Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation
Abstract
1. Introduction
2. Results and Discussion
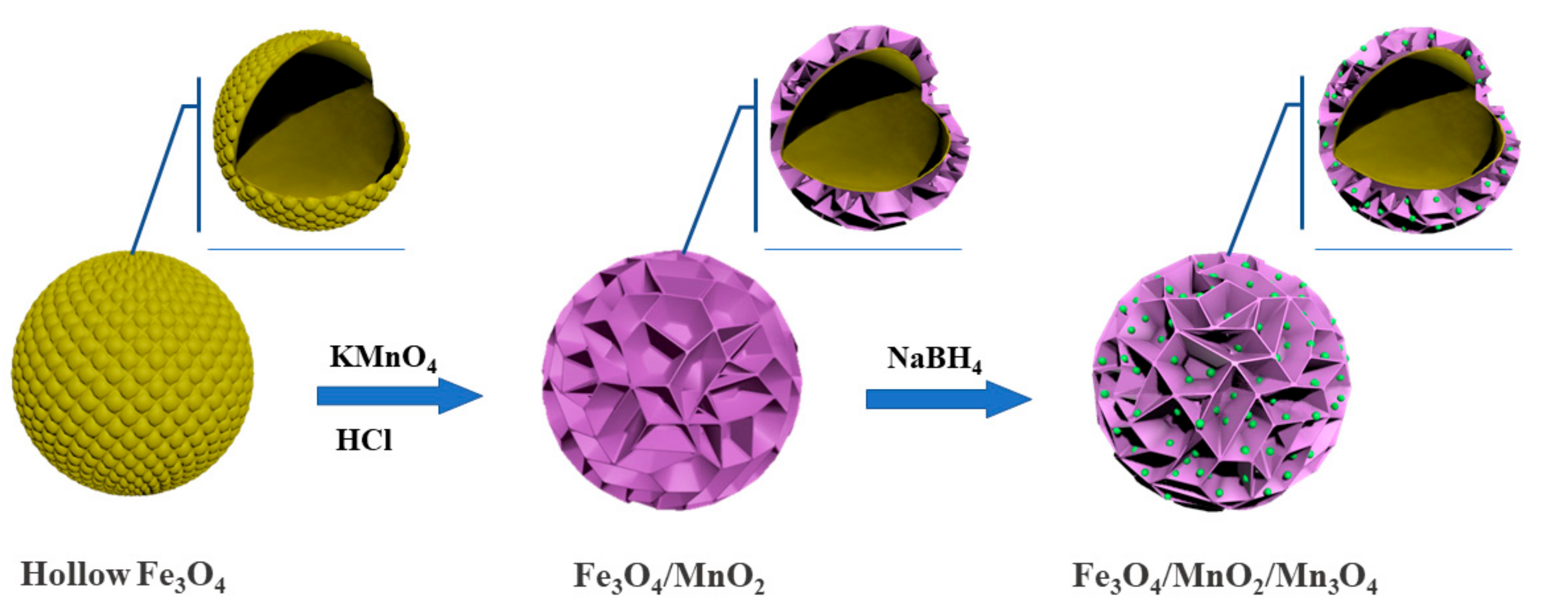
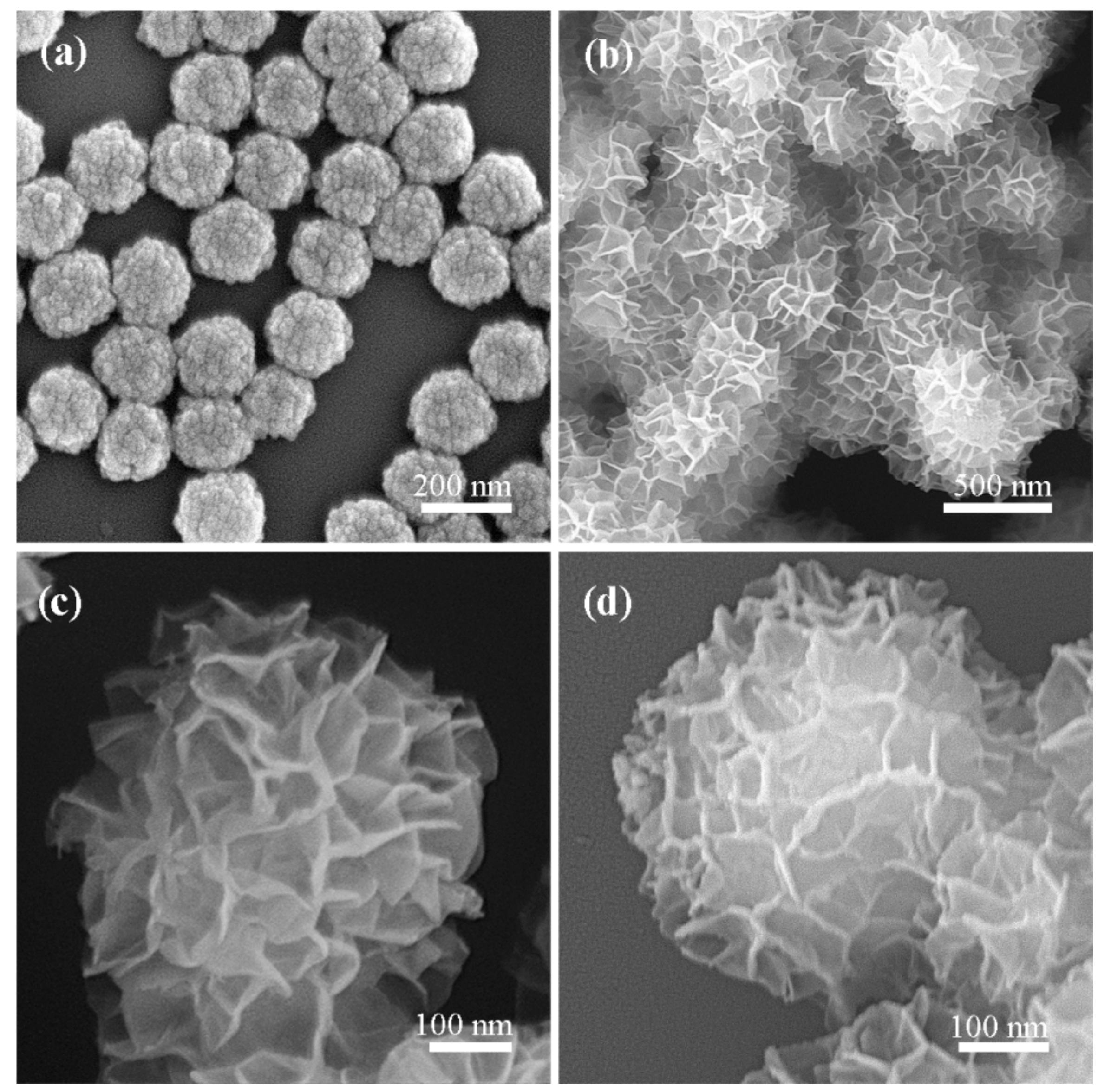
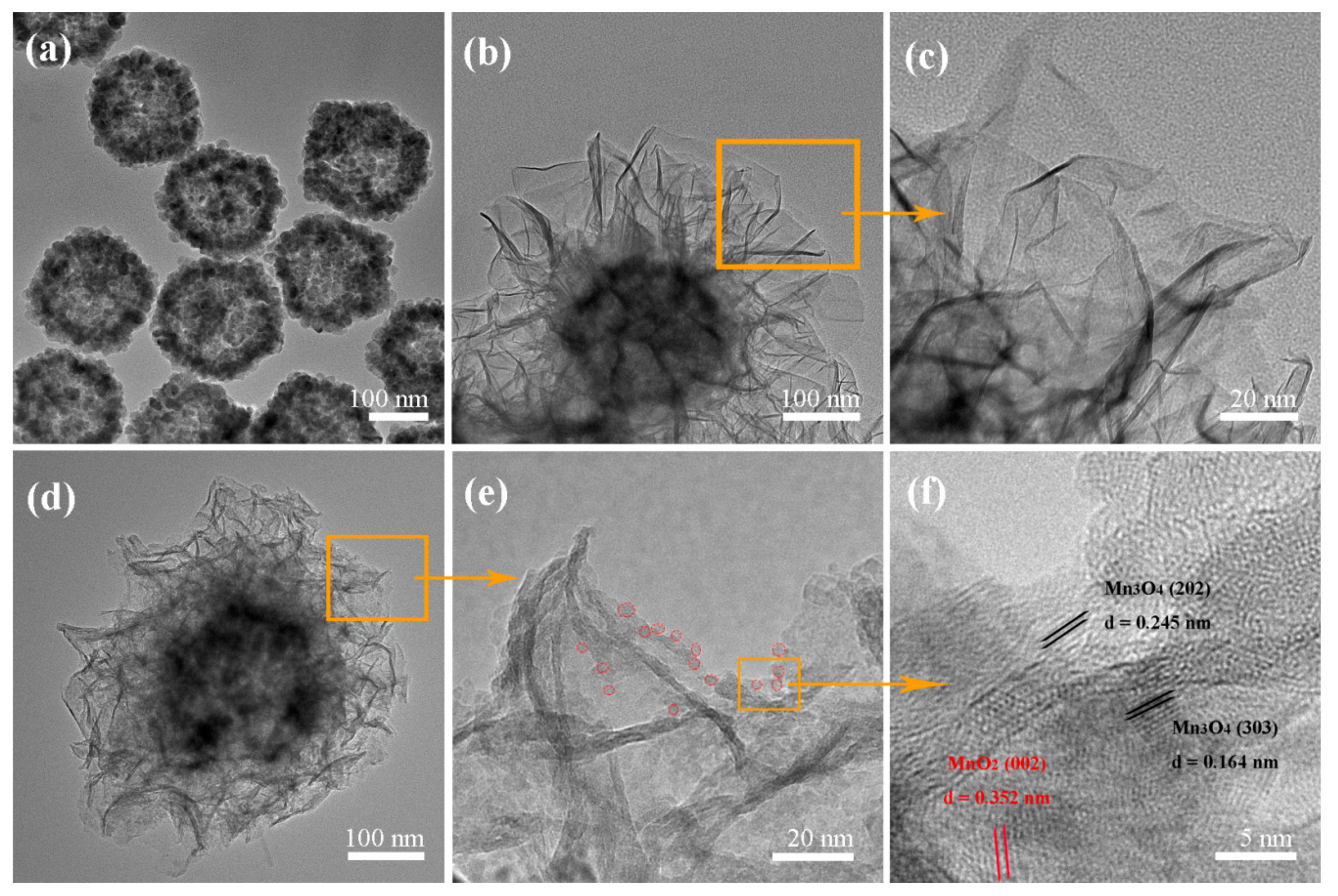
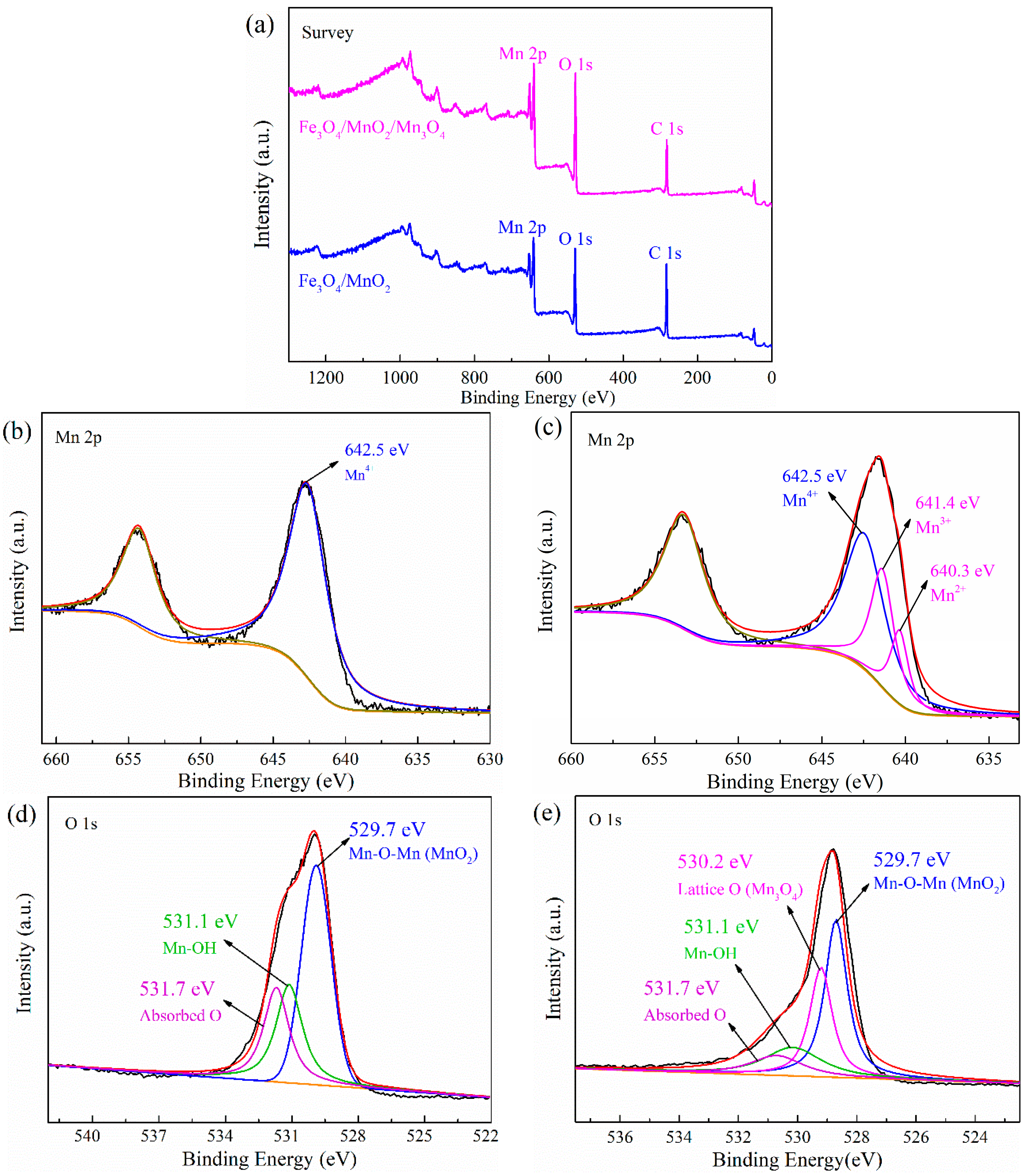
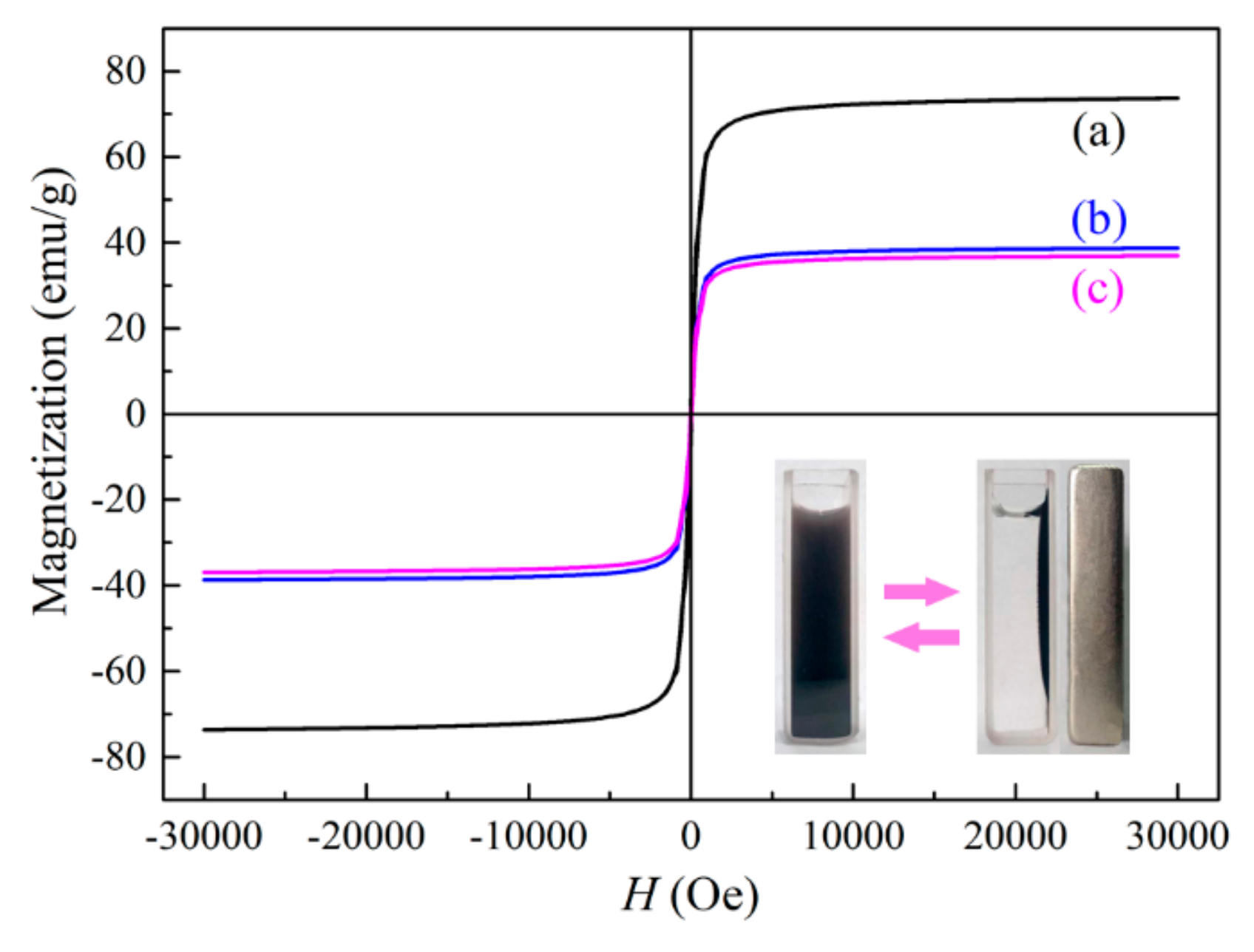
2.1. Characterization of the Photocatalyst
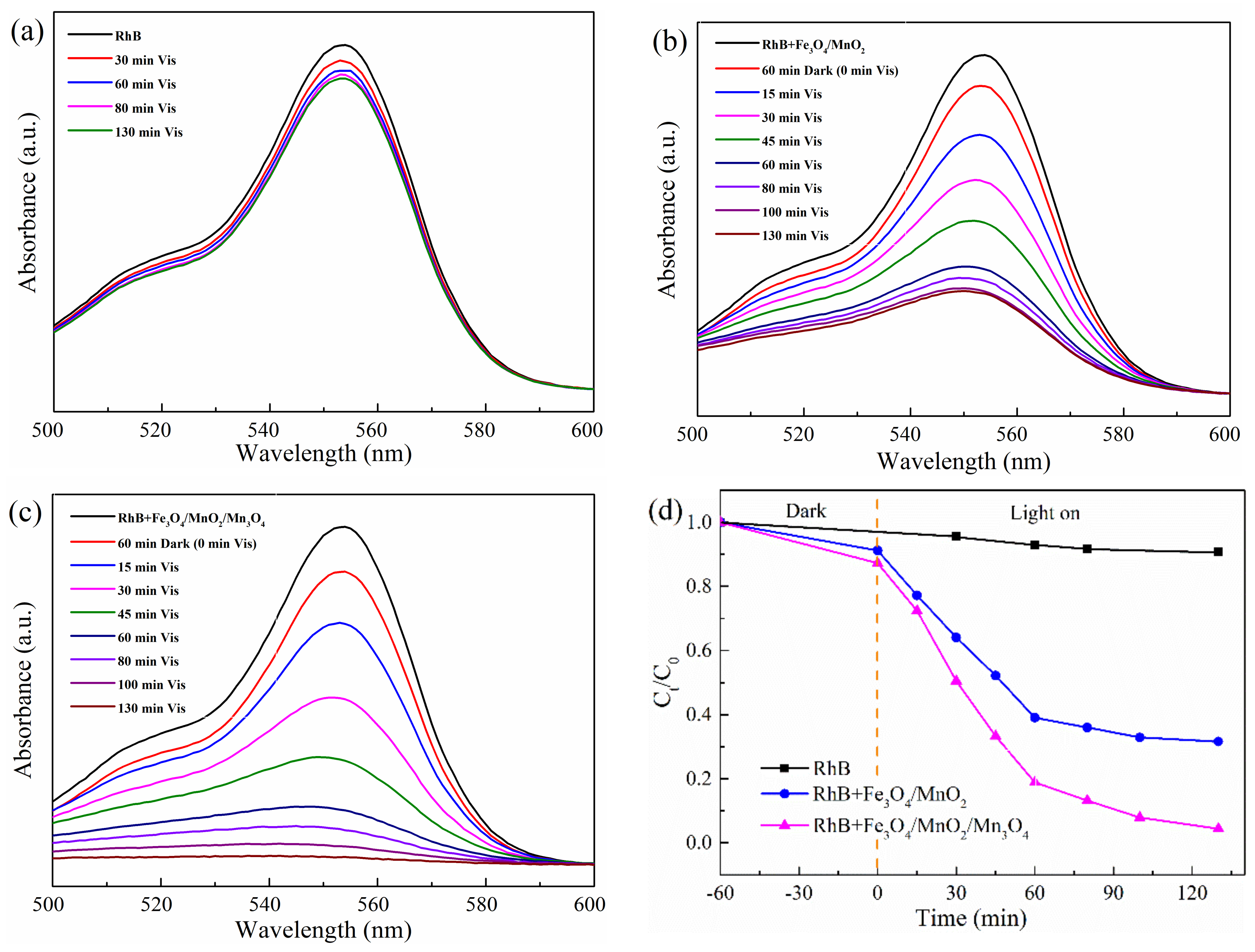
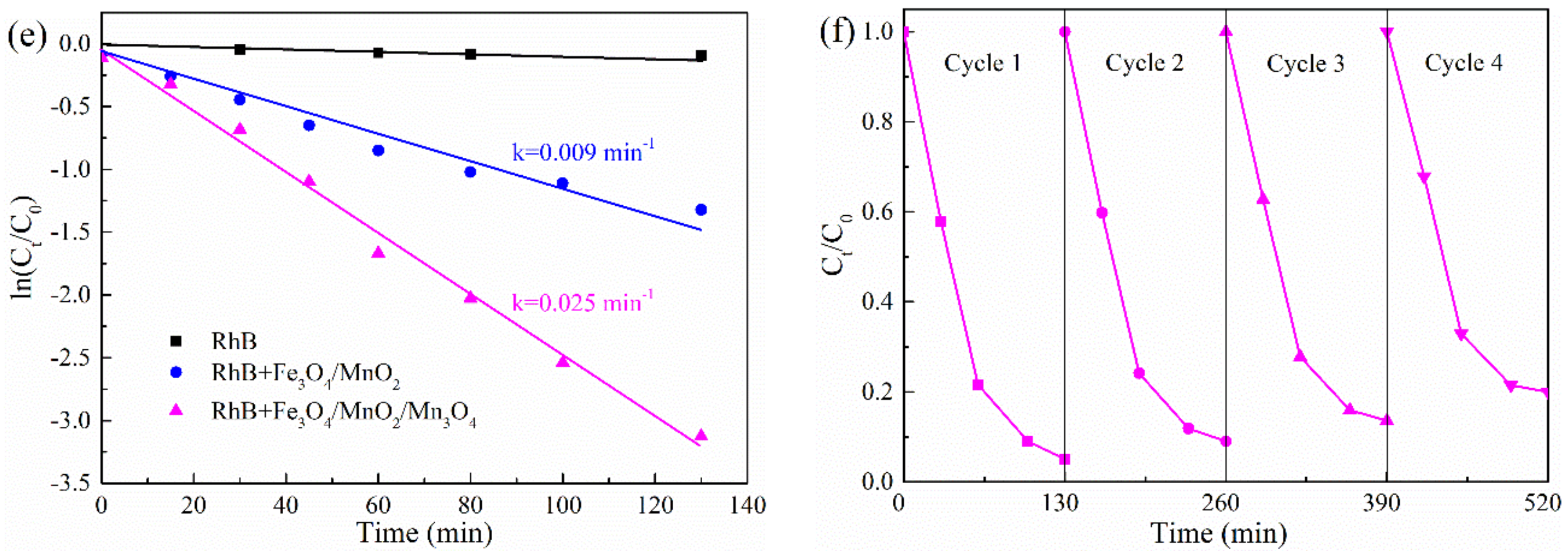
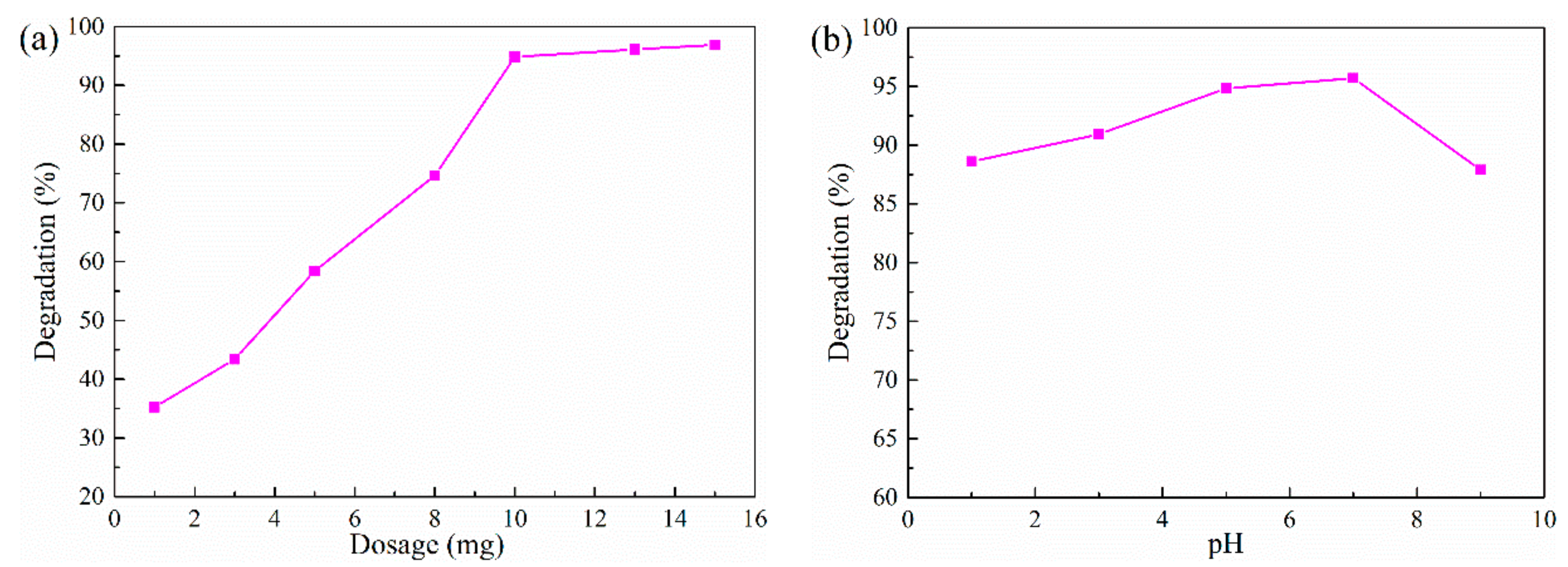
2.2. Photocatalytic Tests
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Flower-Like Fe3O4/MnO2 Microspheres
3.3. Synthesis of Flower-Like Fe3O4/MnO2/Mn3O4 Microspheres
3.4. Photocatalytic Tests
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, R.; Jiao, T.; Li, R.; Chen, Y.; Guo, W.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Kotsedi, L.; Simo, A.; Fuku, X.; Mola, G.T.; Kennedy, J.; Maaza, M. Photocatalytic activity of ZrO 2 doped lead dioxide nanocomposites: Investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 2017, 421, 234–239. [Google Scholar] [CrossRef]
- Dong, W.-H.; Wu, D.-D.; Luo, J.-M.; Xing, Q.-J.; Liu, H.; Zou, J.-P.; Luo, X.-B.; Min, X.-B.; Liu, H.-L.; Luo, S.-L.; et al. Coupling of photodegradation of RhB with photoreduction of CO2 over rGO/SrTi0.95Fe0.05O3-delta catalyst: A strategy for one-pot conversion of organic pollutants to methanol and ethanol. J. Catal. 2017, 349, 218–225. [Google Scholar] [CrossRef]
- Chitiphon, C.; Radheshyam, P.; Keiko, S. Dye-sensitized photocatalyst of sepiolite for organic dye degradation. Catalysts 2019, 3, 235. [Google Scholar]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, J.; Wang, J.; Huyan, Y.; Zhang, H.; Zhang, Q. Flowerlike BSA/Zn3(PO4)2/Fe3O4 magnetic hybrid particles: preparation and application to adsorption of copperions. J. Chem. Eng. Data 2018, 63, 3913–3922. [Google Scholar] [CrossRef]
- Zhang, B.; Huyan, Y.; Wang, J.; Chen, X.; Zhang, H.; Zhang, Q. Fe 3 O 4 @SiO 2 @CCS porous magnetic microspheres as adsorbent for removal of organic dyes in aqueous phase. J. Alloy. Compd. 2018, 735, 1986–1996. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, J.; Jiao, Y. Preparation and application of water-soluble TiO2-ionic liquids hybrid nanomaterials. J. Inorg. Mater. 2018, 33, 577–581. [Google Scholar]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Lv, K.; Li, M. Heterojunction construction between TiO2 hollowsphere and ZnIn2S4 flower for photocatalysis application. Appl. Surf. Sci. 2017, 398, 81–88. [Google Scholar] [CrossRef]
- Samuel Osei-Bonsu, O.; Francis, O.; Govender, P.P. Tuning the electronic and structural properties of Gd-TiO2-GO nanocomposites for enhancing photodegradation of IC dye: The role of Gd3+ ion. Appl. Catal. B: Environ. 2019, 243, 106–120. [Google Scholar]
- Xu, Y.; Li, A.; Yao, T.; Ma, C.; Zhang, X.; Shah, J.H.; Han, H. Strategies for Efficient Charge Separation and Transfer in Artificial Photosynthesis of Solar Fuels. ChemSusChem 2017, 10, 4277–4305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, C.; Zhang, H.; Qiao, M.; Chen, Y.; Zhang, H.; Zhang, Q.; Guo, Z. Morphology-dependent electrochemical supercapacitors in multi-dimensional polyaniline nanostructures. J. Mater. Chem. A 2017, 5, 14041–14052. [Google Scholar] [CrossRef]
- Xiong, X.; Ji, Y.; Xie, M.; You, C.; Yang, L.; Liu, Z.; Asiri, A.M.; Sun, X. MnO2-CoP3 nanowires array: An efficient electrocatalyst for alkaline oxygen evolution reaction with enhanced activity. Electrochem. Commun. 2018, 86, 161–165. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over alpha-MnO2, beta-MnO2 and gamma-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Jiang, C.; Ge, Y.; Chen, W.; Hua, L.; Li, H.; Zhang, Y.; Cao, S. Hierarchically-structured TiO2/MnO2 hollow spheres exhibiting the complete mineralization of phenol. Catalysts 2019, 9, 13. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, W.J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced alpha-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Tan, X.; Wan, Y.; Huang, Y.; He, C.; Zhang, Z.; He, Z.; Hu, L.; Zeng, J.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Meng, X.; Tang, X.; Ji, P. Core-Shell MnO2-SiO2 Nanorods for Catalyzing the Removal of Dyes from Water. Catalysts 2017, 7, 19. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Network Structured SnO2/ZnO Heterojunction Nanocatalyst with High Photocatalytic Activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.-H.; Zhang, C.; Shiu, H.-W.; Chuu, C.-P.; Chen, C.-H.; Chang, C.-Y.S.; Chen, C.-H.; Chou, M.-Y.; Shih, C.-K.; Li, L.-J. Determination of band alignment in the single-layer MoS2/WSe2 heterojunction. Nat. Commun. 2015, 6, 7666. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C 3 N 4 /g-C 3 N 4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p-n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, C.; Zhang, H.; Zhang, Q.; Liu, H.; Wu, S.; Guo, Z. Three-dimensional core-shell Fe3O4/Polyaniline coaxial heterogeneous nanonets: Preparation and high performance supercapacitor electrodes. Electrochimica Acta 2019, 315, 114–123. [Google Scholar] [CrossRef]
- Ma, Y.M.; Yang, R.; Feng, L.; Jia, G.; Chen, W.; Li, P.L. Preparation and characterization of magnetic hollow Fe3O4/P(GMA-EGDMA)-SO3H/Au-PPy recyclable catalyst for catalytic reduction of 4-nitrophenol. Appl. Organomet. Chem. 2018, 32, e4534. [Google Scholar]
- Ma, M.; Yang, Y.; Li, W.; Feng, R.; Li, Z.; Lyu, P.; Ma, Y. Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2019, 54, 323–334. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Liao, D.; Lyu, P.; Zhang, J.; Liang, J.; Zhang, L. Synthesis, characterization and catalytic performance of core-shell structure magnetic Fe3O4/P(GMA-EGDMA)-NH2/HPG-COOH-Pd catalyst. Appl. Organometall. Chem. 2019, 33, e4708. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Liu, Y.; Li, W.; Chen, G.; Ma, Y.; Lyu, P.; Li, S.; Wang, Y.; Wu, G. Preparation of magnetic Fe 3 O 4 /P (GMA-DVB)-PEI/Pd highly efficient catalyst with core-shell structure. Appl. Organomet. Chem. 2019, 33, e4850. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.-P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts 2019, 9, 140. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Q.; Dou, J.; Zhang, H.; Geng, W.; Yin, D.; Chen, S. Fabrication of 1D Fe3O4/P(NIPAM-MBA) thermosensitive nanochains by magnetic-field-induced precipitation polymerization. Colloid Polym. Sci. 2012, 290, 1207–1213. [Google Scholar] [CrossRef]
- Qiao, M.; Lei, X.; Ma, Y.; Tian, L.; Wang, W.; Su, K.; Zhang, Q. Facile synthesis and enhanced electromagnetic microwave absorption performance for porous core-shell Fe3O4@MnO2 composite microspheres with lightweight feature. J. Alloy. Compd. 2017, 693, 432–439. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, Y.; Liu, J.; Li, G.; Li, L.; Huang, K.; Yuan, L.; Feng, S. Delta-MnO2-Mn3O4 nanocomposite for photochemical water oxidation: Active structure stabilized in the interface. ACS Appl. Mater. Interfaces 2016, 8, 27825–27831. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Z.; Li, N.; Nan, J.; Yu, R.; Du, J. Visible-light-driven photocatalytic degradation of ciprofloxacin by a ternary Mn2O3/Mn3O4/MnO2 valence state heterojunction. Chem. Eng. J. 2018, 353, 805–813. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, L.; Yuan, Q.; He, J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Controlled synthesis of graphitic carbon nitride/beta bismuth oxide composite and its high visible-light photocatalytic activity. Carbon 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Zhao, J.; Nan, J.; Zhao, Z.; Li, N.; Liu, J.; Cui, F. Energy-efficient fabrication of a novel multivalence Mn3O4-MnO2 heterojunction for dye degradation under visible light irradiation. Appl. Catal. B: Environ. 2017, 202, 509–517. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Yin, X.; Shao, Q.; Lu, N.; Feng, Y.; Lu, Y.; Wujcik, E.K.; Mai, X.; Wang, C.; et al. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polym. 2018, 156, 128–135. [Google Scholar] [CrossRef]
- Sun, H.; He, Q.; She, P.; Zeng, S.; Xu, K.; Li, J.; Liang, S.; Liu, Z. One-pot synthesis of Au@TiO 2 yolk-shell nanoparticles with enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 505, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 965–973. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Han, W.; Yang, F.; Qu, B.; Liu, X.W. 3D Co3O4@MnO2 heterostructures grown on a flexible substrate and their applications in super-capacitor electrodes and photocatalysts. Dalton Trans. 2016, 45, 16850–16858. [Google Scholar]
- Zhou, J.; Wang, Y.; Ma, Y.; Zhang, B.; Zhang, Q. Surface molecularly imprinted thermo-sensitive polymers based on light-weight hollow magnetic microspheres for specific recognition of BSA. Appl. Surf. Sci. 2019, 486, 265–273. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Zhang, H.; Fan, X.; Liu, Y.; Zhang, Q. One-pot hydrothermal synthesis of highly monodisperse water-dispersible hollow magnetic microspheres and construction of photonic crystals. Chem. Eng. J. 2015, 259, 779–786. [Google Scholar] [CrossRef]

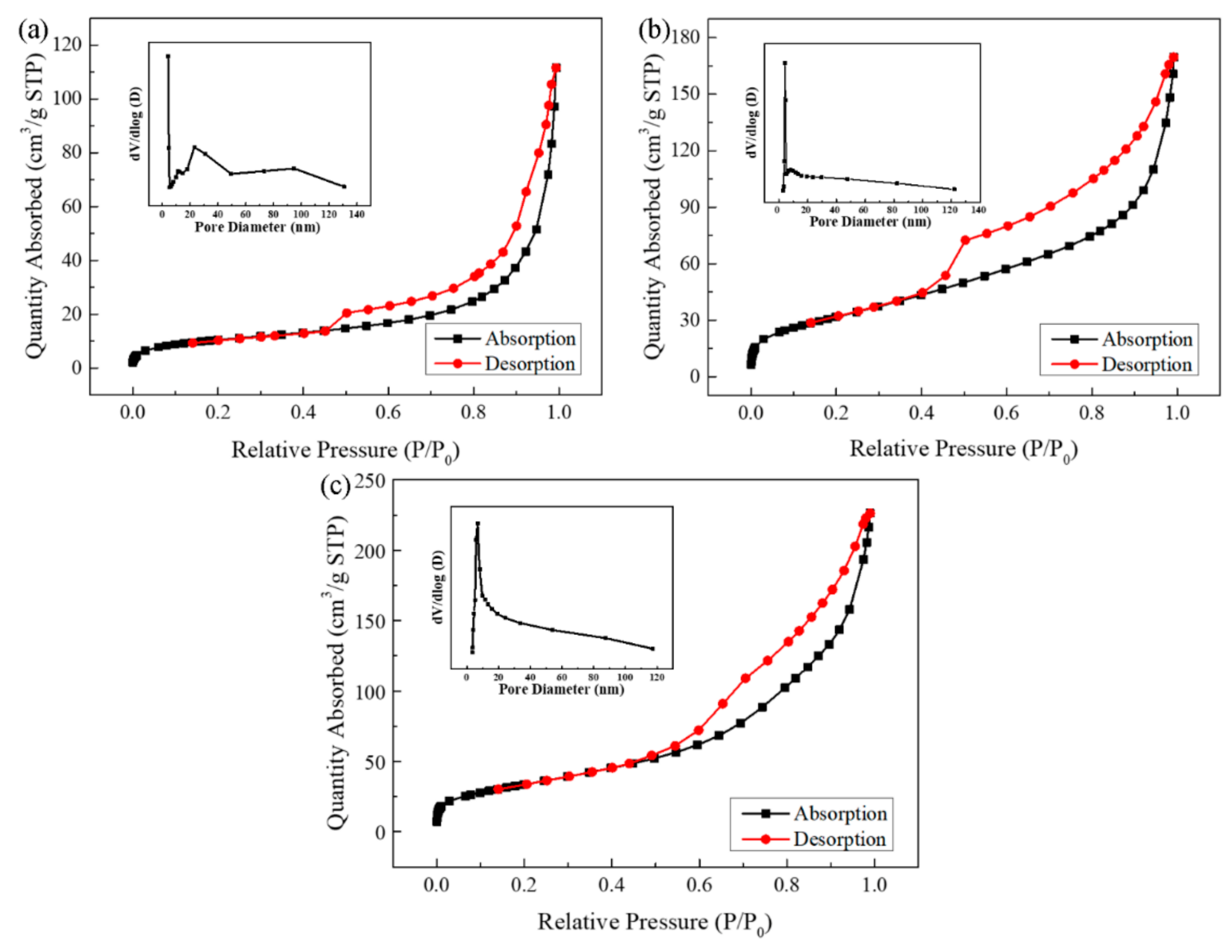

| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Fe3O4 | 38.47 | 0.17 |
| Fe3O4/MnO2 | 117.66 | 0.27 |
| Fe3O4/MnO2/Mn3O4 | 143.03 | 0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Yang, Y.; Chen, Y.; Wu, F.; Li, W.; Lyu, P.; Ma, Y.; Tan, W.; Huang, W. Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts 2019, 9, 589. https://doi.org/10.3390/catal9070589
Ma M, Yang Y, Chen Y, Wu F, Li W, Lyu P, Ma Y, Tan W, Huang W. Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts. 2019; 9(7):589. https://doi.org/10.3390/catal9070589
Chicago/Turabian StyleMa, Mingliang, Yuying Yang, Yan Chen, Fei Wu, Wenting Li, Ping Lyu, Yong Ma, Weiqiang Tan, and Weibo Huang. 2019. "Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation" Catalysts 9, no. 7: 589. https://doi.org/10.3390/catal9070589
APA StyleMa, M., Yang, Y., Chen, Y., Wu, F., Li, W., Lyu, P., Ma, Y., Tan, W., & Huang, W. (2019). Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts, 9(7), 589. https://doi.org/10.3390/catal9070589




