Rapid Microwave-Assisted Polyol Synthesis of TiO2-Supported Ruthenium Catalysts for Levulinic Acid Hydrogenation
Abstract
:1. Introduction
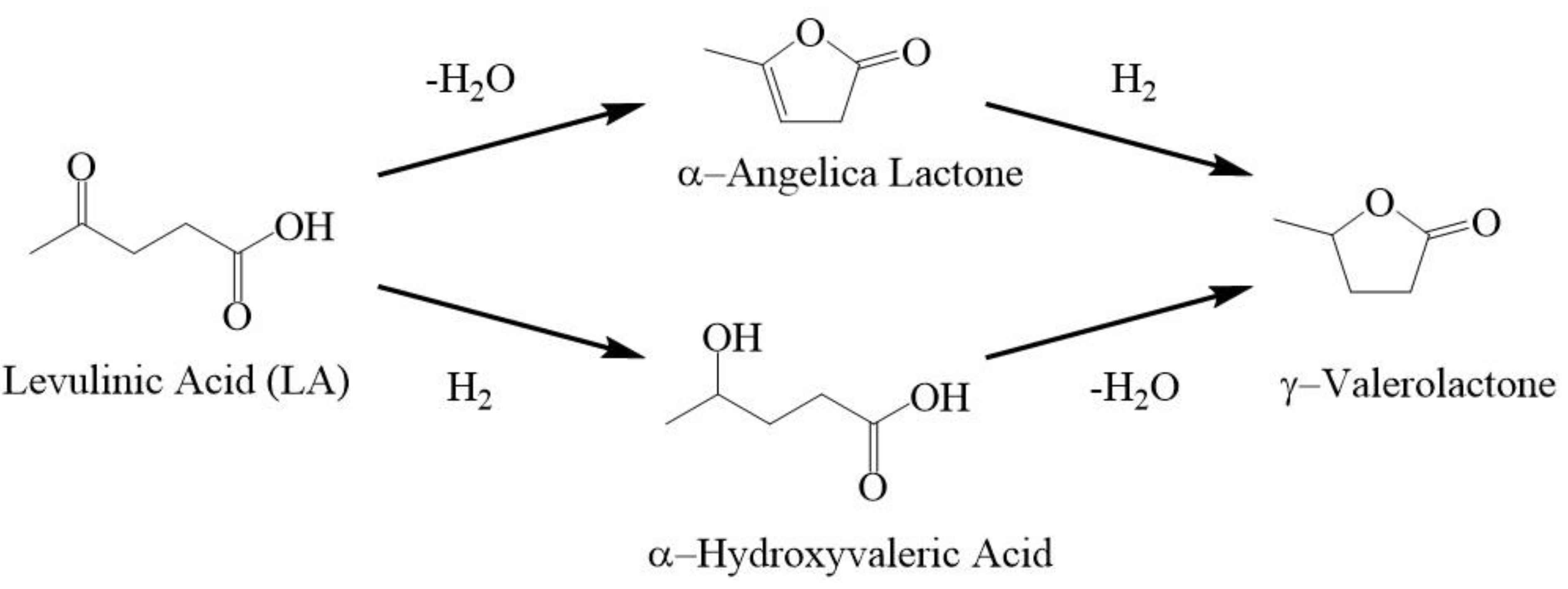
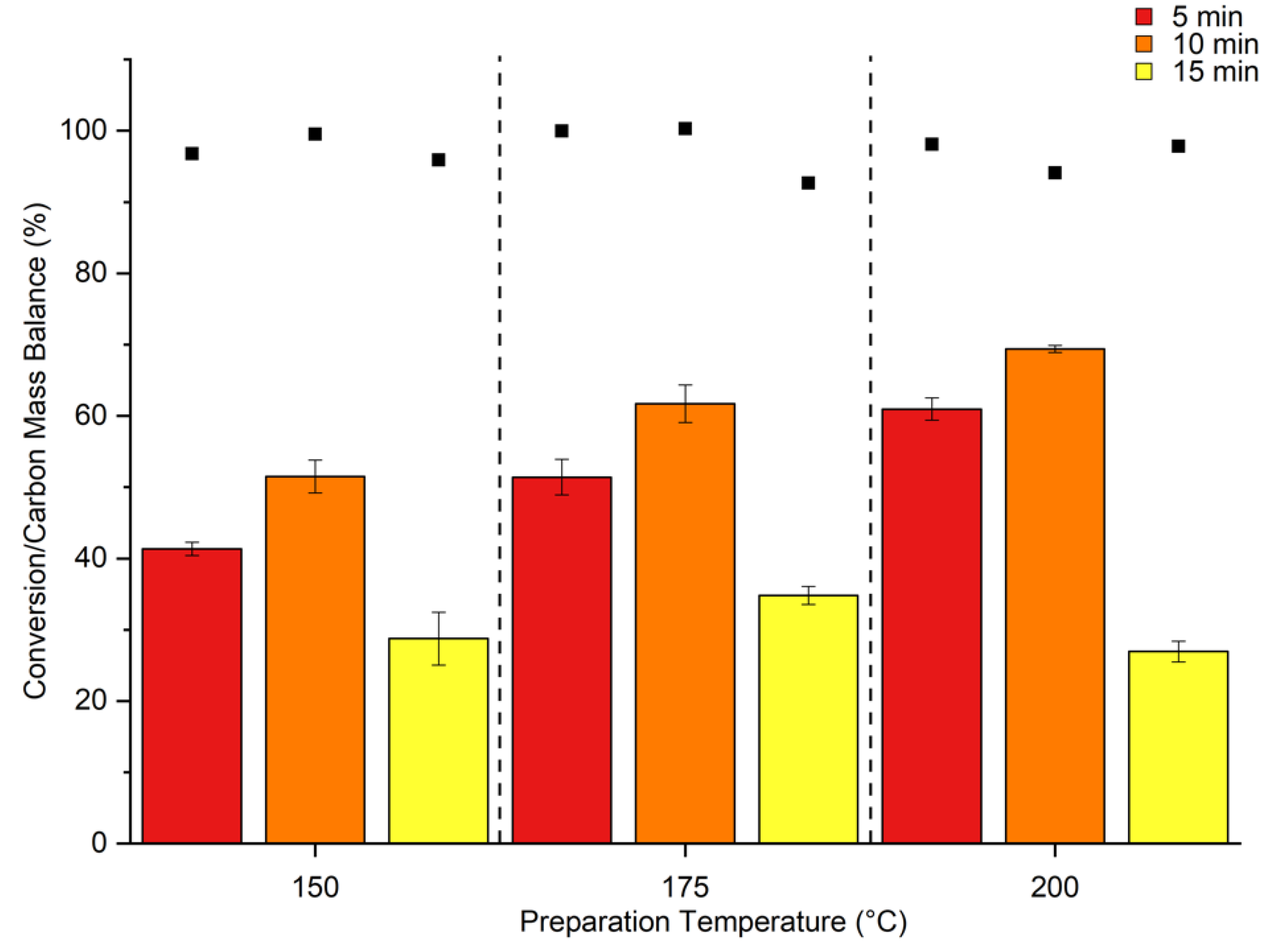
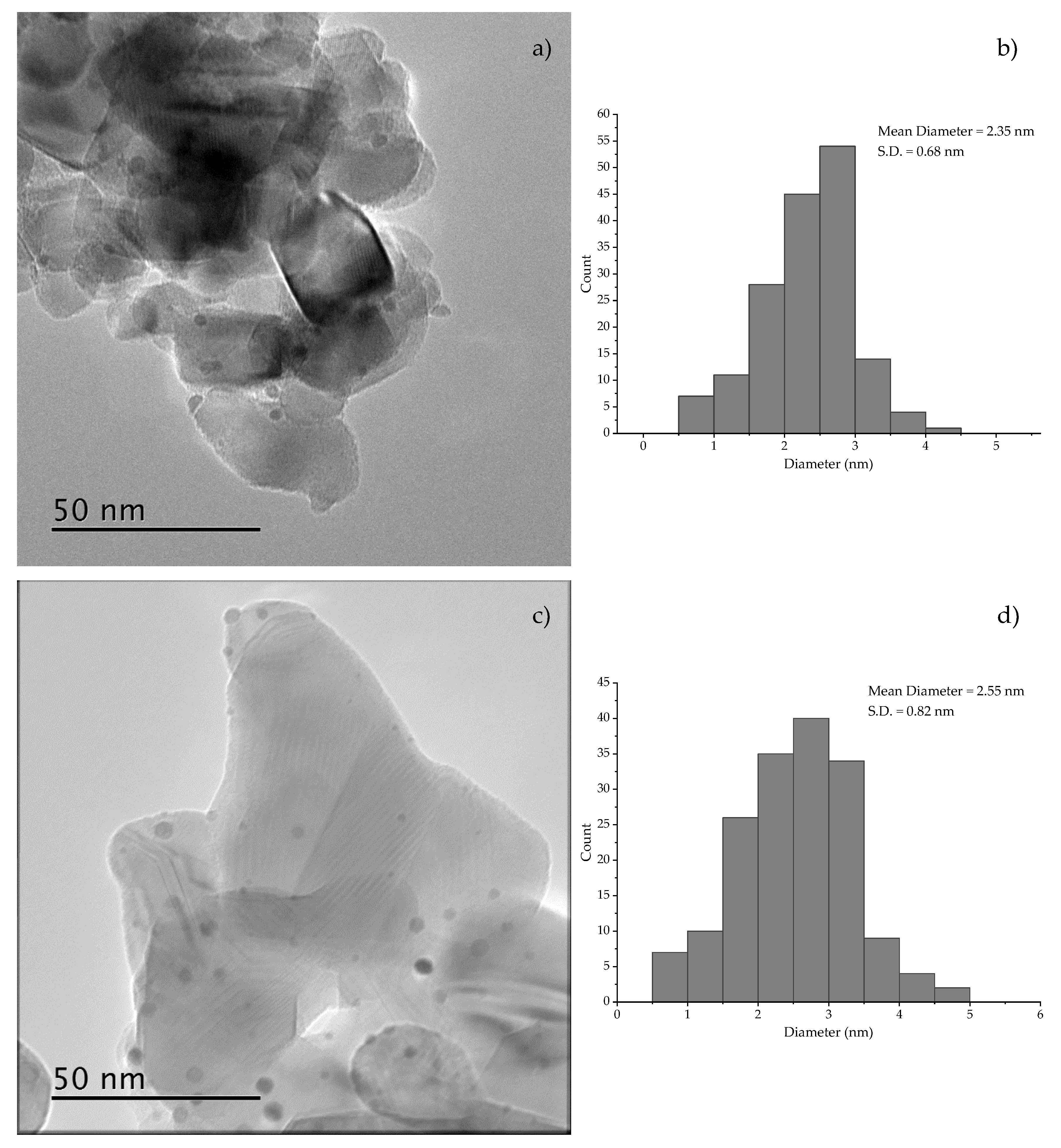
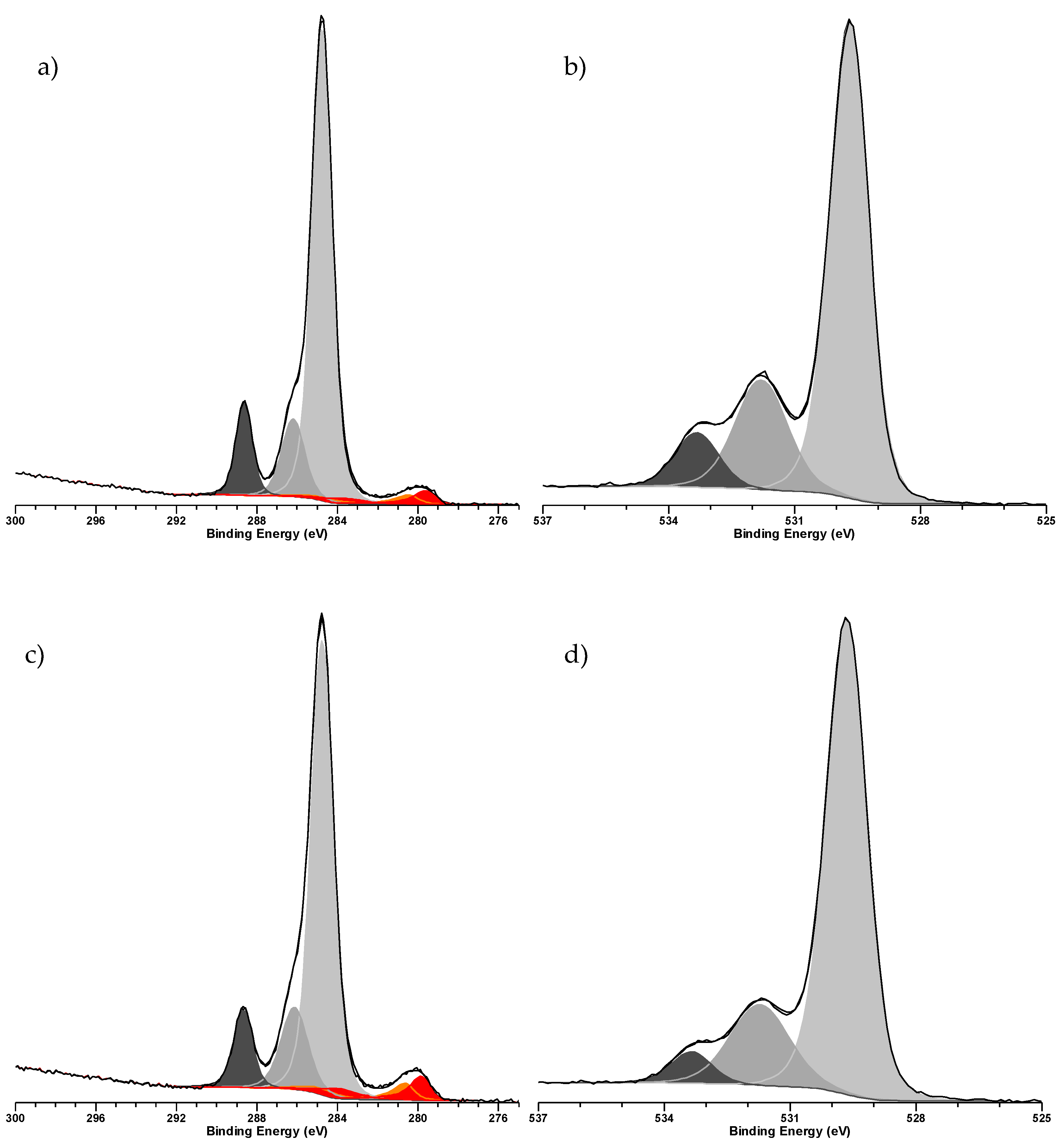
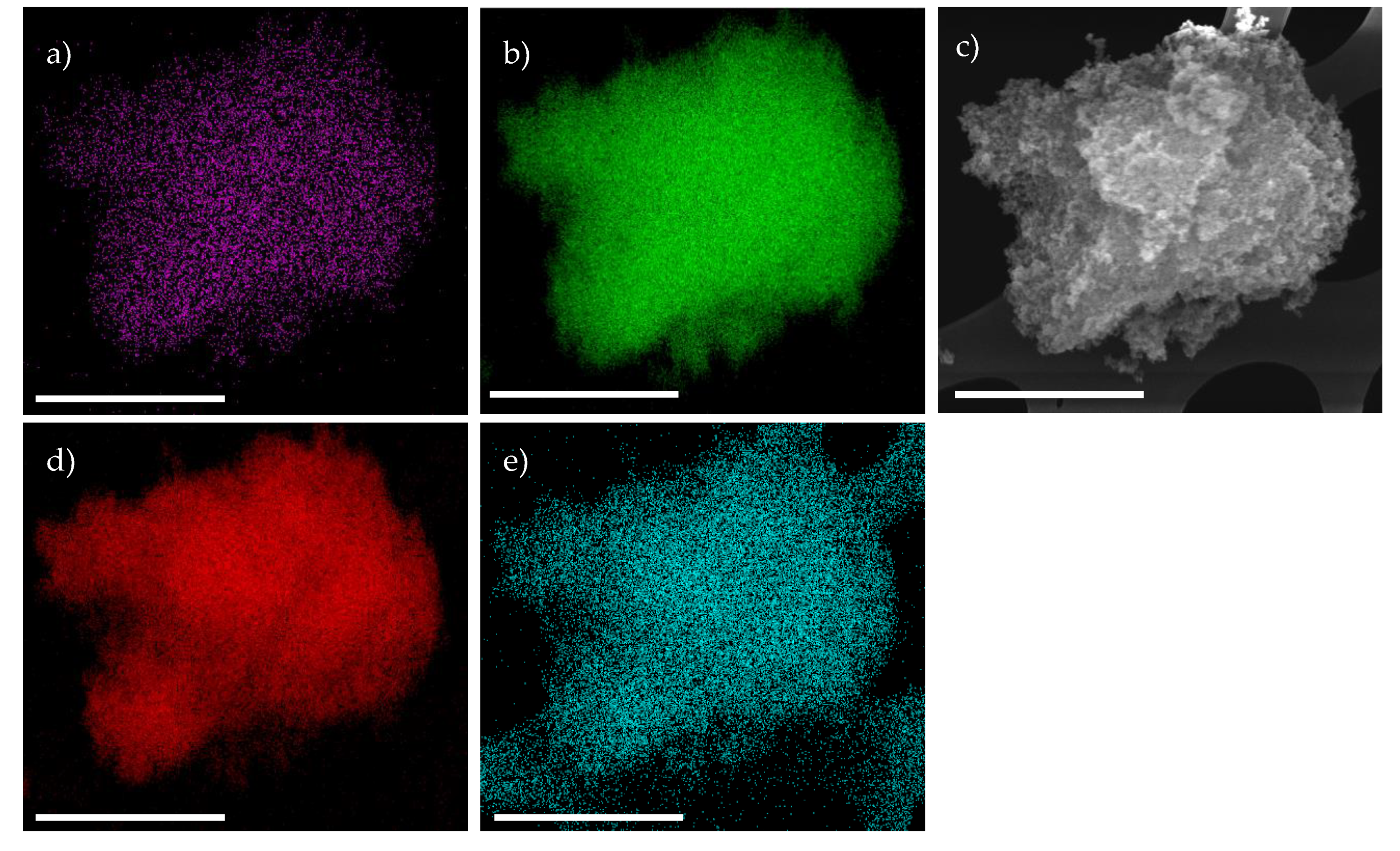
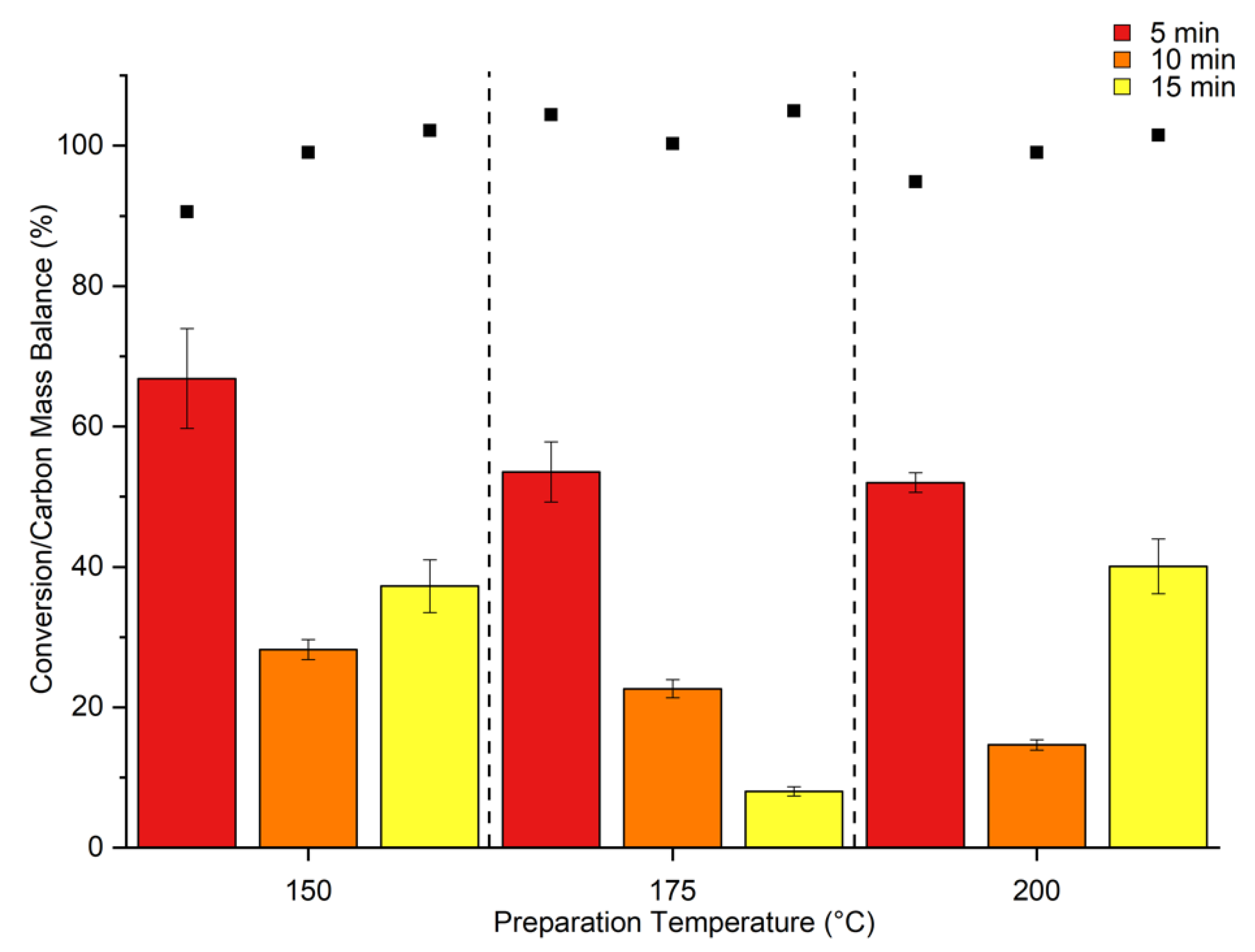
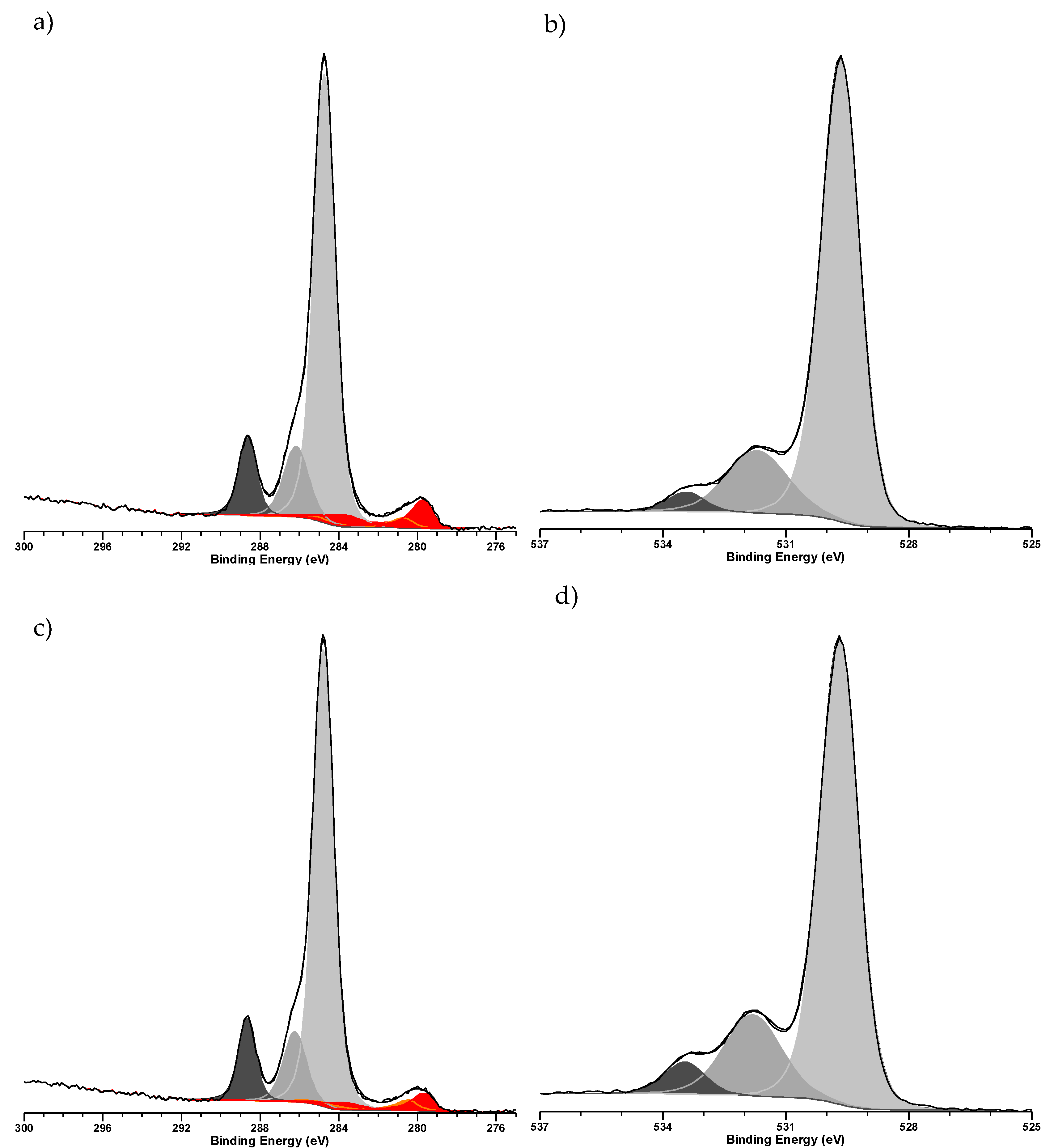
2. Results & Discussion
Catalytic Activity of MW Prepared Ru/TiO2
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Levulinic Acid Hydrogenation
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Statement
References
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Lakewood, CO, USA, 2004. [Google Scholar]
- Corma Canos, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Dunlop, A.P.; Madden, J.W.; Quaker Oats Co. Process of Preparing Gammavalerolactone. U.S. Patent 2,786,852, 26 March 1957. [Google Scholar]
- Schuette, H.A.; Thomas, R.W. Normal valerolactone. III Its preparation by the Catalytic reduction of levulinic acid with hydrogen in the presence of platinum oxide. J. Am. Chem. Soc. 1930, 52, 3010–3012. [Google Scholar] [CrossRef]
- Obregón, I.; Corro, E.; Izquierdo, U.; Requies, J.; Arias, P.L. Levulinic acid hydrogenolysis on Al2O3-based Ni-Cu bimetallic catalysts. Chin. J. Catal. 2014, 35, 656–662. [Google Scholar] [CrossRef]
- Christian, R.V.; Brown, H.D.; Hixon, R.M. Derivatives of γ-Valerolactone, 1,4-Pentanediol and 1,4-Di-(β-cyanoethoxy)-pentane 1. J. Am. Chem. Soc. 1947, 69, 1961–1963. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Wright, W.R.H.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Corbel-Demailly, L.; Ly, B.-K.; Minh, D.-P.; Tapin, B.; Especel, C.; Epron, F.; Cabiac, A.; Guillon, E.; Besson, M.; Pinel, C. Heterogeneous Catalytic Hydrogenation of Biobased Levulinic and Succinic Acids in Aqueous Solutions. ChemSusChem 2013, 6, 2388–2395. [Google Scholar] [CrossRef]
- Chia, M.; Pagán-Torres, Y.J.; Hibbitts, D.; Tan, Q.; Pham, H.N.; Datye, A.K.; Neurock, M.; Davis, R.J.; Dumesic, J.A. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts. J. Am. Chem. Soc. 2011, 133, 12675–12689. [Google Scholar] [CrossRef]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates into biofuels and high-value chemicals. Green. Chem. 2017, 235, 5527–5547. [Google Scholar] [CrossRef]
- Pan, T.; Deng, J.; Xu, Q.; Xu, Y.; Guo, Q.X.; Fu, Y. Catalytic conversion of biomass-derived levulinic acid to valerate esters as oxygenated fuels using supported ruthenium catalysts. Green Chem. 2013, 15, 2967–2974. [Google Scholar] [CrossRef]
- Cao, S.; Monnier, J.R.; Williams, C.T.; Diao, W.; Regalbuto, J.R. Rational nanoparticle synthesis to determine the effects of size, support, and K dopant on Ru activity for levulinic acid hydrogenation to γ-valerolactone. J. Catal. 2015, 326, 69–81. [Google Scholar] [CrossRef]
- Dimitratos, N.; Porta, F.; Prati, L. Au, Pd (mono and bimetallic) catalysts supported on graphite using the immobilisation method. Appl. Catal. A Gen. 2005, 291, 210–214. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Miedziak, P.; Ntainjua, E.; Edwards, J.K.; Morgan, D.; Carley, A.F.; Tiruvalam, R.; Kiely, C.J.; Hutchings, G.J. Au–Pd supported nanocrystals prepared by a sol immobilisation technique as catalysts for selective chemical synthesis. Phys. Chem. Chem. Phys. 2008, 10, 1921. [Google Scholar] [CrossRef]
- Jones, D.R.; Iqbal, S.; Miedziak, P.J.; Morgan, D.J.; Edwards, J.K.; He, Q.; Hutchings, G.J. Selective Hydrogenation of Levulinic Acid Using Ru/C Catalysts Prepared by Sol-Immobilisation. Top. Catal. 2018, 61, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Wang, D.; Veith, G.M.; Vindigni, F.; Prati, L. Sol immobilization technique: A delicate balance between activity, selectivity and stability of gold catalysts. Catal. Sci. Technol. 2013, 3, 3036. [Google Scholar] [CrossRef]
- Kurihara, L.K.; Chow, G.M.; Schoen, P.E. Nanocrystalline metallic powders and films produced by the polyol method. Nanostruct. Mater. 1995, 5, 607–613. [Google Scholar] [CrossRef]
- Varanda, L.C.; Jafelicci, M. Self-assembled FePt nanocrystals with large coercivity: Reduction of the fcc-to-L10 ordering temperature. J. Am. Chem. Soc. 2006, 128, 11062–11066. [Google Scholar] [CrossRef]
- Viau, G.; Fiévet-Vincent, F.; Fiévet, F. Nucleation and growth of bimetallic CoNi and FeNi monodisperse particles prepared in polyols. Solid State Ion. 1996, 84, 259–270. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing: A Tutorial; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9789811064661. [Google Scholar]
- Giancarlo, C.; Diego, C. Microwave Chemistry; Cravotto, G., Carnaroglio, D., Eds.; De Gruyter: Berlin, Germany, 2017; ISBN 9783110479935. [Google Scholar]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-protected metal nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M.; Tylus, W. Microstructure characterization and propane oxidation over supported Ru nanoparticles synthesized by the microwave-polyol method. Appl. Catal. B Environ. 2011, 101, 548–559. [Google Scholar] [CrossRef]
- Wiley, B.J.; Skrabalak, S.E.; Xia, Y.; Kim, M.; Formo, E.V. On the Polyol Synthesis of Silver Nanostructures: Glycolaldehyde as a Reducing Agent. Nano Lett. 2008, 8, 2077–2081. [Google Scholar]
- Matsumoto, T.; Takahashi, K.; Kitagishi, K.; Shinoda, K.; Cuya Huaman, J.L.; Piquemal, J.Y.; Jeyadevan, B. Dissolution and reduction of cobalt ions in the polyol process using ethylene glycol: Identification of the active species and its role. New J. Chem. 2015, 39, 5008–5018. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Salvi, A.M. XPS/XAES study of carbon fibres during thermal annealing under UHV conditions. Carbon 1992, 30, 521–526. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Salvi, A.M.; Cataldi, T.R.I.; Morone, A. XPS investigation of ultra-high-vacuum storage effects on carbon fibre surfaces. Carbon 1992, 30, 527–531. [Google Scholar] [CrossRef]


| Theoretical Ru Loading (wt%) | Actual Ru Loading (wt%) | |
|---|---|---|
| Acac-200-10 | 1 | 1.08 |
| Acac-200-15 | 1 | 0.97 |
| Cl-150-5 | 1 | 0.96 |
| Cl-150-10 | 1 | 1.02 |
| Catalyst | XPS Dervied Surface Concentration (at%) | |||||
|---|---|---|---|---|---|---|
| O | Ti | C | Ru | Cl | Ru(0)/Ru(II) | |
| Acac-200-10 | 38.02 | 13.43 | 48.22 | 0.28 | 0.04 | 1.3 |
| Acac-200-15 | 41.13 | 16.34 | 42.06 | 0.37 | 0.11 | 1.4 |
| Cl-150-5 | 44.17 | 18.24 | 37.16 | 0.36 | 0.09 | 2.6 |
| Cl-150-10 | 41.31 | 16.12 | 42.18 | 0.3 | 0.09 | 1.5 |
| Material | BET Surface Area (m2/g) |
|---|---|
| P25 TiO2 | 51.6 |
| Acac-200-10 | 52.1 |
| Acac-200-15 | 52.1 |
| Cl-150-5 | 51.8 |
| Cl-150-10 | 51.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howe, A.G.R.; Maunder, R.; Morgan, D.J.; Edwards, J.K. Rapid Microwave-Assisted Polyol Synthesis of TiO2-Supported Ruthenium Catalysts for Levulinic Acid Hydrogenation. Catalysts 2019, 9, 748. https://doi.org/10.3390/catal9090748
Howe AGR, Maunder R, Morgan DJ, Edwards JK. Rapid Microwave-Assisted Polyol Synthesis of TiO2-Supported Ruthenium Catalysts for Levulinic Acid Hydrogenation. Catalysts. 2019; 9(9):748. https://doi.org/10.3390/catal9090748
Chicago/Turabian StyleHowe, Alexander G. R., Rhodri Maunder, David J. Morgan, and Jennifer K. Edwards. 2019. "Rapid Microwave-Assisted Polyol Synthesis of TiO2-Supported Ruthenium Catalysts for Levulinic Acid Hydrogenation" Catalysts 9, no. 9: 748. https://doi.org/10.3390/catal9090748
APA StyleHowe, A. G. R., Maunder, R., Morgan, D. J., & Edwards, J. K. (2019). Rapid Microwave-Assisted Polyol Synthesis of TiO2-Supported Ruthenium Catalysts for Levulinic Acid Hydrogenation. Catalysts, 9(9), 748. https://doi.org/10.3390/catal9090748






