Biodiesel Production from Castor oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment
Abstract
1. Introduction
2. Results and Discussion
2.1. Raw Material
2.2. Reaction Conditions and Variables of the Design
2.3. Regression Model Development
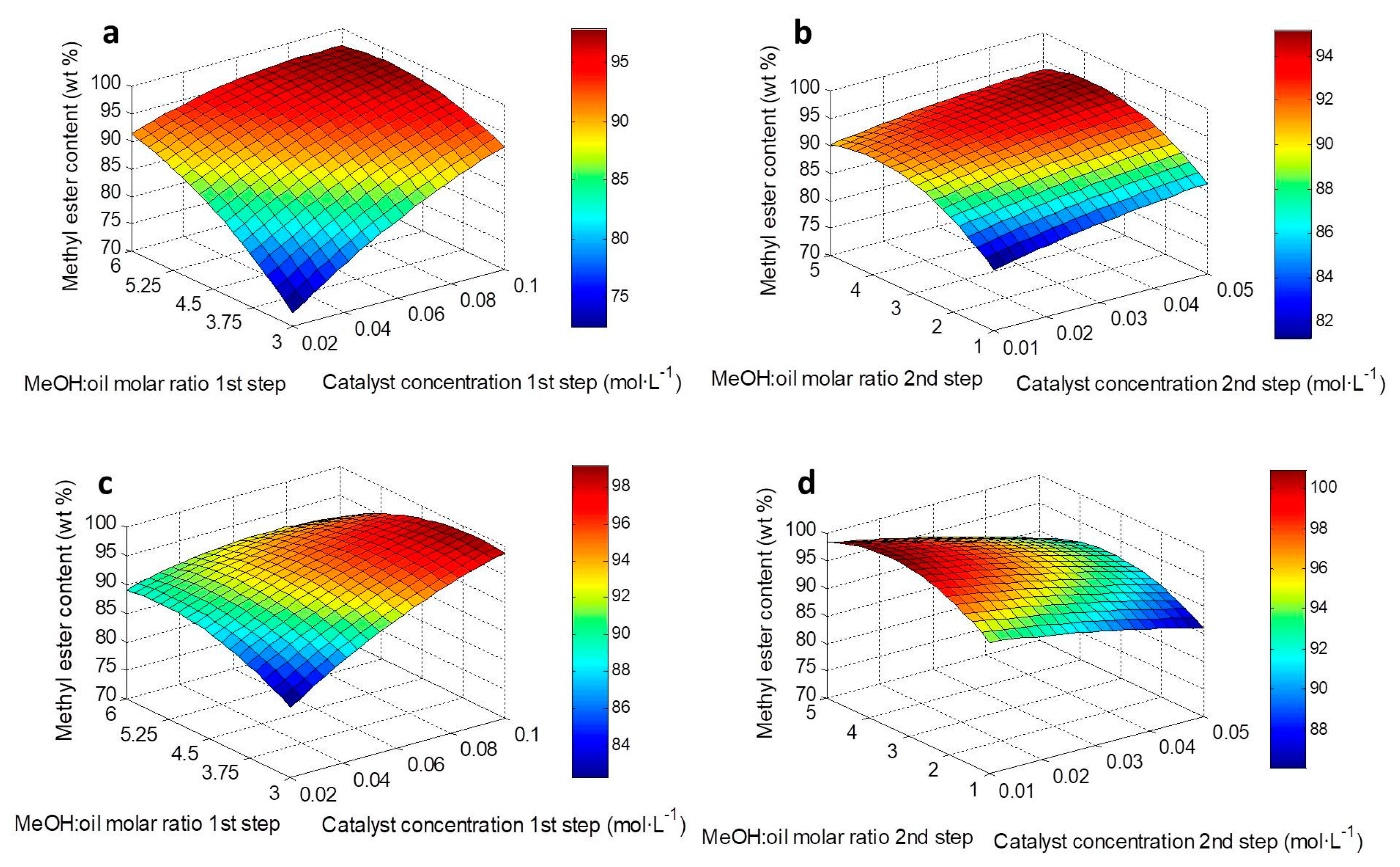
2.4. Response Surface Graphs
2.5. Process Optimization
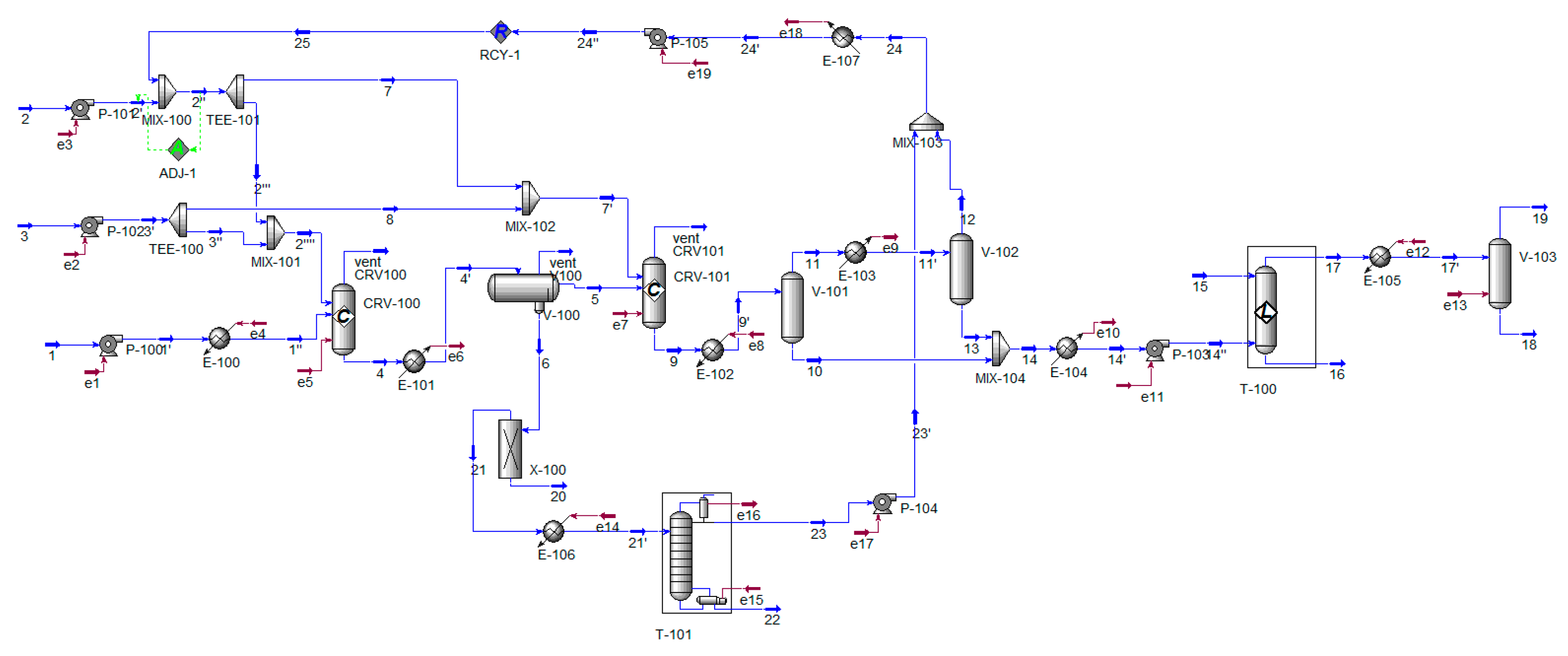
2.6. Process Simulation
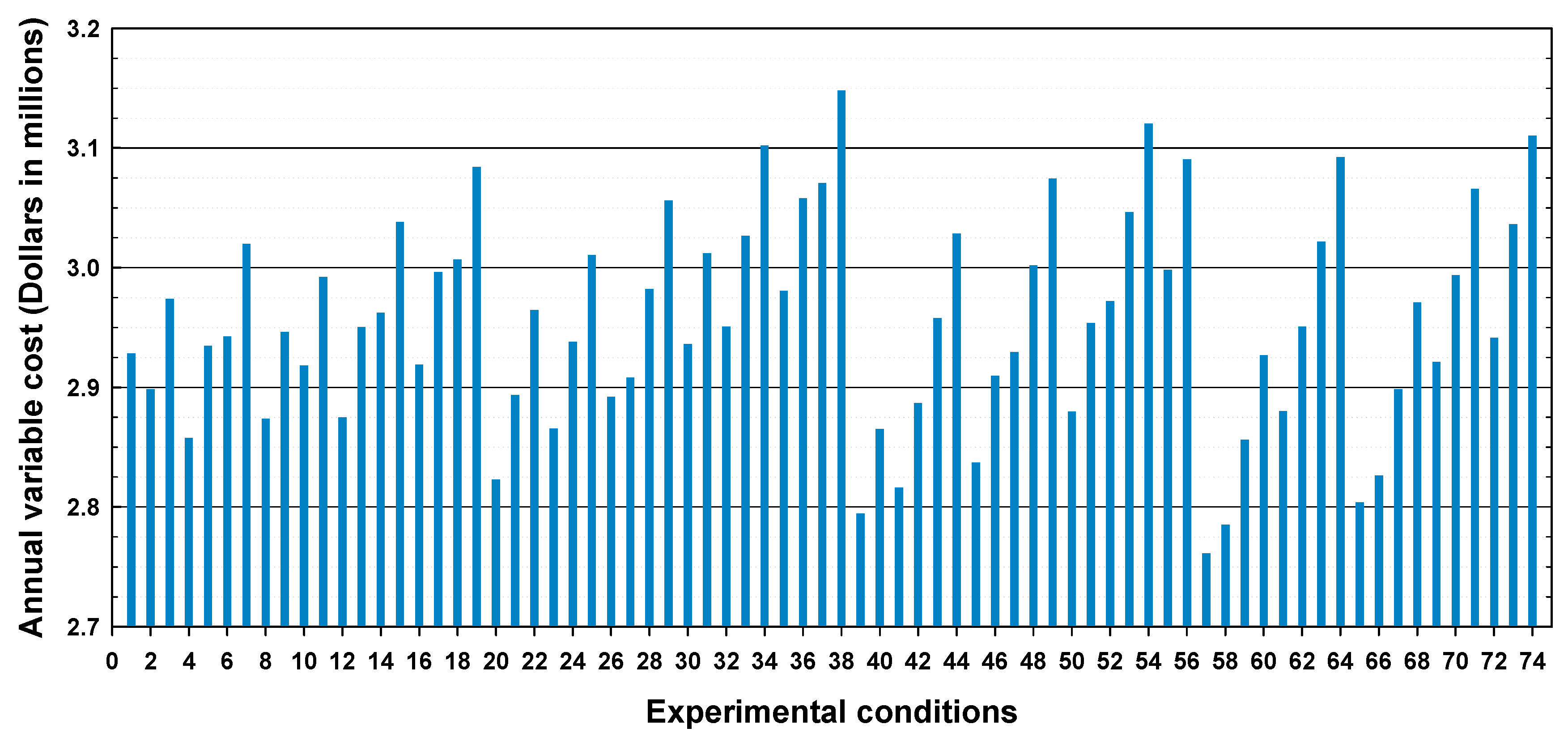
2.7. Cost Evaluation
3. Materials and Methods
3.1. Materials
3.2. Transesterification Reaction
3.3. Experimental Design and Statistical Analysis
3.4. Analytical Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Scholz, V.; da Silva, J.N. Prospects and risks of the use of castor oil as a fuel. Biomass Bioenergy 2008, 32, 95–100. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M.; Kolodnytska, R. The impact of biodiesel fuel on ethanol/diesel blends. Energies 2019, 12, 1804. [Google Scholar] [CrossRef]
- Hurtado, B.; Posadillo, A.; Luna, D.; Bautista, F.M.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.A.; Estevez, R. Synthesis, performance and emission quality assessment of ecodiesel from castor oil in diesel/biofuel/alcohol triple blends in a diesel engine. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Posadillo, A.; Hurtado, B.; Bautista, F.M.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.A.; Luna, D. Performance and emission quality assessment in a diesel engine of straight castor and sunflower vegetable oils, in diesel/gasoline/oil triple blends. Energies 2019, 12, 2181. [Google Scholar] [CrossRef]
- Sánchez, N.; Sánchez, R.; Encinar, J.M.; González, J.F.; Martínez, G. Complete analysis of castor oil methanolysis to obtain biodiesel. Fuel 2015, 147, 95–99. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Serra, T.M.; Barbosa, D.C.; Wolf, C.R. Biodiesel production from vegetable oil mixtures: Cottonseed, soybean, and castor oils. Energy Fuel 2007, 21, 3746–3757. [Google Scholar] [CrossRef]
- Hincapié, G.; Mondragón, F.; López, D. Conventional and in situ transesterification of castor seed oil for biodiesel production. Fuel 2011, 90, 1618–1623. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Pardal, A. Transesterification of castor oil under ultrasonic irradiation conditions. Preliminary results. Fuel Process. Technol. 2012, 103, 9–15. [Google Scholar] [CrossRef]
- Zieba, A.; Matachowski, L.; Gurgul, J.; Bielanska, E.; Drelinkiewicz, A. Transesterification reaction of triglycerides in the presence of Ag-doped H3PW12O40. J. Mol. Catal. A Chem. 2010, 316, 30–44. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gnaneswar Gude, V. Assessment of sustainability indicators for biodiesel production. Appl. Sci. 2017, 7, 869. [Google Scholar] [CrossRef]
- Coronado, C.R.; Tuna, C.E.; Zanzi, R.; Vane, L.F.; Silveira, J.L. Development of a thermoeconomic methodology for optimizing biodiesel production. Part II: Manufacture exergetic cost and biodiesel production cost incorporating carbon credits, a Brazilian case study. Renew. Sustain. Energy Rev. 2014, 29, 565–572. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Torres, C.M.; Jiménez, L.; Font, J.; Bengoa, C. Scale-up and economic analysis of biodiesel production from municipal primary sewage sludge. Bioresour. Technol. 2016, 214, 122–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Mohammadshirazi, A.; Akram, A.; Rafiee, S.; Bagheri Kalhor, E. Energy and cost analyses of biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2014, 33, 44–49. [Google Scholar] [CrossRef]
- Tang, Z.-C.; Lu, Z.; Liu, Z.; Xiao, N. Uncertainty analysis and global sensitivity analysis of techno-economic assessments for biodiesel production. Bioresour. Technol. 2015, 175, 502–508. [Google Scholar] [CrossRef]
- Santana, G.C.S.; Martins, P.F.; de Lima da Silva, N.; Batistella, C.B.; Maciel Filho, R.; Wolf Maciel, M.R. Simulation and cost estimate for biodiesel production using castor oil. Chem. Eng. Res. Des. 2010, 88, 626–632. [Google Scholar] [CrossRef]
- Dias, J.M.; Araújo, J.M.; Costa, J.F.; Alvim-Ferraz, M.C.M.; Almeida, M.F. Biodiesel production from raw castor oil. Energy 2013, 53, 58–66. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. Am. Soc. Agric. Eng. 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Avasthi, K.S.; Reddy, R.N.; Patel, S. Challenges in the production of hydrogen from glycerol—A biodiesel byproduct via steam reforming process. Procedia Eng. 2013, 51, 423–429. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Coronado, C.R.; Tuna, C.E.; Zanzi, R.; Vane, L.F.; Silveira, J.L. Development of a thermoeconomic methodology for the optimization of biodiesel production—Part I: Biodiesel plant and thermoeconomic functional diagram. Renew. Sustain. Energy Rev. 2013, 23, 138–146. [Google Scholar] [CrossRef]
- Encinar, J.M.; Sánchez, N.; Martínez, G.; García, L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011, 102, 10907–10914. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel 2016, 166, 51–58. [Google Scholar] [CrossRef]
- Canoira, L.; García Galeán, J.; Alcántara, R.; Lapuerta, M.; García-Contreras, R. Fatty acid methyl esters (FAMEs) from castor oil: Production process assessment and synergistic effects in its properties. Renew. Energy 2010, 35, 208–217. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; de Lira Silva, L. Biodiesel from castor oil: A comparison of ethanolysis versus methanolysis. Energy Fuel 2006, 20, 2262–2265. [Google Scholar] [CrossRef]
- Barbosa, D.D.C.; Serra, T.M.; Meneghetti, S.M.P.; Meneghetti, M.R. Biodiesel production by ethanolysis of mixed castor and soybean oils. Fuel 2010, 89, 3791–3794. [Google Scholar] [CrossRef]
- Peña, R.; Romero, R.; Martínez, S.L.; Ramos, M.J.; Martínez, A.; Natividad, R. Transesterification of castor oil: Effect of catalyst and co-solvent. Ind. Eng. Chem. Res. 2009, 48, 1186–1189. [Google Scholar] [CrossRef]
- Abuhabaya, A.; Fieldhouse, J.; Brown, D. The optimization of biodiesel production by using response surface methodology and its effect on compression ignition engine. Fuel Process. Technol. 2013, 113, 57–62. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Park, D.-H. Optimization of biodiesel production from castor oil using response surface methodology. Appl. Biochem. Biotechnol. 2009, 156, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.O., Jr.; Maruyama, S.A.; Claus, T.; de Souza, N.E.; Matsushita, M.; Visentainer, J.V. A novel response surface methodology optimization of base-catalyzed soybean oil methanolysis. Fuel 2013, 113, 580–585. [Google Scholar] [CrossRef]
- Methanex Methanol Price Sheet. Available online: http://www.methanex.com (accessed on 28 October 2015).
- Sales-Cruz, M.; Aca-Aca, G.; Sánchez-Daza, O.; López-Arenas, T. Predicting critical properties, density and viscosity of fatty acids, triacylglycerols and methyl esters by group contribution methods. In Proceedings of the 20th European Symposium on Computer Aided Process, Engineering—ESCAPE20, Naples, Italy, 6 June 2010. [Google Scholar]
- França, B.B.; Pinto, F.M.; Pessoa, F.L.P.; Uller, A.M.C. Liquid-Liquid Equilibria for Castor Oil Biodiesel + Glycerol + Alcohol. J. Chem. Eng. Data 2008, 54, 2359–2364. [Google Scholar] [CrossRef]
- Machado, A.B.; Ardila, Y.C.; de Oliveira, L.H.; Aznar, M.; Wolf Maciel, M.R. Liquid−Liquid Equilibrium Study in Ternary Castor Oil Biodiesel + Ethanol + Glycerol and Quaternary Castor Oil Biodiesel + Ethanol + Glycerol + NaOH Systems at (298.2 and 333.2) K. J. Chem. Eng. Data 2011, 56, 2196–2201. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Unit Operations, 3rd ed.; Prentice-Hall International, Inc.: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- West, A.H.; Posarac, D.; Ellis, N. Assessment of four biodiesel production processes using HYSYS. Plant. Bioresour. Technol. 2008, 99, 6587–6601. [Google Scholar] [CrossRef]
- Silla, H. Chemical Process Engineering. Design and Economics; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Treybal, R.E. Mass-Transfer Operations, 3rd ed.; McGraw Hill: Singapore, 1981. [Google Scholar]
- Loh, H.P.; Lyons, J. Process Equipment Cost Estimation; Final Report; DOE/NETL-2002/1169; EG&G Technical Services Inc.: Morgantown, WV, USA, 2002. [Google Scholar]
- Institution Chemical Engineering. A New Guide to Capital Cost Estimating; I Chem E Services: Rugby, Warkwicksire, UK, 1985. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers, 4th ed.; McGraw Hill: New York, NY, USA, 1991. [Google Scholar]
- Castor Oil Industry Reference & Resources. Available online: http://castoroil.in (accessed on 18 December 2015).
- Panwar, N.L.; Shrirame, H.Y.; Rathore, N.S.; Jindal, S.; Kurchania, A.K. Performance evaluation of a diesel engine fueled with methyl ester of castor seed oil. Appl. Therm. Eng. 2010, 30, 245–249. [Google Scholar] [CrossRef]
| Oil | Castor | Rapeseed [25] | Sunflower [25] |
|---|---|---|---|
| Fatty acid profile, % | |||
| C16:0 palmitic | 1.30 | 4.92 | 4.88 |
| C18:0 stearic | 1.22 | 1.63 | 4.78 |
| C18:1 oleic | 3.61 | 66.59 | 67.66 |
| C18:2 linoleic | 4.58 | 17.08 | 21.26 |
| C18:3 linolenic | 0.39 | 7.75 | 0.09 |
| C18:1–OH ricinoleic | 88.9 | N.D. | N.D. |
| Physical and chemical properties | |||
| Density at 15 °C, kg·m−3 | 961 | 919 | 918 |
| Viscosity at 40 °C, cSt | 262 | 38.5 | 38.3 |
| Water content, % | 0.31 | 0.06 | 0.06 |
| Acid value, mgKOH·g−1 | 1.19 | 0.71 | 1.90 |
| Acid number, % | 0.55 | 0.36 | 0.95 |
| Iodine value, gI2·(100 g)−1 | 80.5 | 101.1 | 93.5 |
| Saponification value, mgKOH·g−1 | 179 | 193.2 | 184.0 |
| Source | Sum of Squares | DF | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 676.867 | 14 | 48.348 | 32.190 | 0.0000 |
| A | 239.528 | 1 | 239.528 | 159.476 | 0.0000 |
| B | 207.446 | 1 | 207.446 | 138.116 | 0.0000 |
| C | 26.250 | 1 | 26.250 | 17.477 | 0.0007 |
| D | 98.334 | 1 | 98.334 | 65.470 | 0.0000 |
| AB | 12.781 | 1 | 12.781 | 8.509 | 0.0101 |
| AC | 6.631 | 1 | 6.631 | 4.415 | 0.0518 |
| AD | 3.367 | 1 | 3.367 | 2.242 | 0.1538 |
| BC | 15.642 | 1 | 15.642 | 10.414 | 0.0053 |
| BD | 17.514 | 1 | 17.514 | 11.661 | 0.0035 |
| CD | 0.226 | 1 | 0.226 | 0.150 | 0.7034 |
| AA | 6.326 | 1 | 6.326 | 4.212 | 0.0569 |
| BB | 14.177 | 1 | 14.177 | 9.439 | 0.0073 |
| CC | 0.646 | 1 | 0.646 | 0.430 | 0.5211 |
| DD | 36.699 | 1 | 36.699 | 24.434 | 0.0001 |
| Error | 24.031 | 16 | 1.502 | ||
| Lack of fit | 20.055 | 10 | 2.006 | 3.026 | 0.0941 |
| Pure error | 3.976 | 6 | 0.663 | ||
| Total error | 24.031 | 16 | 1.502 | ||
| R2 = 0.966 | |||||
| A | B | C | D | Predicted Ester Content (wt %) | Experimental Ester Content (wt %) | Relative Error (%) |
|---|---|---|---|---|---|---|
| 0.10 mol·L−1 | 3.00:1 | 0.05 mol·L−1 | 5:1 | 101.2 | 97.6 | 3.6 |
| (A = 2) | (B = −2) | (C = 2) | (D = 2) | |||
| 0.08 mol·L−1 | 5.25:1 | 0.04 mol·L−1 | 4:1 | 96.7 | 96.9 | −0.2 |
| (A = 1) | (B = 1) | (C = 1) | (D = 1) |
| Run | Catalyst Concentration First Step | CH3OH/Oil Molar Ratio First Step | Catalyst Concentration Second Step | CH3OH/Oil Molar Ratio Second Step |
|---|---|---|---|---|
| 57 | 0.06 mol·L−1 | 6.00:1 | 0.01 mol·L−1 | 3:1 |
| 58 | 0.08 mol·L−1 | 6.00:1 | 0.01 mol·L−1 | 2:1 |
| 39 | 0.08 mol·L−1 | 5.25:1 | 0.01 mol·L−1 | 3:1 |
| 65 | 0.06 mol·L−1 | 6.00:1 | 0.02 mol·L−1 | 3:1 |
| Stream | 1″ | 2 | 3 | 4 | 5 | 6 | 7′ | 9 |
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | 45.0 | 20.0 | 20.0 | 45.0 | 25.0 | 25.0 | 20.0 | 45.0 |
| Pressure, kPa | 250 | 101 | 101 | 250 | 250 | 250 | 250 | 250 |
| Molar flow, kmol·h−1 | 6.69 | 20.5 | 3.88 | 42.9 | 30.5 | 12.5 | 20.3 | 50.8 |
| Mass flow, kg·h−1 | 6250 | 56 | 132 | 7418 | 6449 | 970 | 652 | 7100 |
| Component mass fraction | ||||||||
| Ricinolein | 1.000 | 0.000 | 0.000 | 0.091 | 0.078 | 0.177 | 0.000 | 0.016 |
| Methanol | 0.000 | 1.000 | 0.700 | 0.075 | 0.057 | 0.200 | 0.993 | 0.137 |
| NaOH | 0.000 | 0.000 | 0.300 | 0.005 | 0.004 | 0.009 | 0.006 | 0.004 |
| Methyl ricinoleate | 0.000 | 0.000 | 0.000 | 0.755 | 0.859 | 0.060 | 0.000 | 0.836 |
| Glycerol | 0.000 | 0.000 | 0.000 | 0.074 | 0.002 | 0.554 | 0.000 | 0.007 |
| H2O | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Stream | 12 | 15 | 17 | 18 | 19 | 22 | 23 | 25 |
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | 50 | 40.0 | 40.1 | 150.0 | 150.0 | 138.8 | 47.5 | 20.0 |
| Pressure, kPa | 50 | 101 | 101 | 20 | 20 | 60 | 50 | 250 |
| Molar flow, kmol·h−1 | 26.9 | 49.4 | 19.3 | 19.2 | 0.0285 | 6.74 | 5.50 | 32.2 |
| Mass flow, kg·h−1 | 863 | 890 | 6017 | 6016 | 0.942 | 784 | 176 | 1031 |
| Component mass fraction | ||||||||
| Ricinolein | 0.001 | 0.000 | 0.019 | 0.019 | 0.032 | 0.273 | 0.000 | 0.000 |
| Methanol | 0.999 | 0.000 | 0.001 | 0.001 | 0.964 | 0.080 | 1.000 | 1.000 |
| NaOH | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Methyl ricinoleate | 0.000 | 0.000 | 0.980 | 0.980 | 0.002 | 0.027 | 0.000 | 0.000 |
| Glycerol | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.864 | 0.000 | 0.000 |
| H2O | 0.000 | 1.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 |
| Equipment | Cost, Thousands of Dollars | |
|---|---|---|
| 50,000 t·year−1 | 16,000 t·year−1 | |
| Reactor (CRV–100) | 163.13 | 77.78 |
| Reactor (CRV–101) | 158.06 | 75.37 |
| Centrifuge (V–100) | 31.34 | 14.94 |
| Flash distiller (V–101) | 89.41 | 42.63 |
| Flash distiller (V–102) | 52.84 | 25.19 |
| Flash distiller (V–103) | 530.80 | 253.09 |
| Washing column (T–100) | 31.10 | 14.83 |
| Neutralization and removal of the catalyst (X–100) | 138.35 | 65.97 |
| Distillation column (T–101) | 123.00 | 58.65 |
| Heat exchangers | 410.71 | 195.83 |
| Pumps and valves | 112.86 | 53.81 |
| Tanks | 2056.71 | 980.66 |
| Total equipment cost | 3898.30 | 1858.76 |
| Concept | Factor, % | Cost, Thousands of Dollars | |
|---|---|---|---|
| 50,000 t·year−1 | 16,000 t·year−1 | ||
| Equipment costs | 100 | 3898.30 | 1858.76 |
| Installation | 47 | 1832.20 | 873.62 |
| Instrumentation and controls | 18 | 701.69 | 334.58 |
| Piping | 66 | 2572.88 | 1226.78 |
| Electrical systems | 11 | 428.81 | 204.46 |
| Buildings | 18 | 701.69 | 334.58 |
| Civil & structure | 10 | 389.83 | 185.88 |
| Service facilities | 70 | 2728.81 | 1301.13 |
| Total direct cost | 13,254.23 | 6319.78 | |
| Engineering supervision | 33 | 1286.44 | 613.39 |
| Construction costs | 41 | 1598.30 | 762.09 |
| Total indirect cost | 2884.74 | 1375.48 | |
| Legal expenses and contractors fee (about 5% of direct and indirect costs) | 21 | 818.64 | 390.34 |
| Contingency (about 10% of direct and indirect costs) | 42 | 1637.29 | 780.68 |
| Total capital cost (TCC) | 18,594.91 | 8866.28 | |
| Concept | Price, $·kg−1 | Cost, $·kg−1 Biodiesel | |
|---|---|---|---|
| 50,000 t·year−1 | 16,000 t·year−1 | ||
| Castor oil | 0.948 | 0.9849 | |
| Methanol | 0.393 | 0.0490 | |
| KOH | 1.87 | 0.0203 | |
| H3PO4 | 0.40 | 0.0006 | |
| Water | 0.0017 | 0.0002 | |
| Raw material costs | 0.5023 | ||
| K3PO4 | 0.64 | 0.0017 | |
| By-product | 0.0017 | ||
| Waste treatment | 0.21 | 0.0433 | |
| Energetic streams | 0.024 | 0.0144 | |
| Electricity | 0.157 | 0.0295 | |
| Energy costs | 0.0439 | ||
| Variable costs, $·kg−1 biodiesel | 1.1405 | ||
| Depreciation | 0.10·TCC | 0.0386 | 0.0576 |
| Repair | 0.03·TCC | 0.0116 | 0.0173 |
| Administrative costs | 0.03·TCC | 0.0116 | 0.0173 |
| Personal | 530,000 $·year−1 | 0.0111 | 0.0344 |
| Fixed cost, $·kg−1 biodiesel | 0.0729 | 0.1265 | |
| Total manufacturing cost, $·kg−1 biodiesel | 1.2134 | 1.2670 | |
| Total manufacturing cost, $·L−1 biodiesel | 1.1163 | 1.1657 | |
| Variable | Symbol | Coded Factor Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Catalyst concentration first step (mol·L−1) | A | 0.02 | 0.04 | 0.06 | 0.08 | 0.10 |
| Methanol/oil molar ratio first step | B | 3.00:1 | 3.75:1 | 4.50:1 | 5.25:1 | 6.00:1 |
| Catalyst concentration second step (mol·L−1) | C | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 |
| Methanol/oil molar ratio second step | D | 1:1 | 2:1 | 3:1 | 4:1 | 5:1 |
| Runs | A | B | C | D | Predicted Ester Content (wt %) | Experimental Ester Content (wt %) | Residual Value (wt %) |
|---|---|---|---|---|---|---|---|
| 1 | −2 | 0 | 0 | 0 | 84.8 | 85.9 | 1.1 |
| 2 | 2 | 0 | 0 | 0 | 97.4 | 97.8 | 0.4 |
| 3 | 0 | −2 | 0 | 0 | 84.3 | 84.2 | −0.1 |
| 4 | 0 | 2 | 0 | 0 | 96.0 | 97.8 | 1.8 |
| 5 | 0 | 0 | −2 | 0 | 90.3 | 91.3 | 1.0 |
| 6 | 0 | 0 | 2 | 0 | 94.4 | 95.0 | 0.6 |
| 7 | 0 | 0 | 0 | −2 | 84.4 | 84.4 | 0.0 |
| 8 | 0 | 0 | 0 | 2 | 92.5 | 94.0 | 1.5 |
| 9 | −1 | −1 | −1 | −1 | 78.3 | 78.2 | −0.1 |
| 10 | 1 | −1 | −1 | −1 | 86.8 | 85.7 | −1.1 |
| 11 | −1 | 1 | −1 | −1 | 90.1 | 89.2 | −0.9 |
| 12 | 1 | 1 | −1 | −1 | 95.0 | 96.0 | 1.0 |
| 13 | −1 | −1 | 1 | −1 | 83.4 | 84.0 | 0.6 |
| 14 | 1 | −1 | 1 | −1 | 89.4 | 90.1 | 0.7 |
| 15 | −1 | 1 | 1 | −1 | 91.2 | 90.4 | −0.8 |
| 16 | 1 | 1 | 1 | −1 | 93.6 | 92.6 | −1.0 |
| 17 | −1 | −1 | −1 | 1 | 83.3 | 83.0 | −0.3 |
| 18 | 1 | −1 | −1 | 1 | 93.6 | 94.3 | 0.7 |
| 19 | −1 | 1 | −1 | 1 | 90.9 | 89.9 | −1.0 |
| 20 | 1 | 1 | −1 | 1 | 97.6 | 95.7 | −1.9 |
| 21 | −1 | −1 | 1 | 1 | 88.9 | 87.7 | −1.2 |
| 22 | 1 | −1 | 1 | 1 | 96.6 | 96.2 | −0.4 |
| 23 | −1 | 1 | 1 | 1 | 92.5 | 92.4 | −0.1 |
| 24 | 1 | 1 | 1 | 1 | 96.7 | 96.5 | −0.2 |
| 25 | 0 | 0 | 0 | 0 | 93.0 | 92.5 | −0.5 |
| 26 | 0 | 0 | 0 | 0 | 93.0 | 93.5 | 0.5 |
| 27 | 0 | 0 | 0 | 0 | 93.0 | 92.3 | −0.7 |
| 28 | 0 | 0 | 0 | 0 | 93.0 | 91.9 | −1.1 |
| 29 | 0 | 0 | 0 | 0 | 93.0 | 93.5 | 0.5 |
| 30 | 0 | 0 | 0 | 0 | 93.0 | 94.2 | 1.2 |
| 31 | 0 | 0 | 0 | 0 | 93.0 | 92.9 | −0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, N.; Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel Production from Castor oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment. Catalysts 2019, 9, 864. https://doi.org/10.3390/catal9100864
Sánchez N, Encinar JM, Nogales S, González JF. Biodiesel Production from Castor oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment. Catalysts. 2019; 9(10):864. https://doi.org/10.3390/catal9100864
Chicago/Turabian StyleSánchez, Nuria, José María Encinar, Sergio Nogales, and Juan Félix González. 2019. "Biodiesel Production from Castor oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment" Catalysts 9, no. 10: 864. https://doi.org/10.3390/catal9100864
APA StyleSánchez, N., Encinar, J. M., Nogales, S., & González, J. F. (2019). Biodiesel Production from Castor oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment. Catalysts, 9(10), 864. https://doi.org/10.3390/catal9100864








