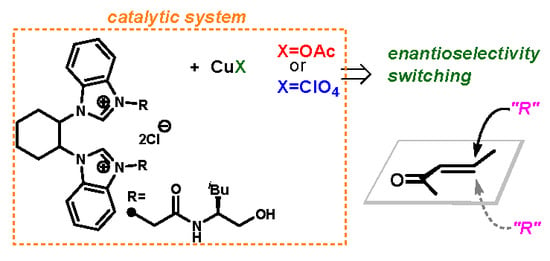
(±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed Asymmetric Conjugate Addition Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Asymmetric Conjugate Addition Reaction of Cyclic Enone
2.2. Catalytic Asymmetric Conjugate Addition Reaction of Acyclic Enone
3. Experimental
3.1. General Procedures
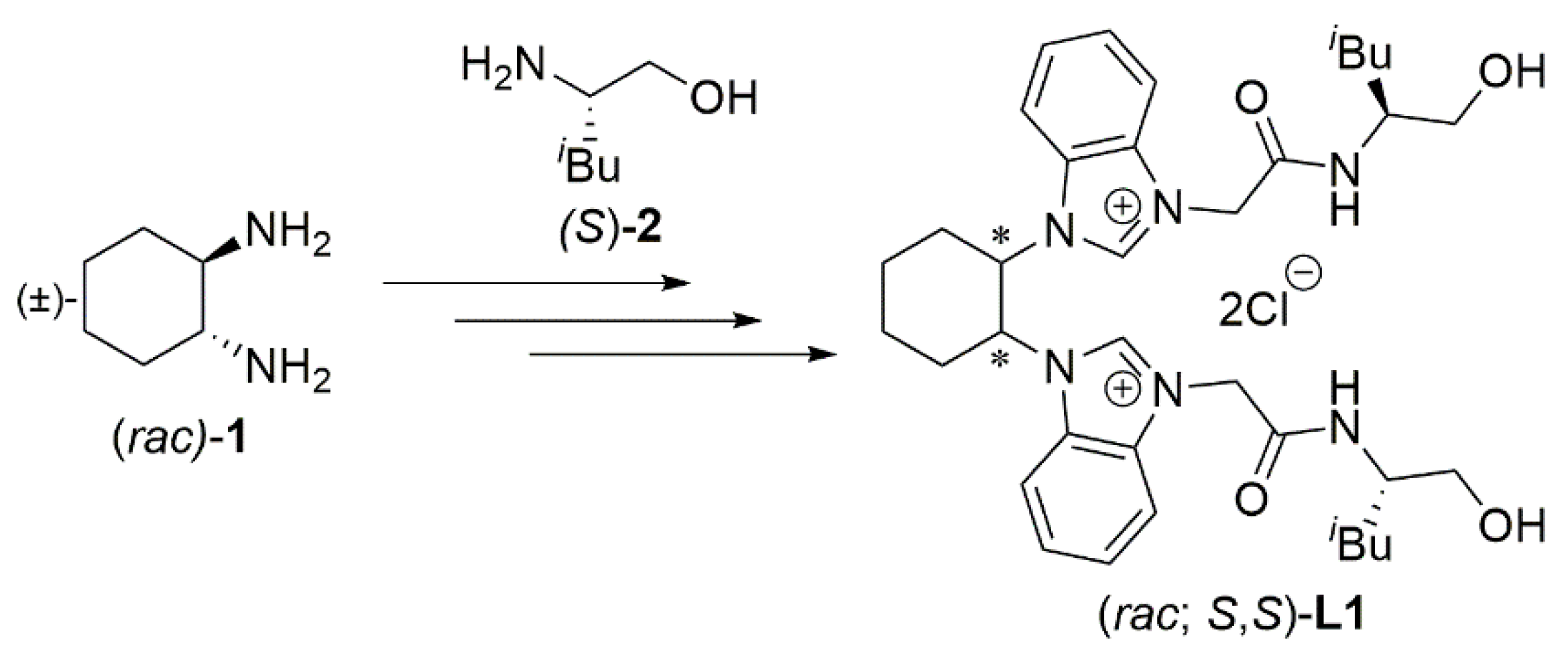
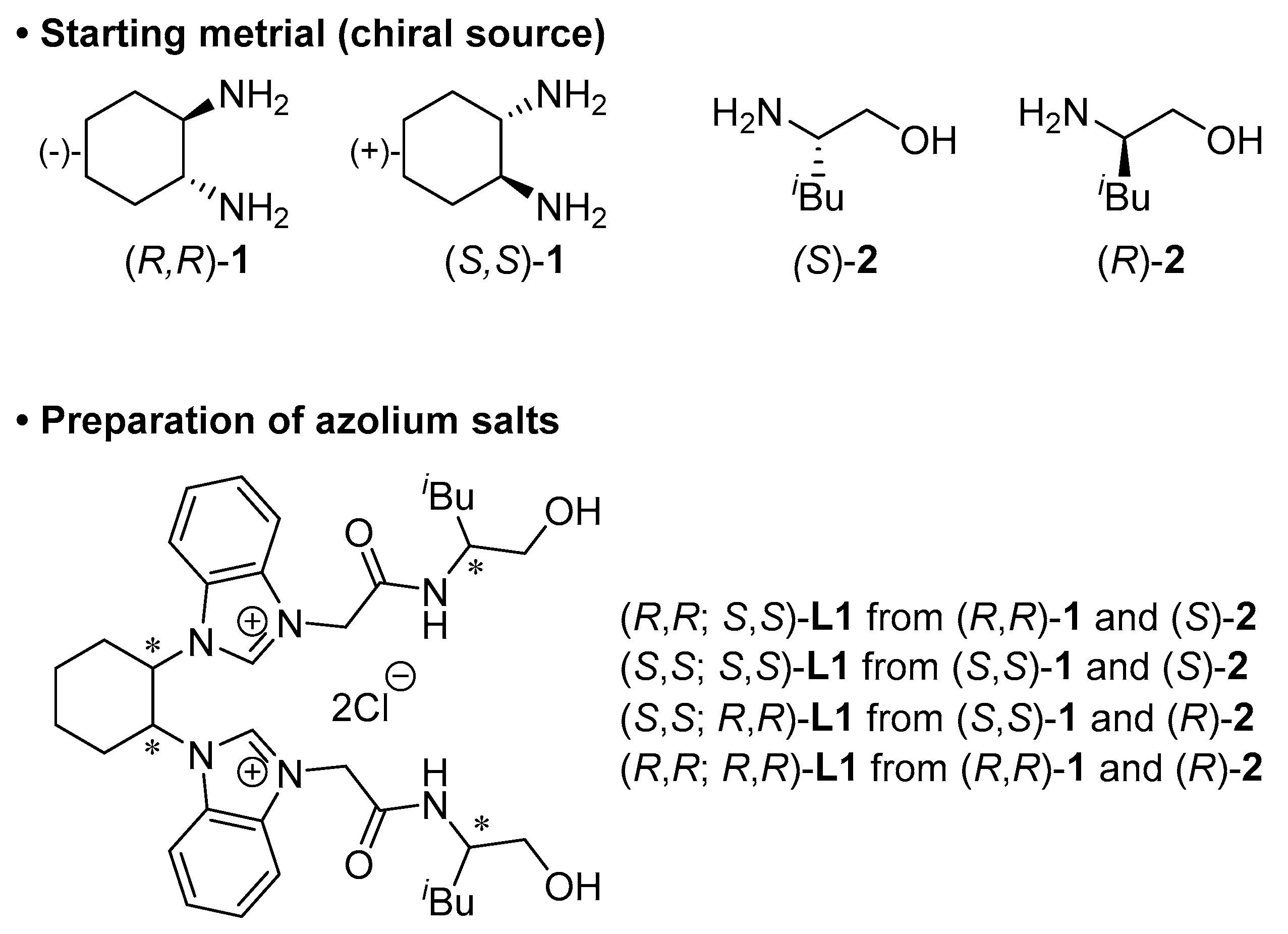
3.2. Procedure for Preparation of Azolium Salt L1
3.3. General Procedure for Cu-Catalyzed Asymmetric Reaction of Enone with Et2Zn
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, G.; Zhang, W. Renaissance of pyridine-oxazolines as chiral ligands for asymmetric catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-L. Privileged Chiral Ligands and Catalysts; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysis. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef] [PubMed]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Perspective on Recycling Strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, 2nd ed.; RSC-Publishing: Cambridge, UK, 2017. [Google Scholar]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper-NHC complexes in catalysis. Coord. Chem. Rev. 2015, 293–294, 48–79. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Schaper, L.A.; Hock, S.J.; Herrmann, W.A.; Kühn, F.E. Synthesis and Application of Water-Soluble NHC Transition-Metal Complexes. Angew. Chem. Int. Ed. 2013, 52, 270–289. [Google Scholar] [CrossRef]
- Hamadd, F.B.; Sun, T.; Xiao, S.; Verpoor, F. Olefin metathesis ruthenium catalysts bearing unsymmetrical heterocylic carbenes. Coord. Chem. Rev. 2013, 257, 2274–2292. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.-J.; Wang, W.; Li, S.; Shi, M. Chiral NHC-metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef] [PubMed]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, P.L.; Casely, I.J. F-Block N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3599–3611. [Google Scholar] [CrossRef]
- De Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes-Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef]
- Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions-A Synthetic Chemist’s Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Boratynski, P.J.; Nowak, A.E.; Skarźewski, J. New Chiral Benzimidazoles Derived from 1,2-Diaminocyclohexane. Synthesis 2015, 47, 3797–3804. [Google Scholar]
- Fliedel, C.; Braunstein, P. Recent advances in S-functionalized N-heterocyclic carbene ligands: From the synthesis of azolium salts and metal complexes to applications. J. Organomet. Chem. 2014, 751, 286–300. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly(N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Hodgson, R.; Douthwaite, R.E. Synthesis and asymmetric catalytic application of chiral imidazolium–phosphines derived from (1R, 2R)-trans-diaminocyclohexane. J. Organomet. Chem. 2005, 690, 5822–5831. [Google Scholar] [CrossRef]
- Bonnet, L.G.; Douthwaite, R.E.; Hodgson, R. Synthesis of Constrained-Geometry Chiral Di-N-Heterocyclic Carbene Ligands and Their Silver(I) and Palladium(II) Complexes. Organometallics 2003, 22, 4384–4386. [Google Scholar] [CrossRef]
- Duan, W.-L.; Shi, M.; Rong, G.-B. Synthesis of novel axially chiral Rh-NHC complexes derived from BINAM and application in the enantioselective hydrosilylation of methyl ketones. Chem. Commun. 2003, 39, 2916–2917. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M. Catalytic Enantioselective Addition of Cyclic β-Keto Esters with Activated Olefins and N-Boc Imines Using Chiral C2-Symmetric Cationic Pd2+ N-Heterocyclic Carbene (NHC) Diaqua Complexes. Organometallics 2010, 29, 2831–2834. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M. Catalytic asymmetric addition of arylboronic acids to N-Boc imines generated in situ using C2-symmetric cationic N-heterocyclic carbenes (NHCs) Pd2+ diaquo complexes. Tetrahedron 2010, 66, 2619–2623. [Google Scholar] [CrossRef]
- Cao, S.-H.; Shi, M. Axially chiral C2-symmetric N-heterocyclic carbene (NHC) palladium complex-catalyzed asymmetric α-hydroxylation of β-keto esters. Tetrahedron Asymmetry 2010, 21, 2675–2680. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Q.; Zhang, X.; Zhang, T.; Shi, M. Axially chiral C2-symmetric N-heterocyclic carbene (NHC) palladium complexes-catalyzed asymmetric arylation of aldehydes with arylboronic acids. Tetrahedron Asymmetry 2010, 21, 1928–1935. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Zhang, T.; Shi, M. Asymmetric 1,4-Addition of Arylboronic Acids to 2,3-Dihydro-4-pyridones Catalyzed by Axially Chiral NHC-Pd(II) Complexes. J. Org. Chem. 2010, 75, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, F.; Shi, M. Synthesis of Chiral Bis(N-heterocyclic carbene) Palladium and Rhodium Complexes with 1,10-Biphenyl Scaffold and Their Application in Asymmetric Catalysis. Organometallics 2009, 28, 4416–4420. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.; Shi, M. Chiral Bis(NHC)-Palladium(II) Complex Catalyzed and Diethylzinc-Mediated Enantioselective Umpolung Allylation of Aldehydes. Organometallics 2009, 28, 2640–2642. [Google Scholar] [CrossRef]
- Ma, G.-N.; Zhang, T.; Shi, M. Catalytic Enantioselective Arylation of N-Tosylarylimines with Arylboronic Acids Using C2-Symmetric Cationic N-Heterocyclic Carbene Pd2+ Diaquo Complexes. Org. Lett. 2009, 11, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M. Efficient chirality switching in the asymmetric addition of indole to N-tosylarylimines in the presence of axially chiral cyclometalated bidentate N-heterocyclic carbene palladium(II) complexes. Tetrahedron Asymmetry 2009, 20, 119–123. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M. Chiral Bidentate Bis(N-Heterocyclic Carbene)-Based Palladium Complexes Bearing Carboxylate Ligands: Highly Effective Catalysts for the Enantioselective Conjugate Addition of Arylboronic Acids to Cyclic Enones. Chem. Eur. J. 2008, 14, 3759–3764. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M.; Zhao, M. Bis(NHC)-Pd(II) complexes as highly efficient catalysts for allylation of aldehydes with allyltributyltin. Tetrahedron 2008, 64, 2412–2418. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, X.; Liu, S.; Dou, Q.; Shi, M. The Use of Chiral BINAM NHC-Rh(III) Complexes in Enantioselective Hydrosilylation of 3-Oxo-3-arylpropionic Acid Methyl or Ethyl Esters. J. Org. Chem. 2007, 72, 2240–2242. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, J.-J.; Xu, Q.; Shi, M. Axially Chiral NHC-Pd(II) Complexes in the Oxidative Kinetic Resolution of Secondary Alcohols Using Molecular Oxygen as a Terminal Oxidant. Org. Lett. 2007, 9, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Qian, H.-X. A new dimeric bidentated NHC-Pd(II) complex from trans-cyclohexane-1,2-diamine for Suzuki reaction and Heck reaction. Tetrahedron 2005, 61, 4949–4955. [Google Scholar] [CrossRef]
- Diez, C.; Nagel, U. Chiral iridium(I) bis(NHC) complexes as catalysts for asymmetric transfer hydrogenation. Appl. Organomet. Chem. 2010, 24, 509–516. [Google Scholar] [CrossRef]
- Nagel, U.; Diez, C. Modular Synthesis of a New Type of Chiral Bis(carbene) Ligand from L-Valinol and Iridium(I) and Rhodium(I) Complexes Thereof. Eur. J. Inorg. Chem. 2009, 2009, 1248–1255. [Google Scholar] [CrossRef]
- Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Chiral NHC-Complexes with Dioxolane Backbone Heterogenized on MCM-41. Catalytic Activity. ChemCatChem 2011, 3, 1320–1328. [Google Scholar] [CrossRef]
- Arnanz, A.; González-Arellano, C.; Juan, A.; Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. New chiral ligands bearing two N-heterocyclic carbene moieties at a dioxolane backbone. Gold, palladium and rhodium complexes as enantioselective catalysts. Chem. Commun. 2010, 46, 3001–3003. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Lowry, R.J.; Swails, J.M.; Ghiviriga, I.; Veige, A.S. Synthesis and characterization of κ-2-bis-N-heterocyclic carbene rhodium(I) catalysts: Application in enantioselective arylboronic acid addition to cyclohex-2-enones. J. Organomet. Chem. 2011, 696, 3127–3134. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Ghiviriga, I.; Abboud, K.A.; Veige, A.S. A new chiral di-N-heterocyclic carbene (NHC) cyclophane ligand and its application in palladium enantioselective catalysis. Dalton Trans. 2010, 39, 6392–6394. [Google Scholar] [CrossRef]
- Gigler, P.; Bechlars, B.; Herrmann, W.A.; Kühn, F.E. Hydrosilylation with Biscarbene Rh(I) Complexes: Experimental Evidence for a Silylene-Based Mechanism. J. Am. Chem. Soc. 2011, 133, 1589–1596. [Google Scholar] [CrossRef]
- Riederer, S.K.U.; Bechlars, B.; Herrmann, W.A.; Kühn, F.E. Chiral N-heterocyclic biscarbenes based on 1,2,4-triazole as ligands for metal-catalyzed asymmetric synthesis. Dalton Trans. 2011, 40, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, Y.; Yu, J.; Zhang, D. N,N’-Bisaryl Substituted Chiral Linker-Bridged Bis(N-Heterocyclic Carbene) Palladium Complexes: Design, Synthesis, and Catalytic Properties. Organometallics 2017, 36, 1372–1382. [Google Scholar] [CrossRef]
- Zhang, D.; He, Y.; Tang, J. Chiral linker-bridged bis-N-heterocyclic carbenes: Design, synthesis, palladium complexes, and catalytic properties. Dalton Trans. 2016, 45, 11699–11709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Tang, J.; Gu, J.; Wang, Q.; Sun, P.; Zhang, D. Chiral 1,2-Cyclohexane-Bridged Bis-NHC Palladium Catalysts for Asymmetric Suzuki-Miyaura Coupling: Synthesis, Characterization, and Steric Effects on Enantiocontrol. Organometallics 2014, 33, 876–884. [Google Scholar] [CrossRef]
- Shigeng, G.; Tang, J.; Zhang, D.; Wang, Q.; Chen, Z.; Weng, L. Synthesis, structure, and catalytic activity of palladium complexes with new chiral cyclohexane-1,2-based di-NHC-ligands. J. Organomet. Chem. 2012, 700, 223–229. [Google Scholar] [CrossRef]
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral benzimidazoles and their applications in stereodiscrimination processes. Tetrahedron Asymmetry 2017, 28, 1233–1289. [Google Scholar] [CrossRef]
- Zhao, D.; Candish, L.; Paul, D.; Glorius, F. N-Heterocyclic Carbenes in Asymmetric Hydrogenation. ACS Catal. 2016, 6, 5978–5988. [Google Scholar] [CrossRef]
- Tornatzky, J.; Kannenberg, A.; Blechert, S. New catalysts with unsymmetrical N-heterocyclic carbene ligands. Dalton Trans. 2012, 41, 8215–8225. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.K.; May, T.L.; Baxter, C.A.; Hoveyda, A.H. All-Carbon Quaternary Stereogenic Centers by Enantioselective Cu-Catalyzed Conjugate Additions Promoted by a Chiral N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2007, 46, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, R.E. Metal-mediated asymmetric alkylation using chiral N-heterocyclic carbenes derived from chiral amines. Coord. Chem. Rev. 2007, 251, 702–717. [Google Scholar] [CrossRef]
- Gade, L.H.; Bellemin-Laponnaz, S. Mixed oxazoline-carbenes as stereodirecting ligands for asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 718–725. [Google Scholar] [CrossRef]
- César, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-heterocyclic carbenes as stereodirecting ligands in asymmetric catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.C.; Burgess, K. Chiral N-heterocyclic carbene-transition metal complexes in asymmetric catalysis. Tetrahedron Asymmetry 2003, 14, 951–961. [Google Scholar] [CrossRef]
- Nakano, Y.; Sakaguchi, S. Inversions in asymmetric conjugate addition reaction of cyclic enones catalyzed by the Cu/NHC-AgX system: Factors affecting the stereoselective formation of both enantiomers. J. Organomet. Chem. 2017, 846, 407–416. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakano, Y.; Shibata, N.; Sakaguchi, S. Enantioselectivity switch in copper-catalyzed conjugate addition reactions under the influence of a chiral N-heterocyclic carbene-silver complex. RSC Adv. 2016, 6, 7755–7759. [Google Scholar] [CrossRef]
- Kondo, J.; Harano, A.; Dohi, K.; Sakaguchi, S. C2-symmetric functionalized azolium salt from serine ester for Cu-catalyzed asymmetric conjugate addition reaction. J. Mol. Catal. A Chem. 2014, 395, 66–71. [Google Scholar] [CrossRef]
- Dohi, K.; Kondo, J.; Yamada, H.; Arakawa, R.; Sakaguchi, S. Functionalized N-Heterocyclic Carbene Ligands for Dual Enantioselective Control in the Cu-Catalyzed Conjugate Addition of Dialkylzinc Compounds to Acyclic Enones. Eur. J. Org. Chem. 2012, 2012, 7143–7152. [Google Scholar] [CrossRef]
- Yoshimura, M.; Shibata, N.; Kawakami, M.; Sakaguchi, S. Ligand design for dual enantioselective control in Cu-catalyzed asymmetric conjugate addition of R2Zn to cyclic enone. Tetrahedron 2012, 68, 3512–3518. [Google Scholar] [CrossRef]
- Shibata, N.; Yoshimura, M.; Yamada, H.; Arakawa, R.; Sakaguchi, S. Hydroxy-amide Functionalized Azolium Salts for Cu-Catalyzed Asymmetric Conjugate Addition: Stereocontrol Based on Ligand Structure and Copper Precatalyst. J. Org. Chem. 2012, 77, 4079–4086. [Google Scholar] [CrossRef]
- Shibata, N.; Okamoto, M.; Yamamoto, Y.; Sakaguchi, S. Reversal of Stereoselectivity in the Cu-Catalyzed Conjugate Addition Reaction of Dialkylzinc to Cyclic Enone in the Presence of a Chiral Azolium Compound. J. Org. Chem. 2010, 75, 5707–5715. [Google Scholar] [CrossRef]
- Harano, A.; Sakaguchi, S. A new C2-symmetric azolium compound for Cu-catalyzed asymmetric conjugate addition of R2Zn to cyclic enone. J. Organomet. Chem. 2011, 696, 61–67. [Google Scholar] [CrossRef]
- Okamoto, M.; Yamamoto, Y.; Sakaguchi, S. A new approach to switching of enantioselectivity in NHC–Cu-catalyzed conjugate addition of alkylzincs to cyclic enones. Chem. Commun. 2009, 45, 7363–7365. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Matsubara, R. Recent topics on catalytic asymmetric 1,4-addition. Tetrahedron Lett. 2017, 58, 1793–1805. [Google Scholar] [CrossRef]
- Alexakis, A.; Krause, N.; Woodward, S. Copper-Catalyzed Asymmetric Synthesis; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Jerphagnon, T.; Pizzuti, M.G.; Minnaard, A.J.; Feringa, B.L. Recent advances in enantioselective copper-catalyzed 1,4-addition. Chem. Soc. Rev. 2009, 38, 1039–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexakis, A.; Bäckvall, J.E.; Krause, N.; Pàmies, O.; Diéguez, M. Enantioselective Copper-Catalyzed Conjugate Addition and Allylic Substitution Reactions. Chem. Rev. 2008, 108, 2796–2823. [Google Scholar] [CrossRef] [PubMed]
- Kamihigashi, S.; Shibata, N.; Sakaguchi, S. Cu-Catalyzed Asymmetric Conjugate Addition of Dialkylzincs to Enones Using a (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Derived from L-Leucinol. Synlett 2014, 25, 2933–2937. [Google Scholar] [CrossRef]
- Endo, K.; Yakeishi, S.; Hamada, D.; Shibata, T. Functionalized BINOL-mono-PHOS for Multinuclear Cu-Catalysts in Asymmetric Conjugate Addition of Organozinc Reagents. Chem. Lett. 2013, 42, 547–549. [Google Scholar] [CrossRef]
- Wang, L.; Meng, W.; Zhu, C.-L.; Zheng, Y.; Nie, J.; Ma, J.-A. The Long-Arm Effect: Influence of Axially Chiral Phosphoramidite Ligands on the Diastereo- and Enantioselectivity of the Tandem 1,4-Addition/Fluorination. Angew. Chem. Int. Ed. 2011, 50, 9442–9446. [Google Scholar] [CrossRef]
- Uchida, T.; Katsuki, T. Construction of a new type of chiral bidentate NHC ligands: Copper-catalyzed asymmetric conjugate alkylation. Tetrahedron Lett. 2009, 50, 4741–4743. [Google Scholar] [CrossRef]
- García, J.M.; González, A.; Kardak, B.G.; Odriozola, J.M.; Oiarbide, M.; Razkin, J.; Palomo, C. Copper-Catalyzed Enantioselective Conjugate Addition of Dialkylzinc Reagents to α’-Oxy Enones. Chem. Eur. J. 2008, 14, 8768–8771. [Google Scholar] [CrossRef]
- Šebesta, R.; Pizzuti, M.G.; Minnaard, A.J.; Feringa, B.L. Copper-Catalyzed Enantioselective Conjugate Addition of Organometallic Reagents to Acyclic Dienones. Adv. Synth. Catal. 2007, 349, 1931–1937. [Google Scholar] [CrossRef]
- Li, K.; Alexakis, A. Copper-Catalyzed Enantioselective Intramolecular Conjugate Addition/Trapping Reactions: Synthesis of Cyclic Compounds with Multichiral Centers. Chem. Eur. J. 2007, 13, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Cao, P.; Wang, M.; Li, L.; Li, D.; Liao, J. Copper-catalyzed enantioselective conjugate addition of diethylzinc to acyclic enones with chiral sulfoxideephosphine ligands. Tetrahedron 2016, 72, 2707–2711. [Google Scholar] [CrossRef]
- Harutyunyan, S.R.; López, F.; Browne, W.R.; Correa, A.; Peňa, D.; Badorrey, R.; Meetsma, A.; Minnaard, A.J.; Feringa, B.L. On the Mechanism of the Copper-Catalyzed Enantioselective 1,4-Addition of Grignard Reagents to α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2006, 128, 9103–9118. [Google Scholar] [CrossRef] [PubMed]
- Hajra, A.; Yoshikai, N.; Nakamura, E. Aminohydroxyphosphine Ligand for the Copper-Catalyzed Enantioselective Conjugate Addition of Organozinc Reagents. Org. Lett. 2006, 8, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, Y.; Katagiri, K.; Danjo, H.; Yamaguchi, K.; Imamoto, T. P-Chiral o-Phosphinophenol as a P/O Hybrid Ligand: Preparation and Use in Cu-Catalyzed Asymmetric Conjugate Addition of Diethylzinc to Acyclic Enones. J. Org. Chem. 2005, 70, 9009–9012. [Google Scholar] [CrossRef] [PubMed]
- Börner, C.; Dennis, M.R.; Sinn, E.; Woodward, S. Copper-Catalysed Asymmetric 1,4-Addition of Organozinc Compounds to Linear Aliphatic Enones Using 2,2′-Dihydroxy 3,3′-Dithioether Derivatives of 1,1′-Binaphthalene. Eur. J. Org. Chem. 2001, 2001, 2435–2446. [Google Scholar] [CrossRef]
- Börner, C.; König, W.A.; Woodward, S. Enone structure as a probe to Lewis acid carbonyl binding in copper-catalysed asymmetric conjugate addition. Tetrahedron Lett. 2001, 42, 327–329. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Nájera, C.; Yus, M. Stereodivergent Catalysis. Chem. Rev. 2018, 118, 5080–5200. [Google Scholar] [CrossRef]
- Blanco, V.; Leigh, D.A.; Marcos, V. Artificial switchable catalysts. Chem. Soc. Rev. 2015, 44, 5341–5370. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Oh, K. Stereodivergent Asymmetric Reactions Catalyzed by Brucine Diol. Synlett 2015, 26, 2067–2087. [Google Scholar]
- Escorihuela, J.; Burguete, M.I.; Luis, S.V. New advances in dual stereocontrol for asymmetric reactions. Chem. Soc. Rev. 2013, 42, 5595–5617. [Google Scholar] [CrossRef] [PubMed]
- Bartók, M. Unexpected Inversions in Asymmetric Reactions: Reactions with Chiral Metal Complexes, Chiral Organocatalysts, and Heterogeneous Chiral Catalysts. Chem. Rev. 2010, 110, 1663–1705. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hayashi, M. New Approach for Complete Reversal of Enantioselectivity Using a Single Chiral Source. Synthesis 2008, 2008, 3361–3376. [Google Scholar]
- Zanoni, G.; Castronovo, F.; Franzini, M.; Vidari, G.; Giannini, E. Toggling enantioselective catalysis-a promising paradigm in the development of more efficient and versatile enantioselective synthetic methodologies. Chem. Soc. Rev. 2003, 32, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cao, P.; Wang, B.; Jia, T.; Lou, Y.; Wang, M.; Liao, J. Copper(I)-Catalyzed Asymmetric Pinacolboryl Addition of N-Bocimines Using a Chiral Sulfoxide−Phosphine Ligand. Org. Lett. 2015, 17, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Spahn, E.; Albright, A.; Shevlin, M.; Pauli, L.; Pfaltz, A.; Gawley, R.E. Double-Asymmetric Hydrogenation Strategy for the Reduction of 1,1-Diaryl Olefins Applied to an Improved Synthesis of CuIPhEt, a C2-Symmetric N-Heterocyclic Carbenoid. J. Org. Chem. 2013, 78, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-Y.; Chen, F.; Qin, J.; He, Y.-M.; Fan, Q.-H. Asymmetric Hydrogenation of 2,4-Disubstituted 1,5-Benzodiazepines Using Cationic Ruthenium Diamine Catalysts: An Unusual Achiral Counteranion Induced Reversal of Enantioselectivity. Angew. Chem. Int. Ed. 2012, 51, 5706–5710. [Google Scholar] [CrossRef]
- Kim, H.Y.; Li, J.-Y.; Kim, S.; Oh, K. Stereodivergency in Catalytic Asymmetric Conjugate Addition Reactions of Glycine (Ket)imines. J. Am. Chem. Soc. 2011, 133, 20750–20753. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Zhou, Y.; Nie, Z.; Luo, S.; Cheng, J.-P. Asymmetric Binary Acid Catalysis: A Regioselectivity Switch between Enantioselective 1,2- and 1,4-Addition through Different Counteranions of InIII. Angew. Chem. Int. Ed. 2011, 50, 6610–6614. [Google Scholar] [CrossRef]
- Frölander, A.; Moberg, C. Ag+-Assisted Hydrosilylation: Complementary Behavior of Rh and Ir Catalysts (Reversal of Enantioselectivity). Org. Lett. 2007, 9, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Kozlowski, M.C.; Murry, J.A.; Burgey, C.S.; Campos, K.R.; Connell, B.T.; Staples, R.J. C2-Symmetric Copper(II) Complexes as Chiral Lewis Acids. Scope and Mechanism of Catalytic Enantioselective Aldol Additions of Enolsilanes to (Benzyloxy)acetaldehyde. J. Am. Chem. Soc. 1999, 121, 669–685. [Google Scholar] [CrossRef]

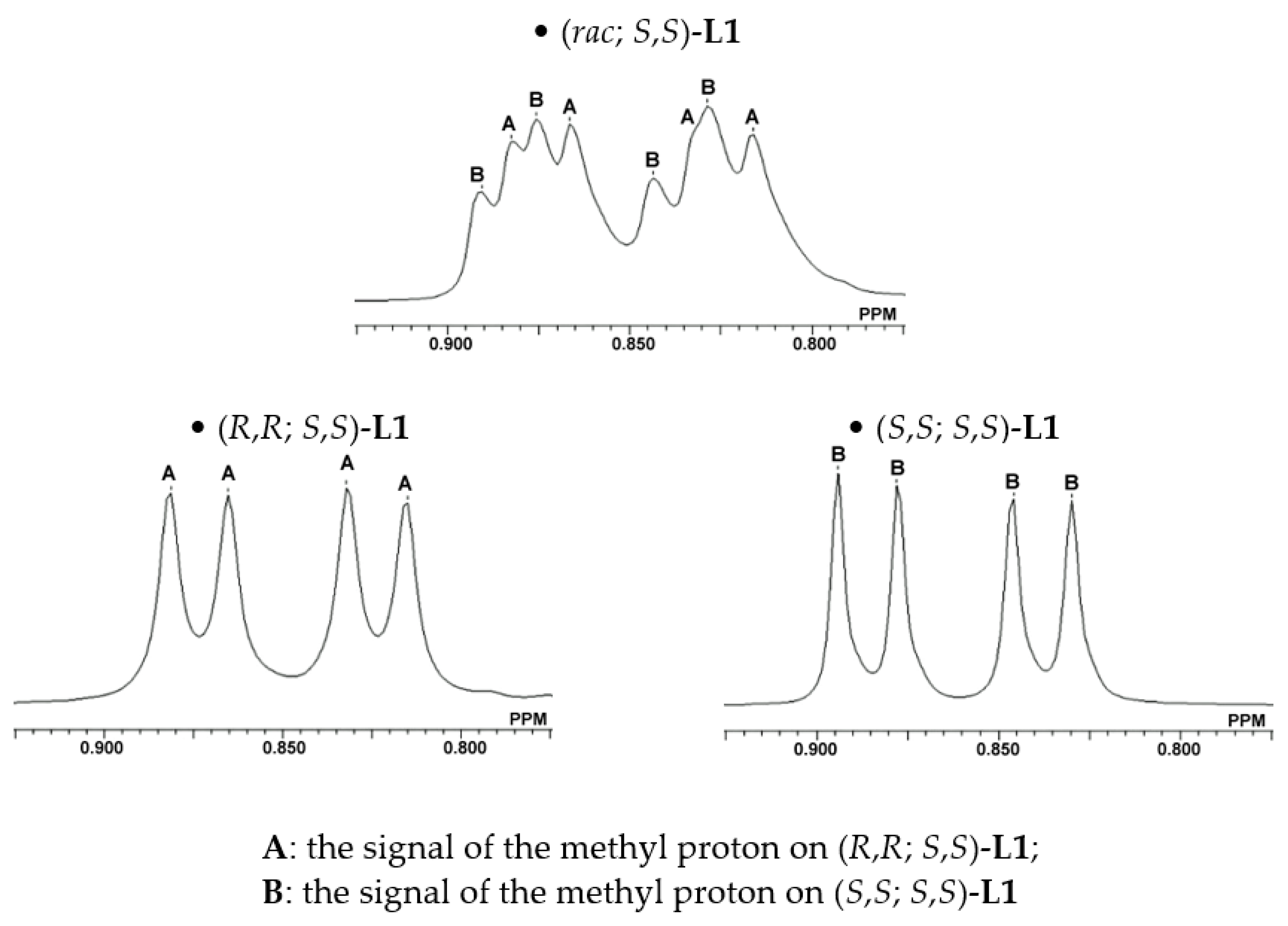
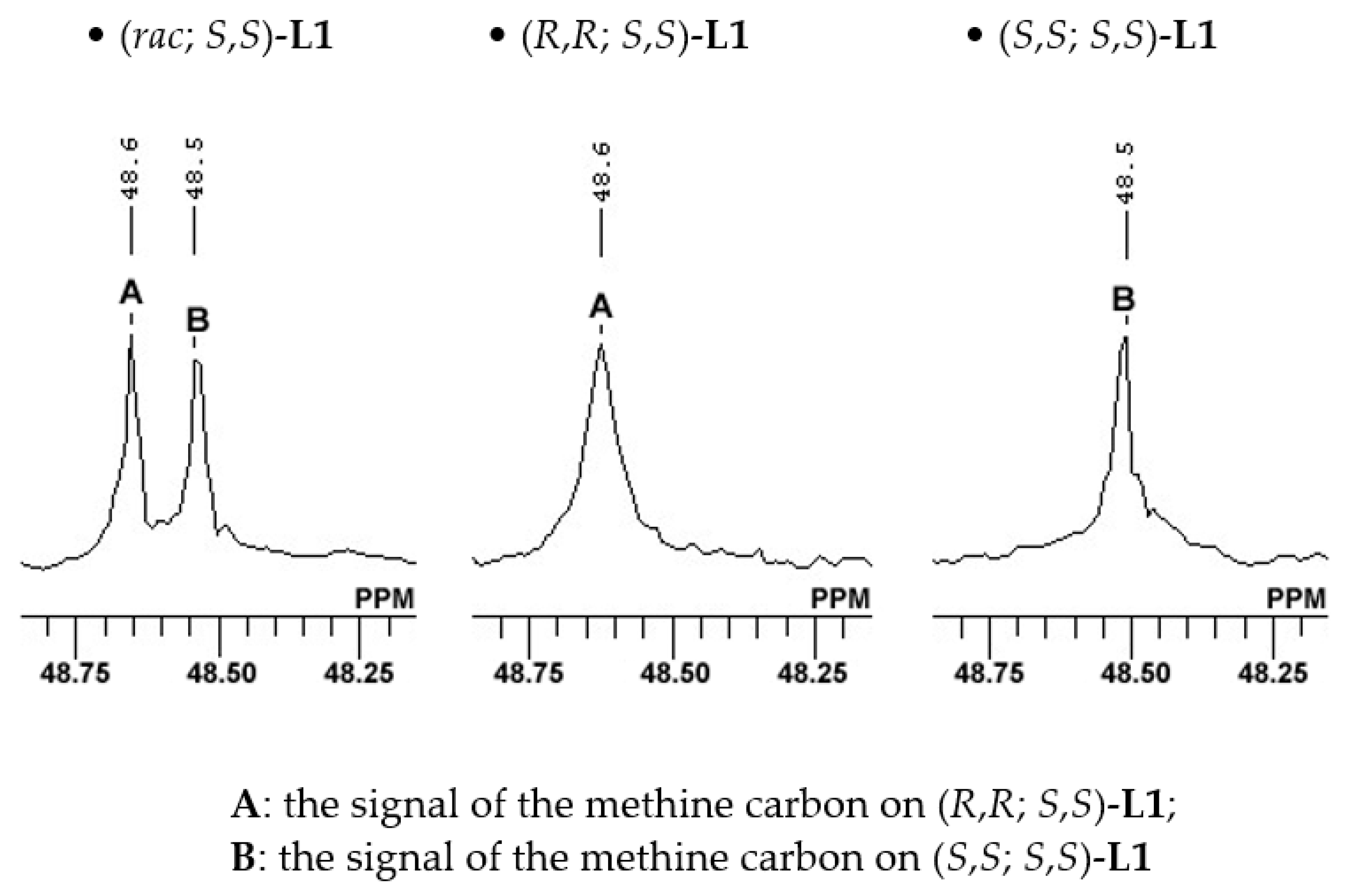


| Entry a | (R,R; S,S)-L1:(S,S; S,S)-L1 | Yield (%) b | ee (%) c |
|---|---|---|---|
| 1 | 1:0 | 57 | 16 |
| 2 | 1:1 ≡ (rac; S,S)-L1 | 99 | 97 |
| 3 | 0:1 | 34 | 26 |

| Entry a | (S,S; R,R)-L1:(R,R; R,R)-L1 | Yield (%) b | ee (%) c |
|---|---|---|---|
| 4 | 1:0 | 59 | 12 |
| 5 | 1:1 ≡ (rac; R,R)-L1 | 91 | 97 |
| 6 | 0:1 | 39 | 24 |

| Entry | (rac; S,S)-L1 (mol%) | Yield (%) b | ee (%) c |
|---|---|---|---|
| 1 d | 4.5 | 99 | 97 |
| 2 | 1.8 | 97 | 93 |
| 3 | 1.35 | 91 | 96 |
| 4 | 0.9 | 82 | 97 |
| 5 | 0.45 | 13 | Nd e |
| 6 | L2, 4.5 | 48 | ent-27 |
| 7 | L3, 4.5 | 18 | 0 |
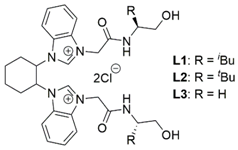
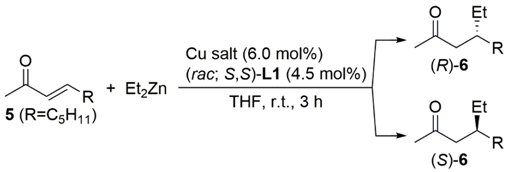
| Entry | Cu Salt | Product | Yield (%) b | ee (%) c |
|---|---|---|---|---|
| 1 d | Cu(hfacac)(btmsa) | (R)-6 | 90 | 49 |
| 2 d | Cu(hfacac)(cod) | (R)-6 | 87 | 61 |
| 3 | Cu(hfacac)2 | (R)-6 | 85 | 53 |
| 4 | CuOCOCF3 | (R)-6 | 73 | 27 |
| 5 | CuOAc | (R)-6 | 78 | 60 |
| 6 | Cu(OAc)2 | (R)-6 | 37 | 63 |
| 7 | CuCl2 | (R)-6 | 50 | 39 |
| 8 | Cu(OTf)2 | (S)-6 | 58 | 33 |
| 9 | CuOTf·0.5C6H6 | (S)-6 | 57 | 2 |
| 10 | Cu(NO3)2 | (S)-6 | 62 | 9 |
| 11 | Cu(ClO4)2 | (S)-6 | 31 | 50 |
| Entry | Cu/L1 (mol%) | Solv | Temp./Time | Product | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 b,c | 6/4.5 | THF | r.t./3 h | (R)-6 | 78 | 60 |
| 2 b | 4.5/6 | THF | r.t./3 h | (R)-6 | 72 | 56 |
| 3 b | 4/4 | THF | r.t./3 h | (R)-6 | 79 | 60 |
| 4 b | 4/4 | Et2O | r.t./3 h | (R)-6 | 46 | 39 |
| 5 b | 4/4 | DME | r.t./3 h | (R)-6 | 43 | 70 |
| 6 b | 4/4 | DME | 0 °C/24 h | (R)-6 | 49 | 78 |
| 7 b | 4/4 | DME | 0 °C/48 h | (R)-6 | 78 | 73 |
| 8 b | 4/4 | DME | −10 °C/48 h | (R)-6 | 69 | 75 |
| 9 d,e | 6/4.5 | THF | r.t./3 h | (S)-6 | 31 | 50 |
| 10 d | 4.5/6 | THF | r.t./3 h | (S)-6 | 44 | 34 |
| 11 d | 6/3 | THF | r.t./3 h | (S)-6 | 39 | 46 |
| 12 d | 6/3 | 2-MeTHF | r.t./3 h | (S)-6 | 26 | 43 |
| 13 d | 6/3 | DME | r.t./3 h | (S)-6 | 28 | 34 |
| 14 d | 6/3 | THF | 0 °C/24 h | (S)-6 | 89 | 70 |
| 15 d | 6/3 | THF | −5 °C/24 h | (S)-6 | 82 | 67 |
| 16 d | 6/3 | THF | −10 °C/24 h | (S)-6 | 90 | 69 |
| 17 d,f | 6/3 | DME | 0 °C/24 h | (S)-6 | 93 | 69 |
| Entry | Cu salt | Additive | (mol%) | Product | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 a,b | CuOAc | none | (R)-6 | 49 | 78 | |
| 2 a | CuCl | none | (R)-6 | 12 | 35 | |
| 3 a | CuCl | AgOAc | 4 | (R)-6 | 47 | 86 |
| 4 a | CuCl | AgOAc | 6 | (R)-6 | 54 | 85 |
| 5 a | CuCl | AgOAc | 8 | (R)-6 | 21 | 74 |
| 6 c,d | Cu(ClO4)2 | (S)-6 | 89 | 70 | ||
| 7 c | CuCl2 | none | (R)-6 | 79 | 48 | |
| 8 c | CuCl2 | AgClO4 | 12 | (S)-6 | 93 | 68 |
| 9 c | CuCl2 | AgClO4 | 15 | (S)-6 | 90 | 66 |
| 10 c | CuCl2 | AgClO4 | 6 | (S)-6 | 80 | 66 |
| Entry | Enone | Conjugate Adduct | Yield (%) | ee (%) |
|---|---|---|---|---|
| 1 a,b | 5 | (R)-6 | 49 | 78 |
| 2 c,d | 5 | (S)-6 | 89 | 70 |
| 3 a | iPrCH = CHCOMe (7) | (S)-8 | 15 | 69 |
| 4 c | 7 | (R)-8 | 44 | 82 |
| 5 c,e | 7 | (R)-8 | 59 | 80 |
| 6 c,e,f | 7 | (R)-8 | 64 | 75 |
| 7 a | PhCH = CHCOMe (9) | (S)-10 | 55 | 63 |
| 8 c | 9 | (R)-10 | 21 | 57 |
| 9 a | PhCH = CHCOPh (11) | (S)-12 | 33 | 55 |
| 10 c | 11 | (R)-12 | 16 | 15 |
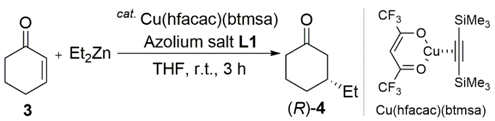
| 11 a | 3 | (R)-4 | 38 | 57 |
| 12 c | 3 | (S)-4 | 64 | 47 |
| Entry | (R,R; S,S)-L1 (mol%) | (S,S; S,S)-L1 (mol%) | Product | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 1 a | 4 | 0 | (R)-6 | 79 | 88 |
| 2 a,b | 2 | 2 | (R)-6 | 49 | 78 |
| 3 a | 0 | 4 | (R)-6 | 17 | 48 |
| 4 a | 3 | 0 | (R)-6 | 70 | 91 |
| 5 c | 3 | 0 | (S)-6 | 70 | 75 |
| 6 c,d | 1.5 | 1.5 | (S)-6 | 89 | 70 |
| 7 c | 0 | 3 | (S)-6 | 82 | 66 |
| Entry | Cu Salt | Ligand | Product | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 1 a | CuOAc | L5 | (R)-6 | 12 | 27 |
| 2 b | Cu(ClO4)2 | L5 | (S)-6 | 73 | 58 |
| 3 a | CuOAc | L6 | (S)-6 | 27 | 16 |
| 4 b | Cu(ClO4)2 | L6 | (S)-6 | 94 | 42 |
| 5 a | CuOAc | L7 | (S)-6 | 31 | 5 |
| 6 b | Cu(ClO4)2 | L7 | (S)-6 | 87 | 69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, A.; Kamihigashi, S.; Iwai, Y.; Sakaguchi, S. (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed Asymmetric Conjugate Addition Reaction. Catalysts 2019, 9, 780. https://doi.org/10.3390/catal9090780
Ishibashi A, Kamihigashi S, Iwai Y, Sakaguchi S. (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed Asymmetric Conjugate Addition Reaction. Catalysts. 2019; 9(9):780. https://doi.org/10.3390/catal9090780
Chicago/Turabian StyleIshibashi, Azusa, Shun Kamihigashi, Yuuki Iwai, and Satoshi Sakaguchi. 2019. "(±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed Asymmetric Conjugate Addition Reaction" Catalysts 9, no. 9: 780. https://doi.org/10.3390/catal9090780
APA StyleIshibashi, A., Kamihigashi, S., Iwai, Y., & Sakaguchi, S. (2019). (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed Asymmetric Conjugate Addition Reaction. Catalysts, 9(9), 780. https://doi.org/10.3390/catal9090780