMFI3 | DS-GaMFI2 |
|---|---|---|---|---|---|
| Aromatic hydrocarbons | |||||
| Benzene | 1.96 ± 0.01 | 2.40 ± 0.03 | 1.72 ± 0.01 | 1.81 ± 0.13 | 1.92 ± 0.14 |
| Toluene | 4.91 ± 0.06 | 6.28 ± 0.03 | 4.56 ± 0.01 | 4.77 ± 0.36 | 4.55 ± 0.35 |
| Ethylbenzene | 0.15 ± 0.01 | 0.35 ± 0.00 | 0.27 ± 0.00 | 0.32 ± 0.02 | 0.38 ± 0.02 |
| p-Xylene | 1.64 ± 0.06 | 3.34 ± 0.05 | 2.79 ± 0.01 | 2.98 ± 0.12 | 2.20 ± 0.15 |
| m-Xylene | 2.43 ± 0.05 | 1.05 ± 0.09 | 0.62 ± 0.02 | 0.64 ± 0.08 | 2.42 ± 0.20 |
| o-Xylene | 0.85 ± 0.00 | 0.24 ± 0.00 | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.86 ± 0.08 |
| 1,2,4-Trimethylbenzene | 0.47 ± 0.01 | 0.10 ± 0.01 | 0.07 ± 0.00 | 0.07 ± 0.01 | 0.78 ± 0.06 |
| Indane | 0.21 ± 0.01 | 0.24 ± 0.01 | 0.17 ± 0.00 | 0.20 ± 0.01 | 0.31 ± 0.02 |
| Indene | 0.16 ± 0.01 | 0.27 ± 0.00 | 0.20 ± 0.00 | 0.23 ± 0.01 | 0.28 ± 0.02 |
| Naphthalene | 1.53 ± 0.01 | 1.65 ± 0.05 | 1.05 ± 0.02 | 1.14 ± 0.11 | 0.69 ± 0.06 |
| 1-methylnaphthalene | 0.13 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.00 |
| 2-methylnaphthalene | 1.82 ± 0.05 | 1.15 ± 0.05 | 0.63 ± 0.01 | 0.85 ± 0.06 | 0.66 ± 0.05 |
| 2,7-dimethylnaphthalene | 1.17 ± 0.05 | 0.47 ± 0.03 | 0.26 ± 0.00 | 0.35 ± 0.02 | 0.52 ± 0.04 |
| Olefins | |||||
| Ethylene | 2.25 ± 0.08 | 2.13 ± 0.05 | 2.14 ± 0.05 | 1.99 ± 0.05 | 2.22 ± 0.10 |
| Propene | 1.00 ± 0.04 | 1.14 ± 0.03 | 1.25 ± 0.01 | 1.19 ± 0.08 | 1.46 ± 0.08 |
| C4 olefins | 0.31 ± 0.02 | 0.36 ± 0.01 | 0.38 ± 0.00 | 0.42 ± 0.01 | 0.45 ± 0.02 |
| C5 olefins | 0.09 ± 0.00 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.16 ± 0.02 |
| Alkanes | |||||
| Methane | 0.64 ± 0.02 | 0.74 ± 0.01 | 0.73 ± 0.02 | 0.70 ± 0.04 | 0.63 ± 0.01 |
| Ethane | 0.24 ± 0.01 | 0.12 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.09 ± 0.00 |
| Propane | 1.15 ± 0.04 | 0.48 ± 0.02 | 0.37 ± 0.02 | 0.36 ± 0.04 | 0.35 ± 0.02 |
| C4 alkanes | 0.19 ± 0.01 | 0.11 ± 0.00 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 |
| C5 alkanes | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| CO and CO2 | 24.28 ± 1.45 | 24.20 ± 3.55 | 20.73 ± 0.24 | 19.55 ± 0.46 | 20.47 ± 0.03 |
| Char/Coke | 50.2 ± 0.71 | 46.5 ± 0.58 | 47.0 ± 0.55 | 45.5 ± 0.21 | 50.9 ± 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Hua, D.; Lu, X.; Wang, Y. Optimizing the Aromatic Product Distribution from Catalytic Fast Pyrolysis of Biomass Using Hydrothermally Synthesized Ga-MFI Zeolites. Catalysts 2019, 9, 854. https://doi.org/10.3390/catal9100854
Li J, Li X, Hua D, Lu X, Wang Y. Optimizing the Aromatic Product Distribution from Catalytic Fast Pyrolysis of Biomass Using Hydrothermally Synthesized Ga-MFI Zeolites. Catalysts. 2019; 9(10):854. https://doi.org/10.3390/catal9100854
Chicago/Turabian StyleLi, Jian, Xiangyu Li, Derun Hua, Xinning Lu, and Yujue Wang. 2019. "Optimizing the Aromatic Product Distribution from Catalytic Fast Pyrolysis of Biomass Using Hydrothermally Synthesized Ga-MFI Zeolites" Catalysts 9, no. 10: 854. https://doi.org/10.3390/catal9100854
APA StyleLi, J., Li, X., Hua, D., Lu, X., & Wang, Y. (2019). Optimizing the Aromatic Product Distribution from Catalytic Fast Pyrolysis of Biomass Using Hydrothermally Synthesized Ga-MFI Zeolites. Catalysts, 9(10), 854. https://doi.org/10.3390/catal9100854





