One-Pot Solvent-Free Synthesis of N,N-Bis(2-Hydroxyethyl) Alkylamide from Triglycerides Using Zinc-Doped Calcium Oxide Nanospheroids as a Heterogeneous Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Brunauer–Emmett–Teller (BET) Surface Area and Hammett Indicator Test
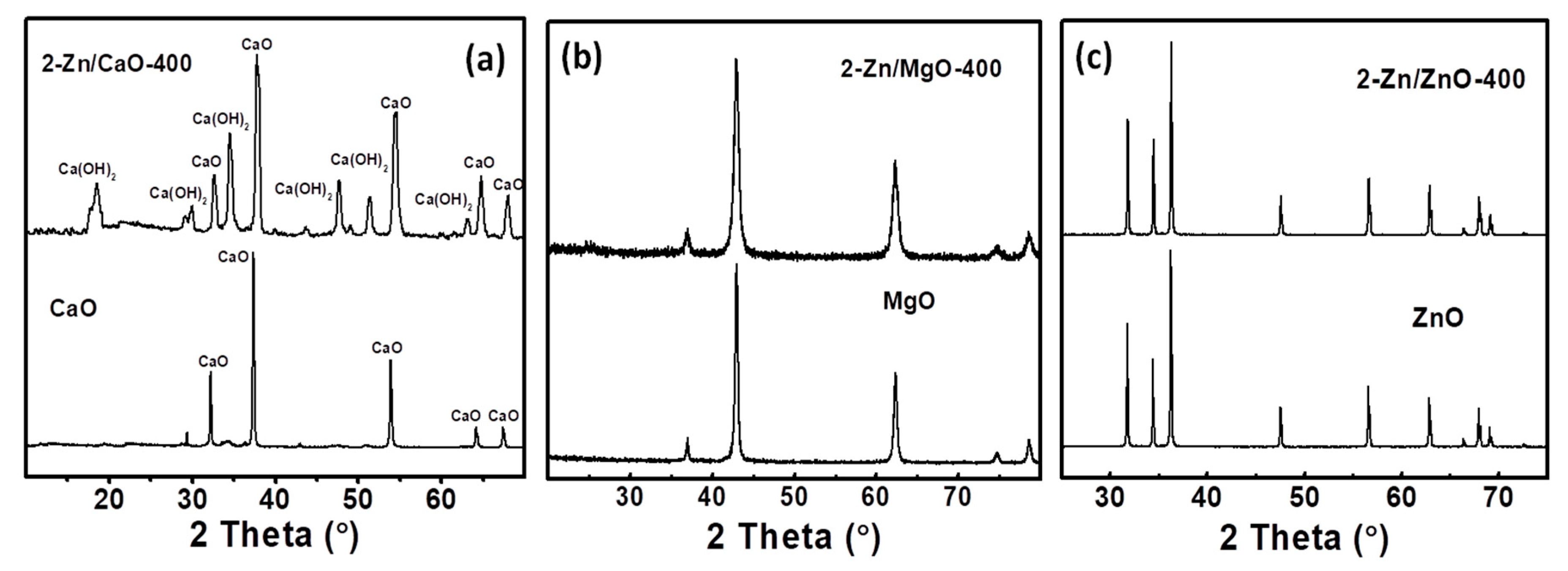
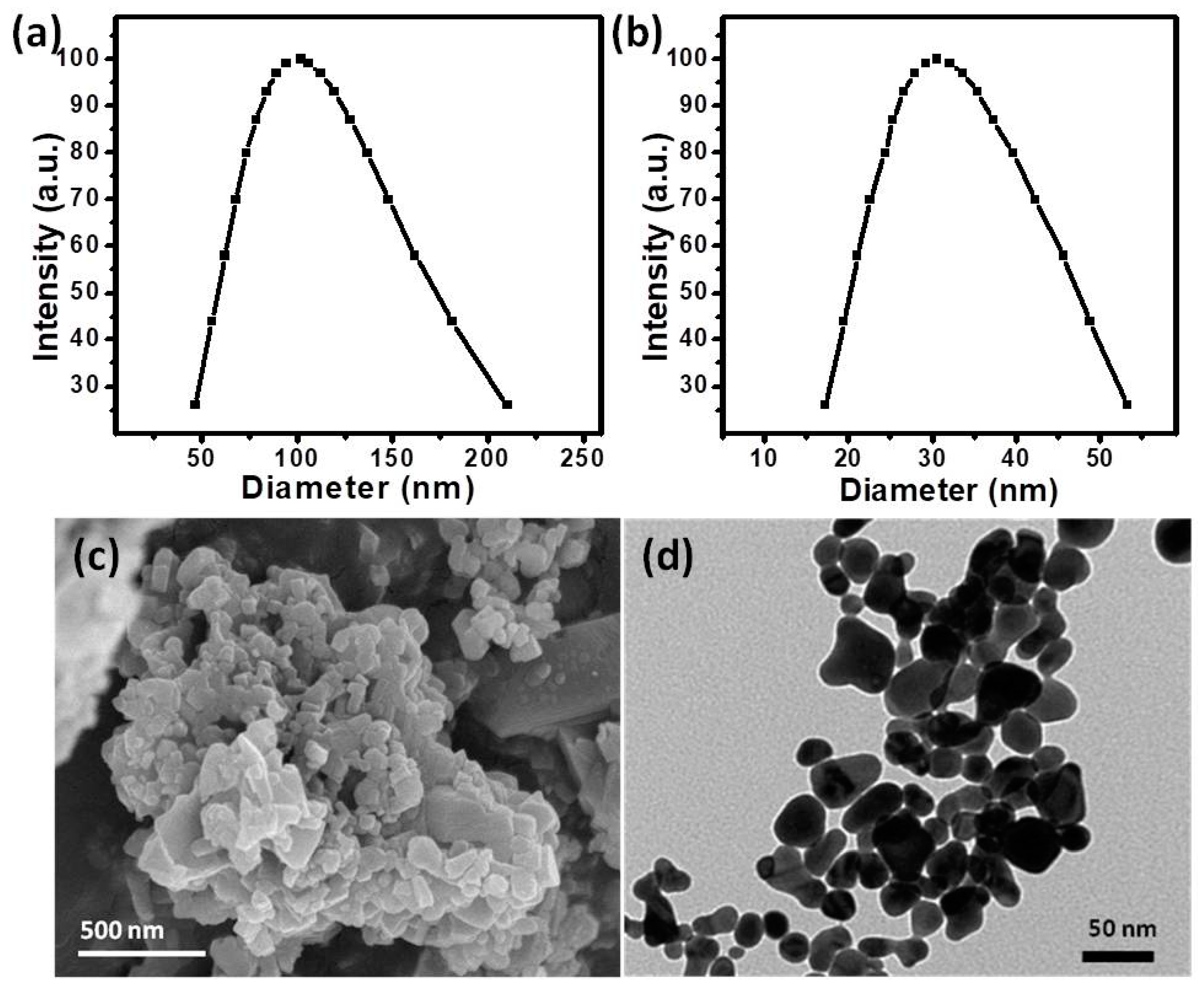
2.2. Structural Analysis of Catalyst
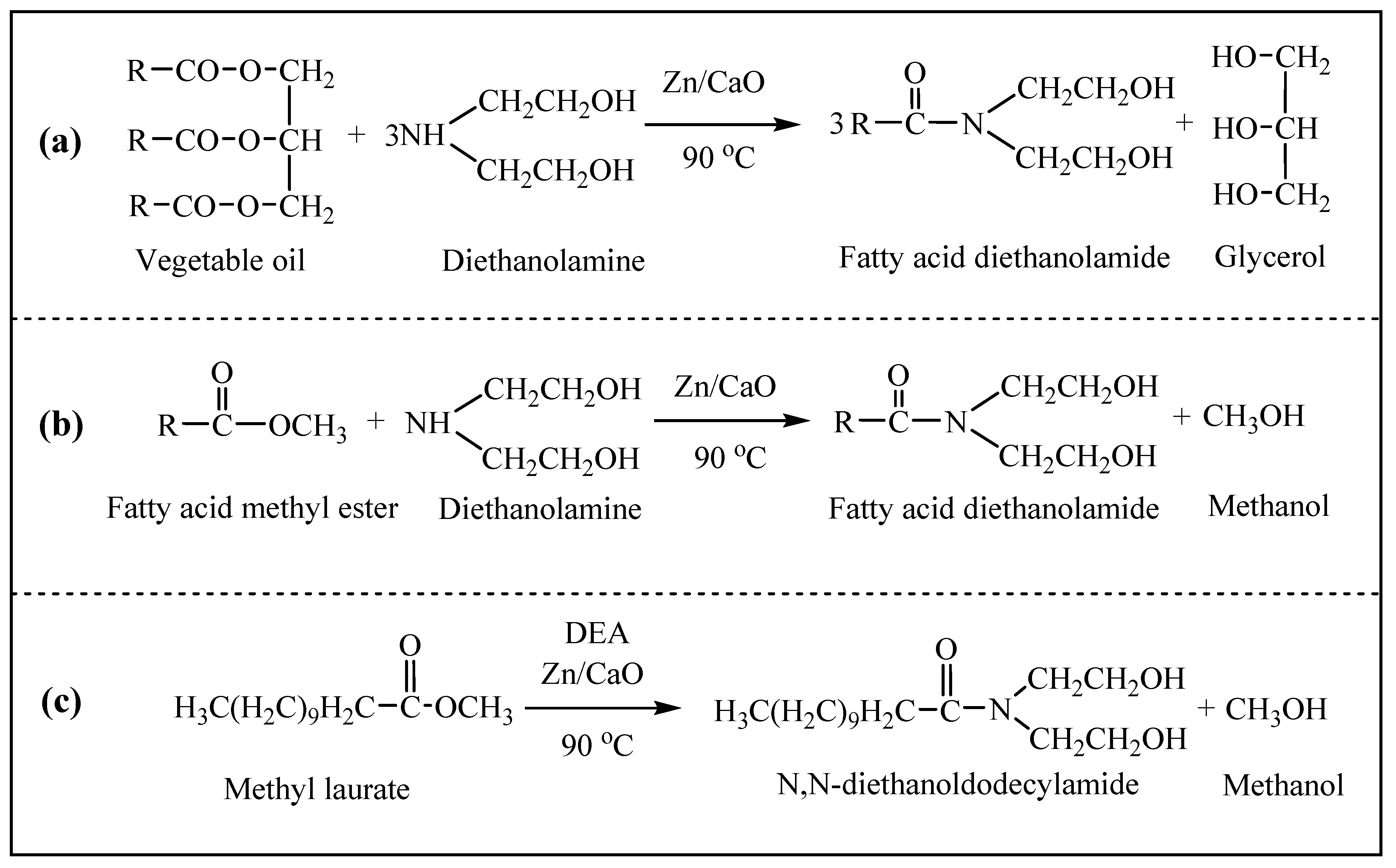
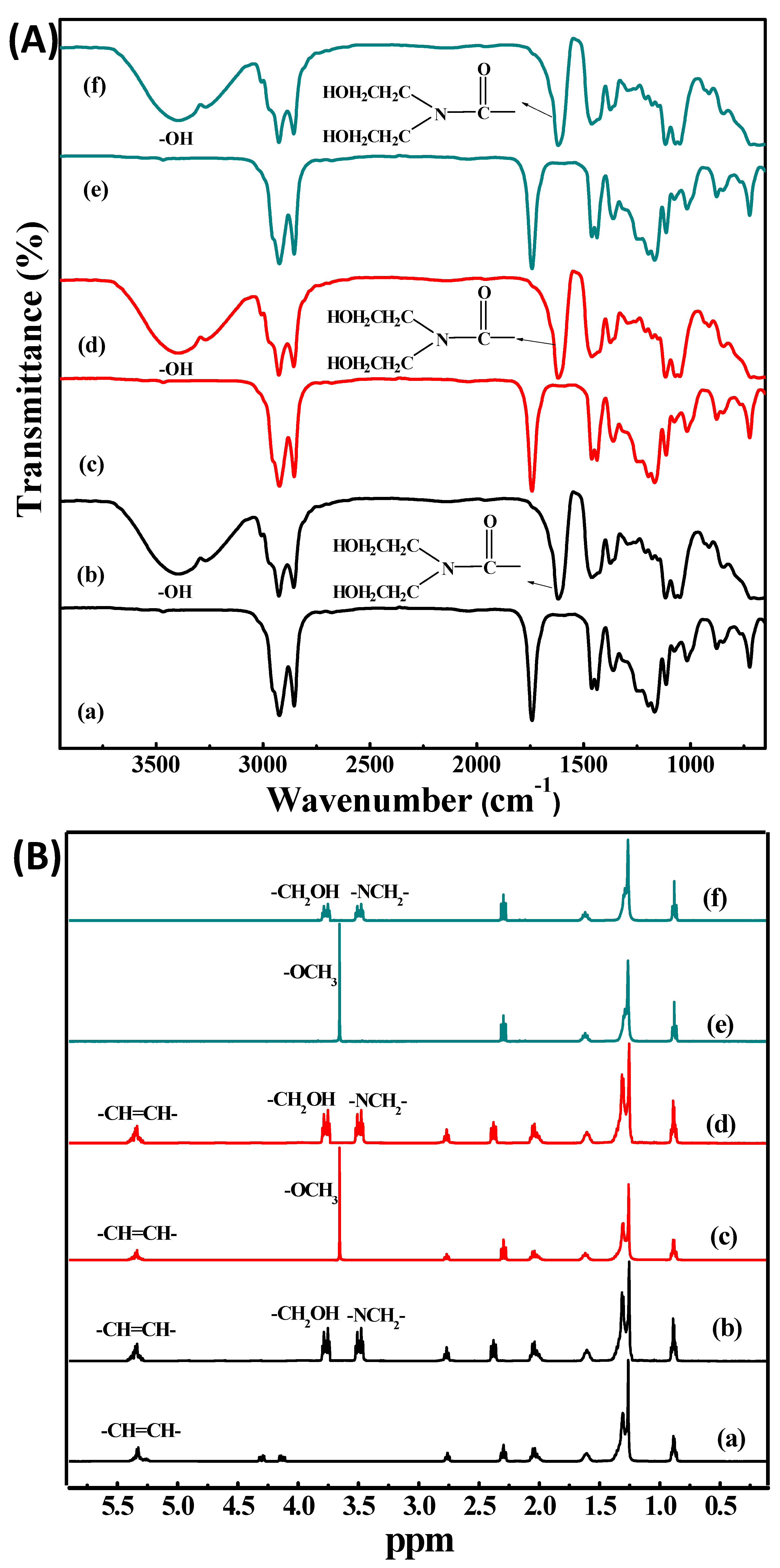
2.3. Aminolysis Reaction
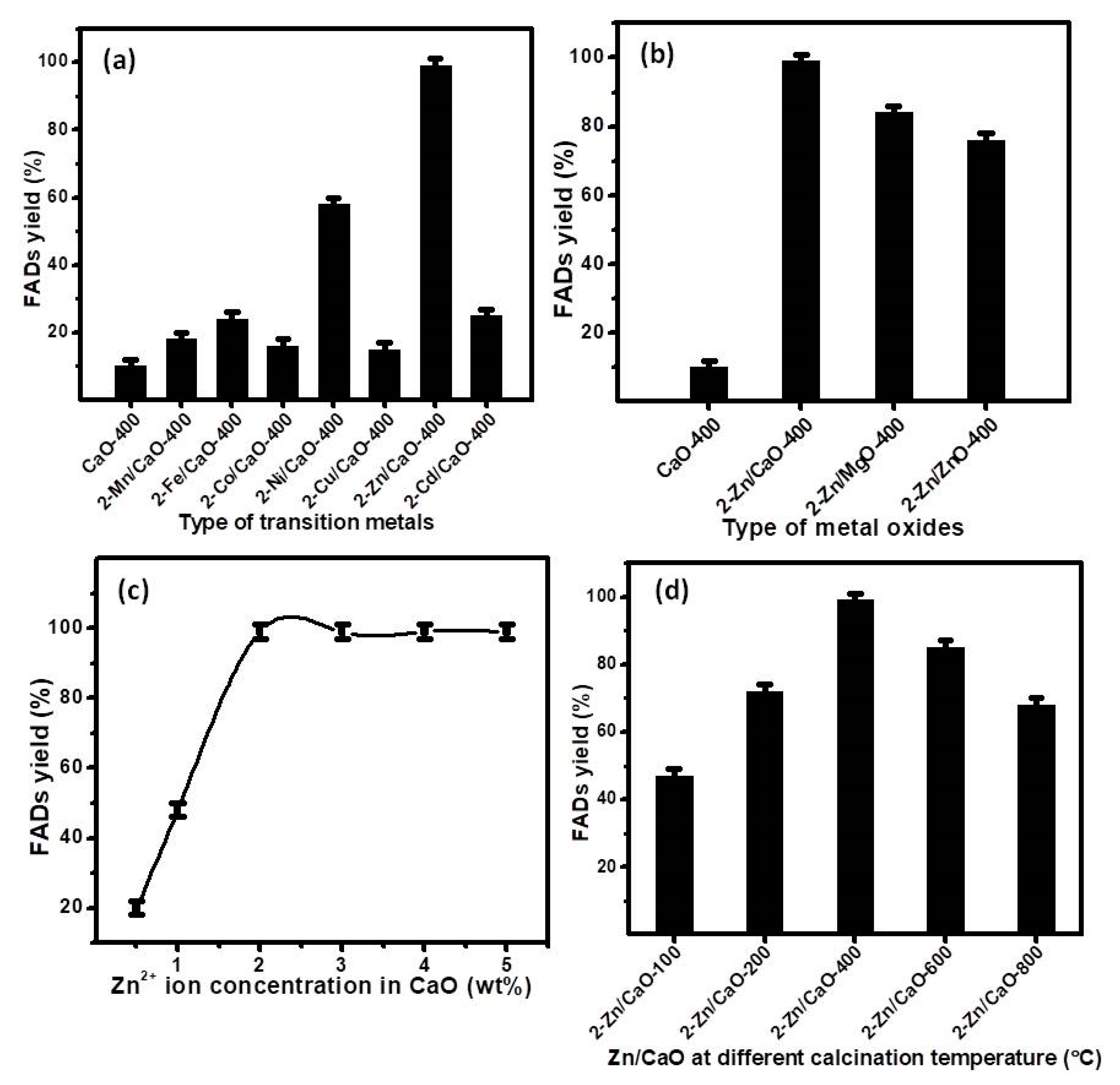
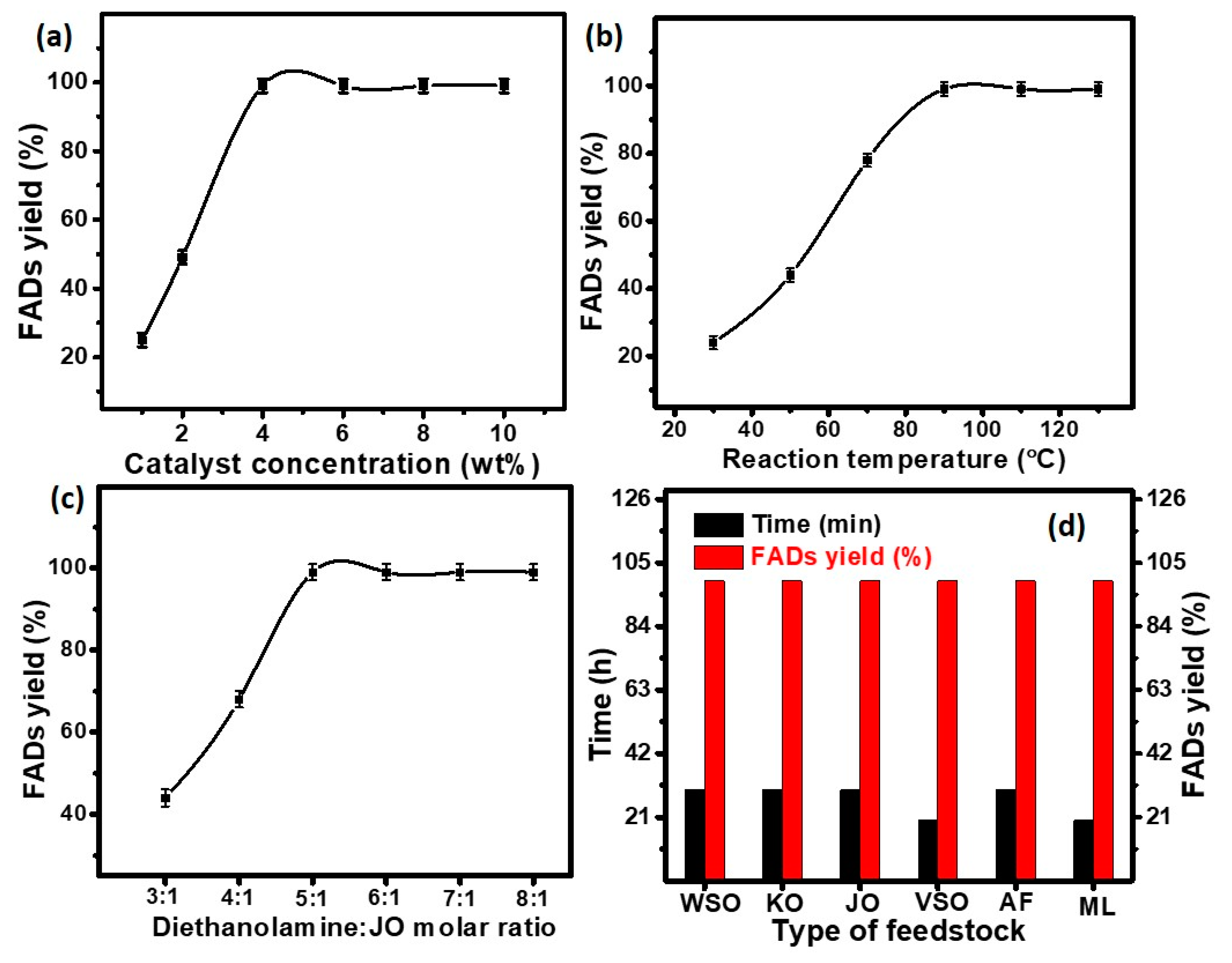
2.3.1. Optimization of Different Parameters
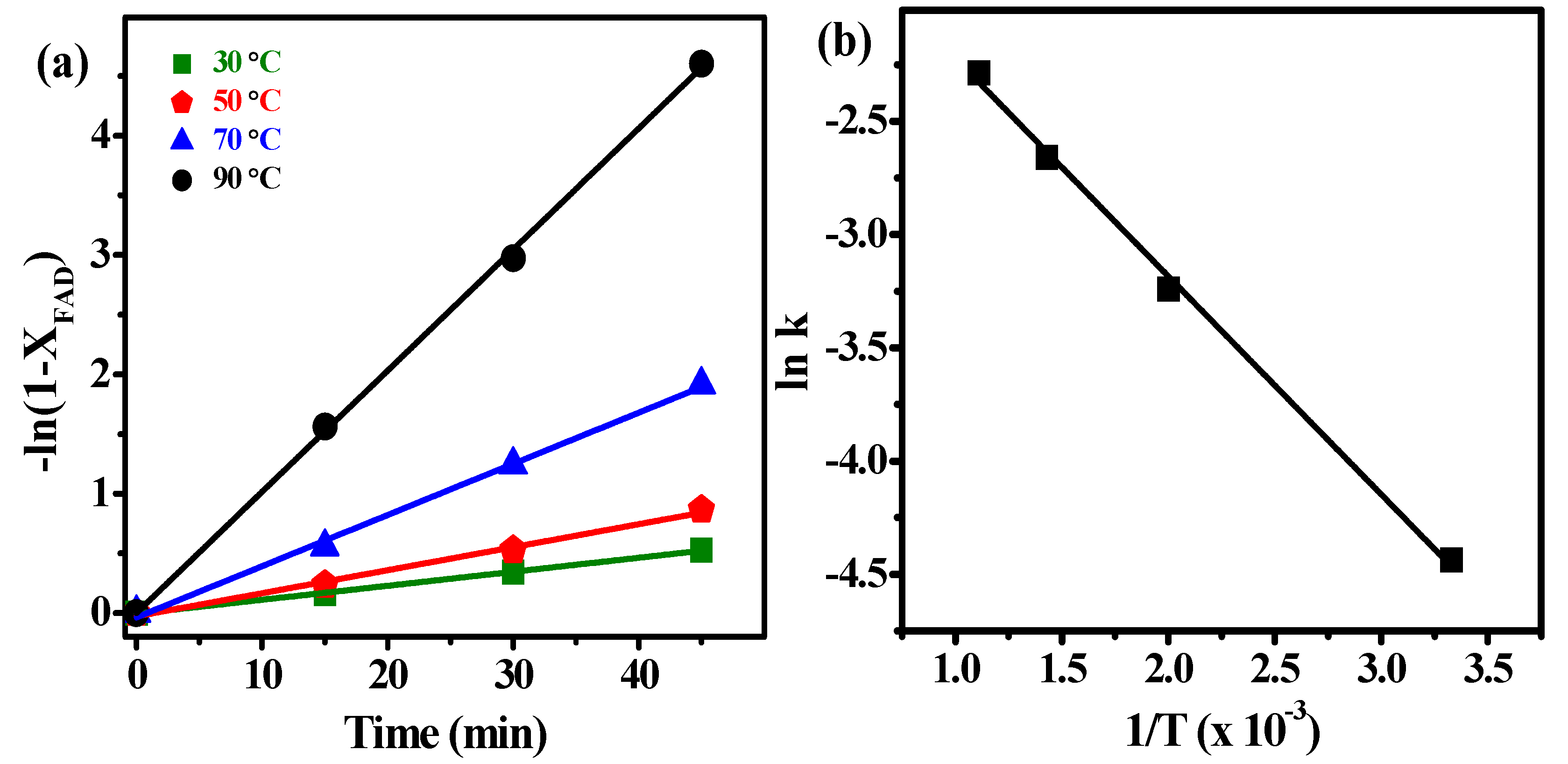
2.3.2. Kinetic Study
3. Materials and Methods
3.1. General
3.2. Experimental Section
3.2.1. Preparation of Catalyst
3.2.2. Aminolysis Reaction
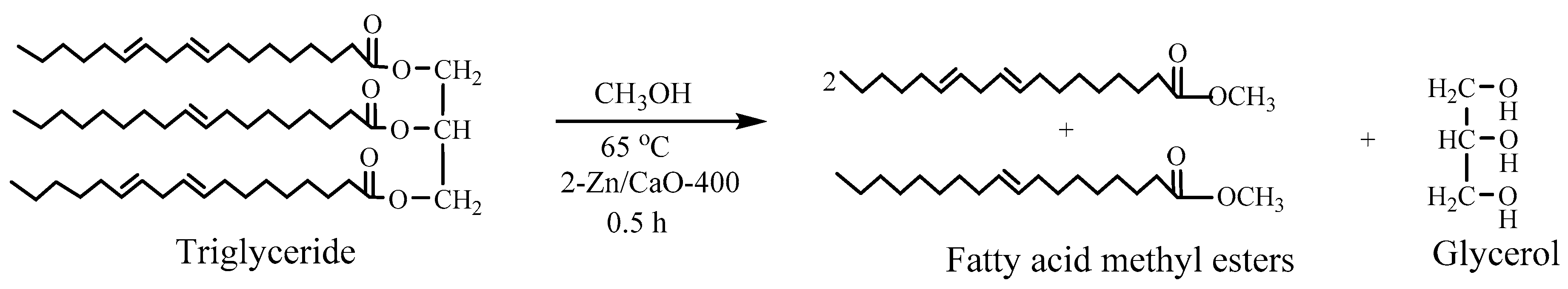
3.2.3. Synthesis of Fatty Acid Methyl Esters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem. Int. Ed. 2012, 51, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Martin Alonso, D.; Dumesic, J.A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 2014, 16, 4816–4838. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Foley, P.; Kermanshahi pour, A.; Beach, E.S.; Zimmerman, J.B. Derivation and synthesis of renewable surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518. [Google Scholar] [CrossRef]
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, J.O.; Rüsch gen. Klaas, M.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Jamil, M.A.R.; Siddiki, S.M.A.H.; Touchy, A.S.; Rashed, M.N.; Poly, S.S.; Jing, Y.; Ting, K.W.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. Selective transformations of triglycerides into fatty amines, amides, and nitriles by using heterogeneous catalysis. ChemSusChem 2019, 12, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Serdari, A.; Lois, E.; Stournas, S. Tertiary fatty amides as diesel fuel substitutes. Int. J. Energy Res. 2000, 24, 455–466. [Google Scholar] [CrossRef]
- Awasthi, N.P.; Singh, R.P. Microwave-assisted facile and convenient synthesis of fatty acid amide (erucamide): Chemical-catalyzed rapid method. Eur. J. Lipid Sci. Technol. 2009, 111, 202–206. [Google Scholar] [CrossRef]
- Rawlins, J.; Pramanik, M.; Mendon, S. Synthesis and characterization of soyamide ferulate. J. Am. Oil Chem. Soc. 2008, 85, 783–789. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Yunus, W.M.Z.W.; Ibrahim, N.A.B.; Rahman, M.Z.A. Synthesis and characterization of n,n′-carbonyl difatty amides from palm oil. J. Oleo Sci. 2009, 58, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Yapa Mudiyanselage, A.; Yao, H.; Viamajala, S.; Varanasi, S.; Yamamoto, K. Efficient production of alkanolamides from microalgae. Ind. Eng. Chem. Res. 2015, 54, 4060–4065. [Google Scholar] [CrossRef]
- Bundesmann, M.W.; Coffey, S.B.; Wright, S.W. Amidation of esters assisted by Mg(OCH3)2 or CaCl2. Tetrahedron Lett. 2010, 51, 3879–3882. [Google Scholar] [CrossRef]
- Litjens, M.J.J.; Sha, M.; Straathof, A.J.J.; Jongejan, J.A.; Heijnen, J.J. Competitive lipase-catalyzed ester hydrolysis and ammoniolysis in organic solvents; equilibrium model of a solid–liquid–vapor system. Biotechnol. Bioeng. 1999, 65, 347–356. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, T. Synthesis of oleoylethanolamide using lipase. J. Agric. Food Chem. 2012, 60, 451–457. [Google Scholar] [CrossRef]
- Karis, N.D.; Loughlin, W.A.; Jenkins, I.D. A facile and efficient method for the synthesis of novel pyridone analogues by aminolysis of an ester under solvent-free conditions. Tetrahedron 2007, 63, 12303–12309. [Google Scholar] [CrossRef]
- Sabot, C.; Kumar, K.A.; Meunier, S.; Mioskowski, C. A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (tbd) under solvent-free conditions. Tetrahedron Lett. 2007, 48, 3863–3866. [Google Scholar] [CrossRef]
- Ishii, Y.; Takeno, M.; Kawasaki, Y.; Muromachi, A.; Nishiyama, Y.; Sakaguchi, S. Acylation of alcohols and amines with vinyl acetates catalyzed by cp*2sm(thf)2. J. Org. Chem. 1996, 61, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H.; Delbridge, E.E.; Gallucci, J.C. Modeling the catalyst resting state in aryl tin(iv) polymerizations of lactide and estimating the relative rates of transamidation, transesterification and chain transfer. New J. Chem. 2004, 28, 145–152. [Google Scholar] [CrossRef]
- Kangani, C.O.; Kelley, D.E. One pot direct synthesis of amides or oxazolines from carboxylic acids using deoxo-fluor reagent. Tetrahedron Lett. 2005, 46, 8917–8920. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.N.; Sreeramamurthy, K.; Palle, S.; Mukkanti, K.; Das, P. Dithiocarbamate and dbu-promoted amide bond formation under microwave condition. Tetrahedron Lett. 2010, 51, 899–902. [Google Scholar] [CrossRef]
- Pan, J.; Devarie-Baez, N.O.; Xian, M. Facile amide formation via s-nitrosothioacids. Org. Lett. 2011, 13, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Crich, D. Facile amide bond formation from carboxylic acids and isocyanates. Org. Lett. 2011, 13, 2256–2259. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Shanmugavelan, P.; Nagarajan, S.; Maheswari, M.; Dinesh, M.; Ponnuswamy, A. Solvent-free protocol for amide bond formation via trapping of nascent phosphazenes with carboxylic acids. Tetrahedron Lett. 2011, 52, 2830–2833. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M. Application of a novel catalyst in the esterification of mixed industrial palm oil for biodiesel production. BioEnergy Res. 2014, 8, 459–463. [Google Scholar] [CrossRef]
- Kumar, D.; Kim, S.M.; Ali, A. One step synthesis of fatty acid diethanolamides and methyl esters from triglycerides using sodium doped calcium hydroxide as a nanocrystalline heterogeneous catalyst. New J. Chem. 2015, 39, 7097–7104. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ali, A. Transesterification of low-quality triglycerides over a Zn/CaO heterogeneous catalyst: Kinetics and reusability studies. Energy Fuels 2013, 27, 3758–3768. [Google Scholar] [CrossRef]
- Kumar, D.; Kim, S.M.; Ali, A. Solvent-free one step aminolysis and alcoholysis of low-quality triglycerides using sodium modified cao nanoparticles as a solid catalyst. RSC Adv. 2016, 6, 55800–55808. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Direct synthesis of fatty acid alkanolamides and fatty acid alkyl esters from high free fatty acid containing triglycerides as lubricity improvers using heterogeneous catalyst. Fuel 2015, 159, 845–853. [Google Scholar] [CrossRef]
- Degirmenbasi, N.; Boz, N.; Kalyon, D.M. Biofuel production via transesterification using sepiolite-supported alkaline catalysts. Appl. Catal. B Environ. 2014, 150–151, 147–156. [Google Scholar] [CrossRef]
| Catalyst Type | Basic Strength (pKBH+) | BET Surface Area (m2/g) | Average Crystallite Size (nm)* |
|---|---|---|---|
| CaO | 9.8 < pKBH+ < 10.1 | 3.56 | 108 |
| 2-Zn/CaO-100 | 11.1 < pKBH+ < 15.0 | 4.51 | 35 |
| 2-Zn/CaO-200 | 15.0 < pKBH+ < 18.4 | 6.03 | 37 |
| 2-Zn/CaO-400 | 18.4 < pKBH+ | 16.87 | 33 |
| 2-Zn/CaO-600 | 15.0 < pKBH+ < 18.4 | 10.12 | 35 |
| 2-Zn/CaO-800 | 11.1 < pKBH+ < 15.0 | 5.25 | 55 |
| 1-Zn/CaO-400 | 11.1 < pKBH+ < 15.0 | 11.72 | 37 |
| 3-Zn/CaO-400 | 15.0 < pKBH+ < 18.4 | 16.24 | 34 |
| 4-Zn/CaO-400 | 15.0 < pKBH+ < 18.4 | 16.54 | 39 |
| 5-Zn/CaO-400 | 15.0 < pKBH+ < 18.4 | 14.36 | 35 |
| 2-Zn/MgO-400 | 15.0 < pKBH+ < 18.4 | 14.89 | 35 |
| 2-Zn/ZnO-400 | 15.0 < pKBH+ < 18.4 | 12.13 | 38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Park, C.H.; Kim, C.S. One-Pot Solvent-Free Synthesis of N,N-Bis(2-Hydroxyethyl) Alkylamide from Triglycerides Using Zinc-Doped Calcium Oxide Nanospheroids as a Heterogeneous Catalyst. Catalysts 2019, 9, 774. https://doi.org/10.3390/catal9090774
Kumar D, Park CH, Kim CS. One-Pot Solvent-Free Synthesis of N,N-Bis(2-Hydroxyethyl) Alkylamide from Triglycerides Using Zinc-Doped Calcium Oxide Nanospheroids as a Heterogeneous Catalyst. Catalysts. 2019; 9(9):774. https://doi.org/10.3390/catal9090774
Chicago/Turabian StyleKumar, Dinesh, Chan Hee Park, and Cheol Sang Kim. 2019. "One-Pot Solvent-Free Synthesis of N,N-Bis(2-Hydroxyethyl) Alkylamide from Triglycerides Using Zinc-Doped Calcium Oxide Nanospheroids as a Heterogeneous Catalyst" Catalysts 9, no. 9: 774. https://doi.org/10.3390/catal9090774





