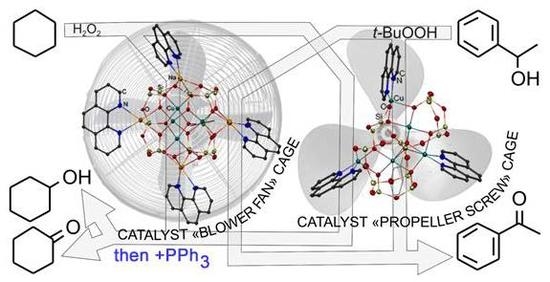
New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile
Abstract
:1. Introduction
2. Experimental
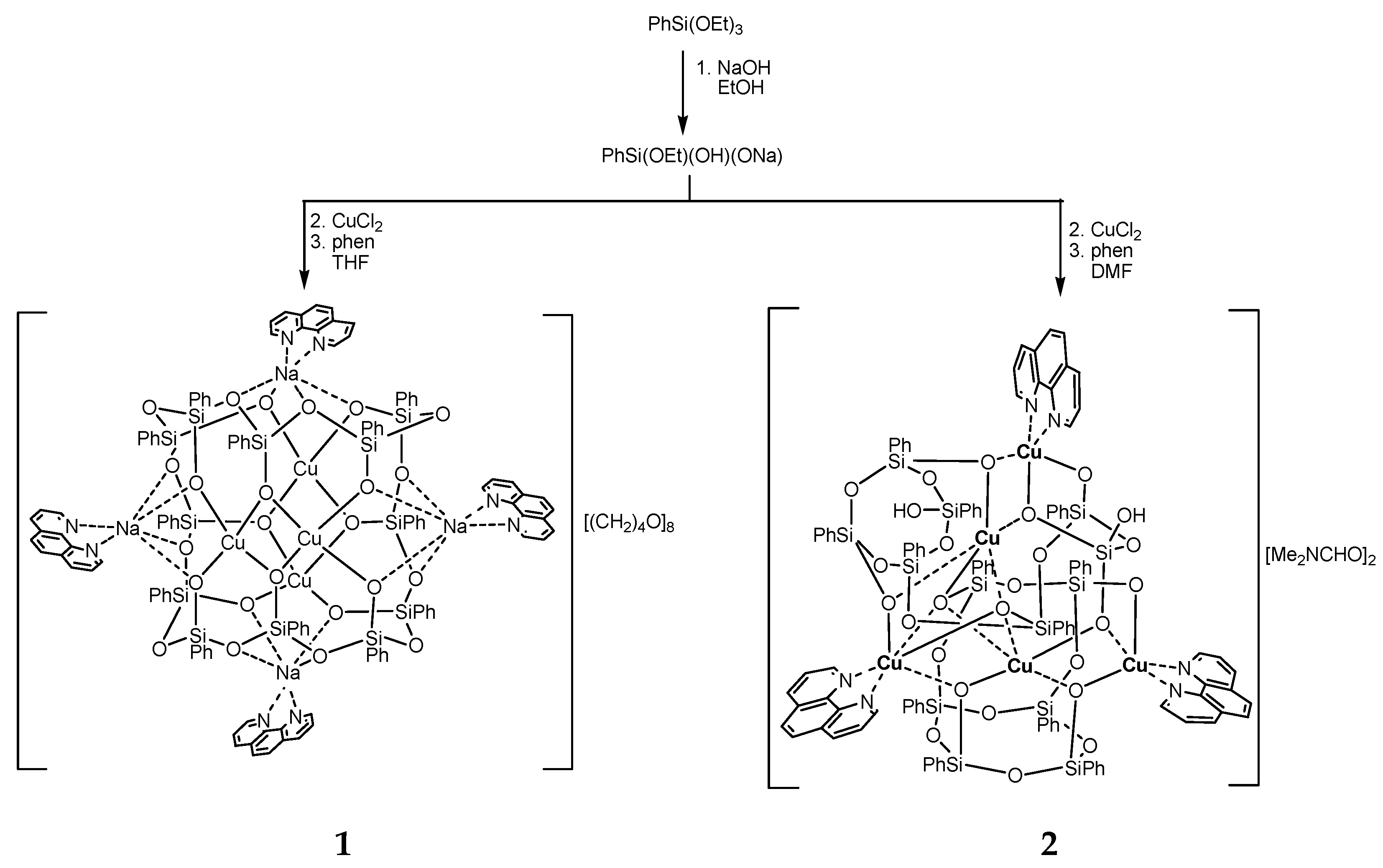
2.1. Synthesis
2.2. Oxidation of Alkanes and Alcohols
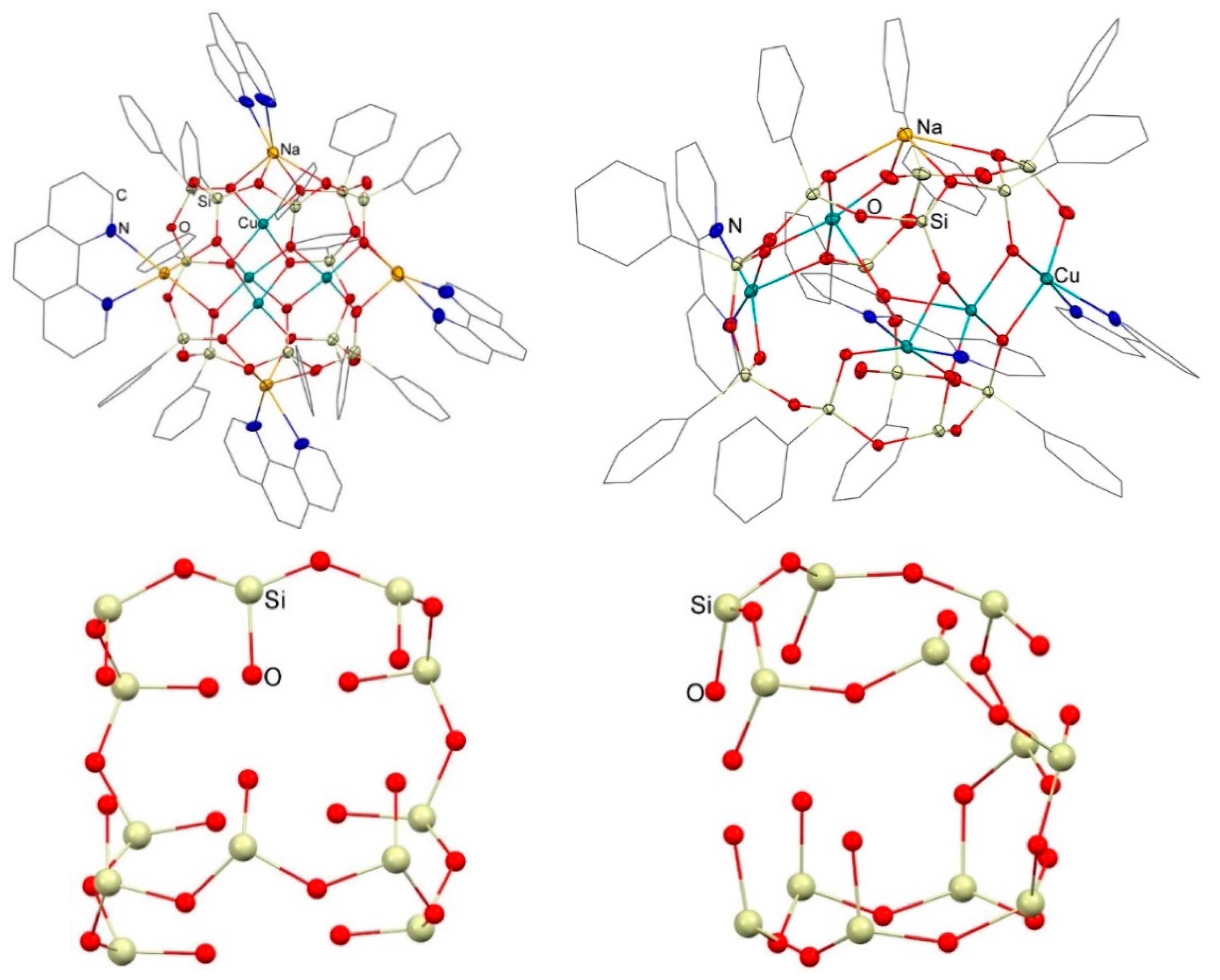
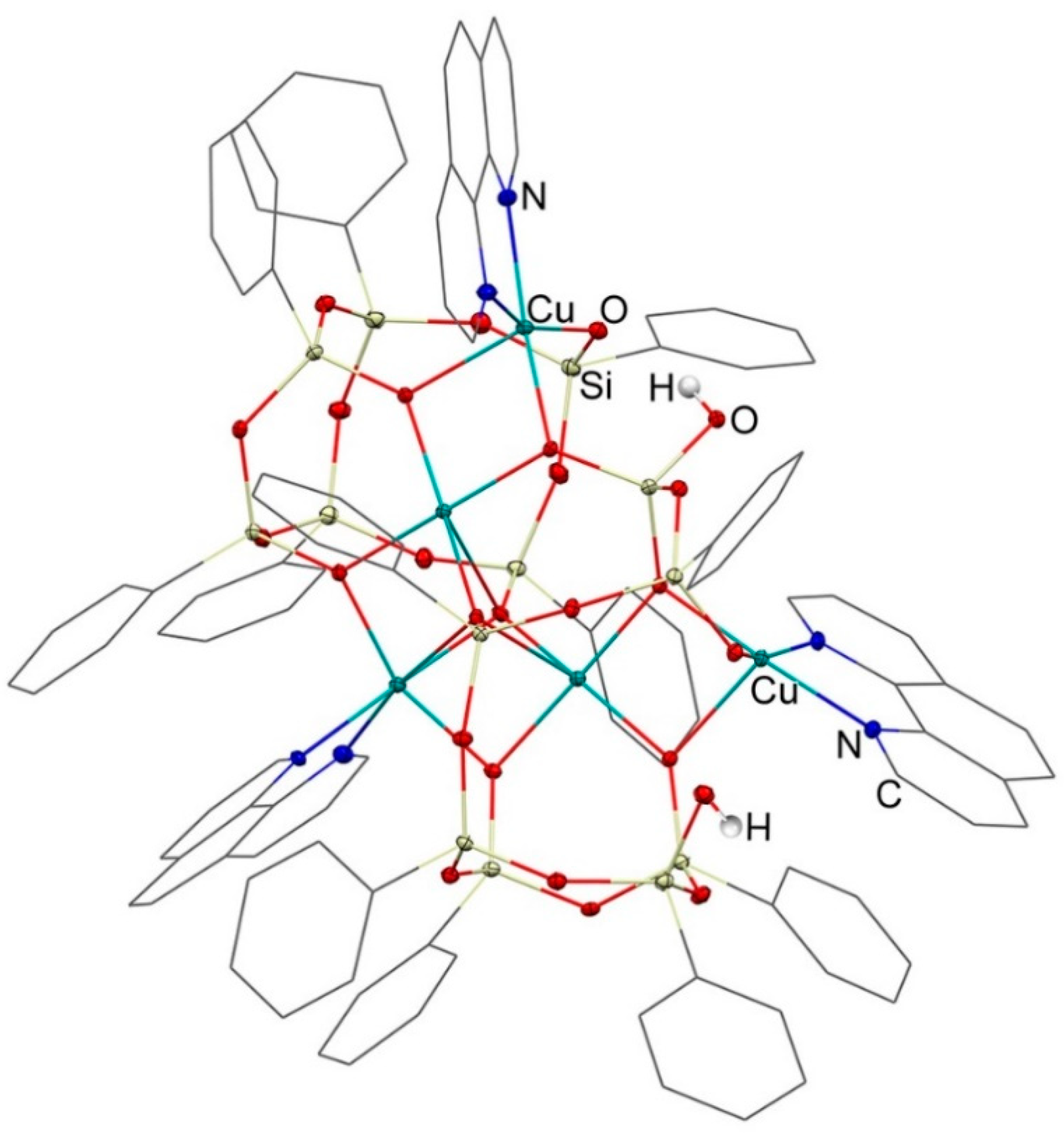
3. Results and Discussion
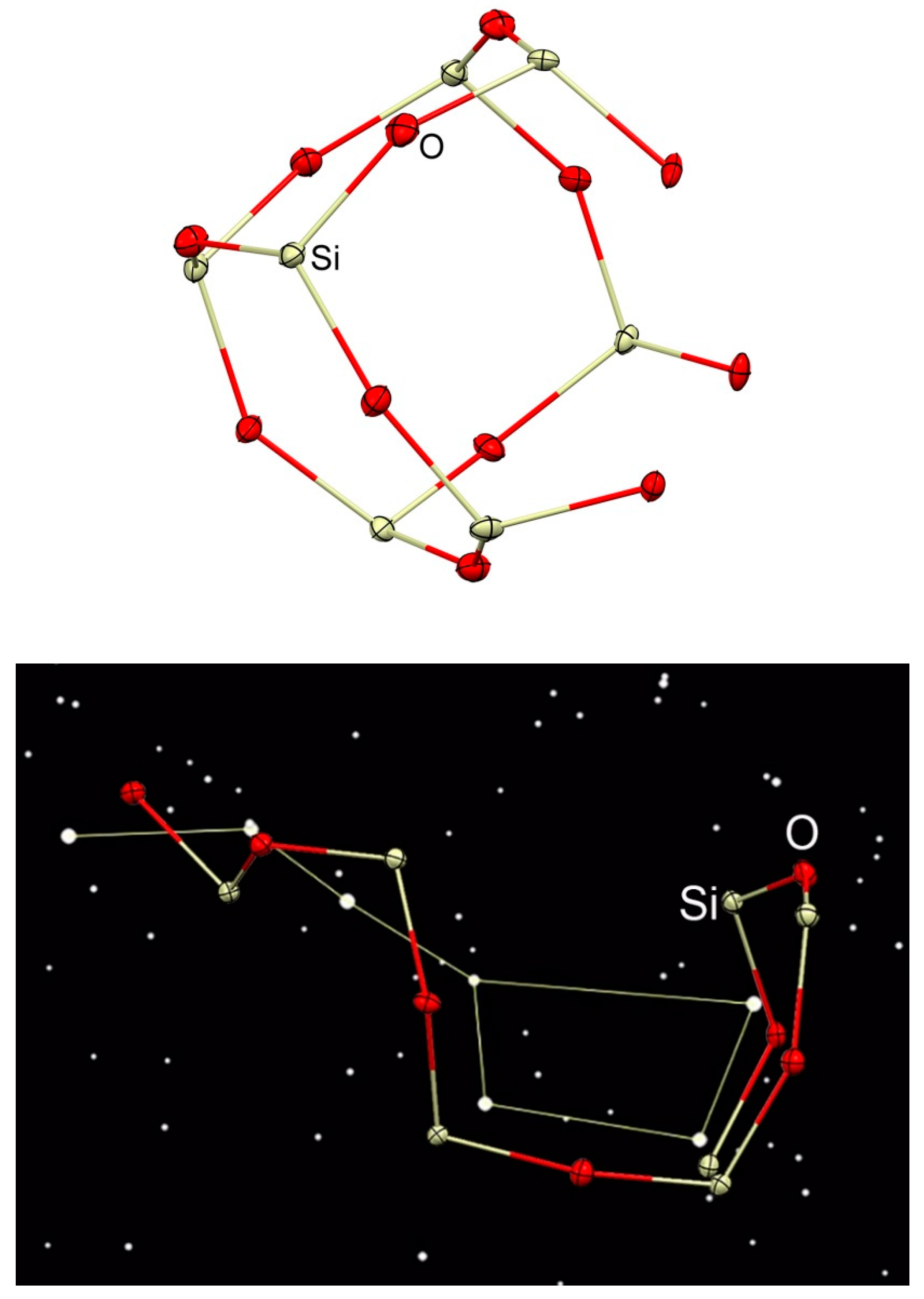
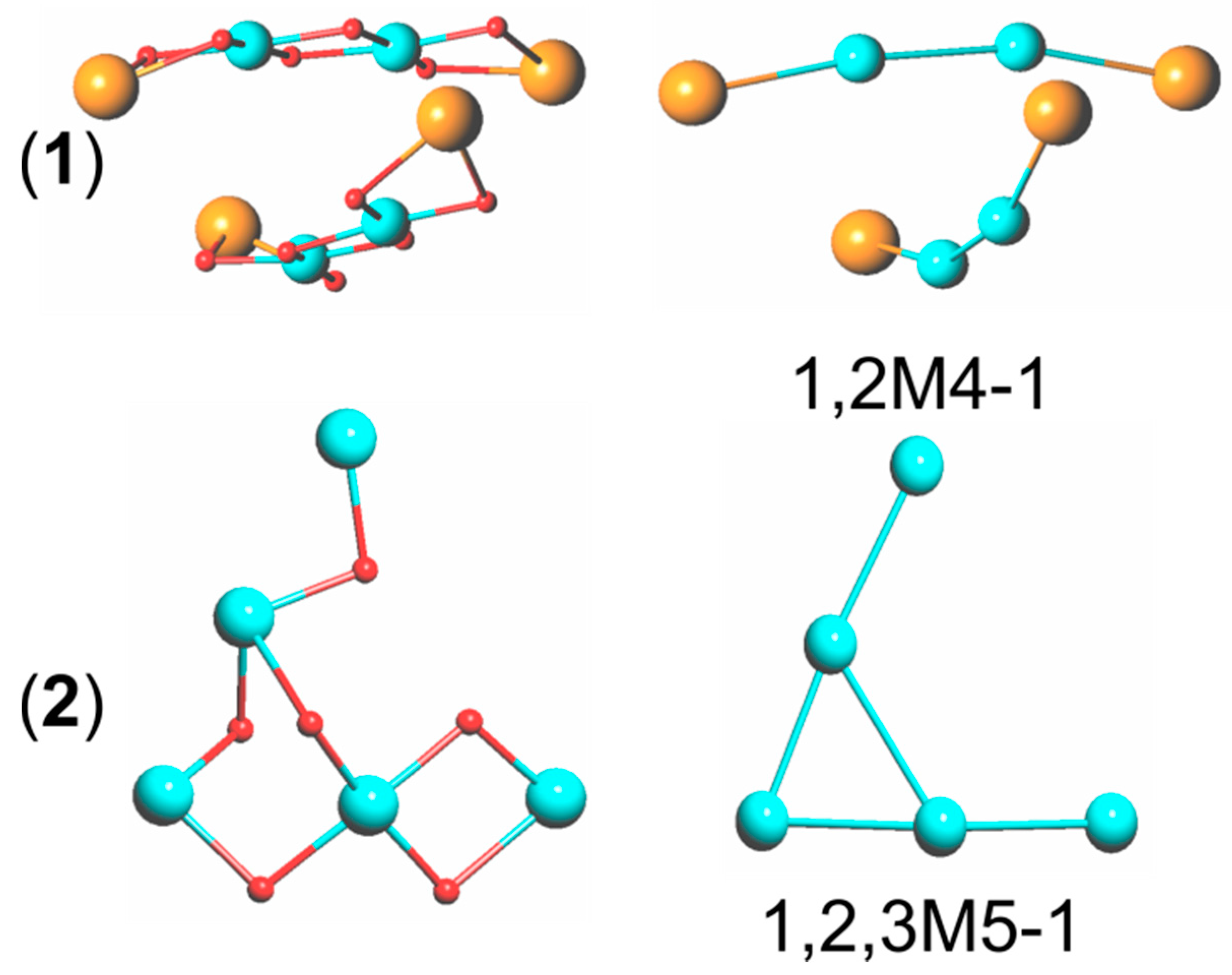
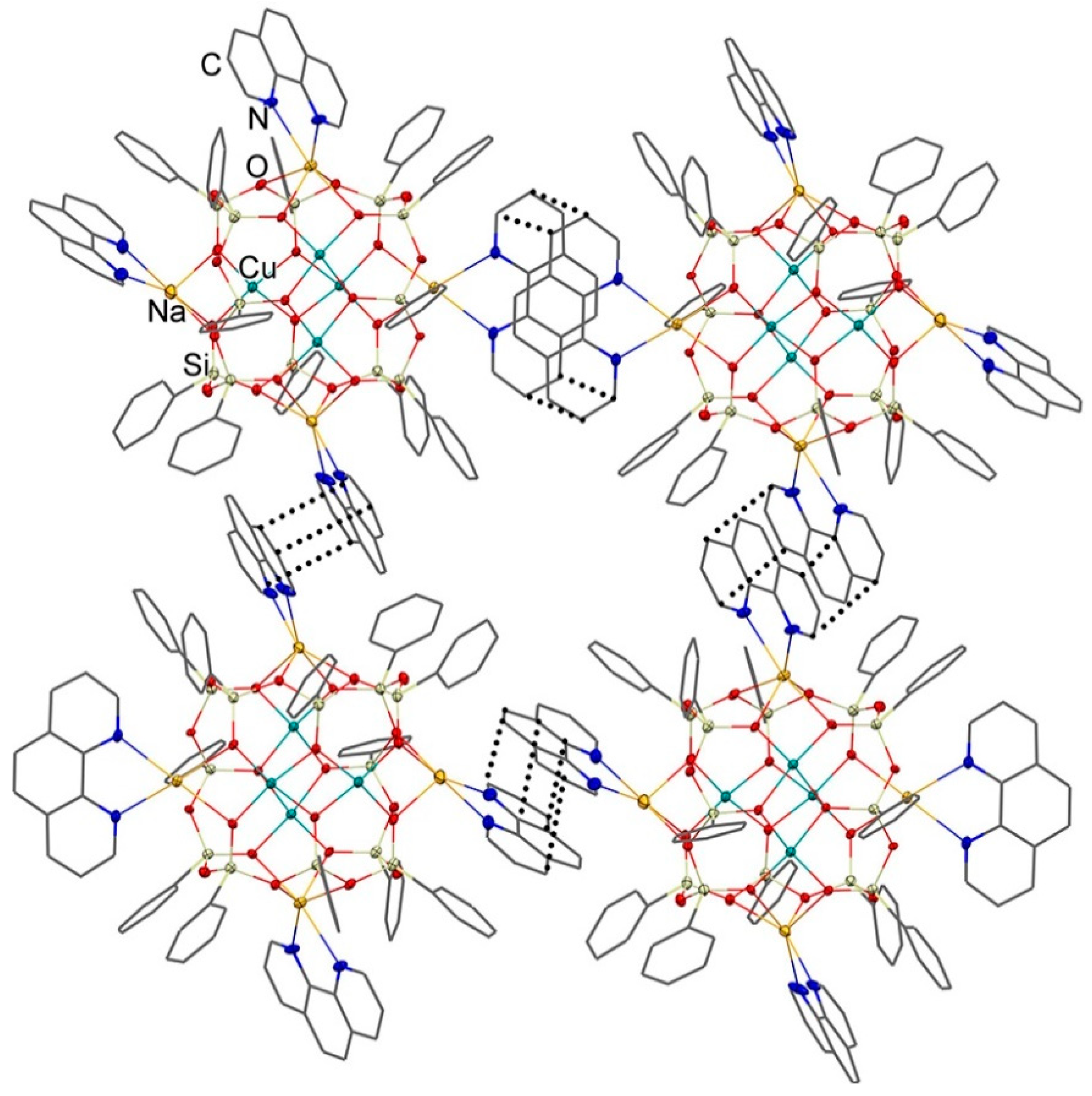
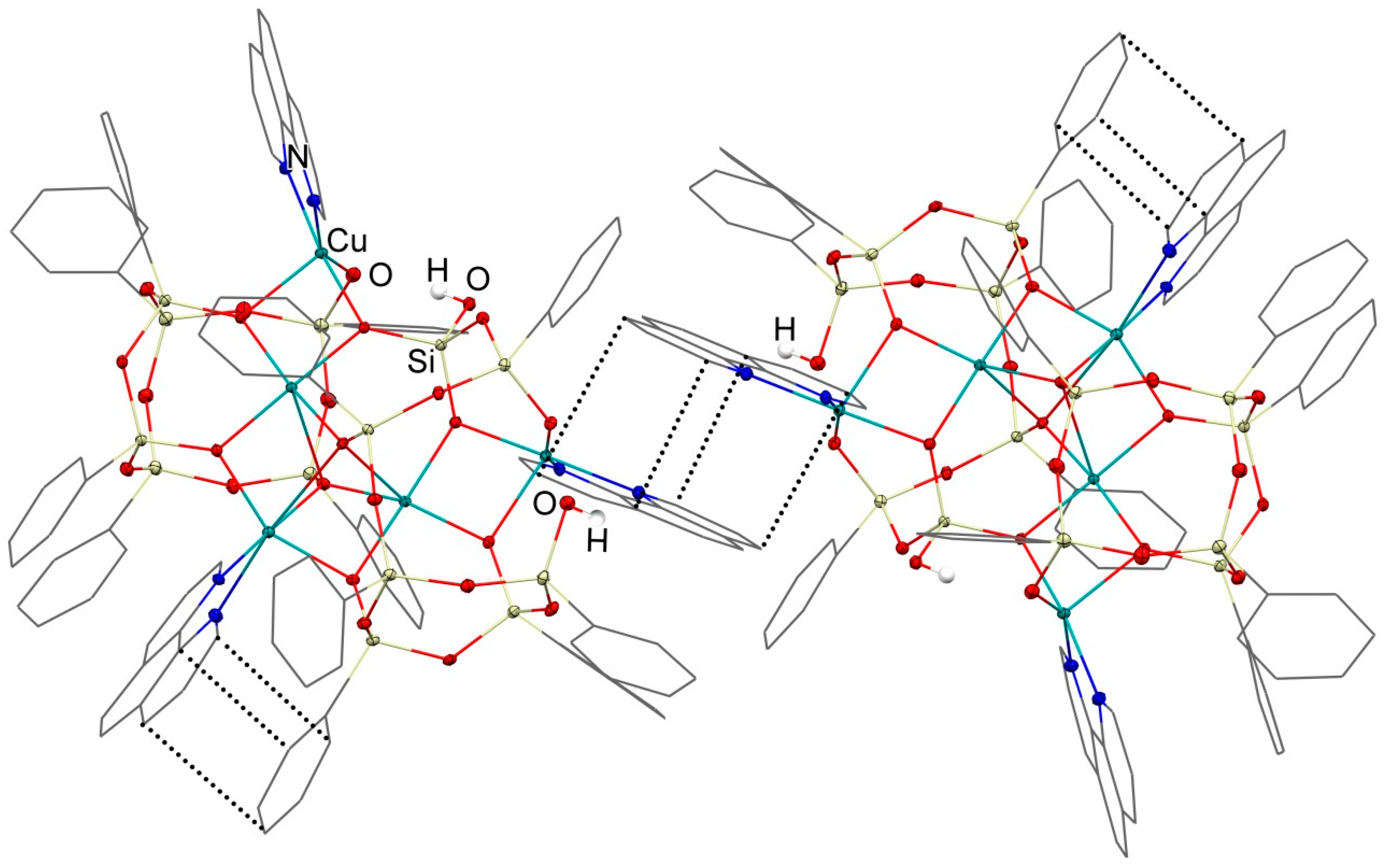
3.1. Description of Compounds 1 and 2
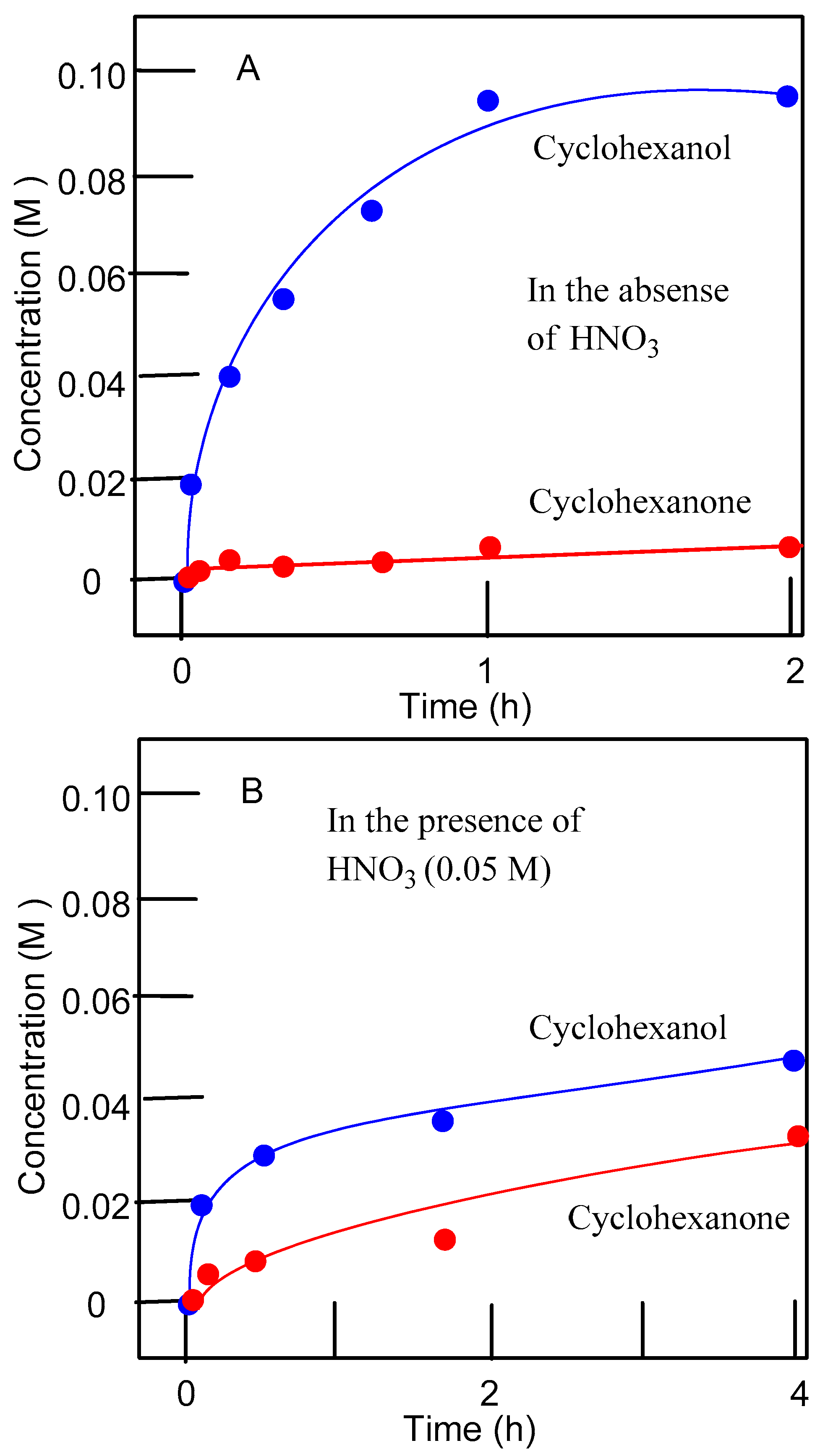
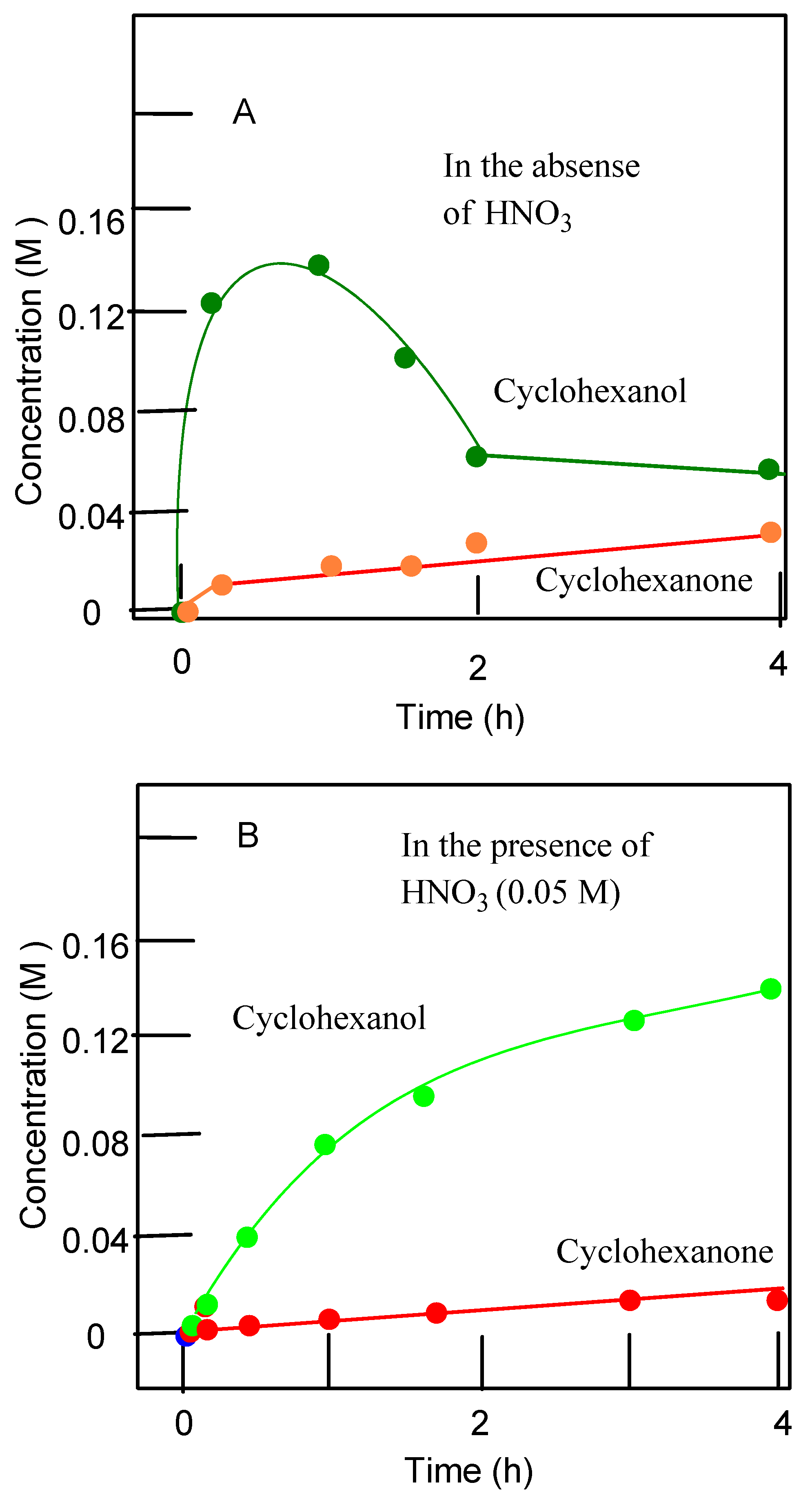
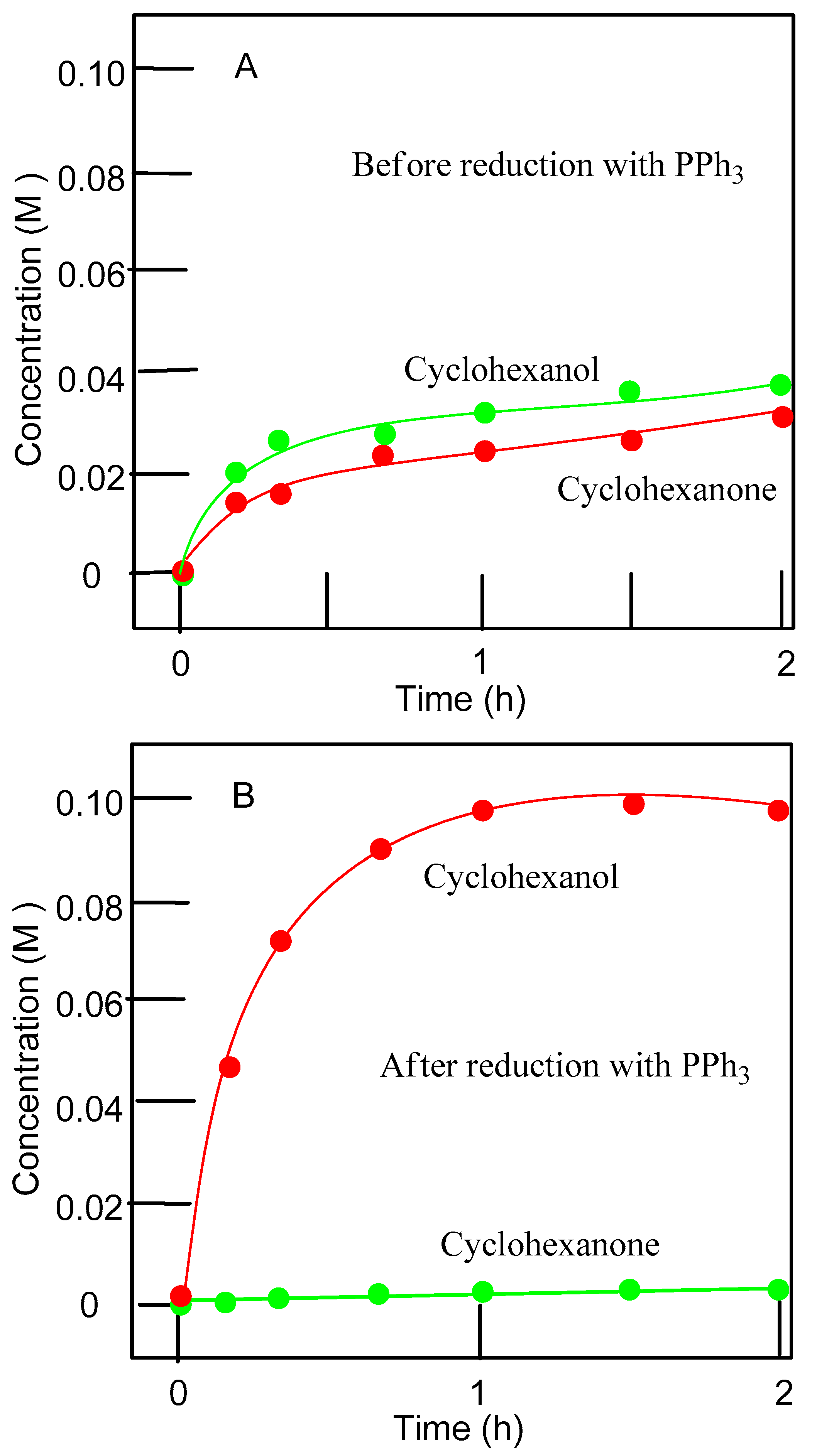
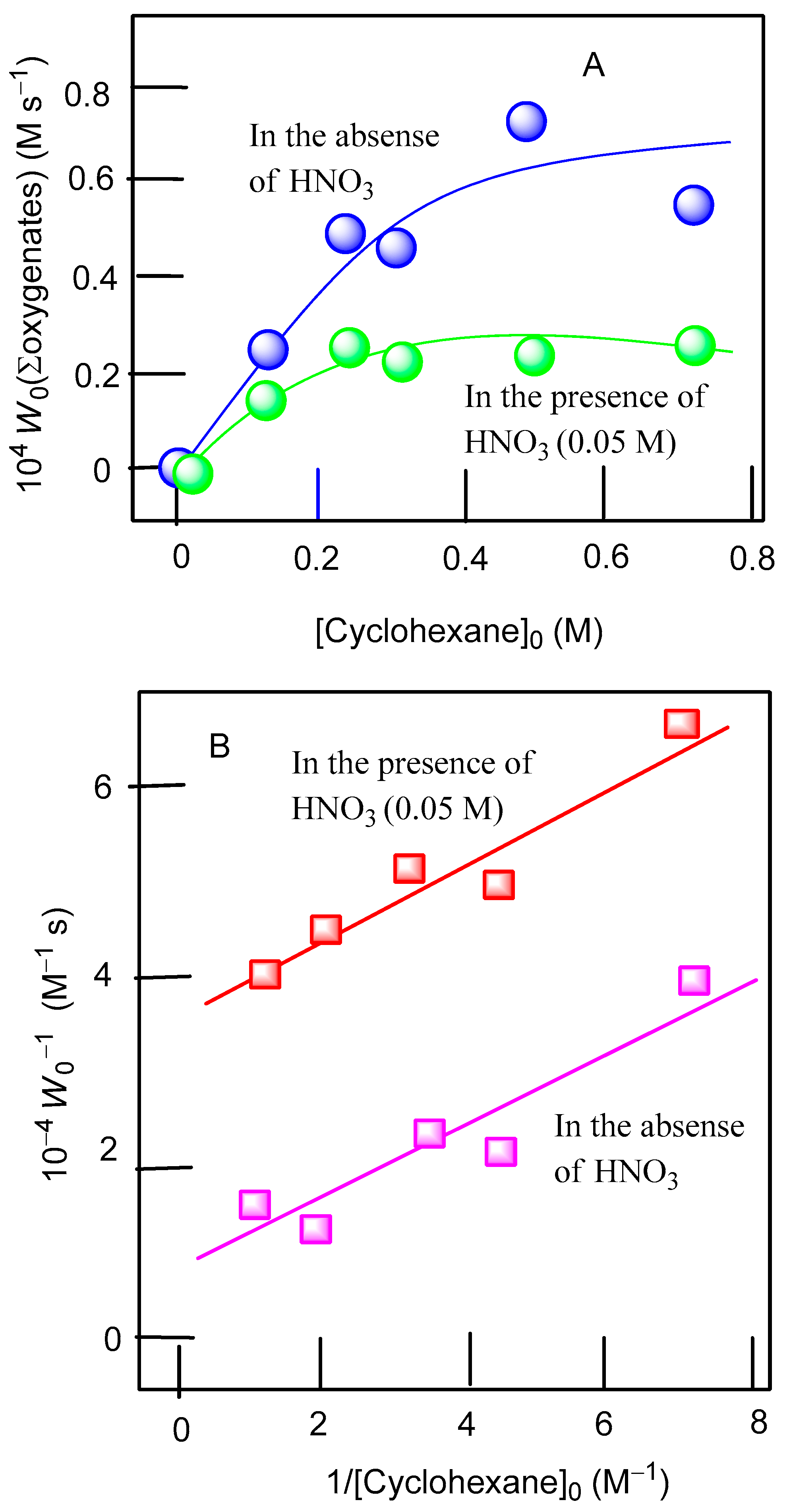
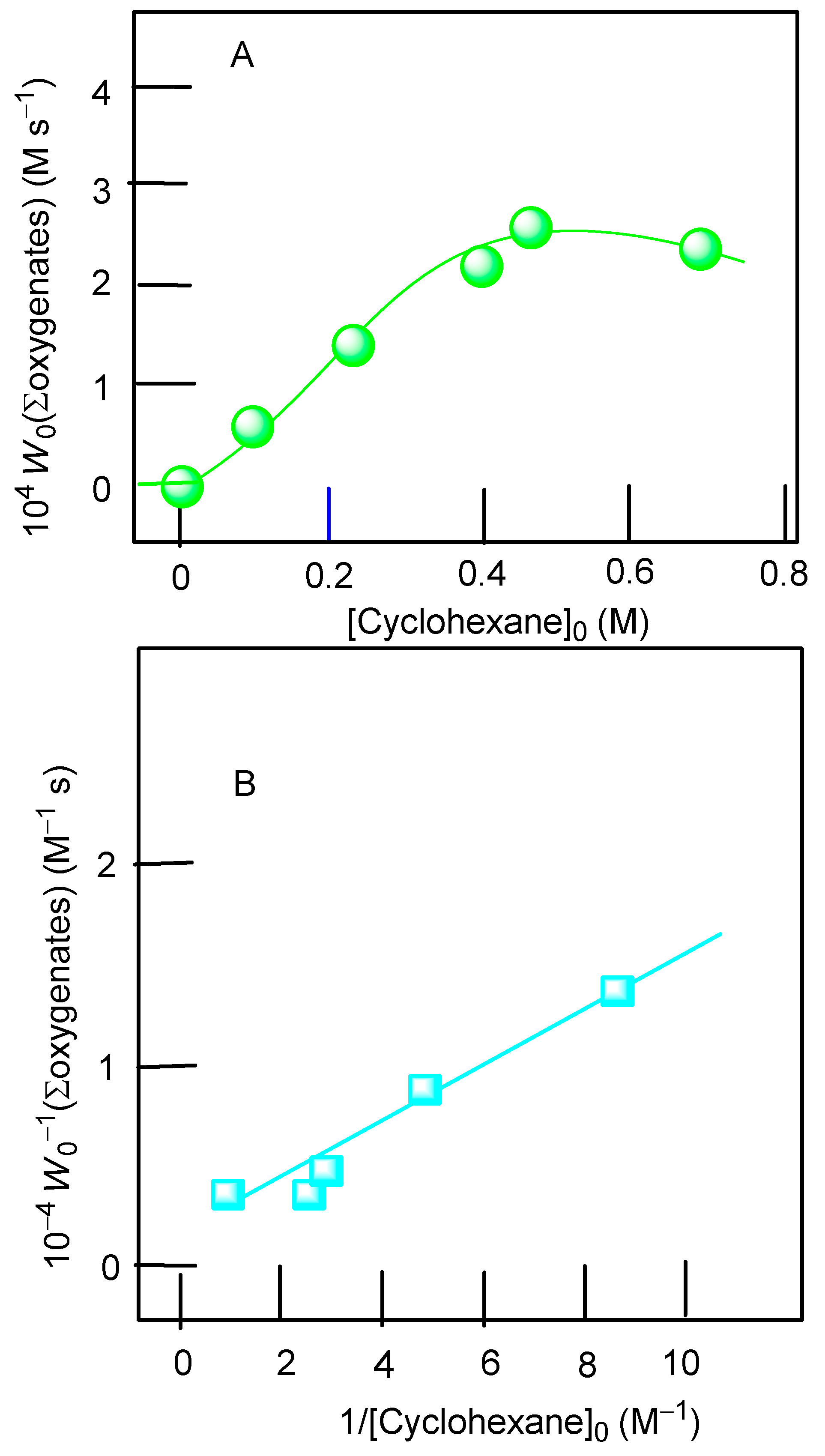
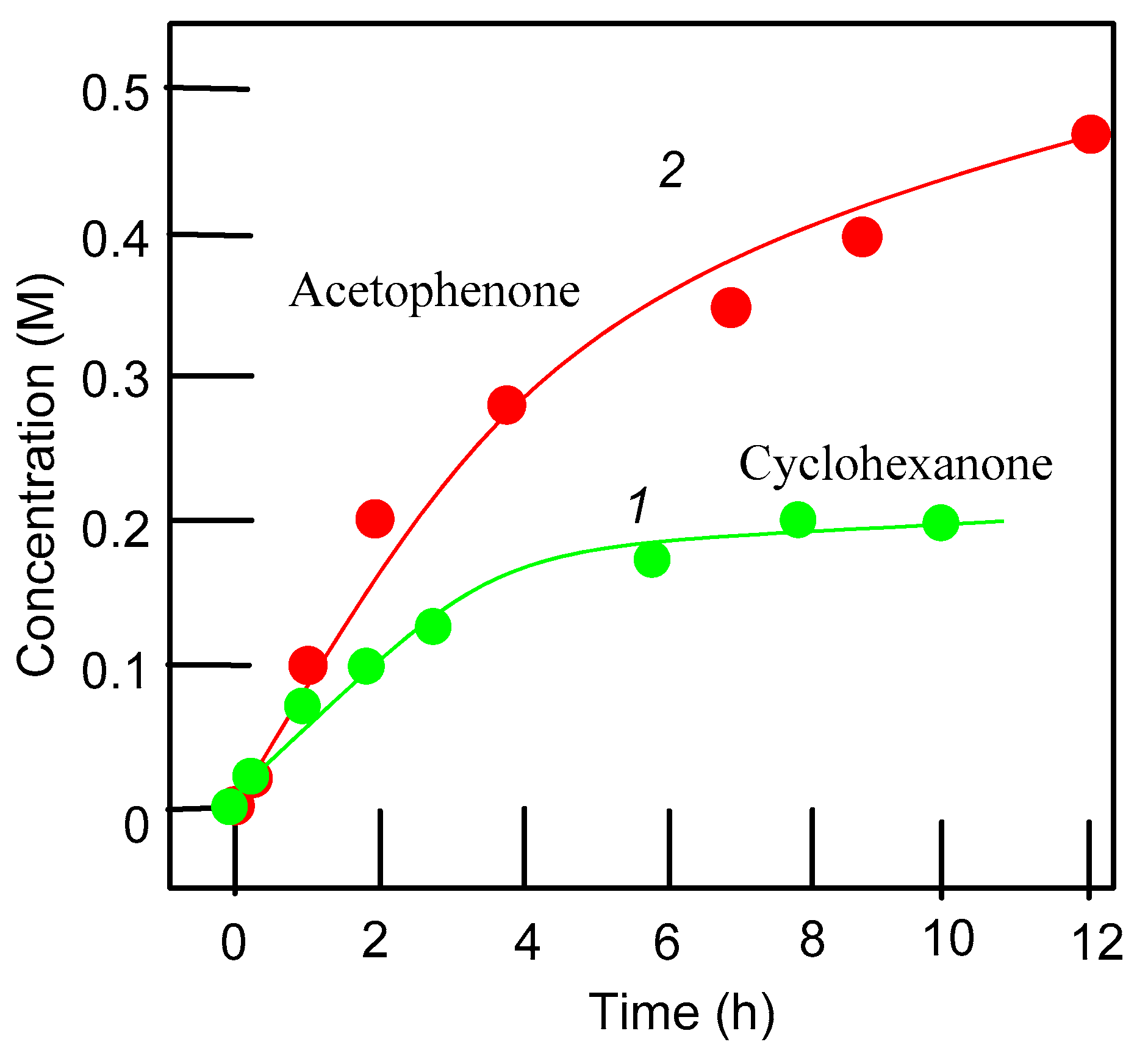
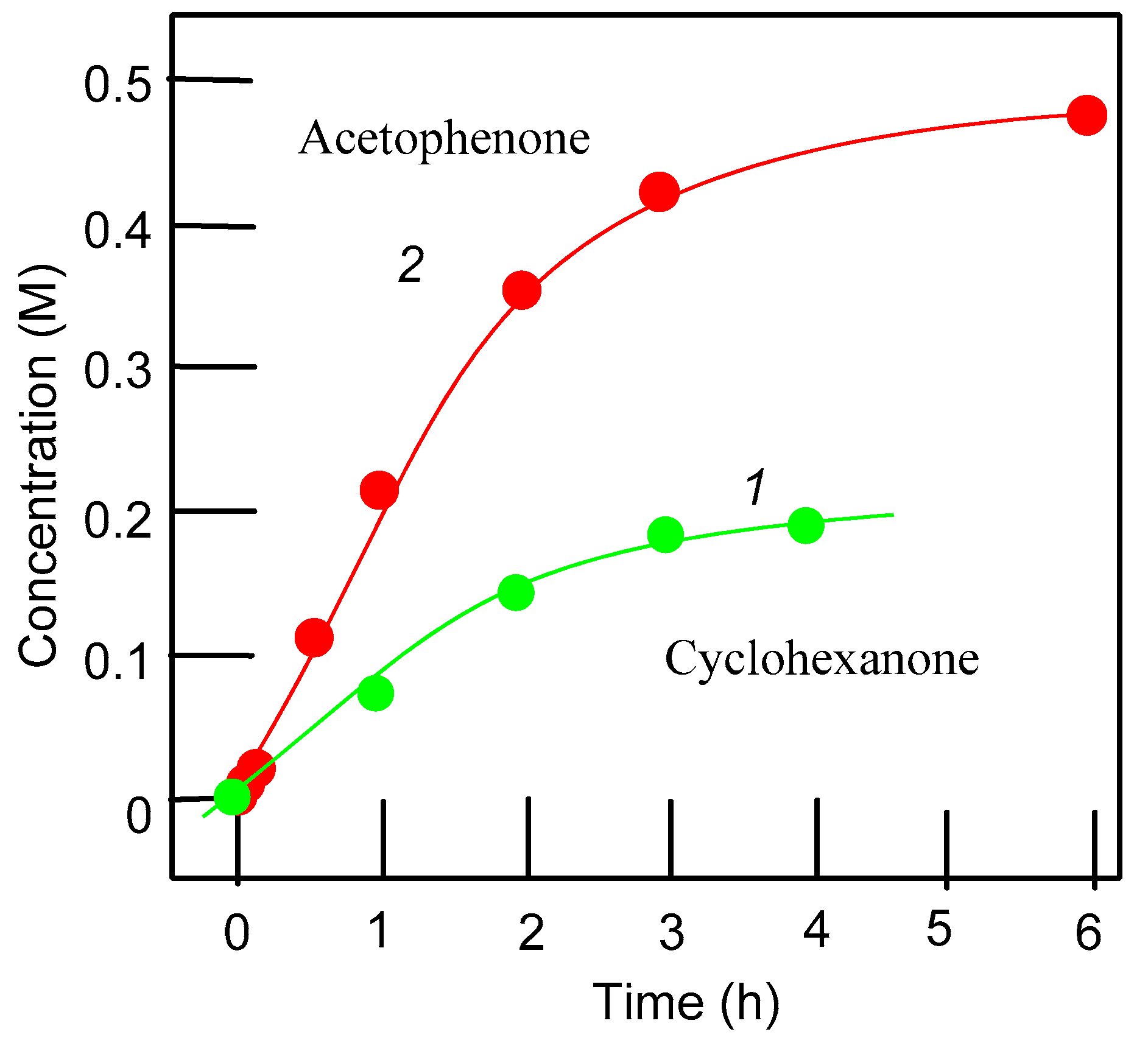
3.2. Catalytic Oxidations of Alkanes and Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and metallasiloxanes derived from silanediols, disilanols, silanetriols, and trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206, 321–368. [Google Scholar] [CrossRef]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of molecular topology and metal nuclearity in multimetallic assemblies: Designer metallosiloxanes derived from silanetriols. Chem. Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. Adv. Organomet. Chem. 2005, 53, 101–153. [Google Scholar]
- Levitsky, M.M.; Zavin, B.G.; Bilyachenko, A.N. Chemistry of metallasiloxanes. Current trends and new concepts. Russ. Chem. Rev. 2007, 76, 847–866. [Google Scholar] [CrossRef]
- Edelmann, F.T. Metallasilsesquioxanes-Synthetic and structural studies. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 383–394. [Google Scholar]
- Pinkert, D.; Limberg, C. Iron silicates, iron-modulated zeolite catalysts, and molecular models thereof. Chem. Eur. J. 2014, 20, 9166–9175. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Zubavichus, Y.V.; Korlyukov, A.A.; Khrustalev, V.N.; Shubina, E.S.; Bilyachenko, A.N. Silicon and Germanium-Based Sesquioxanes as Versatile Building Blocks for Cage Metallacomplexes. J. Clust. Sci. 2019. [Google Scholar] [CrossRef]
- Nehete, U.N.; Roesky, H.W.; Jancik, V.; Pal, A.; Magull, J. Polyhedral antimony(III) and bismuth(III) siloxanes: Synthesis, spectral studies, and structural characterization of [Sb(O3SiR)]4 and [Bi12(O3SiR)8(μ3-O)4Cl4(THF)8] (R = (2,6-iPr2C6H3)N(SiMe3)). Inorg. Chim. Acta 2007, 360, 1248–1257. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Titov, A.A.; Dorovatovskii, P.V.; Shul’pina, L.S.; Lamaty, F.; et al. Ionic Complexes of Tetra and Nonanuclear Cage Copper(II) Phenylsilsesquioxanes: Synthesis and High Activity in Oxidative Catalysis. ChemCatChem 2017, 9, 4437–4447. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’Pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High-Cluster (Cu9) Cage Silsesquioxanes: Synthesis, Structure, and Catalytic Activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Korlyukov, A.A.; Vologzhanina, A.V.; Buzin, M.I.; Sergienko, N.V.; Zavin, B.G.; Muzafarov, A.M. Cu(II)-Silsesquioxanes as Secondary Building Units for Construction of Coordination Polymers: A Case Study of Cesium-Containing Compounds. Cryst. Growth Des. 2016, 16, 1968–1977. [Google Scholar] [CrossRef] [Green Version]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’Pina, L.S.; Nesterov, D.S.; Pombeiro, A.J.L.; Lamaty, F.A.; et al. heterometallic (Fe6Na8) cage-like silsesquioxane: Synthesis, structure, spin glass behavior and high catalytic activity. RSC Adv. 2016, 6, 48165–48180. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Vologzhanina, A.V.; Astakhov, G.S.; Gutsul, E.I.; Shubina, E.S.; Levitsky, M.M. High-Nuclearity (Cu8-Based) Cage Silsesquioxanes: Synthesis and Structural Study. Cryst. Growth Des. 2018, 18, 2452–2457. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Korlyukov, A.A.; Levitsky, M.M.; Antipin, M.Y.; Zavin, B.G. Cage-like manganesephenylsiloxane with an unusual structure. Russ. Chem. Bull. Int. Ed. 2011, 60, 1762–1765. [Google Scholar] [CrossRef]
- Tan, G.; Yang, Y.; Chu, C.; Zhu, H.; Roesky, H.W. Cu24O24Si8R8: Organic soluble 56-membered copper(I) siloxane cage and its use in homogeneous catalysis. J. Am. Chem. Soc. 2010, 132, 12231–12233. [Google Scholar] [CrossRef] [PubMed]
- Abbenhuis, H.C.L. Advances in Homogeneous and Heterogeneous Catalysis with Metal-Containing Silsesquioxanes. Chem. Eur. J. 2000, 6, 25–32. [Google Scholar] [CrossRef]
- Duchateau, R. Incompletely Condensed Silsesquioxanes: Versatile Tools in Developing Silica-Supported Olefin Polymerization Catalysts. Chem. Rev. 2002, 102, 3525–3542. [Google Scholar] [CrossRef]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: New York, NY, USA, 2011; Volume 3, pp. 135–166. [Google Scholar]
- Levitskii, M.M.; Smirnov, V.V.; Zavin, B.G.; Bilyachenko, A.N.; Rabkina, A.Y. Metalasiloxanes: New structure formation methods and catalytic properties. Kinet. Catal. 2009, 50, 490–507. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si-or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849, 201–218. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S.; Long, J.; Guari, Y.; Larionova, J. Magnetic cage-like metallasilsesquioxanes. Coord. Chem. Rev. 2019, 398, 213015. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.; Dronova, M.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’Kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Dorovatovskii, P.V.; et al. Family of Polynuclear Nickel Cagelike Phenylsilsesquioxanes; Features of Periodic Networks and Magnetic Properties. Inorg. Chem. 2017, 56, 12751–12763. [Google Scholar] [CrossRef] [PubMed]
- Beletskiy, E.V.; Hou, X.; Shen, Z.; Gallagher, J.R.; Miller, J.T.; Wu, Y.; Li, T.; Kung, M.C.; Kung, H.H. Supported Tetrahedral Oxo-Sn Catalyst: Single Site, Two Modes of Catalysis. J. Am. Chem. Soc. 2016, 138, 4294–4297. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Shiba, H.; Kanezashi, M.; Wada, H.; Shimojima, A.; Tsuru, T.; Kuroda, K. Synthesis of a 12-membered cyclic siloxane possessing alkoxysilyl groups as a nanobuilding block and its use for preparation of gas permeable membranes. RSC Adv. 2017, 7, 48683–48691. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Zheng, S. Synthesis and self-assembly behavior of organic–inorganic macrocyclic molecular brushes composed of macrocyclic oligomeric silsesquioxane and poly(N-isopropylacrylamide). RSC Adv. 2014, 4, 28439–28450. [Google Scholar] [CrossRef]
- Wei, K.; Liu, N.; Lia, L.; Zheng, S. A stereoregular macrocyclic oligomeric silsesquioxane bearing epoxide groups: Synthesis and its nanocomposites with polybenzoxazine. RSC Adv. 2015, 5, 77274–77287. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shiba, H.; Wada, H.; Shimojima, A.; Kuroda, K. Polymerization of cyclododecasiloxanes with Si-H and Si-OEt side groups by the piers-Rubinsztajn reaction. B Chem. Soc. Jpn. 2018, 91, 747–753. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ikawa, H.; Wada, H.; Shimojima, A.; Kuroda, K. Self-assembly of Cyclohexasiloxanes Possessing Alkoxysilyl Groups and Long Alkyl Chains. Chem. Lett. 2018, 47, 1203–1206. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Polycyclic and Cage Siloxanes by Hydrolysis and Intramolecular Condensation of Alkoxysilylated Cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.-L.; O’Brien, J.; Gun’ko, Y.K. Rare Earth Doped Silica Nanoparticles via Thermolysis of a Single Source Metallasilsesquioxane Precursor. Sci. Rep. 2017, 7, 45862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirotsu, M.; Taruno, S.; Yoshimura, T.; Ueno, K.; Unno, M.; Matsumoto, H. Synthesis and Structures of the First Titanium(IV)Complexes with Cyclic Tetrasiloxide Ligands: Incomplete andComplete Cage Titanosiloxanes. Chem. Lett. 2005, 34, 1542–1543. [Google Scholar] [CrossRef]
- Kishore, P.V.V.N.; Baskar, V. Hexa- and Trinuclear Organoantimony Oxo Clusters Stabilized by Organosilanols. Inorg. Chem. 2014, 53, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Lokare, K.S.; Frank, N.; Braun-Cula, B.; Goikoetxea, I.; Sauer, J.; Limberg, C. Trapping Aluminum Hydroxide Clusters with Trisilanols during Speciation in Aluminum(III)−Water Systems: Reproducible, Large Scale Access to Molecular Aluminate Models. Angew. Chem. Int. Ed. 2016, 55, 12325–12329. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, G.S.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Vologzhanina, A.V.; Shubina, E.S. Tridecanuclear CuII 11Na2 Cagelike Silsesquioxanes. Cryst. Growth Des. 2018, 18, 5377–5384. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shul’pin, G.B. A new “bicycle helmet”-like copper(ii), sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Su, H.-F.; Li, Y.-W.; Liu, Q.-Y.; Jagličić, Z.; Wang, W.-G.; Tung, C.-H.; Sun, D. Space Craft-like Octanuclear Co(II)-Silsesquioxane Nanocages: Synthesis, Structure, Magnetic Properties, Solution Behavior, and Catalytic Activity for Hydroboration of Ketones. Inorg. Chem. 2019, 58, 4574–4582. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Bilyachenko, A.N.; Kozlov, Y.N.; Shul’pin, G.B. Palanquin-like Cu 4 Na 4 silsesquioxane synthesis (via oxidation of 1,1-bis(diphenylphosphino)methane), structure and catalytic activity in alkane or alcohol oxidation with peroxides. Catalysts 2019, 9, 154. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Petrov, A.A.; Korlyukov, A.A.; Shul’pina, L.S.; Khrustalev, V.N.; Dorovatovskii, P.V.; Vologzhanina, A.V.; Tsareva, U.S.; et al. Unusual Tri-, Hexa-, and Nonanuclear Cu(II) Cage Methylsilsesquioxanes: Synthesis, Structures, andCatalytic Activity in Oxidations with Peroxides. Inorg. Chem. 2017, 56, 4093–4103. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4Cage Hexacoppersilsesquioxanes Containing N-Ligands: Synthesis, Structure, and High Catalytic Activity in Peroxide Oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shul’pin, G.B.; Shubina, E.S. Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N, O-Ligated Cu6 Cage Phenylsilsesquioxane. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(μ-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Gradinaru, J.; Kozlov, Y.N. Alkane hydroperoxidation with peroxides catalysed by copper complexes. Org. Biomol. Chem. 2003, 1, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bosch, I.; Siege, M.A. Copper-Catalyzed Oxidation of Alkanes with H2O2 under a Fenton-like Regime. Angew. Chem. Int. Ed. 2016, 55, 12873–12876. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Kardasheva, Y.S.; Predeina, V.V.; Kluev, M.V.; Ramazanov, D.N.; Talanova, M.Y.; Karakhanov, E.A. Iron and copper complexes with nitrogen-containing ligands as catalysts for cyclohexane oxidation with hydrogen peroxide under mild reaction conditions. Pet. Chem. 2012, 52, 318–326. [Google Scholar] [CrossRef]
- Kononevich, Y.N.; Anisimov, A.A.; Korlyukov, A.A.; Tsareva, U.S.; Shchegolikhina, O.I.; Muzafarov, A.M. Synthesis and structures of novel tetra- and pentanuclear copper sandwich-like metallasiloxanes with pyridine ligands. Mendeleev Commun. 2017, 27, 332–334. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Vologzhanina, A.V.; Shul’pin, G.B. Heptanuclear Cage Cu(II)-Silsesquioxanes. Features of Synthesis, Structure and Catalytic Activity. Eur. J. Inorg. Chem. 2018, 22, 2505–2511. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Shul’pina, L.S.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Tsareva, U.S.; Shubina, E.S.; et al. Family of penta- and hexanuclear metallasilsesquioxanes: Synthesis, structure and catalytic properties in oxidations. J. Organomet. Chem. 2018, 867, 133–141. [Google Scholar] [CrossRef]
- Igonin, V.A.; Lindeman, S.V.; Struchkov, Y.T.; Shchegolikhina, O.I.; Zhdanov, A.A.; Molodtsova, Y.A.; Razumovskaya, I.V. The structure of the copper complexes with macrocyclic organosiloxanolate ligands. Organomet. Chem. USSR (Engl. Transl.) 1991, 4, 672. [Google Scholar]
- Zherlitsyna, L.; Auner, N.; Bolte, M. Bis(μ6-cis-2,4,6,8,10,12,14,16-octamethylcyclooctasiloxane-2, 4,6,8,10,12,14,16-octolato)octakis[(dimethylformamide)copper(II)] dimethylformamide solvate enclosing a pyrazine molecule. Acta Crystallogr. Sect. A Crystal Struct. Commun. 2006, 62, m199–m200. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, N.V.; Trankina, E.S.; Pavlov, V.I.; Zhdanov, A.A.; Lyssenko, K.A.; Antipin, M.Yu.; Akhmet’eva, E.I. Reactions of cage-like copper/sodium organosiloxanes with CuCl2. Russ. Chem. Bull. Int. Ed. 2004, 53, 351–357. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’Pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’Pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(ii) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Korlyukov, A.A.; Vologzhanina, A.V.; Khrustalev, V.N.; Kulakova, A.N.; Long, J.; Larionova, J.; Guari, Y.; Dronova, M.S.; Tsareva, U.S.; et al. Tuning linkage isomerism and magnetic properties of bi- and tri-metallic cage silsesquioxanes by cation and solvent effects. Dalton Trans. 2017, 46, 12935–12949. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Blaurock, S.; Görls, H.; Edelmann, F.T. The First Niobasilsesquioxanes. Organometallics 2006, 25, 5922–5926. [Google Scholar] [CrossRef]
- Giovenzana, T.; Guidotti, M.; Lucenti, E.; Biroli, A.O.; Sordelli, L.; Sironi, A.; Ugo, R. Synthesis and Catalytic Activity of Titanium Silsesquioxane Frameworks as Models of Titanium Active Surface Sites of Controlled Nuclearity. Organometallics 2010, 29, 6687–6694. [Google Scholar] [CrossRef]
- Maxim, N.; Abbenhuis, H.C.L.; Stobbelaar, P.J.; Mojet, B.L.; van Santen, R.A. Chromium silsesquioxane based synthesis and characterization of a microporous Cr–Si–O material. Phys. Chem. Chem. Phys. 1999, 1, 4473–4477. [Google Scholar] [CrossRef]
- Maxim, N.; Magusin, P.C.M.M.; Kooyman, P.J.; van Wolput, J.H.M.C.; van Santen, R.A.; Abbenhuis, H.C.L. Microporous Mg−Si−O and Al−Si−O Materials Derived from Metal Silsesquioxanes. Chem. Mater. 2001, 13, 2958–2964. [Google Scholar] [CrossRef]
- Maxim, N.; Overweg, A.; Kooyman, P.J.; Van Wolput, J.H.M.C.; Hanssen, R.W.J.M.; Van Santen, R.A.; Abbenhuis, H.C.L. Synthesis and Characterization of Microporous Fe−Si−O Materials with Tailored Iron Content from Silsesquioxane Precursors. J. Phys. Chem. B 2002, 106, 2203–2209. [Google Scholar] [CrossRef]
- Murugavel, R.; Davis, P.; Shete, V.S. Reactivity Studies, Structural Characterization, and Thermolysis of Cubic Titanosiloxanes: Precursors to Titanosilicate Materials Which Catalyze Olefin Epoxidation. Inorg. Chem. 2003, 42, 4696–4706. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, G.E.; Powell, A.K. An approach to describing the topology of polynuclear clusters. Coord. Chem. Rev. 2009, 253, 2686–2697. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 147, 3576–3586. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Perlepe, S.P.; Blatov, V.A.; Proserpio, D.M.; Powell, A.K. High-nuclearity cobalt coordination clusters: Synthetic, topological and magnetic aspects. Coord. Chem. Rev. 2012, 256, 1246–1278. [Google Scholar] [CrossRef] [Green Version]
- Kostakis, G.E.; Blatov, V.A.; Proserpio, D.M. A method for topological analysis of high nuclearity coordination clusters and its application to Mn coordination compounds. Dalton Trans. 2012, 41, 4634–4640. [Google Scholar] [CrossRef]
- Wix, P.; Kostakis, G.E.; Blatov, V.A.; Proserpio, D.M.; Perlepes, S.P.; Powell, A.K. A database of topological representations of polynuclear nickel compounds. Eur. J. Inorg. Chem. 2013, 9, 520–526. [Google Scholar] [CrossRef]
- Qian, C.; Wang, B.; Xin, Y.; Lin, Y. Studies on organolanthanide complexes. Part 56. Formation of ion-pair complexes [Na·3phen]+[Ln(C5H5)3Cl]–phen (Ln = La, Pr or Nd; phen = 1,10-phenanthroline) and crystal structure for Ln = Pr. J. Chem. Soc. Dalton Trans. 1994, 14, 2109–2112. [Google Scholar] [CrossRef]
- Tseng, C.-K.; Lee, C.-R.; Han, C.-C.; Shyu, S.-G. Copper(I)–Anilide Complex [Na(phen)3] [Cu(NPh2)2]: An Intermediate in the Copper-Catalyzed N-Arylation of N-Phenylaniline. Chem. Eur. J. 2011, 17, 2716–2723. [Google Scholar] [CrossRef]
- Doi, R.; Ohashi, M.; Ogoshi, S. Copper-Catalyzed Reaction of Trifluoromethylketones with Aldehydes via a Copper Difluoroenolate. Angew. Chem. Int. Ed. 2016, 55, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, C.; Wang, X.; Shen, G.; Shen, D. A novel heterometallic FeII–Na+ phase: 1,10-phenanthrolinium aqua-bis(1,10-phenanthroline)-sodium(I) pentacyanonitrosoiron(II) monohydrate. Acta Crystallogr. Sect. E 2004, 60, m1293–m1295. [Google Scholar] [CrossRef]
- Li, F.; Yin, H.; Wang, D. Di-μ-iodo-1:2κ4I: I-diiodo-2κ2I-tris-(1,10-phenanthroline)-1κ2N, N′;2κ2N, N′-bis-muth(III)sodium(I). Acta Crystallogr. Sect. E 2006, 62, m437–m439. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Turner, D.R.; Langley, S.K.; Moubaraki, B.; Murray, K.S.; Deacon, G.B.; Batten, S.R. Synthesis and Structure of New Lanthanoid Carbonate “Lanthaballs”. Inorg. Chem. 2015, 54, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Alkane-oxidizing systems based on metal complexes. Radical versus non-radical mechanisms. In Alkane Functionalization; Pombeiro, A.J.L., Guedes da Silva, F.C., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 47–72. [Google Scholar]
- Shul’pin, G.B. C-H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. New trends in oxidative functionalization of carbon–hydrogen bonds: A review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Reetz, M.T. Directed Evolution of Artificial Metalloenzymes: A Universal Means to Tune the Selectivity of Transition Metal Catalysts? Acc. Chem. Res. 2019, 52, 336–344. [Google Scholar] [CrossRef]
- Hartwig, J.F.; Ward, T.R. New “Cats” in the House: Chemistry Meets Biology in Artificial Metalloenzymes and Repurposed Metalloenzymes. Acc. Chem. Res. 2019, 52, 1145. [Google Scholar]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Cuervo, L.G.; Süss-Fink, G. Hydrogen peroxide oxygenation of alkanes including methane and ethane catalyzed by iron complexes in acetonitrile. Adv. Synth. Catal. 2004, 346, 317–332. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent O2-H2O2-vanadium derivative-pyrazine-2-carboxylic acid. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2 2001, 8, 1351–1371. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G.; Lindsay Smith, J.R. Oxidations by the system hydrogen peroxide-manganese(IV) complex- acetic acid-Part II: Hydroperoxidation and hydroxylation of alkanes in acetonitrile. Tetrahedron 1999, 55, 5345–5358. [Google Scholar] [CrossRef]
- Mandelli, D.; Chiacchio, K.C.; Kozlov, Y.N.; Shul’pin, G.B. Hydroperoxidation of alkanes with hydrogen peroxide catalyzed by aluminium nitrate in acetonitrile. Tetrahedron Lett. 2008, 49, 6693–6697. [Google Scholar] [CrossRef]
- Olivo, G.; Lanzalunga, O.; Di Stefano, S. Non-Heme Imine-Based Iron Complexes as Catalysts for Oxidative Processes (Review). Adv. Synth. Catal. 2016, 358, 843–863. [Google Scholar] [CrossRef]
| Entry | Catalytic System | C(1):C(2):C(3):C(4) | 1°:2°:3° | trans/cis |
|---|---|---|---|---|
| n-Heptane | MCH | cis-1,2-DMCH | ||
| 1 | Catalyst 1 | 1.0:5.9:6.2:5.6 | 1.0:5.6:14.0 | 0.8 |
| 2 | Catalyst 1 + HNO3 | 1.0:4.5:4.3:3.9 | 1.0:4.7:11.3 | 0.8 |
| 3 | Catalyst 2 | 1.0:5.3:5.3:5.4 | 1.0:6.2:17.4 | 0.9 |
| 4 | Catalyst 2 + HNO3 | 1.0:5.7:5.7:5.2 | 1.0:5.4:15.0 | |
| 5 | VO3−/PCA b | 1.0:6.0:7.0:5.0 | 1:9:37 | 0.75 |
| 6 | [Mn2L2(O)3]2+/MeCO2H c | 1:46:35:34 | 1:26:200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astakhov, G.S.; Levitsky, M.M.; Korlyukov, A.A.; Shul’pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Vologzhanina, A.V.; Bilyachenko, A.N.; Dorovatovskii, P.V.; Kozlov, Y.N.; et al. New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts 2019, 9, 701. https://doi.org/10.3390/catal9090701
Astakhov GS, Levitsky MM, Korlyukov AA, Shul’pina LS, Shubina ES, Ikonnikov NS, Vologzhanina AV, Bilyachenko AN, Dorovatovskii PV, Kozlov YN, et al. New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts. 2019; 9(9):701. https://doi.org/10.3390/catal9090701
Chicago/Turabian StyleAstakhov, Grigorii S., Mikhail M. Levitsky, Alexander A. Korlyukov, Lidia S. Shul’pina, Elena S. Shubina, Nikolay S. Ikonnikov, Anna V. Vologzhanina, Aleksey N. Bilyachenko, Pavel V. Dorovatovskii, Yuriy N. Kozlov, and et al. 2019. "New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile" Catalysts 9, no. 9: 701. https://doi.org/10.3390/catal9090701
APA StyleAstakhov, G. S., Levitsky, M. M., Korlyukov, A. A., Shul’pina, L. S., Shubina, E. S., Ikonnikov, N. S., Vologzhanina, A. V., Bilyachenko, A. N., Dorovatovskii, P. V., Kozlov, Y. N., & Shul’pin, G. B. (2019). New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts, 9(9), 701. https://doi.org/10.3390/catal9090701