Recent Advances on Visible Light Metal-Based Photocatalysts for Polymerization under Low Light Intensity
Abstract
:1. Introduction
2. Organometallic Photocatalysts
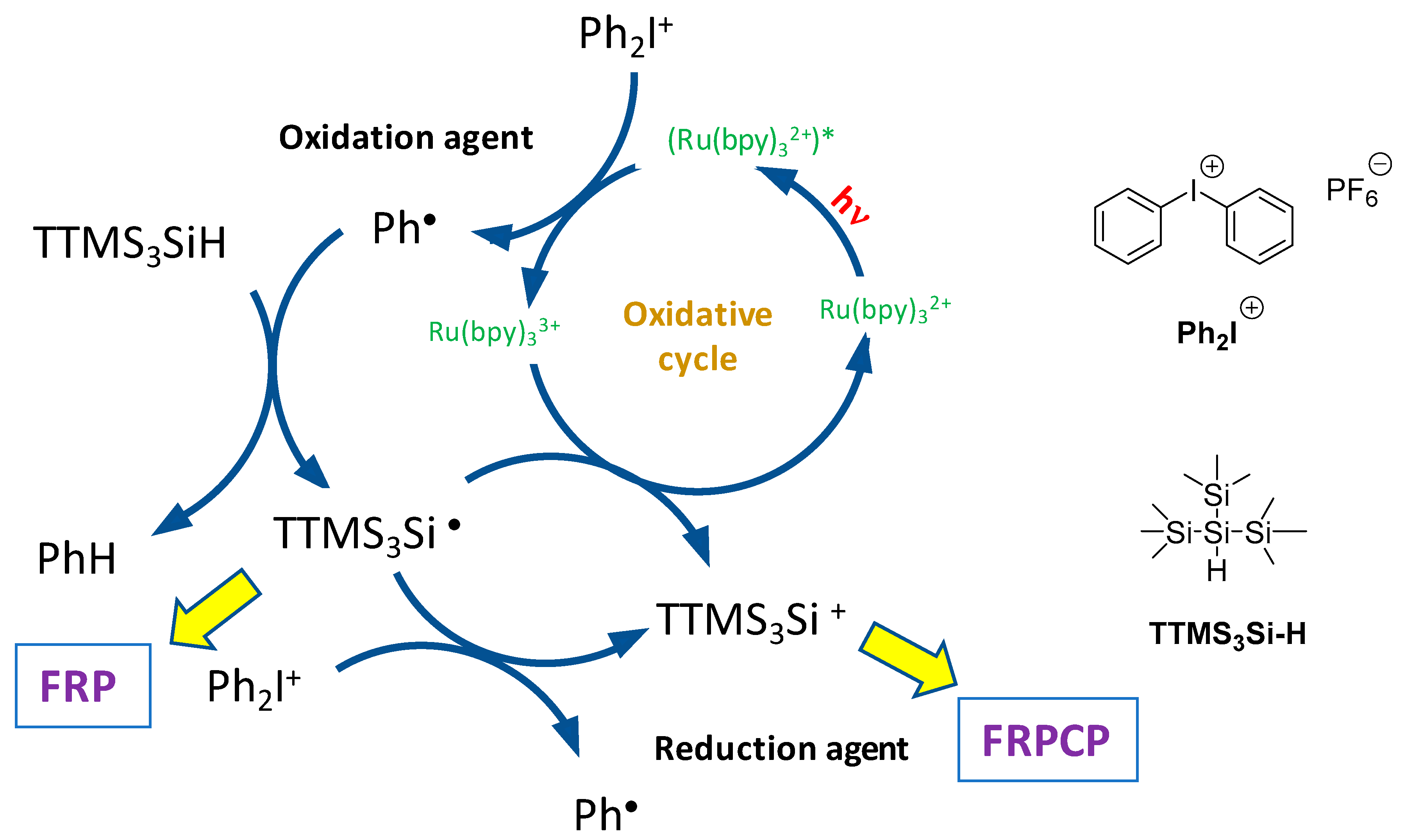
2.1. Ruthenium Complexes
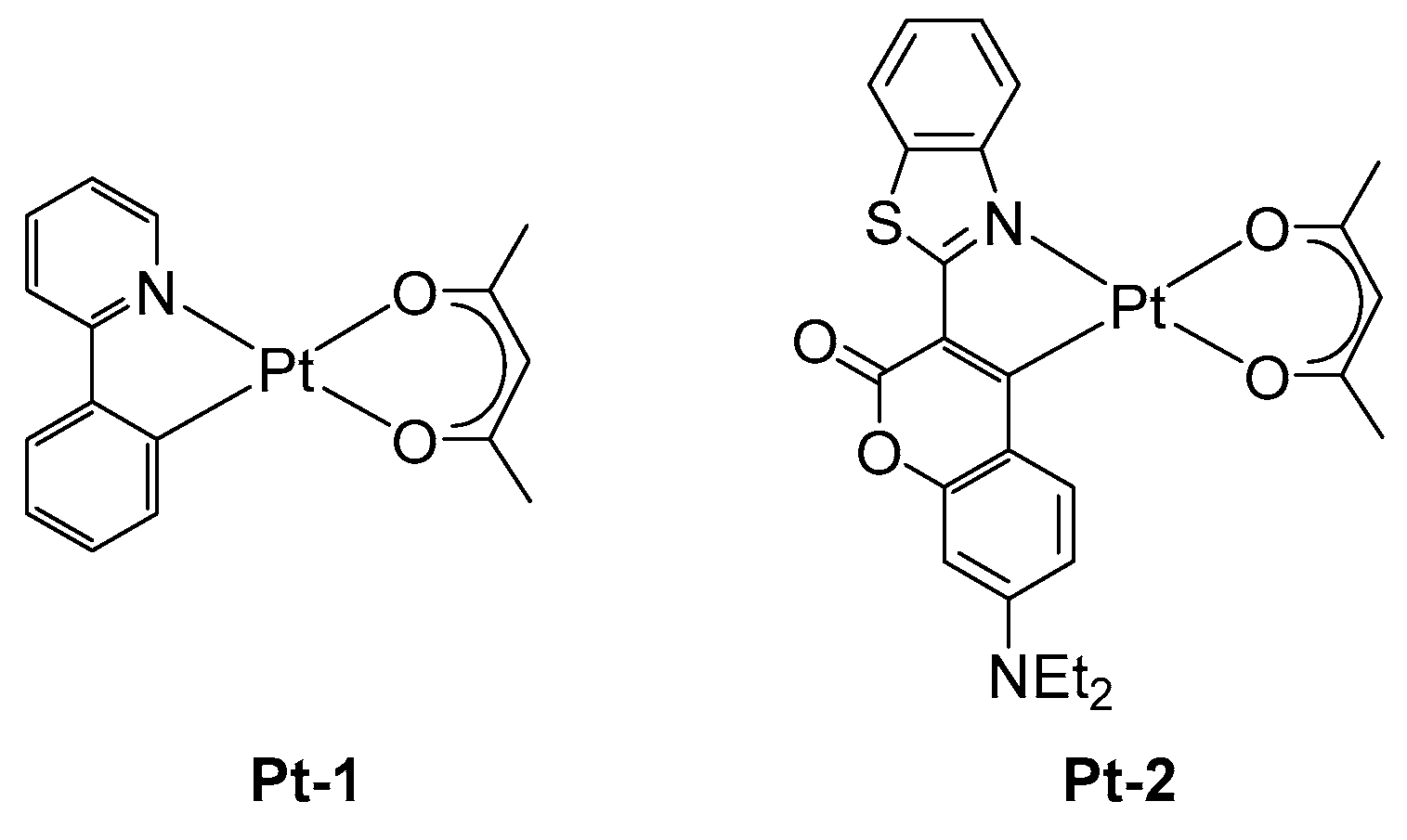
2.2. Platinum Complexes
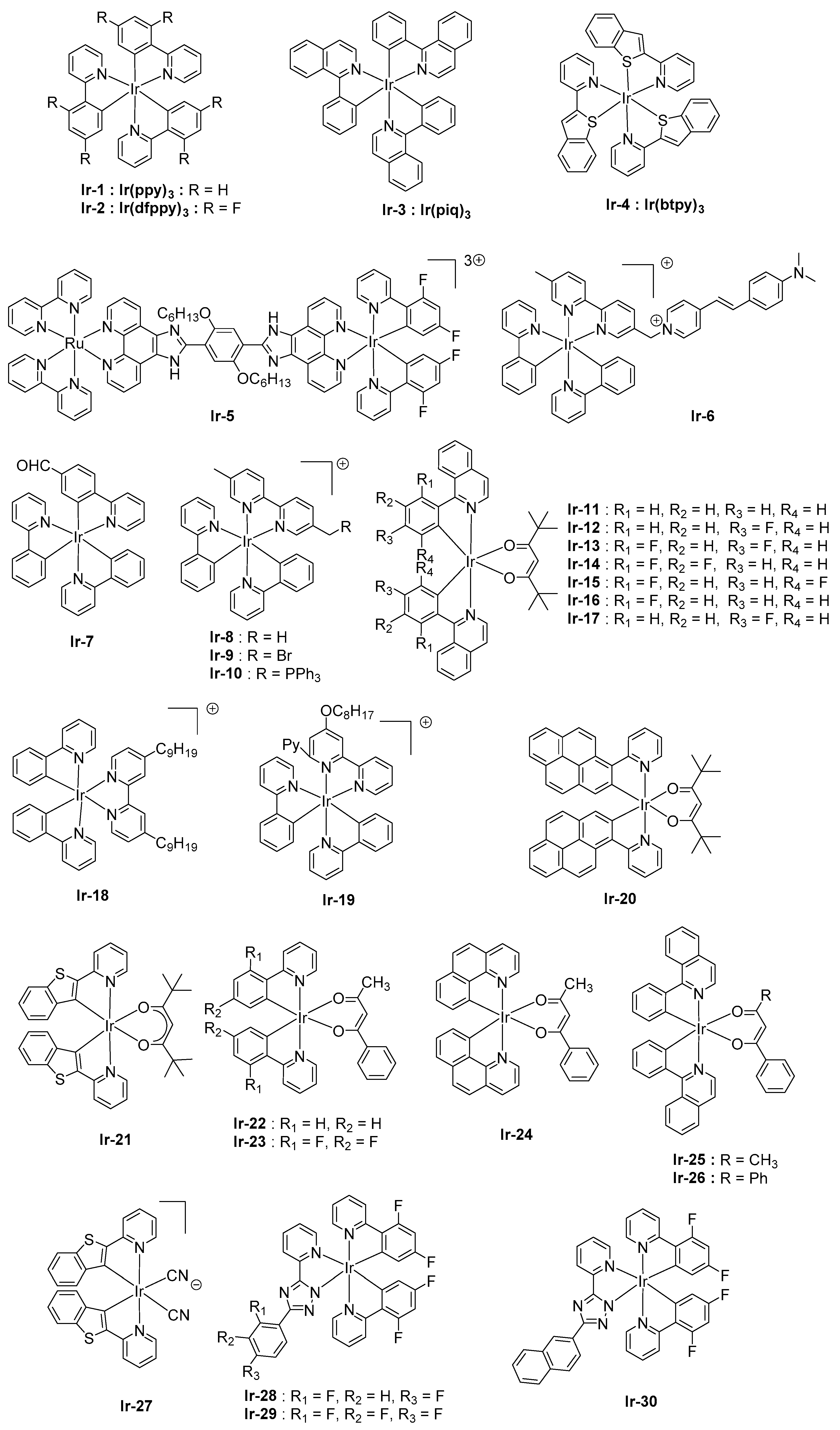
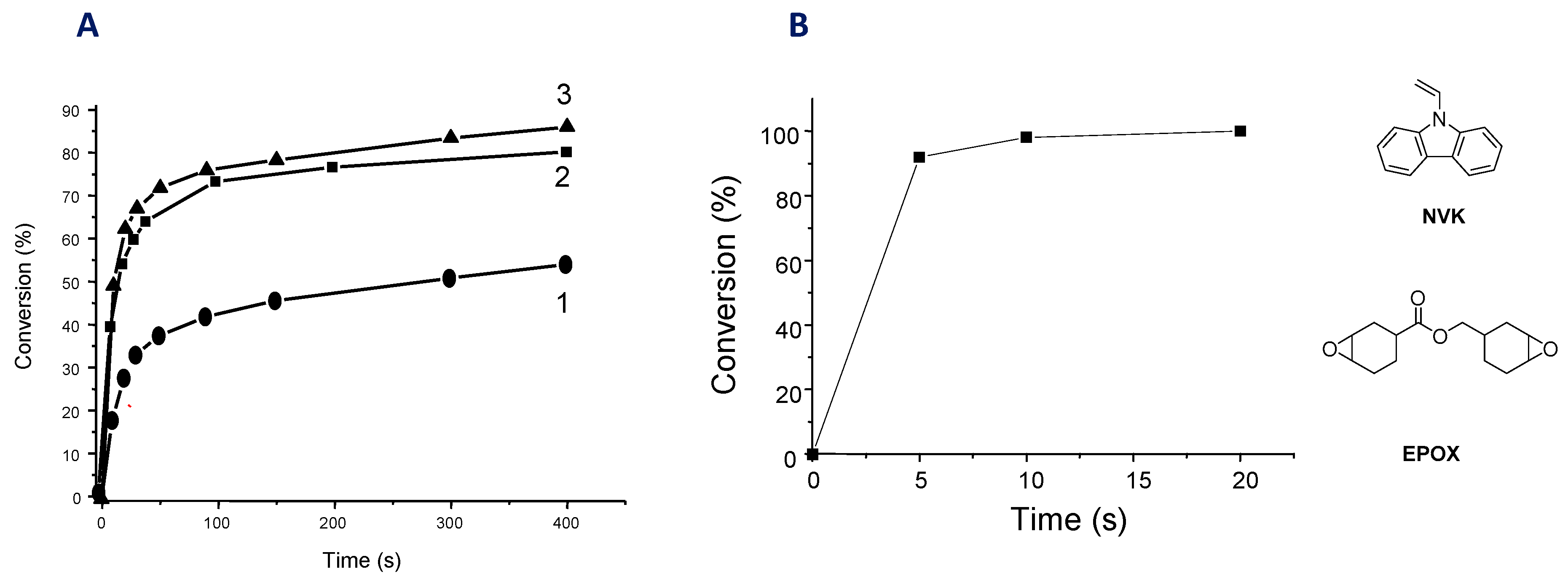
2.3. Iridium Complexes
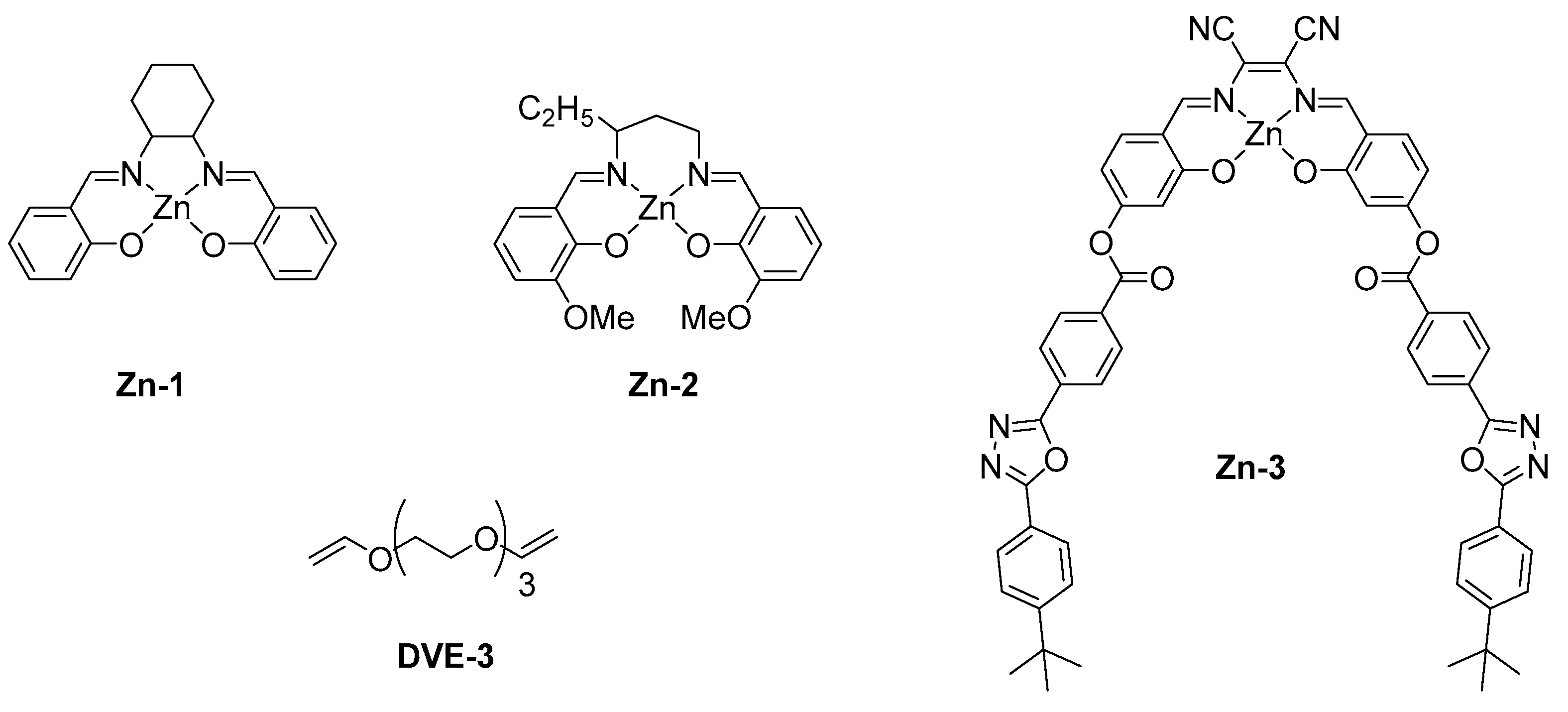
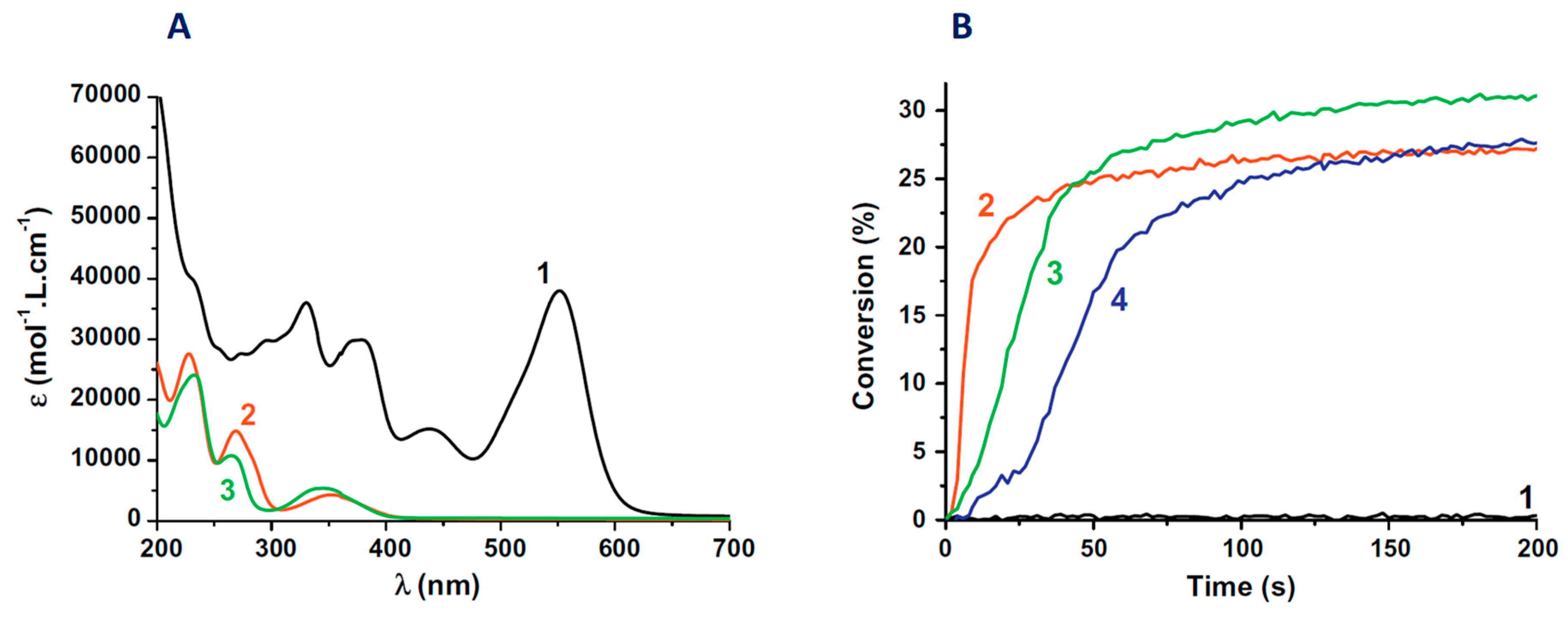
2.4. Zinc Complexes
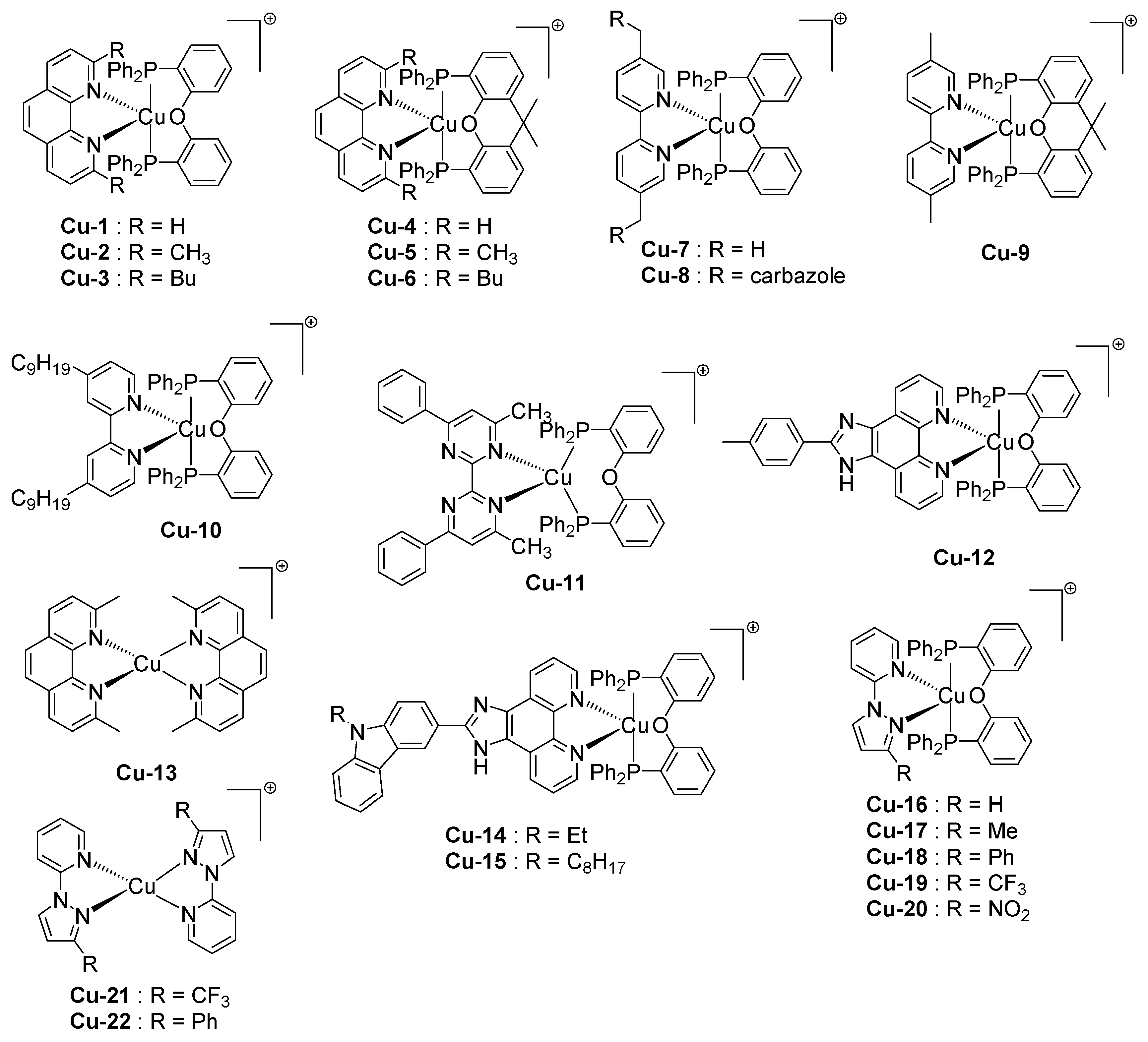
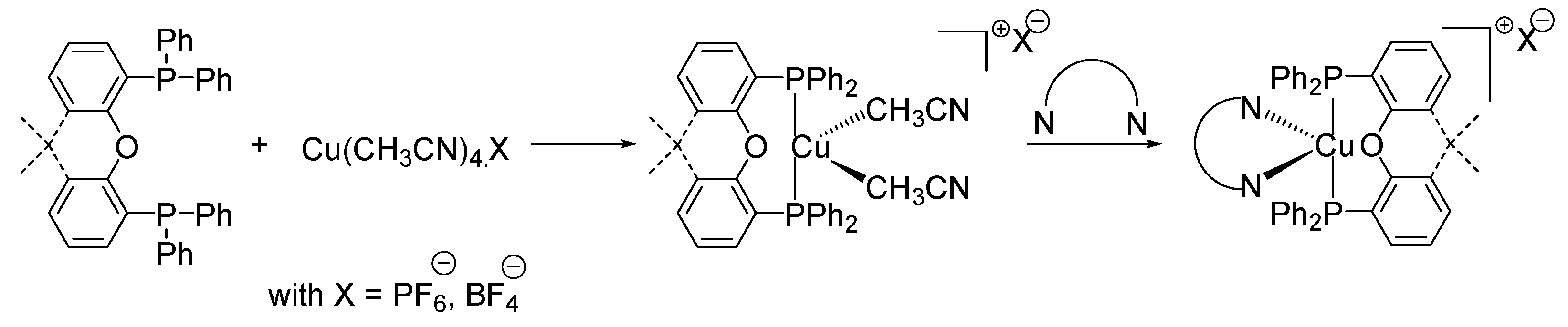
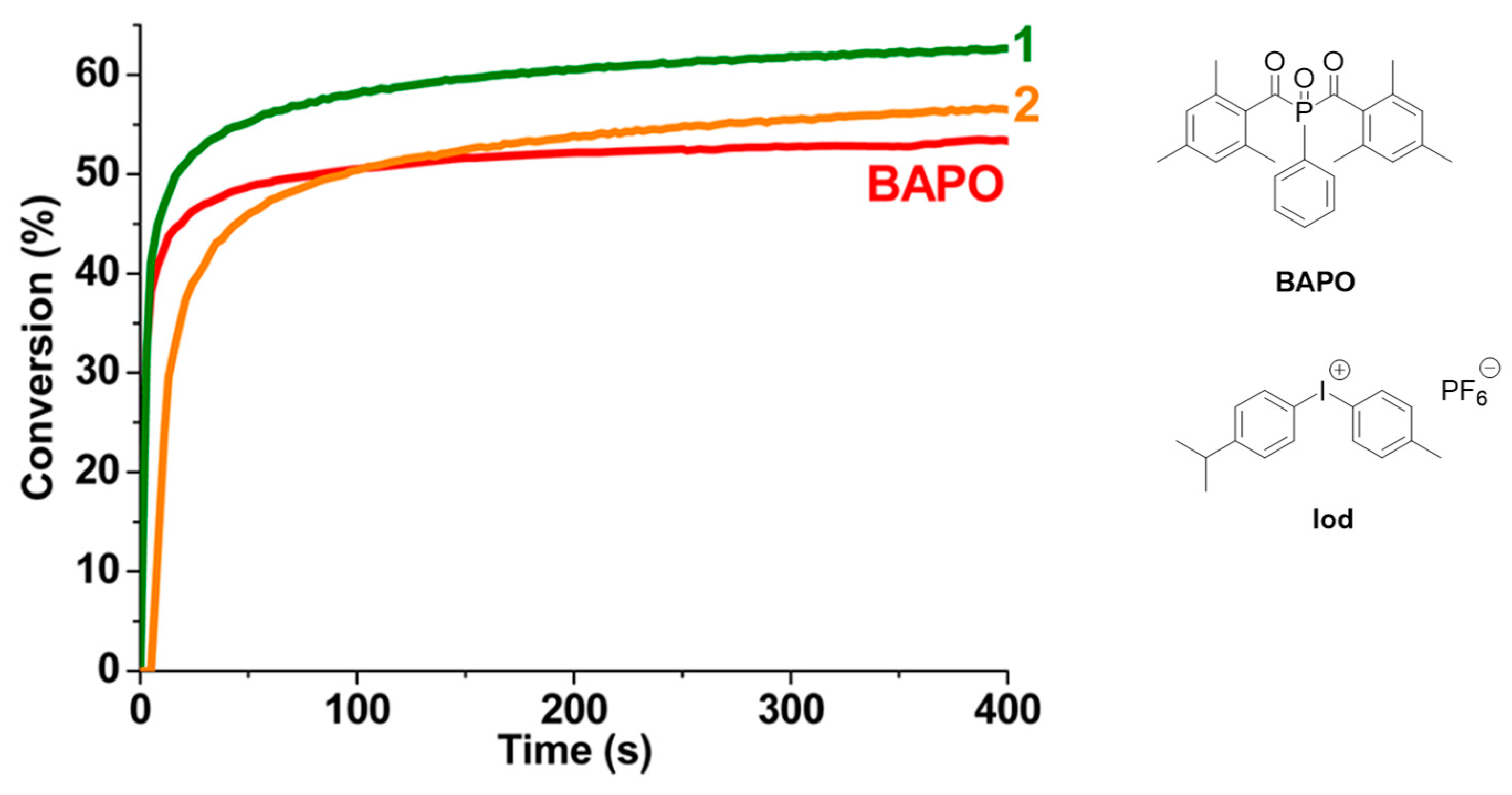
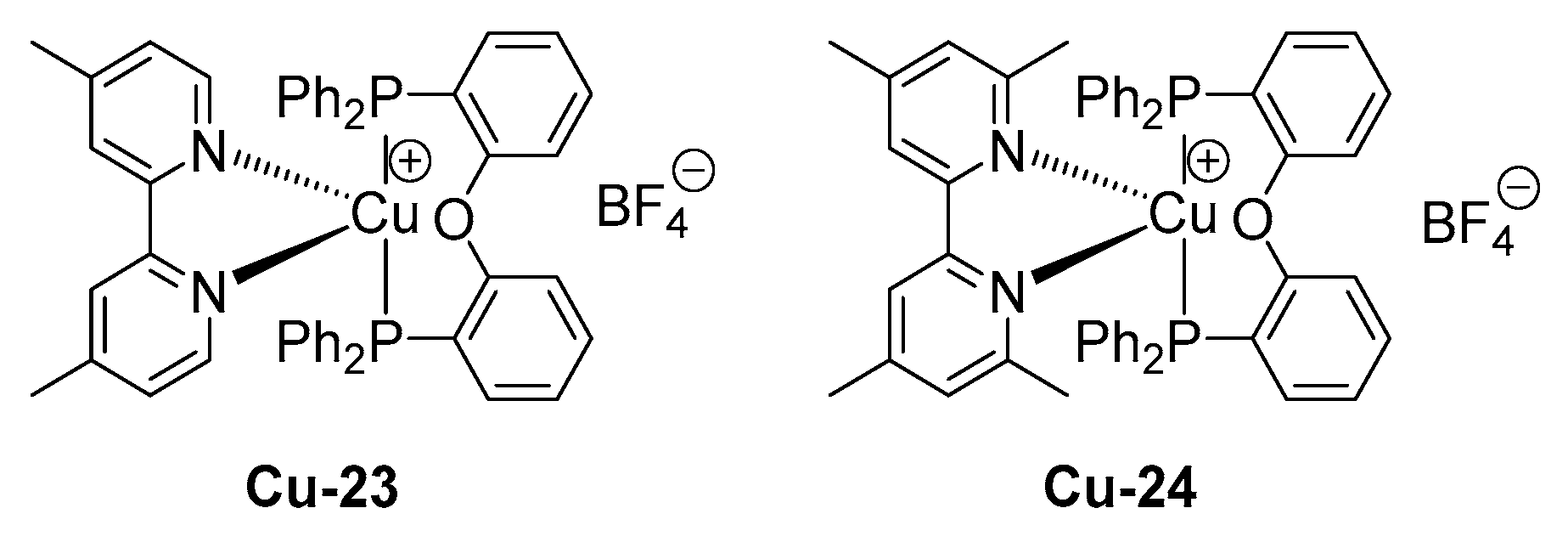
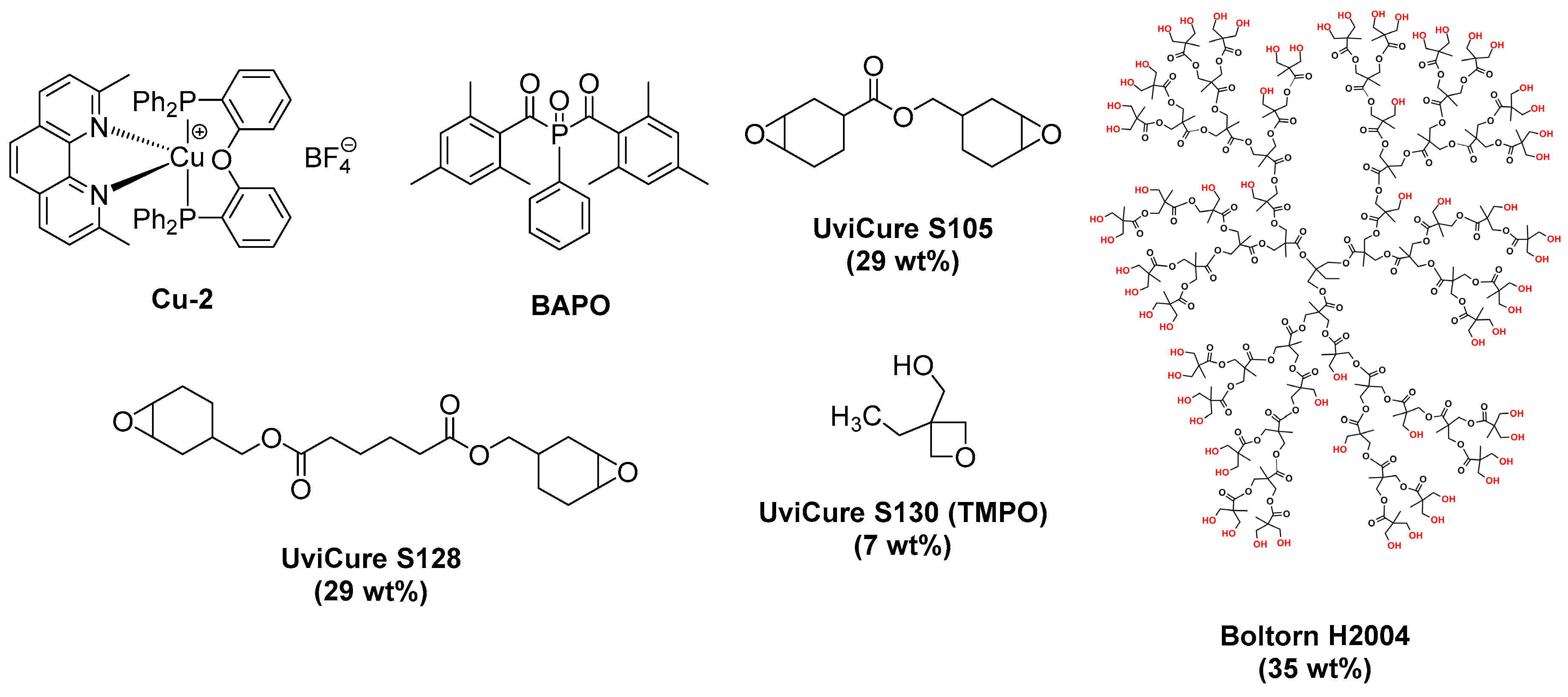
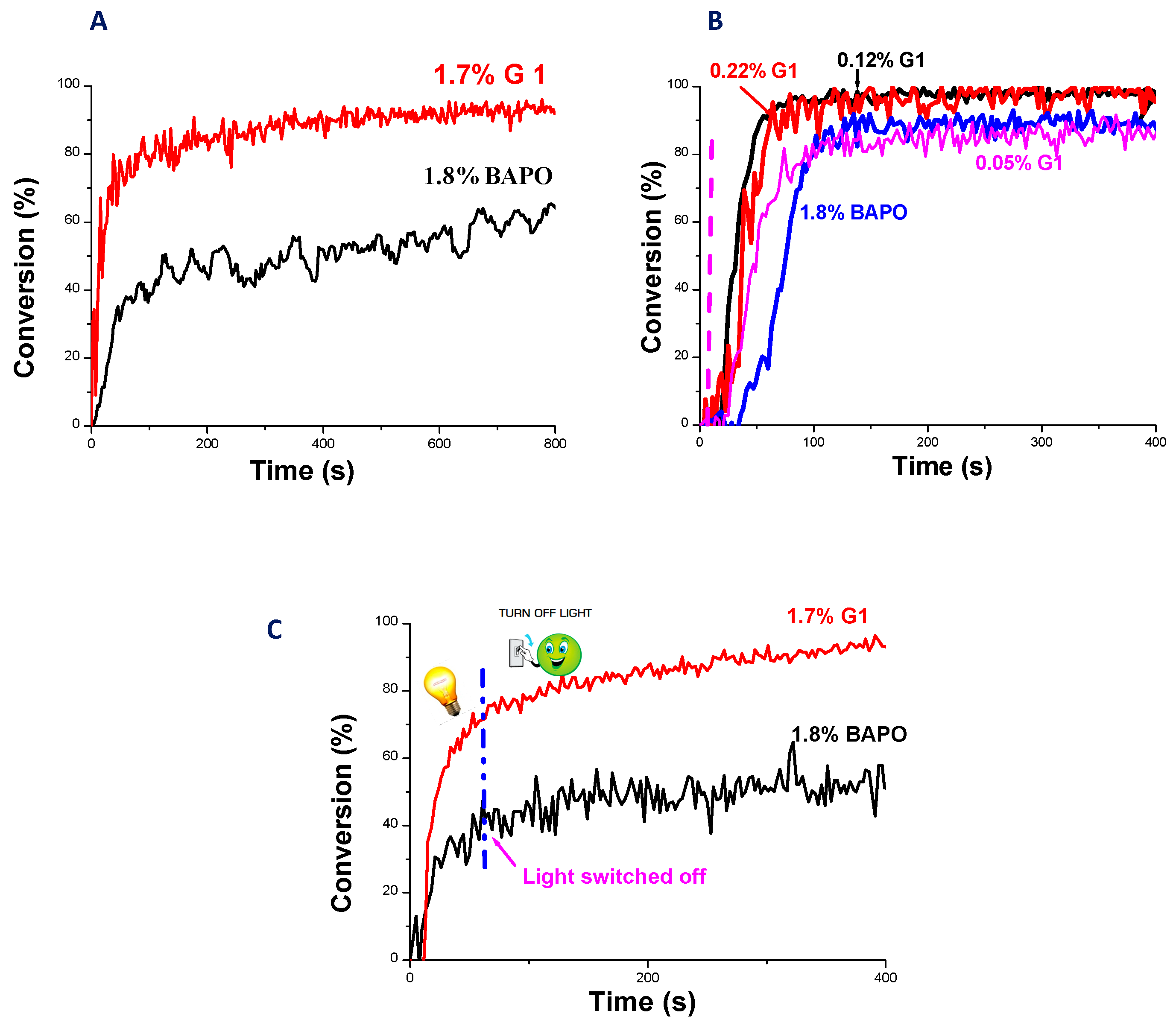
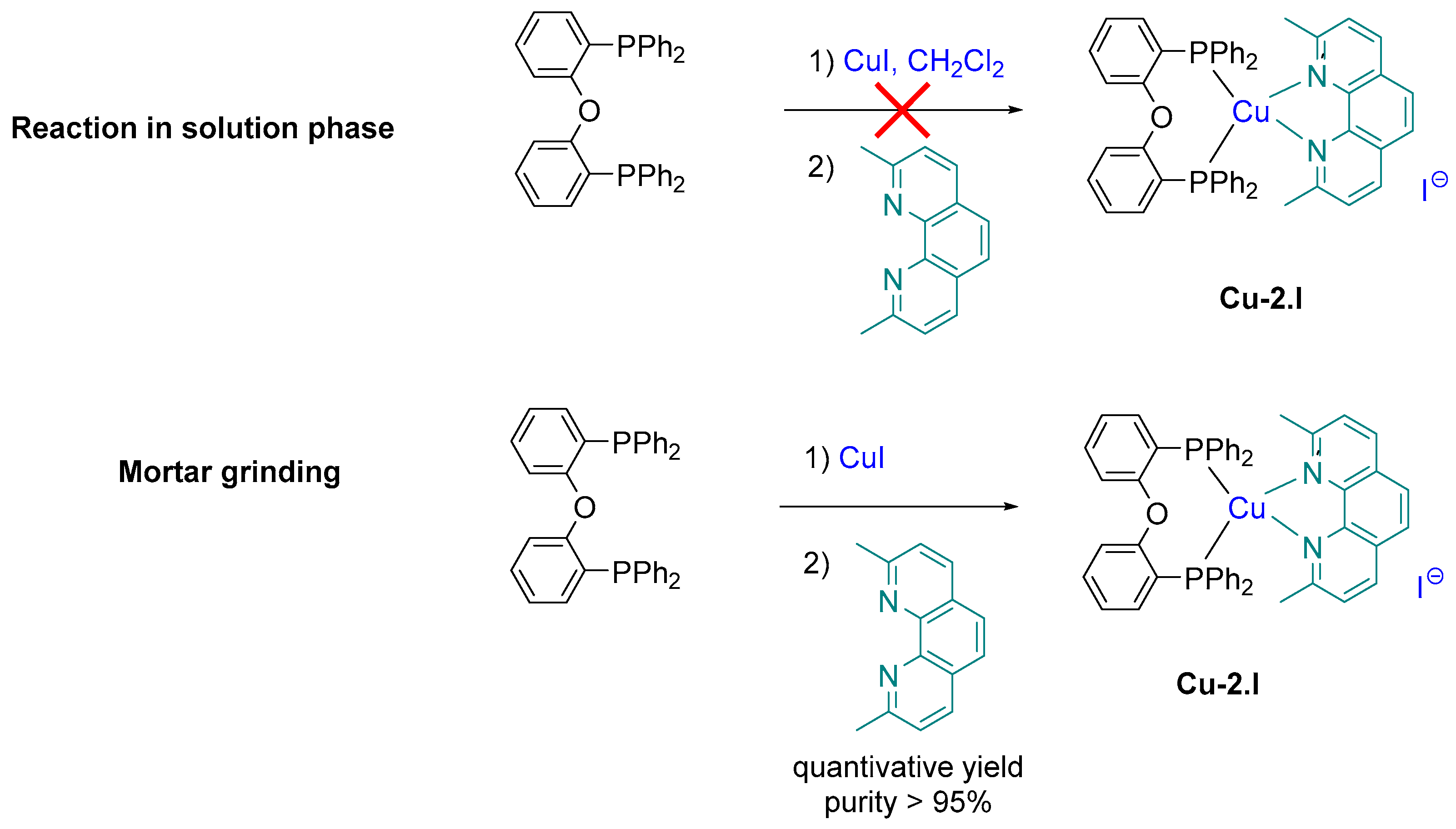
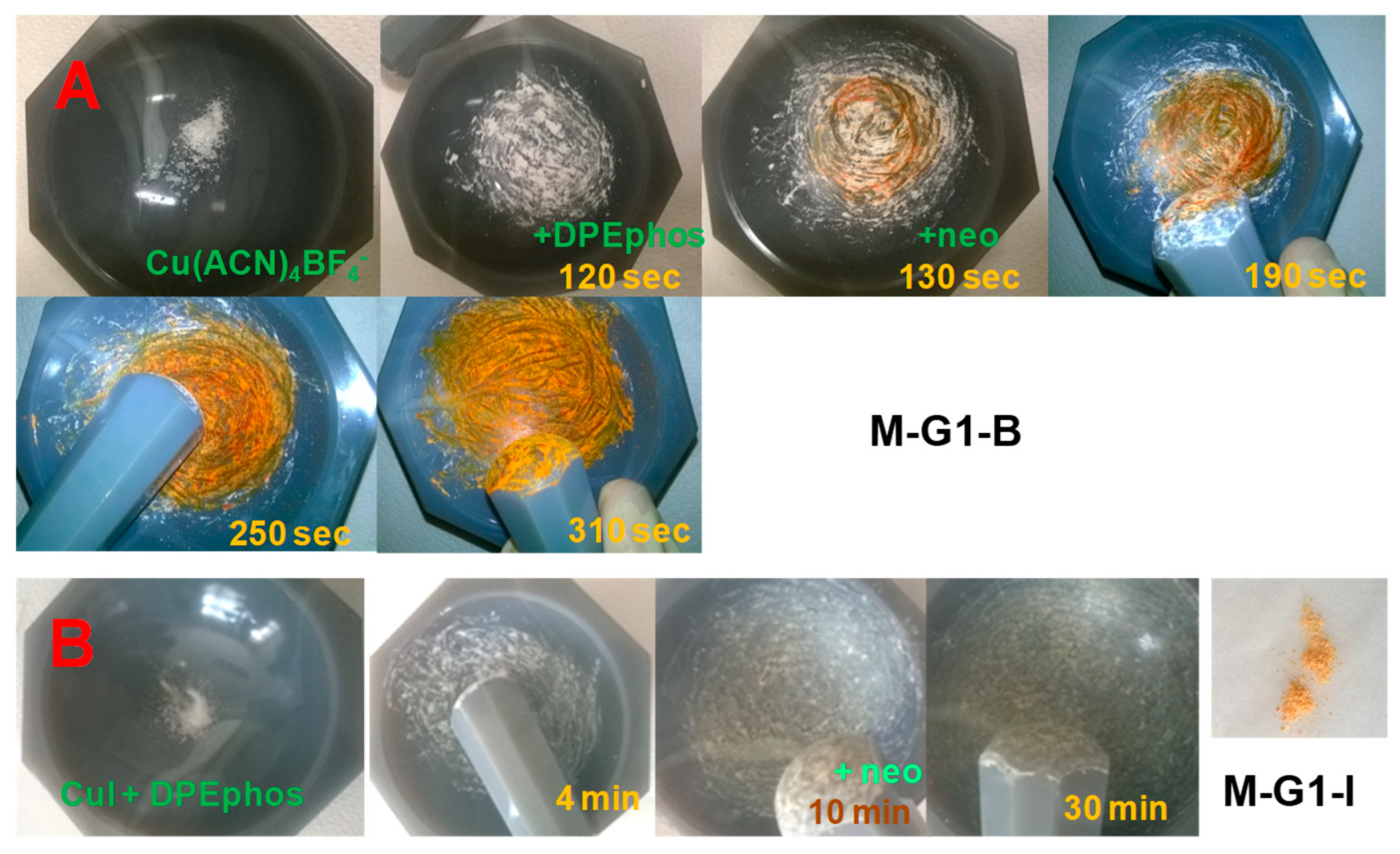
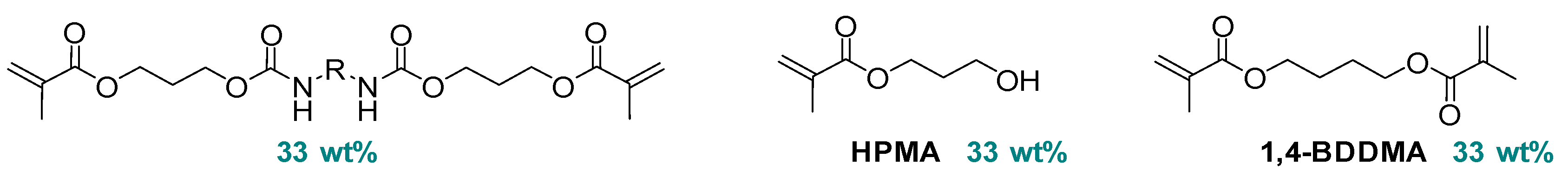
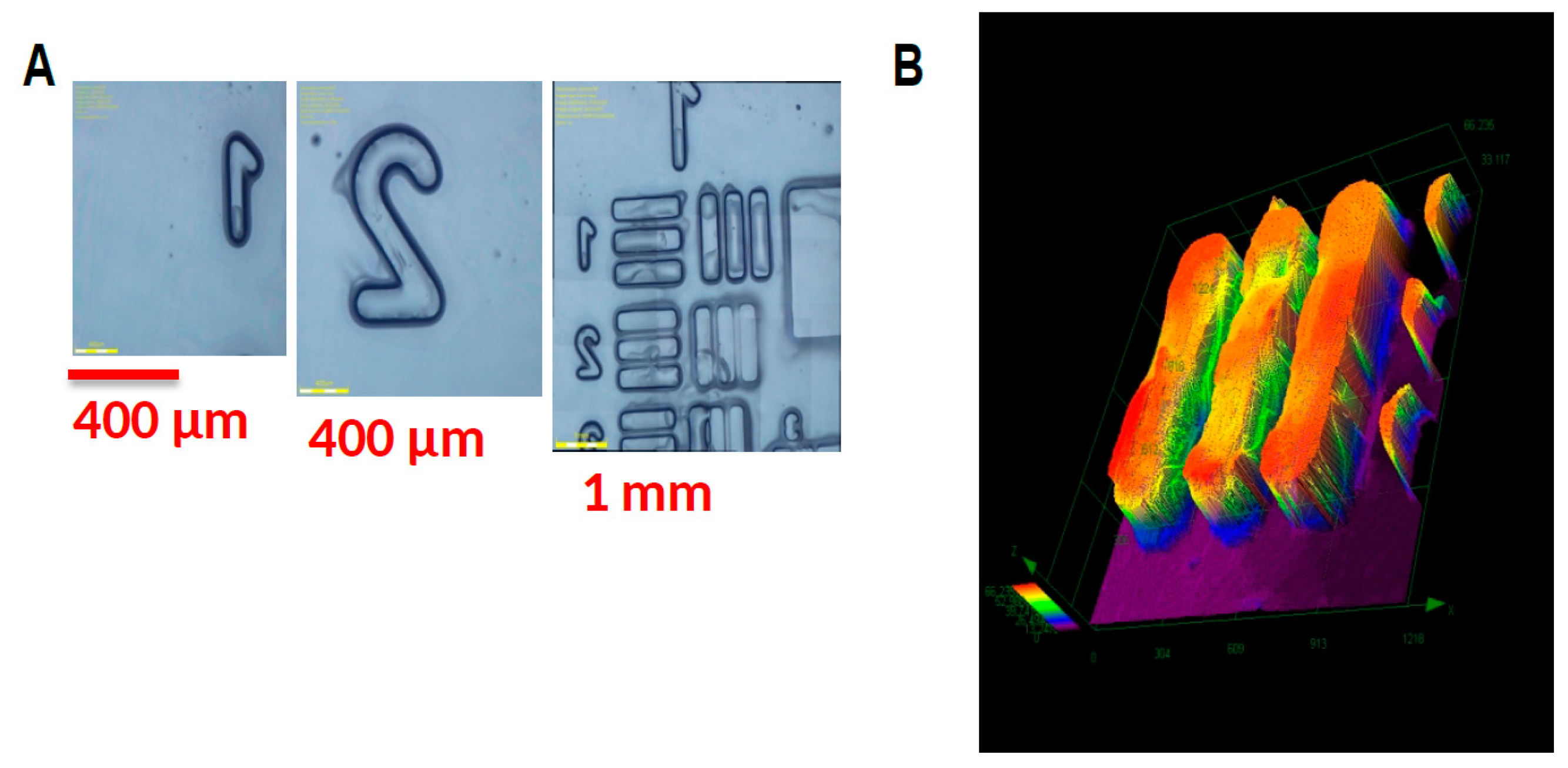
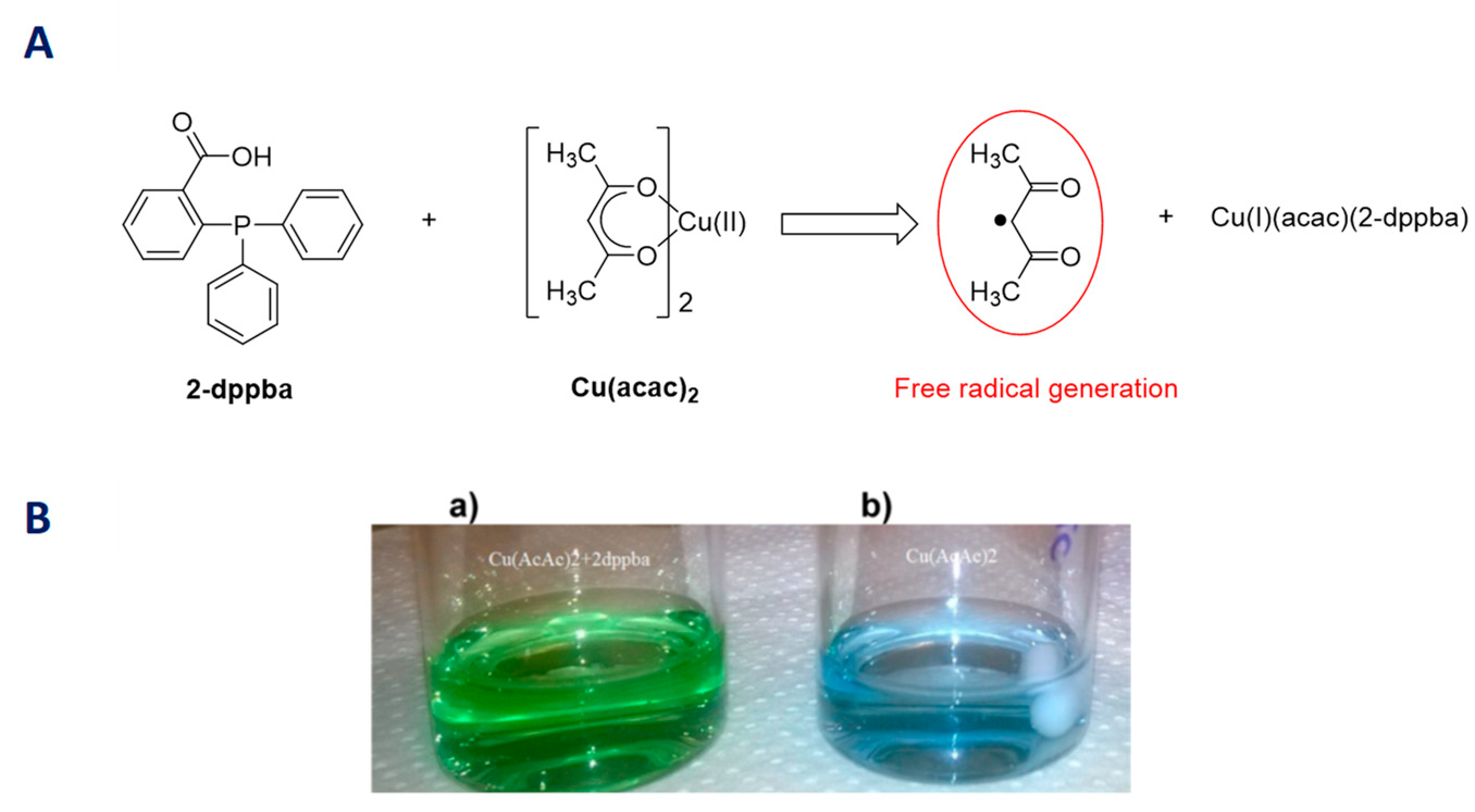
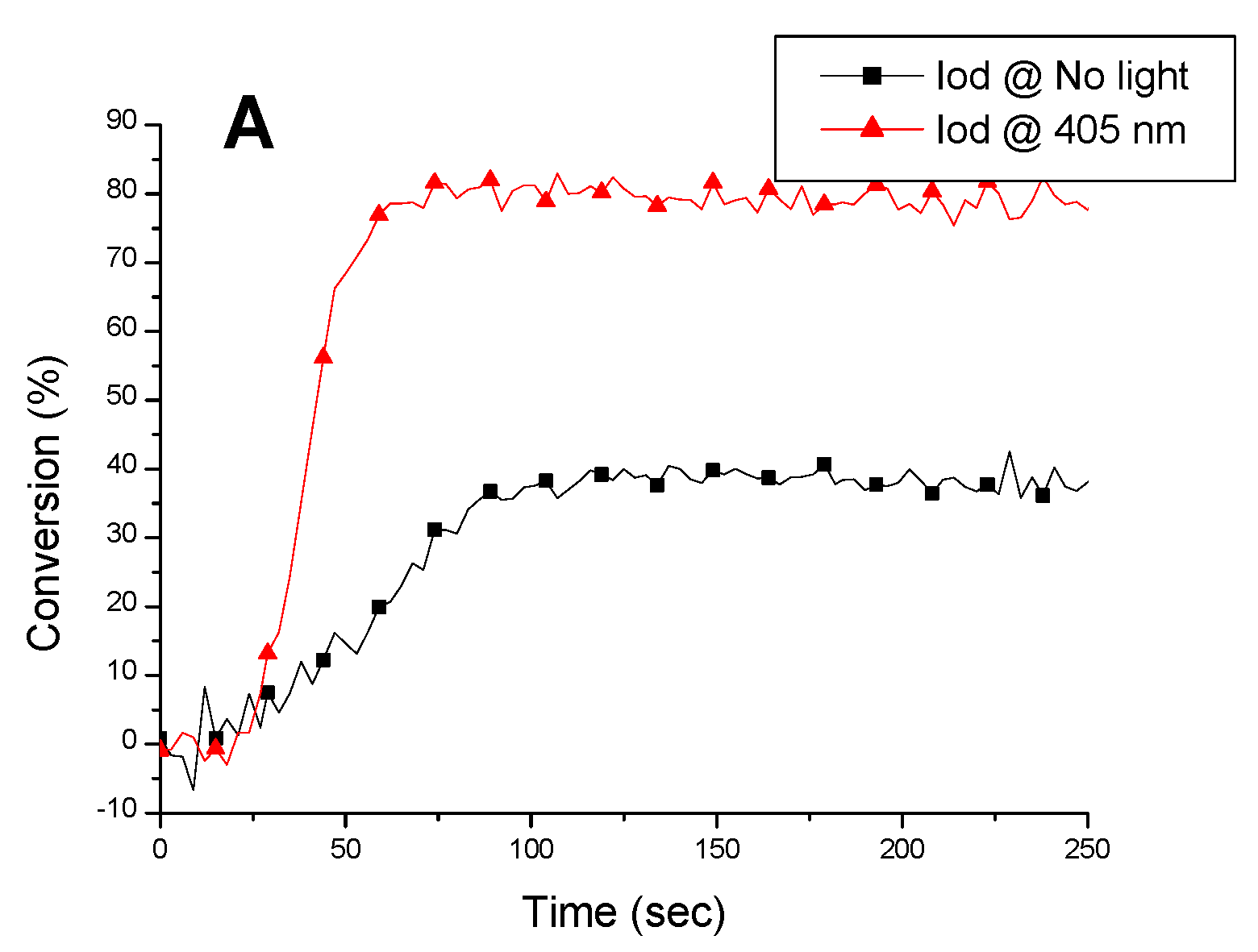
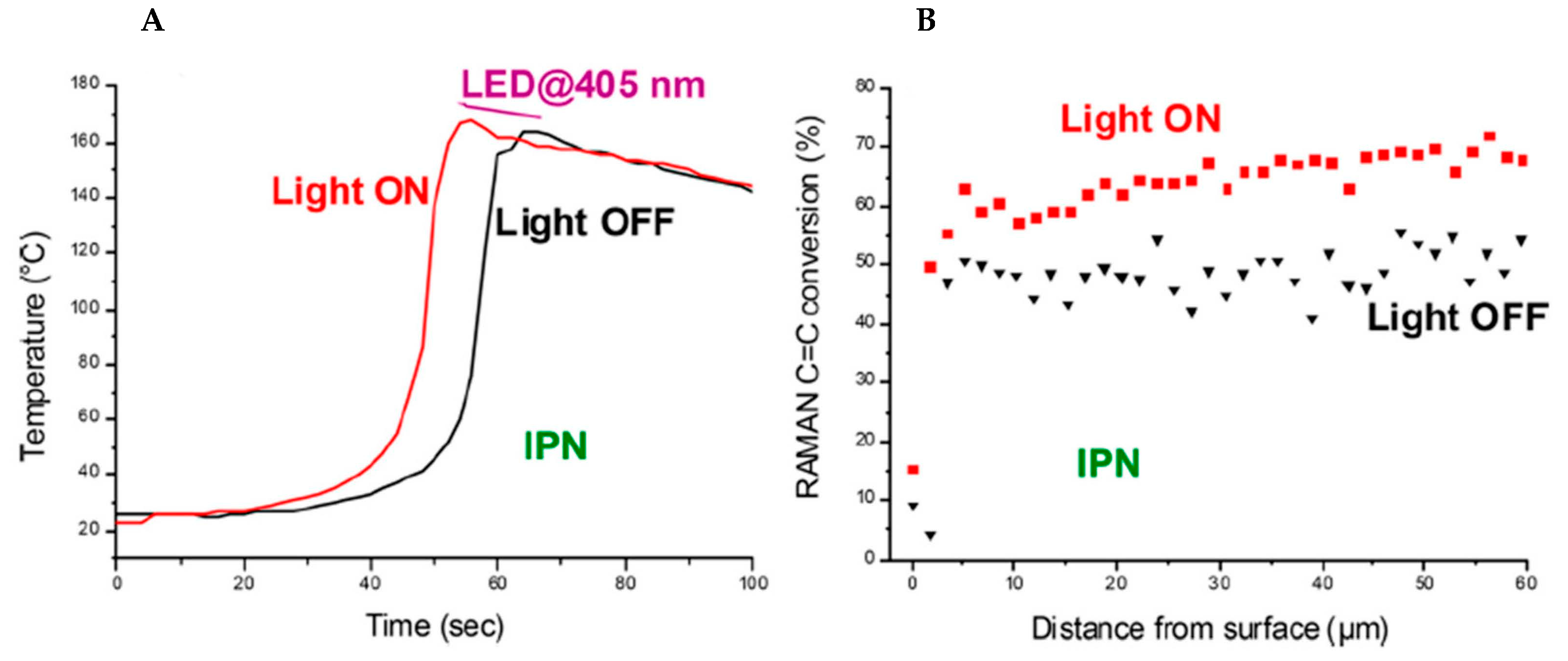
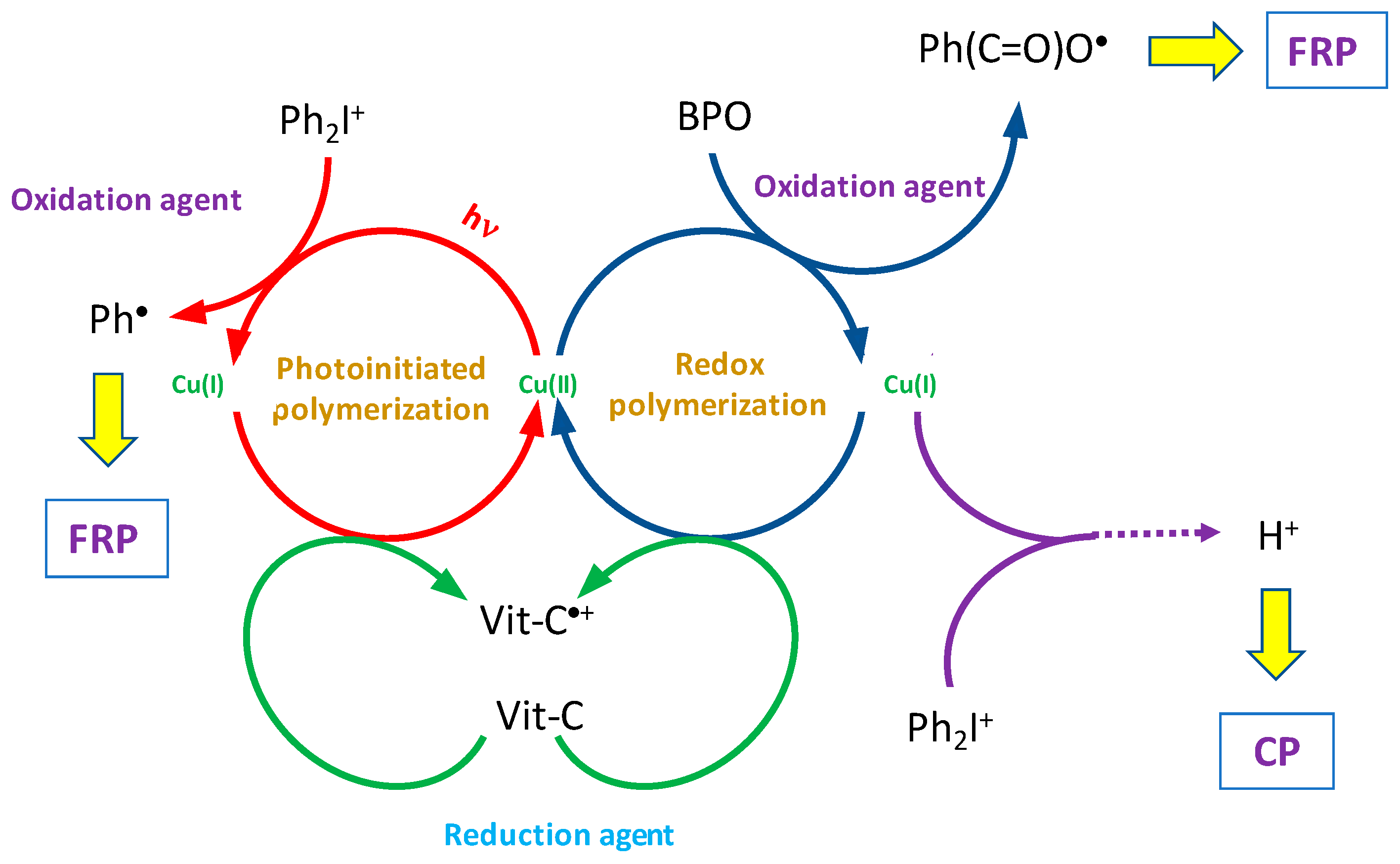
2.5. Copper Complexes
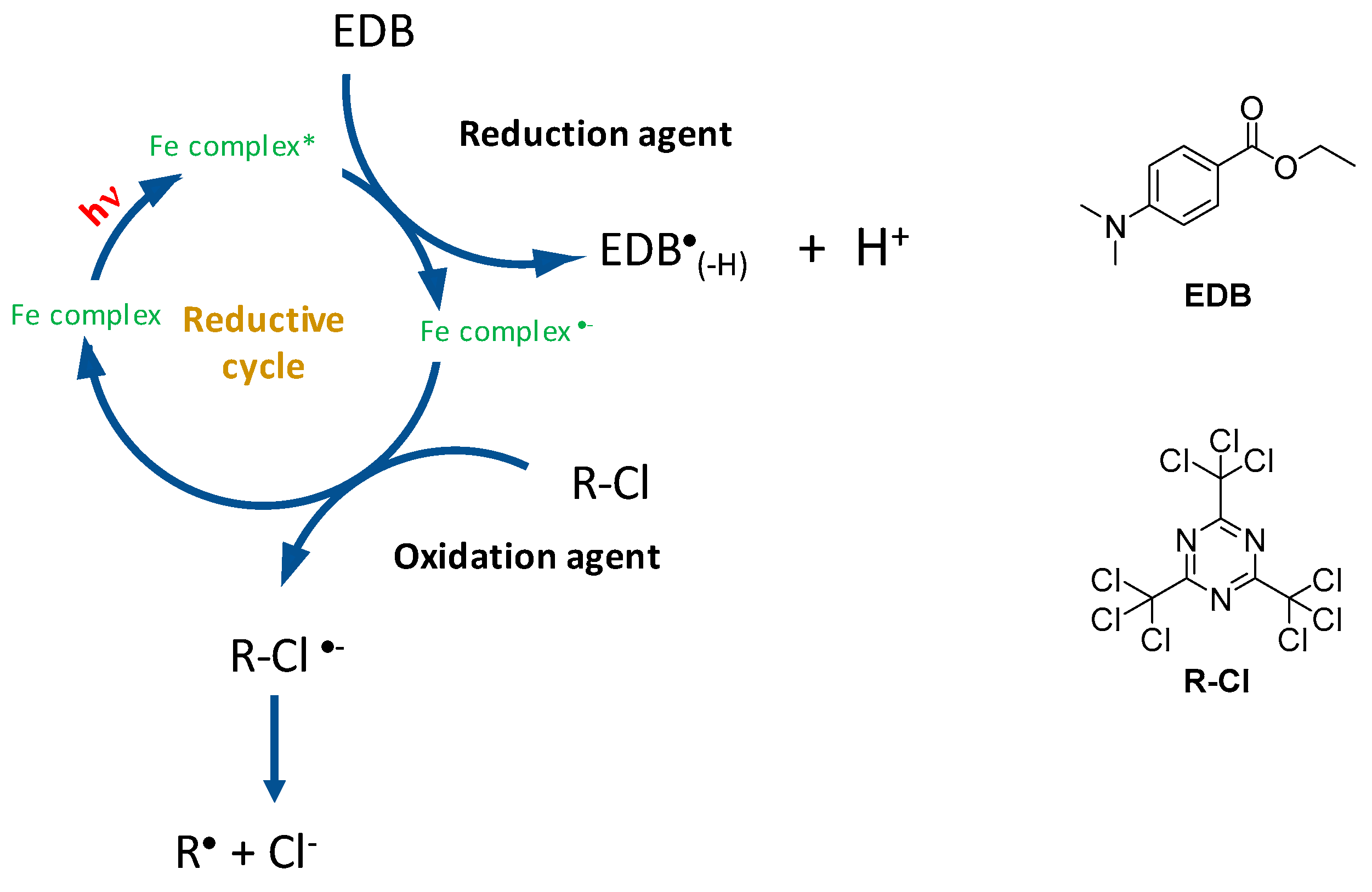
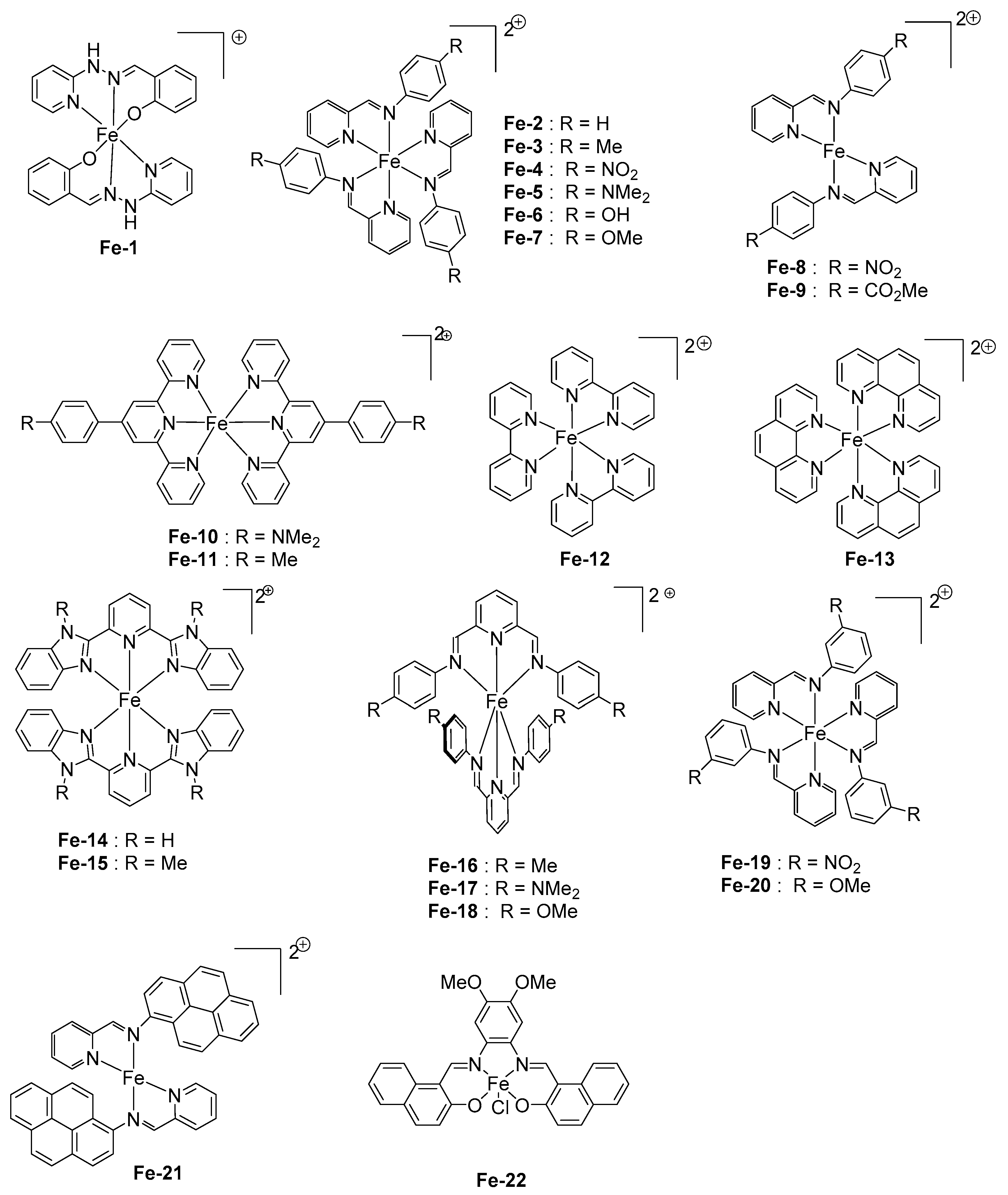
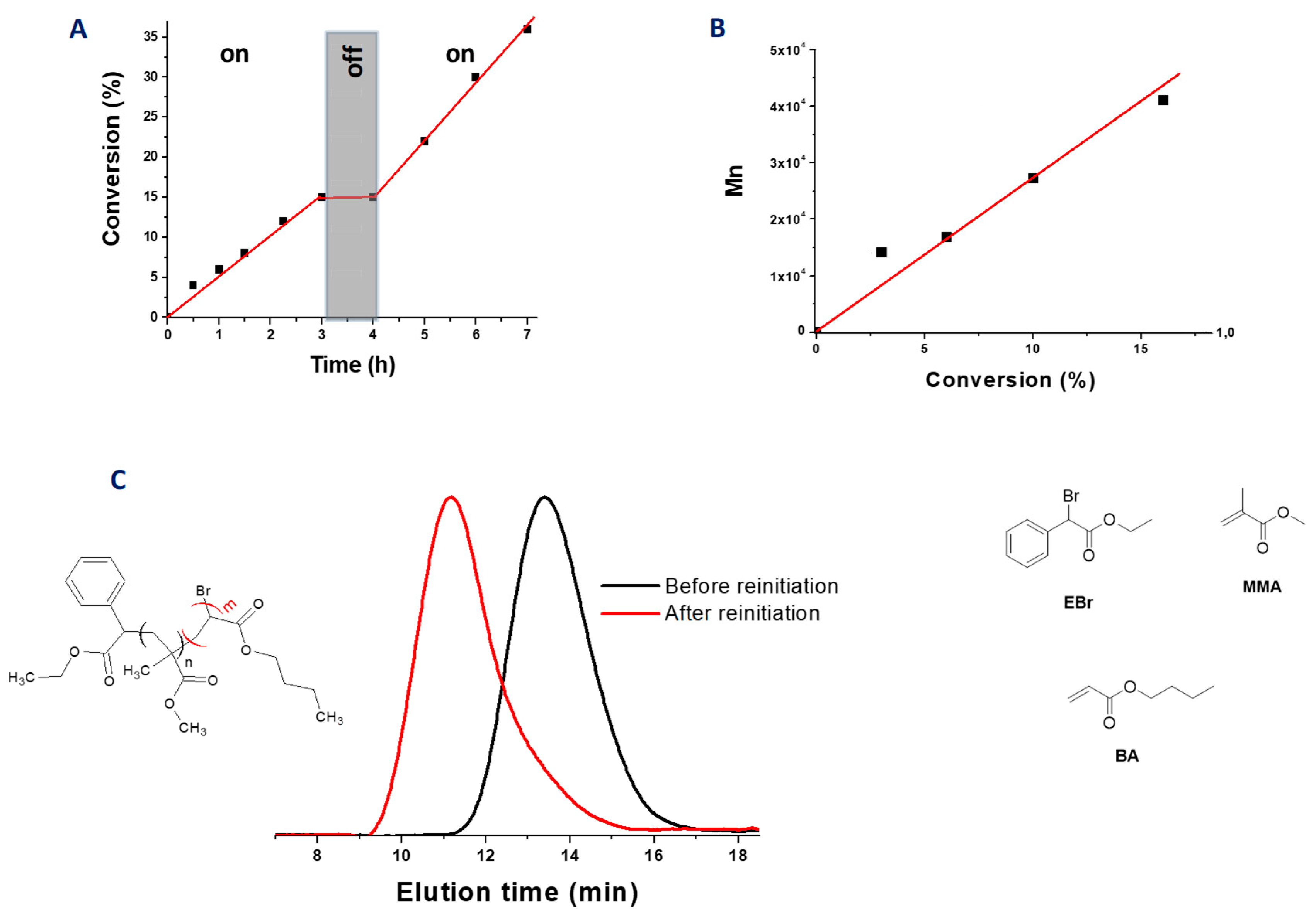
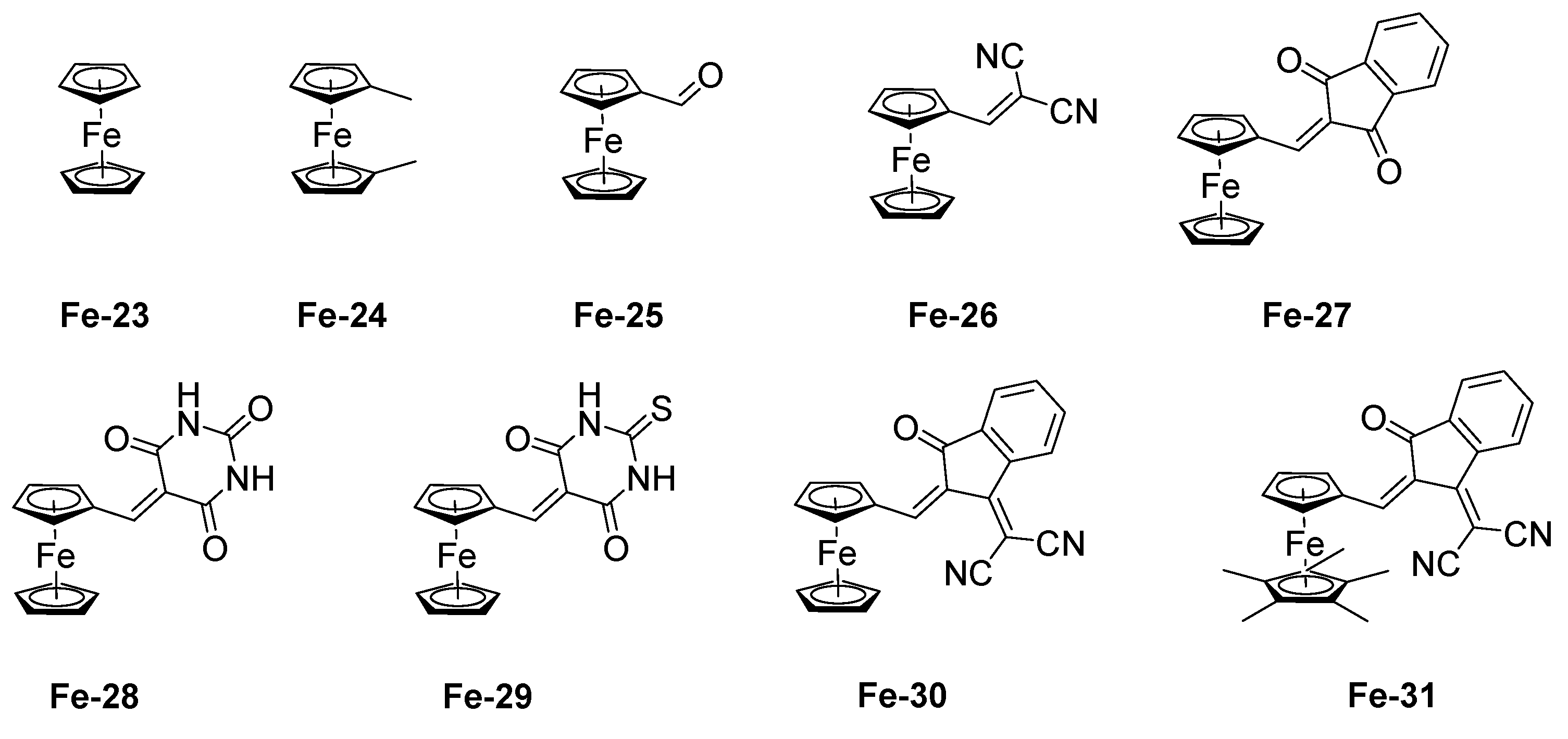
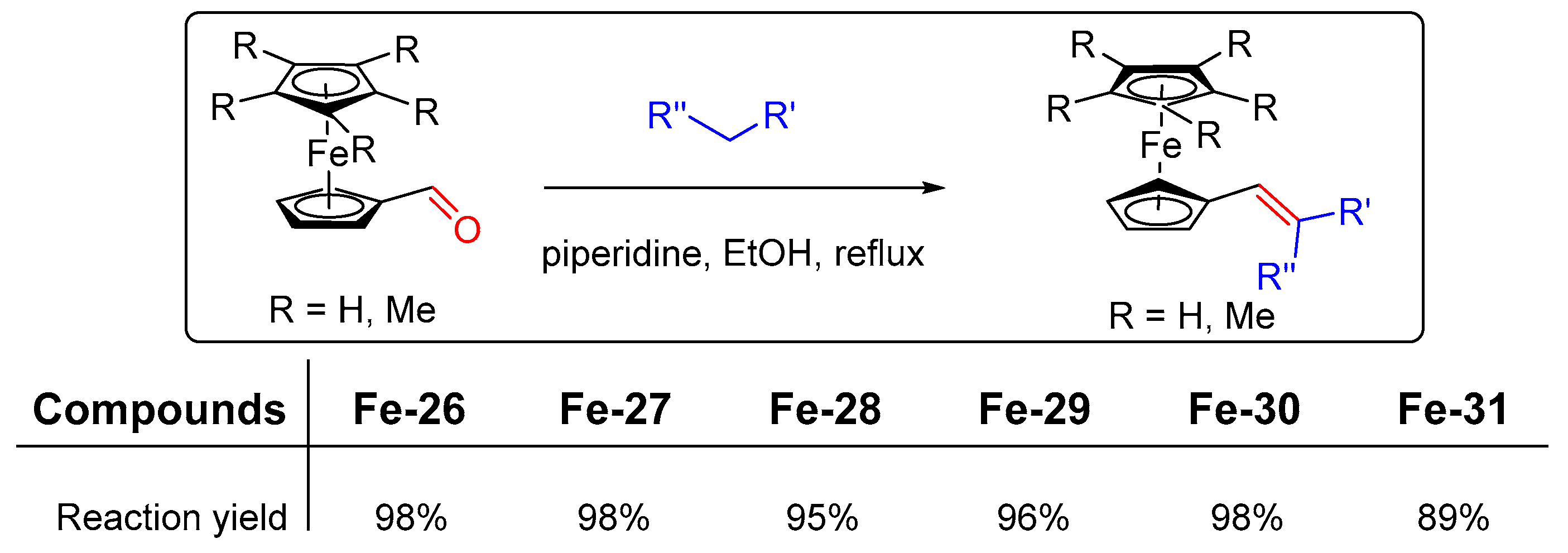
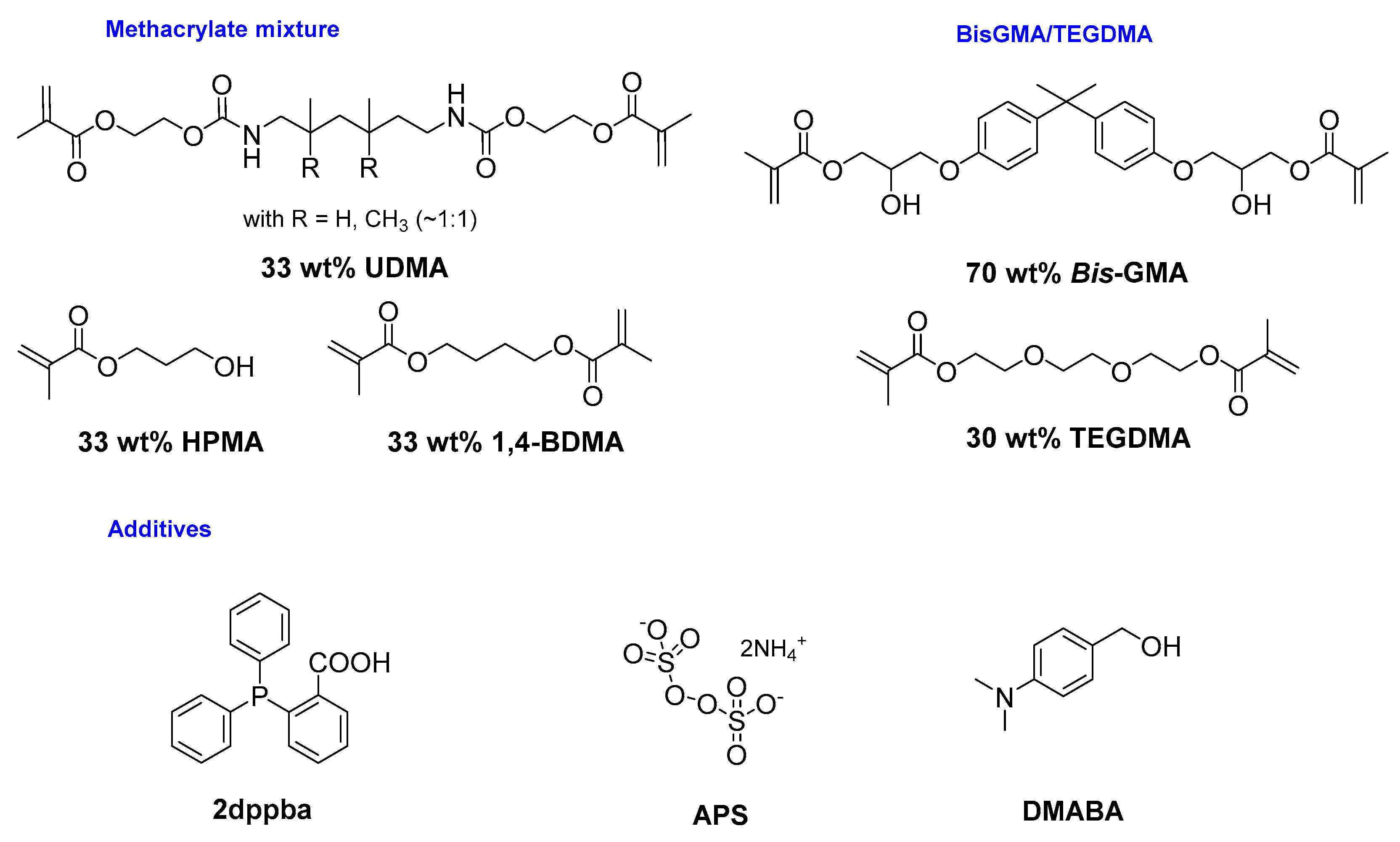
2.6. Iron Complexes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, N. Electron and hydrogen transfer in organic photochemical reactions. J. Phys. Org. Chem. 2015, 28, 121–136. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N. Studies in organic and physical photochemistry—an interdisciplinary approach. Org. Biomol. Chem. 2016, 14, 7392–7442. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, W.; Devalankar, D.; Ding, Z. Photochemical formation and cleavage of C–N bond. Org. Lett. 2015, 17, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, N.; Mizuno, K.; Otsuji, Y.; Caldwell, R.A.; Helms, A.M. Photochemical C−C bond cleavage of 1,2-diarylcyclopropanes bearing an acetylphenyl group. generation and observation of triplet 1,3-biradicals. J. Org. Chem. 1998, 63, 3176–3184. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5323. [Google Scholar] [CrossRef]
- Narayanama, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Bogdos, M.K.; Pinard, E.; Murphy, J.A. Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry. Beilstein J. Org. Chem. 2018, 14, 2035–2064. [Google Scholar] [CrossRef]
- Parasram, M.; Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: Beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef] [PubMed]
- Islamova, R.M.; Dobrynin, M.V.; Vlasov, A.V.; Eremina, A.A.; Kinzhalov, M.A.; Kolesnikov, I.E.; Zolotarev, A.A.; Masloborodova, E.A.; Luzyanin, K.V. Iridium (III)-catalysed cross-linking of polysiloxanes leading to the thermally resistant luminescent silicone rubbers. Catal. Sci. Technol. 2017, 7, 5843–5846. [Google Scholar] [CrossRef]
- Gee, J.C.; Fuller, B.A.; Lockett, H.M.; Sedghi, G.; Robertson, C.M.; Luzyanin, K.V. Visible light accelerated hydrosilylation of alkynes using platinum-[acyclic diaminocarbene] photocatalysts. Chem. Commun. 2018, 54, 9450–9453. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient visible light photocatalysis of [2 + 2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Tucker, J.W.; Stephenson, C.R.J. Electron-transfer photoredox catalysis: Development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. [Google Scholar] [CrossRef]
- Oelgemöller, M. Solar Photochemical synthesis: From the beginnings of organic photochemistry to the solar manufacturing of commodity chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef]
- Poelma, J.E.; Fors, B.P.; Meyers, G.F.; Kramer, J.W.; Hawker, C.J. Fabrication of complex three-dimensional polymer brush nanostructures through light-mediated living radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 6844–6848. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Brandford-Adams, F.; Nurumbetov, G.; Zhang, Q.; Clarkson, G.J.; Fox, D.J.; Wilson, P.; Kempe, K.; Haddleton, D.M. Photo-induced living radical polymerization ofacrylates utilizing a discrete copper(II)-formate complex. Chem. Commun. 2015, 51, 5626–5629. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.P.; Lalevée, J. High Performance Near-infrared (NIR) photoinitiating systems operating under low light intensity and in the presence of oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green bulb light source induced epoxy cationic polymerization under air using tris(2,2′-bipyridine)ruthenium(II) and silyl radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.H.; Dumur, F.; Noirbent, G.; Lalevée, J.; Gigmes, D. Organometallic vs organic photoredox catalysts for photocuring reactions in the visible region. Beilstein J. Org. Chem. 2018, 14, 3025–3046. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; Nguyen, T.M. Recent Applications of Organic Dyes as Photoredox Catalysts in Organic Synthesis. ACS Catal. 2014, 4, 355–360. [Google Scholar] [CrossRef]
- Tucker, J.W.; Stephenson, C.R.J. Shining light on photoredox catalysis: Theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C. Organosilanes in Radical Chemistry; John Wiley & Sons: Chicheste, UK, 2004. [Google Scholar]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. Household LED irradiation under air: Cationic polymerization using iridium or ruthenium complex photocatalysts. Polym. Bull. 2012, 68, 341–347. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green chemistry: Sunlight-induced cationic polymerization of renewable epoxy monomers under air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Combination of transition metal carbonyls and silanes: New photoinitiating systems. J. Polym. Sci. A Polym. Chem. 2010, 48, 1830–1837. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Ma, L.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Zhao, J.; Lalevée, J. Cyclometallated Pt(II) complexes in visible-light photoredox catalysis: New polymerization initiating systems. Macromol. Chem. Phys. 2012, 213, 2282–2286. [Google Scholar] [CrossRef]
- Crivello, J.V. Silane reduction of onium salts. Appl. Organomet. Chem. 2010, 24, 169–178. [Google Scholar] [CrossRef]
- Bamford, C.H.; Mullik, S.U.; Puddephatt, R.J. Photo-initiation of free-radical polymerization by dimethyl-(2,2′-bipyridyl)platinum(II)+ tetrafluoroethylene. J. Chem. Soc. Faraday Trans. 1975, 71, 2213–2225. [Google Scholar] [CrossRef]
- Ziessel, R.; Diring, S.; Retailleau, P. Terpyridine-platinum(II) acetylide complexes bearing pendent coordination units. Dalton Trans. 2006, 3285–3329. [Google Scholar] [CrossRef]
- Li, K.; Ming Tong, G.S.; Wan, Q.; Cheng, G.; Tong, W.Y.; Ang, W.H.; Kwong, W.L.; Che, C.M. Highly phosphorescent platinum (II) emitters: photophysics, materials and biological applications. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef]
- Kappaun, S.; Slugovc, C.; List, E.J.W. Phosphorescent organic light-emitting devices: Working principle and iridium-based emitter materials. Int. J. Mol. Sci. 2008, 9, 1527–1547. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structural effects in the iridium complex series: Photoredox catalysis and photoinitiation of polymerization reactions under visible lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Aspley, C.J.; Williams, J.A.G. Palladium-catalysed cross-coupling reactions of ruthenium bis-terpyridyl complexes: Strategies for the incorporation and exploitation of boronic acid functionality. New J. Chem. 2001, 25, 1136–1147. [Google Scholar] [CrossRef]
- Arm, K.J.; Williams, J.A.G. Boronic acid-substituted metal complexes: Versatile building blocks for the synthesis of multimetallic assemblies. Chem. Commun. 2005, 2, 230–232. [Google Scholar] [CrossRef]
- Arm, K.J.; Williams, J.A.G. A cross-coupling strategy for the synthesis of dimetallic assemblies containing mixed bipyridine-terpyridine bridging ligands: Luminescence and energy transfer properties. Dalton Trans. 2006, 18, 2172–2174. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.; Batsanov, A.S.; Howard, J.A.K.; Williams, J.A.G. Cross-couplings in the elaboration of luminescent bis-terpyridyl iridium complexes: The effect of extended or inhibited conjugation on emission. Dalton Trans. 2004, 4, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Whittle, V.L.; Williams, J.A.G. A new class of iridium complexes suitable for stepwise incorporation into linear assemblies: Synthesis, electrochemistry, and luminescence. Inorg. Chem. 2008, 47, 6596–6607. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.G.; Wilkinson, A.J.; Whittle, V.L. Light-emitting iridium complexes with tridentate ligands. Dalton Trans. 2008, 16, 2081–2099. [Google Scholar] [CrossRef] [PubMed]
- Whittle, V.L.; Williams, J.A.G. Cyclometallated, bis-terdentate iridium complexes as linearly expandable cores for the construction of multimetallic assemblies. Dalton Trans. 2009, 20, 3929–3940. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wei, L.; Wang, J.; Li, Y.; Wu, H.; Yang, W.; Cao, Y. Novel heteroleptic iridium(III) complexes with a 2-(1H-pyrazol-5-yl)pyridine derivative containing a carbazole group as ancillary ligand: Synthesis and application for polymer light-emitting diodes. Synth. Met. 2014, 187, 209–216. [Google Scholar] [CrossRef]
- Lepeltier, M.; Dumur, F.; Marrot, J.; Contal, E.; Bertin, D.; Gigmes, D.; Mayer, C.R. Unprecedented combination of regioselective hydrodefluorination and ligand exchange reaction during the syntheses of tris-cyclometalated iridium(III) complexes. Dalton Trans. 2013, 42, 4479–4486. [Google Scholar] [CrossRef] [PubMed]
- Lepeltier, M.; Dumur, F.; Graff, B.; Xiao, P.; Gigmes, D.; Lalevée, J.; Mayer, C.R. Tris-cyclometalated iridium(III) complexes with three different ligands: A new example with 2-(2,4-difluorophenyl)pyridine-based complex. Helv. Chim. Acta 2014, 97, 939–956. [Google Scholar] [CrossRef]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) complexes as promising emitters for solid-state Light-Emitting Electrochemical Cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Dumur, F.; Yuskevitch, Y.; Wantz, G.; Mayer, C.R.; Bertin, D.; Gigmes, D. Light-emitting electrochemical cells based on a solution-processed multilayered device and an anionic iridium (III) complex. Synth. Met. 2013, 177, 100–104. [Google Scholar] [CrossRef]
- Dumur, F.; Nasr, G.; Wantz, G.; Mayer, C.R.; Dumas, E.; Guerlin, A.; Miomandre, F.; Clavier, G.; Bertin, D.; Gigmes, D. Cationic iridium complex for the design of soft salt-based phosphorescent OLEDs and color-tunable light-emitting electrochemical cells. Org. Electron. 2011, 12, 1683–1694. [Google Scholar] [CrossRef]
- Nasr, G.; Guerlin, A.; Dumur, F.; Beouch, L.; Dumas, E.; Clavier, G.; Miomandre, F.; Goubard, F.; Gigmes, D.; Bertin, D.; et al. Iridium(III) soft salts from dinuclear cationic and mononuclear anionic complexes for OLED devices. Chem. Commun. 2011, 47, 10698–10700. [Google Scholar] [CrossRef] [PubMed]
- Lepeltier, M.; Graff, B.; Lalevée, J.; Wantz, G.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Heteroleptic iridium (III) complexes with three different ligands: Unusual triplet emitters for light-emitting electrochemical cells. Org. Electron. 2016, 37, 24–34. [Google Scholar] [CrossRef]
- Lepeltier, M.; Appaix, F.; Liao, Y.Y.; Dumur, F.; Marrot, J.; Le Bahers, T.; Andraud, C.; Monnereau, C. Carbazole-substituted iridium complex as a solid state emitter for two-photon intravital imaging. Inorg. Chem. 2016, 55, 9586–9595. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Peter, M.; Dumur, F.; Gigmes, D.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Subtle ligand effects in oxidative photocatalysis with iridium complexes: Application to photopolymerization. Chem. Eur. J. 2011, 17, 15027–15031. [Google Scholar] [CrossRef] [PubMed]
- Telitel, S.; Dumur, F.; Lepeltier, M.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photoredox process induced polymerization reactions: Iridium complexes for panchromatic photoinitiating systems. Comptes Rendus Chim. 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Z.; Xu, D.; Yu, Y.; Tang, X.; Guo, H. Studies of free radical polymerization initiated by visible light photoredox catalysis. Macromol. Chem. Phys. 2015, 216, 1055–1060. [Google Scholar] [CrossRef]
- Zhang, G.; Song, I.Y.; Ahn, K.H.; Park, T.; Choi, W. Free radical polymerization initiated and controlled by visible light photocatalysis at ambient temperature. Macromolecules 2011, 44, 7594–7599. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.P. Iridium photocatalysts in free radical photopolymerization under visible lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A novel photopolymerization initiating system based on an iridium complex photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and photophysics of coordination compounds: Iridium. Top. Curr. Chem. 2007, 281, 143–203. [Google Scholar] [CrossRef]
- Okada, S.; Okinaka, K.; Iwawaki, H.; Furugori, M.; Hashimoto, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Tsuboyama, A.; Takiguchi, T.; et al. Substituent effects of iridium complexes for highly efficient red OLEDs. Dalton Trans. 2005, 9, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, M. Benzo[h]quinolin-10-yl-N Iridium(III) Complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Cao, Y.; Shi, C.; Tao, P.; Zhao, Q.; Yuan, A. An oxygen-bridged triarylamine polycyclic unit based tris-cyclometalated heteroleptic iridium(III) complex: Correlation between the structure and photophysical properties. Dalton Trans. 2019, 48, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Alleyne, B.D.; Djurovich, P.I.; Lamansky, S.; Tsyba, I.; Ho, N.N.; Bau, R.; Thompson, M.E. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Edkins, R.M.; Wriglesworth, A.; Fucke, K.; Bettington, S.L.; Beeby, A. The synthesis and photophysics of tris-heteroleptic cyclometalated iridium complexes. Dalton Trans. 2011, 40, 9672–9678. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Meng, G.; Zhou, Y.; Tang, H.; Zhao, J.; Wang, Z. The photoluminescent properties of new cationic iridium (III) complexes using different anions and their applications in white light-emitting diodes. Materials 2015, 8, 6105–6116. [Google Scholar] [CrossRef] [PubMed]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H.-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Gigmes, D.; Blanchard, N.; Fouassier, J.P. Photoredox catalysis for polymerization reactions. Chimia 2012, 66, 439–441. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Metal and metal-free photocatalysts: Mechanistic approach and application as photoinitiators of photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of N-Vinylcarbazole Using Visible-Light Harvesting Iridium Complexes as Photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Dumur, F.; Beouch, L.; Tehfe, M.A.; Contal, E.; Lepeltier, M.; Wantz, G.; Graff, B.; Goubard, F.; Mayer, C.R.; Lalevée, J.; et al. Low-cost zinc complexes for white organic light-emitting devices. Thin Solid Films 2014, 564, 351–360. [Google Scholar] [CrossRef]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of zinc complexes: Easily tunable optical properties by variation of the bridge between the imido groups of schiff base ligands. Eur. J. Inorg. Chem. 2014, 25, 4186–4198. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Poli, R.; Allan, L.E.N.; Shaver, M.P. Iron-mediated reversible deactivation controlled radical polymerization. Prog. Polym. Sci. 2014, 39, 1827–1845. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/”living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Stable copper acetylacetonate-based oxidizing agents in redox (NIR photoactivated) polymerization: An opportunity for the one pot grafting from approach and an example on a 3D printed object. Polym. Chem. 2018, 9, 2173–2182. [Google Scholar] [CrossRef]
- Garra, P.; Carré, M.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper-based (photo)redox initiating systems as highly efficient systems for interpenetrating polymer network preparation. Macromolecules 2018, 51, 679–688. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Mechanosynthesis of a Copper complex for redox initiating systems with a unique near infrared light activation. J. Polym. Sci. A Polym. Chem. 2017, 55, 3646–3655. [Google Scholar] [CrossRef]
- Garra, P.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Monnier, V.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Metal Acetylacetonate–Bidentate Ligand Interaction (MABLI) as highly efficient free radical generating systems for polymer synthesis. Polym. Chem. 2018, 9, 1371–1378. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Gree, S.; Fouassier, J.P.; Lalevée, J. Metal acetylacetonate–bidentate ligand interaction (mabli) (photo)activated polymerization: Toward high performance amine-free, peroxide-free redox radical (photo)initiating systems. Macromolecules 2018, 51, 2706–2715. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Copper Complexes in Radical Photoinitiating Systems: Applications to Free Radical and Cationic Polymerization upon Visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper complexes: The effect of ligands on their photoinitiation efficiencies in radical polymerization reactions under visible light. Polym. Chem. 2014, 5, 6350–6357. [Google Scholar] [CrossRef]
- Yang, Q.; Balverde, S.; Dumur, F.; Lalevée, J.; Poly, J. Synergetic effect of the epoxide functional groups in the photocatalyzed atom transfer radical copolymerization of glycidyl methacrylate. Polym. Chem. 2016, 7, 6084–6093. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet exciton confinement in green organic light-emitting diodes containing luminescent charge-transfer cu (i) complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Parker, C.A.; Hatchard, C.G. Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans. Faraday Soc. 1961, 57, 1894–1904. [Google Scholar] [CrossRef]
- Bui, T.T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzrati-Zerelli, M.; Noirbent, G.; Goubard, G.; Bui, T.T.; Villotte, S.; Dietlin, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Dumur, F.; et al. A novel class of photoinitiators with a thermally activated delayed fluorescence (TADF) property. New J. Chem. 2018, 42, 8261–8270. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Lalevée, J.; Zhao, J.; Stenzel, M.H. N-Vinylcarbazole as Versatile Photoinaddimer of Photopolymerization under Household UV LED Bulb (392 nm). Macromol. Rapid Commun. 2015, 36, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Xiao, P.; Dumur, F.; Gigmes, D. Light induced free radical and/or cationic photopolymerization method. Patent WO2015132295 A1, 2015. [Google Scholar]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalyst “G1”: A new high performance photoinitiator for near-UV and visible LEDs. J. Polym. Chem. 2017, 8, 5580–5592. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Mokbel, H.; Monnier, V.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. New synthetic route to an highly efficient photoredox catalyst by mechanosynthesis. ACS Omega 2018, 3, 10938–10944. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific cationic photoinitiators for near UV and visible LEDs: Iodonium versus ferrocenium structures. J. Appl. Polym. Sci. 2015, 42759. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Al Mousawi, A.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Copper (photo)redox catalyst for radical photopolymerization in shadowed areas and access to thick and filled samples. Macromolecules 2017, 50, 3761–3771. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Laleveée, J. A new highly efficient amine-free and peroxide-free redox system for free radical polymerization under air with possible light activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Doronina, E.P.; Sidorkin, V.F.; Gigmes, D.; Fouassier, J.P.; et al. Peroxide-free and amine-free redox free radical polymerization: Metal acetylacetonates/stable carbonyl compounds for highly efficient synthesis of composites. Macromolecules 2018, 51, 6395–6404. [Google Scholar] [CrossRef]
- Achilias, D.S.; Sideridou, I. Study of the effect of two BPO/amine initiation systems on the free-radical polymerization of MMA used in dental resins and bone cements. J. Macromol. Sci. Part A Pure Appl. Chem. 2002, 39, 1435–1450. [Google Scholar] [CrossRef]
- Garra, P.; Kermagoret, A.; Al Mousawi, A.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. New copper(I) complex based initiating systems in redox polymerization and comparison with the amine/benzoyl peroxide reference. Polym. Chem. 2017, 8, 4088–4097. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron complexes as photoinitiators for radical and cationic polymerization through photoredox catalysis processes. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 42–49. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Visible-light-sensitive photoredox catalysis by iron complexes: Applications in cationic and radical polymerization reactions. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron complexes in visible-light-sensitive photoredox catalysis: Effect of ligands on their photoinitiation efficiencies. ChemCatChem 2016, 8, 2227–2233. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper and iron complexes as visible-light-sensitive photoinitiators of polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The carbazole-bound ferrocenium salt as a specific cationic photoinitiator upon near-UV and visible LEDs (365–405 nm). Polym. Bull. 2016, 73, 493–507. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Nechab, M.; Gigmes, D.; Fouassier, J.P. Metal complex based photocatalyst systems: A quest for new possibilities and application to photopolymerization reactions. Trends Photochem. Photobiol. 2012, 14, 27–38. Available online: http://www.researchtrends.net/tia/article_pdf.asp?in=0&vn=14&tid=15&aid=3797 (accessed on 5 August 2019).
- Cunningham, A.F.; Desobry, V. Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 323–374. [Google Scholar]
- Anderson, J.; Hardgrove, E.; Cavitt, T.B.; Reeves, P. Photoinitiation of multifunctional acrylates via ferrocene-alkyl chloride charge transfer complexes. J. Coat. Technol. Res. 2007, 4, 43–49. [Google Scholar] [CrossRef]
- Neumann, M.G.; Schmitt, C.C.; Rigoli, I.C. The photoinitiation of MMA polymerization in the presence of iron complexes. J. Photochem. Photobiol. A 2003, 159, 145–150. [Google Scholar] [CrossRef]
- Burget, D.; Fouassier, J.P. Laser flash photolysis studies of the interaction of Rose Bengal with an iron arene complex. J. Chem. Soc. Faraday Trans. 1998, 94, 1849–1854. [Google Scholar] [CrossRef]
- Grotzinger, C.; Burget, D.; Jacques, P.; Fouassier, J.P. A novel and efficient xanthenic dye–organometallic ion-pair complex for photoinitiating polymerization. J. Appl. Polym. Sci. 2001, 81, 2368–2376. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Morlet-Savary, F.; Yamashita, K.; Imahashi, S. Visible light-induced polymerization reactions: The seven-role of the electron transfer process in the dye/iron arene complex/amine system. J. Appl. Polym. Sci. 1996, 62, 1877–1885. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Morlet-Savary, F.; Yamashita, K.; Imahashi, S. The role of the dye/iron arene complex/amine system as a photoinitiator for photopolymerization reactions. Polymer 1997, 38, 1415–1421. [Google Scholar] [CrossRef]
- Fredin, L.A.; Papai, M.; Rozsalyi, E.; Vanko, G.; Warnmark, K.; Sundstrçm, V.; Persson, P. Exceptional excited-state lifetime of an iron (II)–N-heterocyclic carbene complex explained. J. Phys. Chem. Lett. 2014, 5, 2066–2071. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole scaffold based photoinitiators/photoredox catalysts for new LED projector 3D printing resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Amino and nitro substituted 2-amino-1H-benzo[de]isoquinoline-1,3(2H)-diones: As versatile photo-initiators of polymerization: From violet-blue LEDs absorption to a panchromatic behavior. J. Polym. Chem. 2015, 6, 1171–1179. [Google Scholar] [CrossRef]
- Morris, J.; Telitel, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Lalevée, J.; Clément, J.L.; Guillaneuf, Y.; Gigmes, D. Novel polymer synthesis methodologies using combinations of thermally- and photochemically-induced nitroxide mediated polymerization. Polym. Chem. 2015, 6, 754–763. [Google Scholar] [CrossRef]
- Telitel, S.; Telitel, S.; Bosson, J.; Lalevée, J.; Clément, J.L.; Godfroy, M.; Fillaut, J.L.; Akdas-Kilig, H.; Guillaneuf, Y.; Gigmes, D.; et al. UV-induced micropatterning of complex functional surfaces by photopolymerization controlled by alkoxyamines. Langmuir 2015, 31, 10026–10036. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Schroeder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible light and sunlight photoinduced ATRP with ppm of Cu catalyst. ACS Macro Lett. 2012, 1, 1219–1223. [Google Scholar] [CrossRef]
- Fors, B.P.; Hawker, C.J. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 2012, 51, 8850–8853. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Kumar, P.; Sharma, C.D.; Ray, S.S. Light-induced controlled free radical polymerization of methacrylates using iron-based photocatalyst in visible light. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2739–2746. [Google Scholar] [CrossRef] [Green Version]
- Tasdelen, M.A.; Uygun, M.; Yagci, Y. Photoinduced controlled radical polymerization. Macromol. Rapid Commun. 2011, 32, 58–62. [Google Scholar] [CrossRef]
- Ishizu, K.; Kakinuma, H. Synthesis of nanocylinders consisting of graft block copolymers by the photo-induced ATRP technique. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 63–70. [Google Scholar] [CrossRef]
- Kwak, Y.; Matyjaszewski, K. Photoirradiated atom transfer radical polymerization with an alkyl dithiocarbamate at ambient temperature. Macromolecules 2010, 43, 5180–5183. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Uygun, M.; Yagci, Y. Photoinduced controlled radical polymerization in methanol. Macromol. Chem. Phys. 2010, 211, 2271–2275. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Tasdelen, M.A.; Kiskan, B.; Wang, X.; Antonietti, M.; Yagci, Y. Photochemically mediated atom transfer radical polymerization using polymeric semiconductor mesoporous graphitic carbon nitride. Macromol. Chem. Phys. 2014, 215, 675–681. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Konkolewicz, D.; Pan, X.; Matyjaszewski, K. Contribution of photochemistry to activator regeneration in ATRP. Macromolecules 2014, 47, 6316–6321. [Google Scholar] [CrossRef]
- Chantasirichot, S.; Inoue, Y.; Ishihara, K. Photoinduced atom transfer radical polymerization in a polar solvent to synthesize a water-soluble poly (2-methacryloyloxyethyl phosphorylcholine) and its block-type copolymers. Polymer 2015, 61, 55–60. [Google Scholar] [CrossRef]
- Doran, S.; Taskin, O.S.; Tasdelen, M.A.; Yagci, Y. Controlled photopolymerization and novel architectures. In Dyes and Chromophores in Polymer Science; Lalevee, J., Fouassier, J.P., Eds.; ISTE Wiley: London, UK, 2015. [Google Scholar]
- Zhang, J.; Dumur, F.; Horcajada, P.; Livage, C.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron-based metal-organic frameworks (MOF) as photocatalysts for radical and cationic polymerizations under near UV and visible LEDs (385-405 nm). Macromol. Chem. Phys. 2016, 217, 2534–2540. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Garcia, H.; Ferrer, B. Metal Organic Frameworks as Heterogeneous Catalysts; The Royal Society of Chemistry: London, UK, 2013; p. 365. [Google Scholar]
- Wang, J.L.; Wang, C.; Lin, W. Metal-organic frameworks for light harvesting and photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Stoesser, M.; Ruppel, R.; Baum, E.; Bohres, E.; Sigl, M.; Lobree, L.; Yaghi, O.M.; Eddaoudi, M. Process for the Alkoxylation of Organic Compounds in the Presence of Novel Framework Materials. U.S. Patent US7279517 B2, 10 October 2001. [Google Scholar]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron (III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Muller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Park, J.; Raiff, A.; Wei, Z.; Zhou, H.C. Metal-organic frameworks as biomimetic catalysts. ChemCatChem 2014, 6, 67–75. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Cirujano, F.G.; Leyva-Pérez, A.; Corma, A.; Llabrés i Xamena, F.X. MOFs as multifunctional catalysts: Synthesis of secondary arylamines, quinolines, pyrroles, and arylpyrrolidines over bifunctional MIL-101. ChemCatChem 2013, 5, 538–549. [Google Scholar] [CrossRef]
- Zhao, Z.; Qian, L.; Lv, H.; Wang, Y.; Zhao, G. Introduction of a Fe3O4 core enhances the photocatalytic activity of MIL-100(Fe) with tunable shell thickness in the presence of H2O2. ChemCatChem 2015, 7, 4148–4155. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Li, L.; Jiang, J. Iron terephthalate metal–organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. J. Appl. Catal. B 2014, 148–149, 191–200. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Garra, P.; Brunel, D.; Noirbent, G.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Sidorkin, V.F.; Dumur, F.; Duché, D.; Gigmes, D.; et al. Ferrocene-based (photo)redox polymerization under long wavelengths. Polym. Chem. 2019, 10, 1431–1441. [Google Scholar] [CrossRef]
- Brunel, D.; Noirbent, G.; Dumur, F. Ferrocene: An unrivaled electroactive building block for the design of push-pull dyes with near-infrared and infrared absorptions. Dyes Pigment. 2019, 170, 107611. [Google Scholar] [CrossRef]













© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumur, F. Recent Advances on Visible Light Metal-Based Photocatalysts for Polymerization under Low Light Intensity. Catalysts 2019, 9, 736. https://doi.org/10.3390/catal9090736
Dumur F. Recent Advances on Visible Light Metal-Based Photocatalysts for Polymerization under Low Light Intensity. Catalysts. 2019; 9(9):736. https://doi.org/10.3390/catal9090736
Chicago/Turabian StyleDumur, Frédéric. 2019. "Recent Advances on Visible Light Metal-Based Photocatalysts for Polymerization under Low Light Intensity" Catalysts 9, no. 9: 736. https://doi.org/10.3390/catal9090736





