Modified Nimo Nanoparticles for Efficient Catalytic Hydrogen Generation from Hydrous Hydrazine
Abstract
1. Introduction
2. Results and Discussion
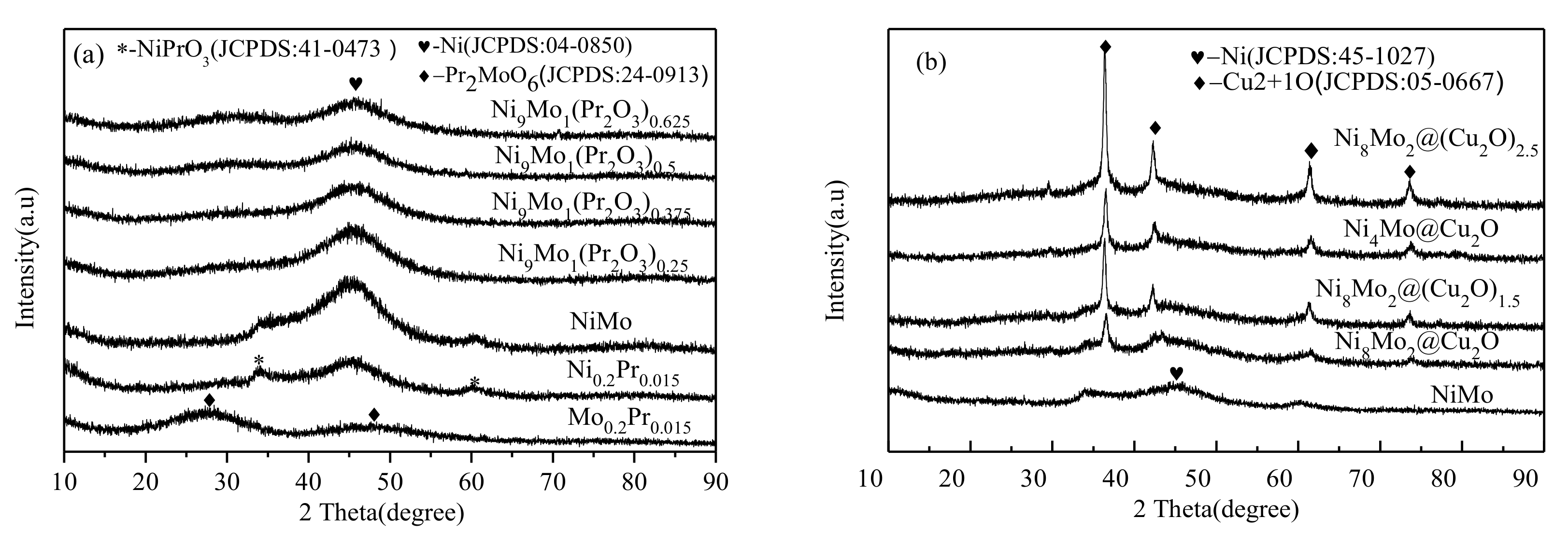
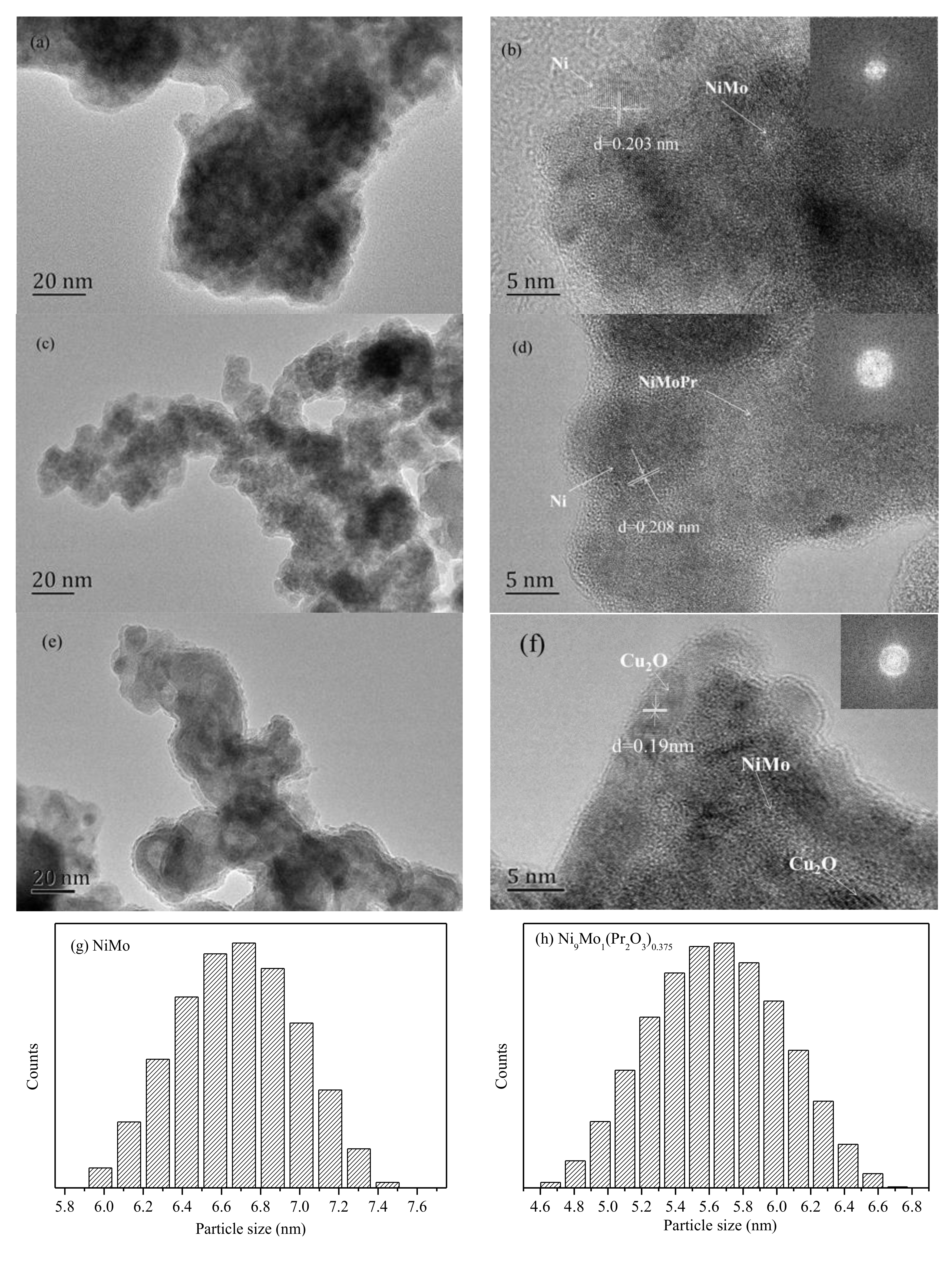
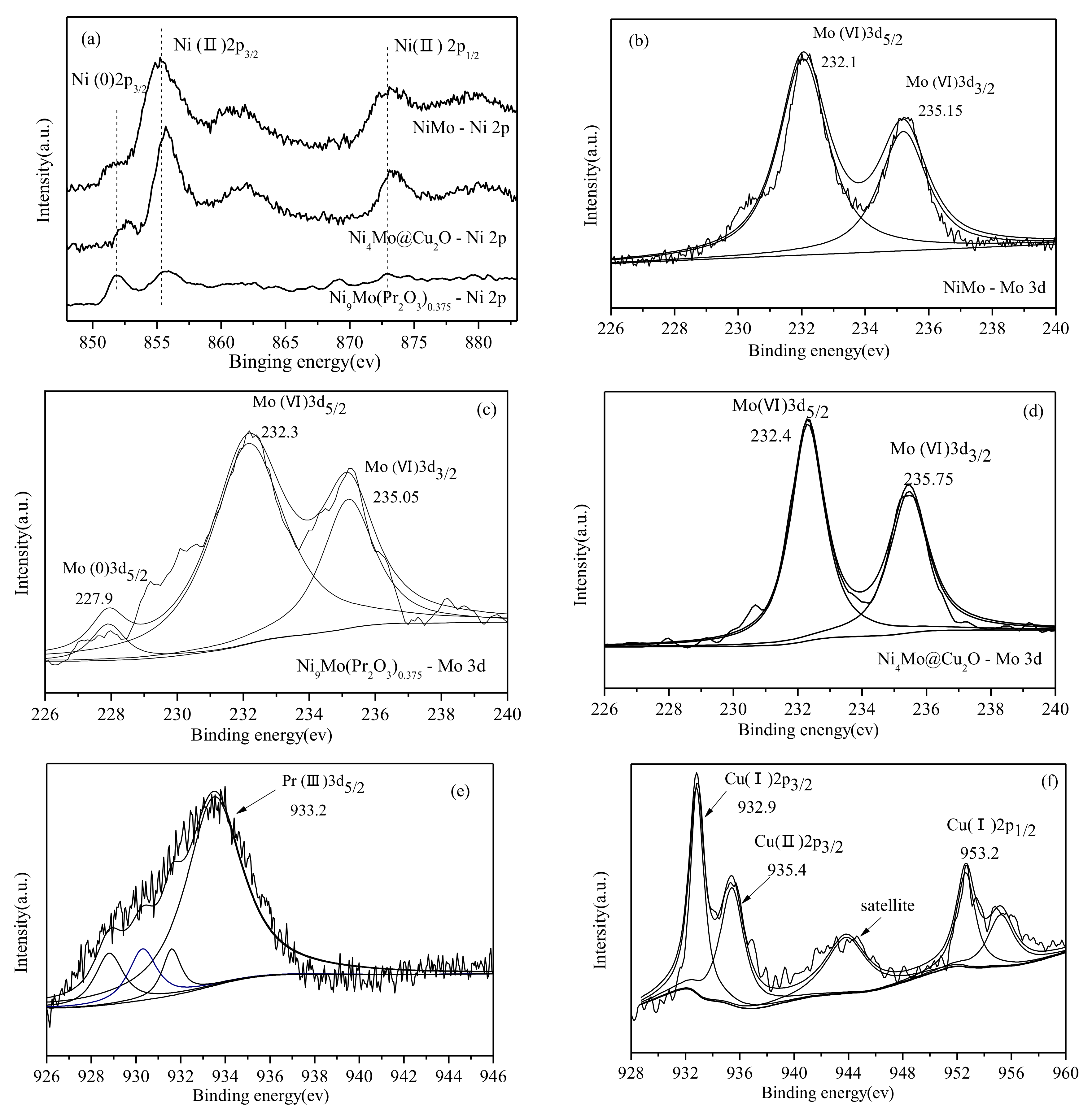
2.1. Physical Characteristics of Ni1-xMox(Pr2O3)y and Ni1-xMox@(Cu2O)y Nanocatalysts
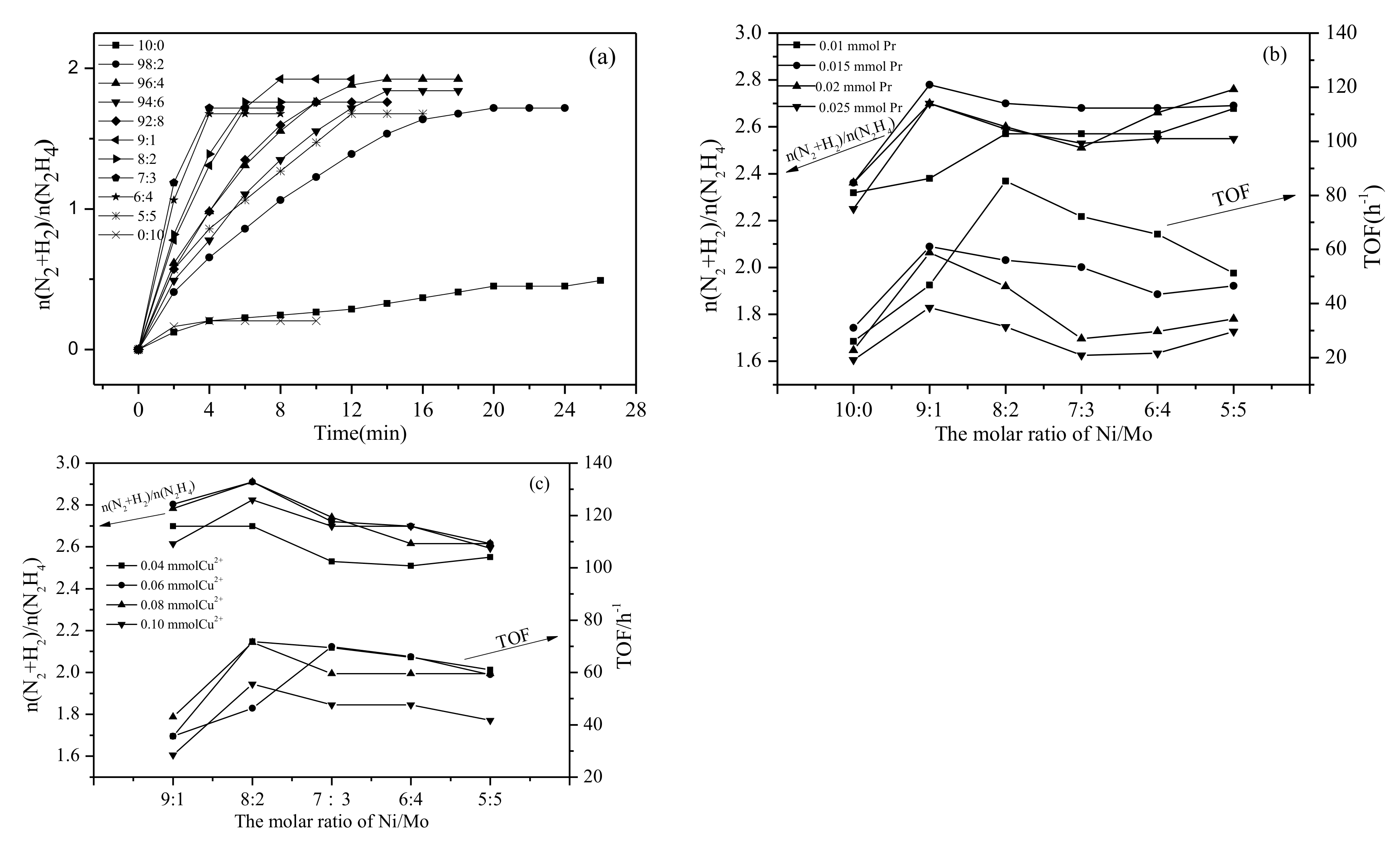
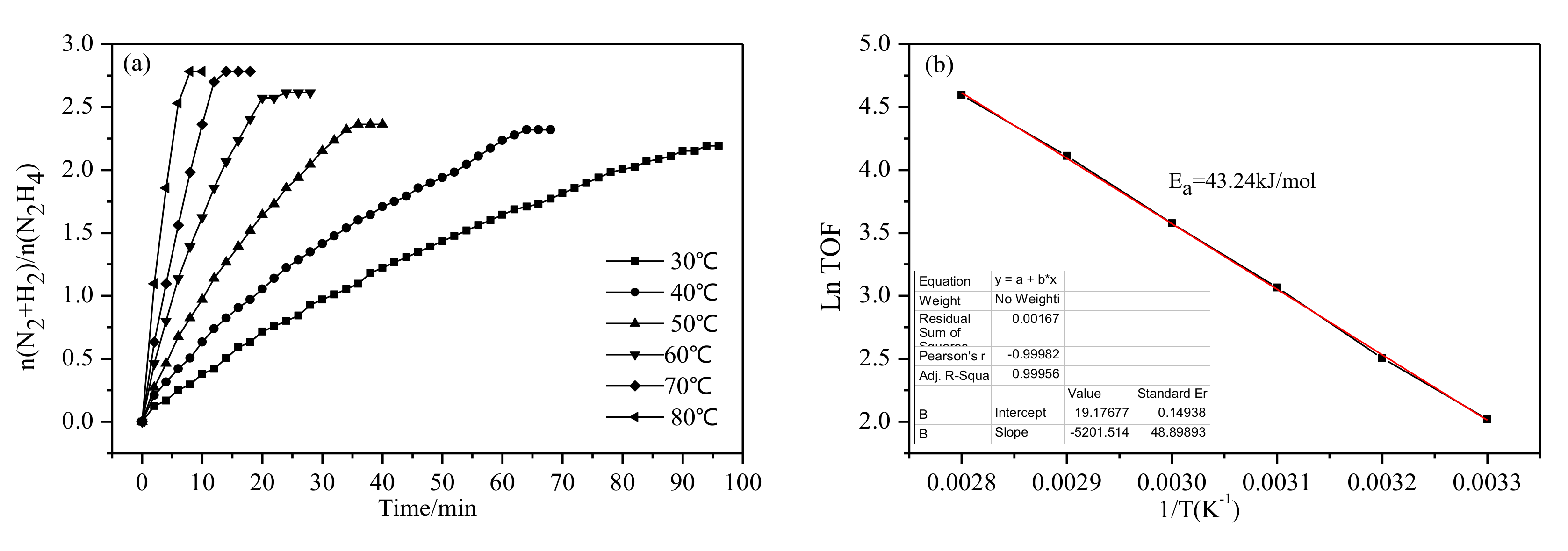
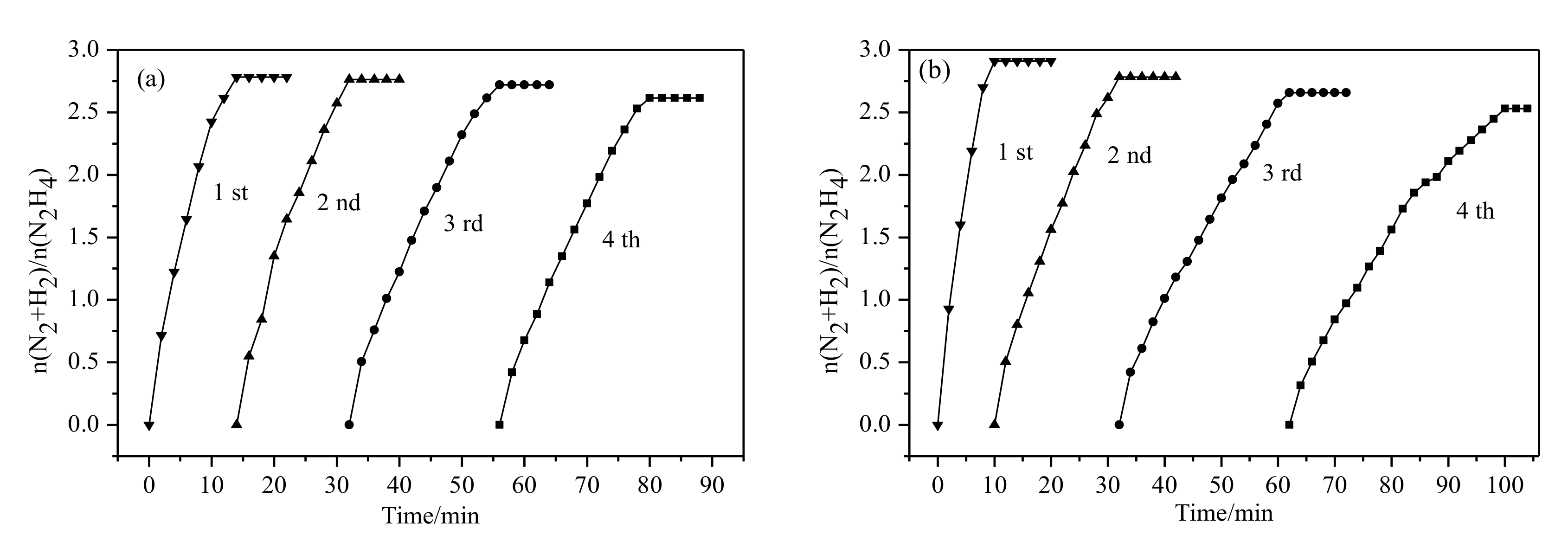
2.2. Catalytic Activities of Ni1-xMox(Pr2O3)y and Ni1-xMox@(Cu2O)y Nanocatalysts
3. Materials and Methods
3.1. Reagents and Materials
3.2. Characterization
3.3. Synthesis of Ni-Based Catalysts: Ni1-xMox(Pr2O3)y and Ni1-xMox@(Cu2O)y
3.4. Catalysis of Hydrazine Hydrate to Produce Hydrogen
3.5. Durability Testing for the Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Lee Tan, K. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Lee, J.W.; Lee, J.H.; Bajgai, J.; Lee, K.J.; Park, S.J. Photocatalytic hydrogen evolution via water splitting: A short review. Catalysts 2018, 8, 655. [Google Scholar] [CrossRef]
- Du, X.; Cai, P.; Luo, W.; Cheng, G. Facile synthesis of P-doped Rh nanoparticles with superior catalytic activity toward dehydrogenation of hydrous hydrazine. Int. J. Hydrog. Energy 2017, 42, 6137–6143. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Singh, S.K.; Xu, Q. Complete conversion of hydrous hydrazine to hydrogen at room temperature for chemical hydrogen storage. J. Am. Chem. Soc. 2009, 131, 18032–18033. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.C.; Aranishi, K.; Singh, A.K.; Demirci, U.B.; Xu, Q. The synergistic effect of Rh-Ni catalysts on the highly-efficient dehydrogenation of aqueous hydrazine borane for chemical hydrogen storage. Chem. Commun. 2012, 48, 11945–11947. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.C.; Mao, Y.L.; Tan, X.; Zhong, P.; Liu, L.X. RhNi nanocatalyst: Spontaneous alloying and high activity for hydrogen generation from hydrous hydrazine. Int. J. Hydrog. Energy 2016, 41, 6362–6368. [Google Scholar] [CrossRef]
- Cao, N.; Su, J.; Luo, W.; Cheng, G. Ni-Pt nanoparticles supported on MIL-101 as highly efficient catalysts for hydrogen generation from aqueous alkaline solution of hydrazine for chemical hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 9726–9734. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Liu, Y.; Liu, X.; Chen, X.; Delgado, J.J.; Wang, X.; Zhang, T. Surface modification of Ni/Al2O3 with Pt: Highly efficient catalysts for H2 generation via selective decomposition of hydrous hydrazine. J. Catal. 2013, 298, 1–9. [Google Scholar] [CrossRef]
- Du, Y.; Su, J.; Luo, W.; Cheng, G. Graphene-supported nickel-platinum nanoparticles as efficient catalyst for hydrogen generation from hydrous hydrazine at room temperature. ACS Appl. Mater. Interfaces 2015, 7, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kang, Q.; Zhang, J.; Dai, H.B.; Wang, P. High-performance nickel-platinum nanocatalyst supported on mesoporous alumina for hydrogen generation from hydrous hydrazine. J. Power Sources 2015, 273, 554–560. [Google Scholar] [CrossRef]
- Singh, S.K.; Lu, Z.H.; Xu, Q. Temperature-induced enhancement of catalytic performance in selective hydrogen generation from hydrous hydrazine with Ni-based nanocatalysts for chemical hydrogen storage. Eur. J. Inorg. Chem. 2011, 2011, 2232–2237. [Google Scholar] [CrossRef]
- Singh, S.K.; Iizuka, Y.; Xu, Q. Nickel-palladium nanoparticle catalyzed hydrogen generation from hydrous hydrazine for chemical hydrogen storage. Int. J. Hydrog. Energy 2011, 36, 11794–11801. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mandal, K.; Dasgupta, S. High performance nickel-palladium nanocatalyst for hydrogen generation from alkaline hydrous hydrazine at room temperature. J. Power Sources 2015, 287, 96–99. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Liu, X.Y.; Li, L.; Wang, A.; Wang, X.; Mou, C.Y.; Zhang, T. Structural and catalytic properties of supported Ni-Ir alloy catalysts for H2 generation via hydrous hydrazine decomposition. Appl. Catal. B Environ. 2014, 147, 779–788. [Google Scholar] [CrossRef]
- Dai, H.; Zhong, Y.; Wang, P. Hydrogen generation from decomposition of hydrous hydrazine over Ni-Ir/CeO2 catalyst. Prog. Nat. Sci. Mater. Int. 2017, 27, 121–125. [Google Scholar] [CrossRef]
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-oxide-modified nickel as a non-noble metal catalyst for selective decomposition of hydrous hydrazine to hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- Men, Y.; Du, X.; Cheng, G.; Luo, W. CeOx-modified NiFe nanodendrits grown on rGO for efficient catalytic hydrogen generation from alkaline solution of hydrazine. Int. J. Hydrog. Energy 2017, 42, 27165–27173. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Cross, A.; Rouvimov, S.; Miller, J.; Mukasyan, A.S.; Wolf, E.E. Low temperature decomposition of hydrous hydrazine over FeNi/Cu nanoparticles. Appl. Catal. A Gen. 2014, 476, 47–53. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhang, Y. Precious-metal-free nanocatalysts for highly efficient hydrogen production from hydrous hydrazine. Adv. Funct. Mater. 2014, 24, 7073–7077. [Google Scholar] [CrossRef]
- Yang, K.; Yao, Q.; Huang, W.; Chen, X.; Lu, Z.H. Enhanced catalytic activity of NiM (M = Cr, Mo, W) nanoparticles for hydrogen evolution from ammonia borane and hydrazine borane. Int. J. Hydrog. Energy 2017, 42, 6840–6850. [Google Scholar] [CrossRef]
- Zhu, L.L.; Lin, X.M.; Qi, L.J. Preparation and properties for solid solution Ce0.8Pr0.2-xSmxO2-δ(x=0.02, 0.05, 0.1). Appl. Mech. Mater. 2015, 723, 596–600. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.H.; Zhang, R.; Zhang, S.; Chen, X.; Jiang, H.L. A noble-metal-free nanocatalyst for highly efficient and complete hydrogen evolution from N2H4·BH3. J. Mater. Chem. A 2018, 6, 4386–4393. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Dai, H.B.; Zhu, M.; Wang, P. Catalytic decomposition of hydrous hydrazine over Ni-Pt/La2O3 catalyst: A high-performance hydrogen storage system. Int. J. Hydrog. Energy 2016, 41, 11042–11049. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.; Wu, Y.; Cheng, J.; Su, H. Construction of bimetallic nanoparticles immobilized by porous functionalized metal-organic frameworks toward remarkably enhanced catalytic activity for the room-temperature complete conversion of hydrous hydrazine into hydrogen. Int. J. Hydrog. Energy 2017, 42, 19096–19105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Yang, K.; Gao, D.; Huang, C.; Chen, Y.; Zhang, F.; Xiong, X.; Li, L.; Xia, Q. Pd-Ni nanoparticles supported on MIL-101 as high-performance catalysts for hydrogen generation from ammonia borane. J. Alloy. Compd. 2016, 677, 87–95. [Google Scholar] [CrossRef]
- Devaiah, D.; Thrimurthulu, G.; Smirniotis, P.G.; Reddy, B.M. Nanocrystalline alumina-supported ceria-praseodymia solid solutions: Structural characteristics and catalytic CO oxidation. RSC Adv. 2016, 6, 44826–44837. [Google Scholar] [CrossRef]
- Devaiah, D.; Tsuzuki, T.; Boningari, T.; Smirniotis, P.G.; Reddy, B.M. Ce0.80M0.12Sn0.08O2-δ (M = Hf, Zr, Pr, and La) ternary oxide solid solutions with superior properties for CO oxidation. RSC Adv. 2015, 5, 30275–30285. [Google Scholar] [CrossRef]
- Wang, H.L.; Yan, J.M.; Li, S.J.; Zhang, X.W.; Jiang, Q. Noble-metal-free NiFeMo nanocatalyst for hydrogen generation from the decomposition of hydrous hydrazine. J. Mater. Chem. A 2015, 3, 121–124. [Google Scholar] [CrossRef]
- Liu, T.; Yu, J.; Bie, H.; Hao, Z. Highly efficient hydrogen generation from hydrous hydrazine using a reduced graphene oxide-supported NiPtP nanoparticle catalyst. J. Alloy. Compd. 2017, 690, 783–790. [Google Scholar] [CrossRef]
- Wang, H.L.; Yan, J.M.; Wang, Z.L.; O, S.I.; Jiang, Q. Highly efficient hydrogen generation from hydrous hydrazine over amorphous Ni0.9Pt0.1/Ce2O3 nanocatalyst at room temperature. J. Mater. Chem. A 2013, 1, 14957–14962. [Google Scholar] [CrossRef]
- Gao, W.; Li, C.; Chen, H.; Wu, M.; He, S.; Wei, M.; Evans, D.G.; Duan, X. Supported nickel-iron nanocomposites as a bifunctional catalyst towards hydrogen generation from N2H4·H2O. Green Chem. 2014, 16, 1560–1568. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. H2 production by selective decomposition of hydrous hydrazine over Raney Ni catalyst under ambient conditions. AIChE J. 2013, 59, 4297–4302. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Delgado, J.J.; Zhang, T. A noble-metal-free catalyst derived from Ni-Al hydrotalcite for hydrogen generation from N2H4 H2O decomposition. Angew. Chem. Int. Ed. 2012, 51, 6191–6194. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Qiu, Y.P.; Dai, H.B.; Wang, P. A study of degradation phenomenon of Ni–Pt/CeO2 catalyst towards hydrogen generation from hydrous hydrazine. Int. J. Hydrog. Energy 2017, 42, 16355–16361. [Google Scholar] [CrossRef]
- Dai, H.; Dai, H.B.; Zhong, Y.J.; Kang, Q.; Sun, L.X.; Wang, P. Kinetics of catalytic decomposition of hydrous hydrazine over CeO2-supported bimetallic Ni–Pt nanocatalysts. Int. J. Hydrog. Energy 2017, 42, 5684–5693. [Google Scholar] [CrossRef]



| Catalyst | Temperature(K) | TOF(h−1) | Ea (kJ mol−1) | Ref. |
|---|---|---|---|---|
| Rh4.4Ni/graphene | 298 | 28 | - | [12] |
| Ni0.9Pt0.1/Ce2O3 | 298 | 28.1 | 42.3 | [33] |
| Ni1.5Fe1.0/(MgO) 3.5 | 299 | 11 | - | [34] |
| NiIr0.059/Al2O3-HT | 303 | 12.4 | 49.3 | [17] |
| NiPt0.057/Al2O3-HT | 303 | 16.5 | 34 | [16] |
| Ni0.6Fe0.4Mo | 323 | 28.8 | 50.7 | [20] |
| Cu@Fe5Ni5 | 343 | 11.9 | - | [27] |
| NiFe/Cu | 343 | 17.6 | 45.95 | [28] |
| Ni9Mo1(Pr2O3)0.375 | 343 | 62 | 43.24 | This work |
| Ni4Mo@Cu2O | 343 | 71.4 | 46.47 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, H.; Ma, C.; Sun, N. Modified Nimo Nanoparticles for Efficient Catalytic Hydrogen Generation from Hydrous Hydrazine. Catalysts 2019, 9, 596. https://doi.org/10.3390/catal9070596
Liu Y, Zhang H, Ma C, Sun N. Modified Nimo Nanoparticles for Efficient Catalytic Hydrogen Generation from Hydrous Hydrazine. Catalysts. 2019; 9(7):596. https://doi.org/10.3390/catal9070596
Chicago/Turabian StyleLiu, Ying, Huan Zhang, Cong Ma, and Nan Sun. 2019. "Modified Nimo Nanoparticles for Efficient Catalytic Hydrogen Generation from Hydrous Hydrazine" Catalysts 9, no. 7: 596. https://doi.org/10.3390/catal9070596
APA StyleLiu, Y., Zhang, H., Ma, C., & Sun, N. (2019). Modified Nimo Nanoparticles for Efficient Catalytic Hydrogen Generation from Hydrous Hydrazine. Catalysts, 9(7), 596. https://doi.org/10.3390/catal9070596





