New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media
Abstract
1. Introduction
2. Results
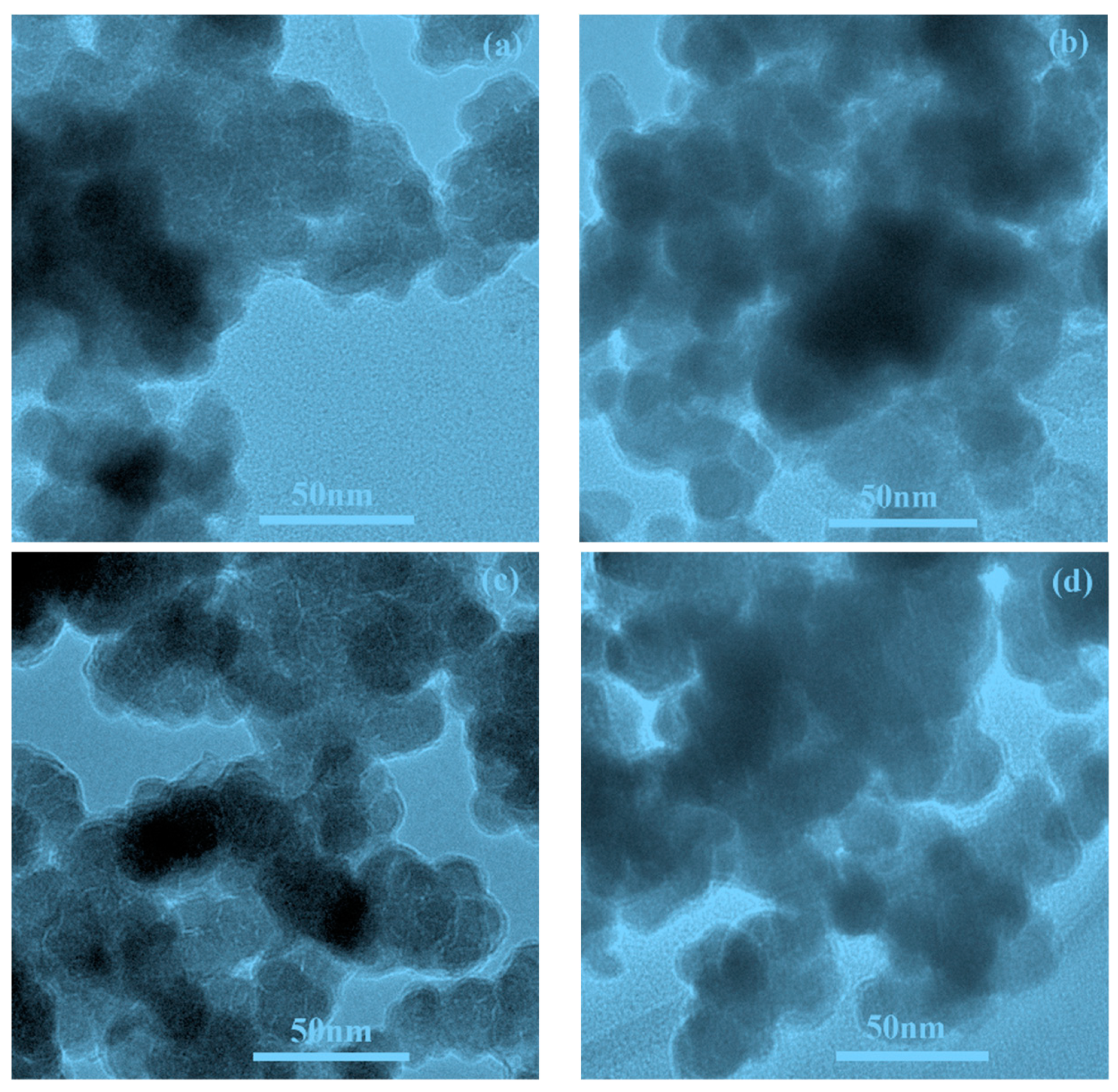
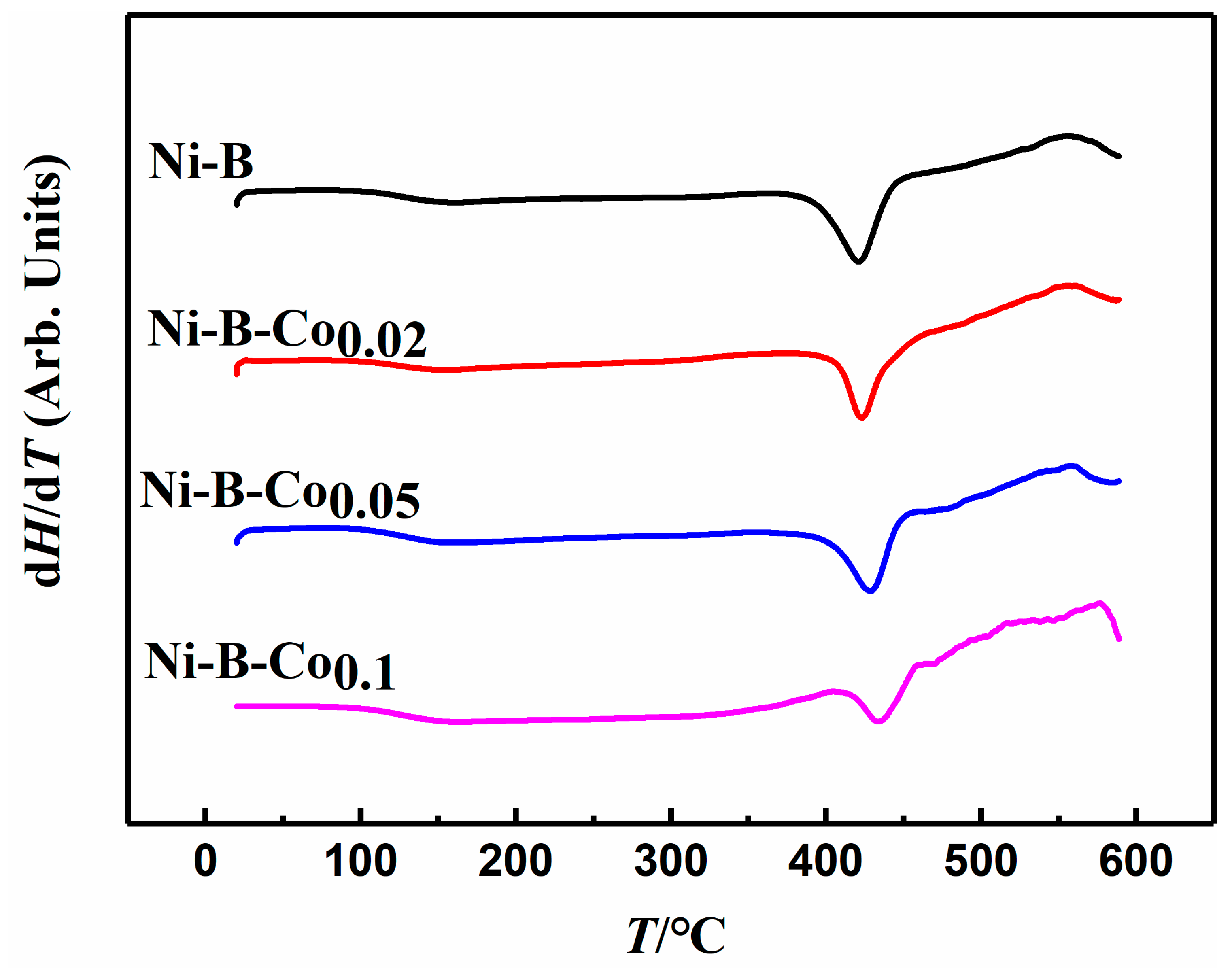
2.1. Physical Characterizations
2.2. Electrochemical Performance
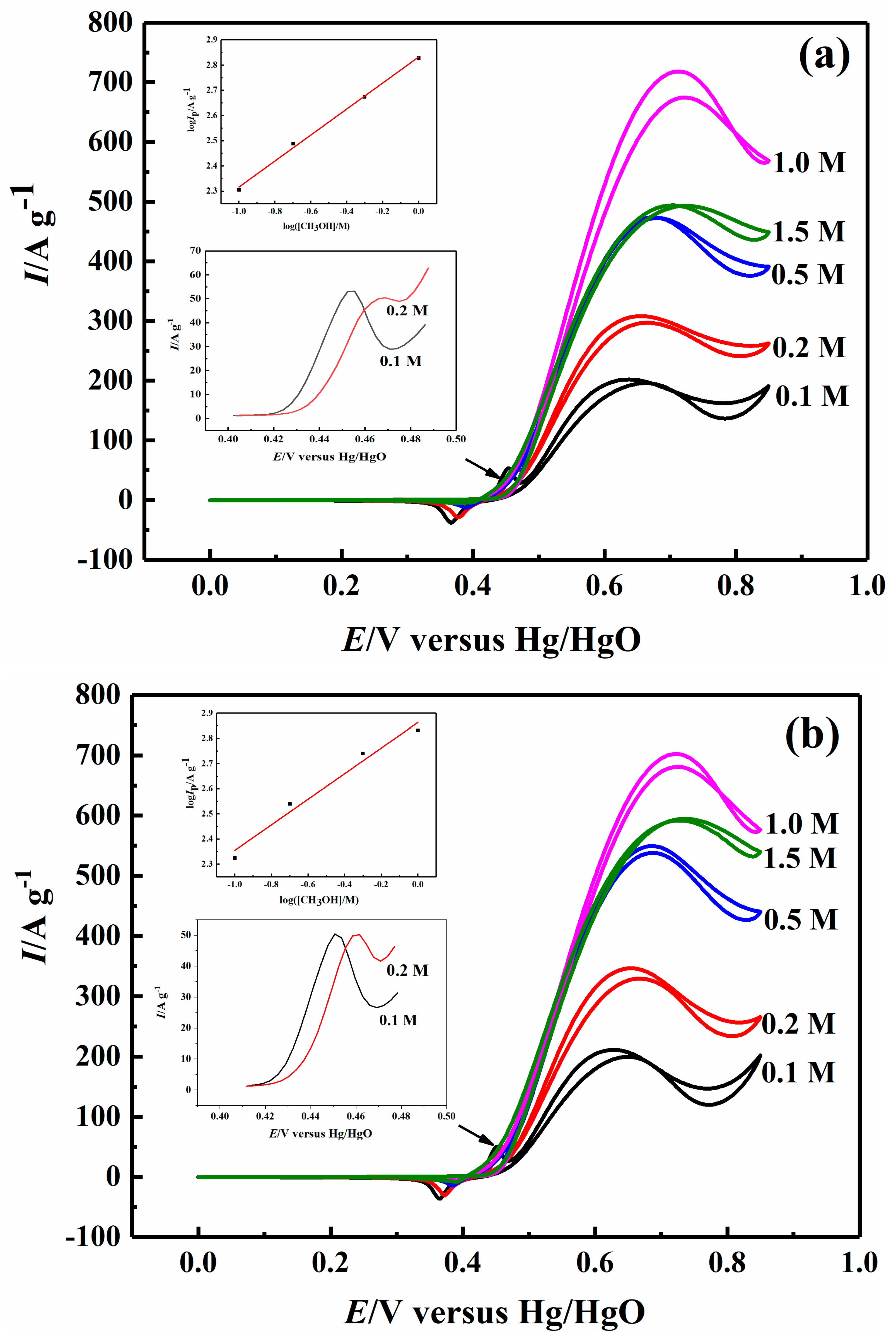
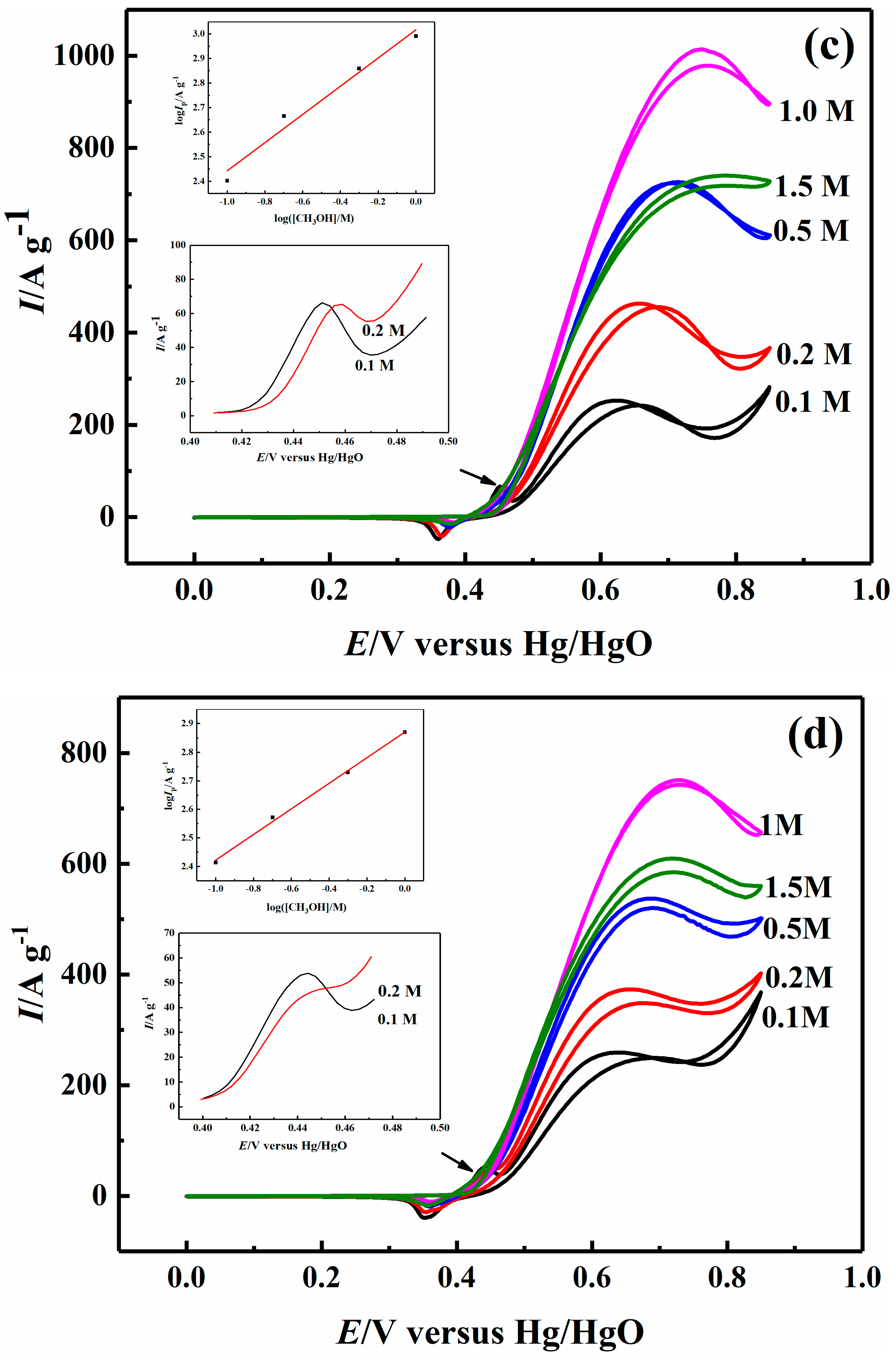
2.3. Effect of Methanol Concentration on Electrocatalytic Properties
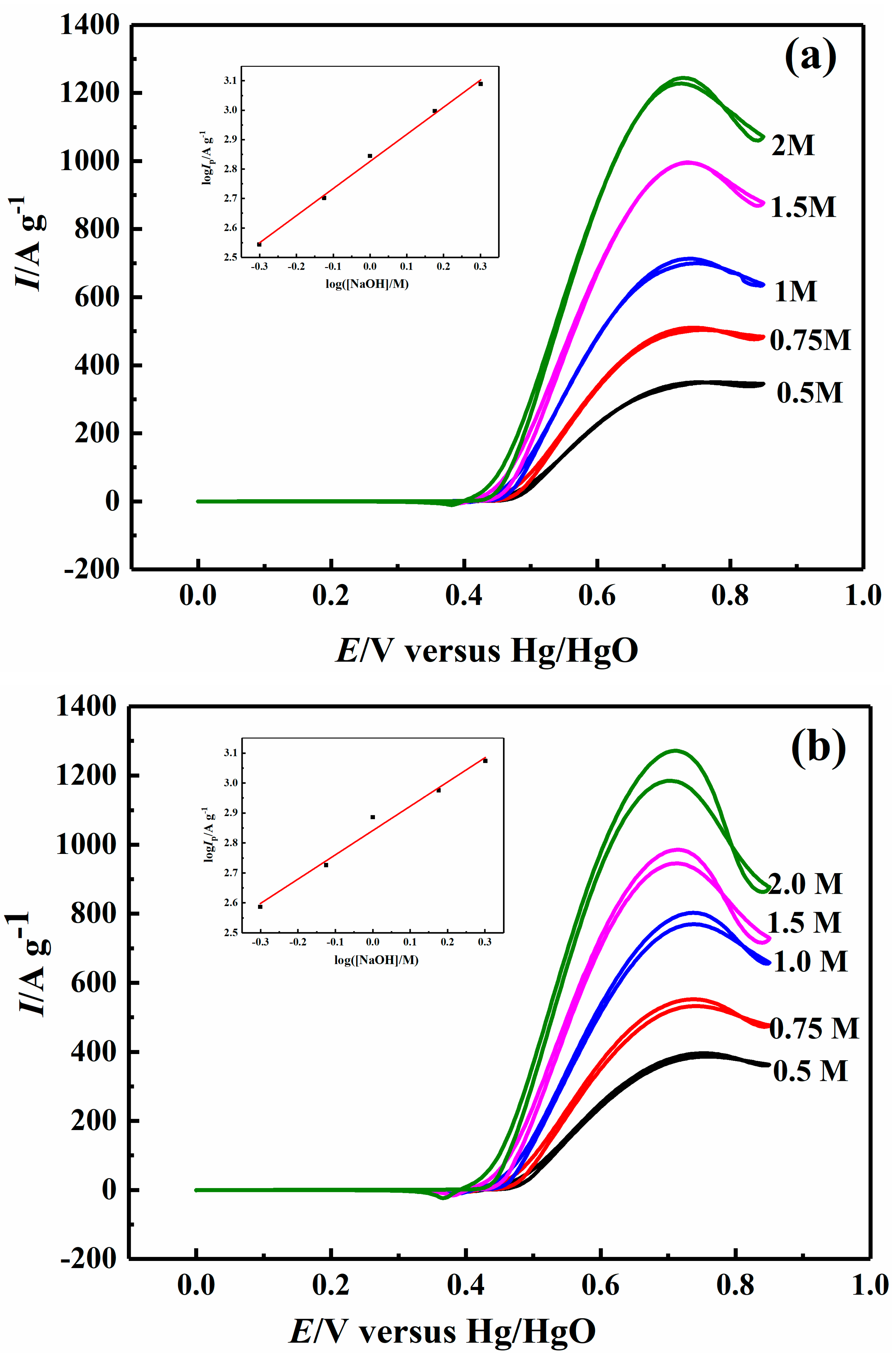
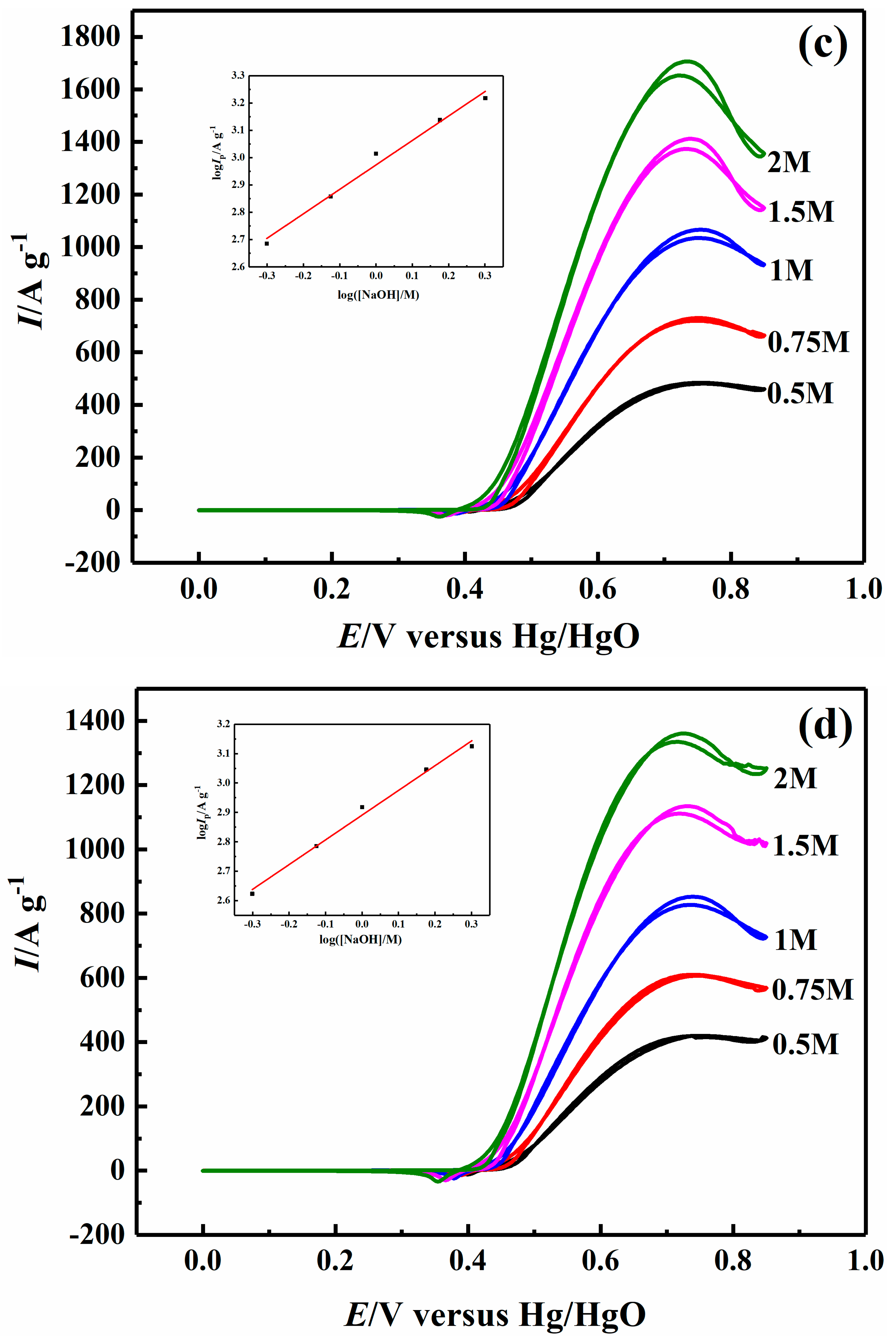
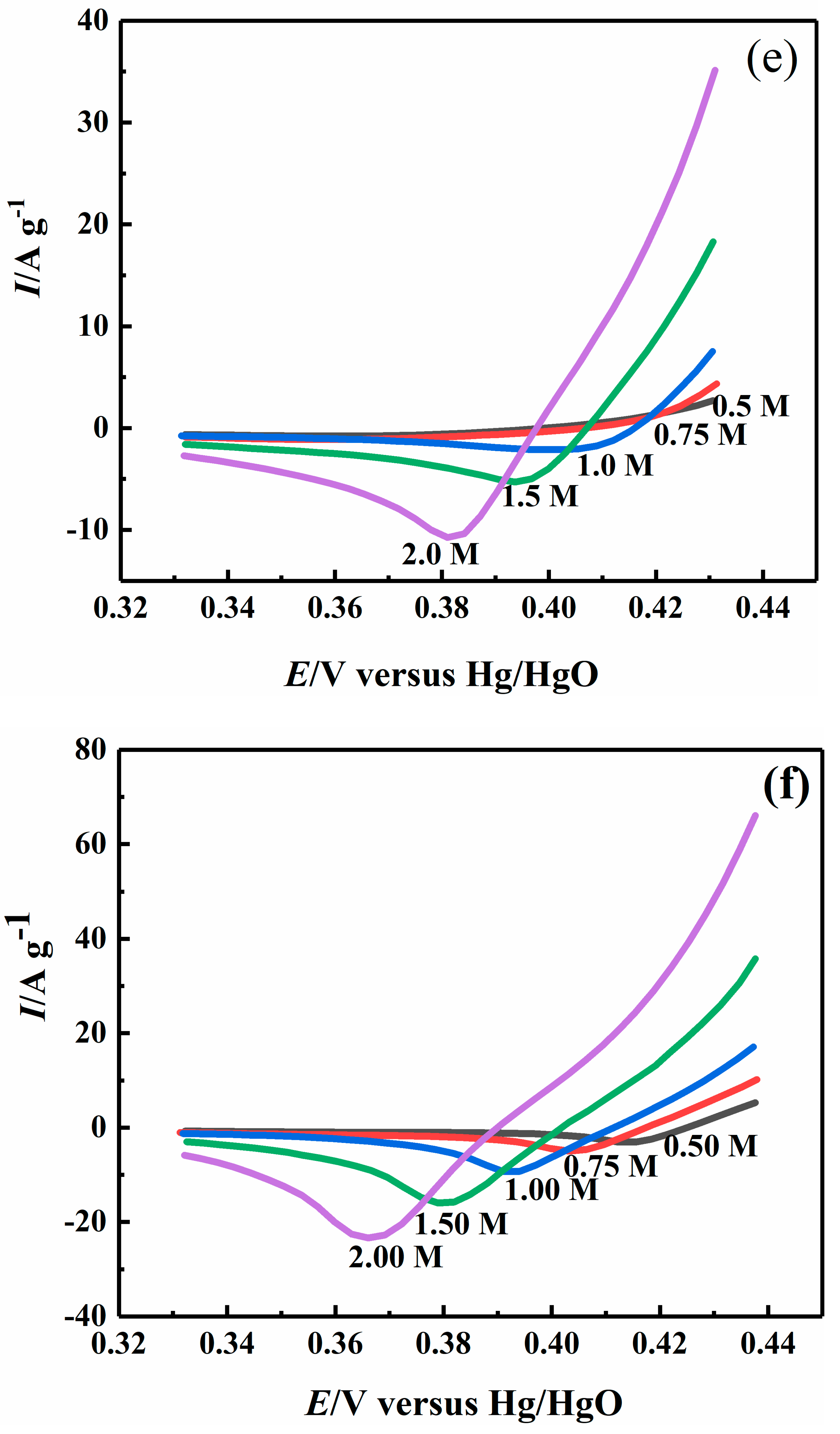
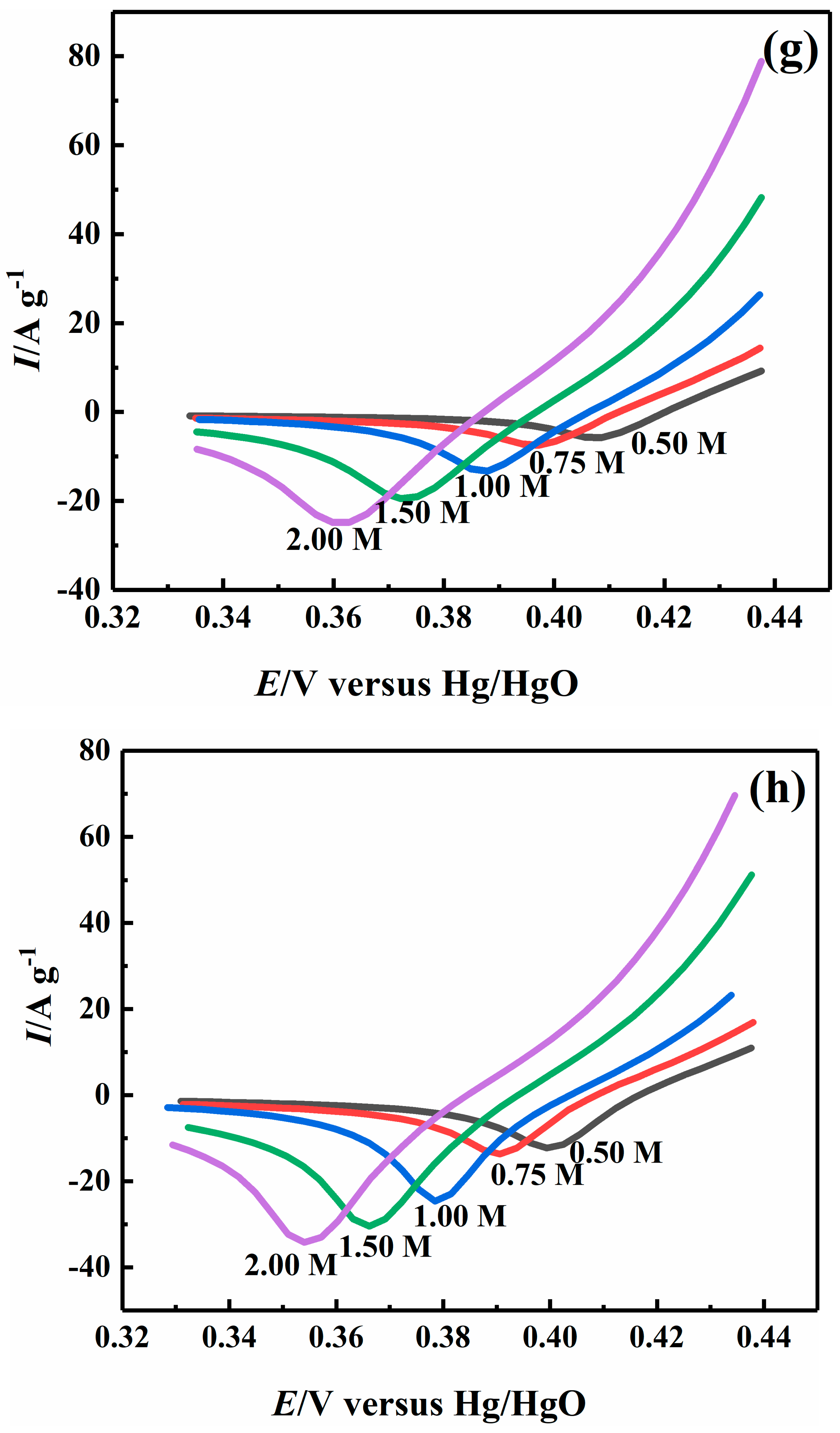
2.4. Effect of Sodium Hydroxide Concentration on Electrocatalytic Properties
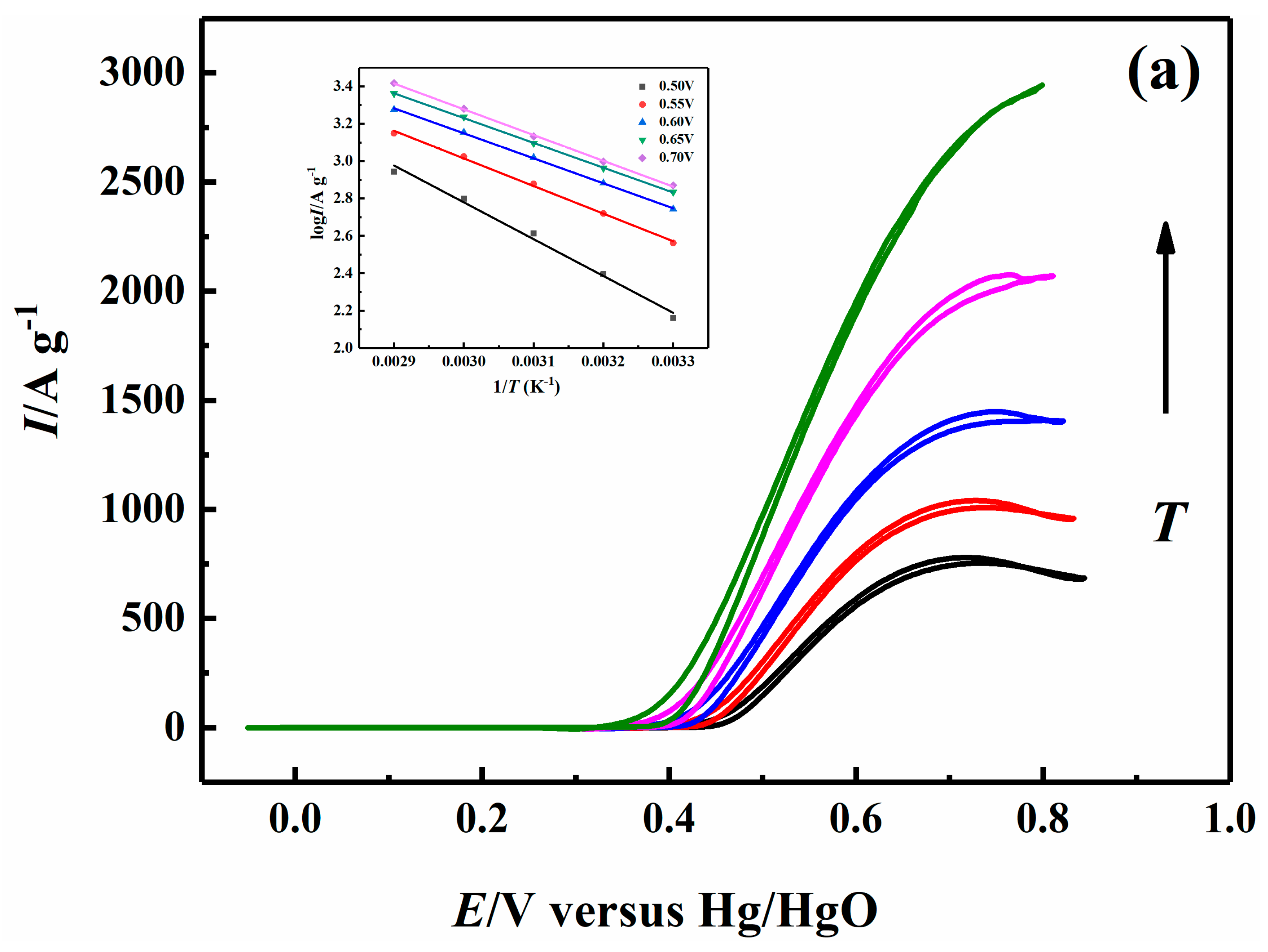
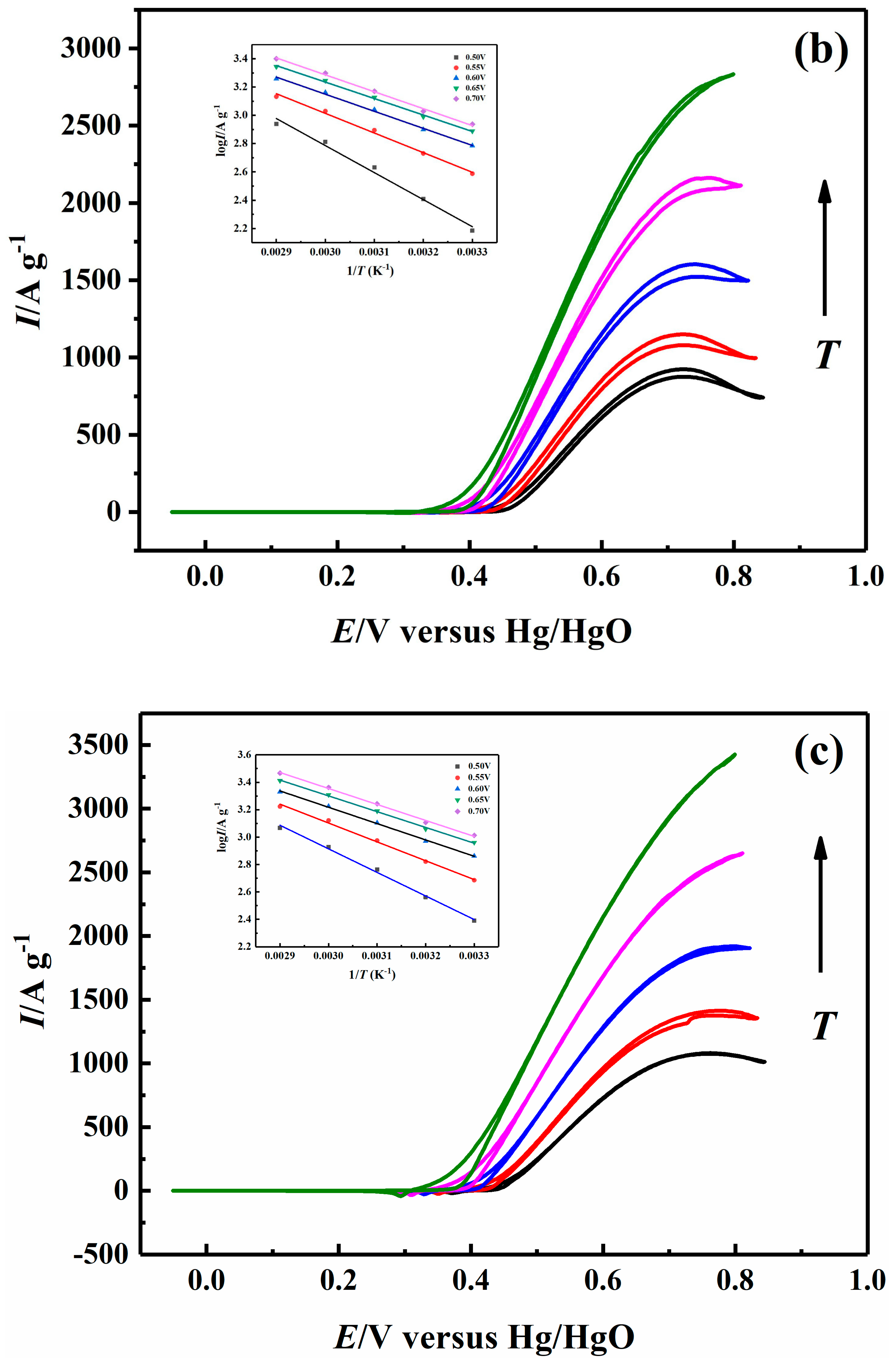
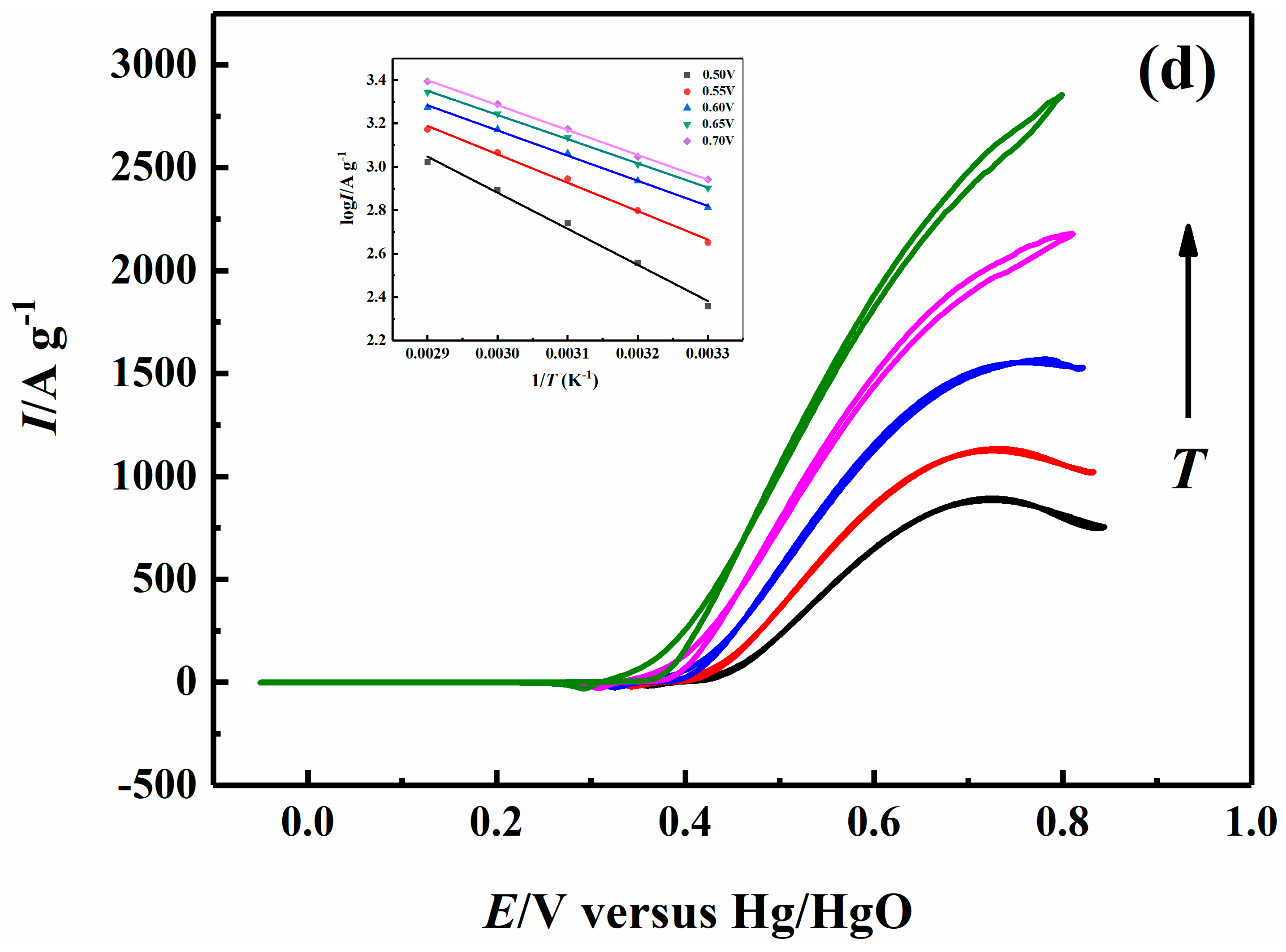
2.5. Effect of Temperature on Electrocatalytic Properties
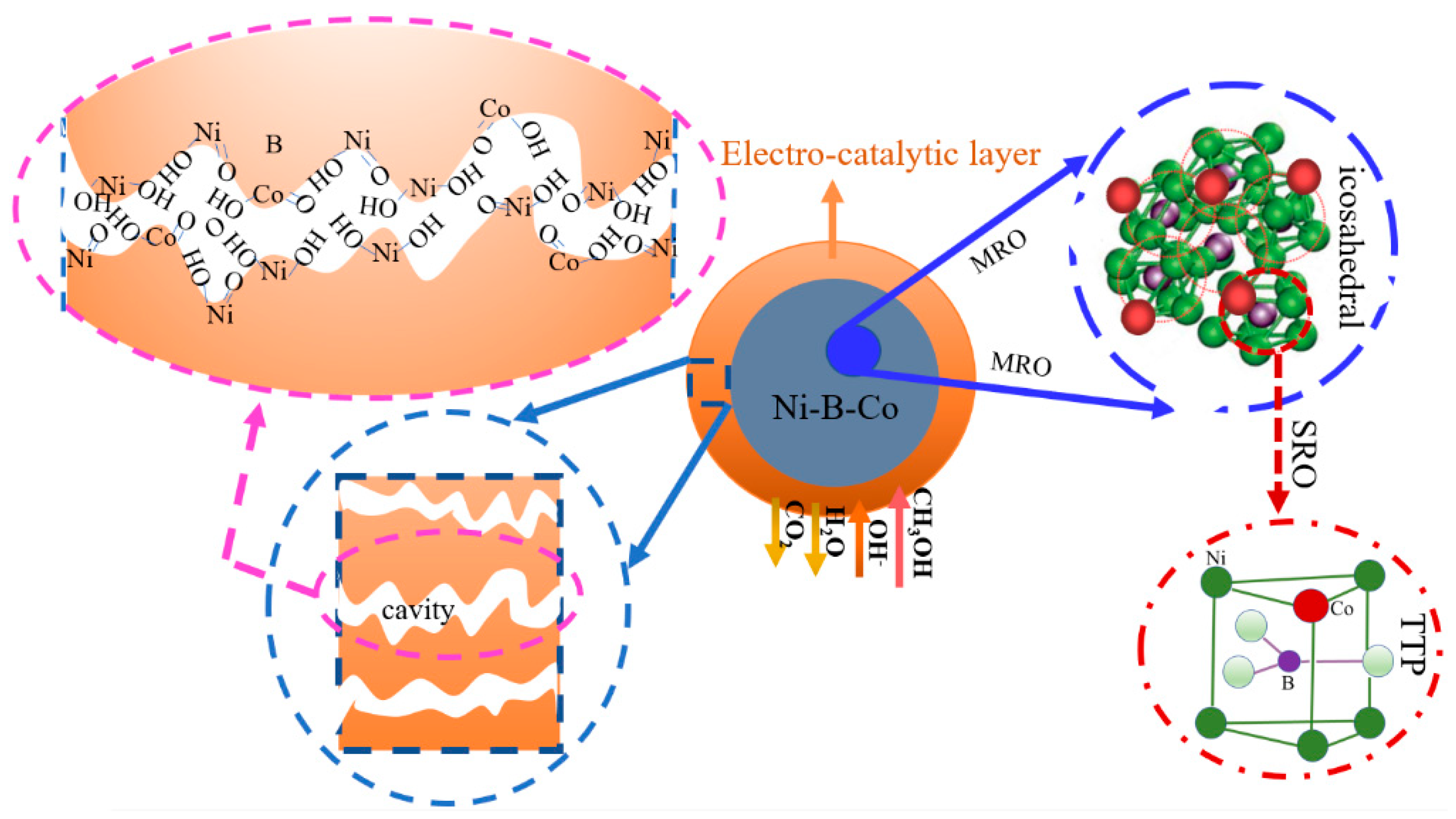
3. Discussion
4. Experimental
4.1. Preparation of the Catalyst Nanoparticles and Working Electrode
4.2. Physical and Electrochemical Characterizations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Joghee, P.; Malik, J.N.; Pylypenko, S.; O’Hayre, R. A review on direct methanol fuel cells –In the perspective of energy and sustainability. MRS Energy Sustain. 2015, 2, 1–31. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Yang, J. A selective electrocatalyst–based direct methanol fuel cell operated at high concentrations of methanol. Sci. Adv. 2017, 3, e1700580. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Wang, H.T.; Zhou, J.G.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.P.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.F.; Li, Z.F.; Wang, S.W.; Yu, X.J. Planar polyphthalocyanine cobalt absorbed on carbon black as stable electrocatalysts for direct methanol fuel cell. J. Power Sources 2010, 195, 4731–4735. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, T.; Singh, A.; Mishra, D.; Tiwari, S.K. Perovskite-type La2-xSrxNiO4 (0 ≤ x ≤ 1) as active anode materials for methanol oxidation in alkaline solutions. Electrochim. Acta 2008, 53, 2322–2330. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zheng, Y.X.; Yuan, L.S.; Zhao, L.H. Ni–B amorphous alloy nanoparticles modified nanoporous Cu toward ethanol oxidation in alkaline medium. J. Power Sources 2014, 247, 428–436. [Google Scholar] [CrossRef]
- Manivasakan, P.; Ramasamy, P.; Kim, J. Use of urchin-like NixCo3-xO4 hierarchical nanostructures based on non-precious metals as bifunctional electrocatalysts for anion-exchange membrane alkaline alcohol fuel cells. Nanoscale 2014, 6, 9665–9672. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The Kinetics and Mechanism of the Oxidation of Amines and Alcohols at Oxide-covered Nickel, Silver, Copper, and Cobalt Electrodes. J. Chem. Soc. Perkin Trans. 1972, 2, 1396–1403. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Yao, Y.Y.; Huang, X.; Willinger, M.; Shao, L.D.; Schlögl, R. Fabrication of nanoscale NiO/Ni heterostructures as electrocatalysts for efficient methanol oxidation. J. Mater. Chem. A 2017, 5, 9946–9951. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; et al. Multi-Component Fe-Ni hydroxide nanocatalyst for oxygen evolution and methanol oxidation reactions under alkaline conditions. ACS Catal. 2017, 7, 365–379. [Google Scholar] [CrossRef]
- Wang, W.Y.; Li, R.F.; Hua, X.; Zhang, R. Methanol electrooxidation on glassy carbon electrode modified with bimetallic Ni(II)Co(II)salen complexes encapsulated in mesoporous zeolite A. Electrochim. Acta 2015, 163, 48–56. [Google Scholar] [CrossRef]
- Wang, W.Y.; Li, R.F.; Zhang, R.; Ma, J.H.; Wang, B.C. Electrocatalytic oxidation of methanol on glassy carbon electrode modified with nickel-manganese salen complexes encapsulated in mesoporous zeolite A. J. Electroanal. Chem. 2015, 742, 110–121. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.Y.; Sun, X.P.; Asiri, A.M. In situ growth of nickel selenide nanowire arrays on nickel foil for methanol electro-oxidation in alkaline media. RSC Adv. 2015, 5, 87051–87054. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.Y.; Sun, X.P.; Asiri, A.M. Hierarchical nickel oxide nanosheet@nanowire arrays on nickel foam: An efficient 3D electrode for methanol electro-oxidation. Catal. Sci. Technol. 2016, 6, 1157–1161. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Duan, D.H.; Ma, Y.H.; Wei, G.Q.; Zhang, Z.L.; Hao, X.G.; Liu, S.B. Performance of nano-nickel core wrapped with Pt crystalline thin film for methanol electro-oxidation. J. Power Sources 2014, 245, 886–891. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hameed, R.M.A.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Yao, S.B.; Zhou, S.M. Electrocatalytic oxidation of methanol at the nano Ni-B amorphous alloy powder microelectrode. Electrochem. Acta Phys. -Chim. Sin. 2007, 13, 307–311. [Google Scholar]
- Yuan, L.S.; Zheng, Y.X.; Jia, M.L.; Zhang, S.J.; Wang, X.L.; Peng, C. Nanoporous nickel-copper-phosphorus amorphous alloy film for methanol electro-oxidation in alkaline medium. Electrochim. Acta 2015, 154, 54–62. [Google Scholar] [CrossRef]
- Hassan, H.B.; Hamid, Z.A. Electroless Ni–B supported on carbon for direct alcohol fuel cell applications. Int. J. Hydrogen Energe 2011, 36, 849–856. [Google Scholar] [CrossRef]
- Cao, H.Z.; Wang, Z.W.; Hou, G.Y.; Zheng, G.Q. TiO2 nanotube-supported amorphous Ni-B electrode for electrocatalytic oxidation of methanol. Surf. Coat. Technol. 2010, 205, 885–889. [Google Scholar] [CrossRef]
- Hameed, R.M.A.; El-Khatib, K.M. Ni-P and Ni-Cu-P modified carbon catalysts for methanol electro-oxidation in KOH solution. Int. J. Hydrogen Energe 2010, 35, 2517–2529. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Tang, H.; Wang, X.L.; Tu, J.P. Three-dimensional astrocyte-network Ni-P-O compound with superior electrocatalytic activity and stability for methanol oxidation in alkaline environments. J. Mater. Chem. A 2015, 3, 4669–4678. [Google Scholar] [CrossRef]
- Wu, F.H.; Zhang, Z.L.; Zhang, F.S.; Duan, D.H.; Li, Y.; Wei, G.Q.; Liu, S.B.; Yuan, Q.B.; Wang, E.Z.; Hao, X.G. Exploring the role of cobalt in promoting the electroactivity of amorphous Ni-B nanoparticles toward methanol oxidation. Electrochim. Acta 2018, 287, 115–123. [Google Scholar] [CrossRef]
- French, H.M.; Henderson, M.J.; Hillman, A.R.; Vieil, E. Ion and solvent transfer discrimination at a nickel hydroxide film exposed to LiOH by combined electrochemical quartz crystal microbalance (EQCM) and probe beam deflection (PBD) techniques. J. Electroanal. Chem. 2001, 500, 192–207. [Google Scholar] [CrossRef]
- French, H.M.; Henderson, M.J.; Hillman, A.R.; Vieil, E. Temporal resolution of ion and solvent transfers at nickel hydroxide films exposed to LiOH. Solid State Ion. 2002, 150, 27–37. [Google Scholar] [CrossRef]
- Taraszewska, J.; Rosłonek, G. Electrocatalytic oxidation of methanol on a glassy carbon electrode modified by nickel hydroxide formed by ex situ chemical precipitation. J. Electroanal. Chem. 1994, 364, 209–213. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Abdelkareem, M.A.; Yousef, A.; Al-Deyab, S.S.; El-Newehy, M.; Kim, H.Y. Cadmium-doped cobalt/carbon nanoparticles as novel nonprecious electrocatalyst for methanol oxidation. Int. J. Hydrogen. Energe. 2013, 38, 3387–3394. [Google Scholar] [CrossRef]
- Deng, J.F.; Yan, J.; Sheng, S.S.; Chen, H.R.; Xiong, G.X. The study of ultrafine Ni-B and Ni-P amorphous alloy powders as catalysits. J. Catal. 1994, 150, 434–438. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ma, A.Z.; Mu, X.H.; Min, E.Z. Selective hydrogenation of reformate oils over amorphous NiB/SiO2 catalyst. Catal. Today 2002, 74, 77–84. [Google Scholar] [CrossRef]
- Das, A.K.; Layek, R.K.; Kim, N.H.; Jung, D.; Lee, J.H. Reduced graphene oxide (RGO)-supported NiCo2O4 nanoparticles: An electrocatalyst for methanol oxidation. Nanoscale 2014, 6, 10657–10665. [Google Scholar] [CrossRef]
- Wang, W.; Chu, Q.X.; Zhang, Y.N.; Zhu, W.; Wang, X.F.; Liu, X.Y. Nickel foam supported mesoporous NiCo2O4 arrays with excellent methanol electro-oxidation performance. New J. Chem. 2015, 39, 6491–6497. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Gu, C.D.; Zhang, J.L.; Tang, H.; Li, Y.; Wang, X.L.; Tu, J.P. Urchin-like Ni-Co-P-O nanocomposite as novel methanol electro-oxidation materials in alkaline environment. Electrochim. Acta 2016, 187, 11–19. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yan, J.; Zhang, Y.S.; Yuan, H.T.; Song, D.Y. Cyclic voltammetric studies of pasted nickel hydroxide electrode microencapsulated by cobalt. J. Appl. Electrochem. 1998, 28, 1377–1382. [Google Scholar] [CrossRef]
- Yu, Z.B.; Qiao, M.H.; Li, H.X.; Deng, J.F. Preparation of amorphous NiCoB alloys and the effect of cobalt on their hydrogenation activity. Appl. Catal. A Gen. 1997, 163, 1–13. [Google Scholar] [CrossRef]
- Xie, S.H.; Li, H.X.; Li, H.; Deng, J.F. Selective hydrogenation of stearonitrile over Ni–B/SiO2 amorphous catalysts in comparison with other Ni-based catalysts. Appl. Catal. A Gen. 1999, 189, 45–52. [Google Scholar] [CrossRef]
- Xie, S.; Tong, X.L.; Jin, G.Q.; Qin, Y.; Guo, X.Y. CNT-Ni/SiC hierarchical nanostructures: Preparation and their application in electrocatalytic oxidation of methanol. J. Mater. Chem. A 2013, 1, 2104–2109. [Google Scholar] [CrossRef]
- Velázquez-Palenzuela, A.; Centellas, F.; Garrido, J.A.; Arias, C.; Rodríguez, R.M.; Brillas, E.; Cabot, P.L. Kinetic analysis of carbon monoxide and methanol oxidation on high performance carbon-supported Pt–Ru electrocatalyst for direct methanol fuel cells. J. Power Sources 2011, 196, 3503–3512. [Google Scholar] [CrossRef]
- Xu, C.W.; Hu, Y.H.; Rong, J.H.; Jiang, S.P.; Liu, Y.L. Ni hollow spheres as catalysts for methanol and ethanol electrooxidation. Electrochem. Commun. 2007, 9, 2009–2012. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K.; Reeve, R.W.; Yang, L.X.; Allen, R.G. Characterisation of platinised Ti mesh electrodes using electrochemical methods: Methanol oxidation in sodium hydroxide solutions. Electrochim. Acta 2004, 49, 2443–2452. [Google Scholar] [CrossRef]
- Döner, A.; Telli, E.; Kardaş, G. Electrocatalysis of Ni-promoted Cd coated graphite toward methanol oxidation in alkaline medium. J. Power Sources 2012, 205, 71–79. [Google Scholar] [CrossRef]
- Cohen, J.L.; Volpe, D.J.; Abruña, H.D. Electrochemical determination of activation energies for methanol oxidation on polycrystalline platinum in acidic and alkaline electrolytes. Phys. Chem. Chem. Phys. 2007, 9, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Julio, P. Physical Chemistry; Oxford University Press: Oxford, UK, 2006; pp. 883–884. [Google Scholar]
- Gaskell, P.H. A new structural model for transition metal–metalloid glasses. Nature 1978, 276, 484–485. [Google Scholar] [CrossRef]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.M.; Gaskell, P.H.; Caër, G.L. A model for the structure of metallic glasses based on chemical twinning. Proc. R. Soc. Lond. A 1985, 402, 323–357. [Google Scholar] [CrossRef]
- Luo, W.K.; Sheng, H.W.; Ma, E. Pair correlation functions and structural building schemes in amorphous alloys. Appl. Phys. Lett. 2006, 89, 131927. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
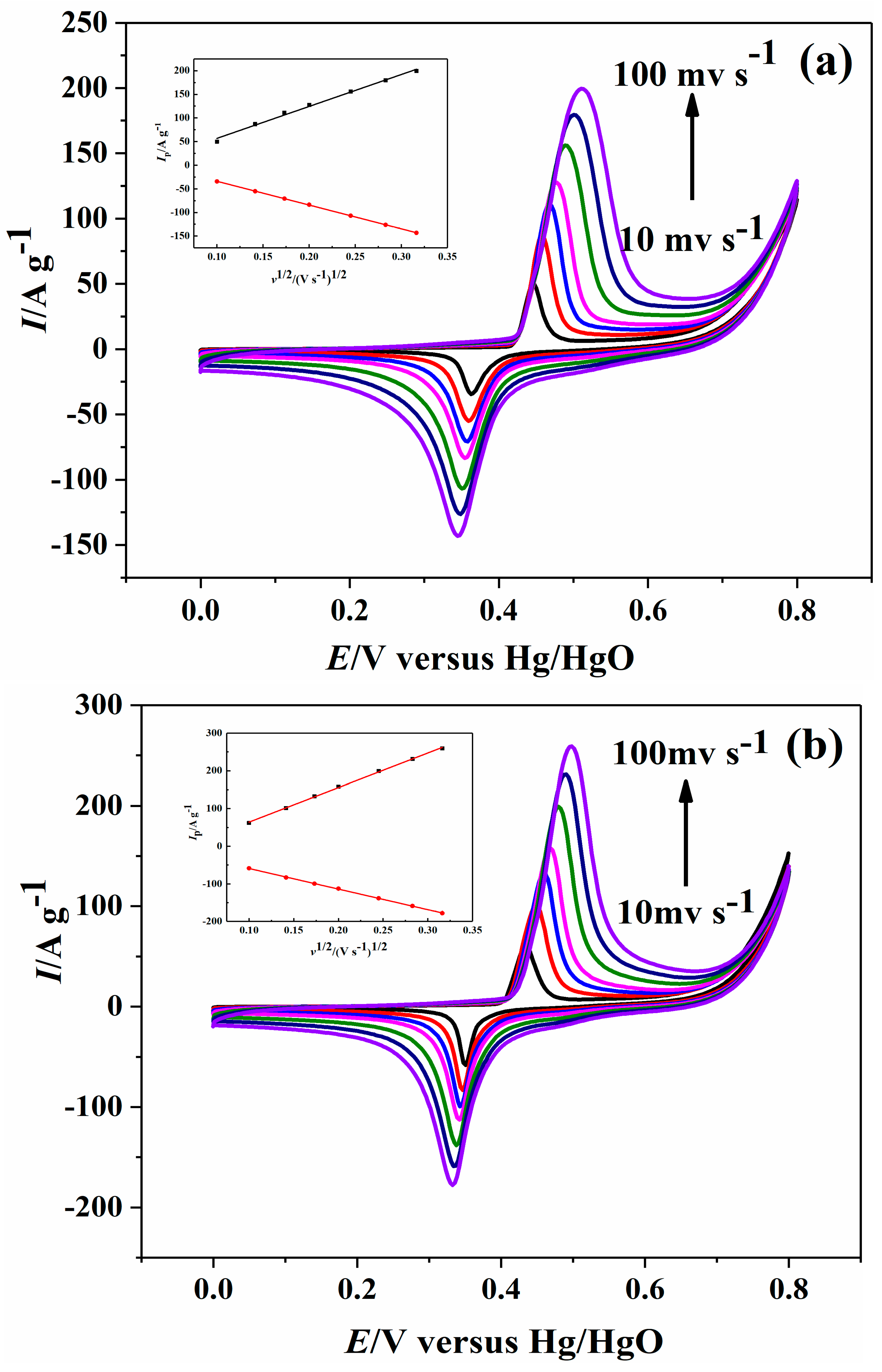
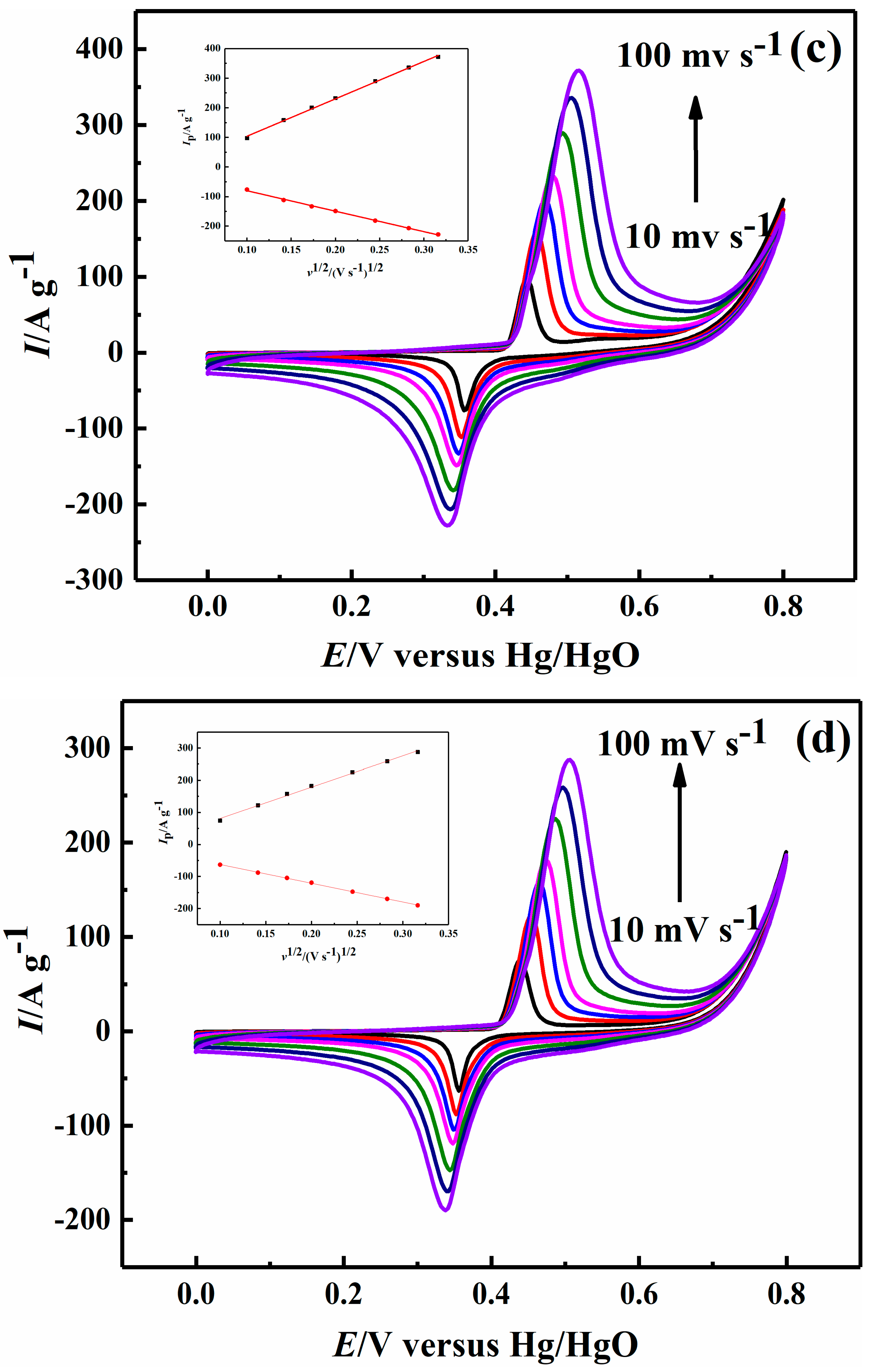
| Sample | Ni-B | Ni-B-Co0.02 | Ni-B-Co0.05 | Ni-B-Co0.1 |
|---|---|---|---|---|
| 10 mV s−1 | 49.98, −34.37 | 61.76, −58.38 | 97.33, −76.42 | 74.29, −63.12 |
| 20 mV s−1 | 86.89, −55.09 | 101.12, −83.09 | 158.27, −111.66 | 122.54, −87.94 |
| 30 mV s−1 | 111.01, −70.68 | 132.91, −99.54 | 200.53, −133.18 | 157.82, −104.20 |
| 40 mV s−1 | 127.64, −83.52 | 158.21, −112.60 | 232.52, −148.67 | 182.08, −119.08 |
| 60 mV s−1 | 155.94, −106.84 | 199.21, −138.24 | 289.39, −181.61 | 225.22, −147.34 |
| 80 mV s−1 | 179.41, −126.28 | 231.28, −158.80 | 335.77, −206.66 | 258.44, −169.62 |
| 100 mV s−1 | 199.47, −143.13 | 259.25, −177.9 | 371.84, −228.04 | 287.28, −189.79 |
| Sample | Ni-B | Ni-B-Co0.02 | Ni-B-Co0.05 | Ni-B-Co0.1 |
|---|---|---|---|---|
| 0.1 M CH3OH | 203.03 | 211.05 | 254.84 | 259.22 |
| 0.2 M CH3OH | 305.39 | 346.50 | 464.11 | 373.31 |
| 0.5 M CH3OH | 472.96 | 549.50 | 723.37 | 537.31 |
| 1 M CH3OH | 673.94 | 680.87 | 978.49 | 742.65 |
| 1.5 M CH3OH | 494.19 | 594.73 | 740.13 | 609.44 |
| Sample | Ni-B | Ni-B-Co0.02 | Ni-B-Co0.05 | Ni-B-Co0.1 |
|---|---|---|---|---|
| 1.0 M NaOH | 34.36 | 58.38 | 76.42 | 63.12 |
| 0.1 M CH3OH | 37.93 | 35.87 | 47.52 | 39.12 |
| 0.2 M CH3OH | 28.76 | 30.48 | 41.34 | 29.28 |
| 0.5 M CH3OH | 12.64 | 14.18 | 22.03 | 18.80 |
| 1 M CH3OH | 2.05 | 6.34 | 11.52 | 10.27 |
| 1.5 M CH3OH | 4.77 | 8.00 | 14.76 | 16.60 |
| Sample | Ni-B | Ni-B-Co0.02 | Ni-B-Co0.05 | Ni-B-Co0.1 |
|---|---|---|---|---|
| 0.50 V | 37.69 | 36.54 | 32.88 | 31.84 |
| 0.55 V | 28.27 | 26.55 | 26.31 | 25.06 |
| 0.60 V | 25.58 | 23.10 | 22.79 | 22.20 |
| 0.65 V | 25.43 | 22.26 | 22.01 | 21.35 |
| 0.70 V | 26.38 | 22.86 | 22.29 | 21.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Zhang, Z.; Zhang, F.; Duan, D.; Li, Y.; Wei, G.; Liu, S.; Yuan, Q.; Wang, E.; Hao, X. New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media. Catalysts 2019, 9, 749. https://doi.org/10.3390/catal9090749
Wu F, Zhang Z, Zhang F, Duan D, Li Y, Wei G, Liu S, Yuan Q, Wang E, Hao X. New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media. Catalysts. 2019; 9(9):749. https://doi.org/10.3390/catal9090749
Chicago/Turabian StyleWu, Fanhua, Zhonglin Zhang, Fusheng Zhang, Donghong Duan, Yu Li, Guoqiang Wei, Shibin Liu, Qinbo Yuan, Enzhi Wang, and Xiaogang Hao. 2019. "New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media" Catalysts 9, no. 9: 749. https://doi.org/10.3390/catal9090749
APA StyleWu, F., Zhang, Z., Zhang, F., Duan, D., Li, Y., Wei, G., Liu, S., Yuan, Q., Wang, E., & Hao, X. (2019). New Insights into the Electrocatalytic Mechanism of Methanol Oxidation on Amorphous Ni-B-Co Nanoparticles in Alkaline Media. Catalysts, 9(9), 749. https://doi.org/10.3390/catal9090749





