1. Introduction
The slow kinetics of oxygen reduction reaction (ORR) is a major bottleneck for the development of proton exchange membrane fuel cells (PEMFCs). Although platinum (Pt) is generally considered to possess the highest ORR catalytic activity among pure metals [
1], the scarcity and high cost greatly restrict its large-scale utilization. Alloying Pt with a transition metal (Fe, Co, Ni, Cu, etc.) has been demonstrated an effective approach to increase the catalytic activity and thus reduce the usage of Pt [
2], due to the electronic and/or strain effect between the non-noble transition metals and Pt [
3,
4,
5]. However, under the acidic ORR environment, the nonprecious metals in Pt alloys will be continuously leached, leading to substantial activity degradation during long-term electrocatalysis [
6,
7]. Recently, compared to conventional disordered Pt alloys, structurally ordered Pt intermetallics have shown much enhanced activity and stability towards ORR due to a higher antidissolution capability of nonprecious metals [
8,
9,
10,
11,
12]. The formation of structurally ordered intermetallics usually relies on the thermal annealing of the disordered counterparts at high temperatures (usually above 600 °C), which, however, could generally result in a detrimental particle sintering effect and thus discounted catalytic performance. To suppress the particle sintering during thermal annealing, tedious processes such as protection by KCl [
13], oxide [
8,
14], or carbon [
12,
15], have been developed yet are difficult to be scaled up.
Compared to binary alloys, ternary alloys generally allow a greater flexibility in adjusting the Pt electronic structure and catalytic properties [
16,
17]. Recent experiments have also found that Pt ternary alloys may be easier to achieve ordering transition compared to Pt binary alloys. For example, adding a small amount of Cu can effectively lower the temperature needed for structurally ordering of Pt–Fe alloy [
18,
19]. Cobalt (Co) was also found to enhance the ordering of a Pt(NiCo) alloy compared to PtNi alloy [
20]. As a result, ordered Pt ternary ORR catalysts have drawn considerable interest recently [
18,
20,
21,
22,
23]. These studies suggest that it is possible to achieve structural ordering of Pt ternary alloys at relatively lower temperatures without the use of tedious surface coating strategies, thus opening a new way to suppress the associated particle sintering at high temperatures. However, the specific roles of the different alloying transition metals during the ordering transformation of Pt ternary alloys are still poorly understood.
To rationally develop ordered Pt ternary intermetallics as durably active ORR electrocatalysts, we present here a systematic and comparative study of the ordering transformation and ORR activity/stability of three representative Pt ternary alloy catalysts (Pt–Fe–Co, Pt–Co–Ni and Pt–Fe–Ni) with a similar Pt atomic content of ~40%. With the aid of X-ray diffraction and (high-resolution) transmission electron microscopy (TEM), we focused on the particle size distributions and ordered/disordered structures of the three ternary catalysts after annealing at 600 °C, in an effort to disclose the roles of different alloying metals. The compositional core–shell structures formed by leaching of surface non-noble metals during ORR electrocatalysis were also probed by electron energy loss spectroscopy (EELS) mapping, aiming to provide some clues on the antidissolution capabilities of different non-noble metals. Based on these studies, we develop a structurally ordered Pt–Fe–Co intermetallic catalyst with both high ordering degree and minimum particle sintering, showing the highest activity and stability among the different Pt ternary catalysts.
2. Results and Discussion
The three ternary alloy nanoparticles (NPs) with Pt atomic content of ~40% (referred to as PtMN, M, N = Fe, Co, Ni, etc.) were synthesized by using organic solution phase reduction methods (see Experimental section for details), and then supported on high surface area carbon (Vulcan XC). Their energy-dispersive spectrometer (EDS) compositions are Pt
44Co
36Fe
20, Pt
37Fe
52Ni
11, and Pt
39Ni
32Co
29, and the compositions obtained from inductively coupled plasma mass spectrometer (ICP-MS) are Pt
41Co
40Fe
19, Pt
36Fe
48Ni
16, and Pt
35Ni
40Co
25, respectively. The realistic Pt contents in the catalysts were also determined by ICP (see
Table S1). The supported catalysts were firstly heated in air at 180 °C to remove residual surfactants used in the nanoparticle synthesis, and then annealed at 400 °C in Ar/4% H
2 for 4 h to obtain disordered Pt ternary alloy catalysts, which were designated as PtMN-H400. The disordered nanoparticle catalysts were further annealed at 600 or 800 °C in an Ar/4% H
2 atmosphere and then naturally cooled down to induce structural ordering (denoted as PtMN-H600 or PtMN-H800).
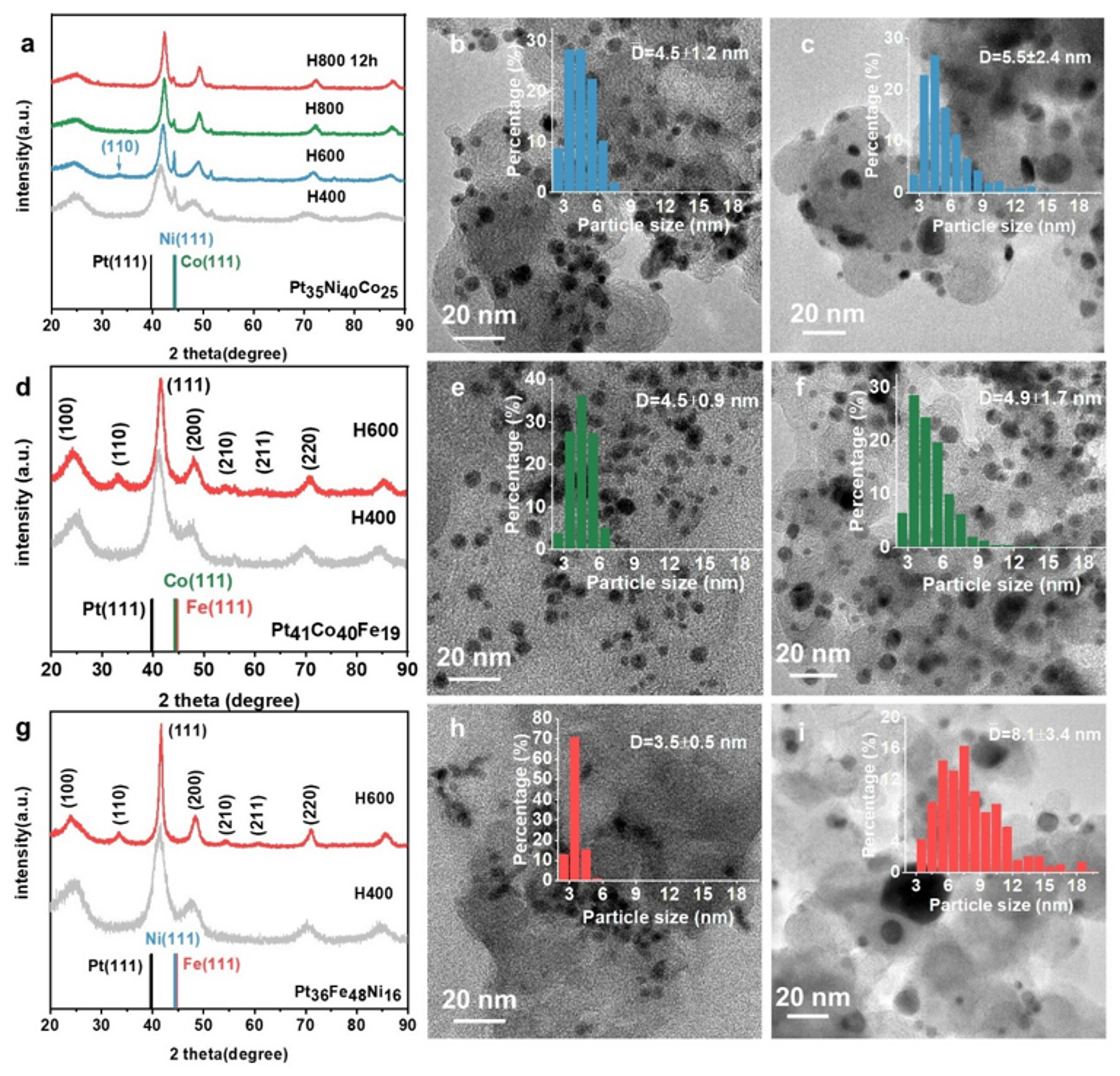
The morphology of the ternary alloy catalysts after heat treatment at 400 and 600 °C and their X-ray diffraction (XRD) patterns are shown in
Figure 1. The XRD profiles in
Figure 1a show that the as-prepared PtNiCo nanoparticles have two phases: a Pt-richer phase ({111} peak at 41.8°) and a Co-richer ({111} peak at 44.2°). After annealing at 600 °C, the PtNiCo-H600 catalyst shows a weak {110} superlattice peak at 33.3°, indicating a slight ordering transformation. After annealing at a higher temperature (800 °C) for 4 h and longer time (12 h), the diffraction from Co-rich phase greatly weakened, indicating decreased lattice parameter (
Table S2), and thus increased alloying degree of PtNiCo. However, the obtained PtNiCo-H800 became completely disordered. For the PtCoFe and PtFeNi catalysts, a disordered single phase after annealing at 400 °C was revealed from the XRD patterns, yet both can achieve obvious ordering transformation and a higher extent of alloying (
Table S2) after annealing at 600 °C (
Figure 1d,g) with a higher ordering degree compared to PtNiCo-H600 catalyst, judged from much higher relative intensity of the {110} diffraction peak.
The different extents of ordering transition of the three alloys indicate a significant influence of the alloying elements during the thermal annealing induced structural ordering. In particular, it appears that Fe could efficiently promote the ordering transformation of Pt alloys. This can be firstly rationalized by the difference in the diffusion rates of the Fe, Co, and Ni atoms as reported by Duhls [
24] et al. It was shown that the diffusion coefficients of Fe, Co, and Ni in the Au matrix decreases in the order of Fe > Co > Ni. While no reference data was reported for the diffusion rates of Fe, Co, and Ni in Pt matrix, it is reasonable to expect a similar trend in Pt. Thus, the much faster diffusion rate of Fe can speed up the rearrangement of different alloying atoms during the structural ordering. Secondly, from the phase diagrams, the disorder–order transformation temperature of L1
0 PtFe intermetallics (~850 °C [
25]) was also higher compared to those of L1
0 PtCo (around 800 °C [
26]) and L1
0 PtNi (~600 °C [
27]). A higher disorder–order transition temperature generally indicates a higher stability of the ordered phase at low temperatures, thus the ordered phase of PtFe should be more easily formed when cooling from high temperatures to room temperature [
28].
According to the XRD patterns of the three alloys, the diffraction peaks of PtNiCo and PtCoFe do not show obvious sharpening after high-temperature annealing, implying that the NPs did not undergo serious growth; in contrast, the XRD peaks of PtFeNi-H600 appear to be significantly narrower. Since both the PtNiCo and PtCoFe contain Co, we speculate that Co can effectively inhibit the agglomeration of NPs during high-temperature annealing.
Figure 1b,e,h presents the TEM images and the corresponding particle size distribution analysis of the three ternary alloys after annealing at 400 °C, while 1c, f, and i show the three alloys annealed at 600 °C, respectively. The particle sizes of the PtNiCo-H600 and PtCoFe-H600 catalysts, particularly the latter with a higher Co content, increased only slightly compared to their counterparts annealed at 400 °C. In contrast, the Co-free PtFeNi NPs grew significantly from 3.5 ± 0.5 nm to 8.1 ± 3.4 nm. The detailed reason for the role of Co in suppressing particle sintering was still unclear. Nevertheless, a strong interaction of Ni on the carbon support was previously shown to be responsible for the significant growth of PtNi
3 nanoparticles during thermal annealing [
29]. Likely, the extent of interaction with the carbon support may be different for Fe, Co, and Ni, leading to the different extent of particle sintering.
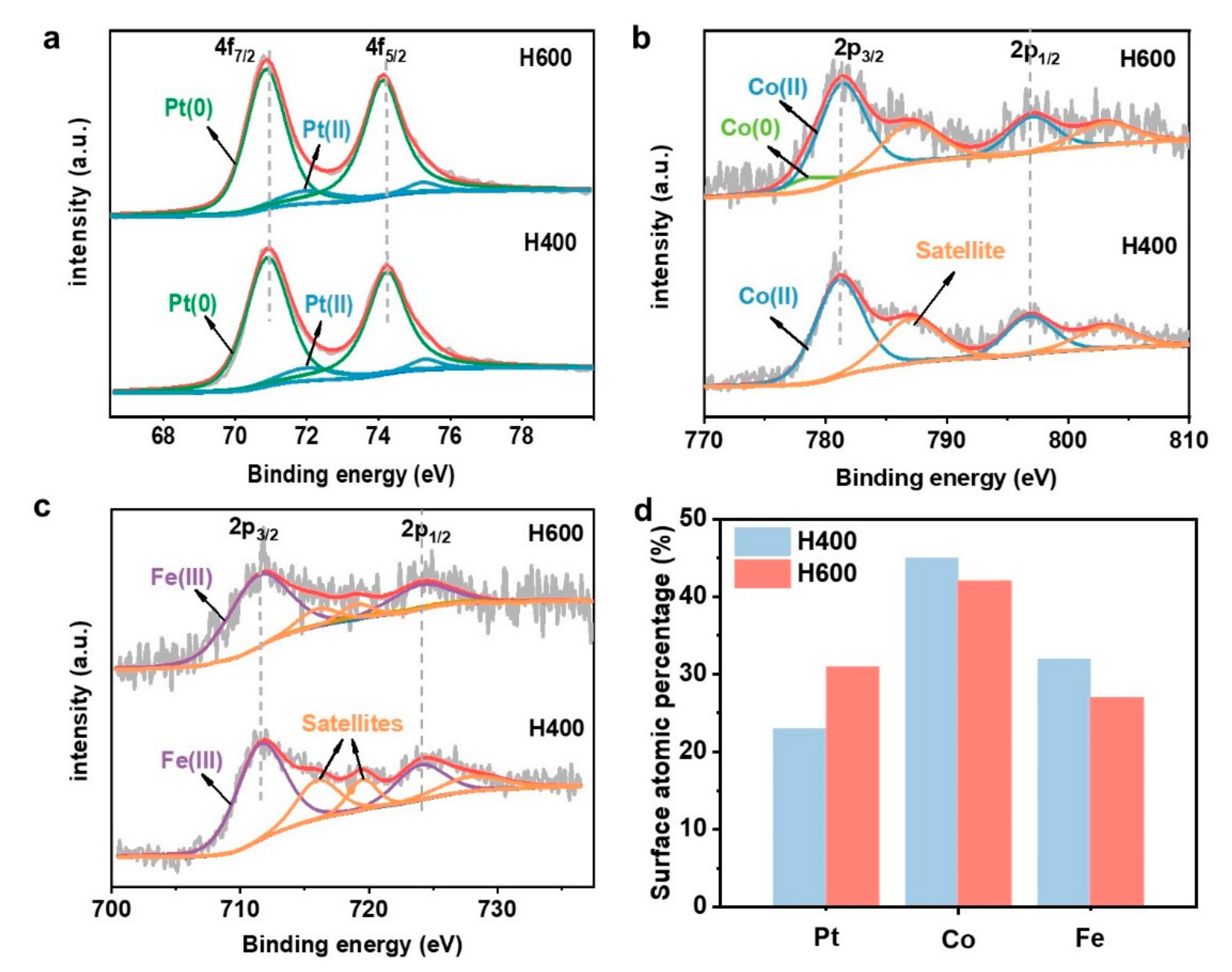
X-ray photoelectron spectroscopy (XPS) was conducted to probe surface composition changes upon annealing at different temperatures, exemplified by the PtCoFe-H400 and the PtCoFe-H600 catalyst (
Figure 2). The Pt 4f spectra of the PtCoFe-H400 catalyst can be fitted by two pairs of peaks corresponding to the Pt(0) and Pt(II) species, respectively (
Figure 2a), showing a majority of metallic Pt(0) on the surface. The less noble metals Co and Fe, however, mainly exist in the oxidized state, showing the majority of Co
2+ and Fe
3+ on the surface (
Figure 2b,c). Importantly, the Pt surface composition substantially increased from 23 at.% in the the PtCoFe-H400 catalyst to 31 at.% in the PtCoFe-H600 catalyst (
Figure 2d), which is closer to the bulk compositions and thus consistent with a higher extent of alloying in the latter. Moreover, a shift of the Pt 4f peak to lower binding energies and a slight shift of Co-/Fe-2p peaks to higher binding energies are also observed in the PtCoFe-H600 catalyst as guided by the dash lines. This indicates increased electron transfer from Co/Fe to Pt in the ordered PtCoFe-H600 catalyst, leading to a downward shift of d-band center which is believed to be beneficial for ORR electrocatalysis [
3].
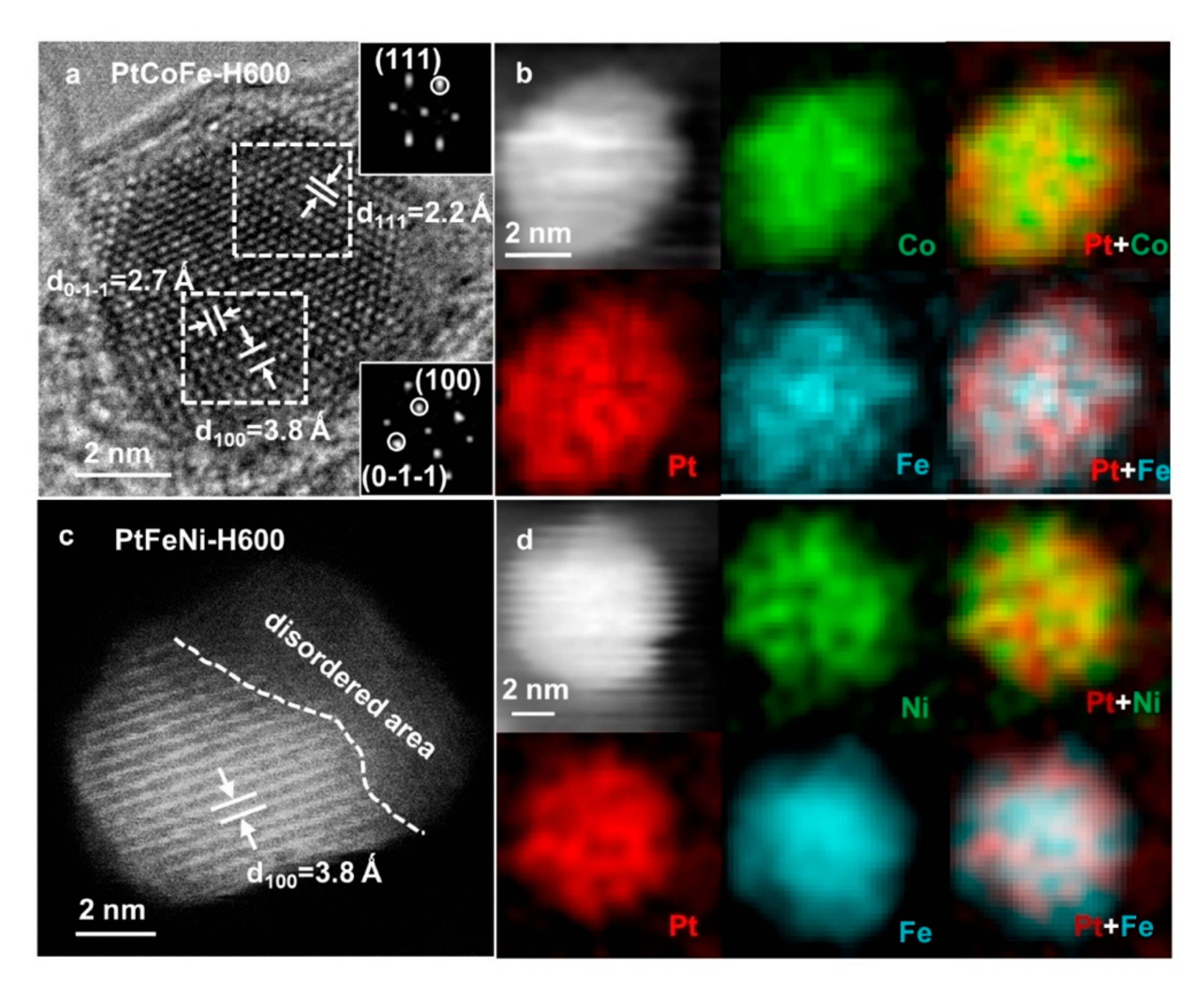
In order to study the atomic ordering structure of the ordered Pt ternary alloys, the PtCoFe-H600 and PtFeNi-H600 were further characterized by high resolution TEM.
Figure 3a presents a high-resolution TEM image of a PtFeCo-H600 nanoparticle oriented along the [01
] zone axis, showing the partially ordered structure where (100) and (
) superlattice diffraction spots emerged, corresponding to an interplanar spacing of 3.8 Å and 2.7 Å, respectively [
30]. The EELS elemental mapping of the PtCoFe-H600 catalyst (
Figure 3b) shows that Pt, Fe, and Co are uniformly distributed. Similar to PtFeCo-H600, the PtFeNi-H600 is also partially ordered (
Figure 3c). Structurally ordered regions and disordered regions coexist inside the particles, and the elements are evenly distributed in the NPs (
Figure 3d).
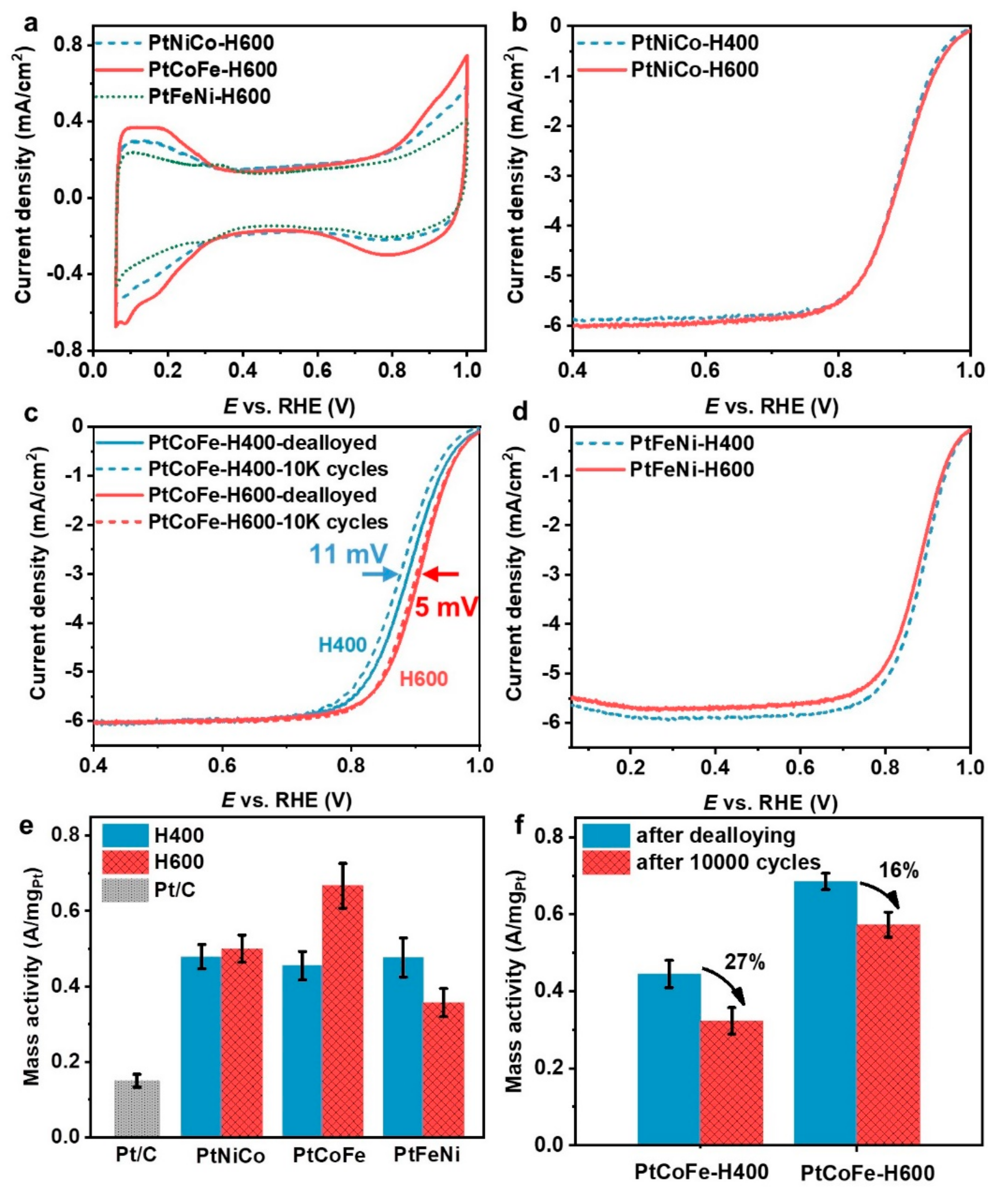
Electrochemical tests were performed to evaluate ORR performances of the different Pt ternary alloys. Before the activity measurement, all the catalysts were subjected to electrochemical dealloying by 200 potential cycles between 0.06 and 1.0 V vs. reversible hydrogen electrode (RHE), which is crucial to achieve the optimum activity (
Figure S1).
Figure 4a presents the cyclic voltammetry (CV) curves of different catalysts annealed at 600 °C (CV curves of catalysts annealed at 400 °C are presented in
Figure S2), which show that the PtCoFe-H600 catalyst has the largest electrochemical surface area (ECSA, see quantitative value in
Table S3), consistent with its minimum particle sintering effect.
Figure 4b–d presents the linear scanning voltammetry (LSV) polarization curves of the dealloyed ternary alloy catalysts on the ORR electrocatalysis, for which the mass activities are compared in
Figure 4e. The half-wave potentials were also listed in
Table S4 for comparison. It can be seen that the ORR activity of PtNiCo-H600 is only slightly higher than that of PtNiCo-H400 due to a rather low degree of ordering transformation in the absence of Fe. Although PtFeNi-H600 exhibits a higher degree of ordering transition, its ORR mass activity is even lower than that of PtFeNi-H400, which is ascribed to the severe nanoparticle sintering in PtFeNi-H600. No significant activity enhancement was observed in Ni-containing ternary species, indicating Ni addition did not favor the performance improvement. Above all, the PtCoFe-H600 catalyst shows the highest mass activity, increasing from 0.44 (for PtCoFe-H400) to 0.65 A/mg
Pt, which can be attributed to its relatively high degree of ordering and the presence of Co inhibiting the NP sintering (
Figure 4e). The activity of the PtCoFe-H600 catalyst is among the highest activities reported in Pt ternary catalysts in the references [
18,
20,
21,
22,
23] (as listed in
Table 1).
We further studied the stability of the disordered and ordered PtCoFe by cycling 10,000 times at a rate of 100 mV/s in the range of 0.6 to 1.0 V (
Figure 4c,f). The half-wave potential of the disordered PtCoFe-H400 catalyst decreased by 11–12 mV after long-term cycling, and the mass activity reduced by 27 ± 2%. In contrast, the half-wave potential of the PtCoFe-H600 catalyst shifted only 5–7 mV and the activity dropped by 16 ± 5%. This demonstrates the ordered PtCoFe-H600 catalyst has both higher catalytic activity and higher durability compared to the disordered PtCoFe-H400 catalyst as well as the other Pt ternary catalysts. EDS analysis (
Table S5) demonstrates that the composition of PtCoFe-H400 changed to Pt
71Co
19Fe
10 after dealloying, while the composition of PtCoFe-H600 became Pt
58Co
24Fe
18 after dealloying and Pt
64Co
18Fe
18 after 10,000 cycles, evidencing that the nonprecious metals in the ordered alloy are more difficult to be leached.
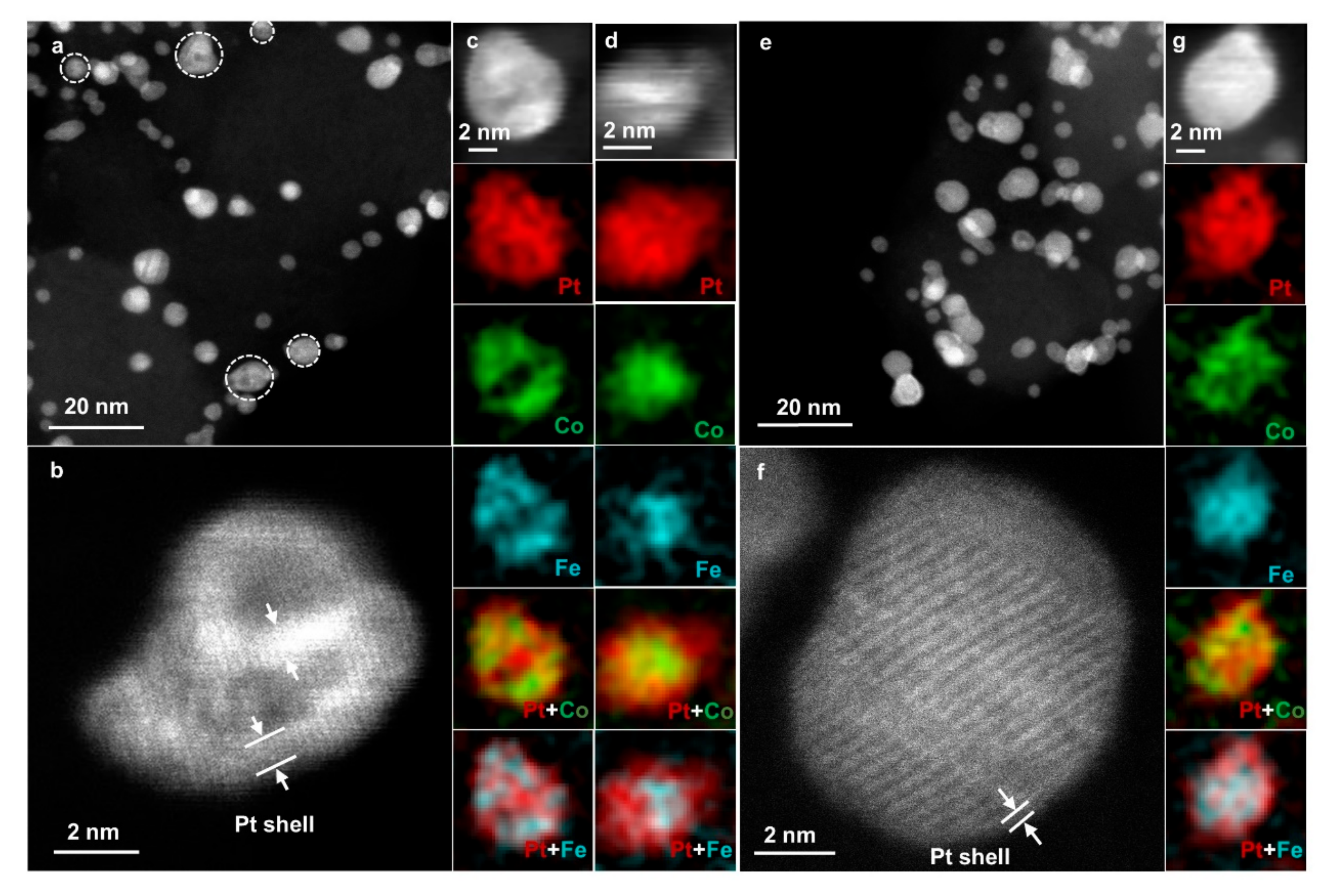
Compositional fine structures of the disordered PtCoFe-H400 and ordered PtCoFe-H600 catalysts after ORR test were further investigated by STEM-EELS to gain further insights into their different activities and stabilities. Some of the particles in the PtCoFe-H400 catalyst were etched into a porous structure (
Figure 5a,b). EELS characterizations confirm that some of the internal Fe and Co were etched (
Figure 5c) to form a hollow structure, in accordance with the sharp decrease in the total content of Fe/Co derived from EDS analysis. For the nonporous solid particles (
Figure 5d), EELS mapping indicates a thick Pt shell up to 1 nm. Both of the porous structure and the thick Pt shell layer in the NPs cause a low compressive stress on the surface Pt [
31], explaining why the activity of PtCoFe-H400 is relatively poorer. In contrast, the ordered PtCoFe-H600 catalyst has almost no pores (
Figure 5e), but a solid core–shell structure with a thin outer layer of Pt (
Figure 5f,g). This reflects the ordered structure of PtCoFe-H600 can effectively protect non-noble metals from being etched and avoid the formation of porous structures. The resulting thinner Pt shell and the higher internal Fe/Co content make the ordered PtCoFe exhibit higher ORR performance.
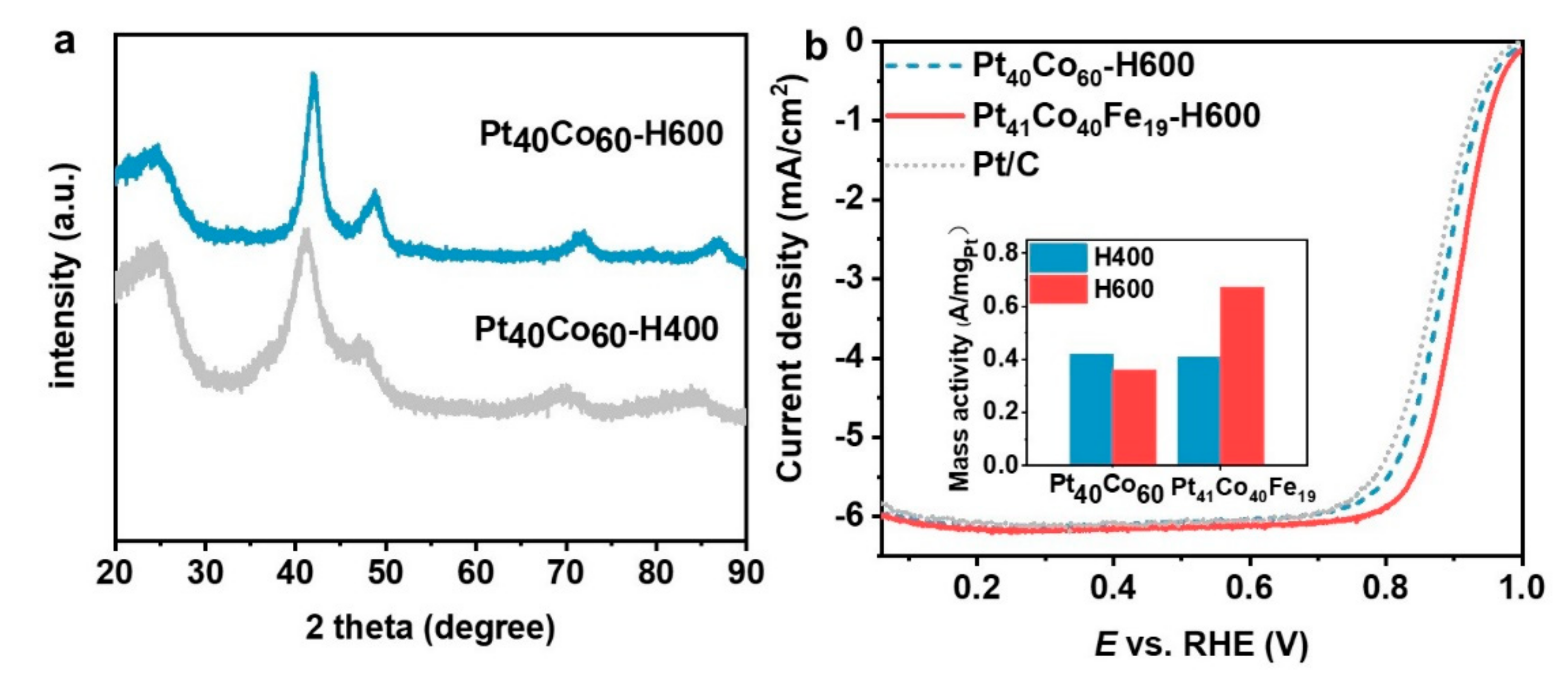
To further evidence the role of Fe in promoting the structural ordering, we also compared the PtCoFe-H600 catalyst (Pt
41Co
40Fe
19) with a homemade Pt
40Co
60 alloy catalyst at a similar Pt content (TEM and EDS results are shown in
Figure S3). The Pt
40Co
60 is still disordered after annealing at 600 °C (
Figure 6a), while Pt
41Co
40Fe
19 can be transformed into ordered phase after annealing at the same temperature for the same time. This suggests that the introduction of Fe with faster atomic diffusion rate can do promote the formation of structurally ordered structure [
24]. The ORR tests of Pt
40Co
60 annealed at 400 °C and 600 °C (Pt
40Co
60-H400 and Pt
40Co
60-H600, respectively) (
Figure S4 and
Figure 6b) indicate the mass activity of Pt
40Co
60-H600 did not increase, but instead decreased slightly (from 0.42 to 0.36 A/mg
Pt). The ORR activity of ordered Pt
41Co
40Fe
19 (0.65 A/mg
Pt) is substantially higher than that of Pt
40Co
60-H600 and four times of Pt/C catalyst (0.15 A/mg
Pt), which evidences the great advantage of ordered ternary alloys as ORR catalysts.
3. Experiments
Chemicals: Platinum acetylacetonate (Pt(acac)2, platinum 48%), nickel acetylacetonate (Ni(acac)2), cobalt acetylacetonate (Co(acac)2), iron acetylacetonate (Fe(acac)3), nickel acetate tetrahydrate (Ni(ac)2·4H2O, 99.999%), and dichlorobenzene (DCB, 99%) were purchased from Alfa Aesar (Heysham, UK). Benzyl ether (BE, 98%), oleylamine (OAm, 70%), oleic acid (OAc, 90%), tetradecanediol (TDD, 90%), and hexadecanediol (HDD, 90%) were bought from Sigma Alrich (Saint Louis, MS, USA). Cobalt acetate tetrahydrate (Co(ac)2·4H2O), trioctylamine (TOAm, 97%), and n-hexane (analytical grade) were bought from Sinopharm (Beijing, China), Acros Organics (Belgium) and Hushi (Shanghai, China), respectively. All reagents were not further purified.
Synthesis of ternary Pt alloys: The synthesis of ternary Pt-alloy nanoparticles (NPs) was carried out by organic liquid phase reduction methods [
32,
33,
34]. The synthesis of Pt–Ni–Co NPs was carried out according to the following procedure; 0.2 mmol Ni(ac)
2·4H
2O, 0.3 mmol Co(ac)
2·4H
2O, 50 mg TDD, 0.2 mL OAm, and 0.2 mL OAc were dissolved in 15 mL BE contained in a 50 mL three-necked flask. Then, the mixture was heated at 110 °C for 30 min to remove residual water in the reaction system; 0.1 mmol of Pt(acac)
2 dissolved in 0.75 mL DCB was injected immediately as soon as the temperature was elevated to 200 °C and the reaction was hold for 1 h, 20 mL ethanol was added, and the mixture was centrifuged at 8000 r/min for 10 min.
The Pt–Co–Fe ternary alloy NPs were prepared by the following method: 0.4 mmol Fe(acac)3 and 0.2 mmol Co(ac)2·4H2O, 50 mg TDD, 0.2 mL OAm, 0.2 mL OAc were dissolved in 15 mL BE; after the water removal step, the temperature was raised to 200 °C, and kept at this temperature for 2 h; 0.1 mmol of Pt(acac)2 dissolved in 0.75 mL DCB was injected, and the temperature was raised to 240 °C for 1 h; the obtained colloid mixed with 20 mL ethanol was centrifuged at 8000 r/min for 10 min.
Synthesis of Pt–Fe–Ni ternary alloy NPs was carried out as follows: 0.175 mmol Pt(acac)2, 0.25 mmol Fe(acac)3, 0.075 mmol Ni(acac)2, 1 mmol HDD, and 0.25 mL OAc were dissolved in 20 mL TOAm; after the water removal step, the temperature was raised to 330 °C for 1 h; the resulted colloid was added with 20 mL ethanol and centrifuged at 8000 r/min for 10 min. The above reactions were all carried out under an argon atmosphere. The centrifuged nanoparticles were separately dispersed in hexane and a calculated amount of carbon black (Vulcan XC72, designed Pt mass percentage of 15%) was added. The mixture was ultrasonicated with a cell pulverizer for 15 min, followed by centrifugation at 8000 r/min for 5 min, and finally the carbon-supported NPs were dried in a vacuum oven at 60 °C for 12 h.
Thermal annealing of nanoparticles: The carbon-supported ternary alloy NPs were first heated in air at 180 °C for 1 h to remove the surfactants on the nanoparticle surface, and then argon was introduced for 30 min to remove the air, followed by purging Ar/4% H2 for another 30 min to create a reductive atmosphere. Then the temperature was elevated to 400 °C and kept for 4 h to realize alloying and disordered alloys were prepared. The disordered ternary alloy catalysts were further annealed at 600 °C for 4 h in an atmosphere of Ar/4% H2 and then naturally cooled down to realize ordering transformation. In addition, the Pt–Ni–Co ternary alloy was annealed at 800 °C for 4 h and 12 h, respectively.
Electrochemical tests: Electrochemical tests were performed on a BioLogic electrochemical workstation (Seyssinet-Pariset, France). The working electrode was a PINE rotating disk electrode loading a catalyst film, the reference electrode was a Hg/Hg2SO4 electrode, and the counter electrode was a Pt mesh electrode. To prepare the working electrode, a catalyst slurry was prepared at catalyst concentration of 1.6 mg/mL, with the solvent consisting of a mixture of isopropanol, ultrapure water, and 5% Nafion (volume ratio: 1:4:0.02). Ten microliters of the uniformly ultrosonicated slurry was dropped on the glassy carbon region of the polished disk electrode, and dried to obtain a uniform and matte catalyst film. The cyclic voltammetry (CV) test was carried out in N2-saturated 0.1 M HClO4 with a test range of 0.06 to 1.0 V (vs. reversible hydrogen electrode, RHE, unless otherwise specified) at 100 mV/s. Electrochemical dealloying was conducted by potential cycling between 0.06 and 1.0 V for 200 cycles at a scanning rate of 500 mV/s. Linear Scanning Voltammetry (LSV) was performed under O2-saturated 0.1 M HClO4 within a range of 0.06 to 1.0 V at a scanning speed of 5 mV/s. The stability test was carried out by scanning CV for 10,000 cycles at a rate of 100 mV/s in the range of 0.6 to 1.0 V.
Materials Characterization: Transmission electron microscopy (TEM) characterizations were performed on FEI TECNAI G2 T12 (acceleration voltage of 120 kV, Netherlands). Scanning transmission electron microscopy (STEM) and electron energy loss spectroscopy (EELS) characterizations were performed on a FEI TECNAI G2 F30 electron microscope (acceleration voltage 300 kV, Netherlands) equipped with a Gatan GIF 965 EELS spectrometer (Pleasanton, CA, USA). X-ray diffraction (XRD) was performed on a Bruker D8 Advance diffractometer with a copper target (wavelength 1.5406 Å, Karlsruhe, Germany), a scanning range of 20 to 90°, and a stepping rate of 5°/min. Inductively Coupled Plasma mass Spectrometry (ICP-MS, SPECTRO, Kleve, Germany) was used to test the compositions of prepared alloys. The standard sample digestion process of ICP tests was carried out by adding 10 mg of catalyst to 12 mL of aqua regia, heating at 120 °C for 30 min, and filtering. Finally, the obtained solution was diluted with water to 250 mL.