Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance
Abstract
:1. Introduction
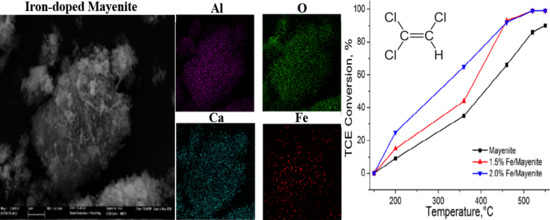
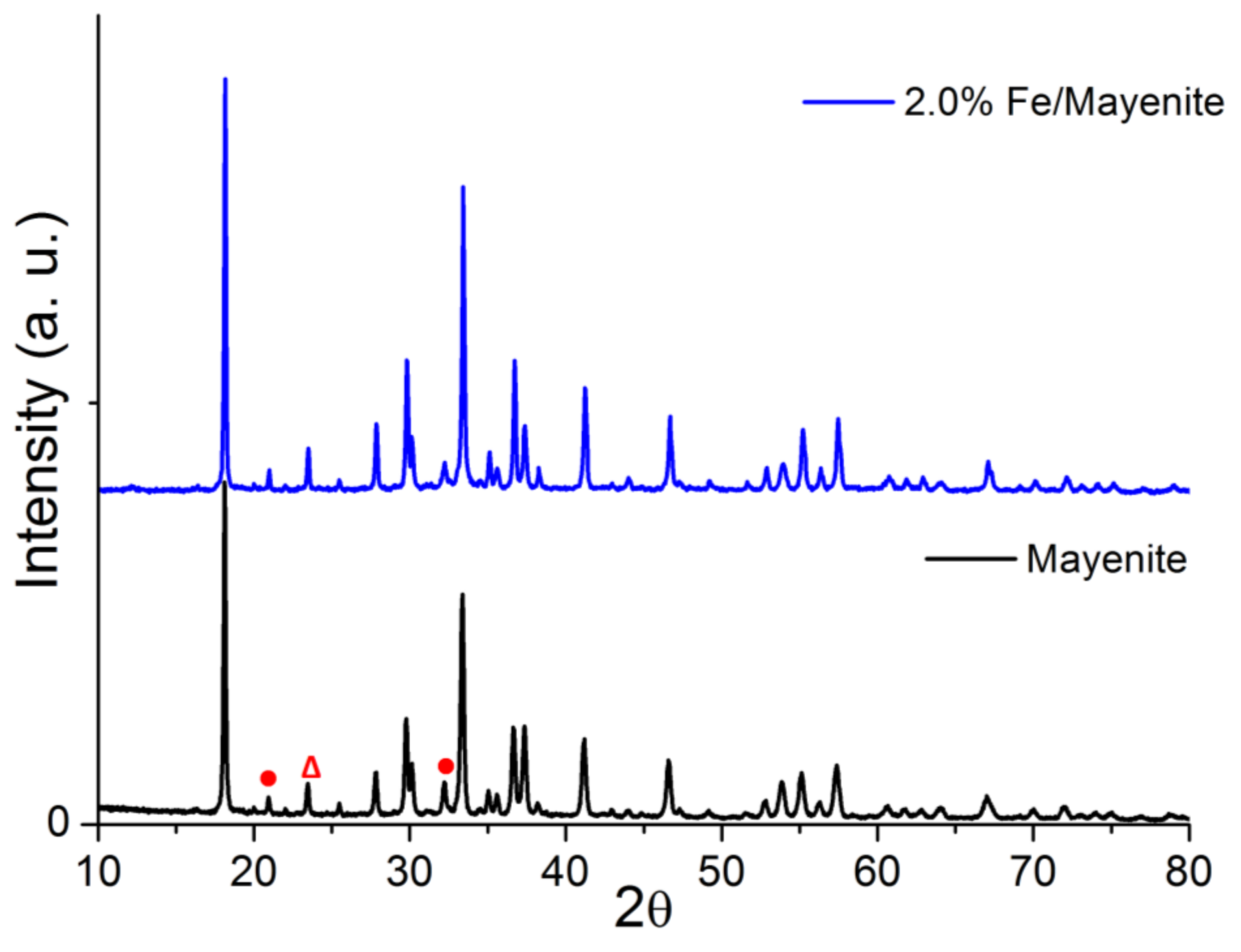
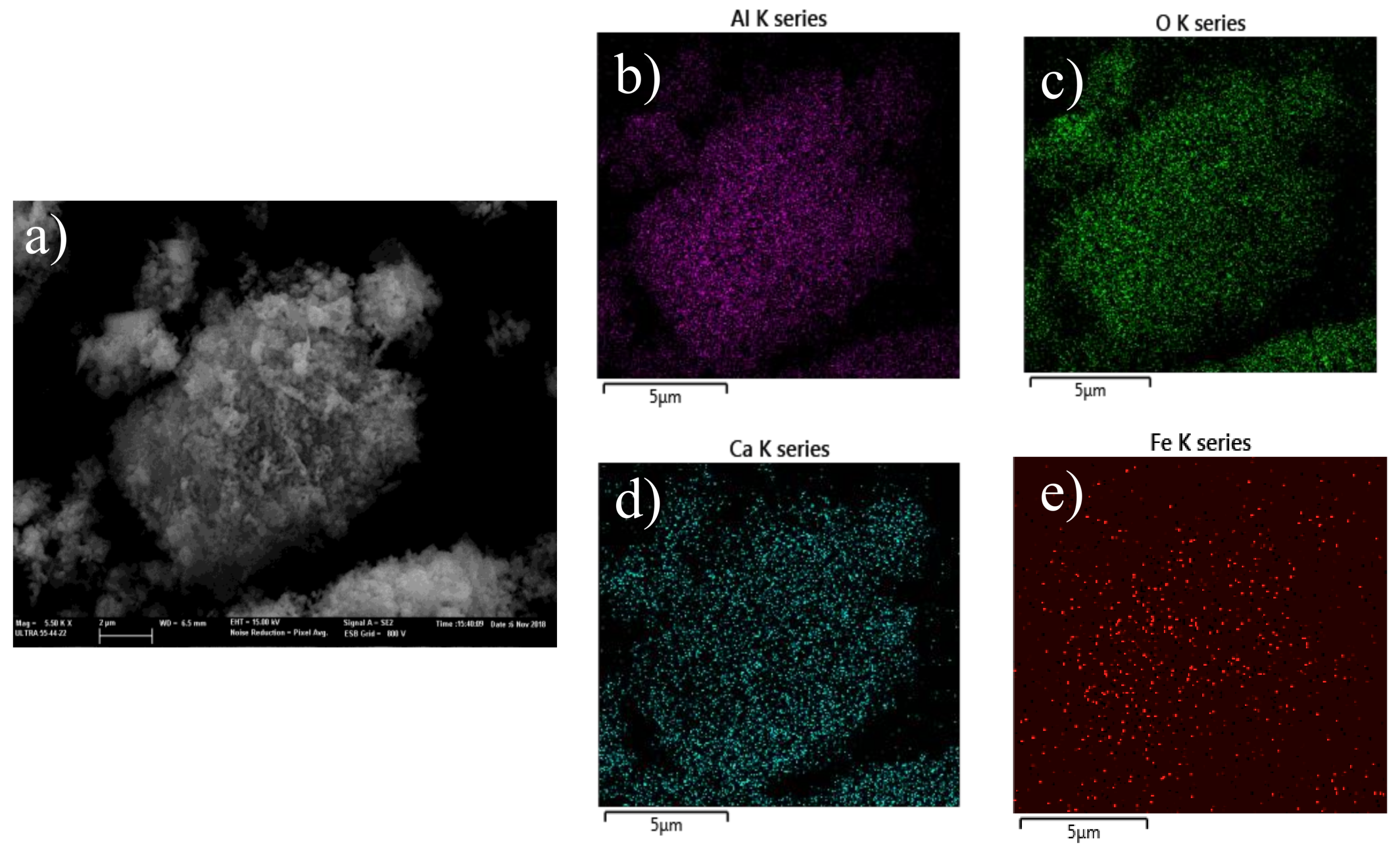
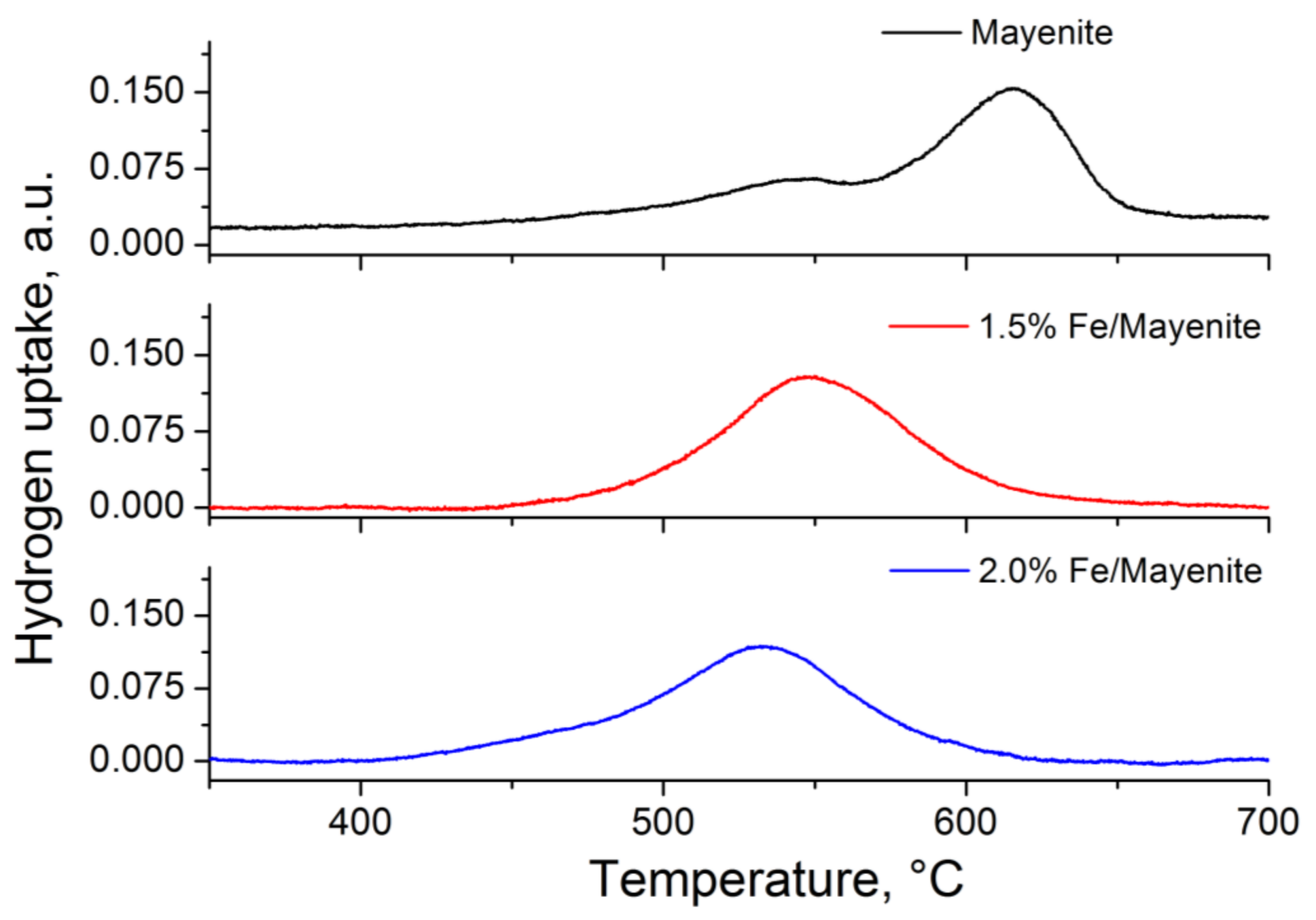
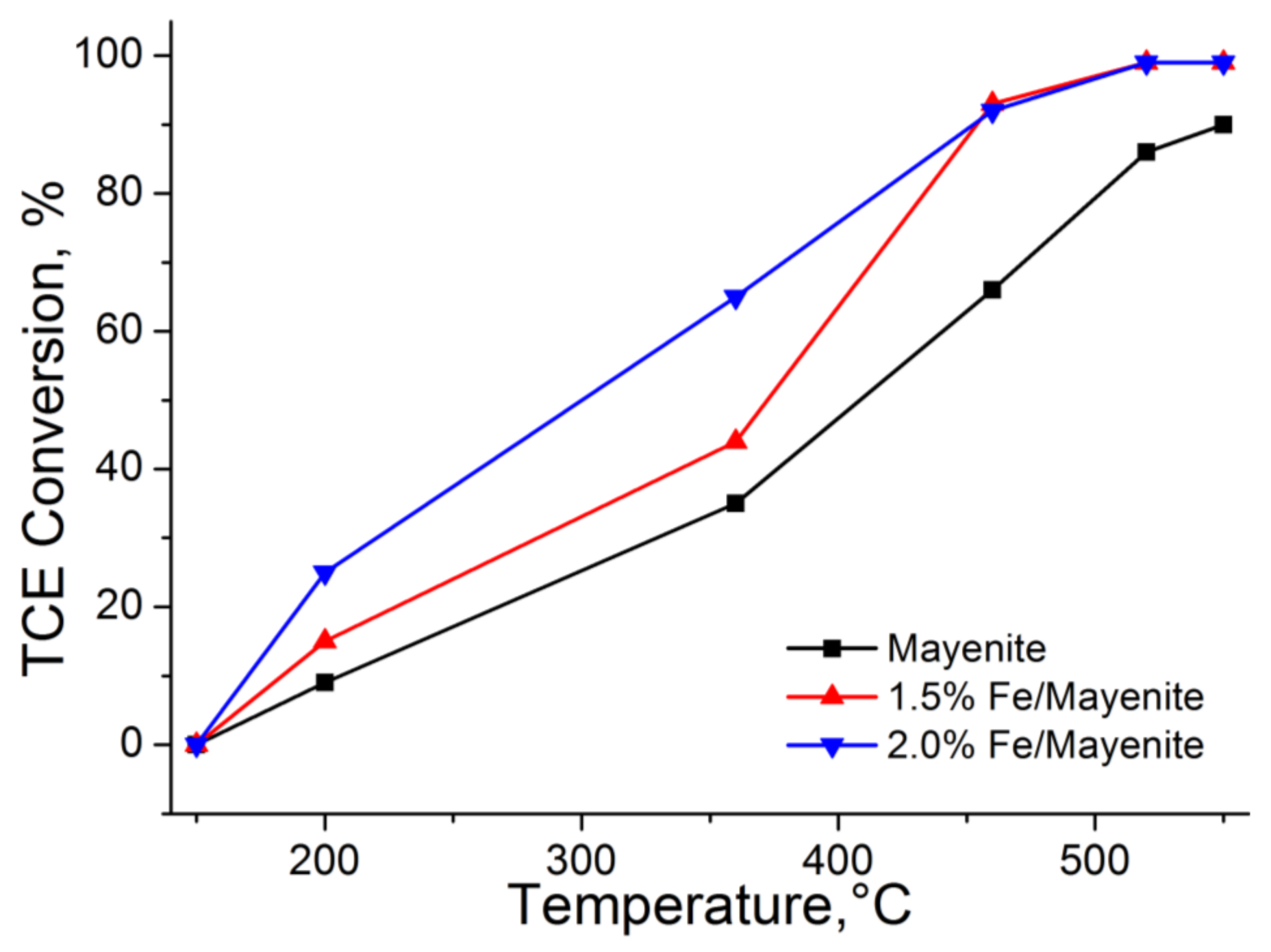
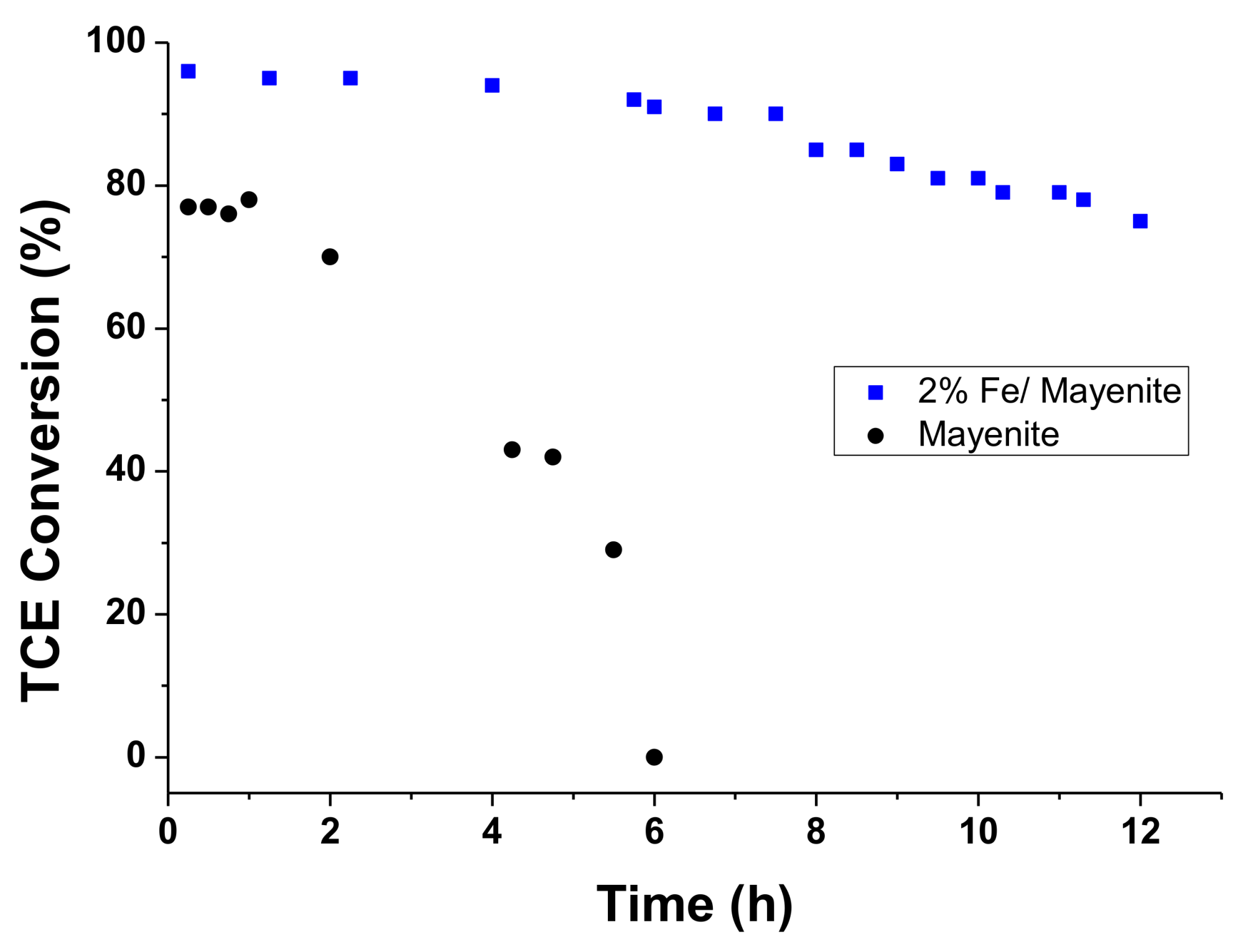
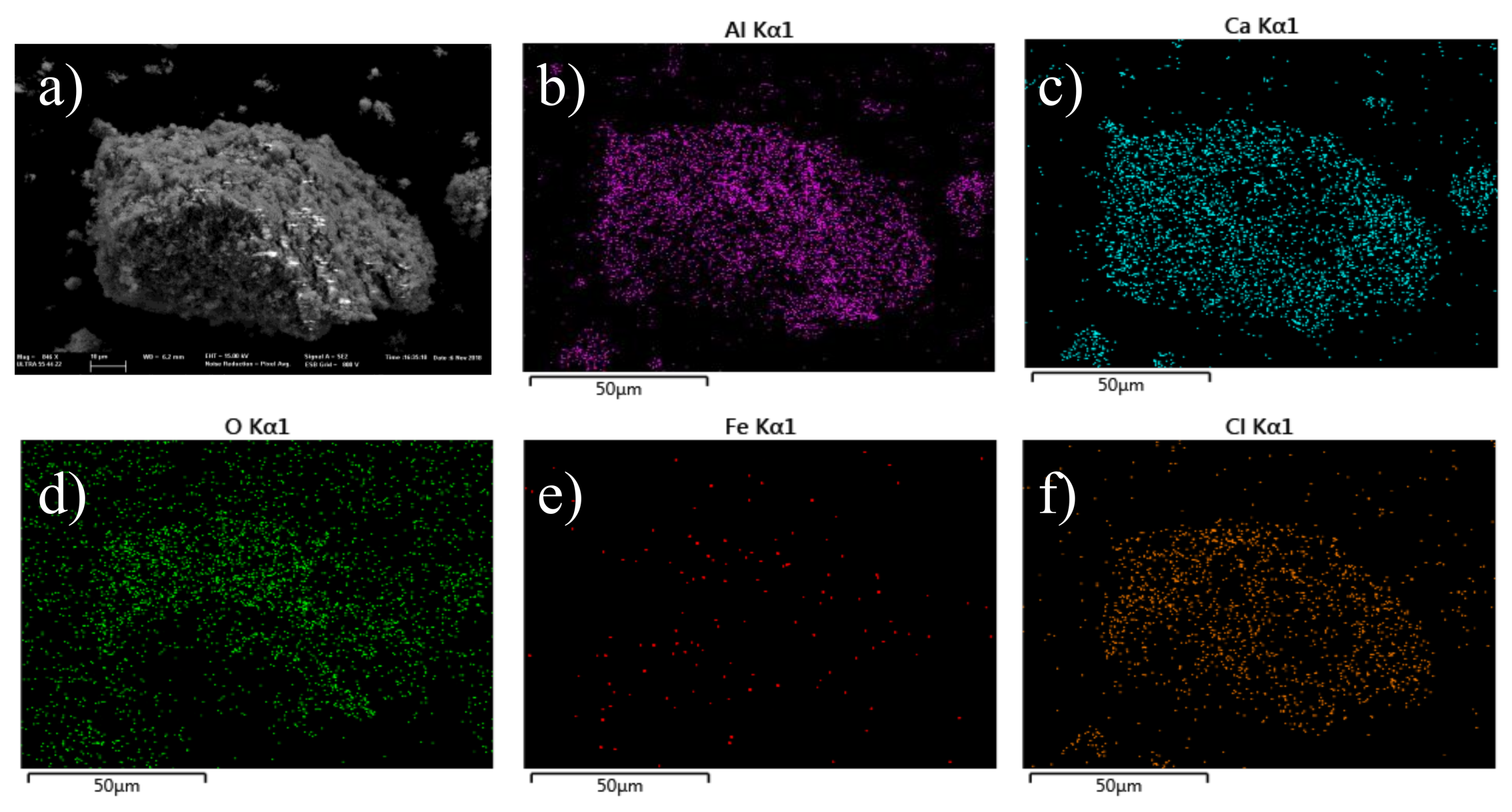
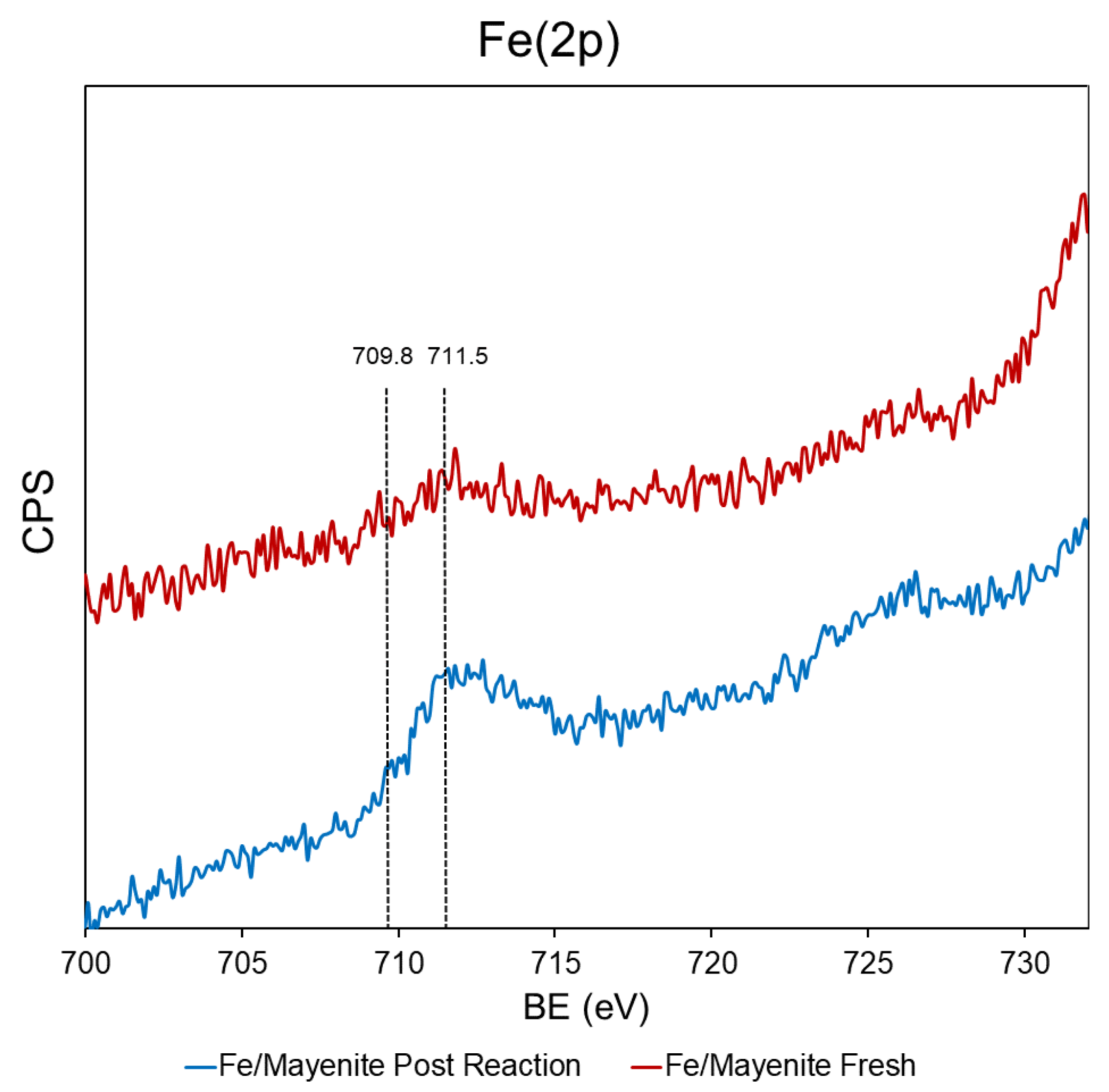
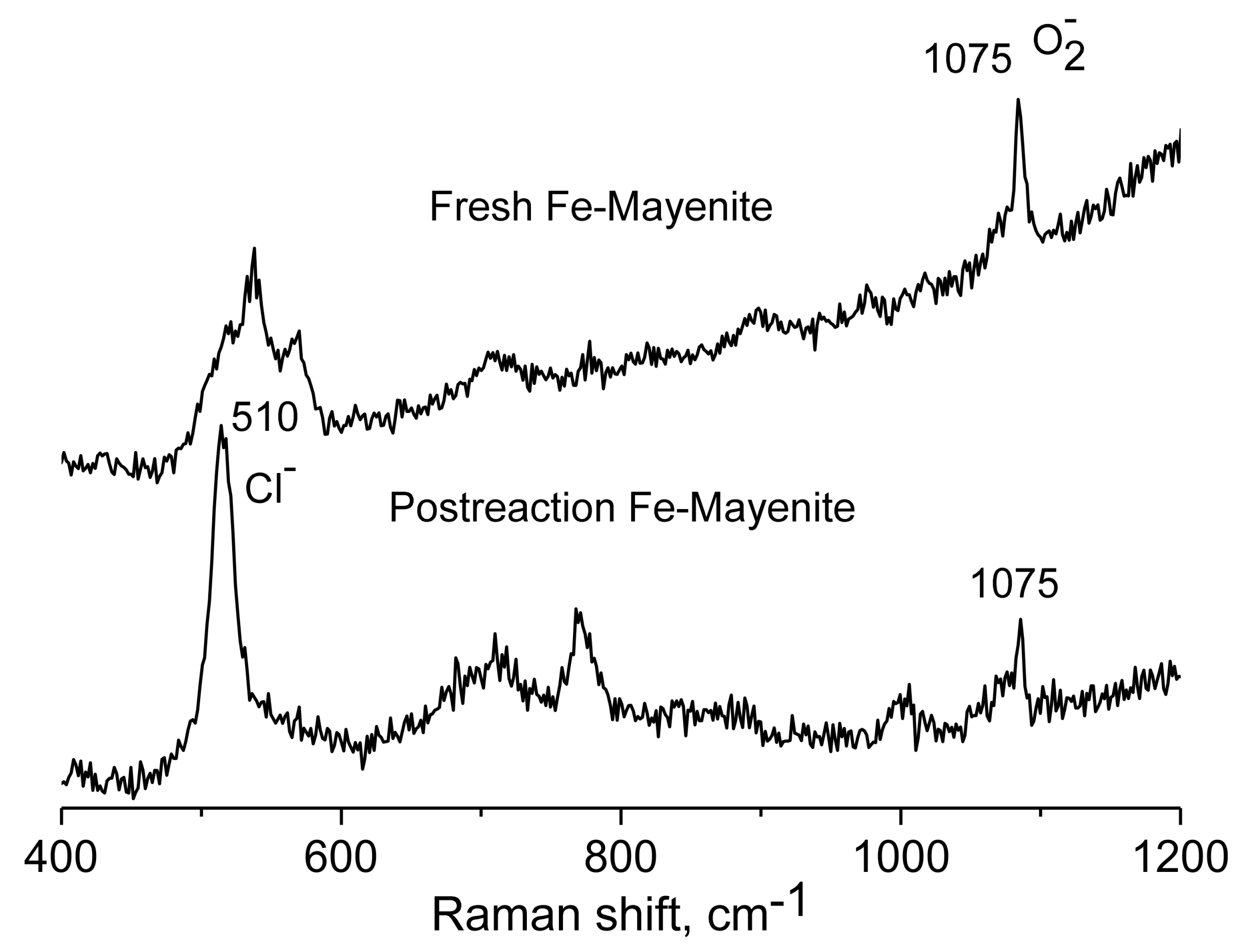
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation and Characterization
3.3. Experimental Setup and Catalytic Reactions
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwille, F. Dense Chlorinated Solvents in Porous and Fractured Media: Model Experiments; Lewis Publishers: Boca Raton, FL, USA, 1988; ISBN 978-0-87371-121-0. [Google Scholar]
- Russell, H.H.; Matthews, J.E.; Guy, W.S. TCE removal from contaminated soil and ground water. In EPA Environmental Engineering Sourcebook; Ann Arbor Press, Inc.: Ann Arbor, MI, USA, 1996. [Google Scholar]
- Rossi, F.; Cucciniello, R.; Intiso, A.; Proto, A.; Motta, O.; Marchettini, N. Determination of the trichloroethylene diffusion coefficient in water. AIChE J. 2015, 61, 3511–3515. [Google Scholar] [CrossRef]
- Ko, J.H.; Musson, S.; Townsend, T. Destruction of trichloroethylene during hydration of calcium oxide. J. Hazard. Mater. 2010, 174, 876–879. [Google Scholar] [CrossRef]
- Ge, J.; Huang, S.; Han, I.; Jaffé, P.R. Degradation of tetra- and trichloroethylene under iron reducing conditions by Acidimicrobiaceae sp. A6. Environ. Pollut. 2019, 247, 248–255. [Google Scholar] [CrossRef]
- Moccia, E.; Intiso, A.; Cicatelli, A.; Proto, A.; Guarino, F.; Iannece, P.; Castiglione, S.; Rossi, F. Use of Zea mays L. in phytoremediation of trichloroethylene. Environ. Sci. Pollut. Res. 2017, 24, 11053–11060. [Google Scholar] [CrossRef]
- Meyer, C.; Borgna, A.; Monzon, A.; Garetto, T. Kinetic study of trichloroethylene combustion on exchanged zeolites catalysts. J. Hazard. Mater. 2011, 190, 903–908. [Google Scholar] [CrossRef]
- Cucciniello, R.; Proto, A.; Rossi, F.; Marchettini, N.; Motta, O. An improved method for BTEX extraction from charcoal. Anal. Methods 2015, 7, 4811–4815. [Google Scholar] [CrossRef]
- Intiso, A.; Miele, Y.; Marchettini, N.; Proto, A.; Sánchez-Domínguez, M.; Rossi, F. Enhanced solubility of trichloroethylene (TCE) by a poly-oxyethylene alcohol as green surfactant. Environ. Technol. Innov. 2018, 12, 72–79. [Google Scholar] [CrossRef]
- Garza-Arévalo, J.I.; Intiso, A.; Proto, A.; Rossi, F.; Sanchez-Dominguez, M. Trichloroethylene solubilization using a series of commercial biodegradable ethoxylated fatty alcohol surfactants. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Aranzabal, A.; Ayo, B.P.; González-Marcos, M.P.; González-Marcos, J.A.; López-Fonseca, R.; González-Velasco, J.R. State of the art in catalytic oxidation of chlorinated volatile organic compounds. Chem. Pap. 2014, 68, 1169–1186. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Suzuki, K. Catalytic oxidation of VOCs over Al- and Fe-pillared montmorillonite. Appl. Clay Sci. 2013, 77, 56–60. [Google Scholar] [CrossRef]
- Tian, W.; Fan, X.; Yang, H.; Zhang, X. Preparation of MnOx/TiO2 composites and their properties for catalytic oxidation of chlorobenzene. J. Hazard. Mater. 2010, 177, 887–891. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Puche, M.; Fetter, G.; Bosch, P. The oxidation of trichloroethylene over different mixed oxides derived from hydrotalcites. Appl. Catal. B Environ. 2014, 160, 129–134. [Google Scholar] [CrossRef]
- Taralunga, M.; Mijoin, J.; Magnoux, P. Catalytic destruction of 1,2-dichlorobenzene over zeolites. Catal. Commun. 2006, 7, 115–121. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.; González-Marcos, J.; Marcos, J.A.G. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior. Appl. Catal. B Environ. 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Triguero, J.M.; Valencia, S. Cu and Co modified beta zeolite catalysts for the trichloroethylene oxidation. Appl. Catal. B Environ. 2016, 187, 90–97. [Google Scholar] [CrossRef]
- Cucciniello, R.; Proto, A.; Rossi, F.; Motta, O. Mayenite based supports for atmospheric NOx sampling. Atmos. Environ. 2013, 79, 666–671. [Google Scholar] [CrossRef]
- Cucciniello, R.; Intiso, A.; Castiglione, S.; Genga, A.; Proto, A.; Rossi, F. Total oxidation of trichloroethylene over mayenite (Ca12Al14O33) catalyst. Appl. Catal. B Environ. 2017, 204, 167–172. [Google Scholar] [CrossRef]
- Intiso, A.; Cucciniello, R.; Castiglione, S.; Proto, A.; Rossi, F. Environmental Application of Extra-Framework Oxygen Anions in the Nano-Cages of Mayenite. In Advances in Bionanomaterials; Lecture Notes in Bioengineering; Springer: Cham, Switzerland, 2018; pp. 131–139. ISBN 978-3-319-62026-8. [Google Scholar]
- Intiso, A.; Martinez-Triguero, J.; Cucciniello, R.; Proto, A.; Palomares, A.E.; Rossi, F. A Novel Synthetic Route to Prepare High Surface Area Mayenite Catalyst for TCE Oxidation. Catalysts 2019, 9, 27. [Google Scholar] [CrossRef]
- Intiso, A.; Martinez-Triguero, J.; Cucciniello, R.; Rossi, F.; Palomares, A.E. Influence of the synthesis method on the catalytic activity of mayenite for the oxidation of gas-phase trichloroethylene. Sci. Rep. 2019, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Proto, A.; Cucciniello, R.; Rossi, F.; Motta, O. Stable carbon isotope ratio in atmospheric CO2 collected by new diffusive devices. Environ. Sci. Pollut. Res. 2013, 21, 3182–3186. [Google Scholar] [CrossRef]
- Eufinger, J.-P.; Schmidt, A.; Lerch, M.; Janek, J. Novel anion conductors—Conductivity, thermodynamic stability and hydration of anion-substituted mayenite-type cage compounds C12A7:X (X = O, OH, Cl, F, CN, S, N). Phys. Chem. Chem. Phys. 2015, 17, 6844–6857. [Google Scholar] [CrossRef]
- Schmidt, A.; Lerch, M.; Eufinger, J.-P.; Janek, J.; Tranca, I.; Islam, M.M.; Bredow, T.; Dolle, R.; Wiemhofer, H.-D.; Boysen, H.; et al. Chlorine ion mobility in Cl-mayenite (Ca12Al14O32Cl2): An investigation combining high-temperature neutron powder diffraction, impedance spectroscopy and quantum-chemical calculations. Solid State Ionics 2014, 254, 48–58. [Google Scholar] [CrossRef]
- Teusner, M.; De Souza, R.A.; Krause, H.; Ebbinghaus, S.G.; Belghoul, B.; Martin, M. Oxygen Diffusion in Mayenite. J. Phys. Chem. C 2015, 119, 9721–9727. [Google Scholar] [CrossRef]
- Ruszak, M.; Inger, M.; Witkowski, S.; Wilk, M.; Kotarba, A.; Sojka, Z. Selective N2O Removal from the Process Gas of Nitric Acid Plants over Ceramic 12CaO·7Al2O3 Catalyst. Catal. Lett. 2008, 126, 72–77. [Google Scholar] [CrossRef]
- Proto, A.; Cucciniello, R.; Genga, A.; Capacchione, C. A study on the catalytic hydrogenation of aldehydes using mayenite as active support for palladium. Catal. Commun. 2015, 68, 41–45. [Google Scholar] [CrossRef]
- Ye, T.-N.; Li, J.; Kitano, M.; Hosono, H. Unique nanocages of 12CaO·7Al2O3 boost heterolytic hydrogen activation and selective hydrogenation of heteroarenes over ruthenium catalyst. Green Chem. 2017, 19, 749–756. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. A crucial role of O2− and O22− on mayenite structure for biomass tar steam reforming over Ni/Ca12Al14O33. Appl. Catal. B Environ. 2009, 88, 351–360. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of tar contained in a raw fuel gas from biomass gasification using nickel-mayenite catalyst. Int. J. Hydrog. Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Mani, S.; Kastner, J.R.; Juneja, A. Catalytic decomposition of toluene using a biomass derived catalyst. Fuel Process. Technol. 2013, 114, 118–125. [Google Scholar] [CrossRef]
- Lacerda, M.; Irvine, J.T.S.; Glasser, F.P.; West, A.R. High oxide ion conductivity in Ca12Al14O33. Nature 1988, 332, 525–526. [Google Scholar] [CrossRef]
- Li, J.; Kitano, M.; Ye, T.-N.; Sasase, M.; Yokoyama, T.; Hosono, H.; Ye, T. Chlorine-Tolerant Ruthenium Catalyst Derived Using the Unique Anion-Exchange Properties of 12CaO·7Al2O3 for Ammonia Synthesis. ChemCatChem 2017, 9, 3078–3083. [Google Scholar] [CrossRef]
- Diwald, O.; Sterrer, M.; Knözinger, E. Site selective hydroxylation of the MgO surface. Phys. Chem. Chem. Phys. 2002, 4, 2811–2817. [Google Scholar] [CrossRef]
- Ruszak, M.; Witkowski, S.; Sojka, Z. EPR and Raman investigations into anionic redox chemistry of nanoporous 12CaO·7Al2O3 interacting with O2, H2 and N2O. Res. Chem. Intermed. 2007, 33, 689–703. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Effects of iron and cerium in La1−yCeyCo1−xFexO3 perovskites as catalysts for VOC oxidation. Appl. Catal. B Environ. 2009, 88, 305–314. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Tang, N.; Pan, S.; Hu, J. FeCl3-modified Co–Ce oxides catalysts for mercury removal from coal-fired flue gas. Chem. Pap. 2017, 71, 2545–2555. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S.; Grosvenor, A. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. Synthesis of higher surface area mayenite by hydrothermal method. Mater. Res. Bull. 2011, 46, 1307–1310. [Google Scholar] [CrossRef]
- Molina, R.; Poncelet, G. α-Alumina-Supported Nickel Catalysts Prepared from Nickel Acetylacetonate: A TPR Study. J. Catal. 1998, 173, 257–267. [Google Scholar] [CrossRef]
- Méndez, M.; Ciuraru, R.; Gosselin, S.; Batut, S.; Visez, N.; Petitprez, D. Reactivity of chlorine radical with submicron palmitic acid particles: Kinetic measurements and products identification. Atmospheric Chem. Phys. Discuss. 2013, 13, 16925–16960. [Google Scholar] [CrossRef]
| Catalyst | Fe Content (wt.%) | BET Surface Area (m2/g) |
|---|---|---|
| Mayenite | - | 11.7 |
| 1.5% Fe/mayenite | 1.72 | 11.5 |
| 2.0% Fe/mayenite | 2.30 | 11.2 |
| Catalyst | T50 (°C) | T90 (°C) | Conversion Rate (mol g−1 s−1) |
|---|---|---|---|
| Mayenite | 410 | 550 | 5.58 × 10−6 |
| 1.5% Fe/mayenite | 375 | 460 | 7.14 × 10−6 |
| 2.0% Fe/mayenite | 300 | 460 | 1.05 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucciniello, R.; Intiso, A.; Siciliano, T.; Palomares, A.E.; Martínez-Triguero, J.; Cerrillo, J.L.; Proto, A.; Rossi, F. Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts 2019, 9, 747. https://doi.org/10.3390/catal9090747
Cucciniello R, Intiso A, Siciliano T, Palomares AE, Martínez-Triguero J, Cerrillo JL, Proto A, Rossi F. Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts. 2019; 9(9):747. https://doi.org/10.3390/catal9090747
Chicago/Turabian StyleCucciniello, Raffaele, Adriano Intiso, Tiziana Siciliano, Antonio Eduardo Palomares, Joaquín Martínez-Triguero, Jose Luis Cerrillo, Antonio Proto, and Federico Rossi. 2019. "Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance" Catalysts 9, no. 9: 747. https://doi.org/10.3390/catal9090747
APA StyleCucciniello, R., Intiso, A., Siciliano, T., Palomares, A. E., Martínez-Triguero, J., Cerrillo, J. L., Proto, A., & Rossi, F. (2019). Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts, 9(9), 747. https://doi.org/10.3390/catal9090747