Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review
Abstract
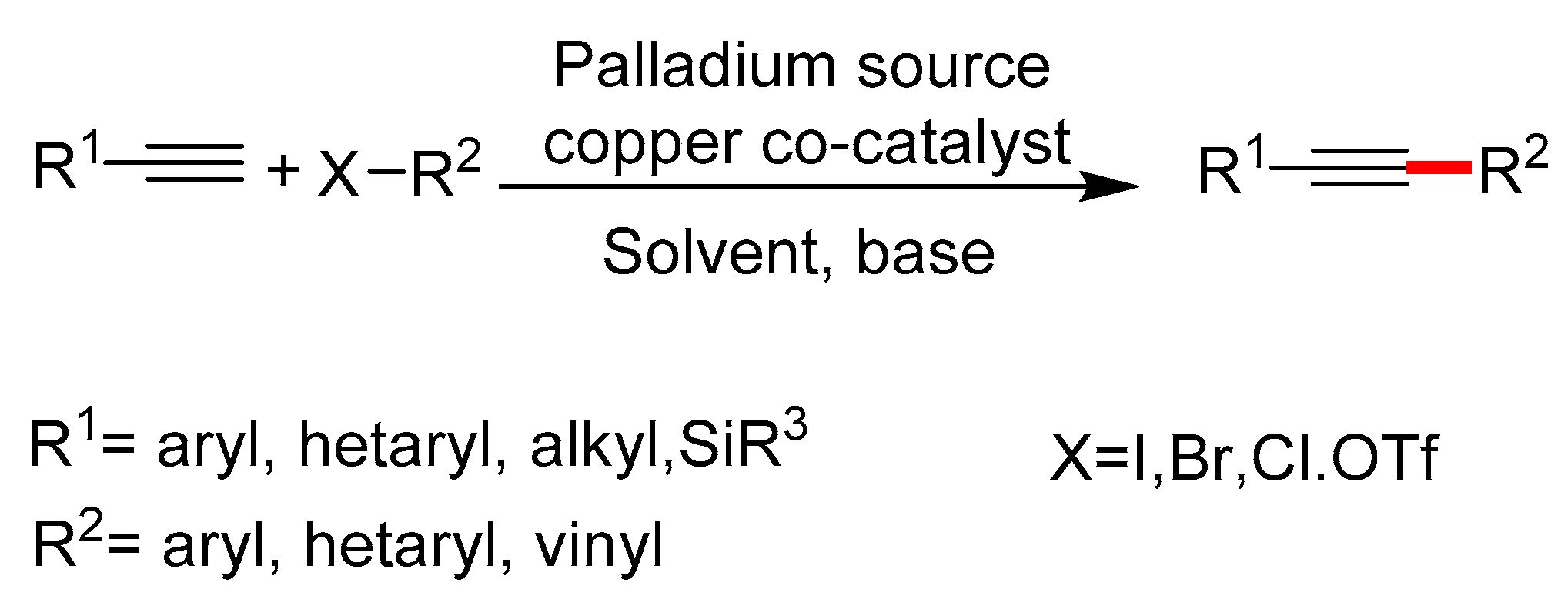
1. Introduction
2. Literature Review
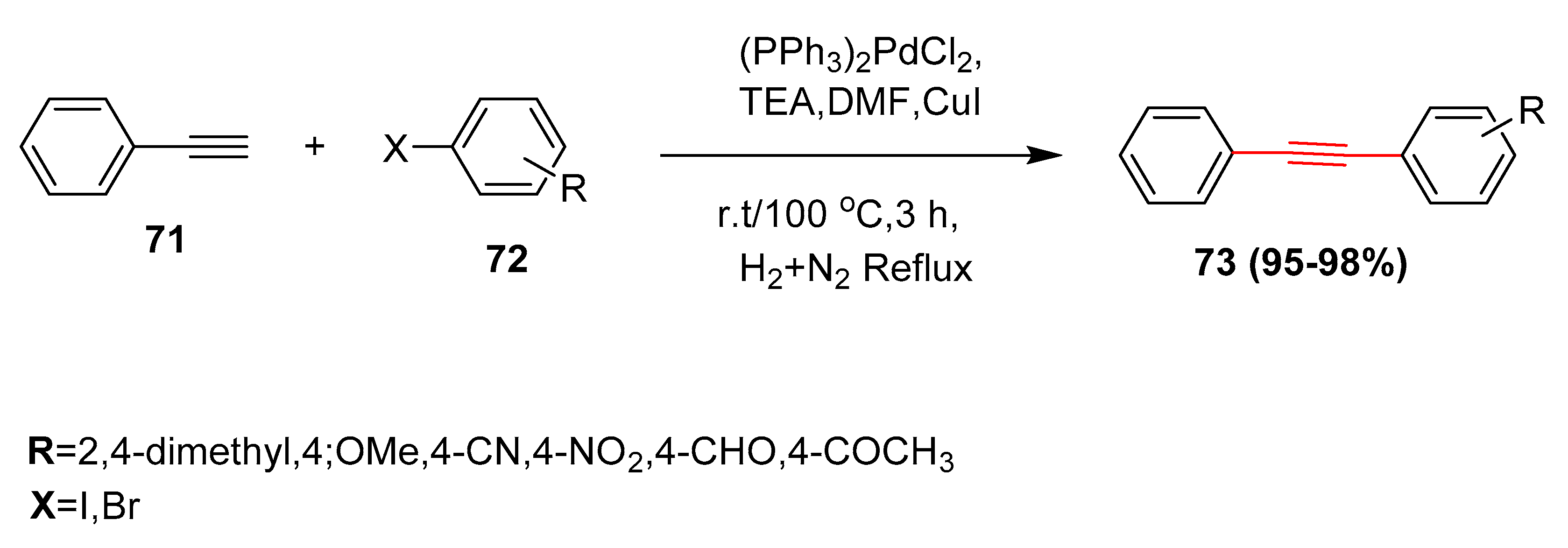
2.1. In 2002
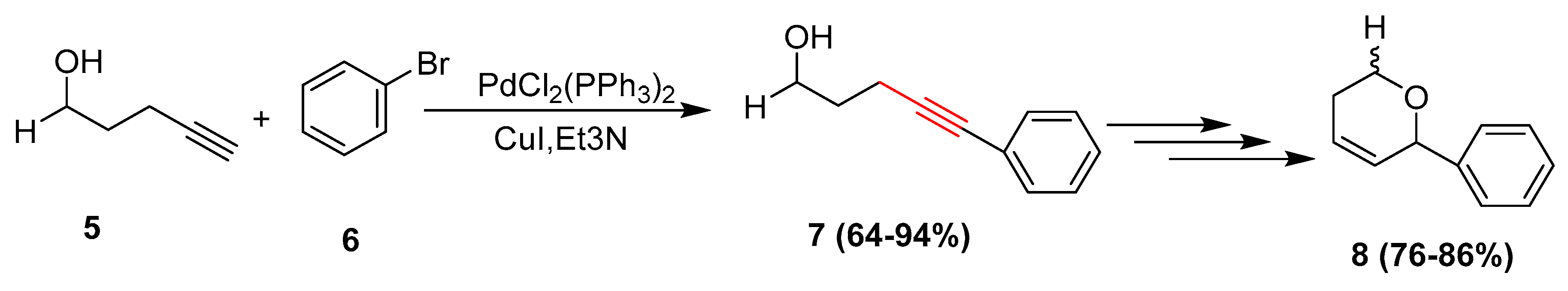
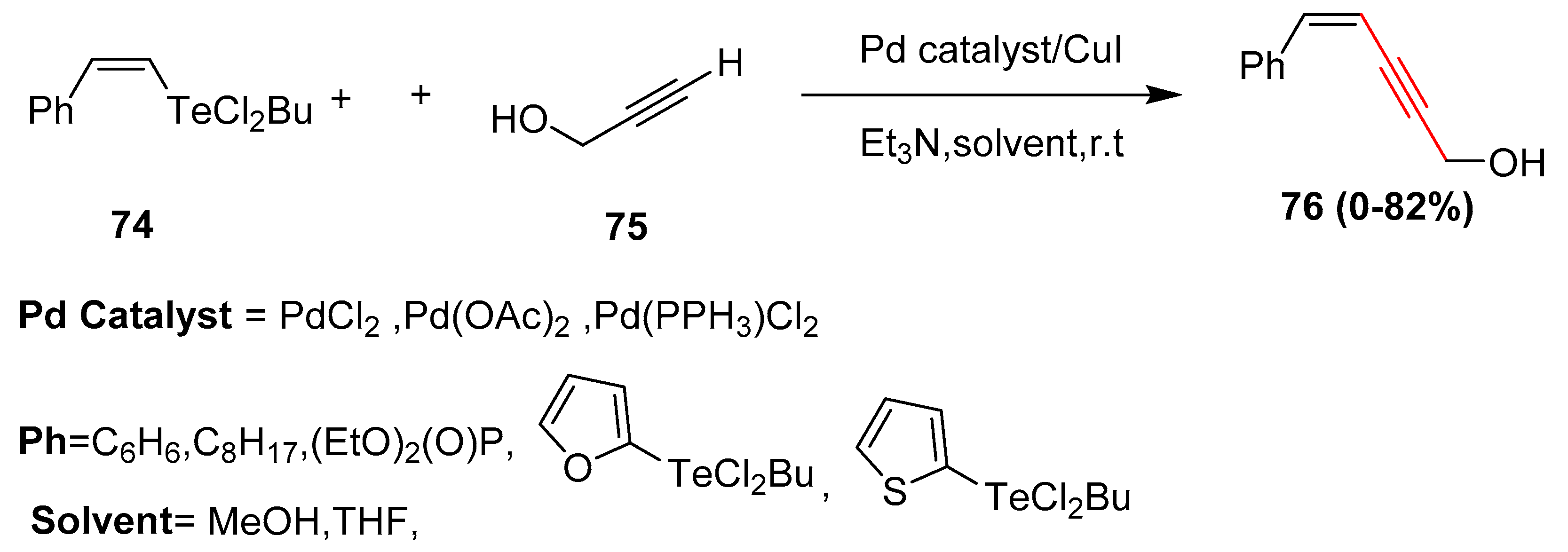
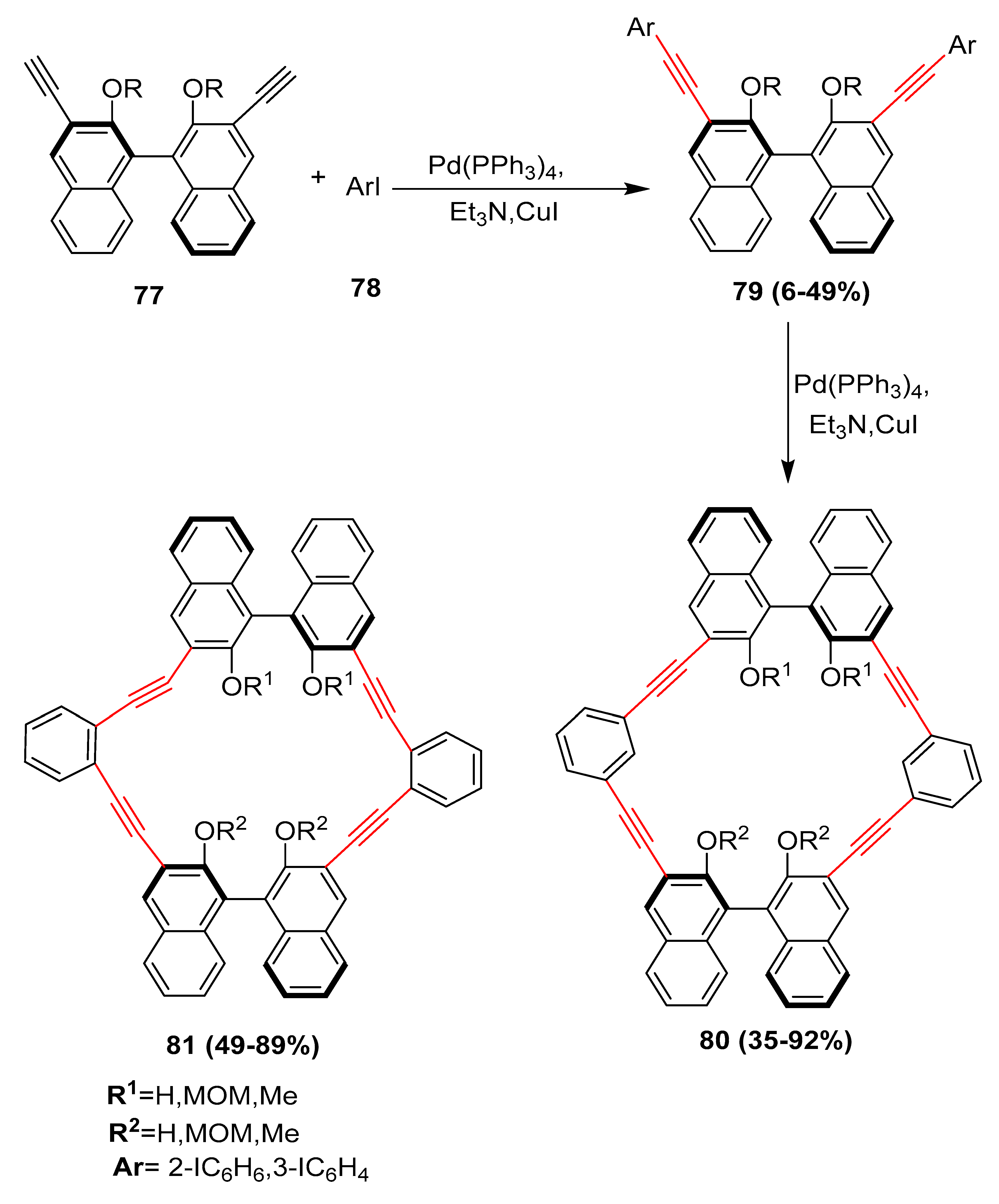
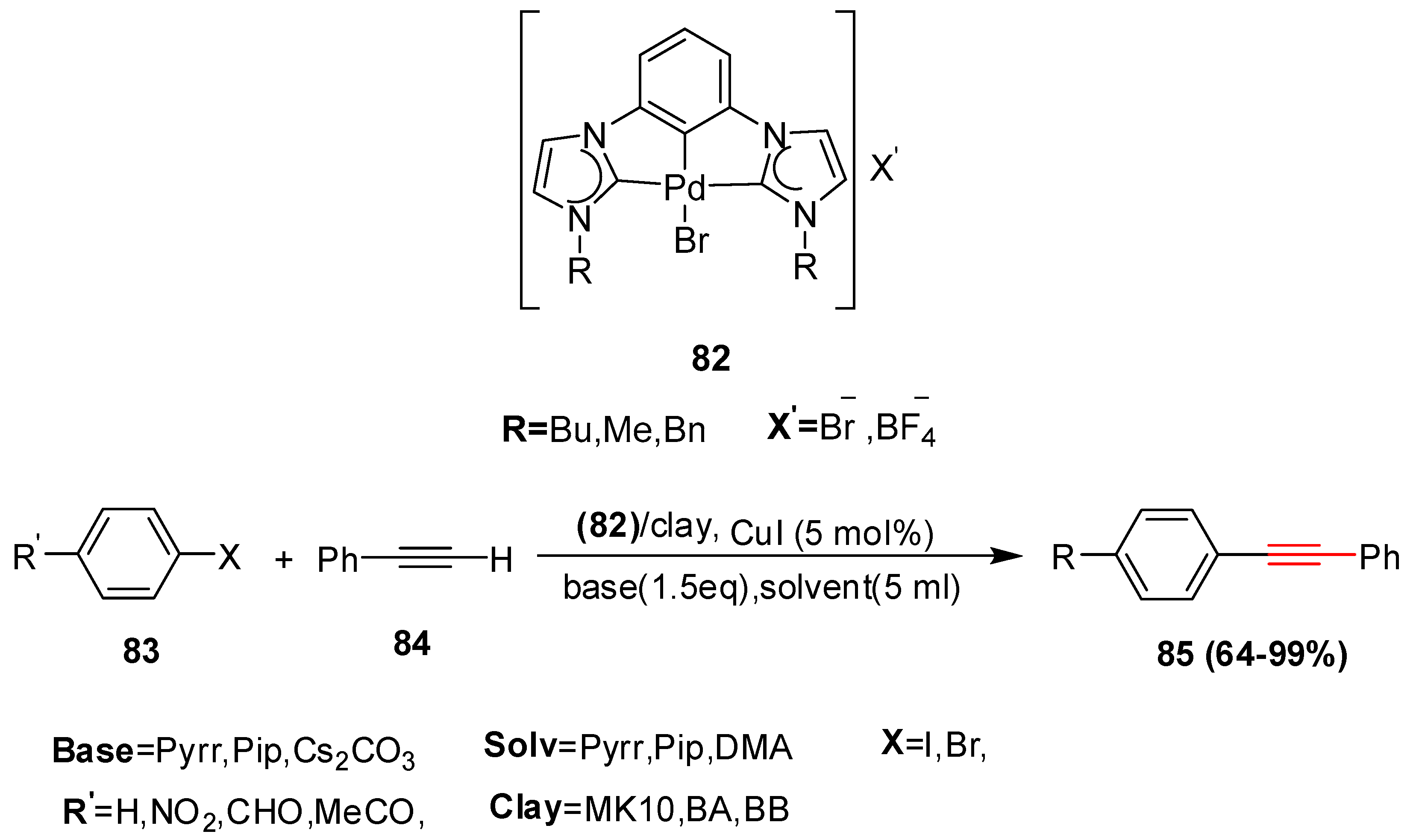
2.2. In 2003
2.3. In 2004
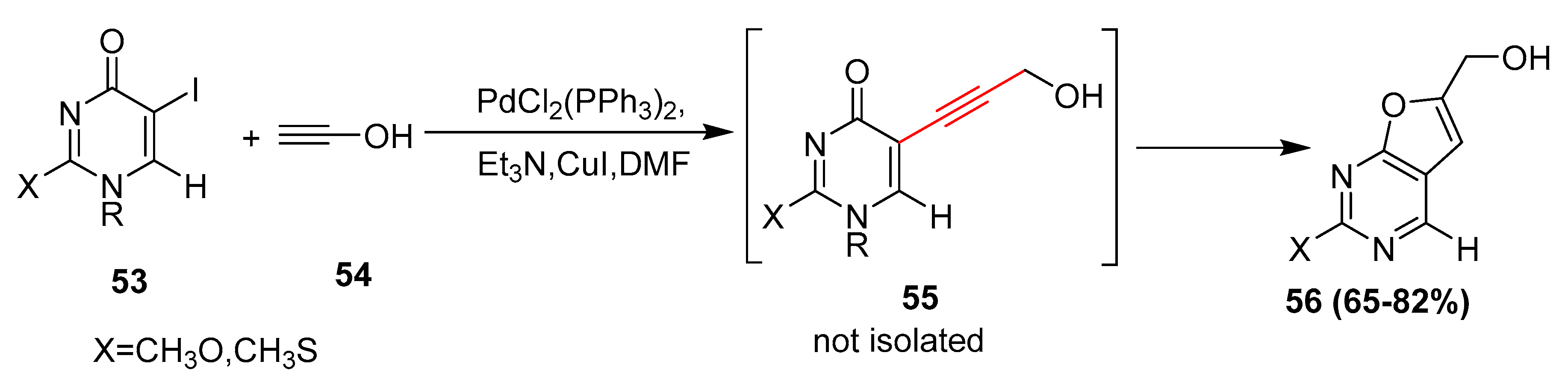
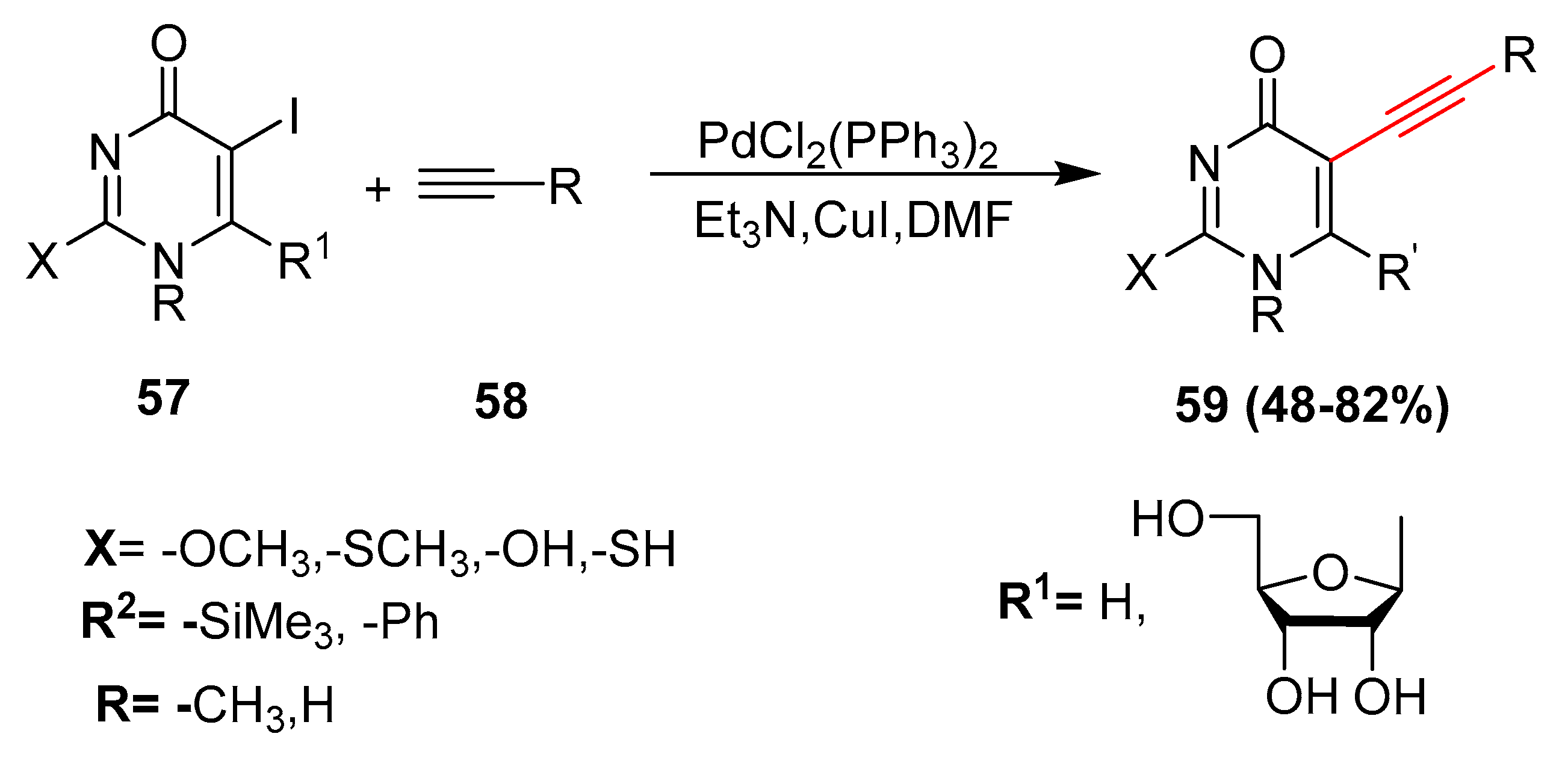
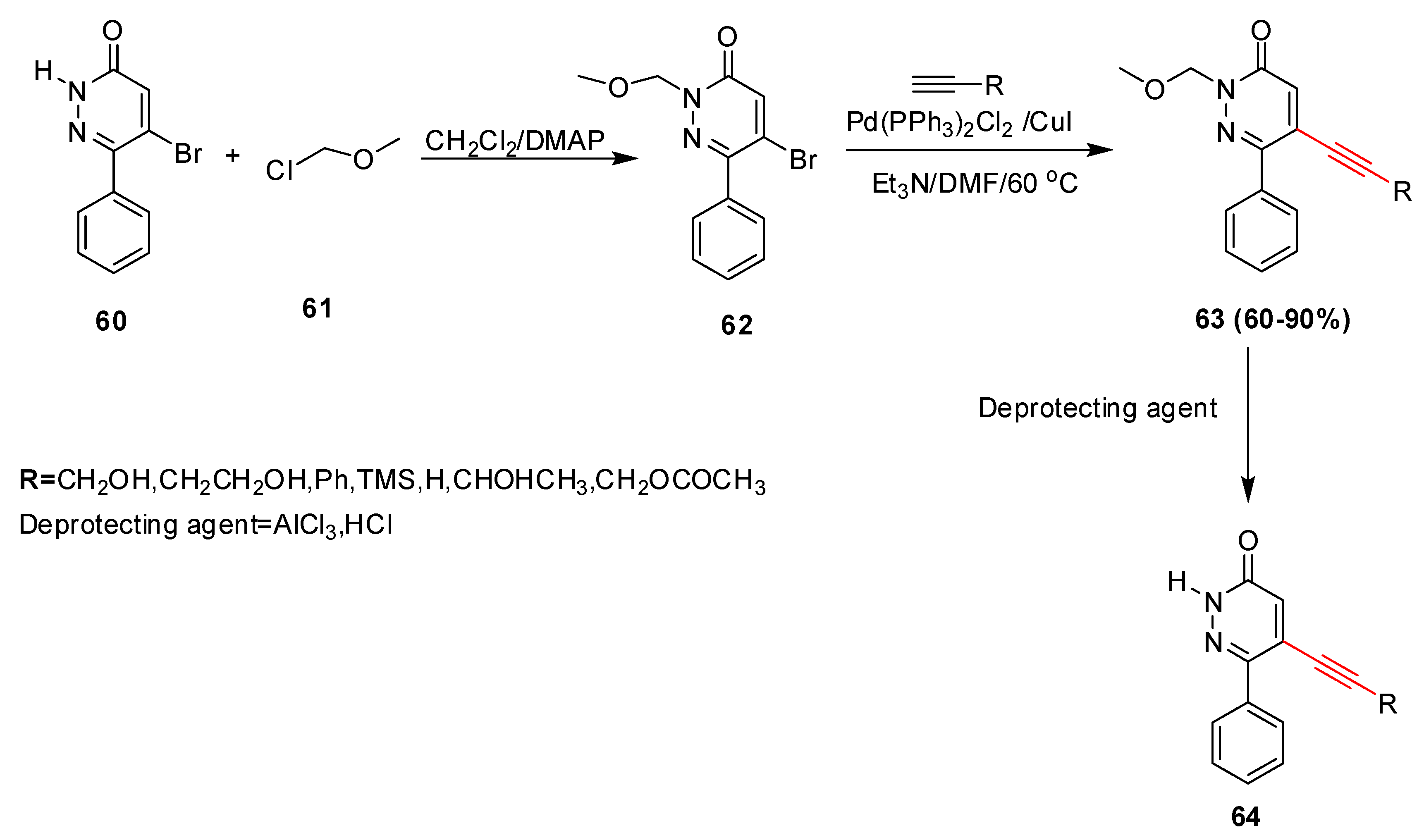
2.4. In 2005
2.5. In 2006
2.6. In 2007
2.7. In 2008
2.8. In 2009
2.9. In 2010
2.10. In 2011
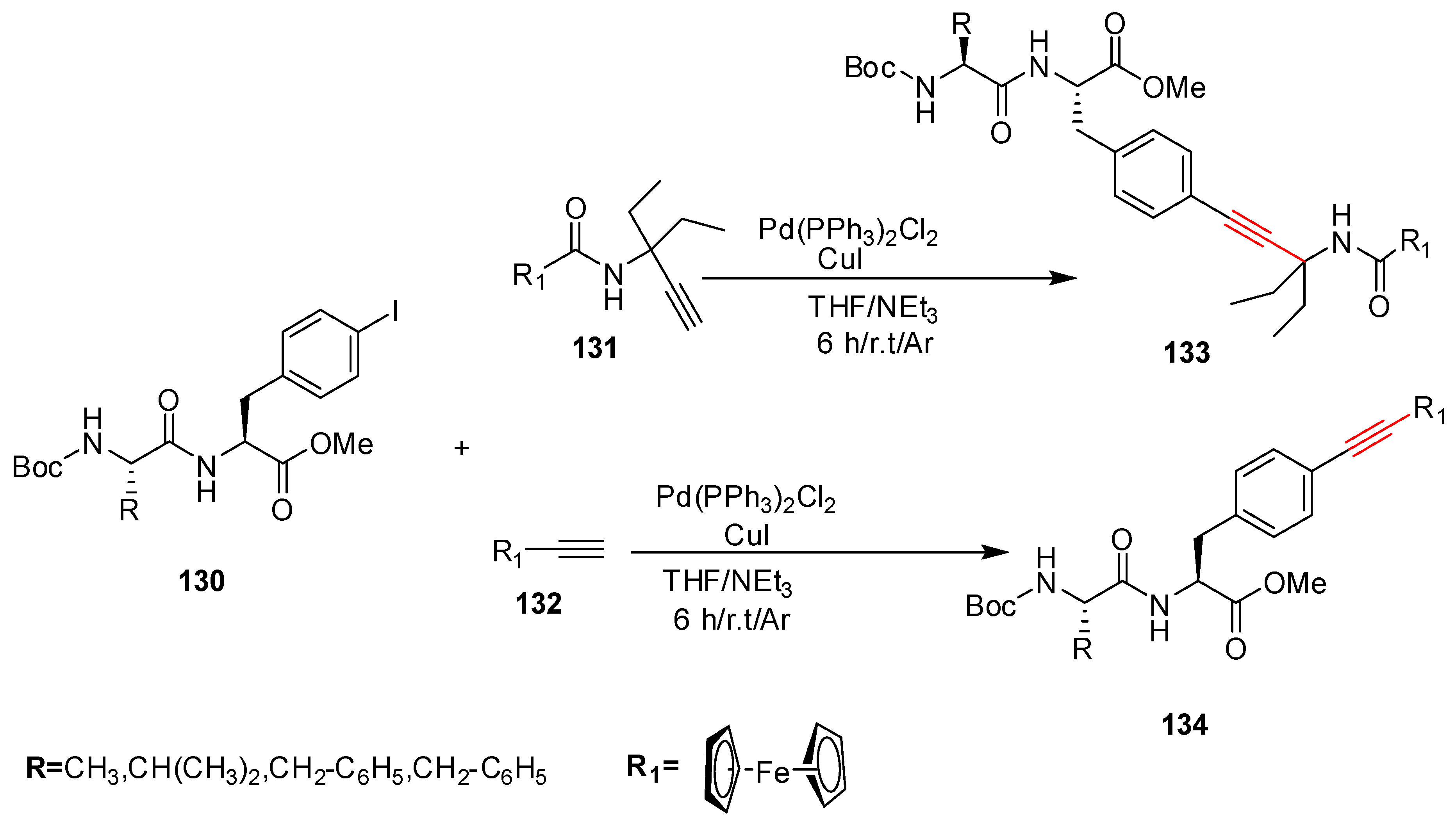
2.11. In 2012
2.12. In 2013
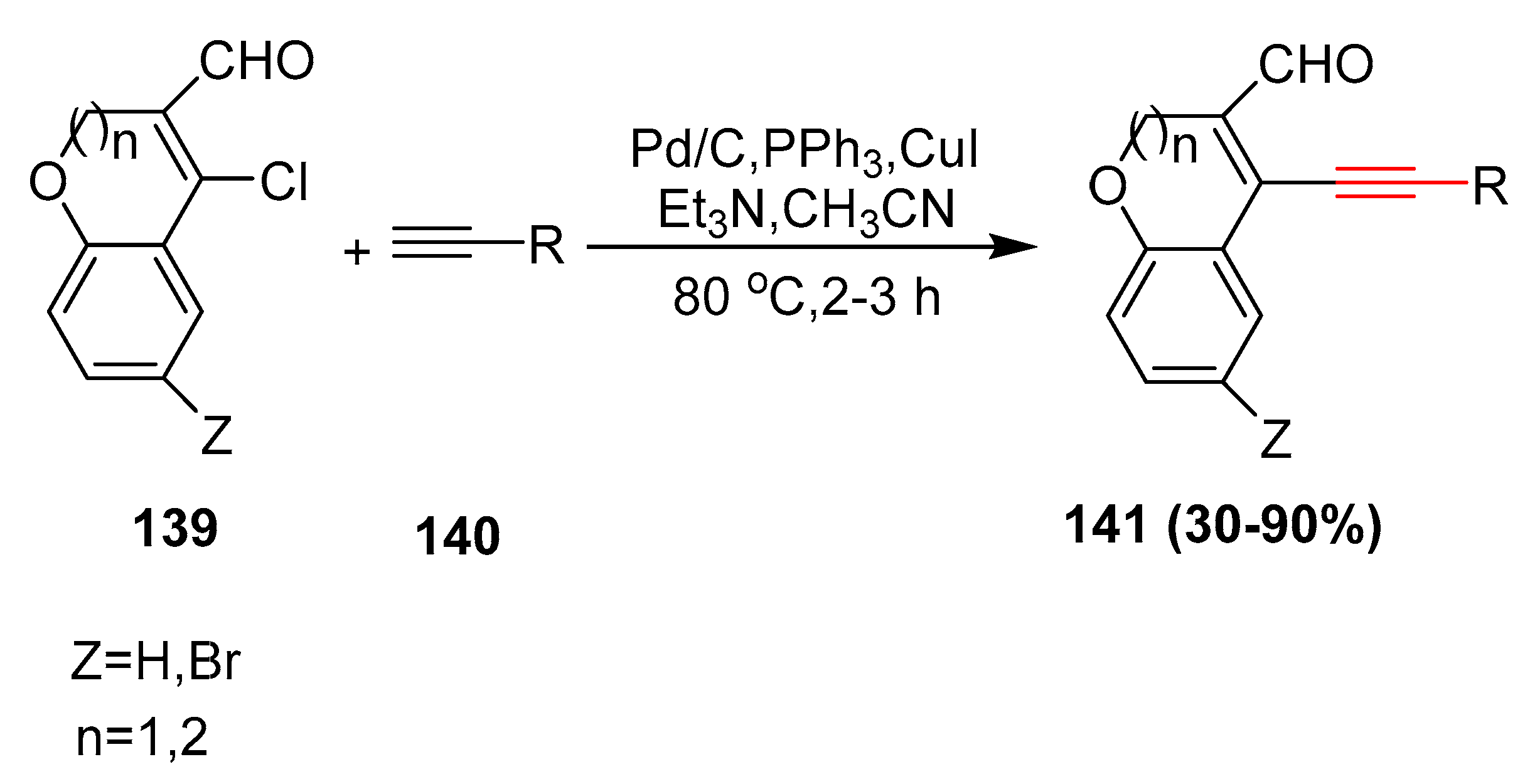
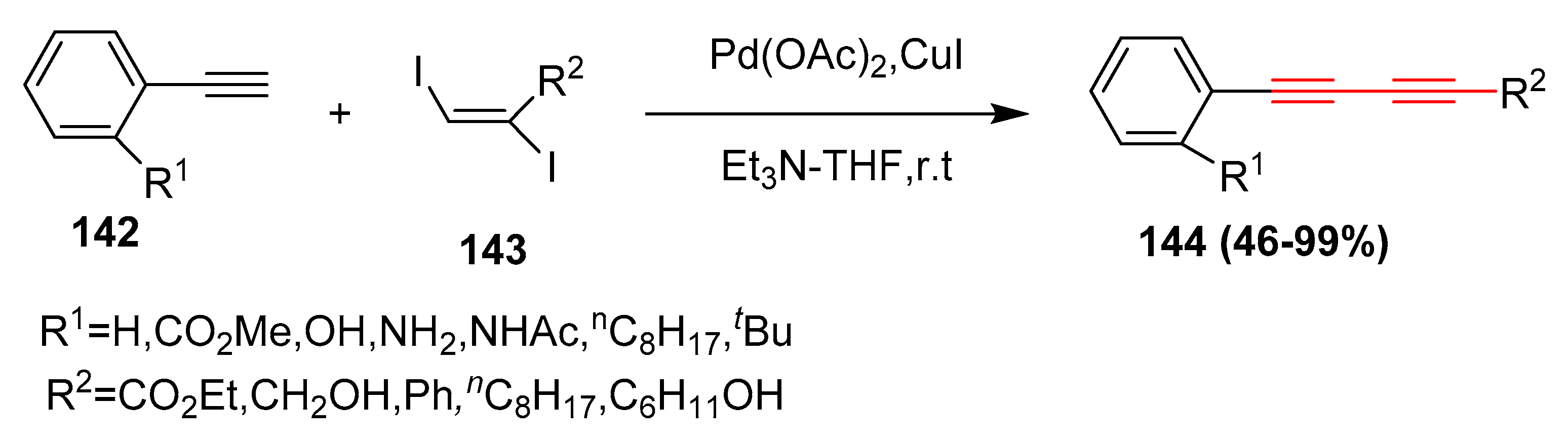
2.13. In 2014
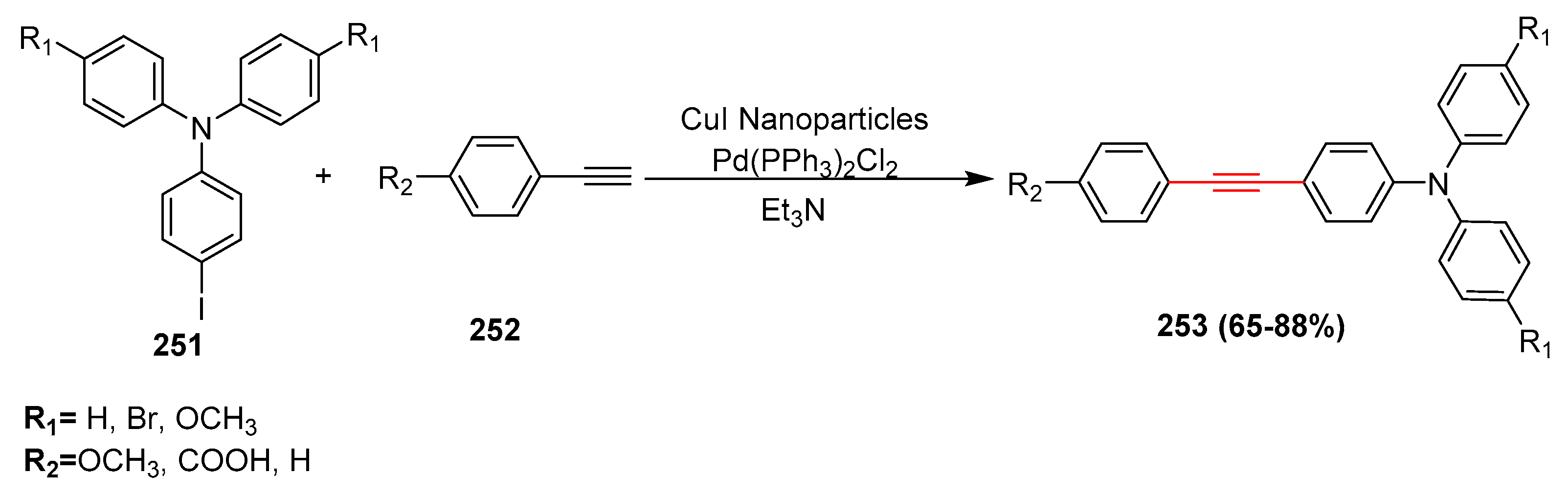
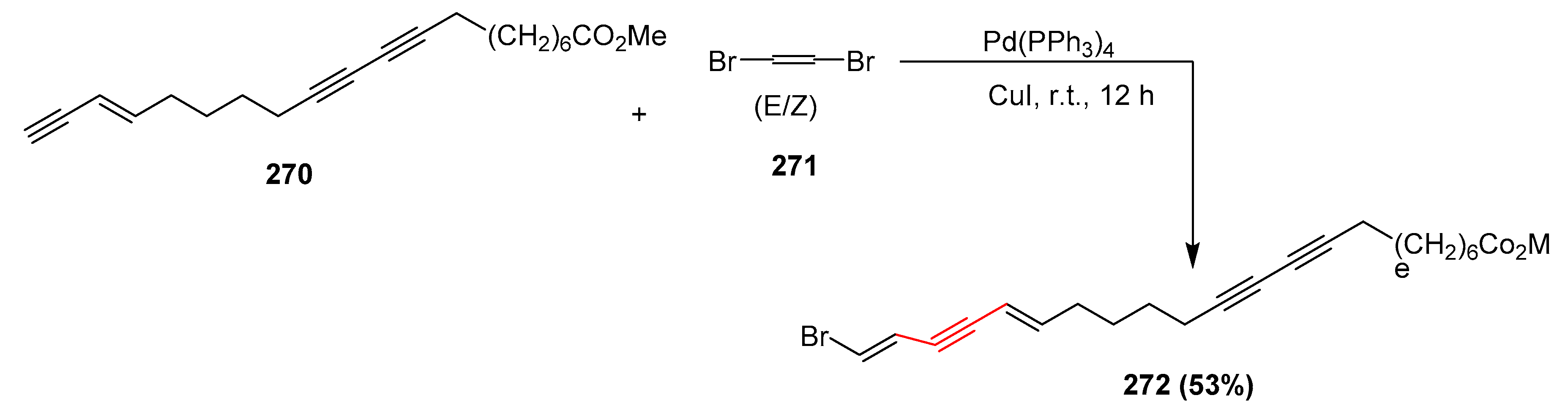
2.14. In 2015
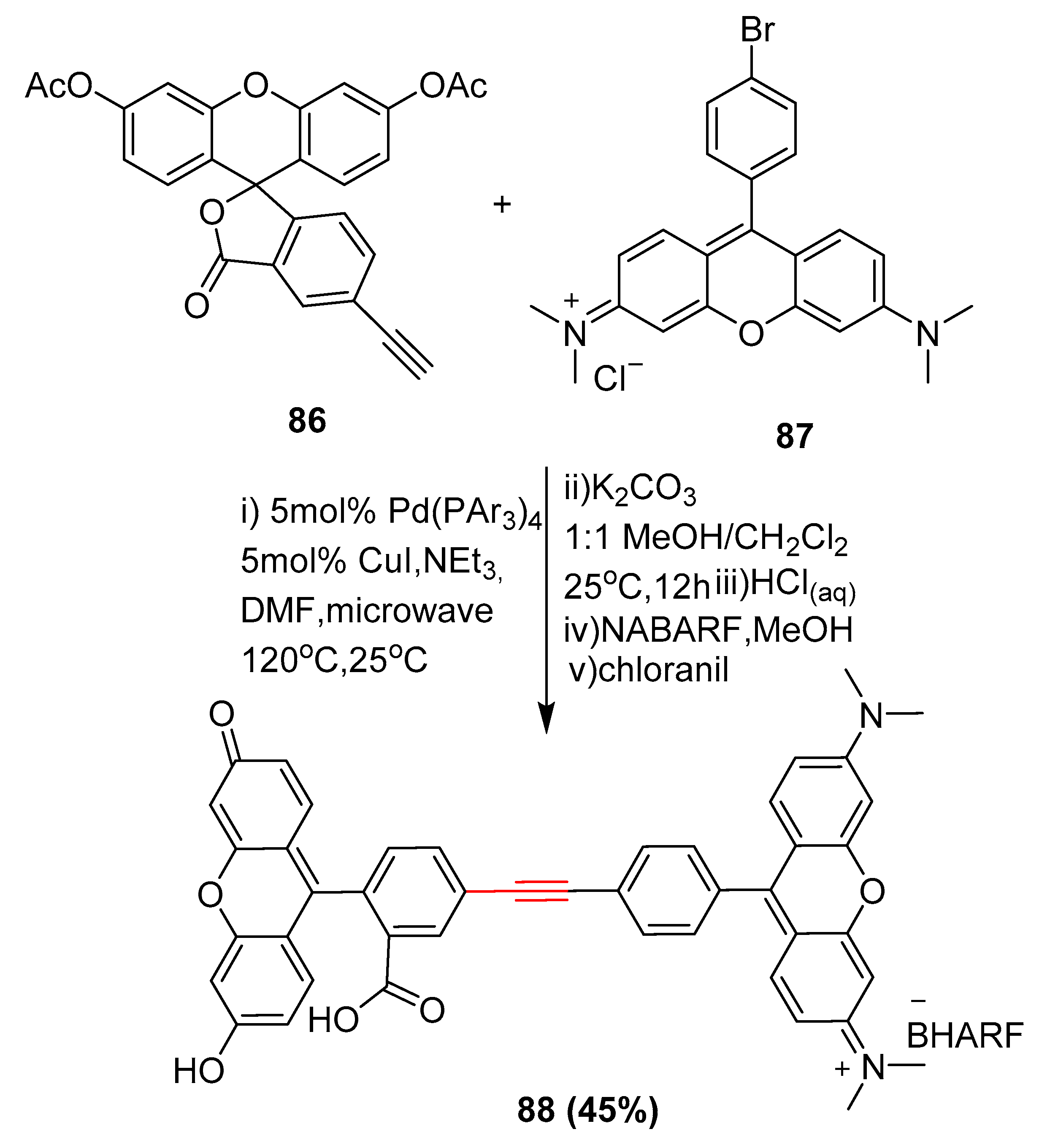
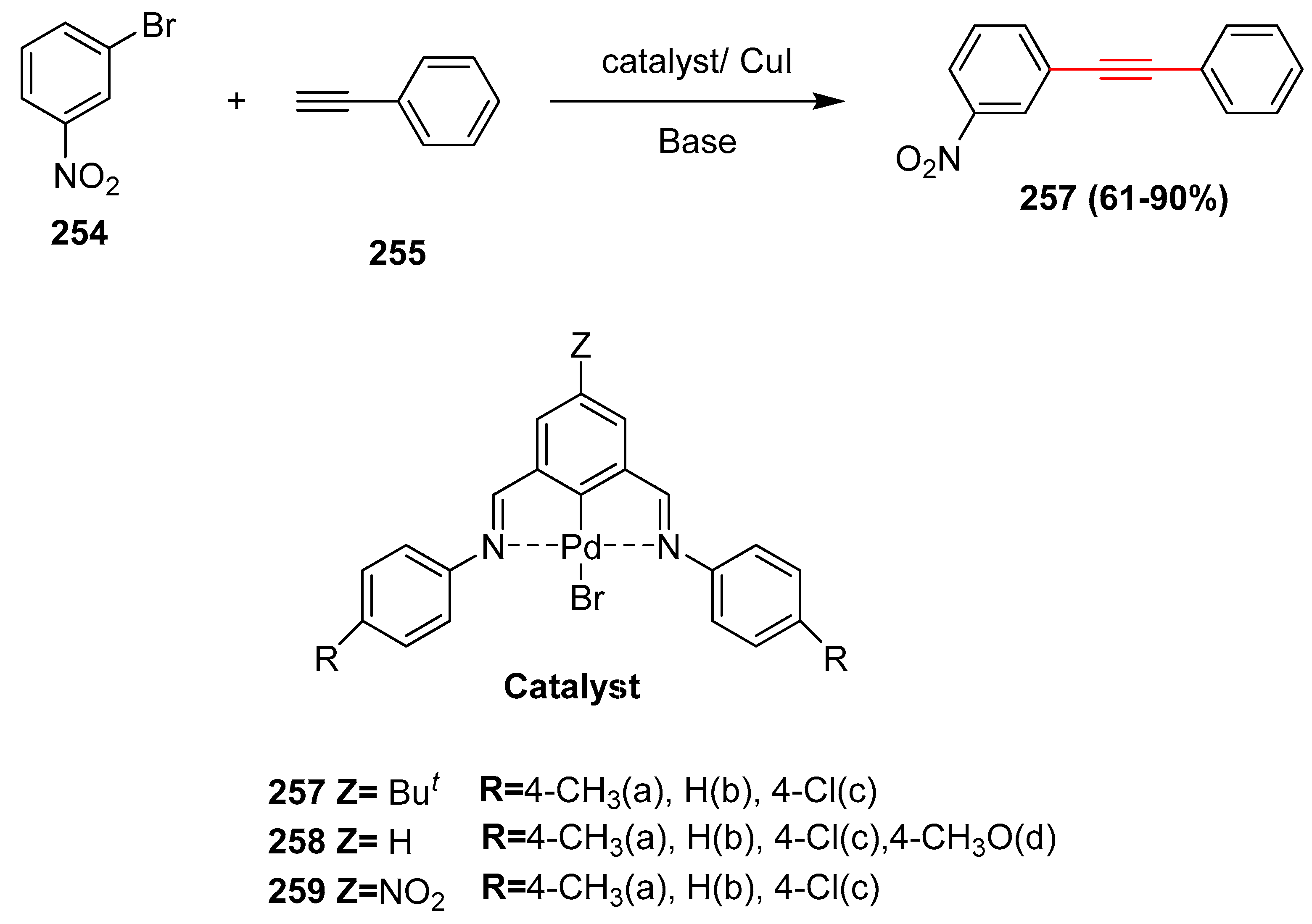
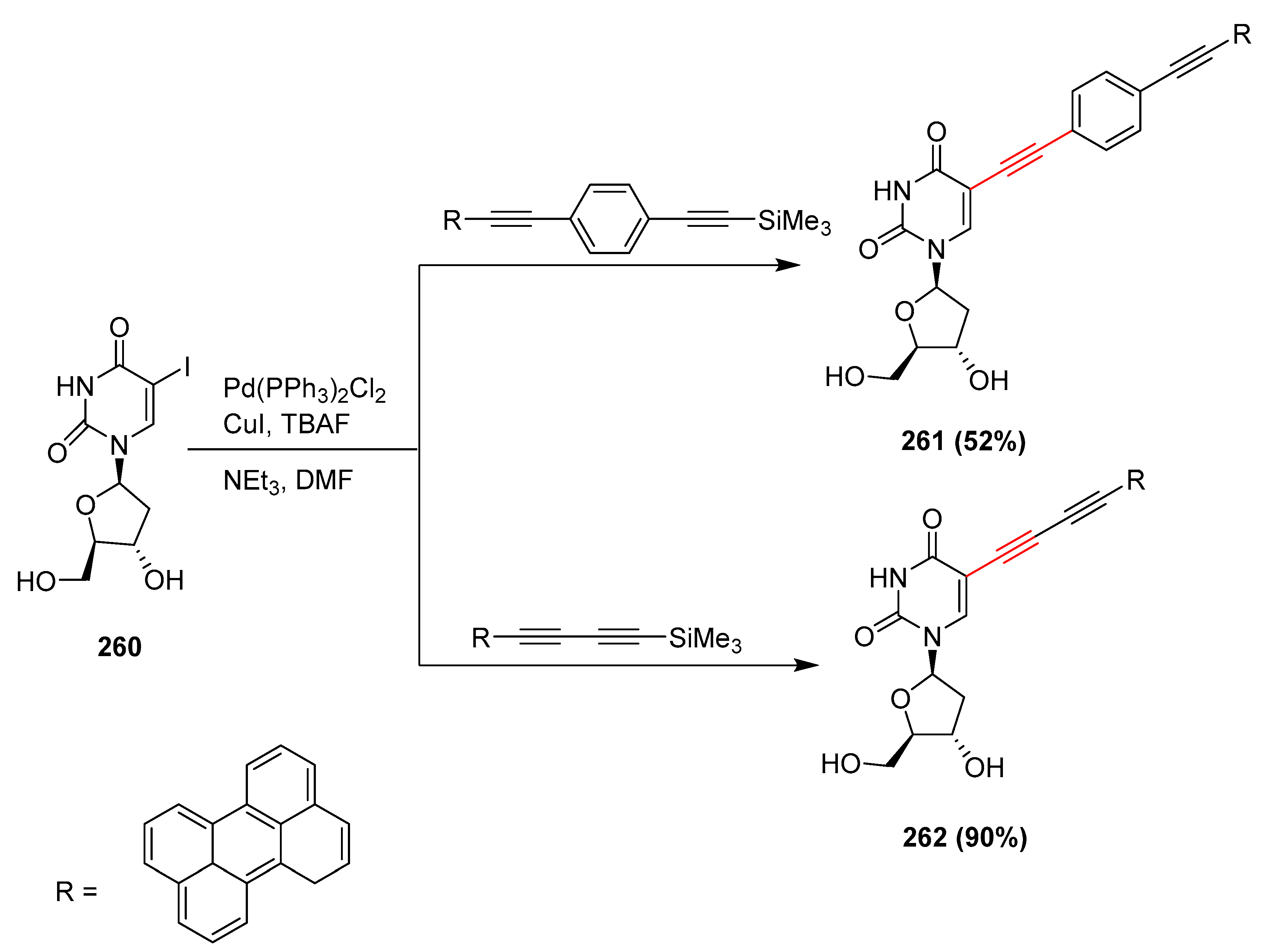
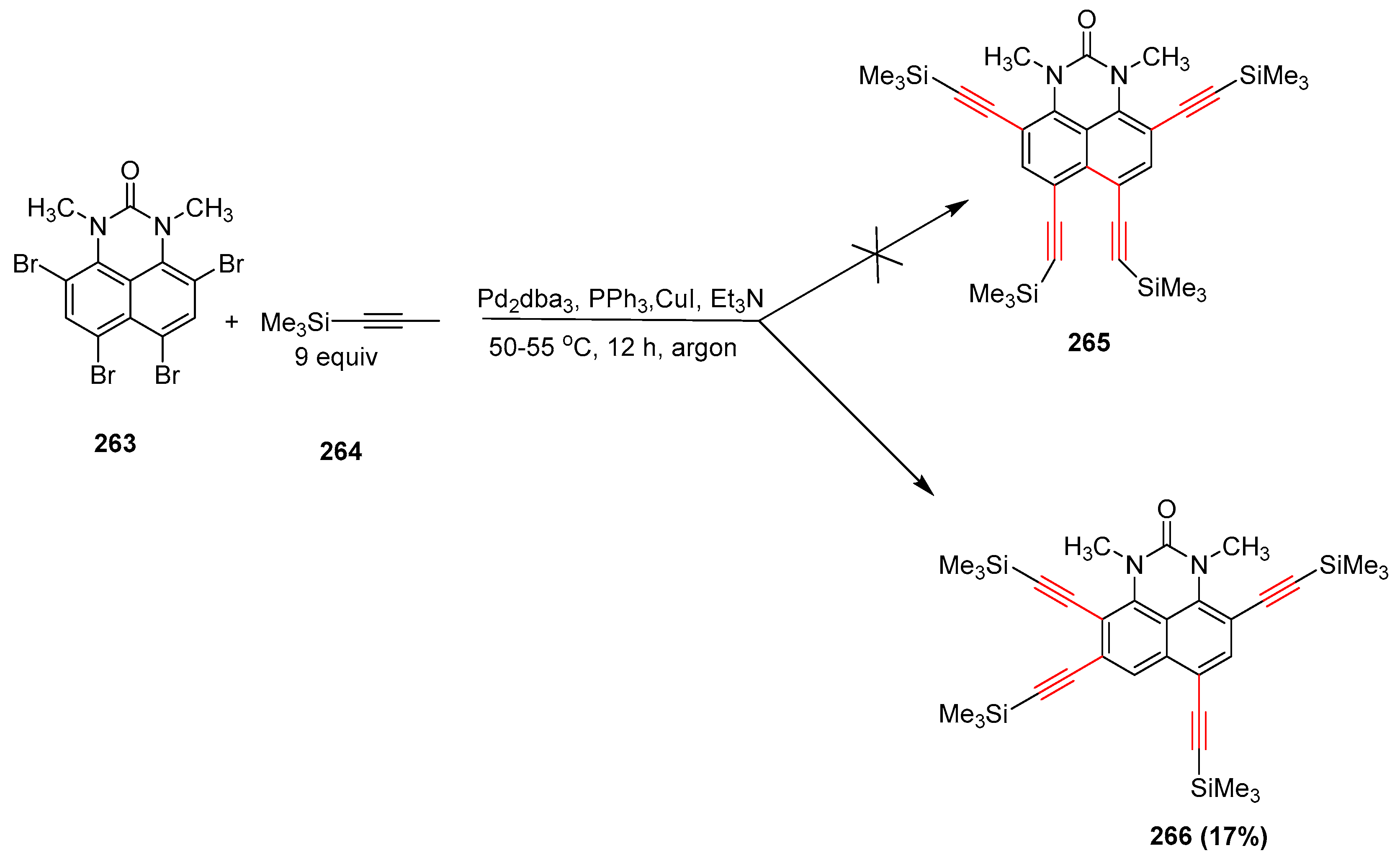
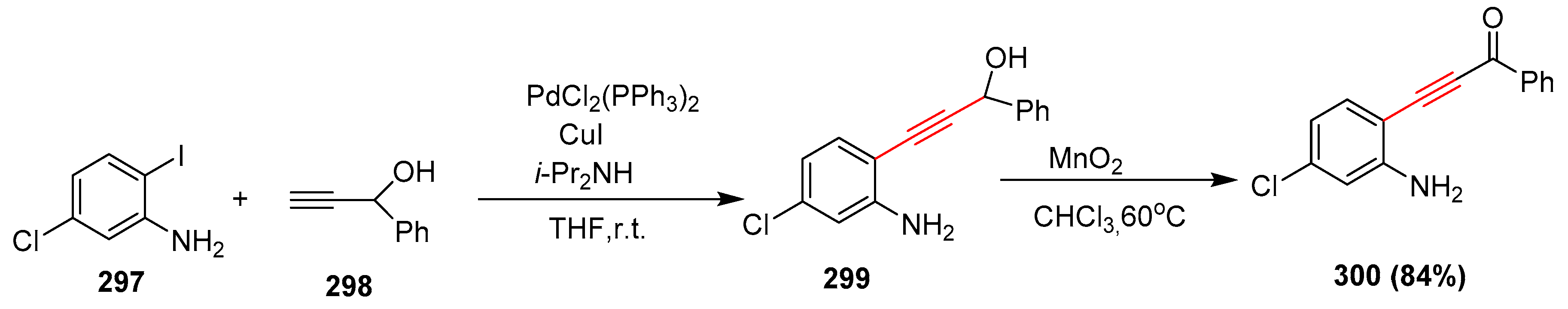
2.15. In 2016
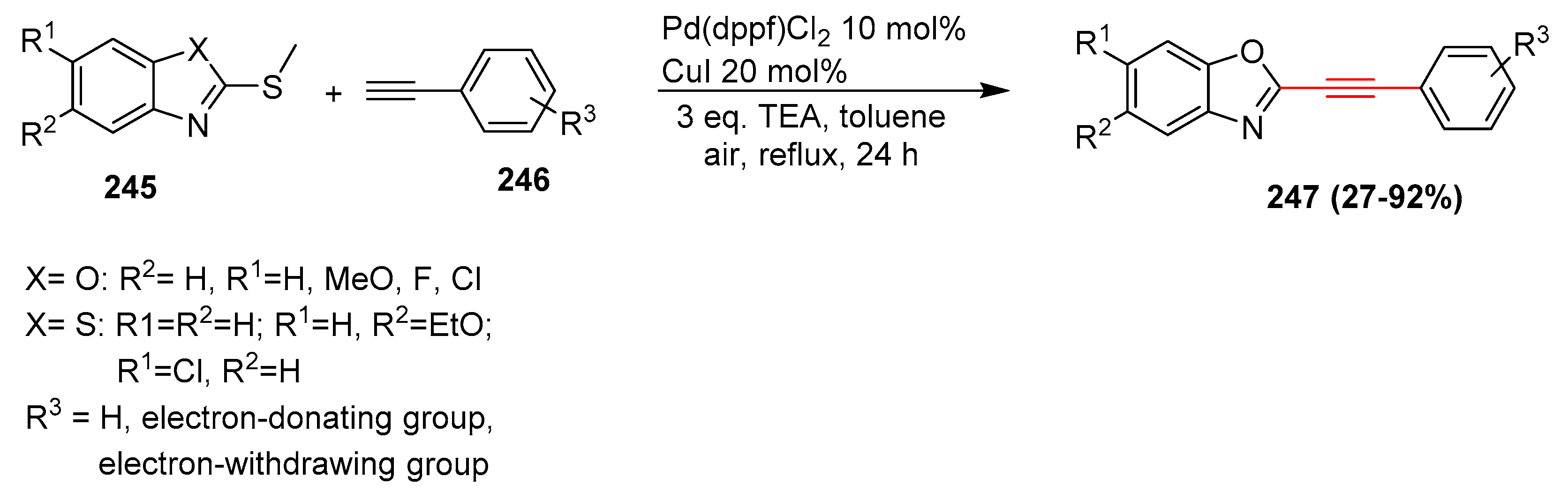
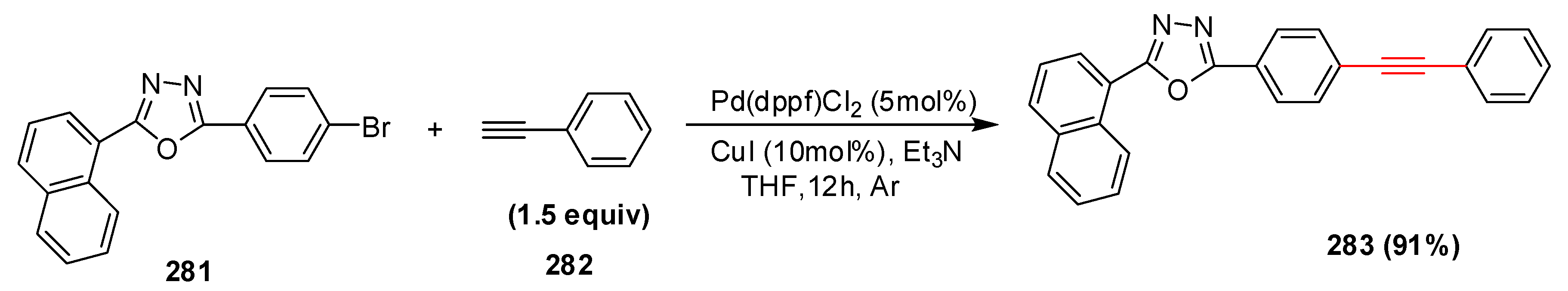
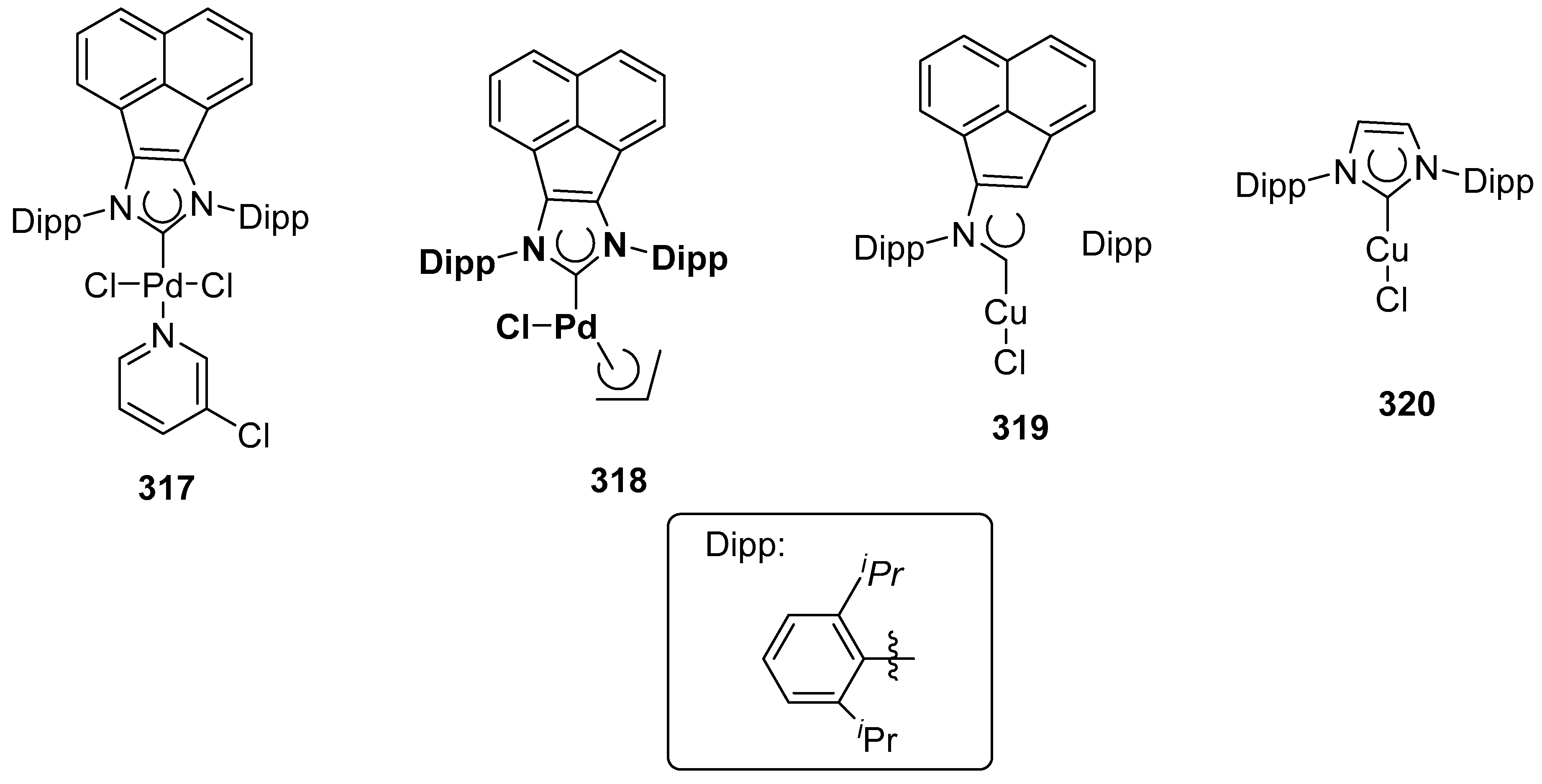
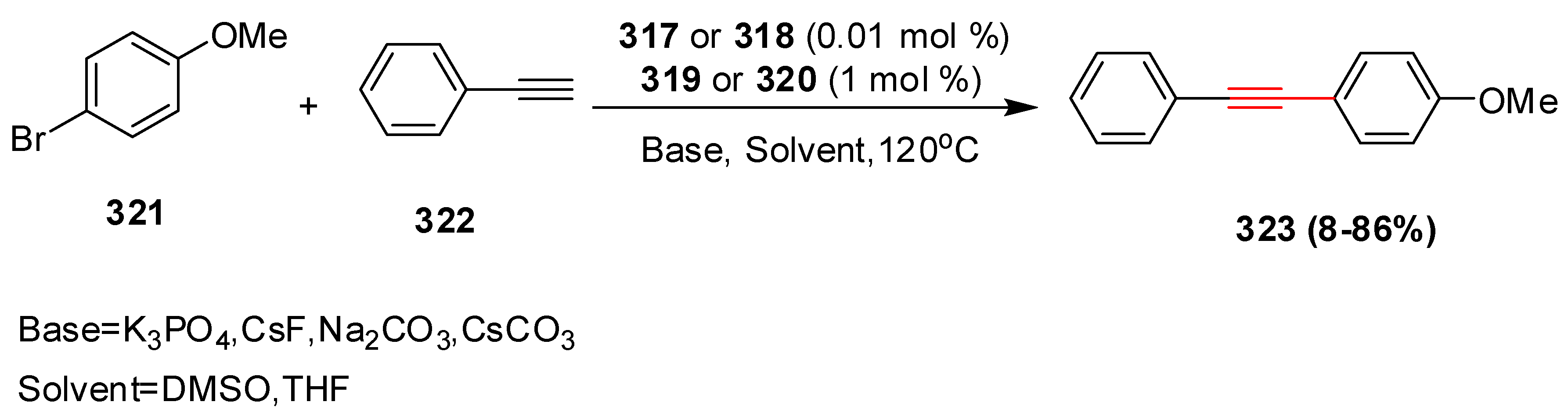
2.16. In 2017
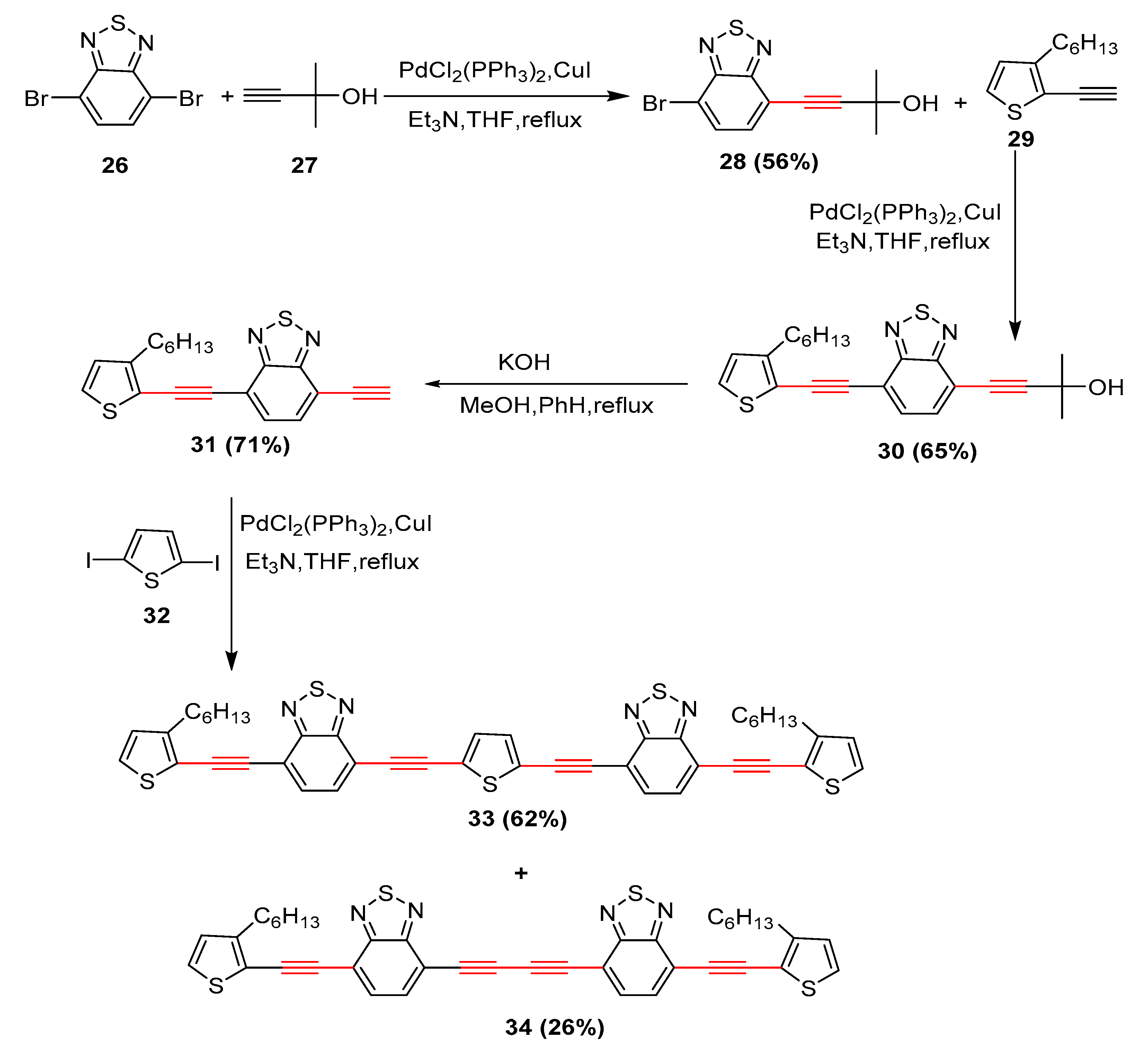
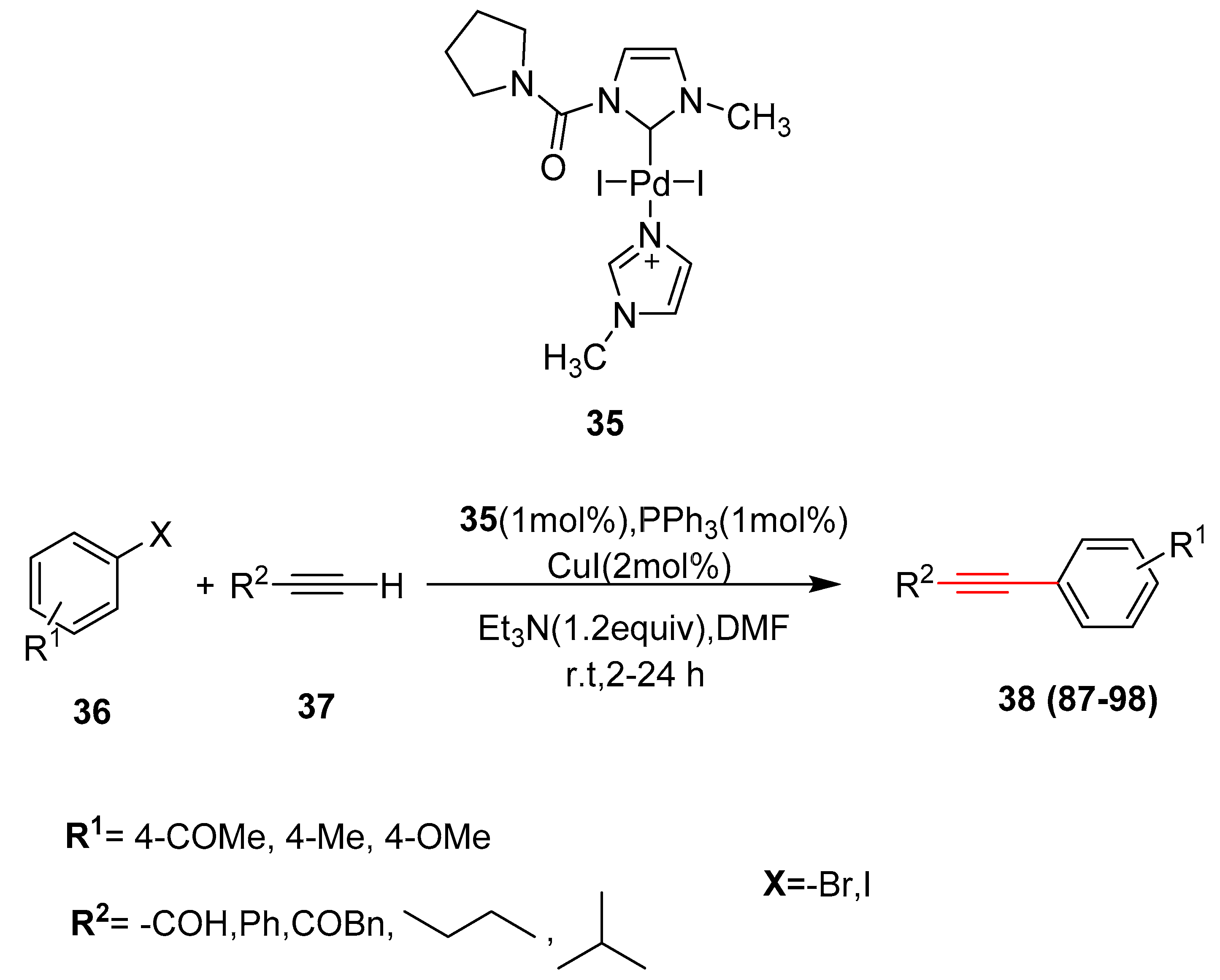
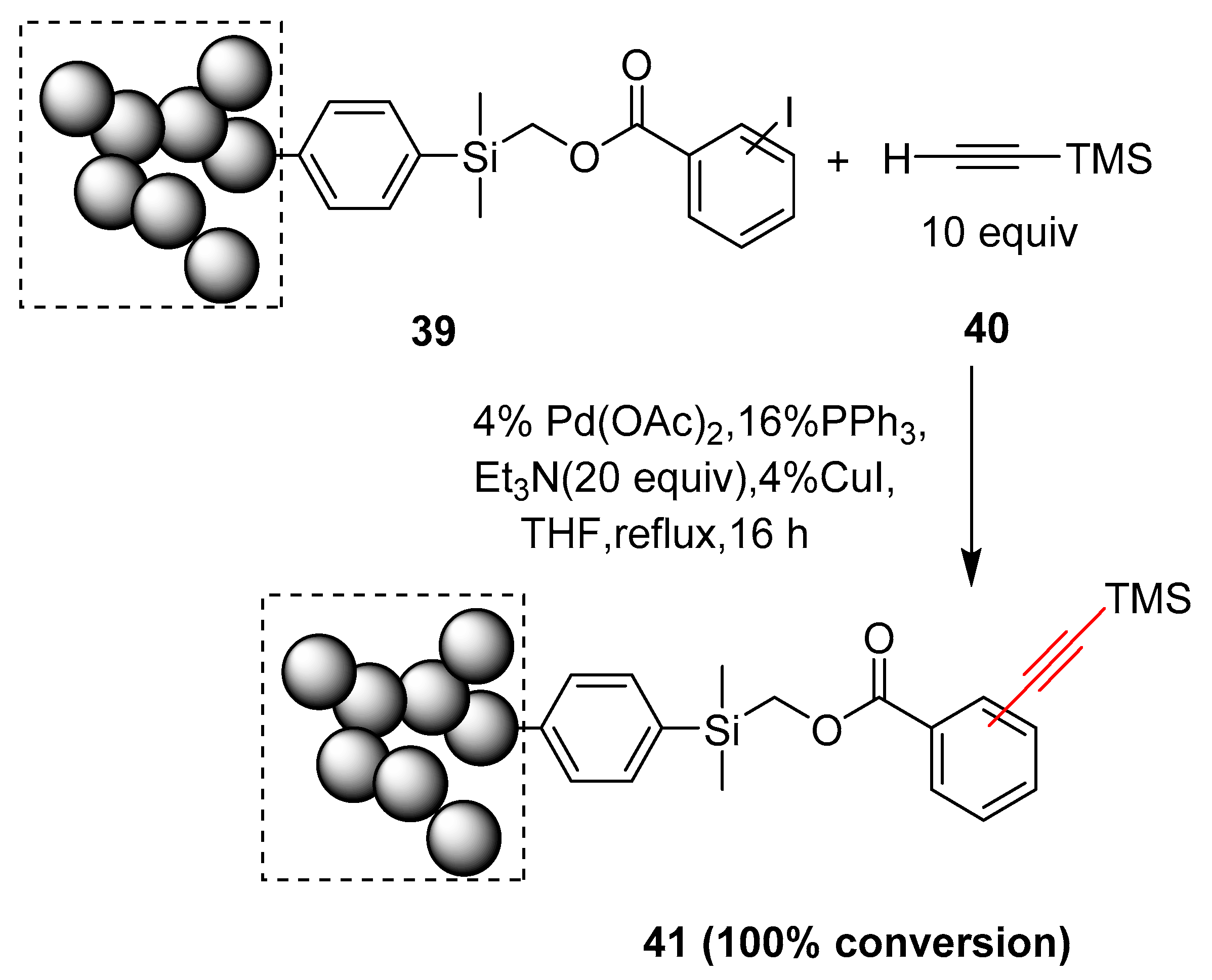
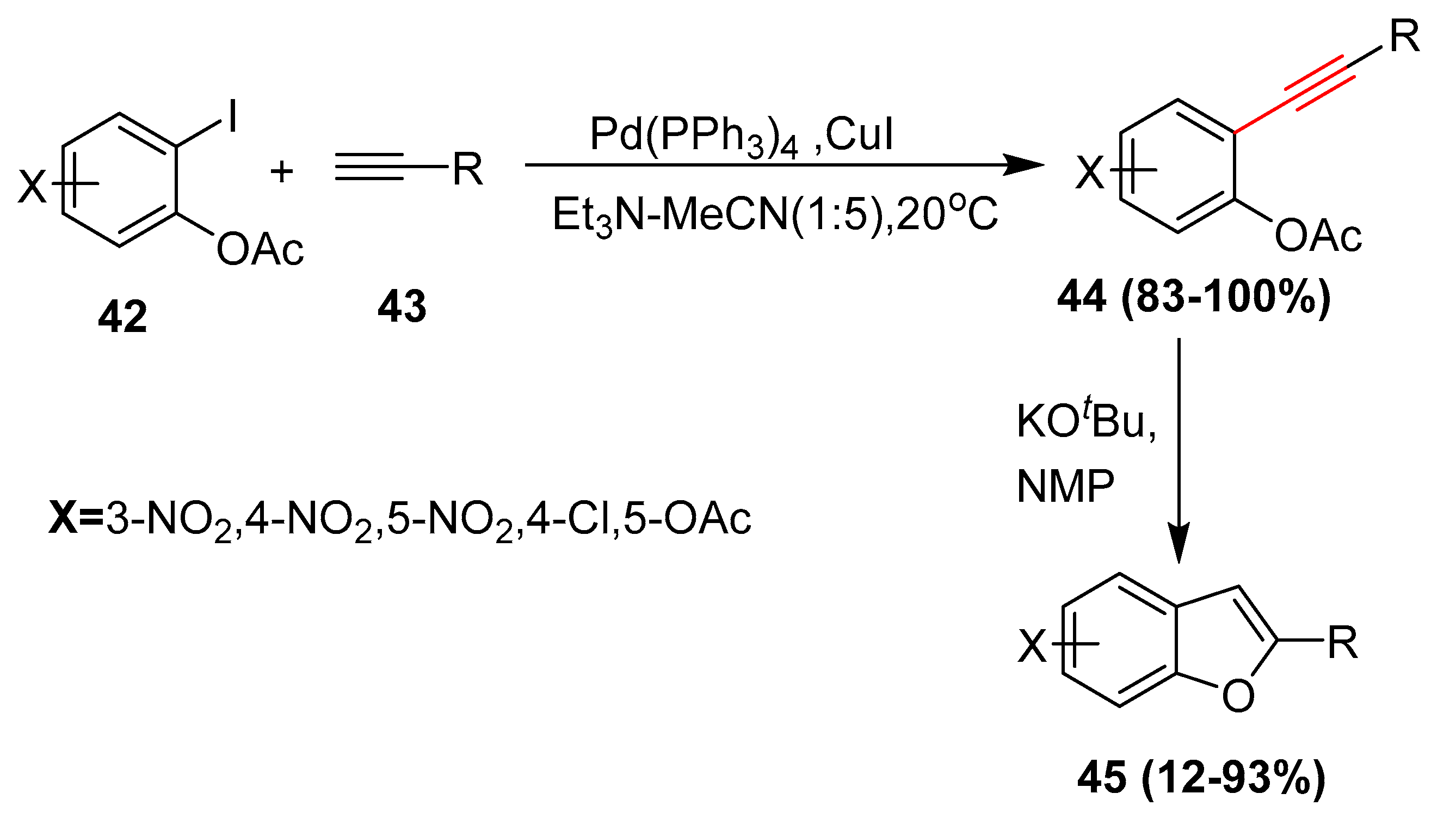
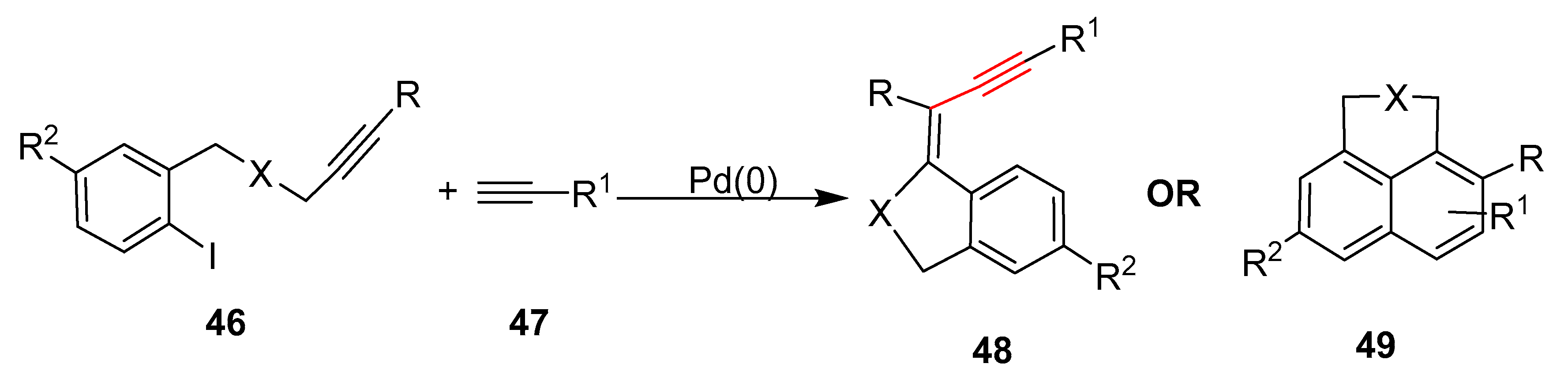
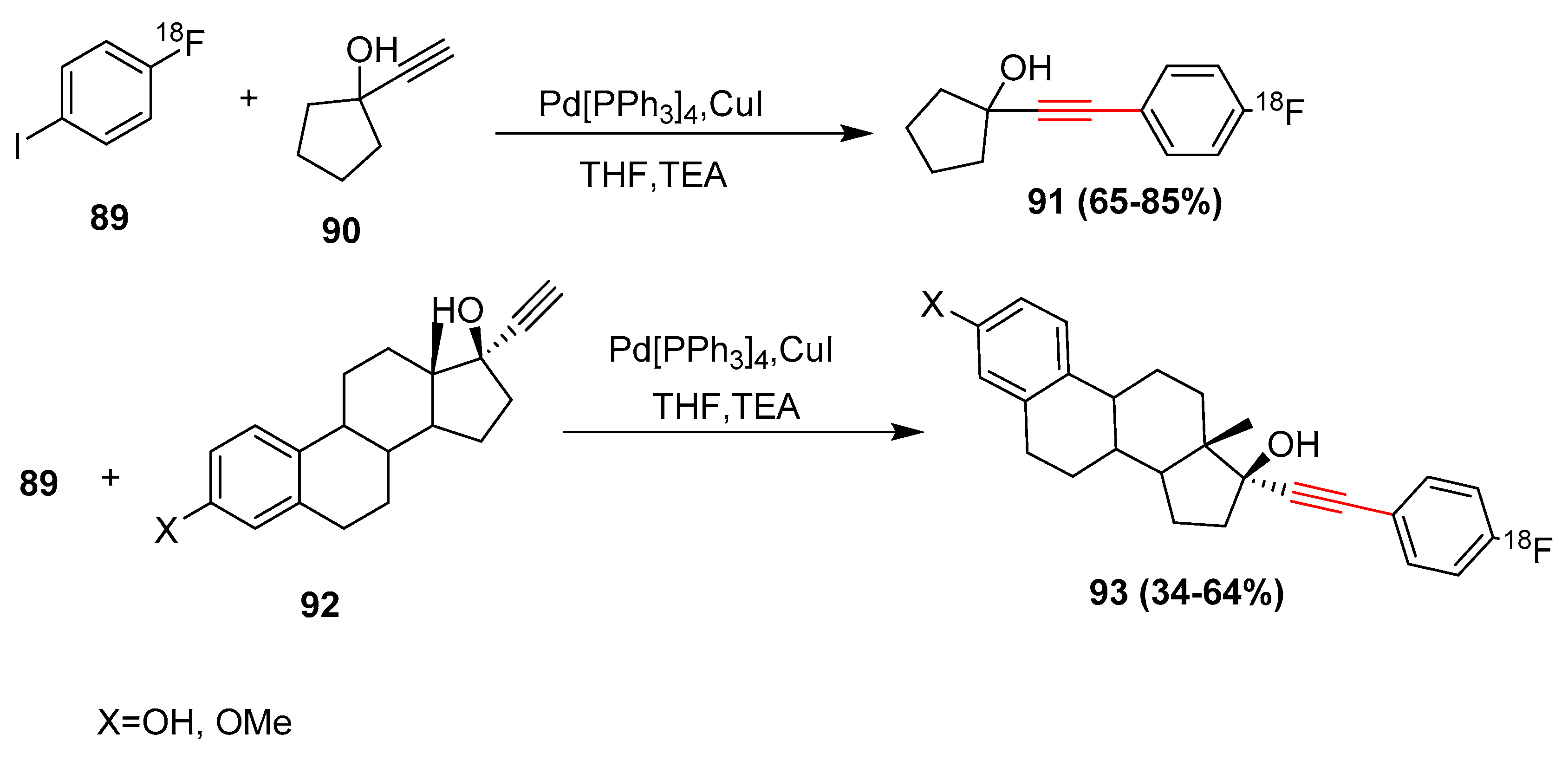
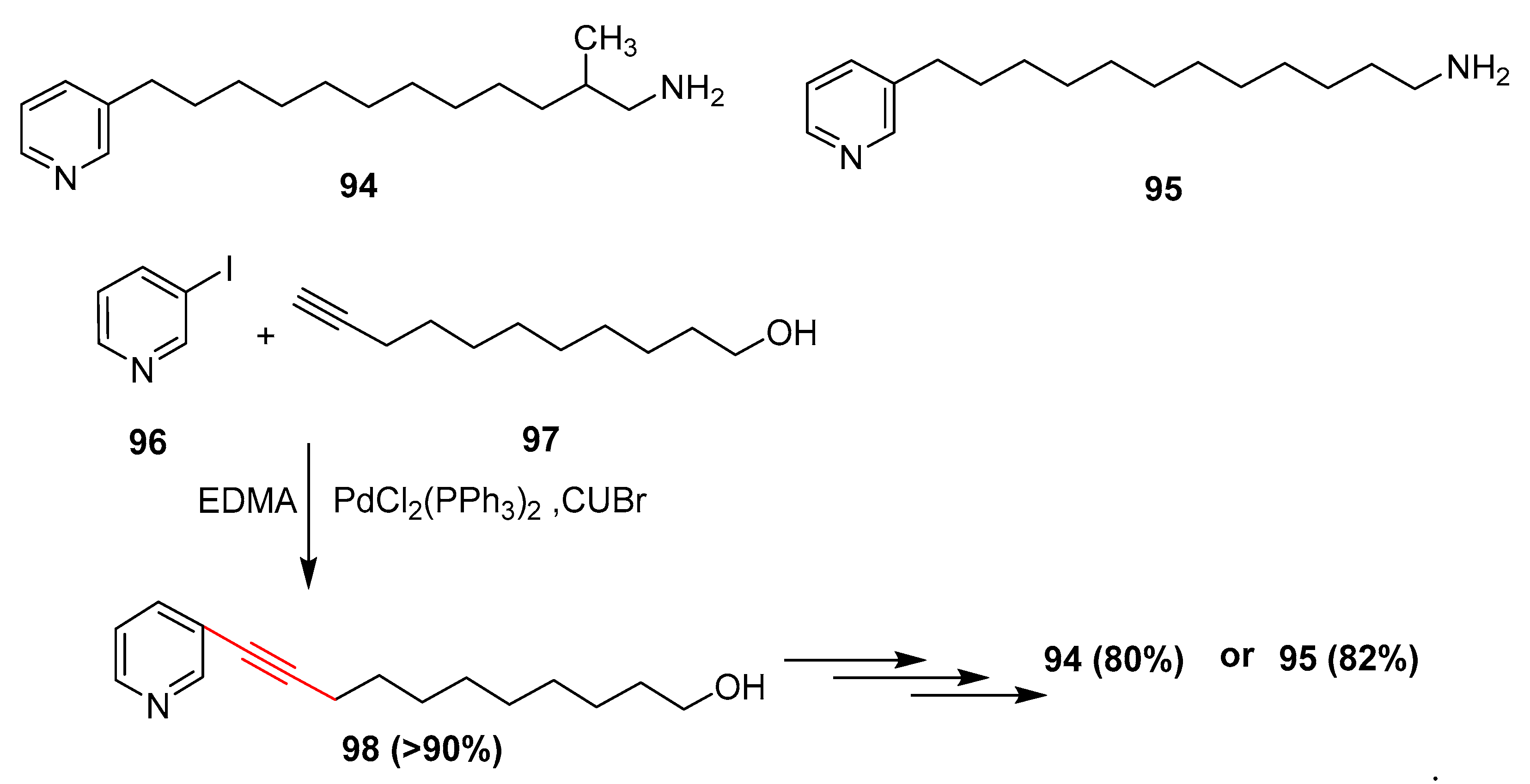
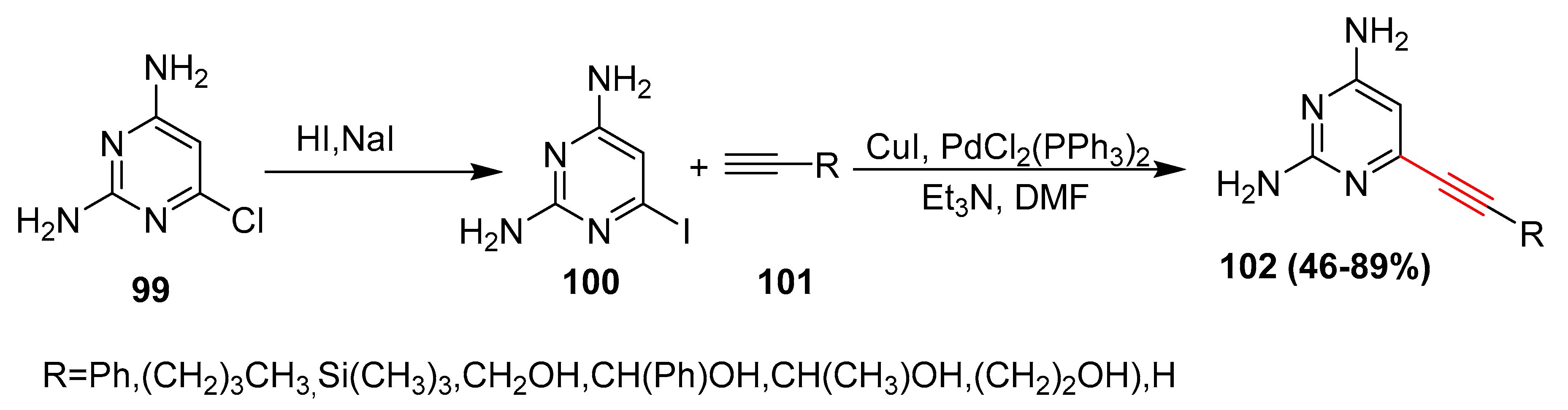
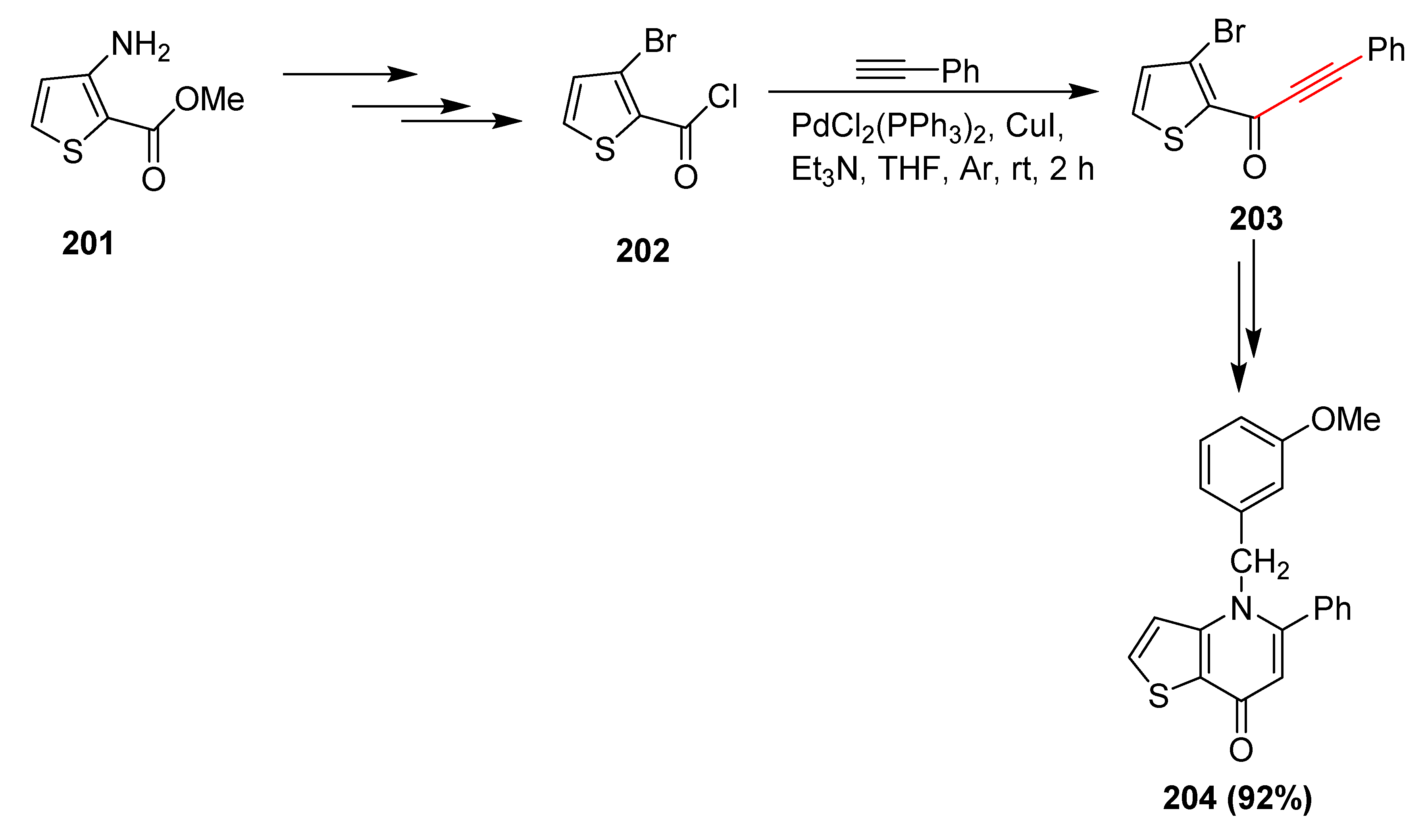
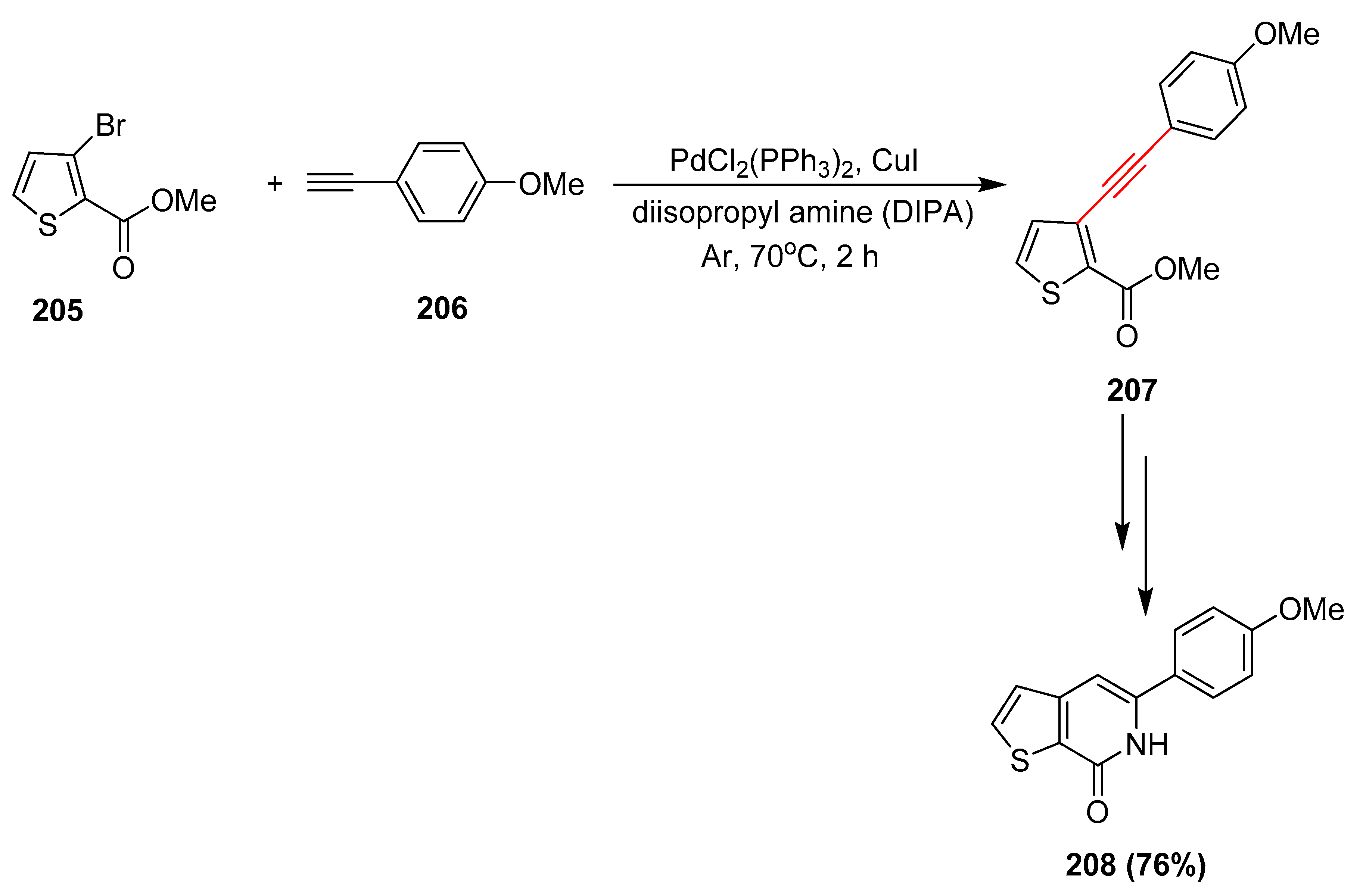
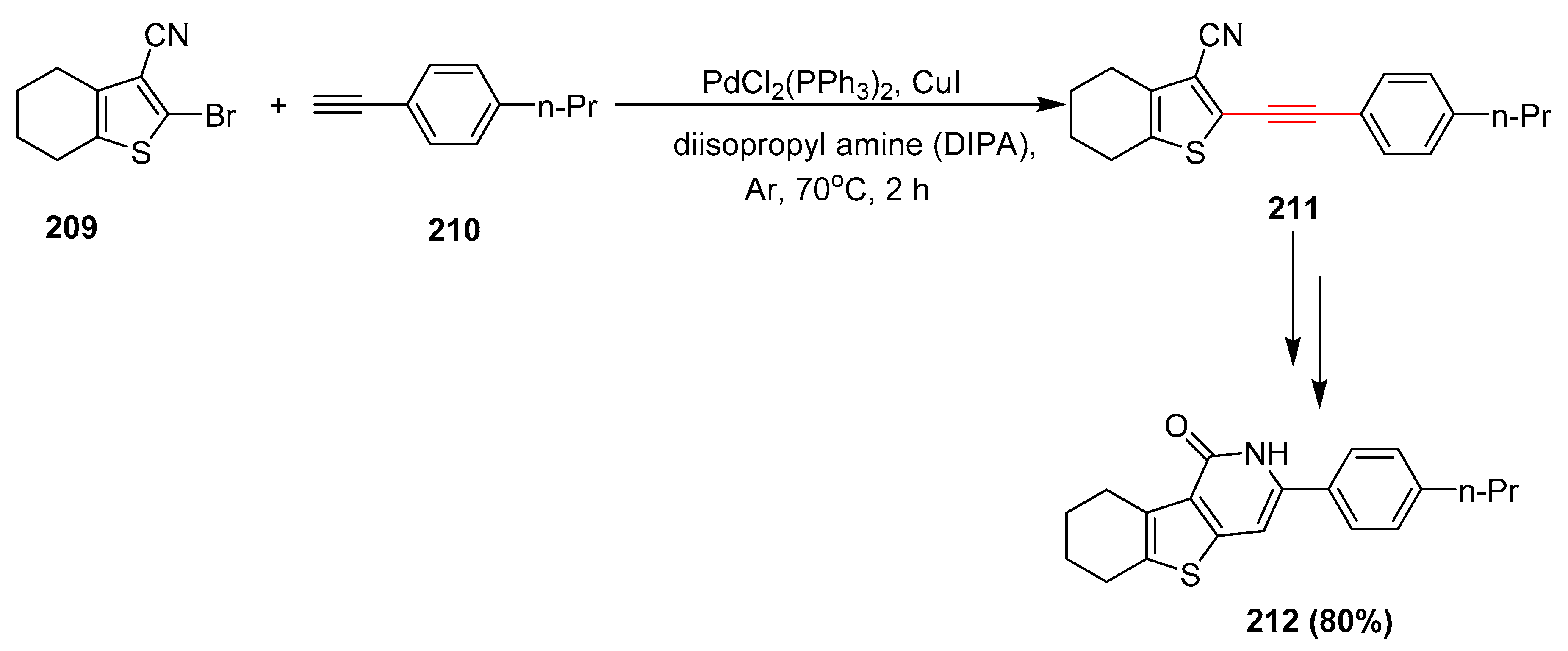
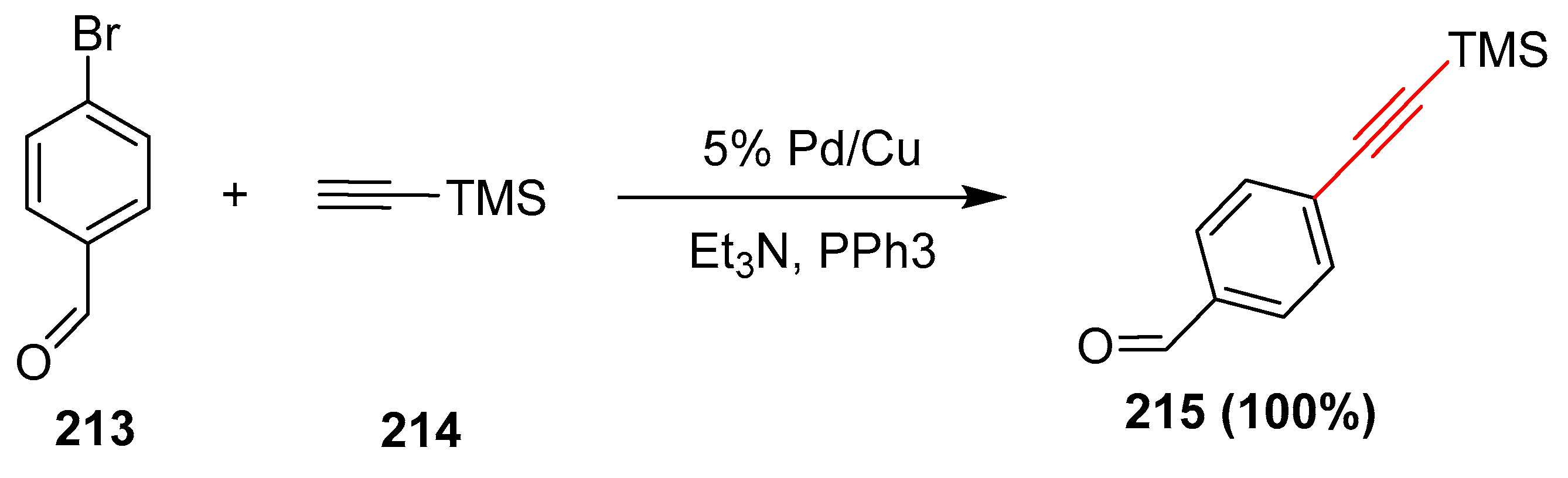
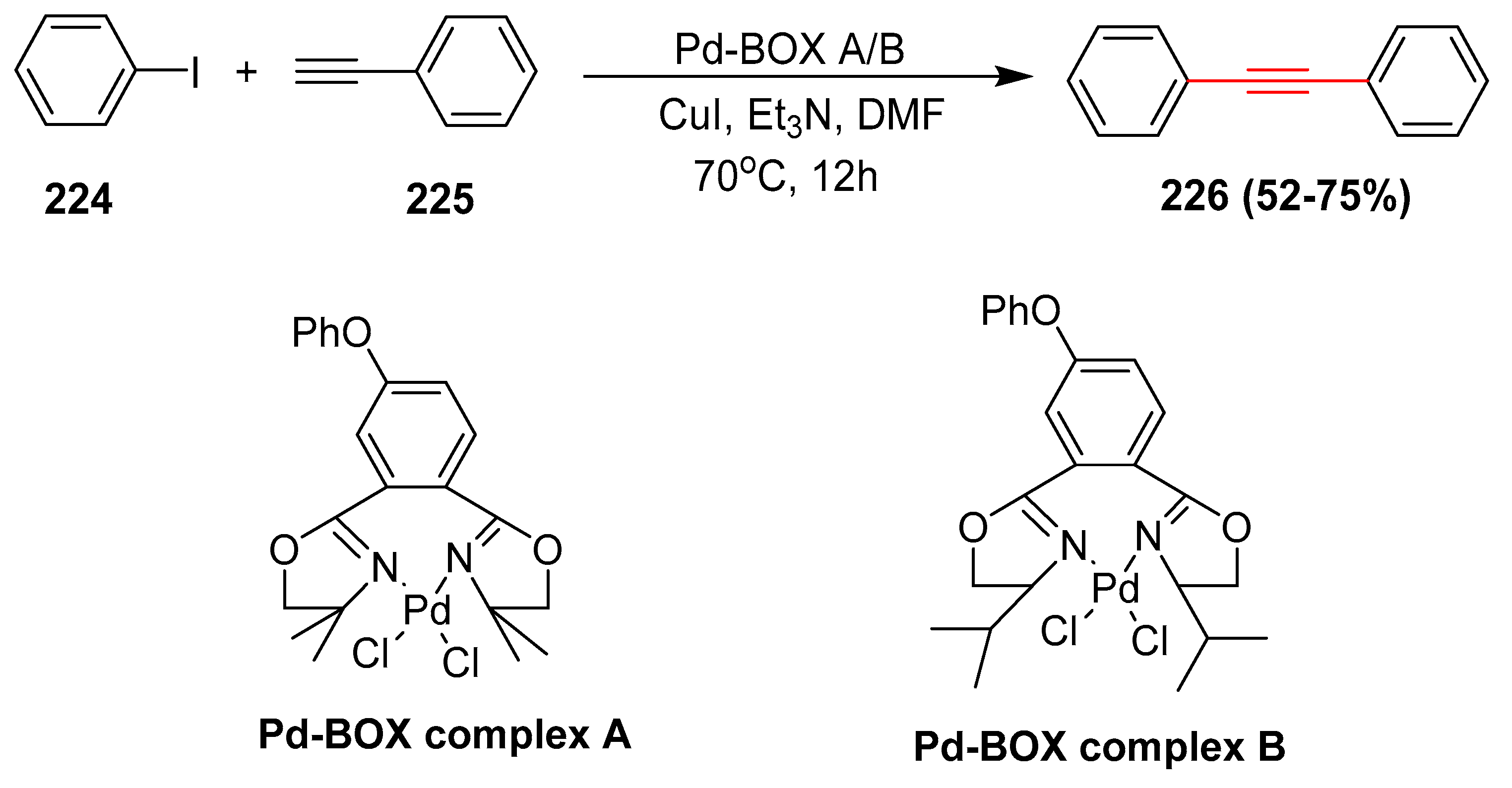
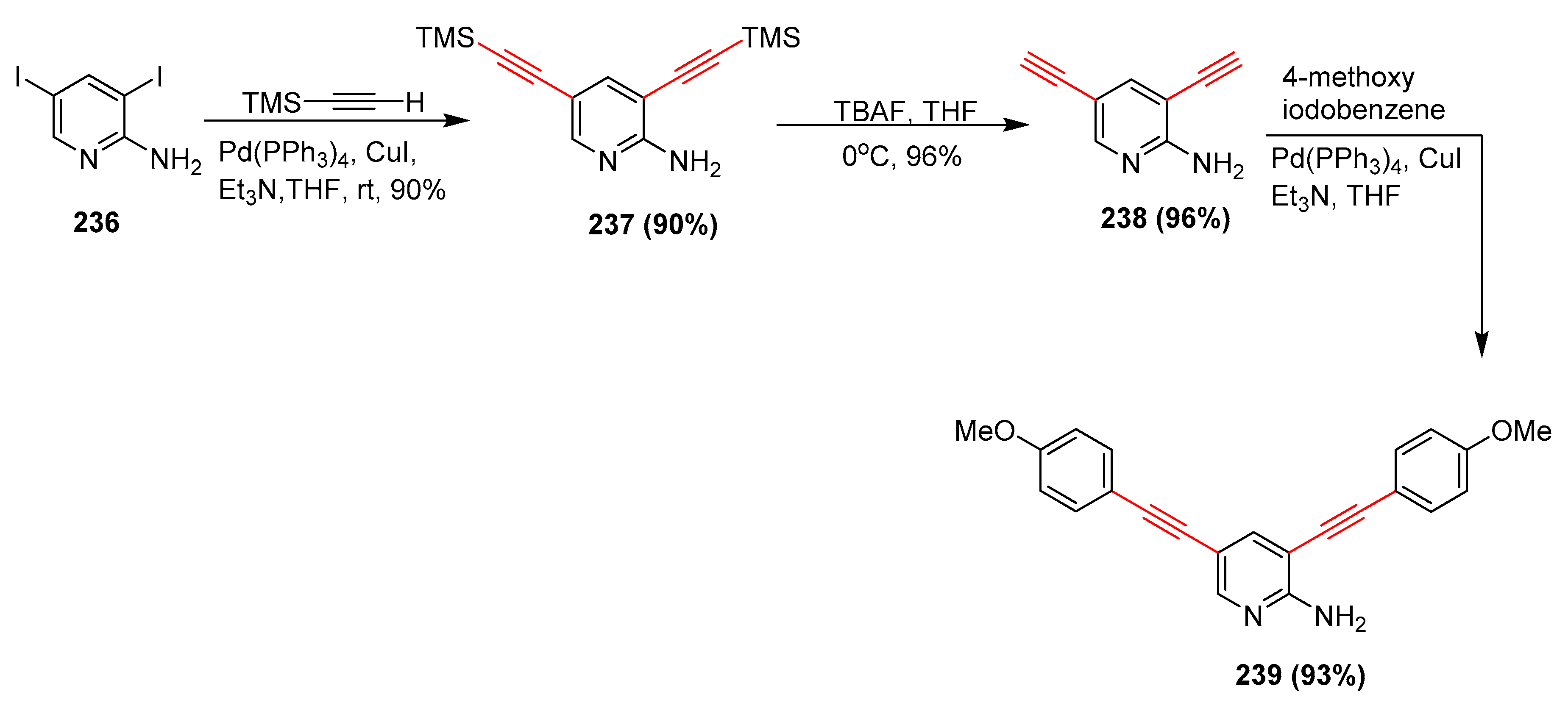
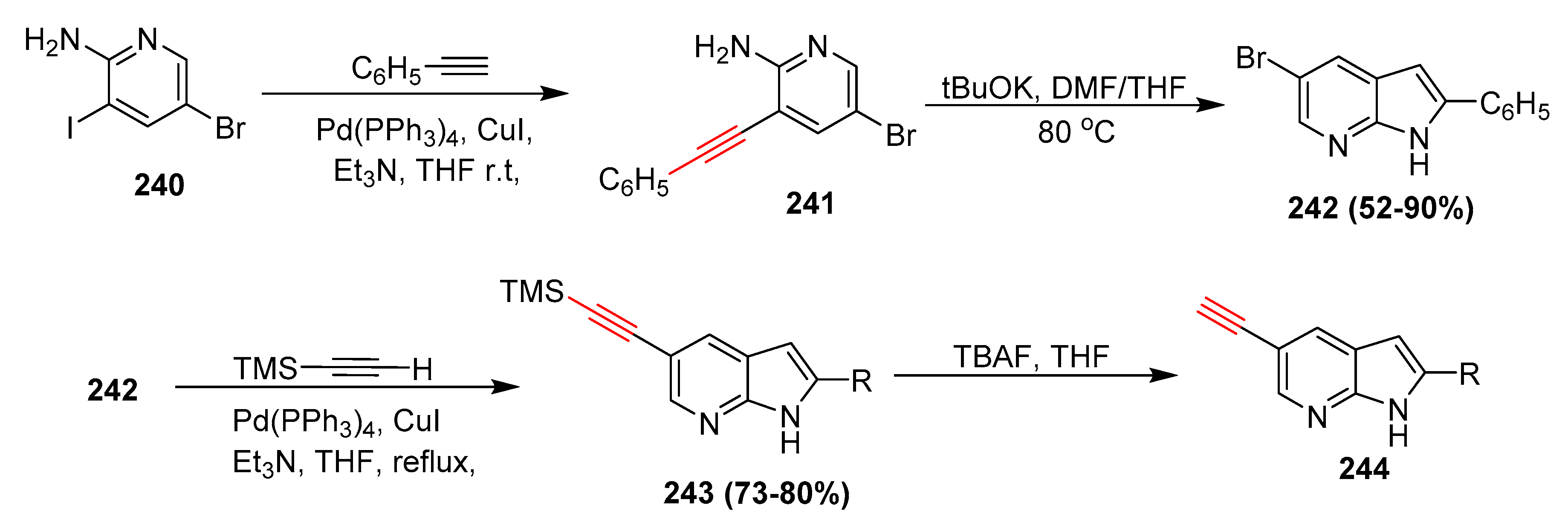
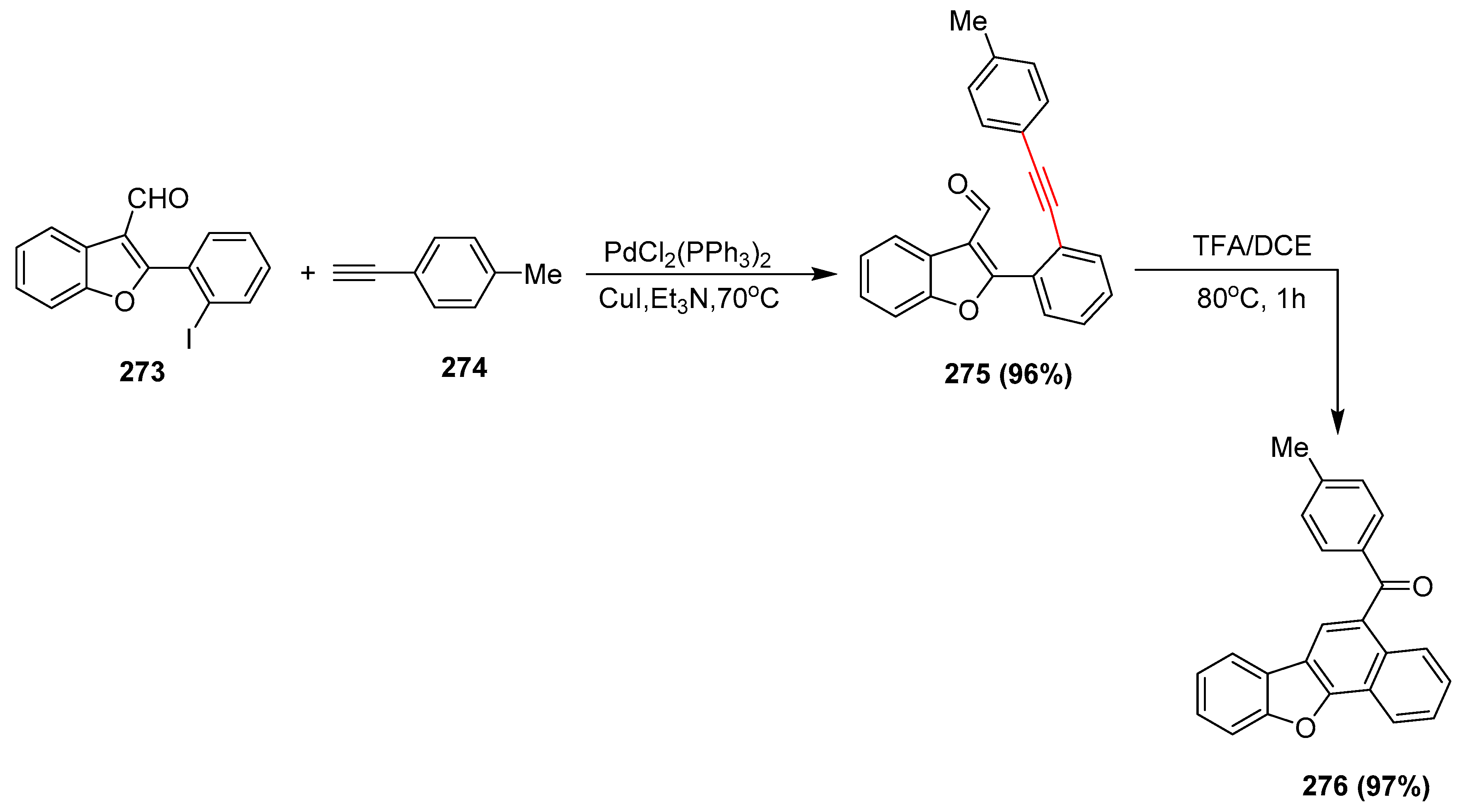
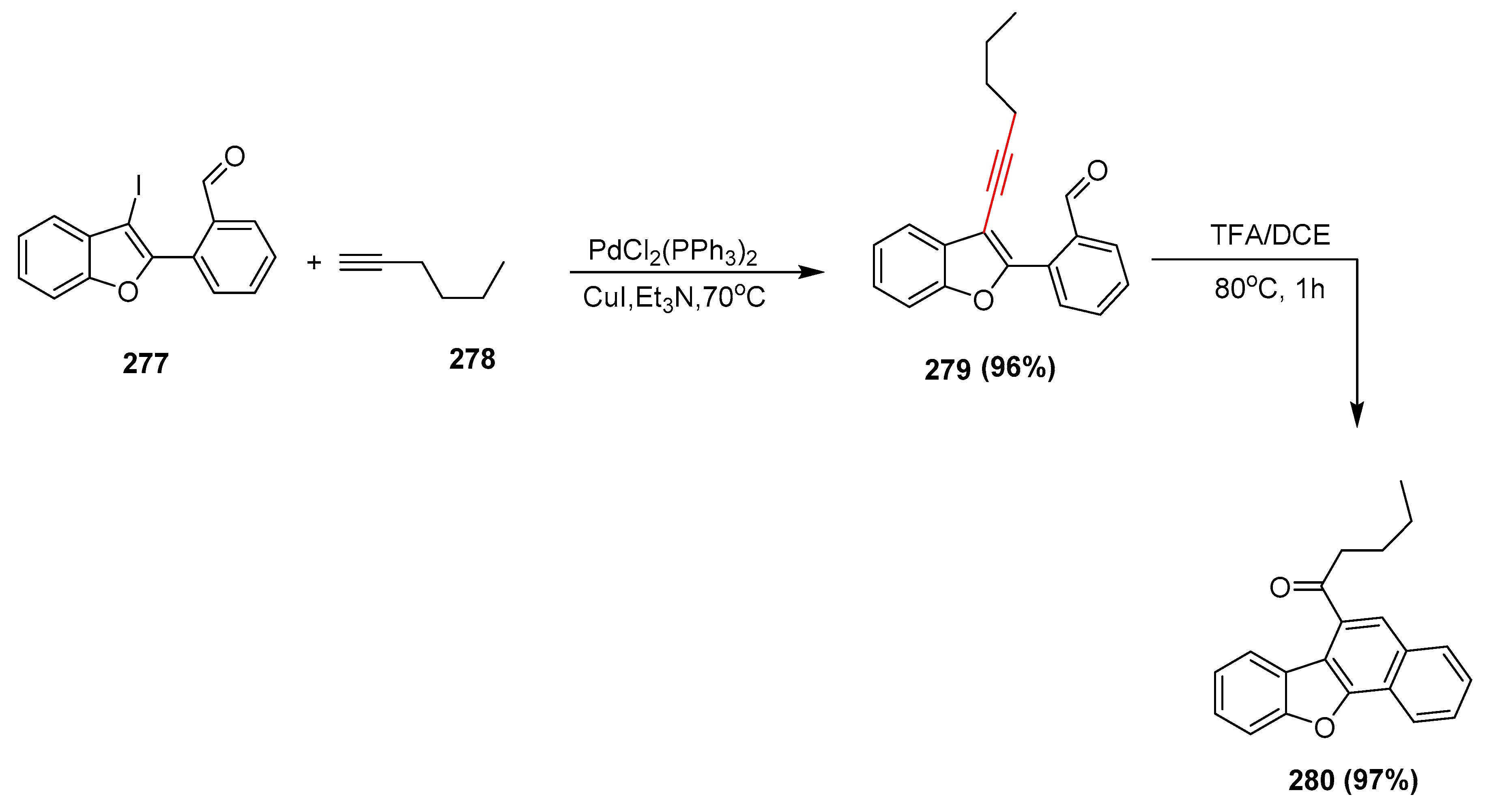
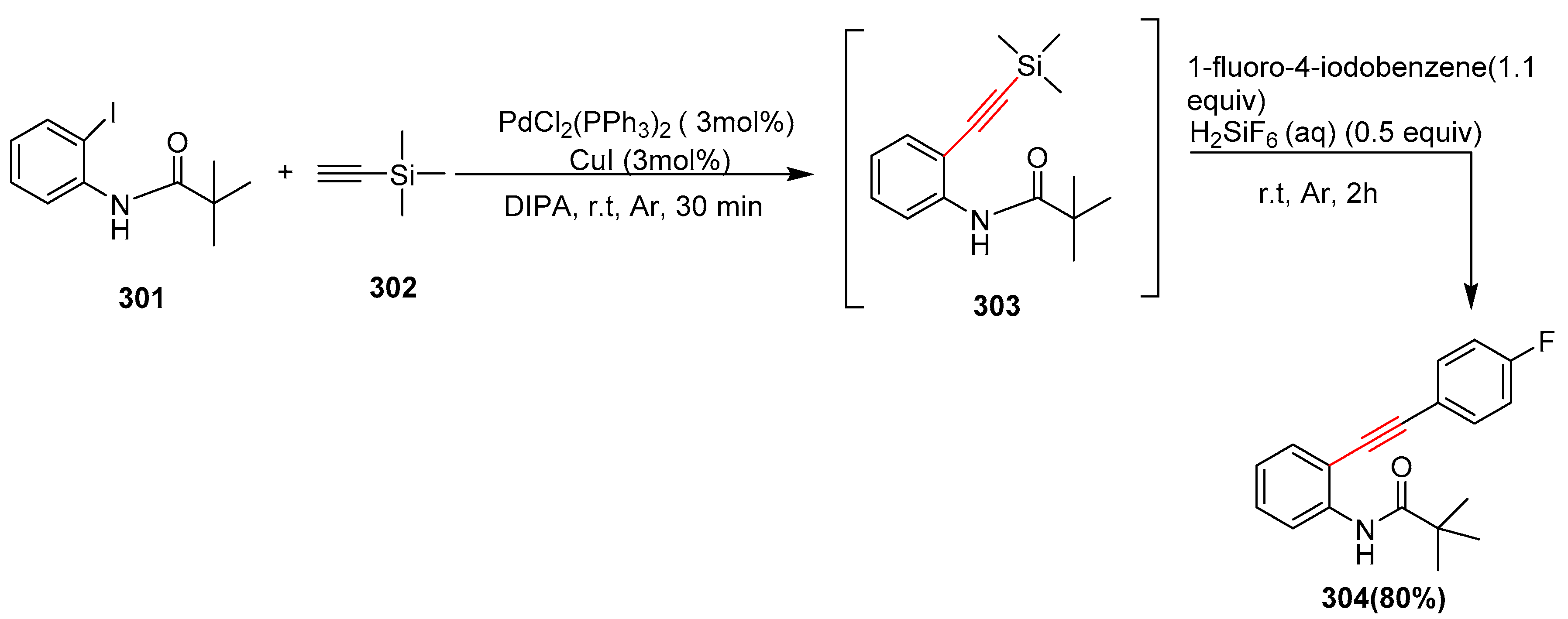
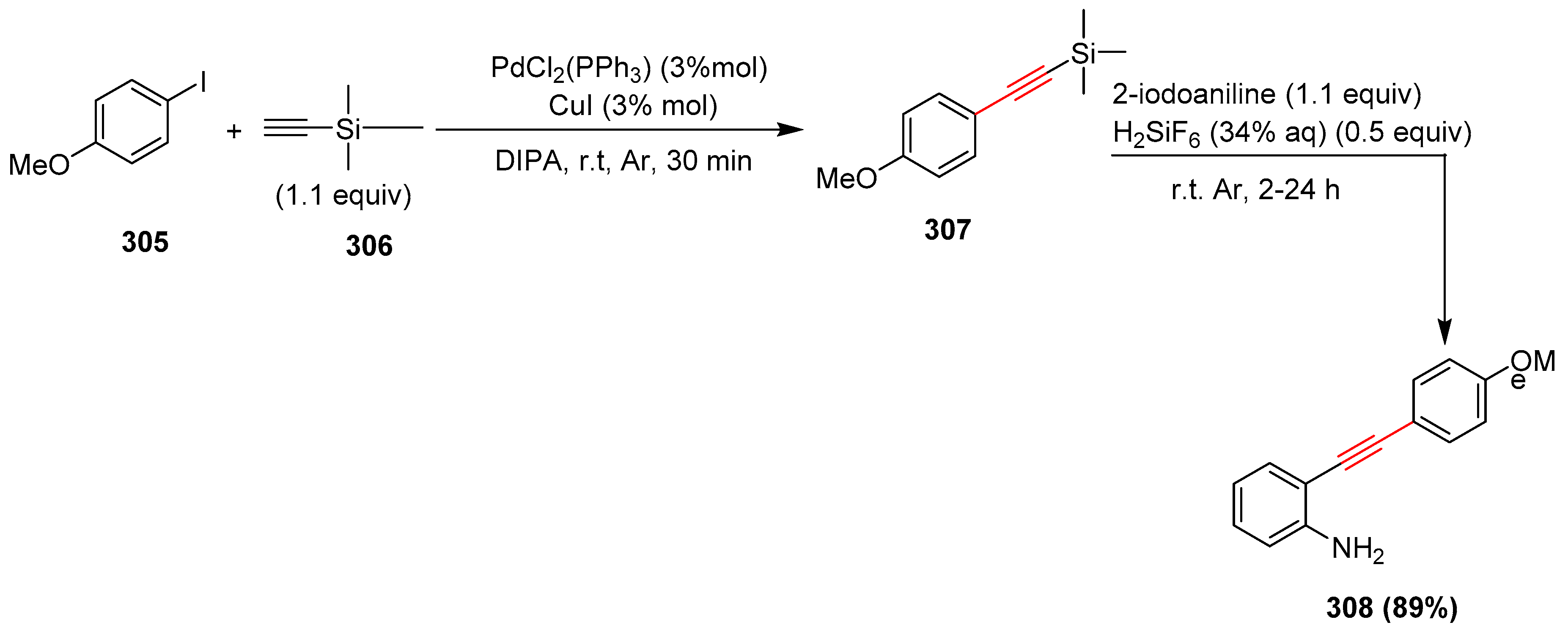
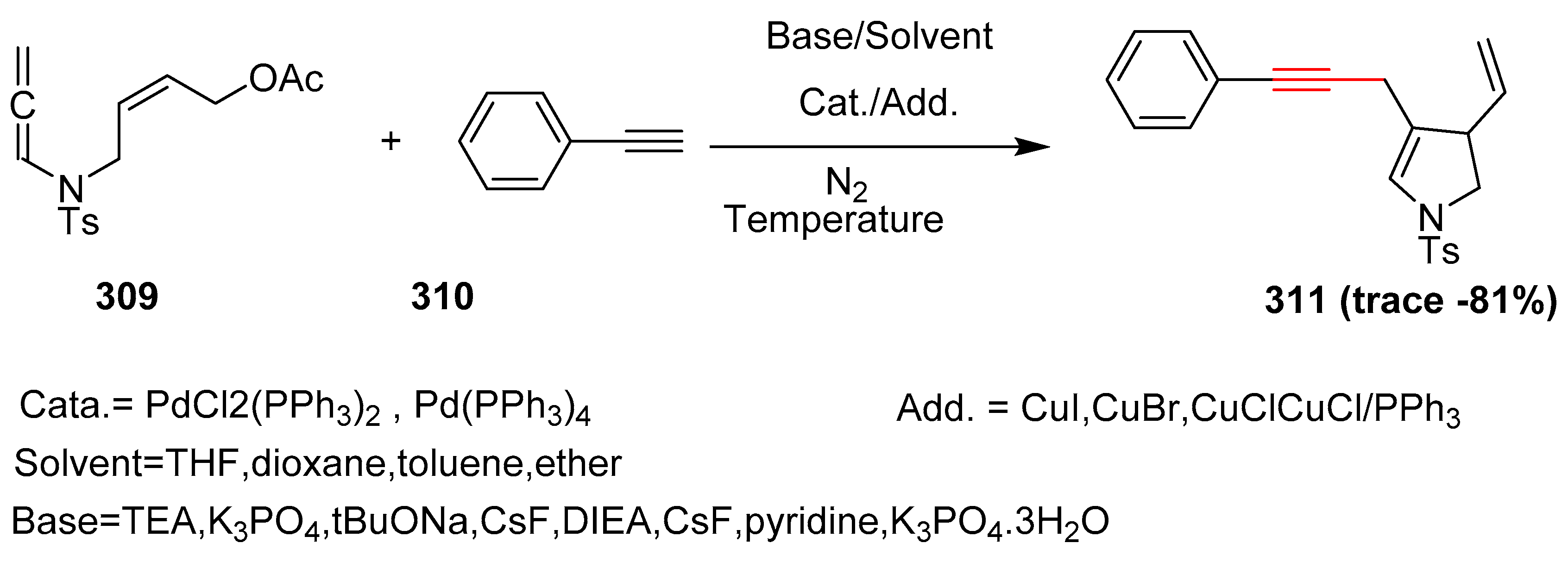
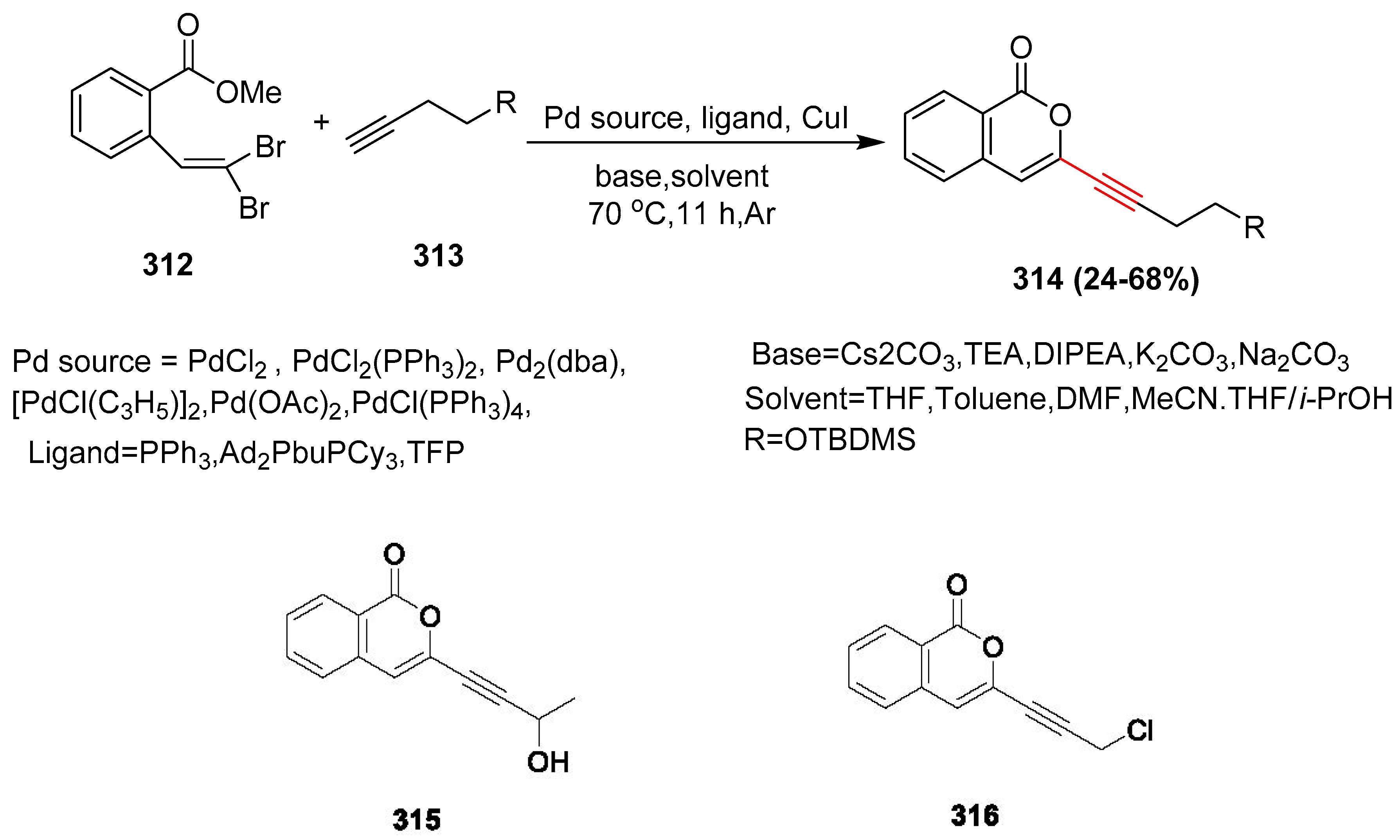
2.17. In 2018
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zapf, A.; Beller, M. Fine chemical synthesis with homogeneous palladium catalysts: Examples, status and trends. Top. Catal. 2002, 19, 101–109. [Google Scholar] [CrossRef]
- Bolm, C.; Beller, M. Transition Metals for Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2004; Volume 1. [Google Scholar]
- Brandsma, L. Synthesis of Acetylenes, Allenes and Cumulenes; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Meijere, A.D.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Tykwinski, R.R. Evolution in the Palladium-catalyzed cross-coupling of sp-and sp2-hybridized carbon atoms. Angew. Chem. Int. Ed. 2003, 42, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Cassar, L. Synthesis of aryl-and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J. Organomet. Chem. 1975, 93, 253–257. [Google Scholar] [CrossRef]
- Dieck, H.; Heck, F. Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene derivatives. J. Organomet. Chem. 1975, 93, 259–263. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Thorand, S.; Krause, N. Improved procedures for the palladium-catalyzed coupling of terminal alkynes with aryl bromides (Sonogashira coupling). J. Org. Chem. 1998, 63, 8551–8553. [Google Scholar] [CrossRef]
- Dieck, H.A.; Heck, F.R. Improved procedures for the palladium-catalyzed coupling of terminal alkynes with aryl bromides (Sonogashira coupling). J. Organomet. Chem. 1975, 93, 259–263. [Google Scholar] [CrossRef]
- Sonogashira, K. Metal-Catalyzed Cross-Coupling Reactions; Diederich, F., Stang, P.J., Eds.; Wiley-VCH: New York, NY, USA, 1998; pp. 203–229. [Google Scholar]
- Abarbri, M.; Thibonnet, J.; Parrain, J.L.; Duchêne, A. Stereocontrolled synthesis of polyenoic acids by a Heck-Sonogashira reaction: Easy access to 9, 10-didehydro retinoic acids. Tetrahedron Lett. 2002, 43, 4703–4705. [Google Scholar] [CrossRef]
- Brimble, M.A.; Pavia, G.S.; Stevenson, R.J. A facile synthesis of aryldihydropyrans using a Sonogashira-selenoetherification strategy. Tetrahedron Lett. 2002, 43, 1735–1738. [Google Scholar] [CrossRef]
- Arnautu, A.; Collot, V.; Ros, J.C.; Alayrac, C.; Witulski, B.; Rault, S. Sonogashira cross-coupling reaction of 3-iodssoindazoles with various terminal alkynes: A mild and flexible strategy to design 2-aza tryptamine. Tetrahedron Lett. 2002, 43, 2695. [Google Scholar] [CrossRef]
- Hudson, R.H.E.; Li, G.; Tse, J. The use of Sonogashira coupling for the synthesis of modified uracil peptide nucleic acid. Tetrahedron Lett. 2002, 43, 1381–1386. [Google Scholar] [CrossRef]
- Shirley, D.A. The use of Sonogashira coupling for the synthesis of modified uracil peptide nucleic acid. Org. React. 1954, 8, 28–58. [Google Scholar]
- Huryn, D.M. Carbanions of alkali and alkaline earth cations: Selectivity of carbonyl addition reactions. Compr. Org. Synth. 1991, 49–75. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Scientific: Oxford, UK, 1997. [Google Scholar]
- Gottardo, C.; Aguirre, A. Palladium-catalyzed carbon-carbon coupling reactions using aryl Grignards. Tetrahedron Lett. 2002, 43, 7091–7094. [Google Scholar] [CrossRef]
- Martin, R.E.; Diederich, F. Linear Monodisperse π-Conjugated Oligomers: Model Compounds for Polymers and More. Angew. Chem. Int. Ed. Engl. 1999, 38, 1350–1377. [Google Scholar] [CrossRef]
- Tour, J.M. Molecular Electronics. Molecular Electronics. Synthesis and Testing of Components. Acc. Chem. Res. 2000, 33, 791–804. [Google Scholar] [CrossRef]
- Kitamura, C.; Saito, K.; Nakagawa, M.; Ouchi, M. Palladium-catalyzed carbon-carbon coupling reactions using aryl Grignards. Tetrahedron Lett. 2002, 43, 3373–3374. [Google Scholar] [CrossRef]
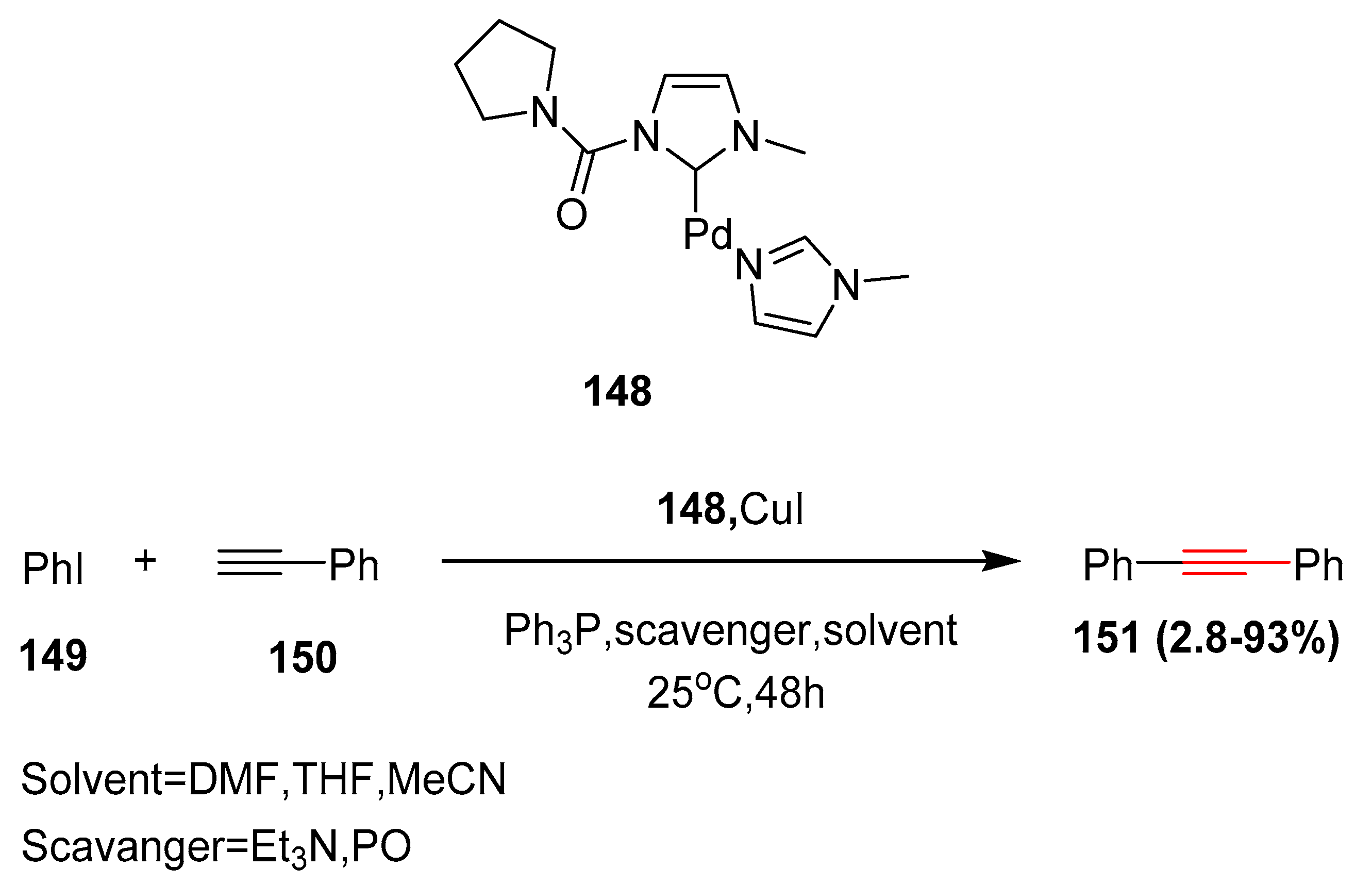
- Batey, R.; Shen, M.; Lough, A.J. Crbamoyl-substituted N-heterocyclic carbene complexes of palladium (II): Application to Sonogashira cross-coupling reactions. Org. Lett. 2002, 4, 1411–1414. [Google Scholar] [CrossRef]
- Obretch, D.; Villargodo, J.M. Solid-Supported Combinatorial and Parallel Synthesis of Small-Molecular-Weight Compound Libraries, 1st ed.; Pergamon: New York, NY, USA, 1998. [Google Scholar]
- Kingsbury, C.L.; Mehrman, S.J.; Takacs, J.M. Combinatorial Chemistry on Solid Supports; Springer: Berlin/Heidelberg, Germany, 1999; p. 497. [Google Scholar]
- Duboc, R. Palladium cross-coupling reactions on solid support using a new silylated linker. J. Organomet. Chem. 2002, 643, 512–515. [Google Scholar] [CrossRef]
- Huang, H.C.; Chamberlain, T.S.; Seibert, K.; Koboldt, C.M.; Isakson, P.C.; Reitz, D.B. Diaryl indenes and benzofurans: Novel classes of potent and selective cyclooxygenase-2 inhibitor. Bioorg. Med. Chem. Lett. 1995, 5, 2377–2380. [Google Scholar] [CrossRef]
- Dai, W.M.; Lai, K.W. Chemistry of aminophenols. Part 3: First synthesis of nitrobenzo[b]furans via a coupling-cyclization approach. Tetrahedron Lett. 2002, 43, 9377–9380. [Google Scholar] [CrossRef]
- Teplý, F.; Stará, I.G.; Starý, I.; Kollárovič, A.; Šaman, D.; Fiedler, P. Transition metal control in the reaction of alkyne-substituted phenyl iodides with terminal alkynes: Sonogashira coupling vs. cyclic carbopalladation. Tetrahedron 2002, 58, 9007–9018. [Google Scholar] [CrossRef]
- Hanna, D.R.; Sherer, E.C.; Davies, R.V.; Titman, R.B.; Laughton, C.A.; Stevens, M.F.G. Structural studies on bioactive compounds Part 29: Palladium catalyzed arylation and alkynylations of sterically hindered immunomodulatory 2-amino-5halo-4,6-(disubstituted)pyrimidines. Bioorg. Med. Chem. 2000, 8, 739–750. [Google Scholar] [CrossRef]
- Khan, S.I.; Grinstaff, M.W. A Facile and convenient solid-phase procedure for synthesizing nucleoside hydroxamic acid. Tetrahedron Lett. 1998, 39, 8031–8034. [Google Scholar] [CrossRef]
- Petricci, E.; Radi, M.; Corelli, F.; Botta, M. Microwave-enhanced Sonogashira coupling reaction of substituted pyrimidinones and pyrimidine nucleosides. Tetrahedron Lett. 2003, 44, 113–116. [Google Scholar] [CrossRef]
- Frank, H.; Heinisch, G. 4 Pharmacologically Active Pyridazine Derivatives. Part 2. Prog. Med. Chem. 1992, 29, 141–183. [Google Scholar]
- Sekhotha, M.M.; Monyeki, K.D.; Sibuyi, M.E. Exposure to agrochemicals and cardiovascular disease: A Review. Int. J. Environ. Res. Public Health 2016, 13, 229. [Google Scholar] [CrossRef]
- Coelho, A.; Sotelo, E.; Ravina, E. Pyridazine derivatives. Part 33: Sonogashira approaches in the synthesis of 5-substituted-6-phenyl-3(2H)-pyridazinones. Tetrahedron 2003, 59, 2477–2484. [Google Scholar] [CrossRef]
- Chanteau, S.H.; Tour, J.M. Synthesis of potential molecular electronic devices containing pyridine units. Tetrahedron Lett. 2001, 42, 3057–3060. [Google Scholar] [CrossRef]
- Takahashi, K.; Kuroyama, Y.; Sonogashira, K.; Hagihara, N. A convenient synthesis of ethynylarenes and diethynylarenes. Synthesis 1980, 1980, 627–630. [Google Scholar] [CrossRef]
- Elangovan, A.; Wang, Y.H.; Ho, T.I. Sonogashira coupling reaction with diminished homocoupling. Org. Lett. 2003, 5, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Petragnani, N.; Stefani, H.A. Advances in organic tellurium chemistry. Tetrahedron 2010, 61, 1613–1679. [Google Scholar] [CrossRef]
- Braga, A.L.; Ludtke, D.S.; Vargas, F.; Donato, R.K.; Ssilveira, C.C.; Stefani, H.A.; Zeni, G. Sonogashira cross-coupling reaction of organotellurium dichlorides with terminal alkynes. Tetrahedron Lett. 2003, 44, 1779–1781. [Google Scholar] [CrossRef]
- Noyori, R. Asymmetric Catalysis in Organic Synthesis; Wiley-Interscience: New York, NY, USA, 1994. [Google Scholar]
- Harada, T.; Matsui, S.I.; Tuyet, T.M.T.; Hatsuda, M.; Ueda, S.; Oku, A.; Shiro, M. Asymmetric synthesis of macrocyclic binaphthol dimers using a Sonogashira coupling reaction. Tetrahedron Asymmetry 2003, 14, 3879–3884. [Google Scholar] [CrossRef]
- Mas-Marza, E.; Segarra, A.M.; Claver, C.; Peris, E.; Fernandez, E. Improved Sonogashira C-C coupling through clay supported palladium complexes with tridentate pincer bis-carbene ligands. Tetrahedron Lett. 2003, 44, 6595–6699. [Google Scholar] [CrossRef]
- Han, J.W.; Castro, J.C.; Burgess, K. Microwave-assisted functionalization of bromo-fluorescein and bromorhodamine derivatives. Tetrahedron Lett. 2003, 44, 9359–9362. [Google Scholar] [CrossRef]
- Park, B.K.; Kitteringham, N.R.O.; Neil, P.M. Metabolism of fluorine-containing drugs. Annu. Rev. Pharmacol. 2001, 41, 443–470. [Google Scholar] [CrossRef]
- Wust, F.R.; Kniess, T. Synthesis of 4-[18F]fluoroiodobenzene and its application in Sonogashira cross-coupling reactions. J. Label. Compd. Radiopharm. 2003, 46, 699–713. [Google Scholar] [CrossRef]
- Kaiser, A.; Billot, X.; Gateau-Olesker, A.; Marazano, C.; Das, B. Selective entry to the dimeric or oligomeric pyridinium sponge macrocycles via aminopentadienal derivatives. Possible biogenetic relevance with Manzamine Alkaloids. J. Am. Chem. Soc. 1998, 120, 8026–8034. [Google Scholar] [CrossRef]
- Hirano, K.; Kubota, T.; Tsuda, M.; Mikami, Y.; Kobayashi, J. Pyrinodemins B-D, potent cytotoxic bis-pyridine alkaloids from marine sponge Amphimedon sp. Chem. Pharm. Bull. 2000, 48, 974–977. [Google Scholar] [CrossRef]
- Krauss, J.; Bracher, F. New total synthesis of niphatesine C and norniphatesine C based on a Sonogashira reaction. Arch. Pharm. 2004, 337, 371–375. [Google Scholar] [CrossRef]
- Gomtsyan, A.; Didomenico, S.; Lee, C.H.; Matulenko, M.A.; Kim, K.; Kowaluk, E.A.; Wismer, C.T.; Mikusa, J.; Yu, H.; Kohlhaas, K.; et al. Design, synthesis and structure-activity relationship of 6-alkynylpyrimidines as potent adenosine kinase inhibitors. J. Med. Chem. 2002, 45, 3639–3648. [Google Scholar] [CrossRef]
- Shen, B.; Liu, W.; Nonaka, K. Enediyne natural products: Biosynthesis and prospect towards engineering novel antitumor agents. Curr. Med. Chem. 2003, 10, 2317–2325. [Google Scholar] [CrossRef]
- Hockova, D.; Holy, A.; Masojidkova, M.; Votruba, I. An efficient synthesis of cytostatic mono and bis-alkynylpyrimidine derivatives by the Sonogashira cross-coupling reactions of 2,4-diamino-6-iodo pyrimidine and 2-amino-4,6-dichloropyrimidine. Tetrahedron 2004, 60, 4983–4987. [Google Scholar] [CrossRef]
- Harvey, S.C.; Foster, K.L.; McKay, P.F.; Carroll, M.R.; Seyoum, R.; Woods, J.E.; Grey, C.; Jones, C.M.; McCane, S.; Cummings, R.; et al. The GABAA receptora1 subtype in the ventral pallidum regulates EtOH-seeking behaviors. J. Neurosci. 2002, 22, 3765–3775. [Google Scholar] [CrossRef]
- Yin, W.; Sarma, P.V.V.S.; Ma, J.; Han, D.; Chen, J.L.; Cook, J.M. Synthesis of bivalent ligands of β-carboline-3-carboxylates via a palladium-catalyzed homocoupling process. Tetrahedron Lett. 2005, 46, 6363–6368. [Google Scholar] [CrossRef]
- Tozer, M.J.; Herpin, T.F. Methods for the synthesis of gem-difluoromethylene compounds. Tetrahedron 1996, 26, 8619–8693. [Google Scholar] [CrossRef]
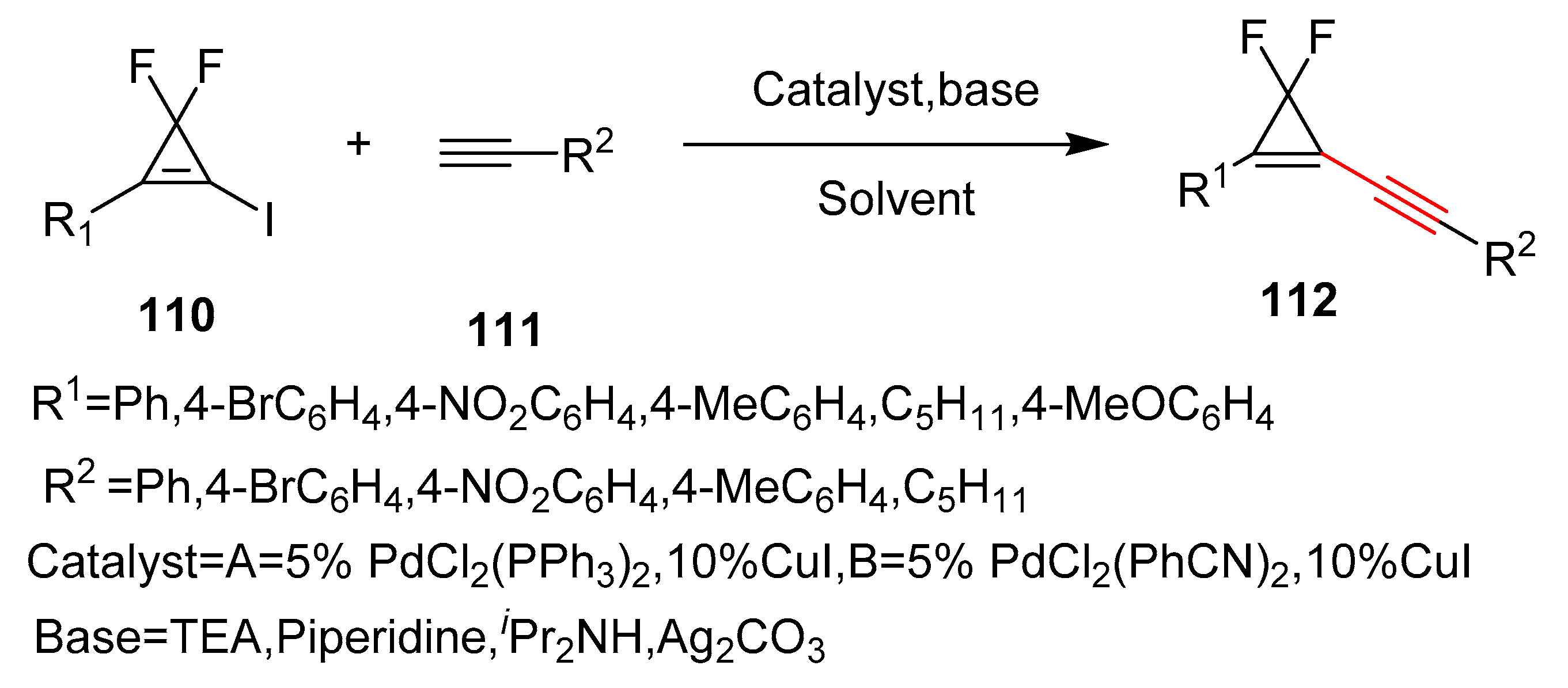
- Cheng, Z.L.; Chen, Q.Y. Difluorocarbene chemistry: Synthesis of gem-difluorocyclopropenylalkynes and 3,3,3′,3′-tetrafluorobicyclopropyl-1,1′-dienes. J. Fluor. Chem. 2005, 126, 93–97. [Google Scholar] [CrossRef]
- Hillier, A.; Grasa, G.; Viciu, M.; Lee, H.; Yang, C.; Nolan, S. Catalytic cross coupling reaction mediated by palladium/nucleophilic carbene system. J. Organomet. Chem. 2002, 653, 69–82. [Google Scholar] [CrossRef]
- Sakamoto, T.; Shiga, F.; Yasuhara, A.; Uchiyama, D.; Kondo, Y.; Yamanaka, H. Preparation of ethyl arylpropiolates from aryl Iodides by palladium-catalyzed cross-coupling reaction. Synthesis 1992, 8, 746–748. [Google Scholar] [CrossRef]
- Lemhadri, M.; Doucet, H.; Santelli, M. Sonogashira reaction of aryl halides with propiolaldehyde diethyl acetal catalyzed by a tetraphosphine/palladium complex. Tetrahedron 2005, 61, 9839–9847. [Google Scholar] [CrossRef]
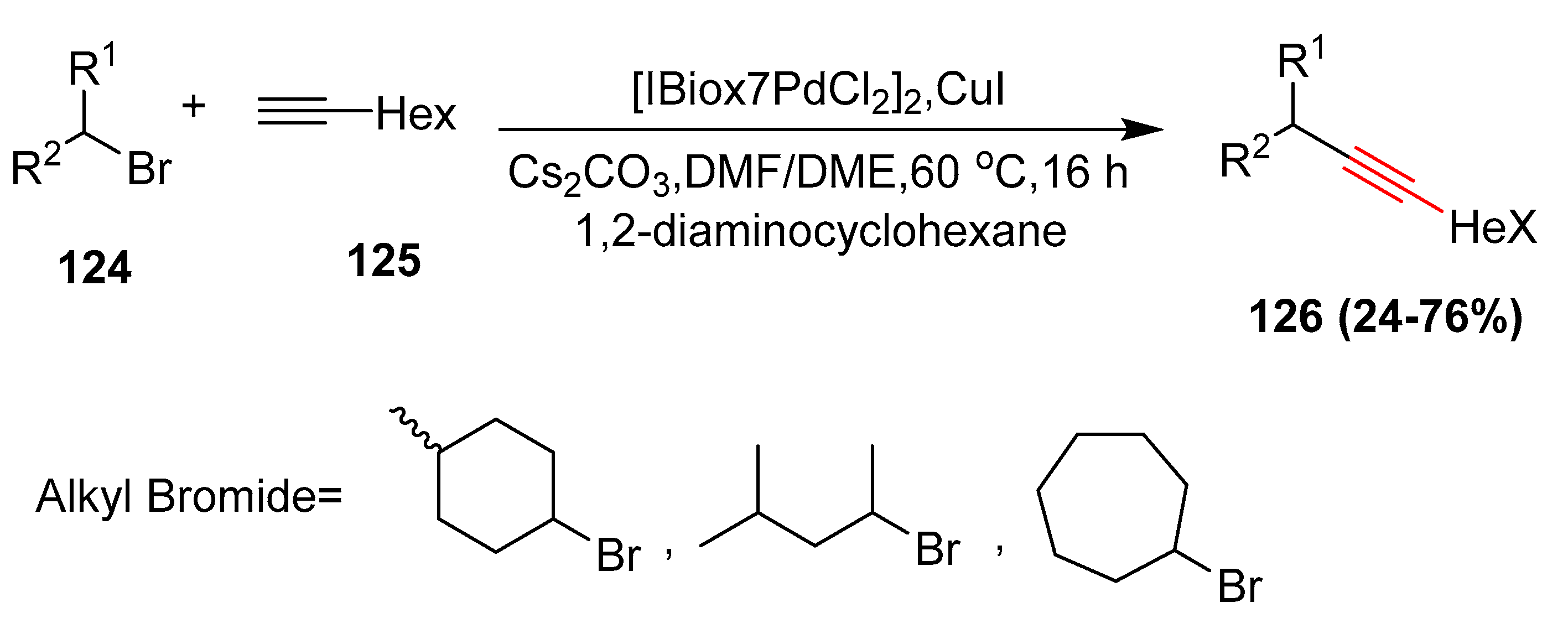
- Altenhoff, G.; Wurtz, S.; Glorius, F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 2006, 47, 2925–2928. [Google Scholar] [CrossRef]
- Shimizu, K.; Koizumi, S.; Hatamachi, T.; Yoshida, H.; Komai, S.; Kodama, T.; Kitayama, Y. Structural investigations of functionalized mesoporous silica-supported palladium catalyst for Heck and Suzuki coupling reactions. J. Catal. 2004, 22, 141–151. [Google Scholar] [CrossRef]
- Cai, M.; Xu, Q.; Wang, P. A novel MCM-41-supported sulfur palladium (0)complex catalyst for Sonogashira coupling reaction. J. Mol. Catal. A Chem. 2006, 250, 199–202. [Google Scholar] [CrossRef]
- Fish, R.H.; Jaouen, G. Structural diversity of organometallic complexes with bioligands and molecular recognition studies of several supramolecular hosts with biomolecules, alkali-metal ions, and organometallic pharmaceuticals. Organometallics 2003, 22, 2166–2177. [Google Scholar] [CrossRef]
- Hoffmanns, U.; Metzler-Nolte, N. Use of the sonogashira coupling reaction for the “Two-Step” labeling of phenylalanine peptide side chains with organometallic compounds. Bio. Conjugate. Chem. 2006, 17, 204–213. [Google Scholar] [CrossRef]
- Hoffmann, H.M.R.; Hartung, I.V. Use of the Sonogashira coupling reaction for the “two-step” labeling of phenylalanine peptide side chains with organometallic compounds. Angew. Chem. Int. Ed. 2004, 116, 1968–1984. [Google Scholar] [CrossRef]
- Hogermeier, J.; Reissig, H.U. Mild and efficient Sonogashira couplings of 8-oxa and 8-thiabicyclo[3.2.1] octanone derived alkenyl nonaflates. Tetrahedron 2007, 63, 8485–8491. [Google Scholar] [CrossRef]
- Konig, B.; Pitsch, W.; Dix, I.; Jones, P.G. Synthesis of macrocyclic enediynes by twofold C-Alkylation. Synthesis 1996, 4, 446–448. [Google Scholar] [CrossRef]
- Bera, R.; Swamy, N.K.; Dhananjaya, G.; Moses Babu, J.; Rajender Kumar, P.; Mukkanti, K.; Pal, M. Pd/C-catalyzed alkynylation of β-chloroacroleins. Tetrahedron 2007, 63, 13018–13023. [Google Scholar] [CrossRef]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic coupling: A powerful tool in molecular construction. Chem. Int. Ed. 2000, 39, 2633–2657. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, L.M.; Zhang, Y.H.; Li, J.H. Palladium-catalyzed cross-coupling reactions of 1,2-diiodoalkenes with terminal alkynes: Selective synthesis of unsymmetrical buta-1,3-diynes and 2-ethynylbenzofurans. Synthesis 2008, 24, 3988–3994. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, P.; Deng, H.; Zhang, L.; Xu, P.F.; Zhai, H. Propylene oxide assisted sonogashira coupling reaction. Synlett 2008, 1, 3239–3241. [Google Scholar]
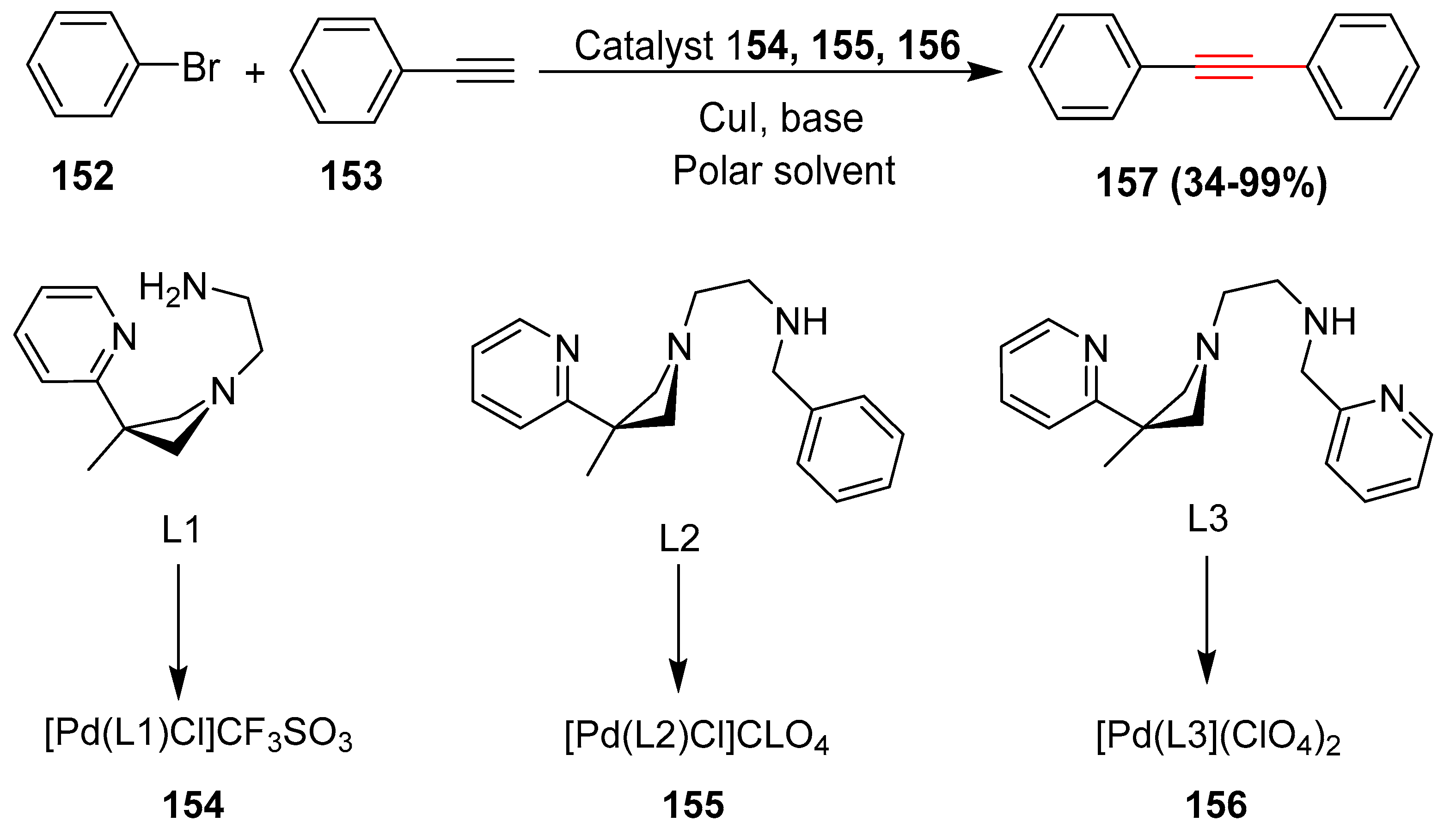
- Lee, D.H.; Lee, Y.H.; Harrowfield, J.M.; Lee, I.M.; Lee, H.I.; Lim, W.T.; Kim, Y.; Jin, M.J. Phosphine-free Sonogashira coupling: Reactions of aryl halides catalysed by palladium(II) complexes of azetidine-derived polyamines under mild conditions. Tetrahedron 2009, 65, 1630–1634. [Google Scholar] [CrossRef]
- Day, S.H.; Chiu, N.Y.; Tsao, L.T.; Wang, J.P.; Lin, C.N. New lignan glycosides with potent antiinflammatory effect, isolated from Justicia ciliata. J. Nat. Prod. 2000, 63, 1560–1562. [Google Scholar] [CrossRef]
- Wellington, K.W.; Qwebani-Ogunleye, T.; Kolesnikova, N.I.; Brady, D.; de Koning, C.B. One-pot laccase-catalysed synthesis of 5,6-dihydroxylatedbenzo[b]furans and catechol derivatives, and their anticancer activity. Arch. Pharm. 2013, 346, 266–277. [Google Scholar] [CrossRef]
- Abdel-motaal, M.; Kandeel, E.M.; Abou-Elzahab, M.; Elghareeb, F. Synthesis and evaluation of antioxidant activity of some new heterocyclic compounds bearing the Benzo[b]furan moiety. Eur. Sci. J. 2017, 13, 297–313. [Google Scholar] [CrossRef]
- Abdelhafez, O.M.; Abedelatif, N.A.; Badria, F.A. DNA binding, antiviral activities and cytotoxicity of new furochromone and Benzofuran derivatives. Arch. Pharm. Res. 2011, 34, 1623–1632. [Google Scholar] [CrossRef]
- Hejchman, E.; Ostrowska, K.; Maciejewska, D.; Kossakowski, J.; Courchesne, W.E. Synthesis and antifungal activity of derivatives of 2- and 3-Benzofurancarboxylic acids. J. Pharmacol. Exp. Ther. 2012, 343, 380–388. [Google Scholar] [CrossRef] [PubMed]
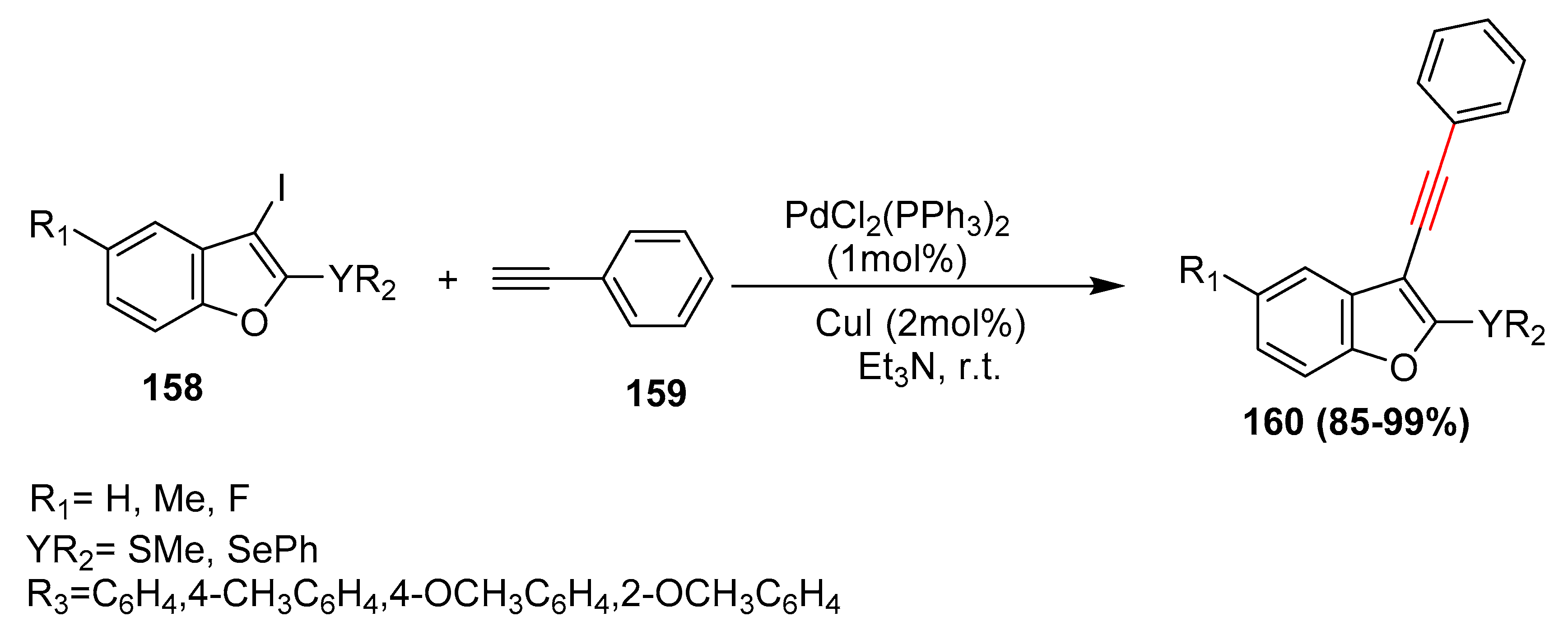
- Manarin, F.; Roehrs, J.A.; Brandao, R.; Nogueira, C.W.; Zeni, G. Synthesis of 3-Alkynyl-2-(methylsulfanyl) benzo[b]furans via Sonogashira Cross-Coupling of 3-Iodo-2-(methylsulfanyl) benzo[b]furans with Terminal Alkynes. Synthesis 2009, 23, 4001–4009. [Google Scholar] [CrossRef]
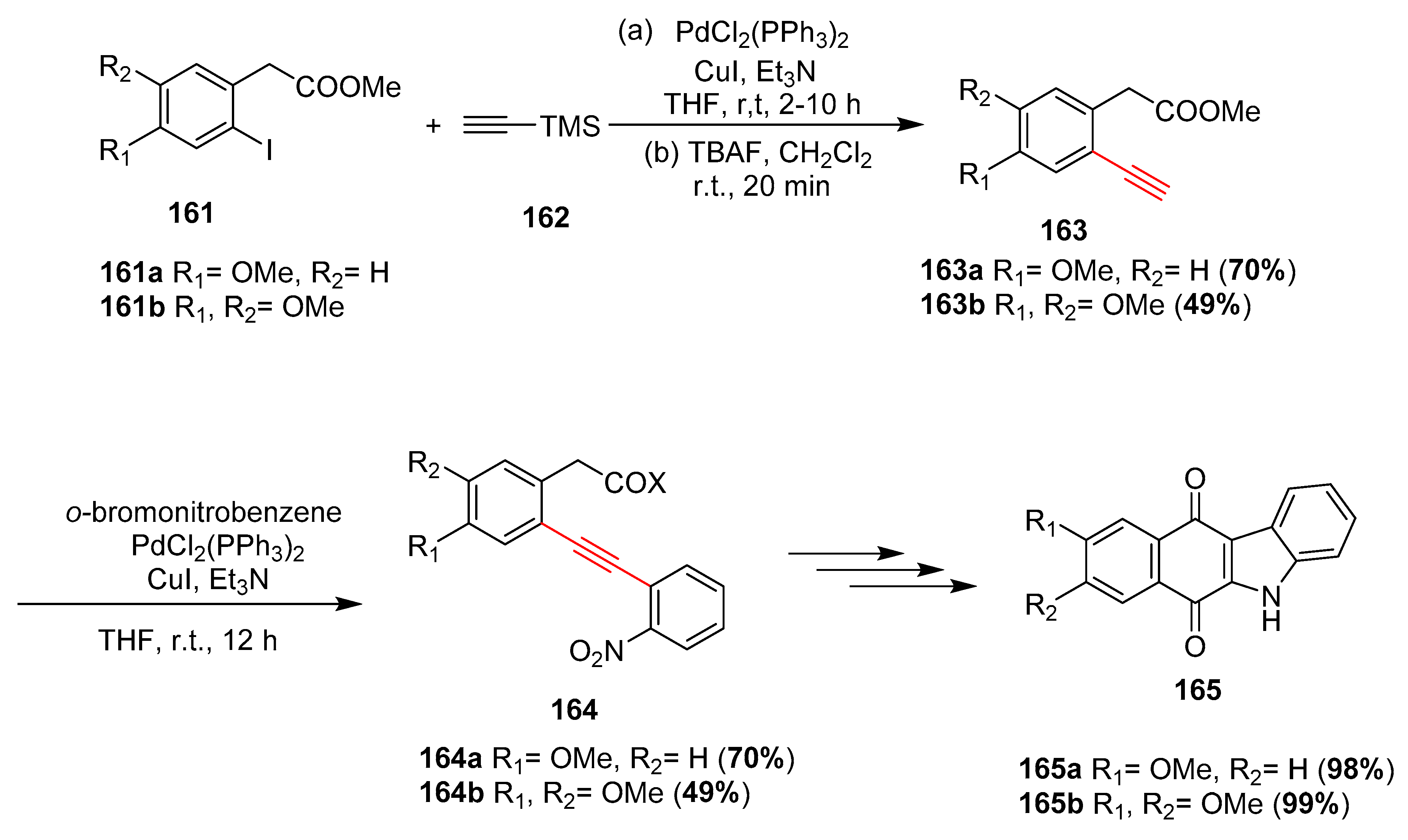
- Salas, C.O.; Reboredo, F.J.; Estévez, J.C.; Tapia, R.A.; Estévez, R.J. Gold-facilitated ‘6-exo-dig’ intramolecular cyclization of 2-[(2-nitrophenyl)ethynyl]phenylacetic acids: General access to 5H-benzo[b]carbazole-6,11-diones. Synlett 2009, 19, 3107–3110. [Google Scholar] [CrossRef]
- Koreca, R.; Senschb, K.H.; Zoukasb, T. Effects of the neoflavonoid coutareagenin, one of the antidiabetic active substances of Hintonia latiflora, on streptozotocin-induced diabetes mellitus in rats. Arzneimittelforschung 2011, 50, 122–128. [Google Scholar] [CrossRef]
- Kang, Y.; Mei, Y.; Du, Y.; Jin, Z. Total synthesis of the highly potent anti-HIV natural product daurichromenic acid along with Its two chromane derivatives, rhododaurichr omanic acids A and B. Org. Lett. 2003, 5, 4481–4484. [Google Scholar] [CrossRef]
- Speranca, A.; Godoi, B.; Souza, A.C.; Zeni, G. 3-Iodo-4-chalcogen-2H-benzopyran as a convenient precursor for the sonogashira cross-coupling: Synthesis of 3-alkynyl-4-chalcogen-2H-benzopyrans. Tetrahedron Lett. 2010, 51, 36–39. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.K.; Mandadapu, A.K.; Gauniyal, H.M.; Kundu, B. Three-component tandem reaction involving acid chlorides, terminal alkynes, and ethyl 2-amino-1H-indole-3-carboxylates: Synthesis of highly diversified pyrimido[1,2-a]indoles via sequential Sonogashira and [3+3] cyclocondensation reactions. Tetrahedron Lett. 2011, 52, 4288–4291. [Google Scholar] [CrossRef]
- Gawande, M.B. Sustainable nanocatalysts for organic synthetic transformations. Org. Chem. Curr. Res. 2014, 3, 100–137. [Google Scholar] [CrossRef]
- Sato, O.; Ikushima, Y.; Yokoyama, T. Noncatalytic Beckmann rearrangement of cyclohexanone-oxime in supercritical water. J. Org. Chem. 1998, 63, 9100–9102. [Google Scholar] [CrossRef]
- Kuhlmann, B.; Arnett, E.M.; Siskin, M. Classical organic reactions in pure superheated water. J. Org. Chem. 1994, 59, 3098–3101. [Google Scholar] [CrossRef]
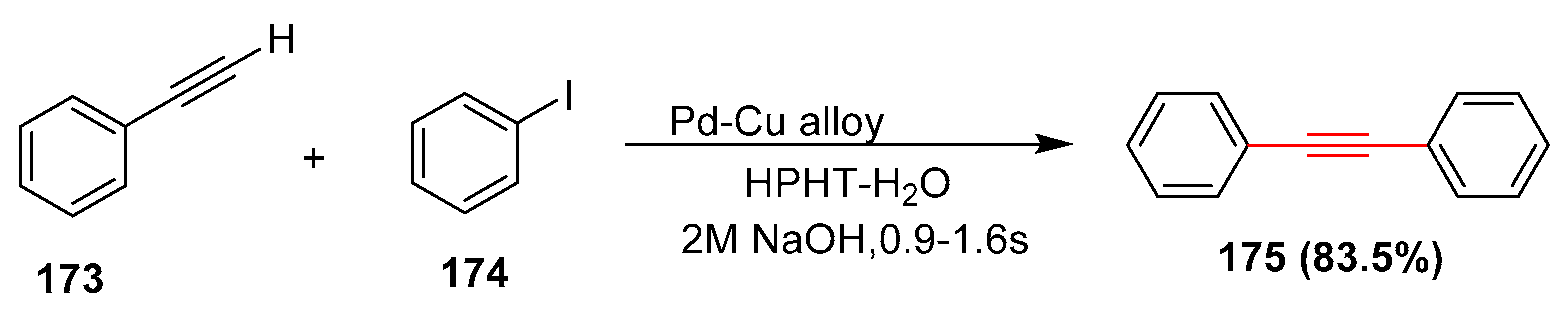
- Javaid, R.; Kawanami, H.; Chatterjee, M.; Ishizaka, T.; Suzuki, A.; Suzuki, T.M. Sonogashira C-C coupling reaction in water using tubular reactors with catalytic metal inner surface. Chem. Eng. J. 2011, 167, 431–435. [Google Scholar] [CrossRef]
- Kawasoko, C.Y.; Nazario, C.E.; Santana, A.S.; Viana, L.H.; Hurtado, G.R.; Marques, F.A.; Baroni, A.C. Hydroalumination of silylacetylenes:A novel and highly stereoselective synthesis of (E)-telluro(silyl)ketene acetals and their applications in Sonogashira cross-coupling reactions. Tetrahedron Lett. 2011, 52, 6067–6071. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Ofele, K.; Preysing, D.V.; Schneider, S.K. Phospha-palladacycles and N-heterocyclic carbene palladium complexes: Efficient catalysts for CC-coupling reactions. J. Organomet. Chem. 2003, 687, 229–248. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Öfele, K.; Elison, M.; Kühn, F.E.; Roesky, P.W. Nucleophilic cyclocarbenes as ligands in metal halides and metal oxides. J. Organomet. Chem. 1994, 480, 7–9. [Google Scholar] [CrossRef]
- Poater, A.; Ragone, F.; Giudice, S.; Costabile, C.; Dorta, R.; Nolan, S.P.; Cavallo, L. Thermodynamics of N-heterocyclic carbene dimerization: The balance of sterics and electronics. Organometallics 2008, 27, 2679–2681. [Google Scholar] [CrossRef]
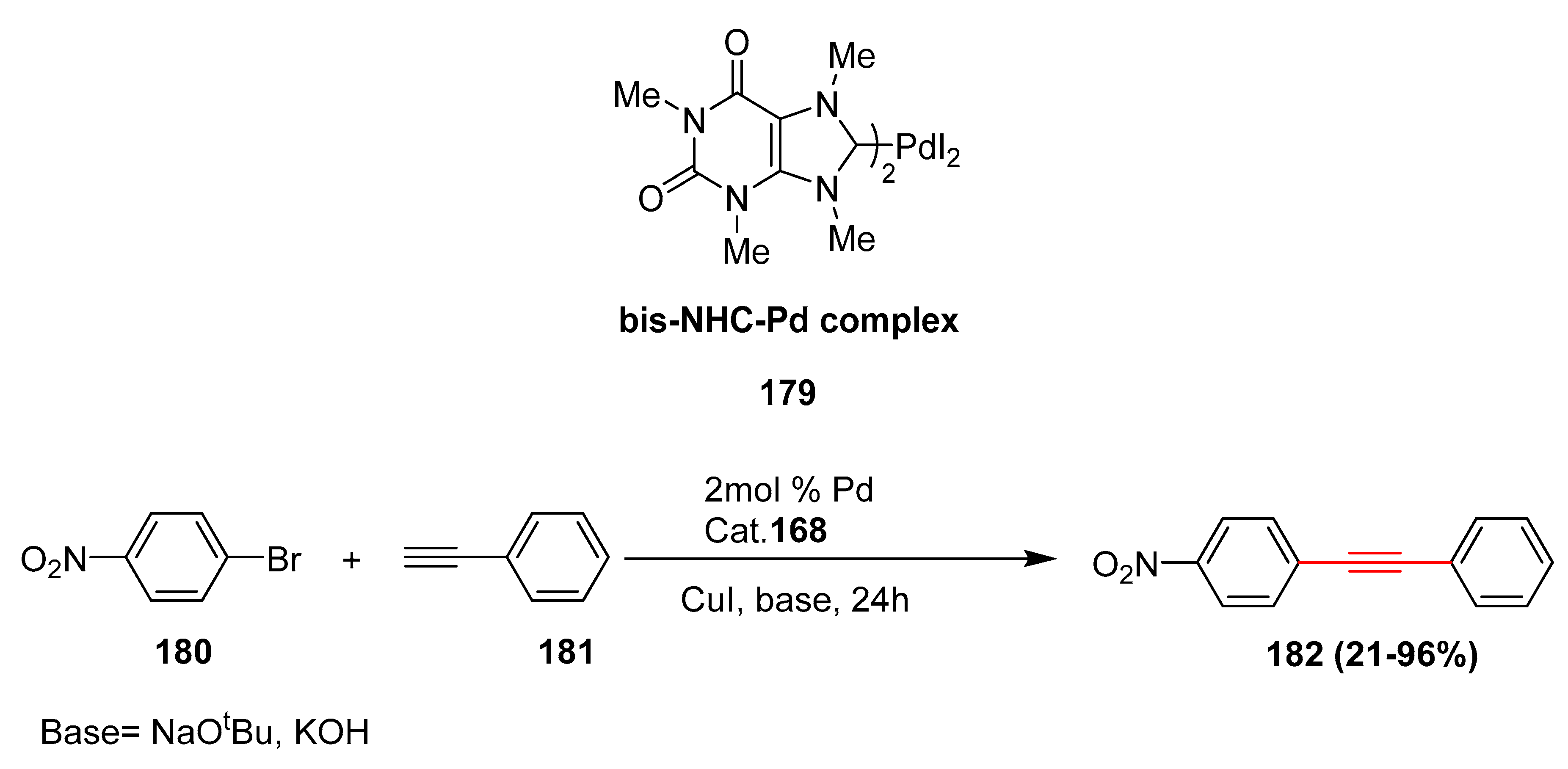
- Luo, F.T.; Lo, H.K. Short synthesis of bis-NHC-Pd catalyst derived from caffeine and its applications to Suzuki, Heck, and Sonogashira reactions in aqueous solution. J. Org. Chem. 2011, 696, 1262–1265. [Google Scholar] [CrossRef]
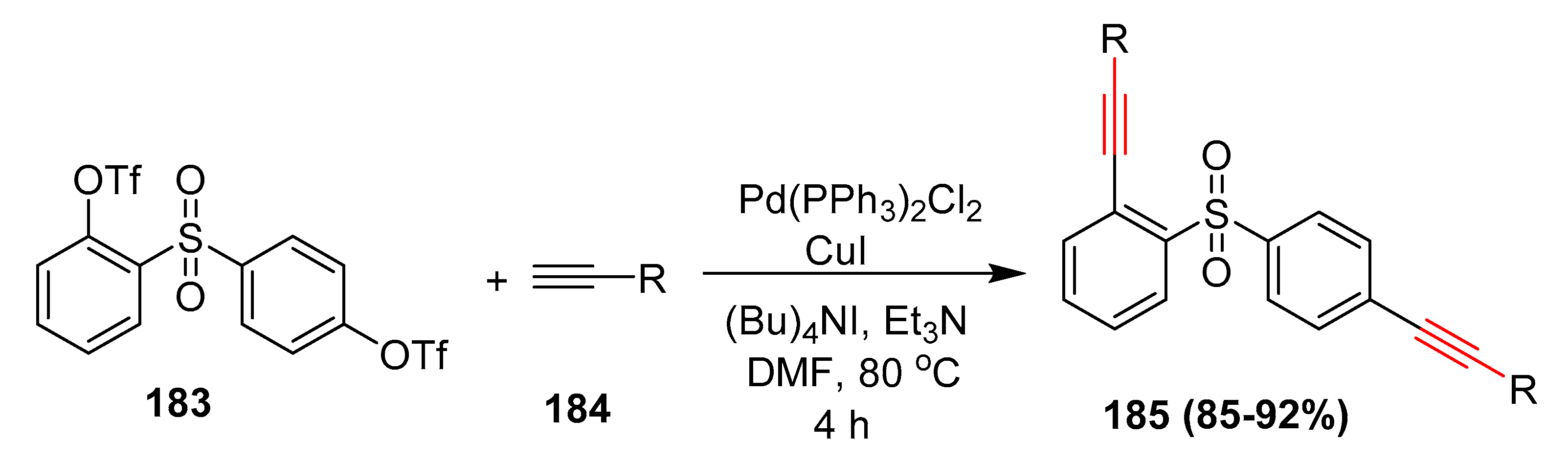
- Khera, R.A.; Ali, A.; Hussain, M.; Ibad, M.F.; Villinger, A.; Langer, P. Site-selective Suzuki-Miyaura and Sonogashira cross coupling reactions of the bis(triflate) of 2,4’-bis(hydroxy)diphenyl sulfone. ChemCatChem 2012, 4, 356–362. [Google Scholar] [CrossRef]
- Shrimali, S.S.; Joshi, B.C.; Kishore, D. Studies on the synthesis and antimicrobial properties of aryl barbituryl sulfones. ChemInform 1989, 20, 438–440. [Google Scholar] [CrossRef]
- Neamati, N.; Mazumder, A.; Zhao, H.; Sunder, S.; Burke, T.R.; Schultz, R.J.; Pommier, Y. Diarylsulfones, a novel class of human immunodeficiency virus type 1 integrase inhibitors. Antimicrob. Agents Chemother. 1997, 41, 385–393. [Google Scholar] [CrossRef]
- Stanton, J.L.; Cahill, E.; Dotson, R.; Tan, J.; Tomaselli, H.C.; Wasvary, J.M.; Steele, R.E. Synthesis and biological activity of phenoxyphenyl oxamic acid derivatives related to L-thyronine. Bioorg. Med. Chem. 2000, 10, 1661–1663. [Google Scholar] [CrossRef]
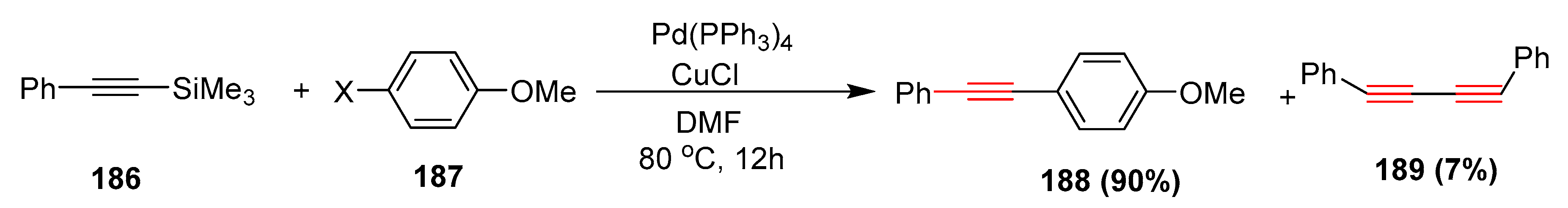
- Nishihara, Y.; Inoue, E.; Noyori, S.; Ogawa, D.; Okada, Y.; Iwasaki, M.; Takagi, K. Synthesis of unsymmetrically disubstituted ethynes by the palladium/copper(I)-cocatalyzed sila-Sonogashira-Hagihara coupling reactions of alkynylsilanes with aryl iodides, bromides and chlorides through a direct activation of a carbon-silicon bond. Tetrahedron 2012, 68, 4869–4881. [Google Scholar] [CrossRef]
- Ahammed, S.; Dey, R.; Ranu, B.C. Palladium and copper catalyzed one-pot Sonogashira reaction of 2-nitroiodobenzenes with aryl acetylenes and subsequent regioselective hydration in water: Synthesis of 2-(2-nitrophenyl)-1-aryl ethanones. Tetrahedron Lett. 2013, 54, 3697–3701. [Google Scholar] [CrossRef]
- Mwaura, J.K.; Zhao, X.; Jiang, H.; Schanze, K.S.; Reynolds, J.R. Spectral broadening in nanocrystalline TiO2 solar cells based on poly(p-phenylene ethynlene) and polythiophene senstitizer. Chem. Matter. 2006, 18, 6109–6111. [Google Scholar] [CrossRef]
- Cremer, J.; Bäuerle, P.; Wienk, M.M.; Janssen, R.A. High open-circuit voltage poly (ethynylene bithienylene): Fullerene solar cells. Chem. Mater. 2006, 18, 5832–5834. [Google Scholar] [CrossRef]
- Li, J.H.; Liang, Y.; Xie, Y.X. Efficient palladium-catalyzed homocoupling reaction and Sonogashira cross-coupling reaction of terminal alkynes under aerobic conditions. J. Org. Chem. 2005, 70, 4393–4396. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-free sonogashira coupling reaction with PdCl2 in water under aerobic conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef]
- Bai, X.; Chen, X.; Barnes, C.; Dias, J.R.; Sandreczki, T.C. Investigation of oxygen-free Sonogashira step growth synthesis of mono-terminated di-tert-butyl-substituted oligo(phenylene ethynylene)s (OPEs). Tetrahedron 2013, 69, 1105–1111. [Google Scholar] [CrossRef]
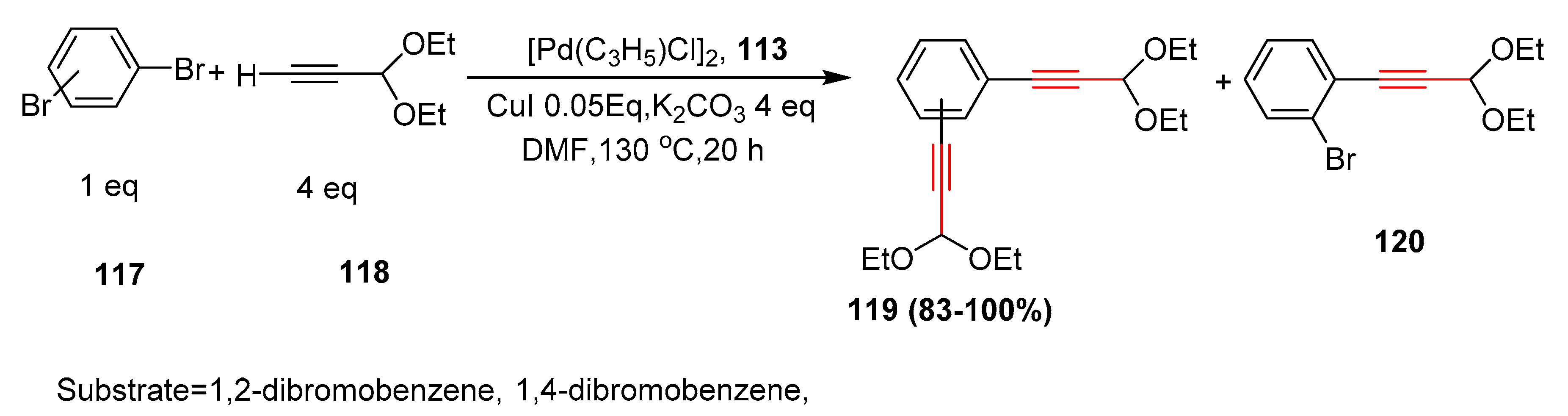
- Laroshenko, V.O.; Ali, S.; Babar, T.M.; Abbasi, M.S.; Sosnovskikh, V.Y.; Villinger, A.; Tolmachev, A.; Langer, P. Efficient synthesis of novel thieno [3,2-b]-,[2,3-c]-and [3,2-c] pyridones by Sonogashira coupling of bromothiophenes with terminal alkynes and subsequent intramolecular C–N bond-forming reaction. Tetrahedron 2013, 69, 3167–3181. [Google Scholar] [CrossRef]
- Korzec, M.; Bartczak, P.; Niemczyk, A.; Szade, J.; Kapkowski, M.; Zenderowska, P.; Polanski, J. Bimetallic nano-Pd/PdO/Cu system as a highly effective catalyst for the Sonogashira reaction. J. Catal. 2014, 313, 1–8. [Google Scholar] [CrossRef]
- Rossy, C.; Majimel, J.; Delapierre, M.T.; Fouquet, E.; Felpin, F.X. Palladium and copper-supported on charcoal: A heterogeneous multi-task catalyst for sequential Sonogashira-Click and Click-Heck reactions. J. Organomet. Chem. 2014, 755, 78–85. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ibrahim, M.B.; Fazal, A.; Suleiman, R.; Fettouhi, M.; El Ali, B. Palladium-bis(oxazoline) complexes with inherent chirality: Synthesis, crystal structures and applications in Suzuki, Heck and Sonogashira coupling reactions. Polyhedron 2014, 70, 39–46. [Google Scholar] [CrossRef]
- Paju, A.; Kanger, T.; Muurisepp, A.M.; Aid, T.; Pehk, T.; Lopp, M. Sonogashira cross-coupling of 3-bromo-1,2-diones: An access to 3-alkynyl-1,2-diones. Tetrahedron 2014, 70, 5843–5848. [Google Scholar] [CrossRef]
- AoReilly, F.; AHabib Jiwan, J.L. A new calix[4]arene-based fluorescent sensor for sodium ion. Chem. Commun. 1999, 795–796. [Google Scholar] [CrossRef]
- Jin, T.; Ichikawa, K.; Koyama, T. A fluorescent calix[4]arene as an intramolecular excimer-forming Na+ sensor in nonaqueous solution. J. Chem. Soc. Chem. Commun. 1992, 499–501. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, F.; Zhang, L.; Luo, L.; Zou, Z.L.; Cao, X.L.; Li, H.B. Synthesis of calix[4]arene derivatives via a Pd-catalyzed Sonogashira reaction and their recognition properties towards phenols. Chin. Chem. Lett. 2014, 25, 226–228. [Google Scholar] [CrossRef]
- Bellina, F.; Lessi, M.; Marianetti, G.; Panattoni, A. Highly regioselective C-5 alkynylation of imidazoles by one-pot sequential bromination and Sonogashira cross coupling. Tetrahedron Lett. 2015, 56, 3855–3857. [Google Scholar] [CrossRef]
- Yamauchi, T.; Shibahara, F.; Murai, T. Facile synthetic method for diverse polyfunctionalized imidazoles by means of pd-catalyzed C-H bond arylation of N -methyl-4,5-dibromoimidazole. J. Org. Chem. 2014, 79, 7185–7192. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, M.; Wu, W.; Huang, H.; Jiang, H. Copper-Catalyzed Intermolecular Oxidative Cyclization of Haloalkynes: Synthesis of 2-Halo-substituted Imidazo[1,2-a]pyridines, Imidazo[1,2-a]pyrazines and Imidazo[1,2-a]pyrimidines. Adv. Synth. Catal. 2013, 355, 2263–2273. [Google Scholar] [CrossRef]
- Leboho, T.C.; Giri, S.; Popova, I.; Cock, I.; Michael, J.P.; de Koning, C.B. Double sonogashira reactions on dihalogenated aminopyridines for the assembly of an array of 7-azaindoles bearing triazole and quinoxaline substituents at C-5: Inhibitory bioactivity against Giardia duodenalis trophozoite. Bioorg. Med. Chem. 2015, 23, 4943–4951. [Google Scholar] [CrossRef]
- Paun, A.; Matache, M.; Enache, F.; Nicolau, I.; Paraschivescu, C.C.; Ionita, P.; Guillaumet, G. Convenient synthesis of 2-alkynylbenzazoles through Sonogashira cross-coupling reaction between thioethers and terminal alkynes. Tetrahedron Lett. 2015, 56, 5349–5352. [Google Scholar] [CrossRef]
- Paterson, I.; Davies, R.D.; Marquez, R. Total synthesis of the callipeltoside aglycon. Angew. Chem. Int. Ed. 2001, 40, 603–607. [Google Scholar] [CrossRef]
- Toyota, M.; Komori, C.; Ihara, M.A. Concise formal total synthesis of mappicine and nothapodytine B via an intramolecular hetero Diels-Alder reaction. J. Org. Chem. 2000, 65, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, L.; Umar, M.; Awut, T.; Mi, H.; Tan, C.; Nurulla, I. Novel poly(arylene ethynylene) derivatives containing main chain triphenylamine and pendent quinoxaline moieties: Synthesis and elementary characterization. Polym. Int. 2009, 58, 800–8006. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Zhen, H.; Zhu, D.; Awut, T.; Mi, H.; Nurulla, I. Novel luminescent polymers containing backbone triphenylamine groups and pendant quinoxaline groups. Dye. Pigment. 2009, 83, 102–110. [Google Scholar] [CrossRef]
- Plater, M.J.; Jackson, T. Polyaromatic amines. Part 4: Synthesis of poly (ethynyl) linked aromatic amines. Tetrahedron 2003, 59, 4687–4692. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Akbarzadeh, Z. Sonochemically synthesis of arylethynyl linked triarylamines catalyzed by CuI nanoparticles: A rapid and green procedure for Sonogashira coupling. Ultrason. Sonochem. 2015, 22, 365–370. [Google Scholar] [CrossRef]
- Wieczorek, B.; Dijkstra, H.P.; Egmond, M.R.; Gebbink, R.J.K.; van Koten, G. Incorporating ECE-pincer metal complexes as functional building blocks in semisynthetic metalloenzymes, supramolecular polypeptide hybrids, tamoxifen derivatives, biomarkers and sensors. J. Organomet. Chem. 2009, 694, 812–822. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, P.; Hu, W.P.; Wang, H.X. Substituent effect of diimino-palladium (II) pincer complexes on the catalysis of Sonogashira coupling reaction. Polyhedron 2015, 96, 107–112. [Google Scholar] [CrossRef]
- Chistov, A.A.; Ivanov, N.M.; Kutyakov, S.V.; Ustinov, A.; Glybin, A.V.; Streshnev, P.P.; Korshun, V.A. Fluorescent nucleosides with an elongated rigid linker: Attaching perylene to a nucleobase via a one-pot desilylation/Sonogashira reaction. Tetrahedron Lett. 2016, 57, 4821–4823. [Google Scholar] [CrossRef]
- Filatova, E.A.; Gulevskaya, A.V.; Pozharskii, A.F.; Ozeryanskii, V.A. Synthesis and some properties of alkynyl derivatives of 1,3-dialkylperimidones. an example of the 1,2-palladium migration in the Sonogashira reaction. Tetrahedron 2016, 72, 1547–1557. [Google Scholar] [CrossRef]
- Gholinejad, M.; Jeddi, N.; Pullithadathil, B. Agarose functionalized phosphorus ligand for stabilization of small-sized palladium and copper nanoparticles: Efficient heterogeneous catalyst for Sonogashira reaction. Tetrahedron 2016, 72, 2491–2500. [Google Scholar] [CrossRef]
- Gong, J.X.; Wang, H.Y.; Yao, L.G.; Li, X.W.; Guo, Y.W. First total synthesis of the marine natural brominated polyunsaturated lipid xestospongenyne as a potent pancreatic lipase inhibitory agent. Synlett 2016, 27, 391–394. [Google Scholar]
- Nayak, M.; Singh, D.K.; Kim, I. Regiospecific synthesis of 5- and 6-acylated naphtho[1,2-b]benzofurans via intramolecular alkyne carbonyl metathesis. Synthesis 2016, 49, 2063–2073. [Google Scholar]
- Paun, A.; Paraschivescu, C.C.; Matache, M.; Parvulescu, V.I. Synthesis of new alkynyl-bridged 2,5-disubstituted 1,3,4-oxadiazoles. Synthesis 2016, 48, 606–614. [Google Scholar]
- Liu, X.T.; Ding, Z.C.; Ju, L.C.; Tang, Z.N.; Wu, F.; Zhan, Z.P. Iron(III) chloride catalyzed nucleophilic substitution of tertiary propargylic alcohols and synthesis of Iodo-3H-pyrazoles. Synlett 2017, 28, 620–624. [Google Scholar] [CrossRef]
- Nayak, S.; Prabagar, B.; Ghosh, N.; Mallick, R.K.; Sahoo, A.K. Ag(I)-catalyzed cycloisomerization and cyclization of ketene aminals: Construction of azepine and 1,2-dihydropyridine derivatives. Synthesis 2017, 49, 4261–4271. [Google Scholar]
- Chittimalla, S.K.; Koodalingam, M.; Gadi, V.K.; Anaspure, P. α-Halogenation as a strategy to functionalize cyclohexa-2,4-dienones. Synlett 2017, 13, 475–480. [Google Scholar] [CrossRef][Green Version]
- Wang, C.C.; Ku, Y.C.; Chuang, G.J. Photoinduced decarbonylative rearrangement of Bicyclo[2.2.2] octenones: Synthesis of the marasmane skeleton. J. Org. Chem. 2015, 80. [Google Scholar] [CrossRef]
- Tsao, K.W.; Devendar, B.; Liao, C.C. Ring rearrangement metathesis of 2-allylbicyclo[2.2.2]octenes: A short entry to cis-hydrindenols from 2-methoxyphenols. Tetrahedron Lett. 2013, 54, 3055–3059. [Google Scholar] [CrossRef]
- Rode, N.D.; Arcadi, A.; Chiarini, M.; Marinelli, F. An improved environmentally friendly approach to 4-Nitro-, 4-Sulfonyl-, and 4-Aminoquinolines and 4-Quinolones through conjugate addition of nucleophiles to β-(2-Aminophenyl)-α,β-ynones. Synthesis 2017, 49, 2501–2512. [Google Scholar] [CrossRef]
- Sinai, A.; Meszaros, A.; Balogh, A.; Zwillinger, M.; Novak, Z. Hexafluorosilicic acid as a novel reagent for the desilylation of silylacetylenes: Application in sequential Sonogashira coupling and Click reaction. Synthesis 2017, 49, 2374–2388. [Google Scholar] [CrossRef]
- Juse, J.; March, A. The aromatic pathways of porphins, chlorins and bacteriochlorins. Phys. Chem. 2000, 2, 2145–2151. [Google Scholar]
- Kaur, M.; Choi, D.H. Diketopyrrolopyrrole: Brilliant red pigment dye-based fluorescent probes and their applications. Chem. Soc. Rev. 2014, 44, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Meng, L.; Chi, X.; Yao, S.; Chen, H.; Jiao, L. Palladium / Copper co-catalyzed cascade metallo-ene / Sonogashira coupling reaction of Allenamides. Asian. J. Org. Chem. 2018, 2, 1793–1796. [Google Scholar]
- Ashraf, Z. Metal-catalyzed synthesis of isocoumarin derivatives. Chem. Heterocycl. Compd. 2016, 52, 149–151. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Zhou, Y.; Liu, P. Palladium/Copper-catalyzed tandem Sonogashira coupling/lactonezation of methyl 2-(2’,2’-dibromovinyl) benzoate with terminal alkynes: Facile access to 3-alkynyl isocoumarins. Tetrahedron Lett. 2018, 59, 3151–3154. [Google Scholar] [CrossRef]
- Viciu, M.S.; Kelly, R.A.; Stevens, E.D.; Naud, F.; Studer, M.; Nolan, S.P. Synthesis, characterization and catalytic activity of N-heterocyclic carbene (NHC) palladacycle complexes. Org. Lett. 2003, 5, 1479–1482. [Google Scholar] [CrossRef]
- Shen, A.; Wu, Z.; Fang, Y.; Yang, J.; Zhu, H.; Tu, T. A collaborative catalytic system for Sonogashira couplings: Combination of N-Heterocyclic carbene palladium and copper complexes. Asian. J. Org. Chem. 2018, 7, 1113–1117. [Google Scholar] [CrossRef]



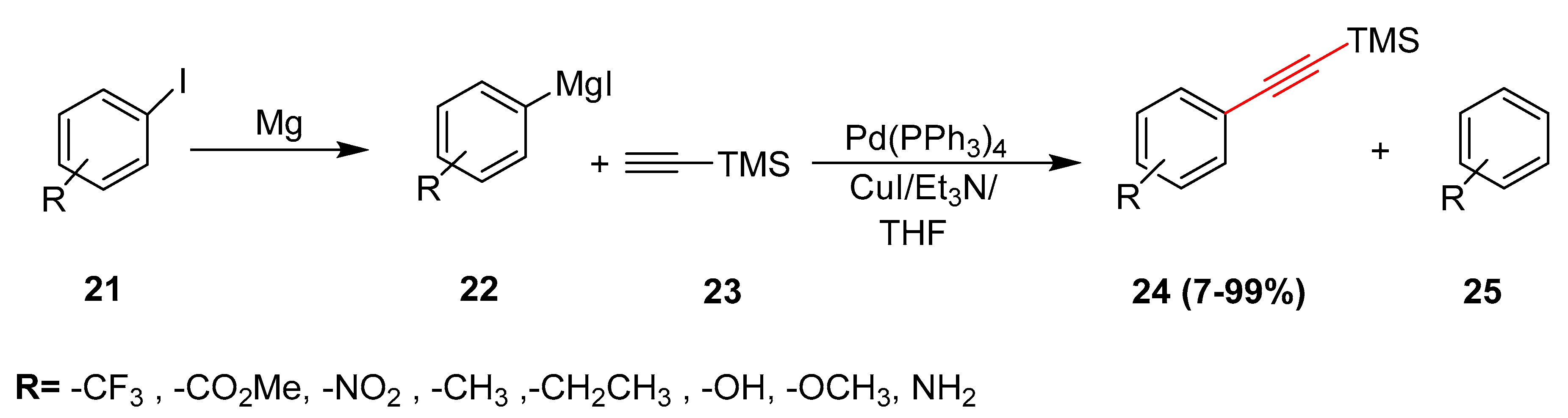
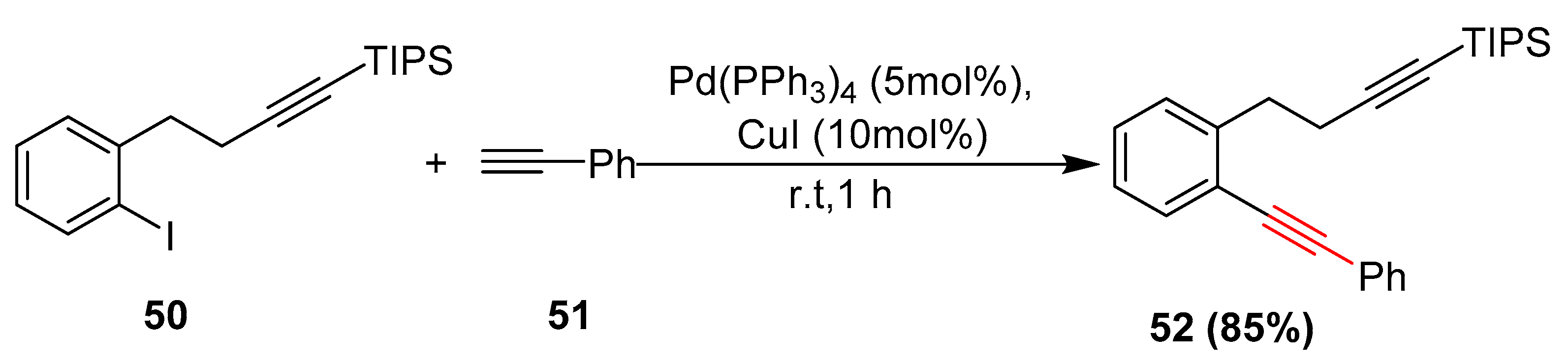




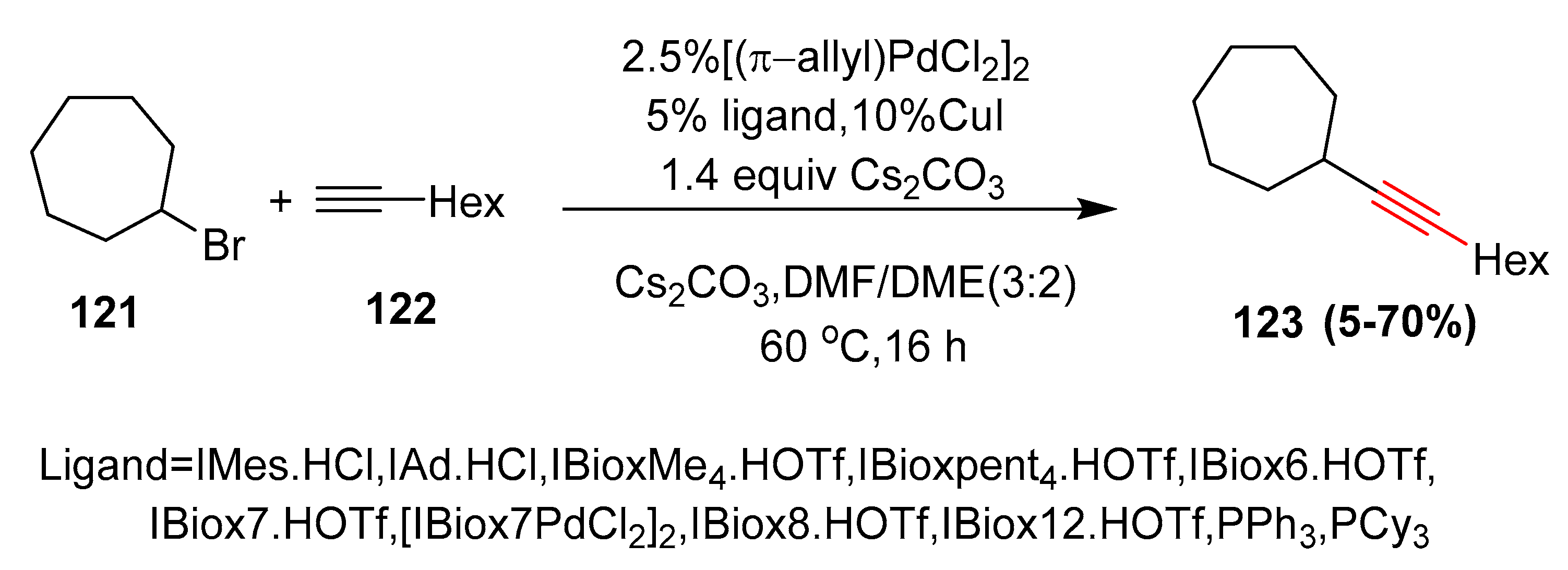

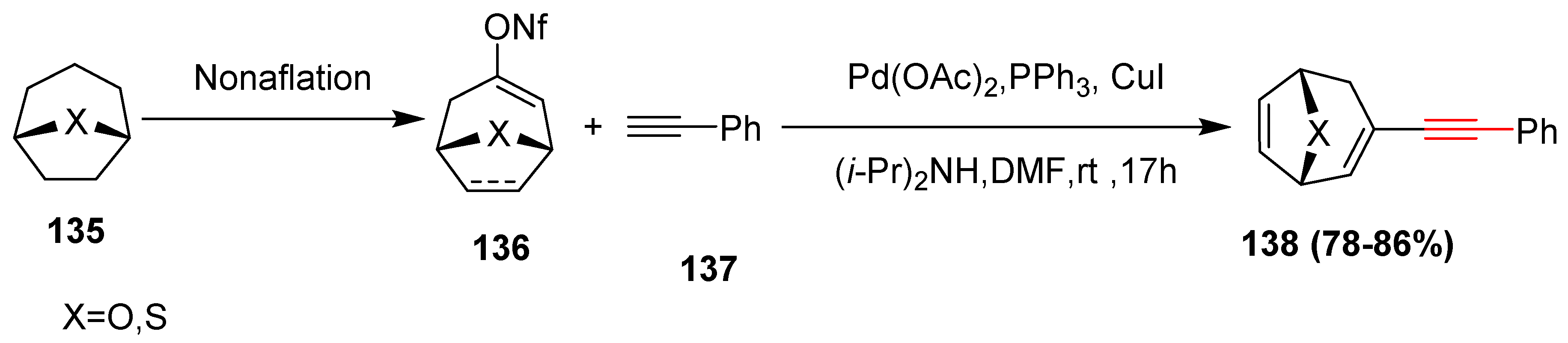
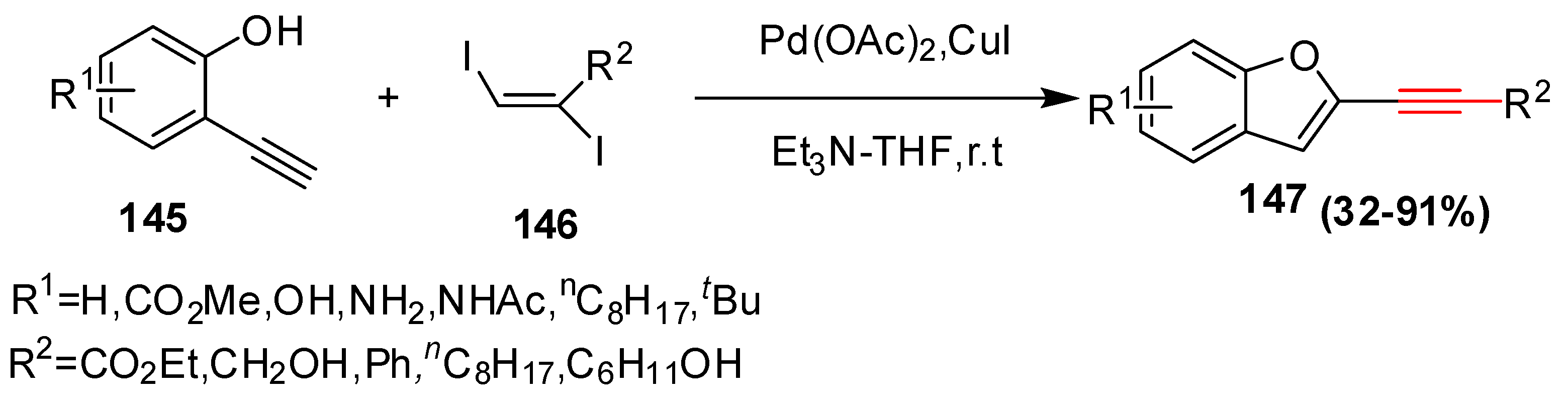
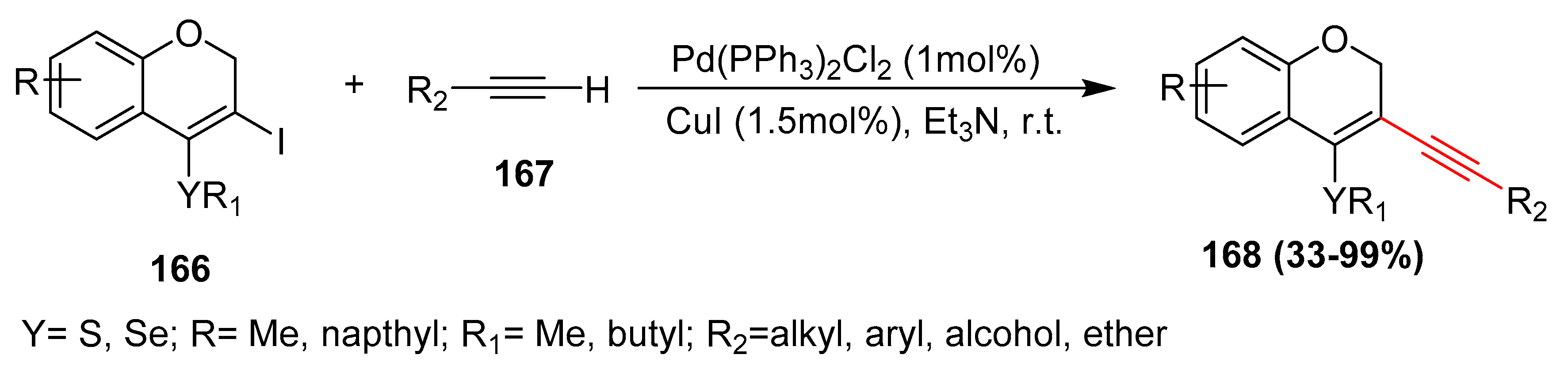

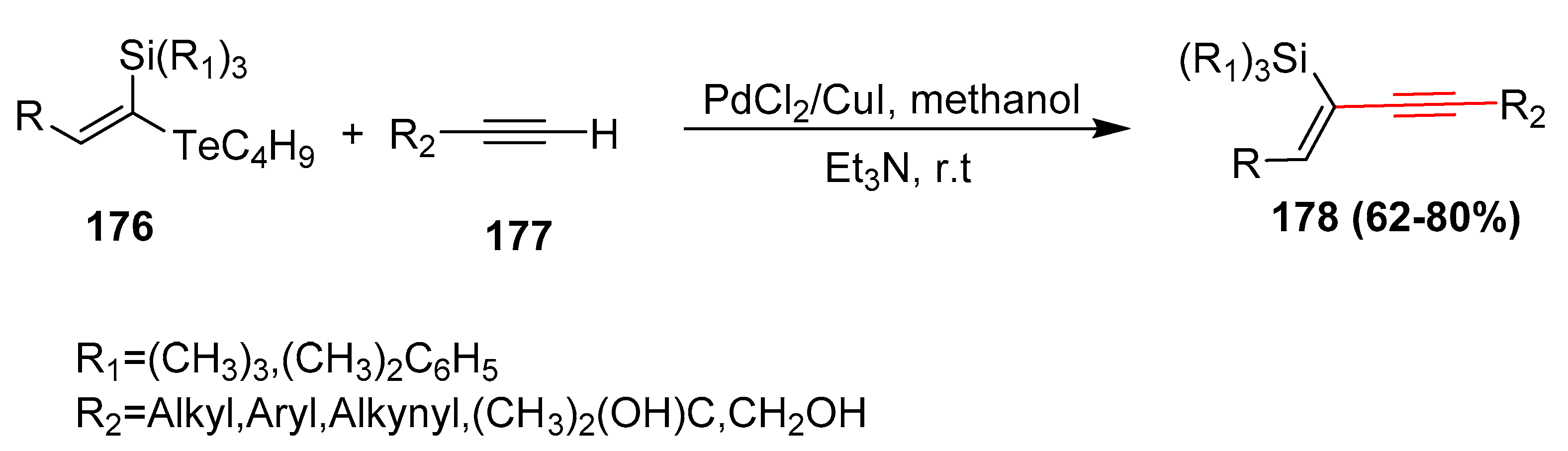
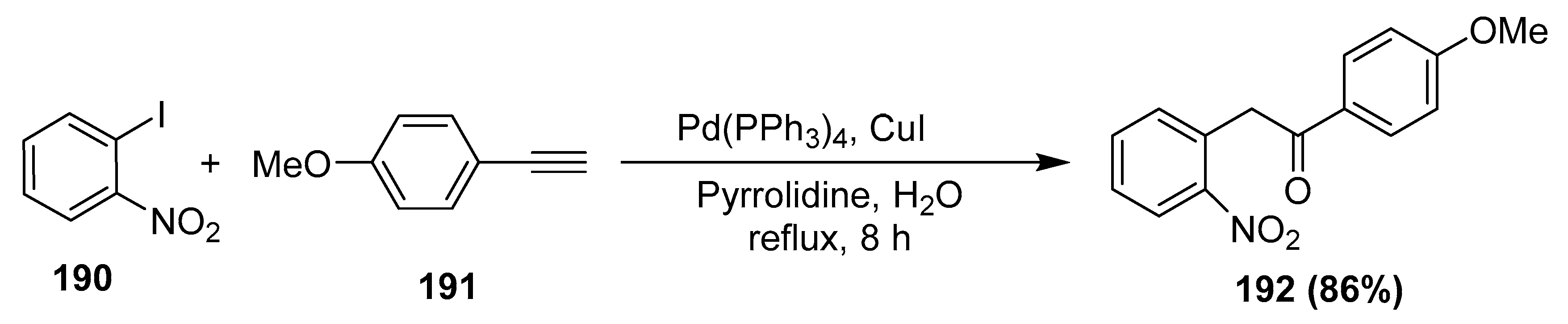



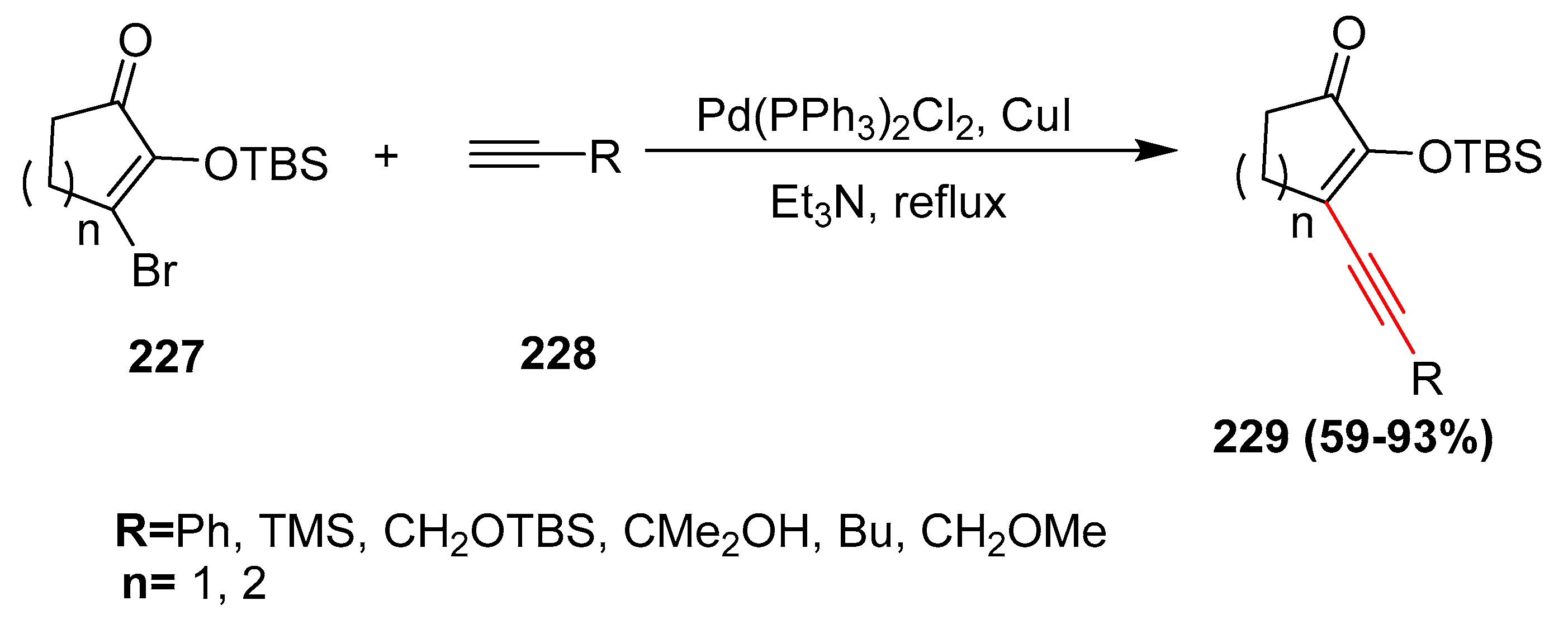




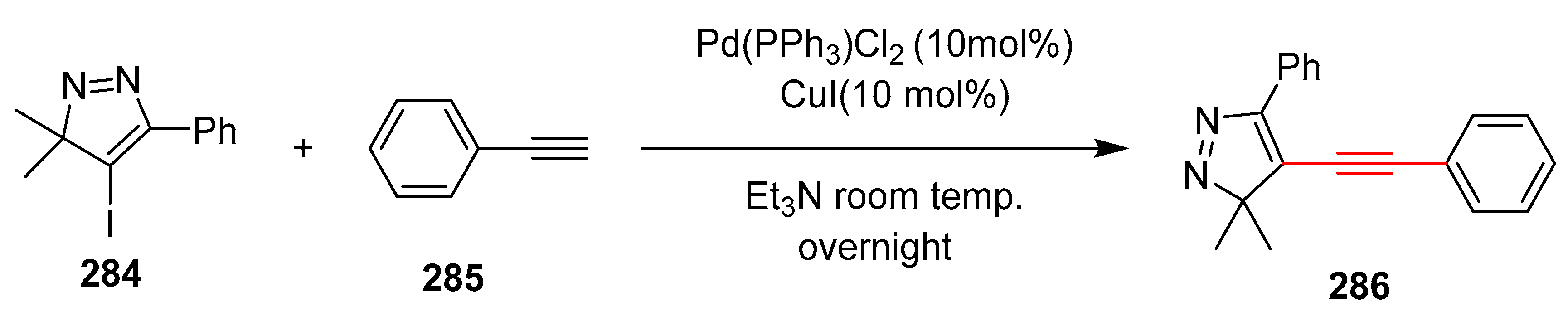
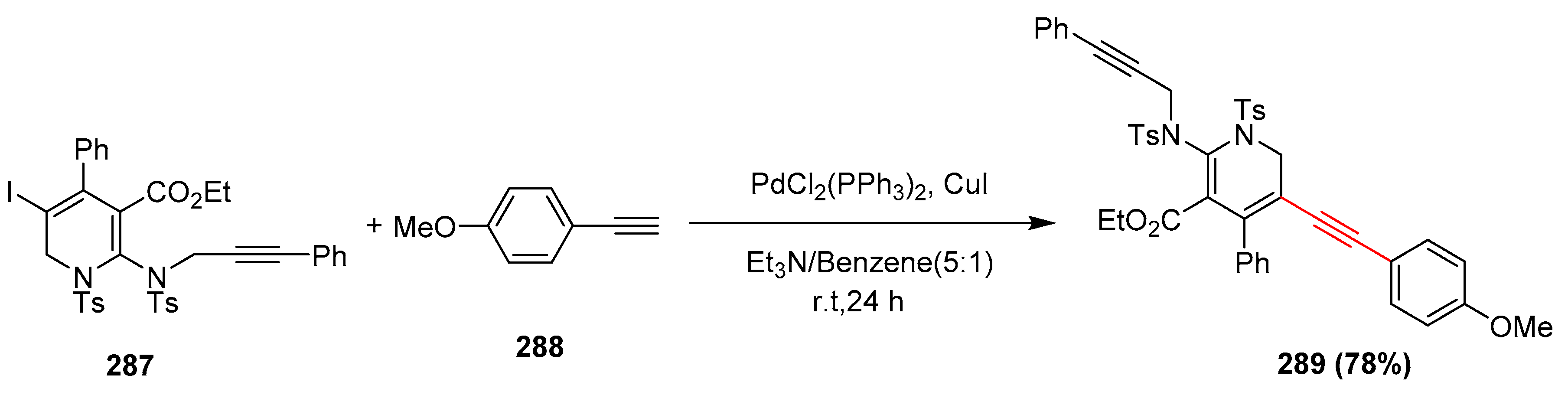

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. https://doi.org/10.3390/catal10040443
Kanwal I, Mujahid A, Rasool N, Rizwan K, Malik A, Ahmad G, Shah SAA, Rashid U, Nasir NM. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts. 2020; 10(4):443. https://doi.org/10.3390/catal10040443
Chicago/Turabian StyleKanwal, Iram, Aqsa Mujahid, Nasir Rasool, Komal Rizwan, Ayesha Malik, Gulraiz Ahmad, Syed Adnan Ali Shah, Umer Rashid, and Nadiah Mad Nasir. 2020. "Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review" Catalysts 10, no. 4: 443. https://doi.org/10.3390/catal10040443
APA StyleKanwal, I., Mujahid, A., Rasool, N., Rizwan, K., Malik, A., Ahmad, G., Shah, S. A. A., Rashid, U., & Nasir, N. M. (2020). Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts, 10(4), 443. https://doi.org/10.3390/catal10040443






