Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction
Abstract
:1. Introduction
2. Results
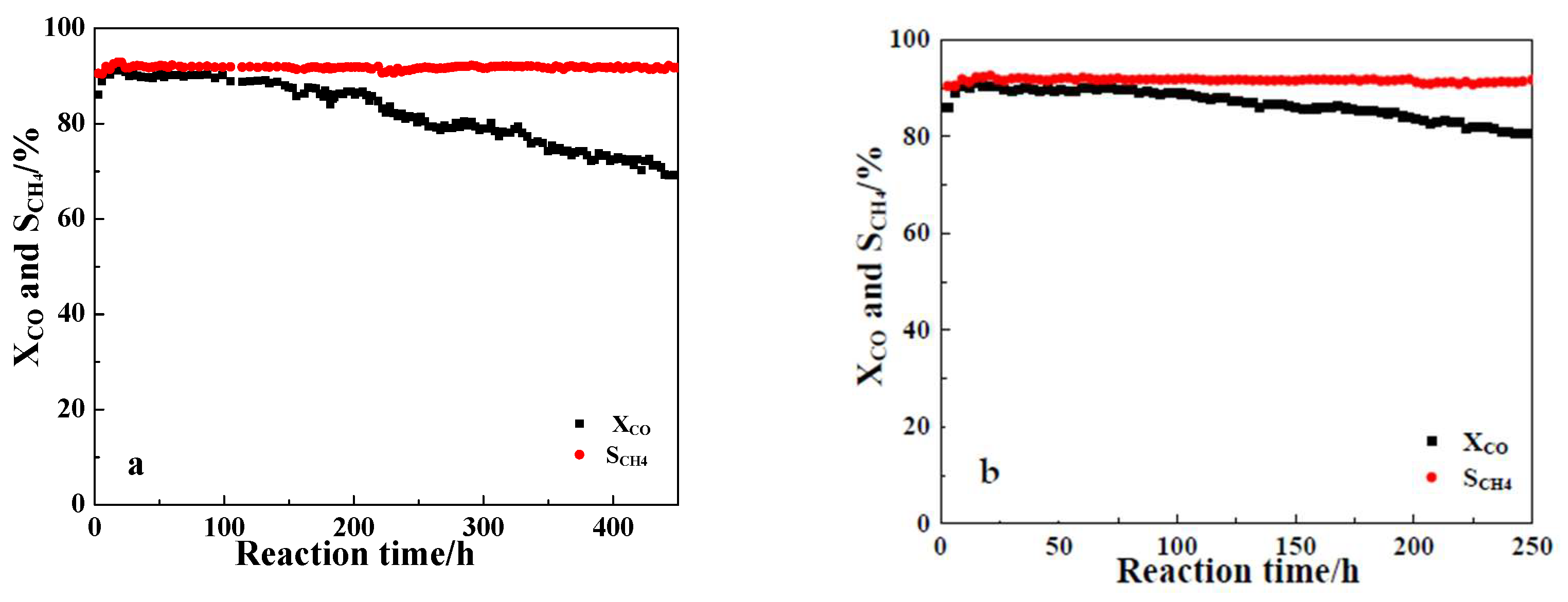
2.1. Catalytic Stability Evaluation in Slurry-bed
2.2. Catalyst Composition
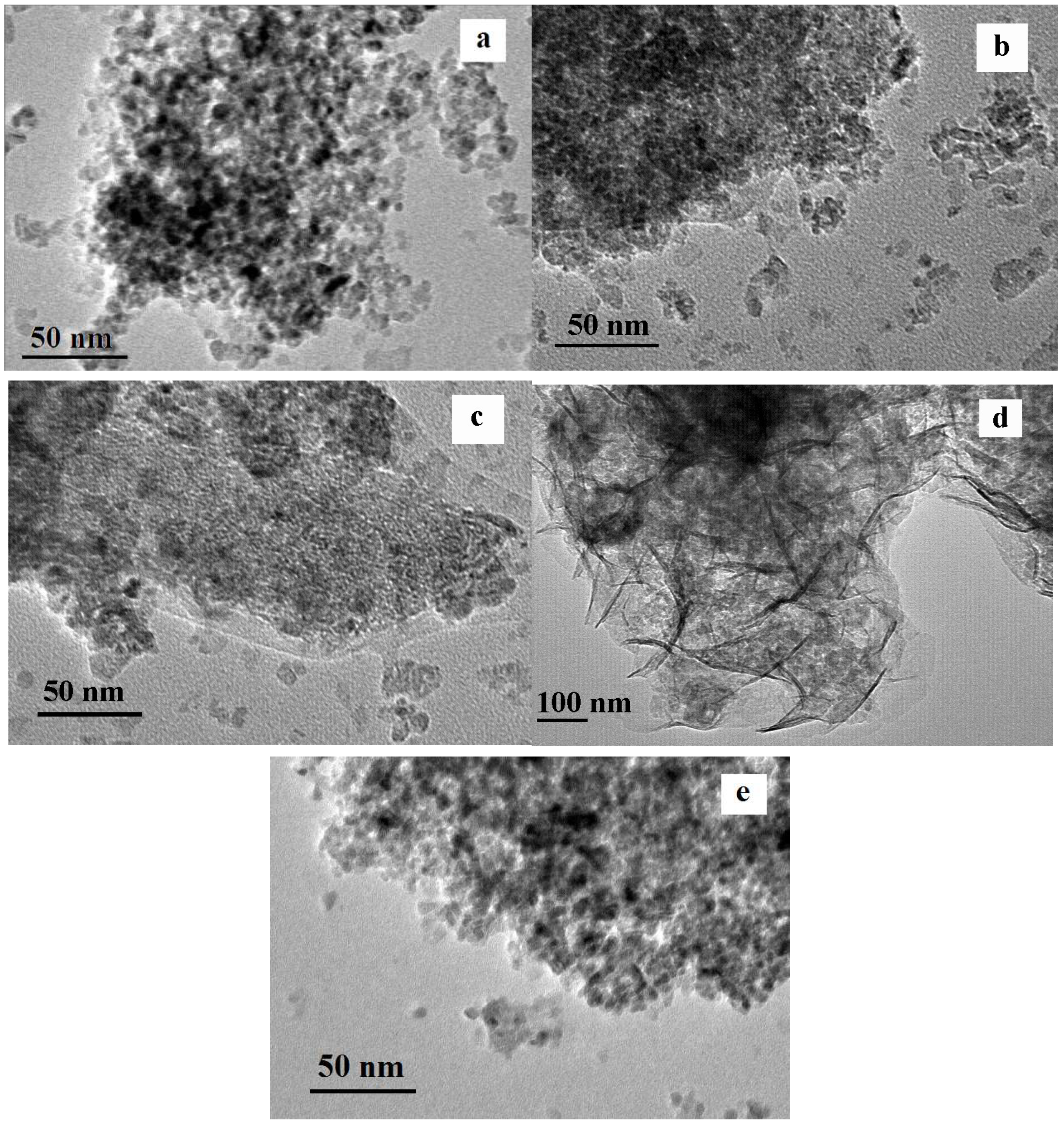
2.3. Microstructure and Morphology of Catalyst
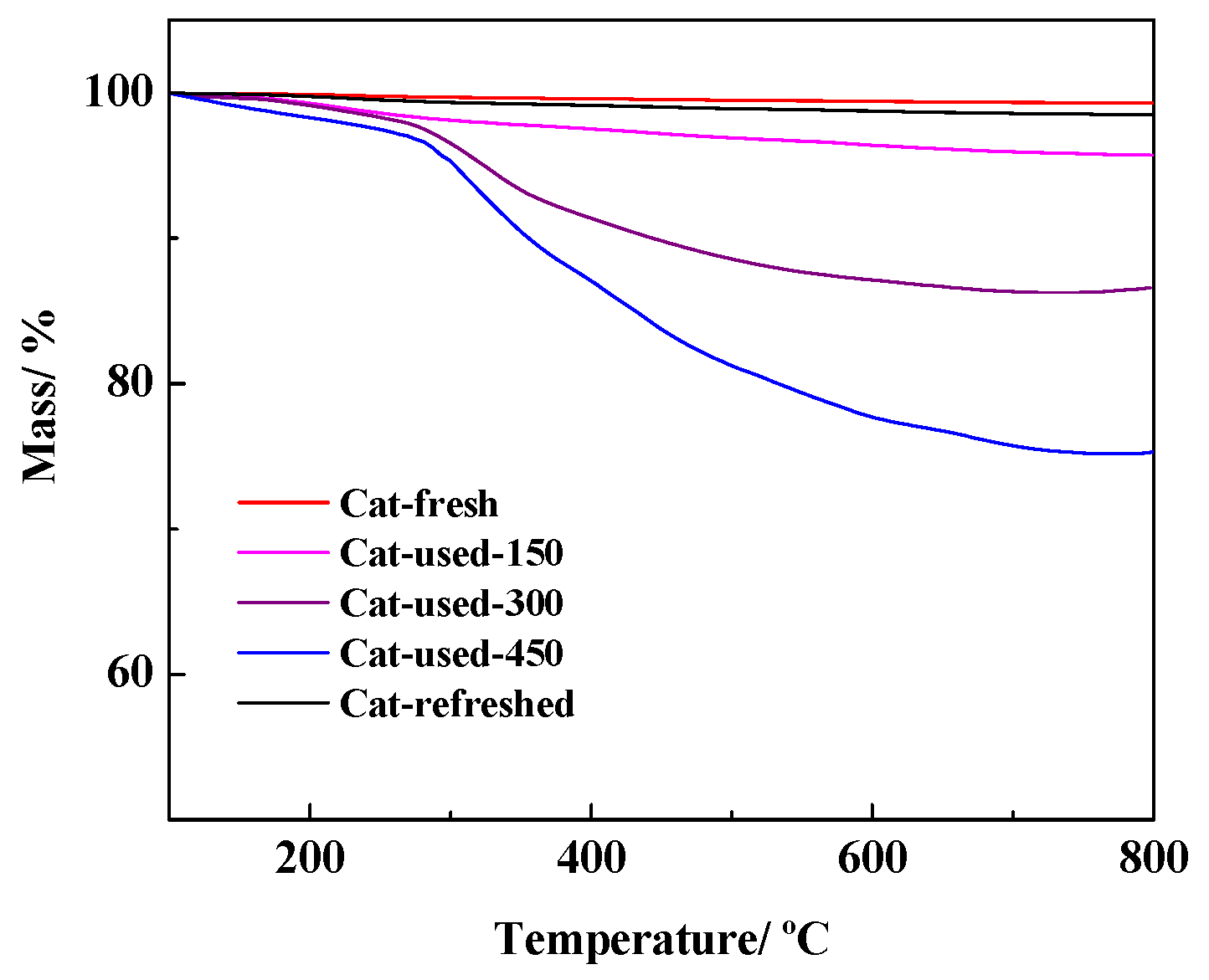
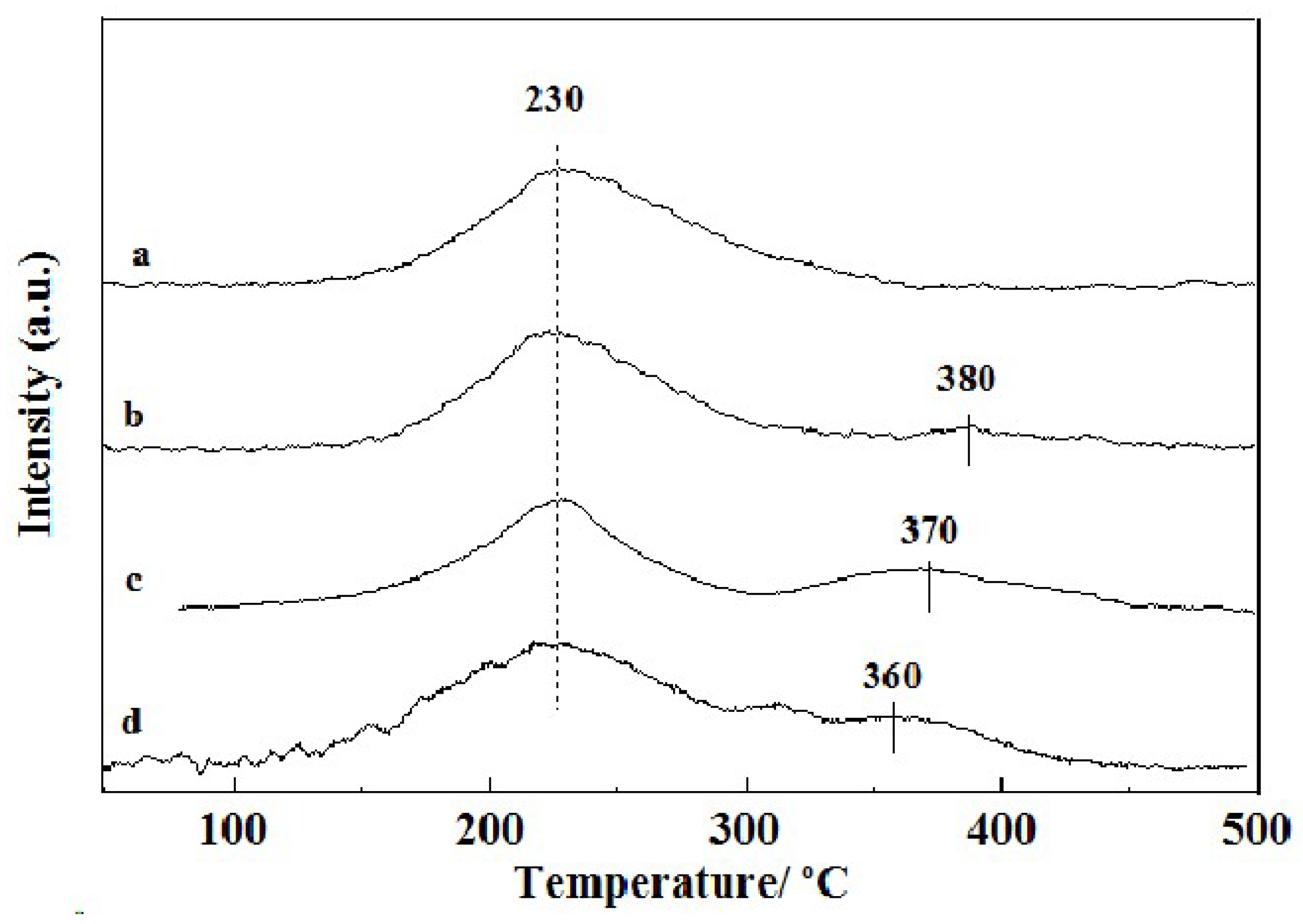
2.4. Carbon Deposition Analysis of Catalyst
2.5. H2 Chemisorption Properties of Catalyst
3. Discussion
4. Experimental Section
4.1. Catalyst Preparation
4.2. Catalyst Activity Evaluation and Regeneration
4.3. Characterization
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Liu, Q.; Shi, L.; Yan, Y.; Liu, Z. Behaviors of coking and radicals during reaction of volatiles generated from fixed-bed pyrolysis of a lignite and a subbituminous coal. Fuel Process. Technol. 2017, 161, 304–310. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Cai, Q.; Cui, Y. Production of aromatic hydrocarbons by hydrogenation-cocracking of bio-oil and methanol. Fuel Process. Technol. 2017, 161, 232–239. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Xiao, R.; Wu, C. Methanation of syngas (H2/CO) over the different Ni-based catalysts. Fuel 2017, 189, 419–427. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.; Jin, Y.; Zhu, J.; Liu, S.; Lin, J.; Li, Z. Silica/titania composite-supported Ni catalysts for CO methanation: effects of Ti species on the activity, anti-sintering, and anti-coking properties. Appl. Catal. B-Environ. 2017, 201, 561–572. [Google Scholar] [CrossRef]
- Tao, M.; Xin, Z.; Meng, X.; Bian, Z.; Lv, Y. Highly dispersed nickel within mesochannels of SBA-15 for CO methanation with enhanced activity and excellent thermostability. Fuel 2017, 188, 267–276. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, I.; Kang, J.Y.; Jeong, H.; Park, J.K.; Park, J.H.; Jung, J.C. Alcohol-assisted low temperature methanol synthesis from syngas over Cu/ZnO catalysts: Effect of pH value in the co-precipitation step. J. Mol. Catal. A Chem. 2015, 400, 132–138. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Wen, Z. Assessment of policy alternatives and key technologies for energy conservation and water pollution reduction in China’s synthetic ammonia industry. J. Cleaner Prod. 2012, 25, 96–105. [Google Scholar] [CrossRef]
- Liu, G.; Larson, E.D. Comparison of coal/biomass co-processing systems with CCS for production of low-carbon synthetic fuels: Methanol-to-Gasoline and Fischer-Tropsch. Energy Procedia 2014, 63, 7315–7329. [Google Scholar] [CrossRef]
- Yang, S.; Qian, Y.; Liu, Y.; Wang, Y.; Yang, S. Modeling, simulation, and techno-economic analysis of Lurgi gasification and BGL gasification for coal-to-SNG. Chem. Eng. Res. Des. 2017, 117, 355–368. [Google Scholar] [CrossRef]
- Yang, S.; Liang, J.; Yang, S.; Qian, Y. A novel cascade refrigeration process using waste heat and its application to coal-to-SNG. Energy 2016, 115, 486–497. [Google Scholar] [CrossRef]
- Li, S.; Ji, X.; Zhang, X.; Gao, L.; Jin, H. Coal to SNG: Technical progress, modeling and system optimization through exergy analysis. Appl. Energy 2014, 136, 98–109. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, T.; Peng, J.; Wang, S.; Wang, S. A comparison of Ni/SiC and Ni/Al2O3 catalyzed total methanation for production of synthetic natural gas. Appl. Catal. A-Gen. 2013, 462–463, 75–81. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Shaw, G.; Smith, P.; Morgan, D.; Perdjon, M.; Li, Z. Sacrificial carbon strategy toward enhancement of slurry methanation activity and stability over Ni-Zr/SiO2 catalyst. Ind. Eng. Chem. Res. 2018, 57, 4798–4806. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Liu, J.; Cui, X.; Zheng, H. Effect of promoter Ce on the structure and catalytic performance of Ni/Al2O3 catalyst for CO methanation in slurry-bed reactor. J. Nat. Gas Sci. Eng. 2015, 23, 250–258. [Google Scholar] [CrossRef]
- Hui, W.; Zhang, J.-f.; Bai, Y.-x.; Wang, W.-f.; Tan, Y.-s.; Han, Y.-Z. NiO@SiO2 core-shell catalyst for low-temperature methanation of syngas in slurry reactor. J. Fuel Chem. and Technol. 2016, 44, 548–556. [Google Scholar]
- Zhang, J.; Bai, Y.; Zhang, Q.; Wang, X.; Zhang, T.; Tan, Y.; Han, Y. Low-temperature methanation of syngas in slurry phase over Zr-doped Ni/γ-Al2O3 catalysts prepared using different methods. Fuel 2014, 132, 211–218. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, F.; Cheng, Y.; Li, Z. Influence of fuel additives in the urea-nitrates solution combustion synthesis of Ni-Al2O3 catalyst for slurry phase CO methanation. Appl. Catal. A-Gen. 2017, 534, 12–21. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, F.; Ji, K.; Song, Y.; Li, Z. Slurry phase methanation of carbon monoxide over nanosized Ni–Al2O3 catalysts prepared by microwave-assisted solution combustion. Appl. Catal. A-Gen. 2016, 510, 74–83. [Google Scholar] [CrossRef]
- Ji, K.; Meng, F.; Li, Z. Ni-based catalysts prepared by impregnation combustion method for CO methanation in a slurry-bed reactor. Asia-Pac. J. Chem. Eng. 2016, 11, 151–157. [Google Scholar] [CrossRef]
- Ke-Ming, J.; Fan-Hui, M.; Yuan, C.; Zhong, L. Solution combustion prepared Ni-based catalysts and their catalytic performance for slurry methanation. Chin. J. Inorg. Chem. 2015, 31, 267–274. [Google Scholar]
- Keming, J.; Fanhui, M.; Yuan, G.; Zhong, L. Effect of fuel on structure and catalytic performance for slurry methanation over Ni-Al2O3 catalysts prepared by combustion method. Chem. J. Chin. Univ. 2016, 37, 134–141. [Google Scholar]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A-Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Guo, H.; Qin, Z.; Zhao, S.; Lu, Y.; Li, Z.; Ren, J. Mechanism studies concerning carbon deposition effect of CO methanation on Ni-based catalyst through DFT and TPSR methods. Int. J. Hydrogen Energy 2016, 41, 8401–8411. [Google Scholar] [CrossRef]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef]
- La Cava, A.I.; Bernardo, C.A.; Trimm, D.L. Studies of deactivation of metals by carbon deposition. Carbon 1982, 20, 219–223. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Wang, H.Y. Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/γ-Al2O3 catalysts. J. Catal. 2002, 205, 289–293. [Google Scholar] [CrossRef]
- Dabros, T.M.H.; Andersen, M.L.; Lindahl, S.B.; Hansen, T.W.; Høj, M.; Gabrielsen, J.; Grunwaldt, J.D.; Jensen, A.D. Hydrodeoxygenation (HDO) of aliphatic oxygenates and phenol over NiMo/MgAl2O4: reactivity, inhibition, and catalyst reactivation. Catalysts 2019, 9, 521. [Google Scholar] [CrossRef]
- Gomes, R.; Costa, D.; Junior, R.; Santos, M.; Rodella, C.; Fréty, R.; Beretta, A.; Brandão, S. Dry reforming of methane over NiLa-based catalysts: influence of synthesis method and Ba addition on catalytic properties and stability. Catalysts 2019, 9, 313. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.; Yang, T.; Liu, W.; Zhang, D. Carbon deposition behavior of a Co–Ni aerogel catalyst in CH4 oxy-CO2 reforming using various types of reactors. Int. J. Hydrogen Energy 2014, 39, 15474–15481. [Google Scholar] [CrossRef]
- Remiro, A.; Valle, B.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Operating conditions for attenuating Ni/La2O3–αAl2O3 catalyst deactivation in the steam reforming of bio-oil aqueous fraction. Fuel Process Technol. 2013, 115, 222–232. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A-Gen. 2012, 417–418, 281–289. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Li, P.; Zhu, Q.; Gao, J.; Gu, F.; Su, F. Enhanced fluidized bed methanation over a Ni/Al2O3 catalyst for production of synthetic natural gas. Chem. Eng. J. 2013, 219, 183–189. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Tao, M. Activity and stability of nickel based MCM-41 methanation catalysts for production of synthetic natural gas. CIESC J. 2014, 65, 160–168. [Google Scholar]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported Ru catalysts. Appl. Catal. B-Environ. 2009, 88, 470–478. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Chen, X.; Tadd, A.R.; Schwank, J.W. Carbon deposited on Ni/CeZrO isooctane autothermal reforming catalysts. J. Catal. 2007, 251, 374–387. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3 Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Z.; Hu, M.; Zuo, C.; Traitangwong, A.; Meeyoo, V.; Li, C.; Zhang, S. Highly active Ni-based catalyst derived from double hydroxides precursor for low temperature CO2 methanation. Ind. Eng. Chem. Res. 2018, 57, 9102–9111. [Google Scholar] [CrossRef]
- Struis, R.P.; Schildhauer, T.J.; Czekaj, I.; Janousch, M.; Biollaz, S.M.; Ludwig, C. Sulphur poisoning of Ni catalysts in the SNG production from biomass: A TPO/XPS/XAS study. Appl. Catal. A-Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]
- Le Valant, A.; Bion, N.; Can, F.; Duprez, D.; Epron, F. Preparation and characterization of bimetallic Rh-Ni/Y2O3-Al2O3 for hydrogen production by raw bioethanol steam reforming: influence of the addition of nickel on the catalyst performances and stability. Appl. Catal. B-Environ. 2010, 97, 72–81. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Srisiriwat, N.; Therdthianwong, A.; Croiset, E. Reforming of bioethanol over Ni/Al2O3 and Ni/CeZrO2/Al2O3 catalysts in supercritical water for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2877–2886. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Guo, L.; Zhang, X. Hydrogen production by biomass gasification in supercritical water over Ni/γAl2O3 and Ni/CeO2-γAl2O3 catalysts. Int. J. Hydrogen Energy 2010, 35, 7161–7168. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Liu, J.; Guo, C.; Wang, Y.; Zhang, J. Ethanol steam reforming reactions over Al2O3·SiO2-supported Ni–La catalysts. Fuel 2009, 88, 511–518. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Carbon deposition in steam reforming and methanation. Catal. Rev. 1982, 24, 67–112. [Google Scholar] [CrossRef]

| Catalyst | Ni Relative Content/wt% | Al Relative Content/wt% | Mass of Ni/Al |
|---|---|---|---|
| (Theoretical value) | 20 | 42.4 | 0.47 |
| Cat-fresh | 20.5 | 45.6 | 0.45 |
| Cat-used-150 | 19.0 | 39.7 | 0.48 |
| Cat-used-300 | 17.1 | 37.1 | 0.46 |
| Cat-used-450 | 15.4 | 32.9 | 0.47 |
| Cat-refreshed | 20.1 | 44.7 | 0.45 |
| Catalyst | SBET (m2·g−1) | Vp (cm3·g−1) | Dp (nm) |
|---|---|---|---|
| Cat-fresh | 167 | 0.278 | 7.1 |
| Cat-used-150 | 152 | 0.236 | 5.4 |
| Cat-used-300 | 128 | 0.214 | 4.9 |
| Cat-used-450 | 134 | 0.219 | 4.8 |
| Cat-refreshed | 158 | 0.273 | 6.8 |
| Catalyst | Metallic Surface Area/(m2·gmetal−1) | Metal Dispersion/% |
|---|---|---|
| Cat-fresh | 16.3 | 2.45 |
| Cat-used-150 | 15.3 | 2.29 |
| Cat-used-300 | 12.6 | 1.89 |
| Cat-used-450 | 9.0 | 1.35 |
| Cat-refreshed | 15.9 | 2.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, K.; Meng, F.; Xun, J.; Liu, P.; Zhang, K.; Li, Z.; Gao, J. Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction. Catalysts 2019, 9, 570. https://doi.org/10.3390/catal9070570
Ji K, Meng F, Xun J, Liu P, Zhang K, Li Z, Gao J. Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction. Catalysts. 2019; 9(7):570. https://doi.org/10.3390/catal9070570
Chicago/Turabian StyleJi, Keming, Fanhui Meng, Jiayao Xun, Ping Liu, Kan Zhang, Zhong Li, and Junhua Gao. 2019. "Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction" Catalysts 9, no. 7: 570. https://doi.org/10.3390/catal9070570
APA StyleJi, K., Meng, F., Xun, J., Liu, P., Zhang, K., Li, Z., & Gao, J. (2019). Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction. Catalysts, 9(7), 570. https://doi.org/10.3390/catal9070570






