Nanostructured Fe-Ni Sulfide: A Multifunctional Material for Energy Generation and Storage
Abstract
1. Introduction
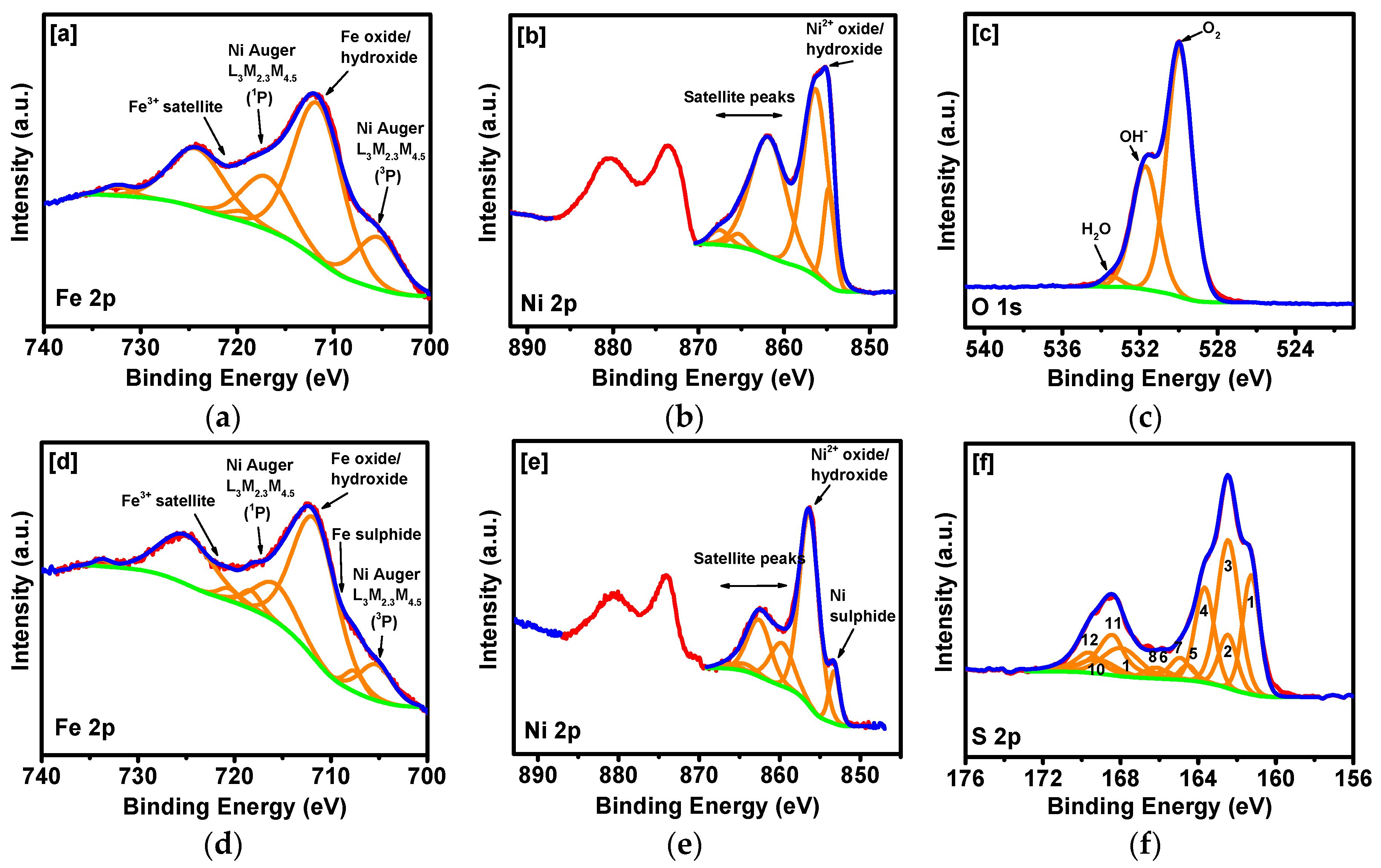
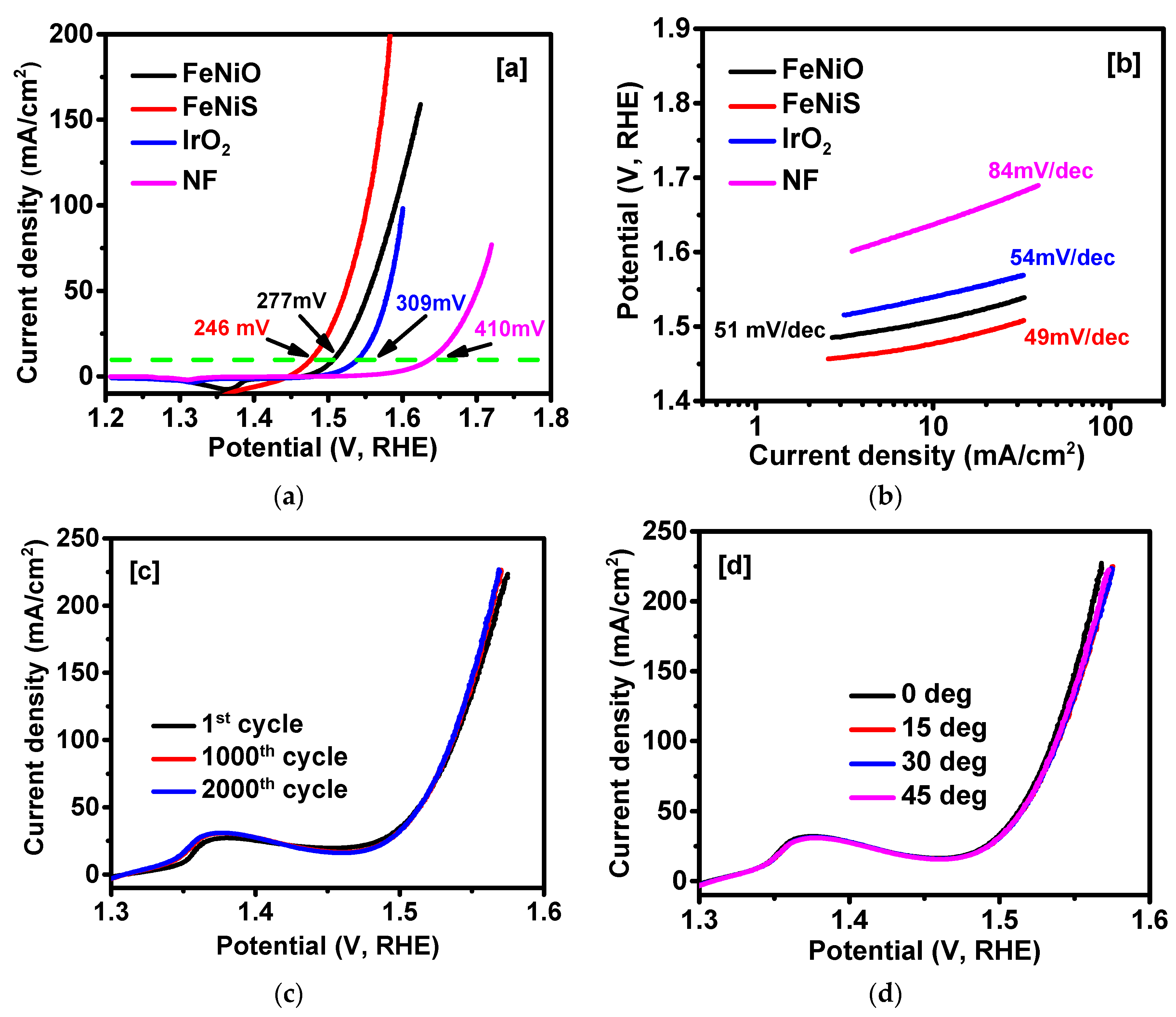
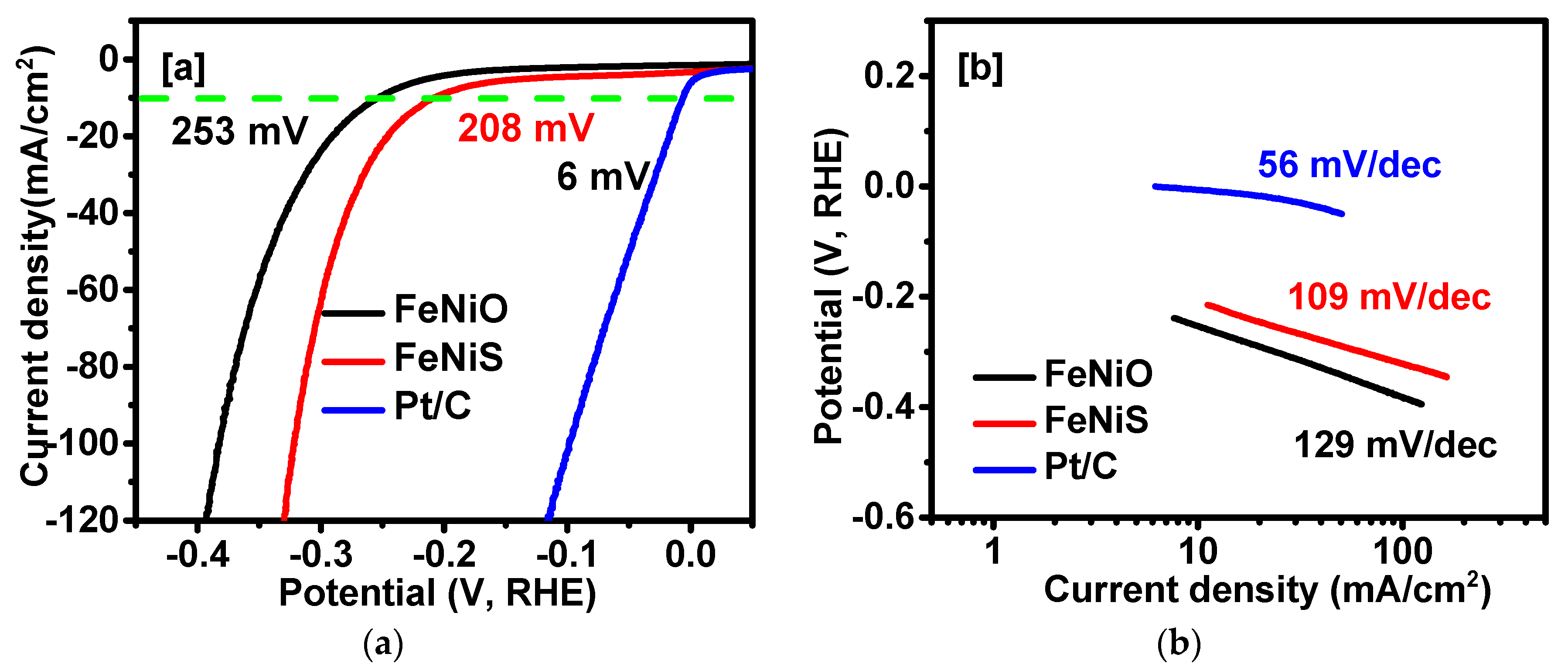
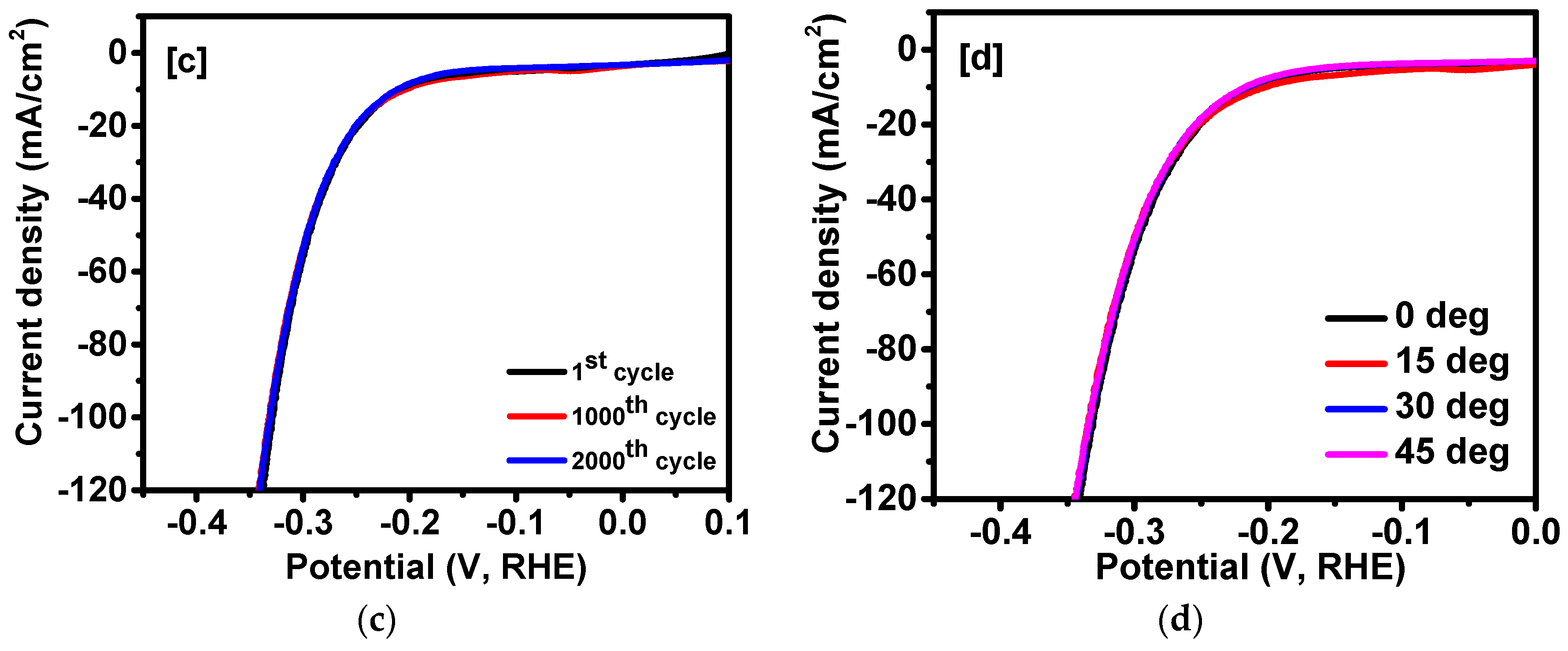
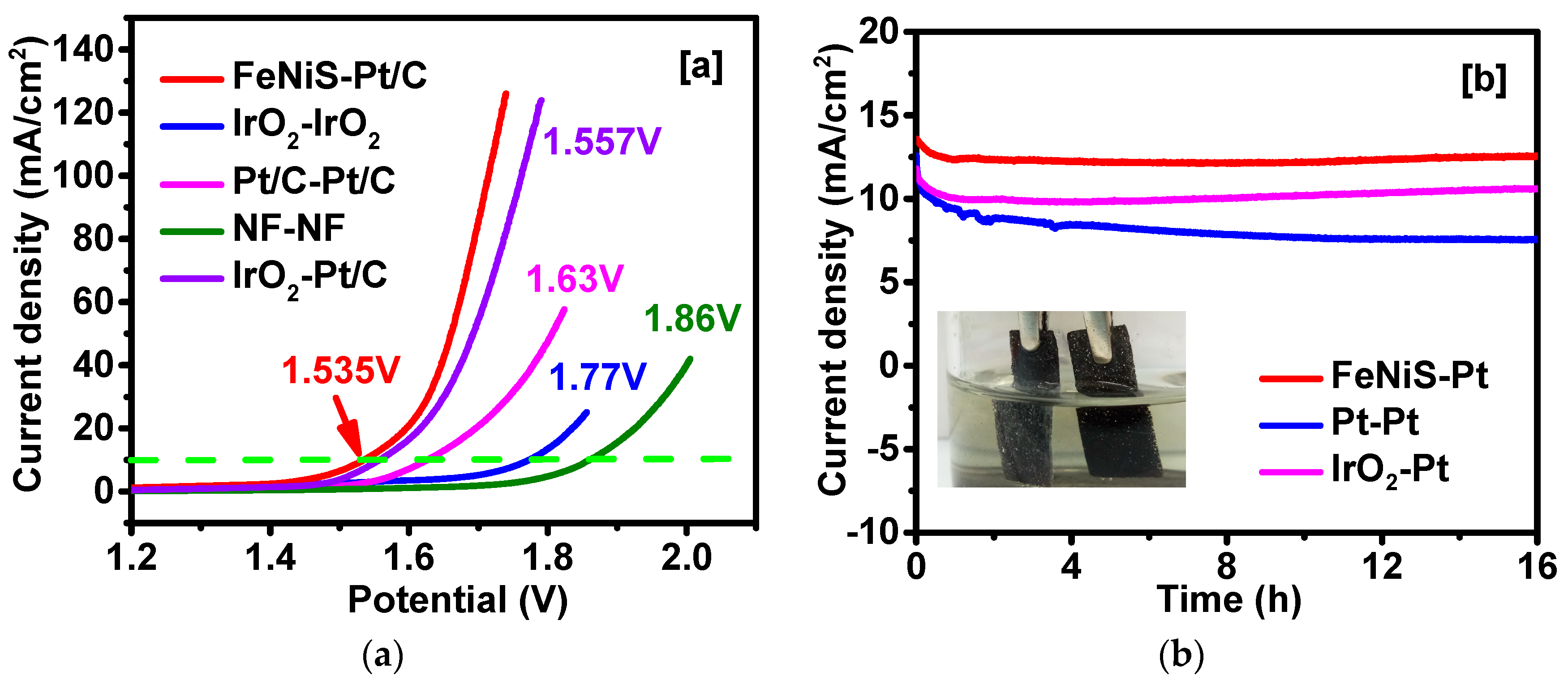
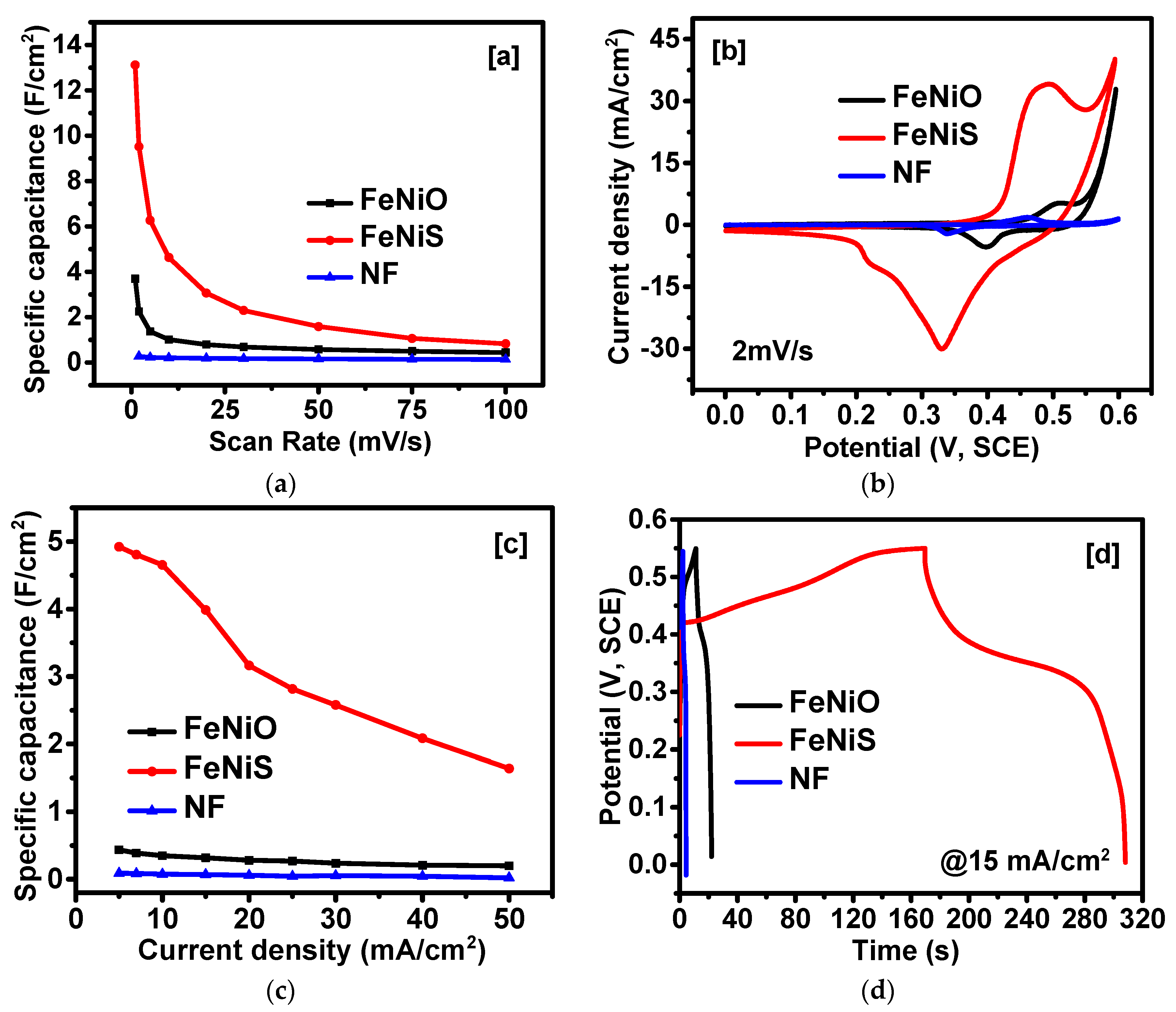
2. Results and Discussion
3. Experimental Details
3.1. Preparation of FeNiO and FeNiS Electrodes
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EIA. International Energy Outlook 2017 Overview; U.S. Energy Information Administration: Washington, DC, USA, 2017.
- Tao, S.; Ru, M.Y.; Du, W.; Zhu, X.; Zhong, Q.R.; Li, B.G.; Shen, G.F.; Pan, X.L.; Meng, W.J.; Chen, Y.L.; et al. Quantifying the rural residential energy transition in China from 1992 to 2012 through a representative national survey. Nat. Energy 2018, 3, 567–573. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Recent Development on Nanocomposites of Graphene for Supercapacitor Applications. Curr. Graphene Sci. 2017, 1, 26. [Google Scholar] [CrossRef]
- Bhoyate, S.; Kahol, P.K.; Sapkota, B.; Mishra, S.R.; Perez, F.; Gupta, R.K. Polystyrene activated linear tube carbon nanofiber for durable and high-performance supercapacitors. Surf. Coat. Technol. 2018, 345, 113–122. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Zhang, C.; Bhoyate, S.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Gupta, R.K. Flower-shaped cobalt oxide nano-structures as an efficient, flexible and stable electrocatalyst for the oxygen evolution reaction. Mater. Chem. Front. 2017, 1, 1580–1584. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Eco-Friendly and High Performance Supercapacitors for Elevated Temperature Applications Using Recycled Tea Leaves. Glob. Chall. 2017, 1, 1700063. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Bhoyate, S.; Morey, T.; Neria, B.L.; Vasiraju, V.; Gupta, G.; Palchoudhury, S.; Kahol, P.K.; Mishra, S.R.; et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. C 2017, 3, 33. [Google Scholar] [CrossRef]
- Adhikari, H.; Ghimire, M.; Ranaweera, C.K.; Bhoyate, S.; Gupta, R.K.; Alam, J.; Mishra, S.R. Synthesis and electrochemical performance of hydrothermally synthesized Co3O4 nanostructured particles in presence of urea. J. Alloy. Compd. 2017, 708, 628–638. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Riley, B.J.; Chun, J.; Um, W.; Lepry, W.C.; Matyas, J.; Olszta, M.J.; Li, X.; Polychronopoulou, K.; Kanatzidis, M.G. Chalcogen-based aerogels as sorbents for radionuclide remediation. Environ. Sci. Technol. 2013, 47, 7540–7547. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, K.; Malliakas, C.D.; He, J.; Kanatzidis, M.G. Selective Surfaces: Quaternary Co(Ni)MoS-Based Chalcogels with Divalent (Pb2+, Cd2+, Pd2+) and Trivalent (Cr3+, Bi3+) Metals for Gas Separation. Chem. Mater. 2012, 24, 3380–3392. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Prasittichai, C.; Hupp, J.T.; Kanatzidis, M.G. Enhanced Electrocatalytic Reduction of CO2with Ternary Ni-Fe4S4and Co-Fe4S4-Based Biomimetic Chalcogels. J. Am. Chem. Soc. 2011, 133, 15854–15857. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bhoyate, S.; Hyatt, M.; Neria, B.L.; Siam, K.; Kahol, P.; Ghimire, M.; Mishra, S.; Perez, F.; Gupta, R.K. Nitrogen-doped flexible carbon cloth for durable metal free electrocatalyst for overall water splitting. Surf. Coat. Technol. 2018, 347, 407–413. [Google Scholar] [CrossRef]
- Tang, D.; Liu, J.; Wu, X.; Liu, R.; Han, X.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon Quantum Dot/NiFe Layered Double-Hydroxide Composite as a Highly Efficient Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 7918–7925. [Google Scholar] [CrossRef] [PubMed]
- May, K.J.; Perry, E.E.; Lee, Y.; Suntivich, J.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO 2 and RuO 2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Zeng, Z.; Shi, W.; Zhu, Y.; Yan, Q.; Liu, H.; Wang, J.; Zhang, H. One-step synthesis of Ni3S2nanorod@Ni(OH)2 nanosheet core–shell nanostructures on a three-dimensional graphene network for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2216–2221. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Xu, J.; Cheng, G.; Yang, W.; Kou, T.; Zhang, Z.; Ding, Y. 3D binder-free Cu2O@Cu nanoneedle arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 18229–18235. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, K.; Zou, R.; Hu, J. A Hybrid Electrode of Co3O4@PPy Core/Shell Nanosheet Arrays for High-Performance Supercapacitors. Nano-Micro Lett. 2016, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of High-Capacitance 3D CoO@Polypyrrole Nanowire Array Electrode for Aqueous Asymmetric Supercapacitor. Nano Lett. 2013, 13, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S.; Grosvenor, A. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, G.; Kartio, I.; Hirsch, D.; Kunze, S.; Szargan, R. Oxidation of Galena in Acetate Buffer Investigated by Atomic Force Microscopy and Photoelectron Spectroscopy. Langmuir 1996, 12, 5709–5721. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Buckley, A.; Woods, R. X-ray photoelectron spectroscopy of oxidized pyrrhotite surfaces. Appl. Surf. Sci. 1985, 22, 280–287. [Google Scholar] [CrossRef]
- Buckley, A.; Woods, R. X-ray photoelectron spectroscopy of oxidised pyrrhotite surfaces. Appl. Surf. Sci. 1985, 20, 472–480. [Google Scholar] [CrossRef]
- Piontek, S.; Andronescu, C.; Zaichenko, A.; Konkena, B.; Puring, K.j.; Marler, B.; Antoni, H.; Sinev, I.; Muhler, M.; Mollenhauer, D.; et al. Influence of the Fe:Ni Ratio and Reaction Temperature on the Efficiency of (FexNi1−x)9S8 Electrocatalysts Applied in the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 987–996. [Google Scholar] [CrossRef]
- Shalvoy, R.B.; Reucroft, P.J. Characterization of a sulfur-resistant methanation catalyst by XPS. J. Vac. Sci. Technol. 1979, 16, 567–569. [Google Scholar] [CrossRef]
- Dickinson, T.; Povey, A.F.; Sherwood, P.M.A. Dissolution and passivation of nickel. An X-ray photoelectron spectroscopic study. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 327. [Google Scholar] [CrossRef]
- Van Der Heide, H.; Hemmel, R.; Van Bruggen, C.; Haas, C. X-ray photoelectron spectra of 3d transition metal pyrites. J. Solid State Chem. 1980, 33, 17–25. [Google Scholar] [CrossRef]
- Seah, M.P. An accurate and simple universal curve for the energy-dependent electron inelastic mean free path. Surf. Interface Anal. 2012, 44, 497–503. [Google Scholar] [CrossRef]
- Sultana, U.K.; He, T.; Du, A.; O’Mullane, A.P. An amorphous dual action electrocatalyst based on oxygen doped cobalt sulfide for the hydrogen and oxygen evolution reactions. RSC Adv. 2017, 7, 54995–55004. [Google Scholar] [CrossRef]
- Jin, C.; Lu, F.; Cao, X.; Yang, Z.; Yang, R. Facile synthesis and excellent electrochemical properties of NiCo2O4 spinel nanowire arrays as a bifunctional catalyst for the oxygen reduction and evolution reaction. J. Mater. Chem. A 2013, 1, 12170. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Ghodake, G.S.; Maile, N.C.; Kadam, A.A.; Lee, D.S.; Fulari, V.J.; Shinde, S.K. Chemical synthesis of hierarchical NiCo2S4 nanosheets like nanostructure on flexible foil for a high performance supercapacitor. Sci. Rep. 2017, 7, 9764. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel-Cobalt Layered Double Hydroxide Nanosheets for High-performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2014, 24, 934. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Qiao, L.; Zhou, M.; Yang, Y.; Xiao, P.; Zhang, Y. Ni–Co sulfide nanowires on nickel foam with ultrahigh capacitance for asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 6540–6548. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhang, C.; Bhoyate, S.; Kahol, P.K.; Kostoglou, N.; Mitterer, C.; Hinder, S.; Baker, M.; Constantinides, G.; Polychronopoulou, K.; et al. Nanostructured Fe-Ni Sulfide: A Multifunctional Material for Energy Generation and Storage. Catalysts 2019, 9, 597. https://doi.org/10.3390/catal9070597
Zhao C, Zhang C, Bhoyate S, Kahol PK, Kostoglou N, Mitterer C, Hinder S, Baker M, Constantinides G, Polychronopoulou K, et al. Nanostructured Fe-Ni Sulfide: A Multifunctional Material for Energy Generation and Storage. Catalysts. 2019; 9(7):597. https://doi.org/10.3390/catal9070597
Chicago/Turabian StyleZhao, Chen, Chunyang Zhang, Sanket Bhoyate, Pawan K. Kahol, Nikolaos Kostoglou, Christian Mitterer, Steve Hinder, Mark Baker, Georgios Constantinides, Kyriaki Polychronopoulou, and et al. 2019. "Nanostructured Fe-Ni Sulfide: A Multifunctional Material for Energy Generation and Storage" Catalysts 9, no. 7: 597. https://doi.org/10.3390/catal9070597
APA StyleZhao, C., Zhang, C., Bhoyate, S., Kahol, P. K., Kostoglou, N., Mitterer, C., Hinder, S., Baker, M., Constantinides, G., Polychronopoulou, K., Rebholz, C., & Gupta, R. K. (2019). Nanostructured Fe-Ni Sulfide: A Multifunctional Material for Energy Generation and Storage. Catalysts, 9(7), 597. https://doi.org/10.3390/catal9070597








