Photocatalytic Decomposition of N2O by Using Nanostructured Graphitic Carbon Nitride/Zinc Oxide Photocatalysts Immobilized on Foam
Abstract
:1. Introduction
2. Results and Discussion
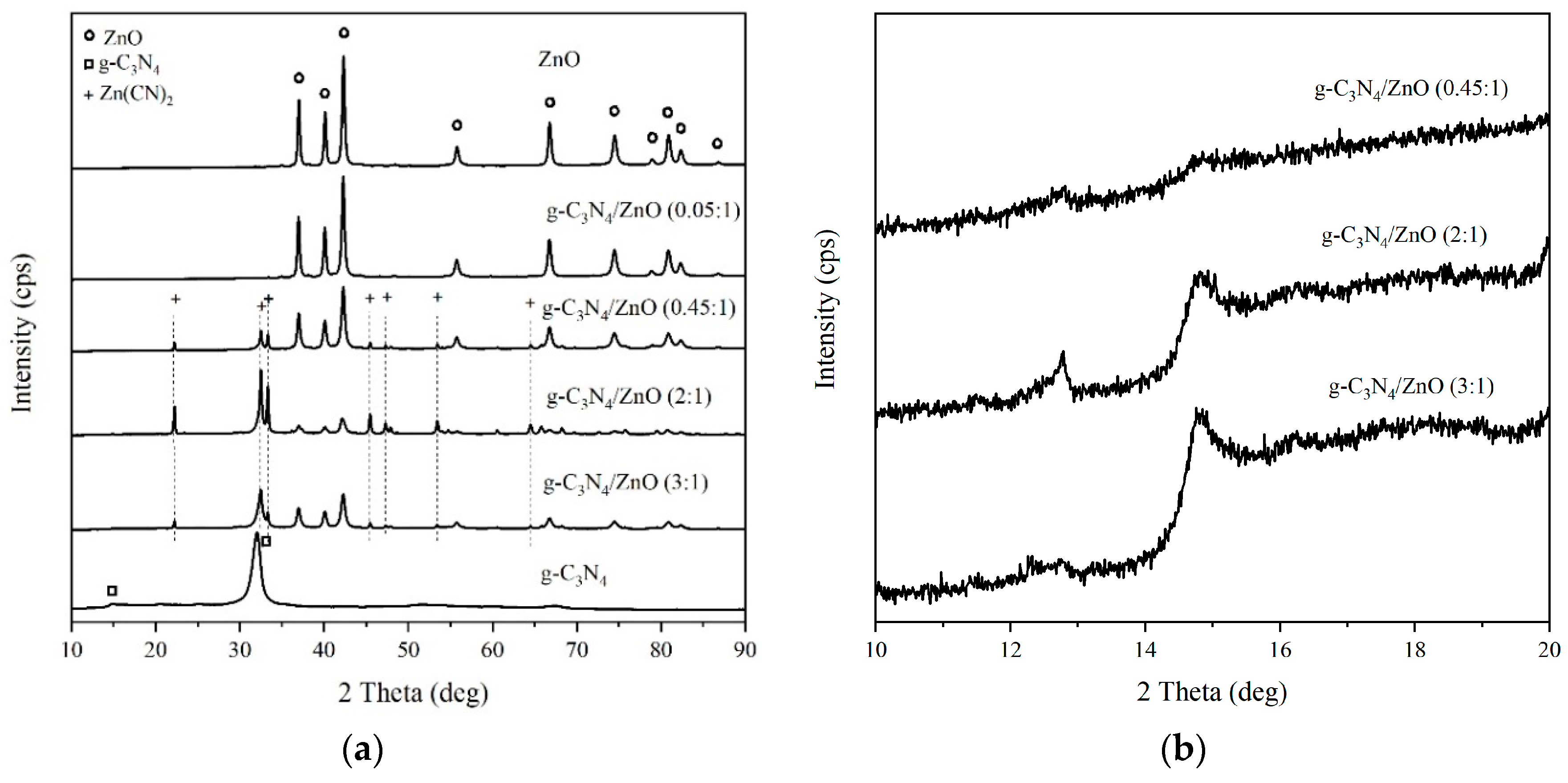
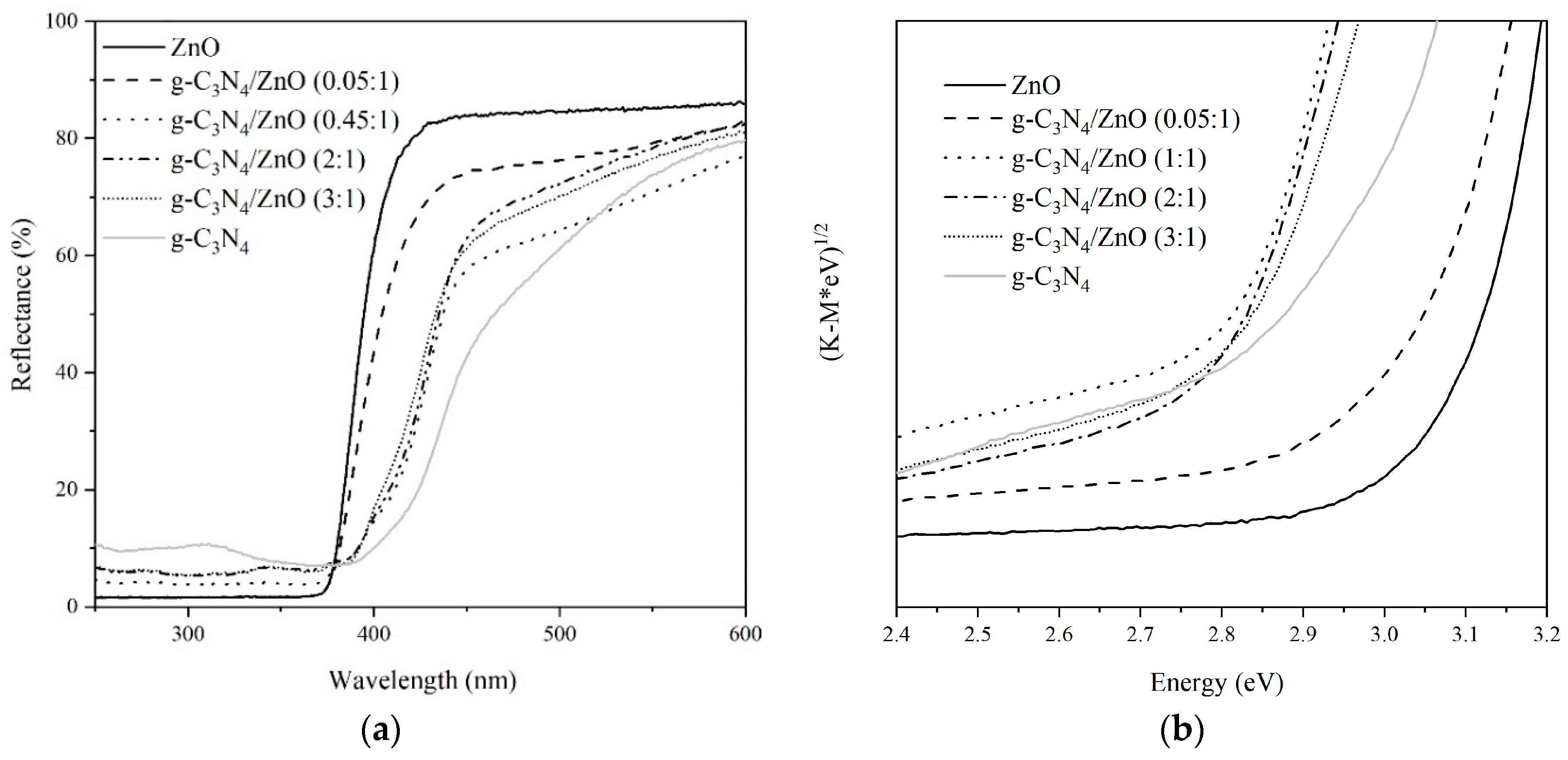
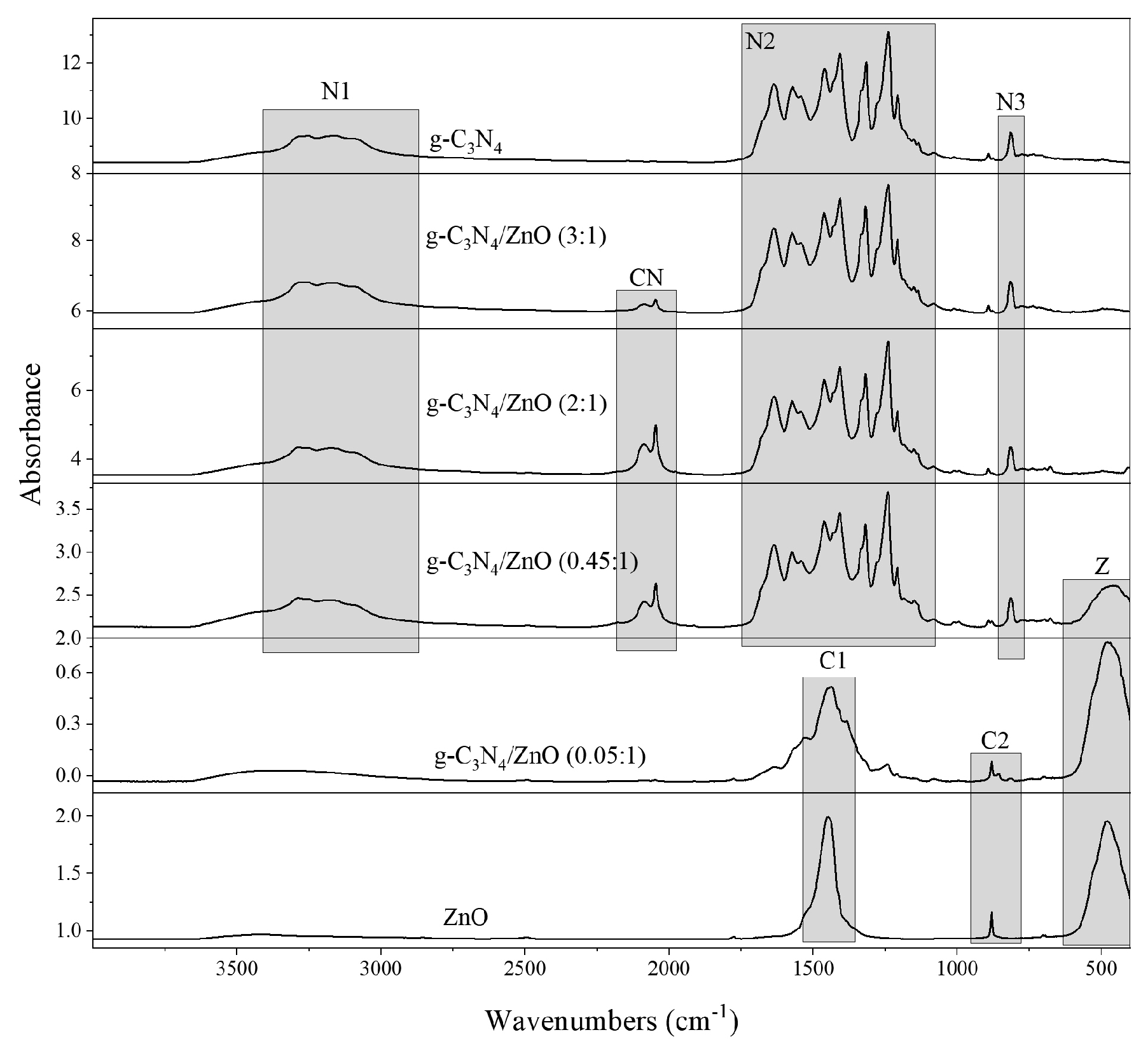
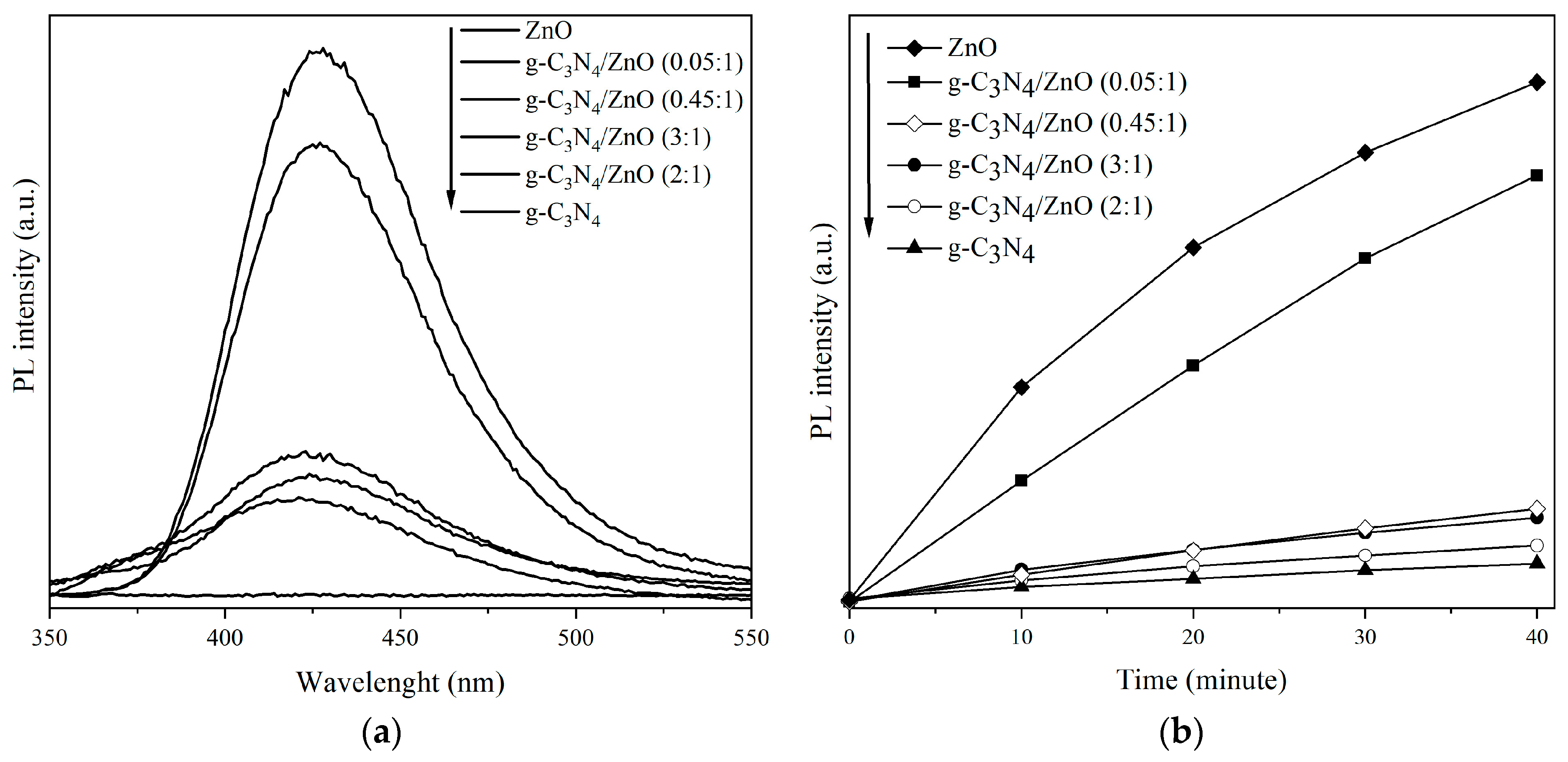
2.1. Photocatalysts Characterization
2.2. Photocatalytic Decomposition of N2O
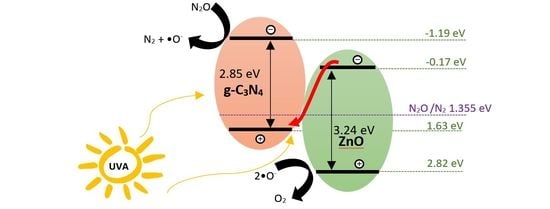
2.3. Proposed Mechanism for Photocatalytic Activity Enhancement
3. Materials and Methods
3.1. Preparation of ZnO, g-C3N4, and g-C3N4/ZnO Nanocomposites
3.2. Characterization of g-C3N4/ZnO Nanocomposite and Photocatalytic Decomposition of N2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ming, T.; de Richter, R.; Shen, S.; Caillol, S. Fighting global warming by greenhouse gas removal: Destroying atmospheric nitrous oxide thanks to synergies between two breakthrough technologies. Environ. Sci. Pollut. Res. Int. 2016, 23, 6119–6138. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Ju, W.S.; Takahashi, K.; Yamashita, H.; Anpo, M. Photocatalytic decomposition of N2O into N2 and O2 at 298 K on Cu(I) ion catalysts anchored onto various oxides. The effect of the coordination state of the Cu(I) ions on the photocatalytic reactivity. J. Phys. Chem. B 2000, 104, 4911–4915. [Google Scholar] [CrossRef]
- Obalová, L.; Šihor, M.; Praus, P.; Reli, M.; Kočí, K. Photocatalytic and photochemical decomposition of N2O on ZnS-MMT catalyst. Catal. Today 2014, 230, 61–66. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Obalová, L.; Čapek, L.; Wu, J.C.S. Preparation, characterization and photocatalytic performance of TiO2 prepared by using pressurized fluids in CO2 reduction and N2O decomposition. J. Sol Gel Sci. Technol. 2015, 76, 621–629. [Google Scholar] [CrossRef]
- Sano, T.; Negishi, N.; Mas, D.; Takeuchi, K. Photocatalytic Decomposition of N2O on Highly Dispersed Ag+ Ions on TiO2 Prepared by Photodeposition. J. Catal. 2000, 194, 71–79. [Google Scholar] [CrossRef]
- Kočí, K.; Krejčíková, S.; Šolcová, O.; Obalová, L. Photocatalytic decomposition of N2O on Ag-TiO2. Catal. Today 2012, 191, 134–137. [Google Scholar] [CrossRef]
- Obalová, L.; Reli, M.; Lang, J.; Matějka, V.; Kukutschová, J.; Lacný, Z.; Kočí, K. Photocatalytic decomposition of nitrous oxide using TiO2 and Ag-TiO2 nanocomposite thin films. Catal. Today 2013, 209, 170–175. [Google Scholar] [CrossRef]
- Matějová, L.; Šihor, M.; Brunátová, T.; Ambrožová, N.; Reli, M.; Čapek, L.; Obalová, L.; Kočí, K. Microstructure-performance study of cerium-doped TiO2 prepared by using pressurized fluids in photocatalytic mitigation of N2O. Res. Chem. Intermed. 2015, 41, 9217–9231. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Ambrožová, N.; Šihor, M.; Troppová, I.; Čapek, L.; Kotarba, A.; Kustrowski, P.; Hospodková, A.; Obalová, L. Optimization of cerium doping of TiO2 for photocatalytic reduction of CO2 and photocatalytic decomposition of N2O. J. Sol Gel Sci. Technol. 2016, 78, 550–558. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Bahadur, I. Graphitic carbon nitride based ternary nanocomposites: From synthesis to their applications in photocatalysis: A recent review. J. Mol. Liq. 2019, 281, 634–654. [Google Scholar] [CrossRef]
- Yin, S.; Han, J.; Zhou, T.; Xu, R. Recent progress in g-C3N4 based low cost photocatalytic system: Activity enhancement and emerging applications. Catal. Sci. Technol. 2015, 5, 5048–5061. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef] [PubMed]
- Reli, M.; Huo, P.; Sihor, M.; Ambrozova, N.; Troppova, I.; Matejova, L.; Lang, J.; Svoboda, L.; Kustrowski, P.; Ritz, M.; et al. Novel TiO2/C3N4 Photocatalysts for Photocatalytic Reduction of CO2 and for Photocatalytic Decomposition of N2O. J. Phys. Chem. A 2016, 120, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupková, J.; Kustrowski, P.; Praus, P. Photocatalytic decomposition of N2O over TiO2/g-C3N4 photocatalysts heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Reli, M.; Svoboda, L.; Šihor, M.; Troppová, I.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/WO3 photocatalysts. Environ. Sci. Pollut. Res. Int. 2018, 25, 34839–34850. [Google Scholar] [CrossRef]
- Alenzi, N.; Liao, W.-S.; Cremer, P.S.; Sanchez-Torres, V.; Wood, T.K.; Ehlig-Economides, C.; Cheng, Z. Photoelectrochemical hydrogen production from water/methanol decomposition using Ag/TiO2 nanocomposite thin films. Int. J. Hydrogen Energy 2010, 35, 11768–11775. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Komatsu, T. The First Synthesis and Characterization of Cyameluric High Polymers. Macromol. Chem. Phys. 2001, 202, 19–25. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Horák, M.; Papoušek, D. Infrared Spectra and Structure of Molecules; Academia: Prague, Czech Republic, 1976. [Google Scholar]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.-C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]
- Kuang, P.-Y.; Su, Y.-Z.; Chen, G.-F.; Luo, Z.; Xing, S.-Y.; Li, N.; Liu, Z.-Q. g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci. 2015, 358, 296–303. [Google Scholar] [CrossRef]
- Troppová, I.; Šihor, M.; Reli, M.; Ritz, M.; Praus, P.; Kočí, K. Unconventionally prepared TiO2/g-C3N4 photocatalysts for photocatalytic decomposition of nitrous oxide. Appl. Surf. Sci. 2018, 430, 335–347. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, Q.; Yang, J. Enhanced visible light activity on direct contact Z-scheme g-C3N4-TiO2 photocatalyst. Appl. Surf. Sci. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Yang, Z.; Du, H.; Jiang, Y.; Shen, C.; Liang, K.; Xu, A. Enhanced visible-light photocatalytic activity of Z-scheme graphitic carbon nitride/oxygen vacancy-rich zinc oxide hybrid photocatalysts. Chin. J. Catal. 2015, 36, 2135–2144. [Google Scholar] [CrossRef]
- Berks, B.C.; Ferguson, S.J.; Moir, J.W.; Richardson, D.J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1995, 1232, 97–173. [Google Scholar] [CrossRef] [Green Version]
- Troppová, I.; Matějová, L.; Sezimová, H.; Matěj, Z.; Peikertová, P.; Lang, J. Nanostructured TiO2 and ZnO prepared by using pressurized hot water and their eco-toxicological evaluation. J. Nanopart. Res. 2017, 19, 198. [Google Scholar] [CrossRef]
- Koci, K.; Reli, M.; Svoboda, L.; Praus, P. In Exfoliated nanosheets of graphitic carbon nitride: Study of optical and photoelectrochemical properties. In Proceedings of the 8th International Conference on Nanomaterials—Research & Application (NANOCON 2016), Brno, Czech Republic, 19–21 October 2016; Tanger Ltd.: Brno, Czech Republic, 2017; pp. 98–103. [Google Scholar]
- Tasbihi, M.; Kočí, K.; Edelmannová, M.; Troppová, I.; Reli, M.; Schomäcker, R. Pt/TiO2 photocatalysts deposited on commercial support for photocatalytic reduction of CO2. J. Photochem. Photobiol. A 2018, 366, 72–80. [Google Scholar] [CrossRef]
- Reli, M.; Troppová, I.; Šihor, M.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/BiVO4 composite. Appl. Surf. Sci. 2019, 469, 181–191. [Google Scholar] [CrossRef]

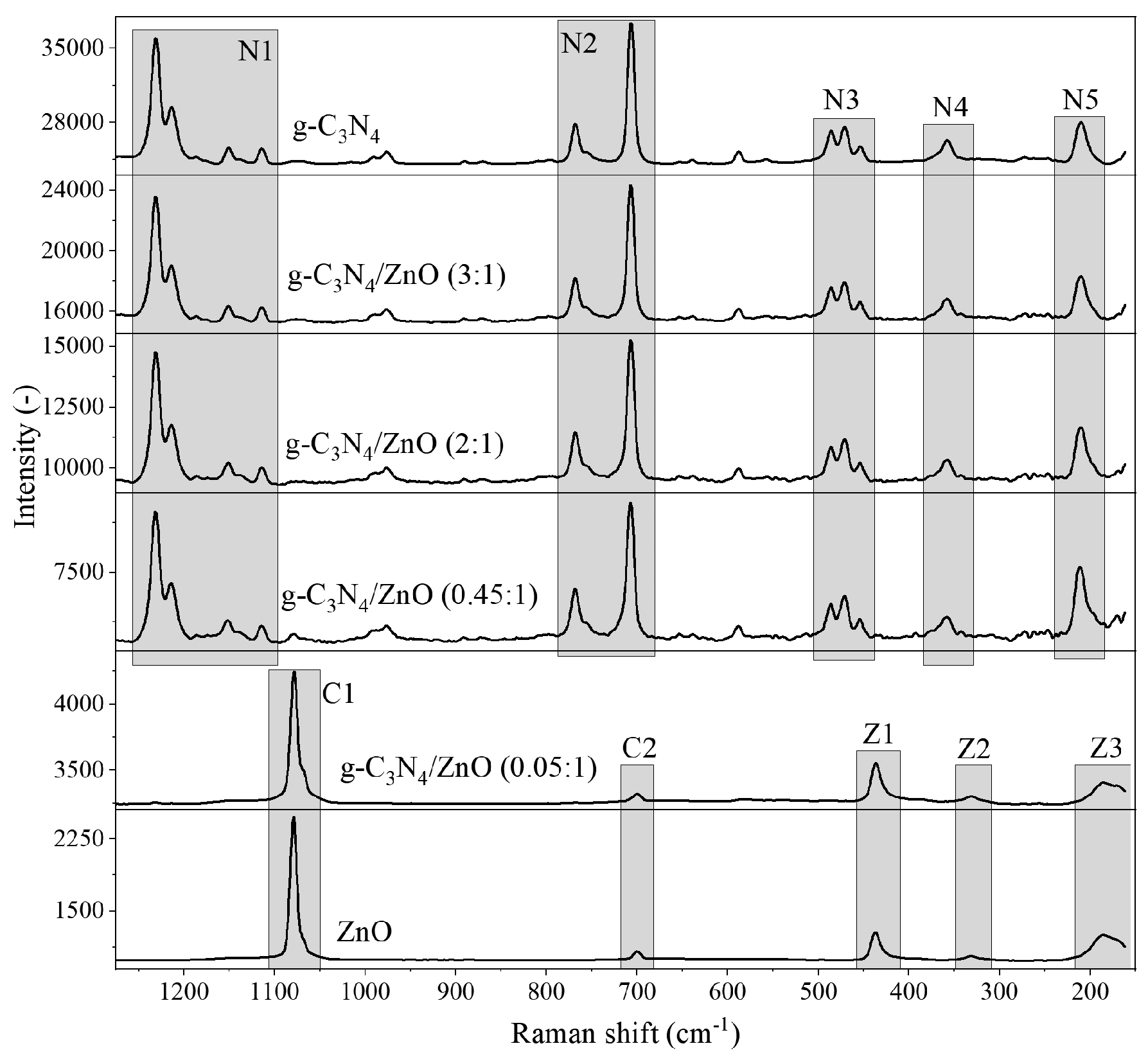
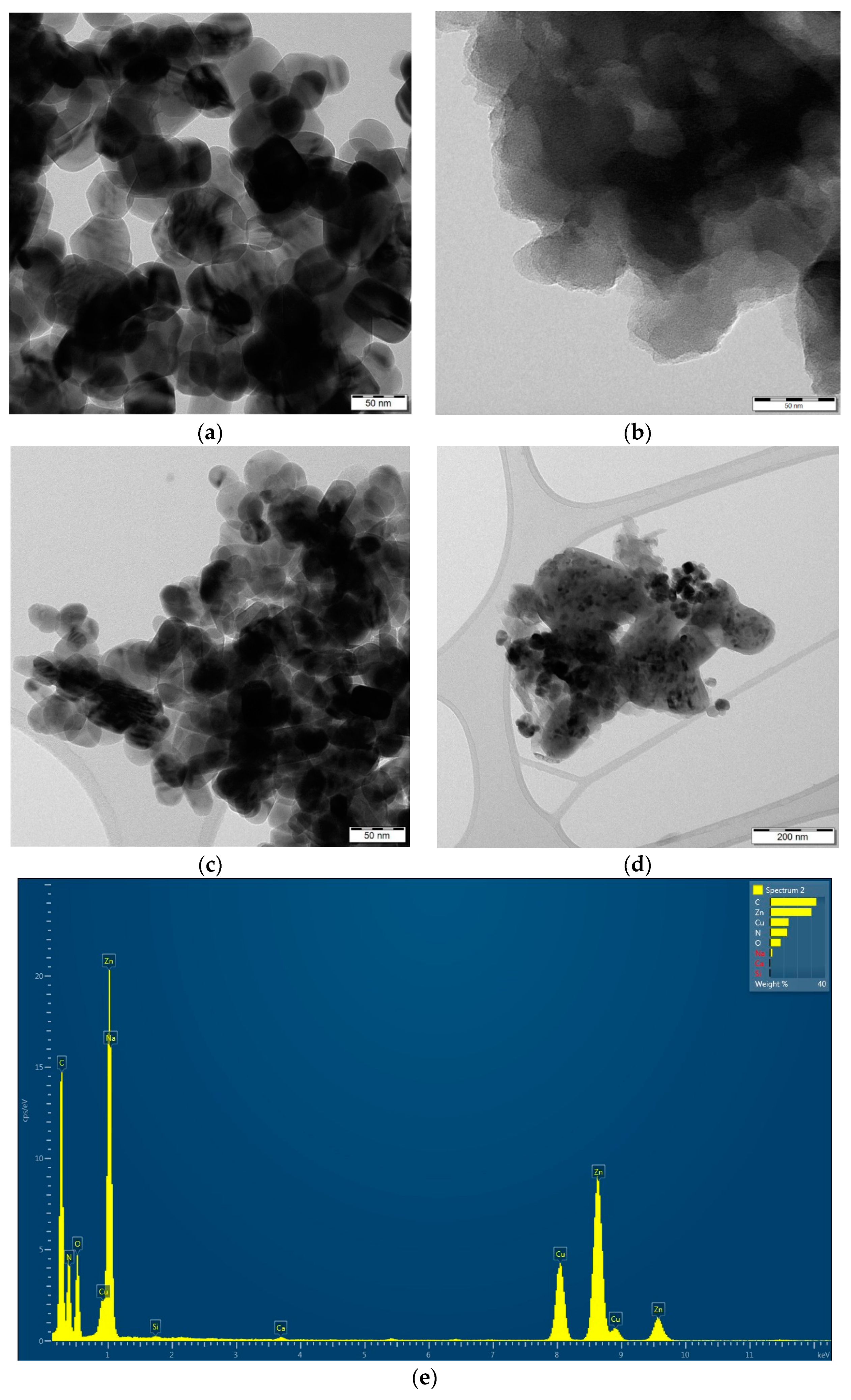
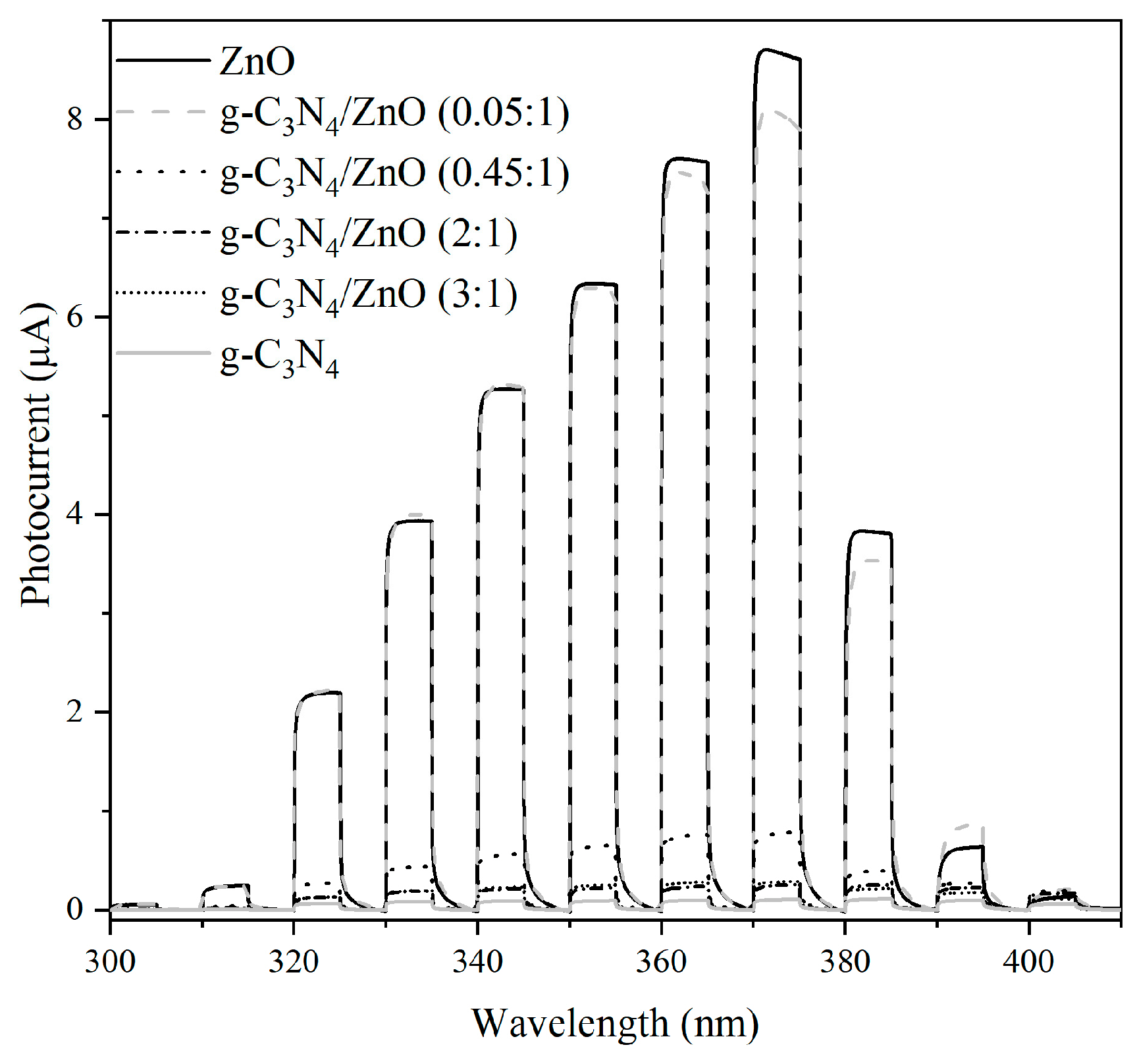
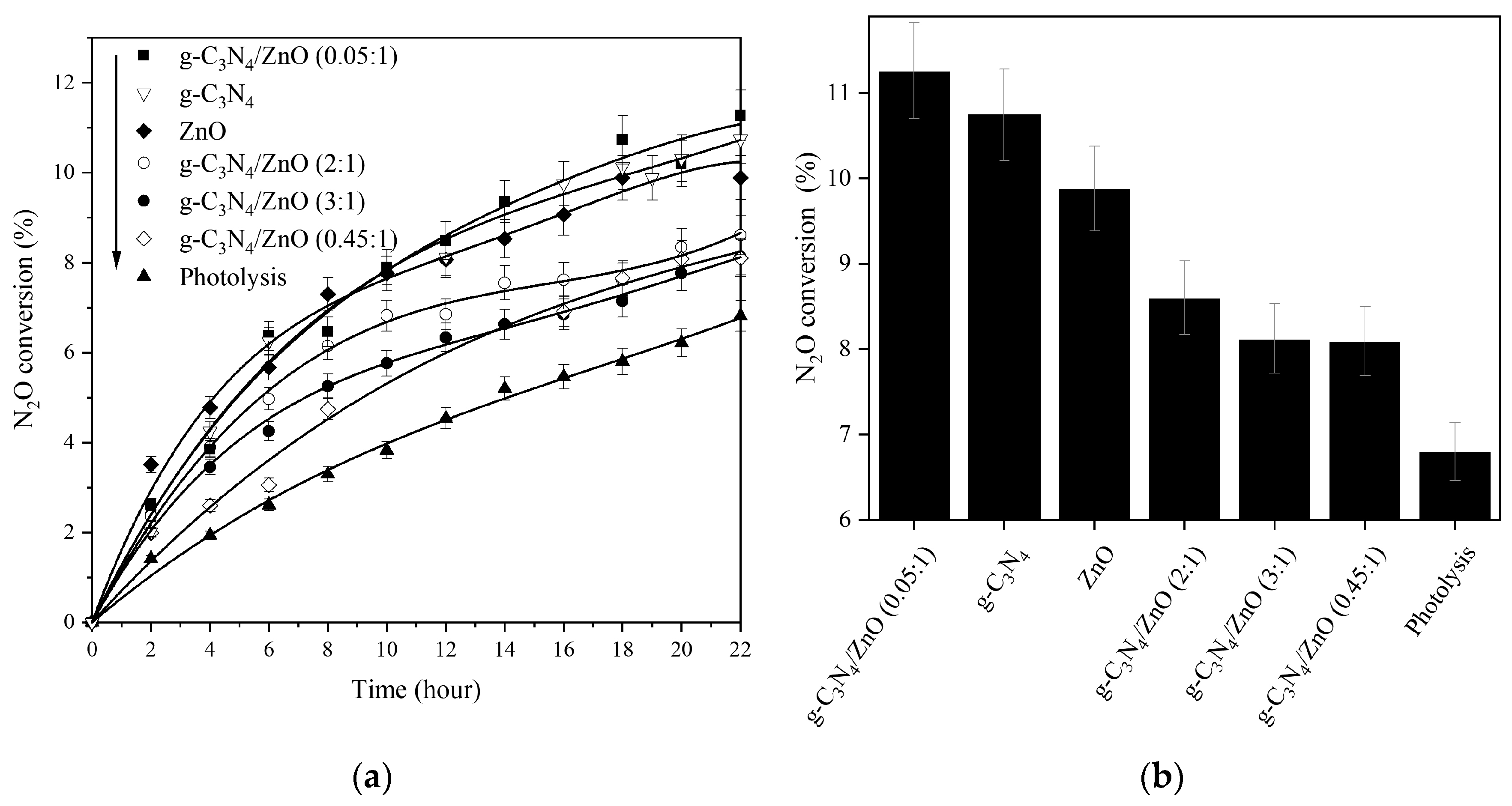
| Photocatalysts | SBET (m2/g) | V net (mm3lig/g) | Content of Zn2+ (wt.%) |
|---|---|---|---|
| ZnO | 7 | 61 | 80 |
| g-C3N4/ZnO (0.05:1) | 8 | 62 | 77 |
| g-C3N4/ZnO (0.45:1) | 37 | 212 | 55 |
| g-C3N4/ZnO (2:1) | 58 | 298 | 26 |
| g-C3N4/ZnO (3:1) | 68 | 339 | 21 |
| g-C3N4 | 85 | 413 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Bajcarová, T.; Ritz, M.; Pavlovský, J.; Praus, P. Photocatalytic Decomposition of N2O by Using Nanostructured Graphitic Carbon Nitride/Zinc Oxide Photocatalysts Immobilized on Foam. Catalysts 2019, 9, 735. https://doi.org/10.3390/catal9090735
Kočí K, Reli M, Troppová I, Šihor M, Bajcarová T, Ritz M, Pavlovský J, Praus P. Photocatalytic Decomposition of N2O by Using Nanostructured Graphitic Carbon Nitride/Zinc Oxide Photocatalysts Immobilized on Foam. Catalysts. 2019; 9(9):735. https://doi.org/10.3390/catal9090735
Chicago/Turabian StyleKočí, Kamila, Martin Reli, Ivana Troppová, Marcel Šihor, Tereza Bajcarová, Michal Ritz, Jiří Pavlovský, and Petr Praus. 2019. "Photocatalytic Decomposition of N2O by Using Nanostructured Graphitic Carbon Nitride/Zinc Oxide Photocatalysts Immobilized on Foam" Catalysts 9, no. 9: 735. https://doi.org/10.3390/catal9090735
APA StyleKočí, K., Reli, M., Troppová, I., Šihor, M., Bajcarová, T., Ritz, M., Pavlovský, J., & Praus, P. (2019). Photocatalytic Decomposition of N2O by Using Nanostructured Graphitic Carbon Nitride/Zinc Oxide Photocatalysts Immobilized on Foam. Catalysts, 9(9), 735. https://doi.org/10.3390/catal9090735