An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye
Abstract
1. Introduction
2. Results
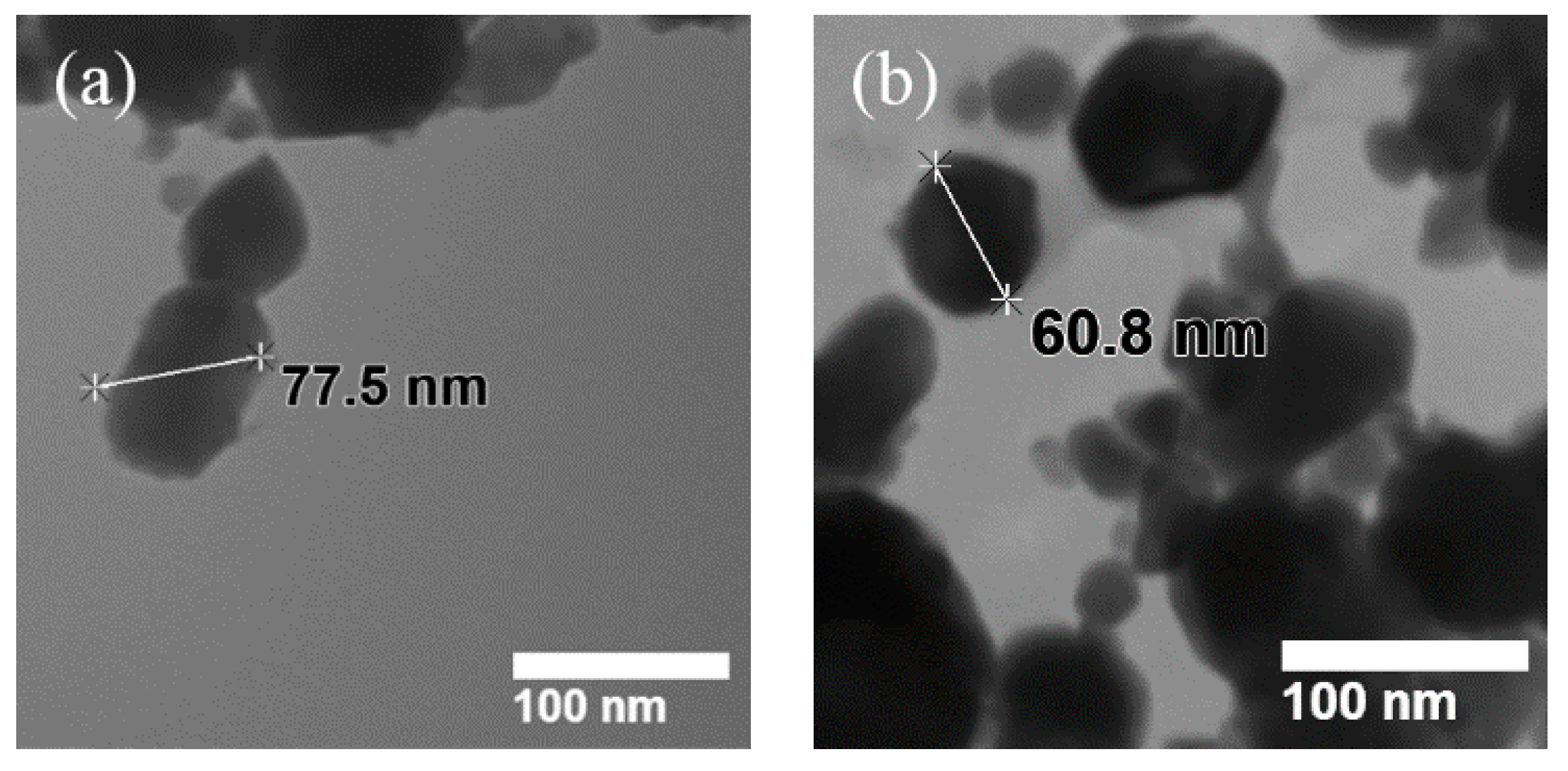
2.1. Morphological and Microstructural Analysis
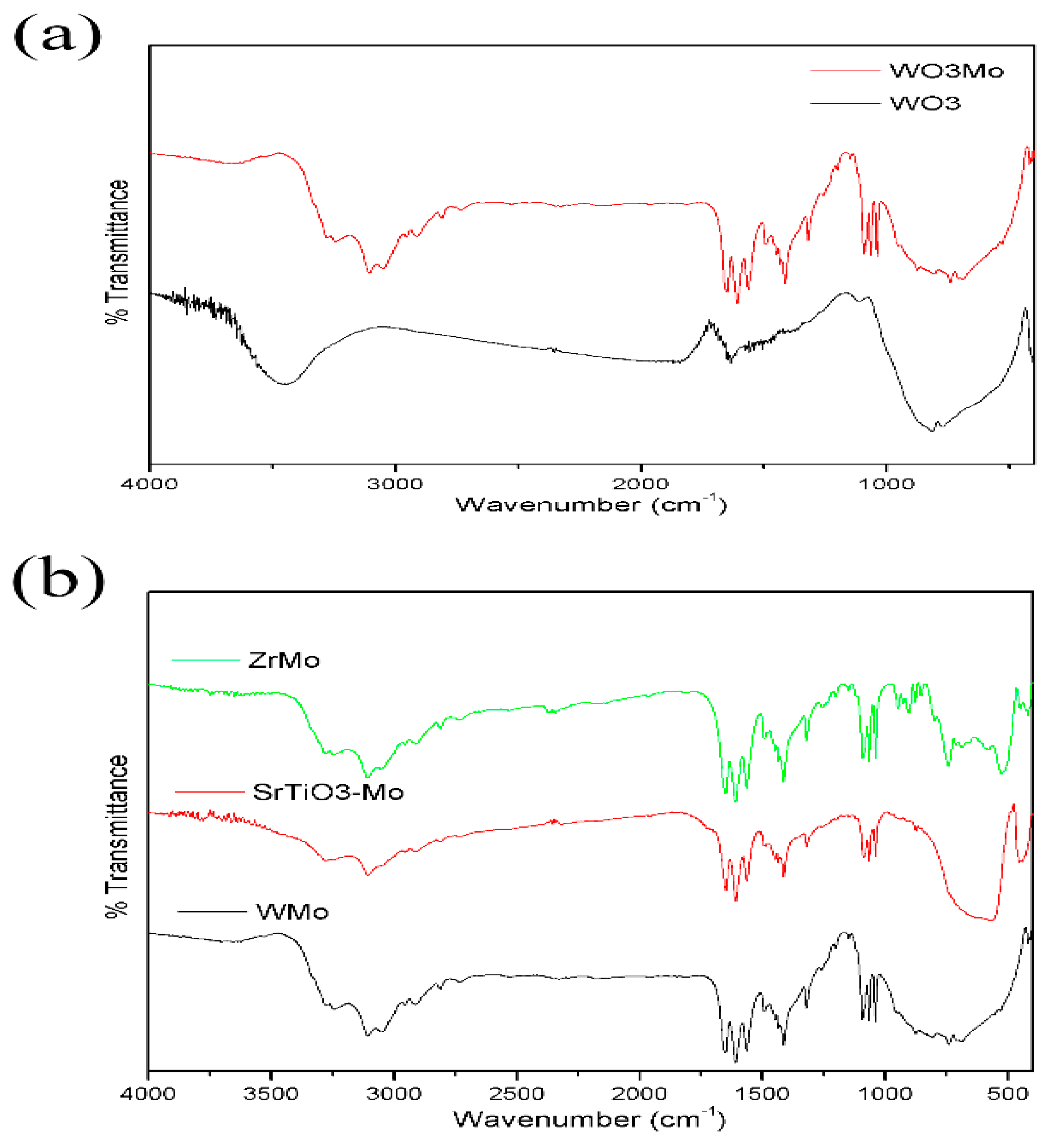
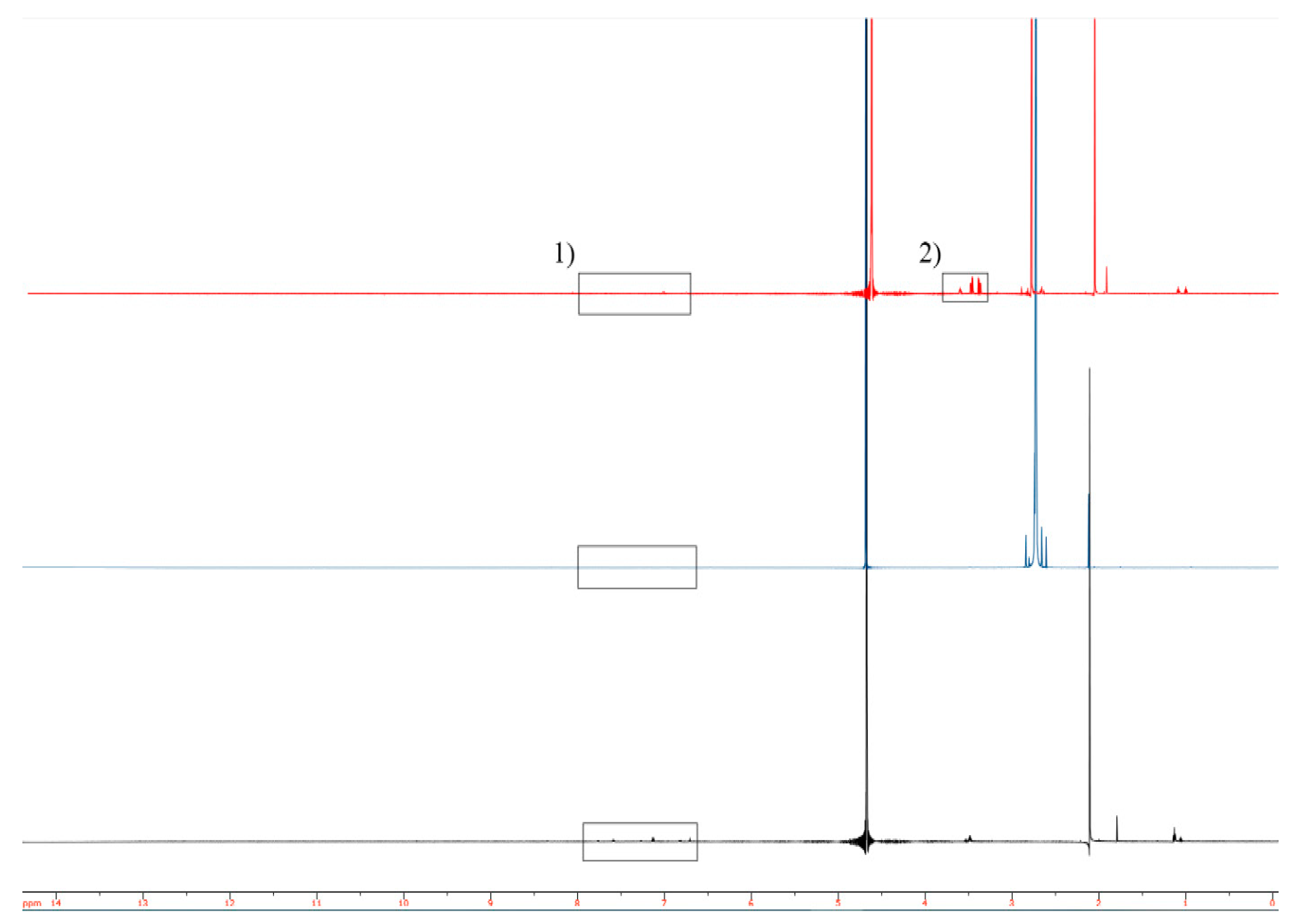
2.2. Structural Analysis
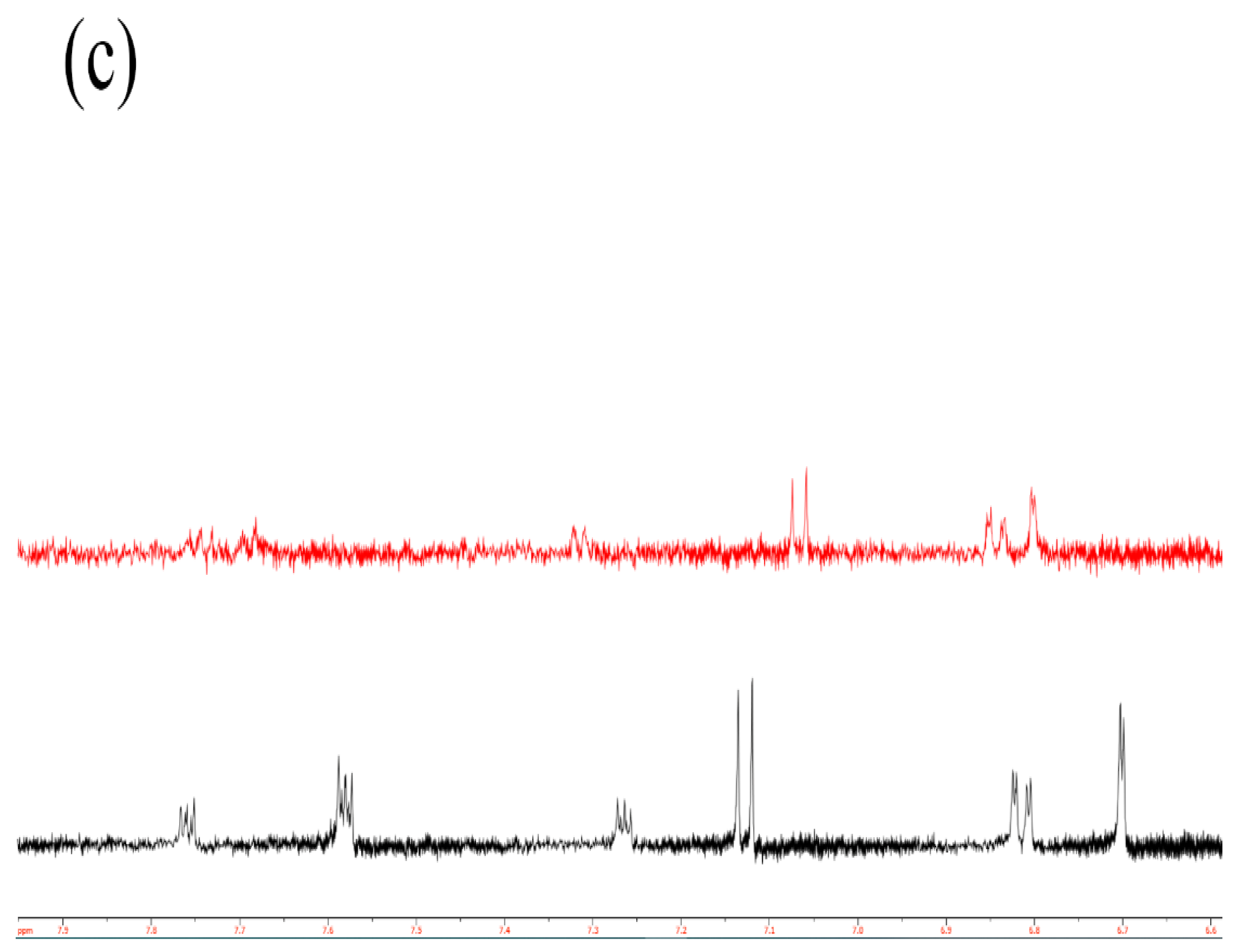
2.3. Catalytic Study
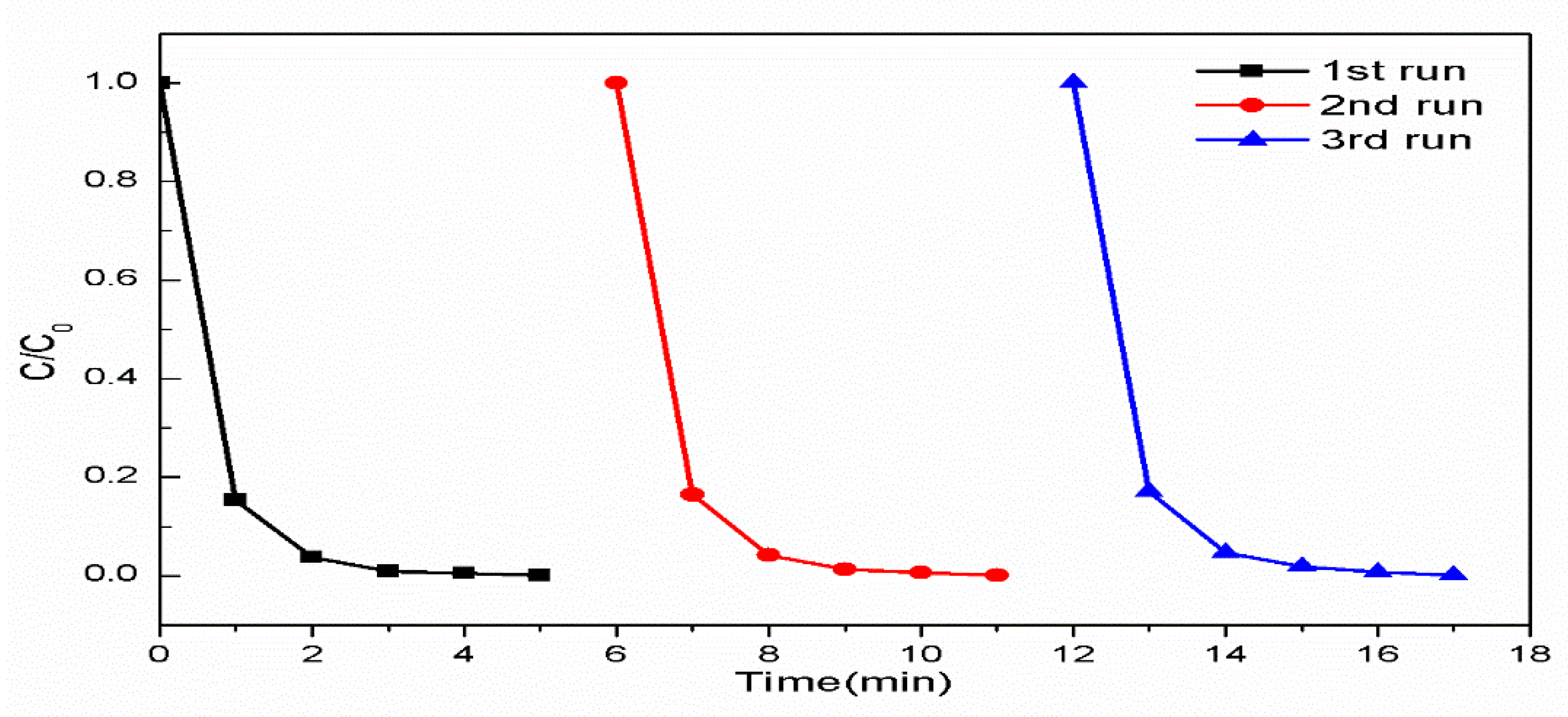
2.4. Reusability of WO3/MoCl5
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Molybdenum(V) Chloride-Tungsten Oxide Complex
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benbachir, H.; Gaffour, H.; Mokhtari, M. Photodegradation of 2, 4, 6-trichlorophenol using natural hematite modified with chloride of zirconium oxide. React. Kinet. Mech. Catal. Lett. 2017, 122, 635–653. [Google Scholar] [CrossRef]
- Zaied, M.; Chutet, E.; Peulon, S.; Bellakhal, N.; Desmazières, B.; Dachraoui, M.; Chaussé, A. Spontaneous oxidative degradation of indigo carmine by thin films of birnessite electrodeposited onto SnO2. App. Catal. B Environ. 2011, 107, 42–51. [Google Scholar] [CrossRef]
- Oeztuerk, A.; Abdullah, M.J. Toxicological effect of indole and its azo dye derivatives on some microorganisms under aerobic conditions. Sci. Total Environ. 2006, 358, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tünay, O.; Kabdasli, I.; Ermektar, G.; Orhon, D. Color removal from textile wastewaters. Water Sci. Technol. 1996, 34, 9–16. [Google Scholar] [CrossRef]
- Carmine, L.; Andrea, A.; Katia, D.C.; Bianca, D.; Nadia, G.; Giancarlo, C.; Simelys, H.; Valentina, C. Sonophotocalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro and nanoparticles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef]
- Rochat, J.; Demenge, P.; Rerat, J.C. Toxicologic study of a fluorescent tracer: Rhodamine B. Toxical. Eur. Res. 1978, 1, 23–26. [Google Scholar]
- IARC. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1–42; WHO: Lyon, France, 1987. [Google Scholar]
- Shimida, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengrich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens, toxic chemicals; studies with liver microsomes of 30 Japanese, 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid. Interf. Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev. Environ. Contam. Toxicol. 2015, 237, 71–104. [Google Scholar]
- Au, W.; Pathak, S.; Collie, C.J.; Hsu, T.C. Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutat. Res. Genet. Toxicol. 1978, 58, 269–276. [Google Scholar] [CrossRef]
- Fernanda, R.G.; Silvia, N.J.M.; Wanderley, D.S.; Fernando, S.C.R.D. The mitochondrion of Trypanosoma cruzi is a target of crystal violet toxicity. Mol. Biochem. Parasitol. 1989, 34, 117–126. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Zaied, M.; Peulon, S.; Bellakhal, N.; Desmazières, B.; Chaussé, A. Studies of N-demethylation oxidative and degradation of methylene blue by thin layers of birnessite electrodeposited onto SnO2. Appl. Catal. B Environ. 2011, 101, 441–450. [Google Scholar] [CrossRef]
- Awad, H.S.; Galwa, N.A. Electrochemical degradation of Acid Blue and Basic Brown dyes on Pb/PbO2 electrode in the presence of different conductive electrolyte and effect of various operating factors. Chemosphere 2005, 61, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Lente, G.; Espenson, J.H. Oxidation of 2, 4, 6-trichlorophenol by hydrogen peroxide. Comparison of different iron-based catalysts. Green Chem. 2005, 7, 28–34. [Google Scholar] [CrossRef]
- Sarma, H.; Bhattacharyya, K.G. Catalytic wet oxidation of 2, 4, 6-trichlorophenol with Fe(III) supported ZSM5. Int. J. Interdiscip. Multidiscip. Stud. 2014, 2, 11–16. [Google Scholar]
- Rengaraj, S.; Li, X.Z. Enhanced photocatalytic activity of TiO2 by doping with Ag for degradation of 2, 4, 6-trichlorophenol in aqueous suspension. J. Mol. Catal. A Chem. 2006, 243, 60–67. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Yang, X.; Tian, P.; Zhang, C.; Deng, Y.; Xu, J.; Gong, J.; Han, Y. Au/carbon as Fenton-like catalysts for the oxidative degradation of bisphenol A. Appl. Catal. B Environ. 2013, 134, 145–152. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, D.; Hu, C.; Shi, Y. Framework Cu-doped AlPO4 as an effective Fenton-like catalyst for bisphenol A degradation. Appl. Catal. B Environ. 2017, 207, 9–16. [Google Scholar] [CrossRef]
- Zbiljic, J.; Vajdle, O.; Guzsvany, V.; Molnar, J.; Agbaba, J.; Dalmacija, B.; Kalcher, K. Hydrodynamic chronoamperometric method for the determination of H2O2 using MnO2-based carbon paste electrodes in groundwater treated by Fenton and Fenton-like reagents for natural organic matter removal. J. Hazard. Mater. 2015, 283, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Yang, B.; Liu, C.; Xu, F.; Xi, X.; Hou, G.; Shao, R. Highly enhanced photocatalytic activity of WO3 thin films loaded with Pt-Ag bimetallic alloy nanoparticles. RSC Adv. 2017, 7, 947–956. [Google Scholar] [CrossRef]
- Jan’aky, C.; Rajeshwar, K.; De Tacconi, N.; Chanmanee, W.; Huda, M. Tungsten-based oxide semiconductors for solar hydrogen generation. Catal. Today 2013, 199, 53–64. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Arakawa, H. The visible light induced photocatalytic activity of tungsten trioxide powders. Appl. Catal. A Gen. 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Sayama, K.; Hayashi, H.; Arai, T.; Yanagida, M.; Gunji, T.; Sugihara, H. Highly active WO3 semiconductor photocatalyst prepared from amorphous peroxo-tungstic acid for the degradation of various organic compounds. Appl. Catal. B Environ. 2010, 94, 150–157. [Google Scholar] [CrossRef]
- Kristina, H.; Gregor, S.; Siegfried, R.W. Novel Domino Oxidative Coupling: C–C Bond Formation Sequence to Highly Functionalized Dibenzo[a,c]cycloheptenes. Org. Lett. 2011, 13, 916–919. [Google Scholar]
- Trosien, S.; Bottger, P.; Waldbogel, S.R. Versatile oxidative approach to carbazoles and related compounds using MoCl5. Org. Lett. 2014, 16, 402–405. [Google Scholar] [CrossRef]
- Tian, W.; Liao, H.; Wu, J.-G.; Yang, G.-D. Study on the coordination of the hydroxyl group: Crystal structure and FT-IR spectra of potassium hydrogen galactarate. Polyhedron 1997, 16, 2055–2058. [Google Scholar] [CrossRef]
- Kee, C.W. Assignment of O–O and Mo=O Stretching Frequencies of Molybdenum/Tungsten Complexes Revisited. J. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Booji, A.S. Infrared Spectra of NbCl5 and MoCl5 in the gas phase. Vib. Spectrosc. 1994, 6, 345–349. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Kats, E. Enhanced electrical properties in sub-10-nm WO3 nanoflakes prepared via a two-step sol-gel-exfoliation method. Nanoscale Res. Lett. 2014, 9, 401–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of the high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Wilke, K.; Breuer, H.D. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A Chem. 1999, 121, 49–53. [Google Scholar] [CrossRef]
- Wei, B.X.; Zhao, L.; Wang, T.J.; Gao, H.; Wu, H.X.; Jin, Y. Photo-stability of TiO2 particles coated with several transition metal oxides and its measurement by rhodamine-B degradation. Adv. Powder Technol. 2013, 24, 708–713. [Google Scholar] [CrossRef]
- Sahoo, C.; Gupta, A.K.; Pal, A. Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO2. Dyes Pigments 2005, 66, 189–196. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Nazim, M.; Shin, H.S. Rapid photocatalytic degradation of crystal violet dye over ZnO flower nanomaterials. Mater. Lett. 2013, 96, 228–232. [Google Scholar] [CrossRef]
- Sajid, M.M.; Khan, S.B.; Shad, N.A.; Amin, N.; Zhang, Z. Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using BiVO4/FeVO4 heterojunction composite. RSC Adv. 2018, 42, 23489–23498. [Google Scholar] [CrossRef]
- Claudia, C.; Hua, X.; Federica, B.; Cinzia, B.; Teresa, S.; Elvio, C.; Valeria, V.; Giuseppe, G.; Giuseppe, C. Controllable One-Pot Synthesis of Anatase TiO2 Nanorods with the Microwave-Solvothermal Method. Sci. Adv. Mater. 2014, 6, 1668–1675. [Google Scholar]
- Catala, M.; Dominguez-Morueco, N.; Migens, A.; Molina, R.; Martinez, F.; Valcarcel, Y.; Mastroianni, N.; Lopez de Alda, M.; Barcelo, D.; Segura, Y. Elimination of drugs of abuse and their toxicity from natural waters by photo-Fenton treatment. Sci. Total Environm. 2015, 520, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Zhang, G.; Zhang, N.; Liu, Y.; Zhu, J.; Huang, T.; Fang, S. Preparation of FeCeOx by ultrasonic impregnation method for heterogeneous Fenton degradation of diclofenac. Ultrason. Sonochem. 2016, 32, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Jaafarzadeh, N.; Kakavandi, B.; Takdastan, A.; Kalantary, R.R.; Azizi, M.; Jorfi, S. Powder activated carbon/Fe3O4 hybrid composite as a highly efficient heterogeneous catalyst for Fenton oxidation of tetracycline: Degradation mechanism and kinetic. RSC Adv. 2015, 5, 84718–84728. [Google Scholar] [CrossRef]
- Arzate-Salgado, S.-Y.; Morales-Perez, A.-A.; Solis-Lopez, M.; Ramirez-Zamora, R.-M. Evaluation of metallurgical slag as a Fenton-type photocatalyst for the degradation of an emerging pollutant: Diclofenac. Catal. Today 2016, 266, 126–135. [Google Scholar] [CrossRef]
- Bobu, M.; Yediler, A.; Siminiceanu, I.; Schulte-Hostede, S. Degradation studies of ciprofloxacin on a pillared iron catalyst. Appl. Catal. B Environ. 2008, 83, 15–23. [Google Scholar] [CrossRef]
- Qamar, M.; Gondal, M.A.; Hayat, K.; Yamani, Z.H.; Al-Hooshani, K. Laser-induced removal of a dye C.I. Acid Red 87 using n-type WO3 semiconductor catalyst. J. Hazard. Mater. 2009, 170, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Gondal, M.A.; Khaled, M.M.; Yamani, Z.H. Laser induced photocatalytic degradation of hazardous dye (Safranin-O) using self synthesized nanocrystallne WO3. J. Hazard. Mater. 2011, 186, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Goldup, S.M.; Dunn, S. Photodegradation of Rhodamine B over Ag modified ferroelectric BaTiO3 under simulated solar light: Pathways and mechanism. RSC Adv. 2015, 5, 30372–30379. [Google Scholar] [CrossRef]
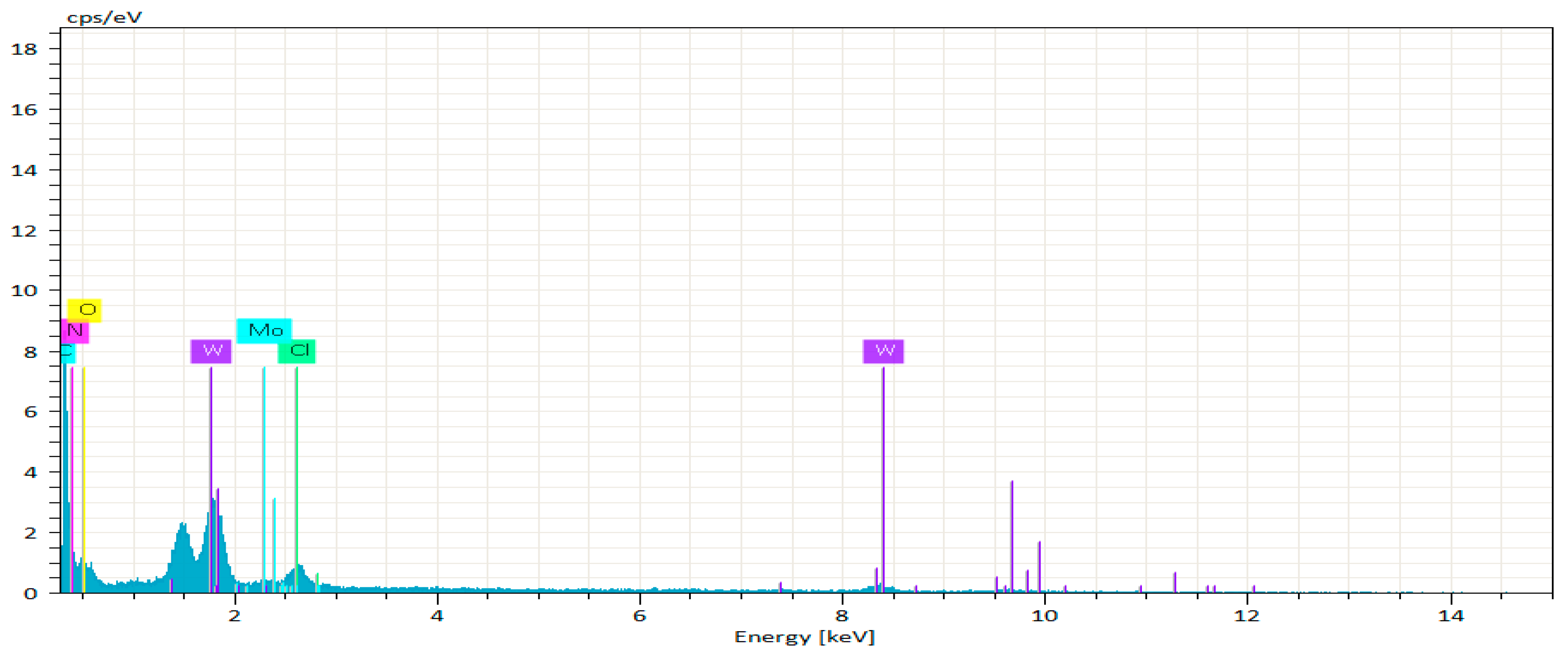




| Element | Atom No. | Mass Norm [%] | Atom [%] | Abs. Error [%] |
|---|---|---|---|---|
| Nitrogen | 7 | 52.3 | 72.17 | 19.38 |
| Chlorine | 17 | 3.97 | 2.16 | 0.52 |
| Oxygen | 8 | 18.97 | 22.92 | 8.62 |
| Carbon | 6 | 0 | 0 | 0 |
| Tungsten | 74 | 23.24 | 2.45 | 3.1 |
| Molybdenum | 42 | 1.52 | 0.31 | 0.31 |
| Dye | CV 30 | CV 50 | RB 30 | RB 50 | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | k [sec−1] | R2 | k [sec−1] | R2 | k [sec−1] | R2 | k [sec−1] | |
| Normal Condition | 0.986 | 1.744 | 0.990 | 1.178 | 0.737 | 1.08 | 0.898 | 0.928 |
| Dark Condition | 0.961 | 1.378 | 0.984 | 1.363 | 0.932 | 1.218 | 0.986 | 0.884 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, G.; Bae, H.; Kim, E.; Moon, B.; Cheon, D.; Tarte, N.H. An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye. Catalysts 2019, 9, 642. https://doi.org/10.3390/catal9080642
Kim D, Kim G, Bae H, Kim E, Moon B, Cheon D, Tarte NH. An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye. Catalysts. 2019; 9(8):642. https://doi.org/10.3390/catal9080642
Chicago/Turabian StyleKim, Dongin, Geonwoo Kim, Hyeonbin Bae, Eunwoo Kim, Byunghyun Moon, Daho Cheon, and Naresh Hiralal Tarte. 2019. "An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye" Catalysts 9, no. 8: 642. https://doi.org/10.3390/catal9080642
APA StyleKim, D., Kim, G., Bae, H., Kim, E., Moon, B., Cheon, D., & Tarte, N. H. (2019). An External Energy Independent WO3/MoCl5 Nano-Sized Catalyst for the Superior Degradation of Crystal Violet and Rhodamine B Dye. Catalysts, 9(8), 642. https://doi.org/10.3390/catal9080642




