Palladium Nanoparticles Supported on Graphene Oxide as Catalysts for the Synthesis of Diarylketones
Abstract
:1. Introduction
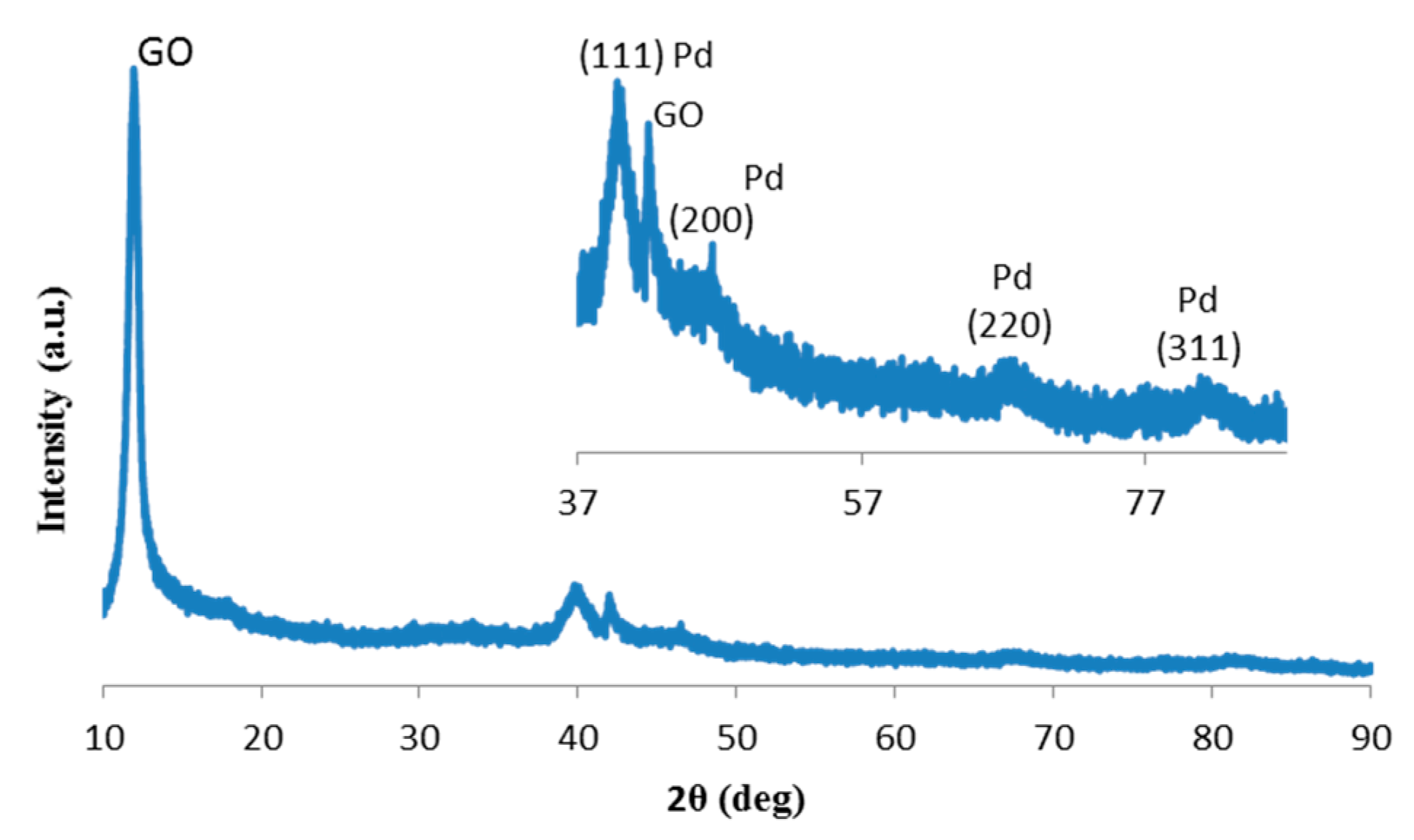
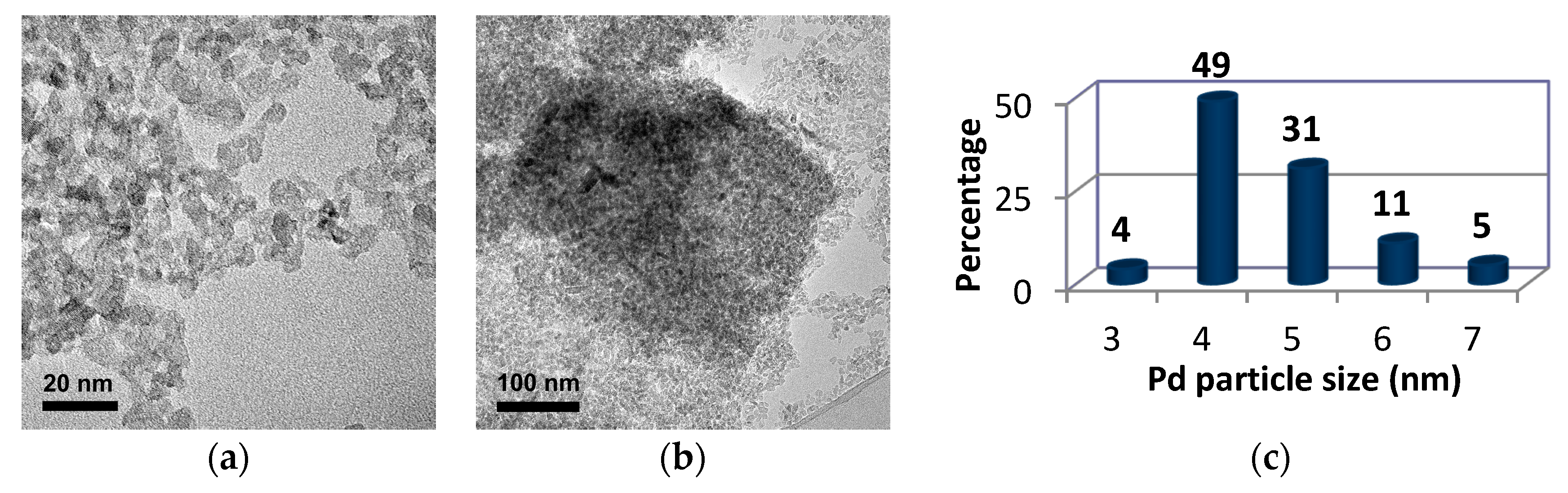
2. Results and Discussion
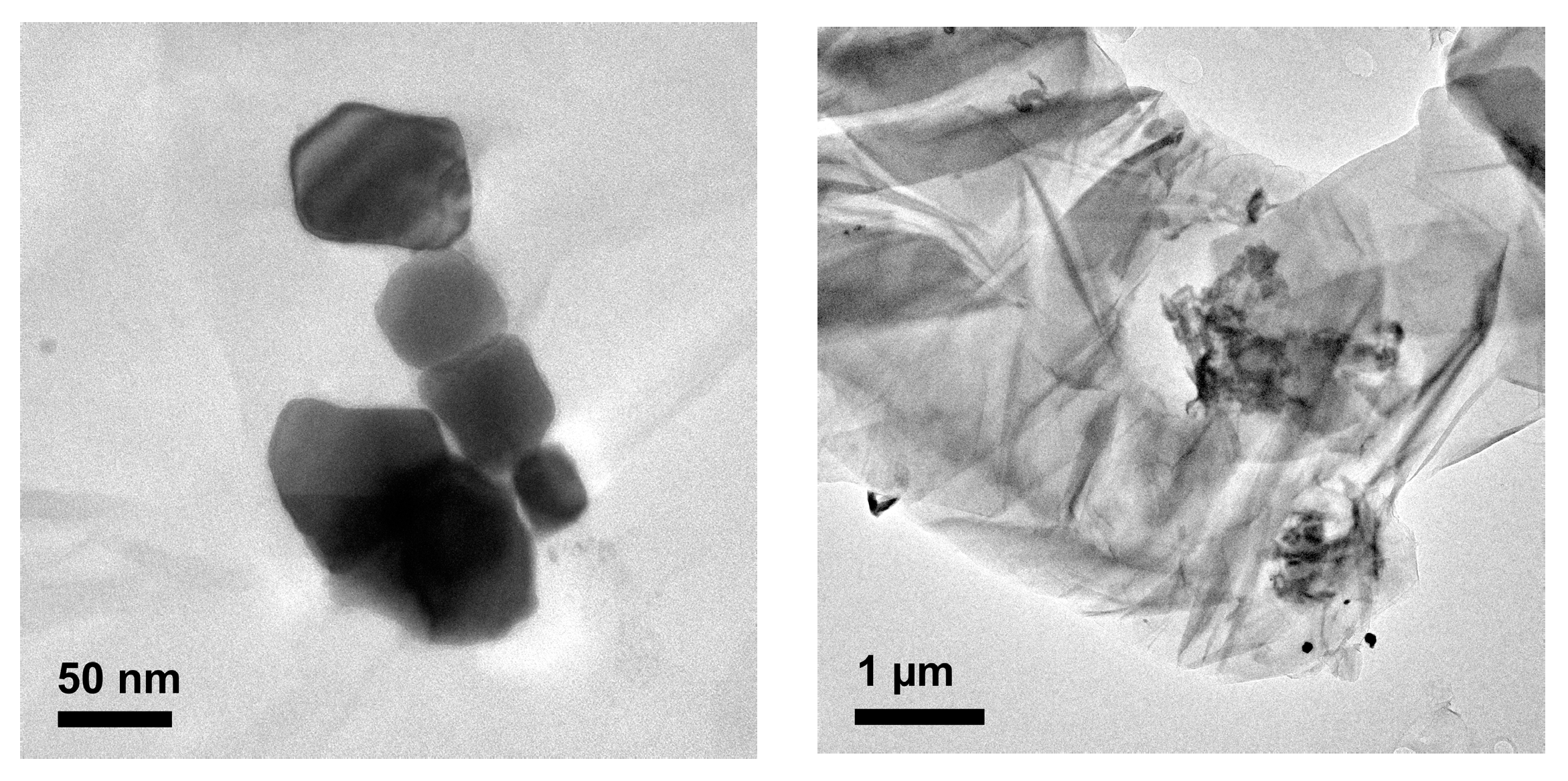
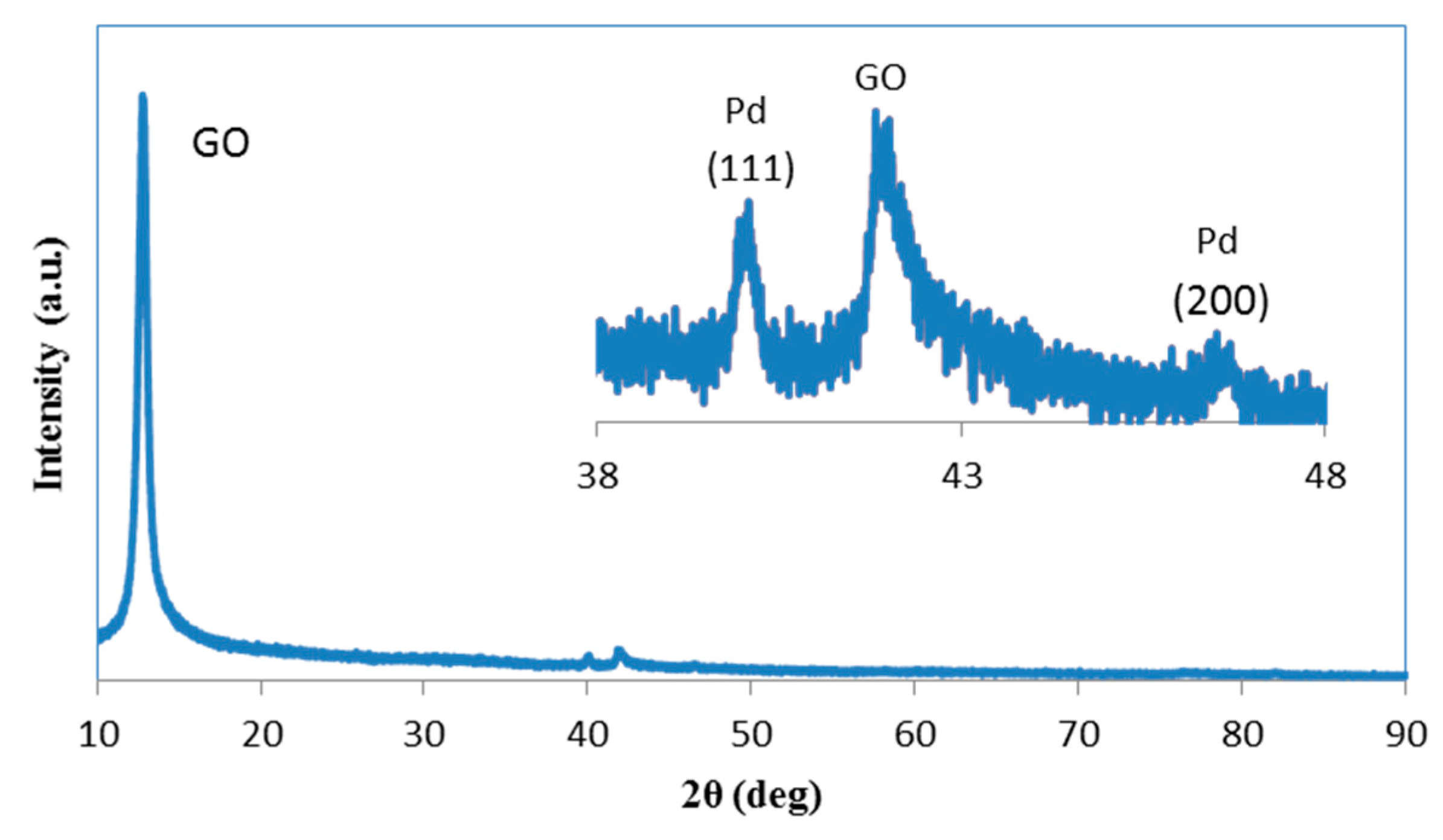
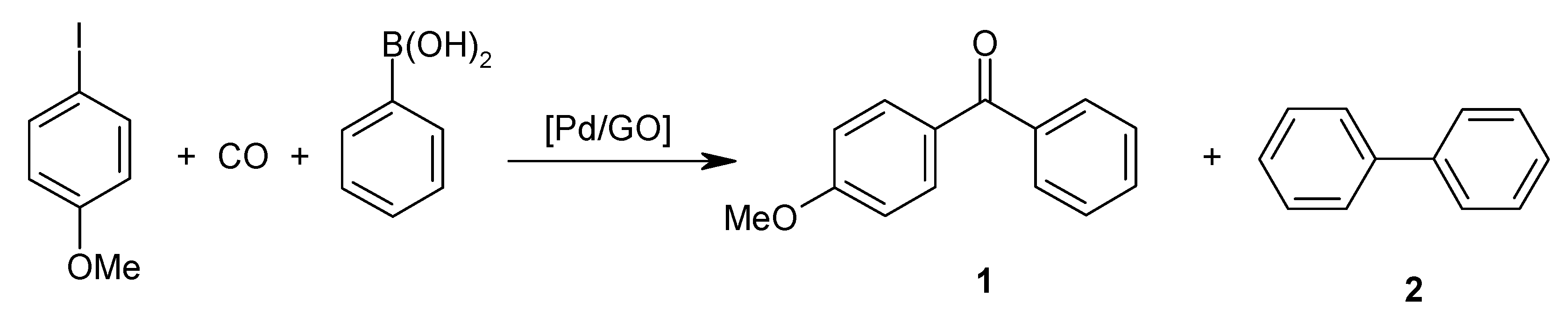
2.1. Catalytic Activity of Pd/GO
2.2. Optimization of the Reaction Conditions for Pd/GO-TiO2
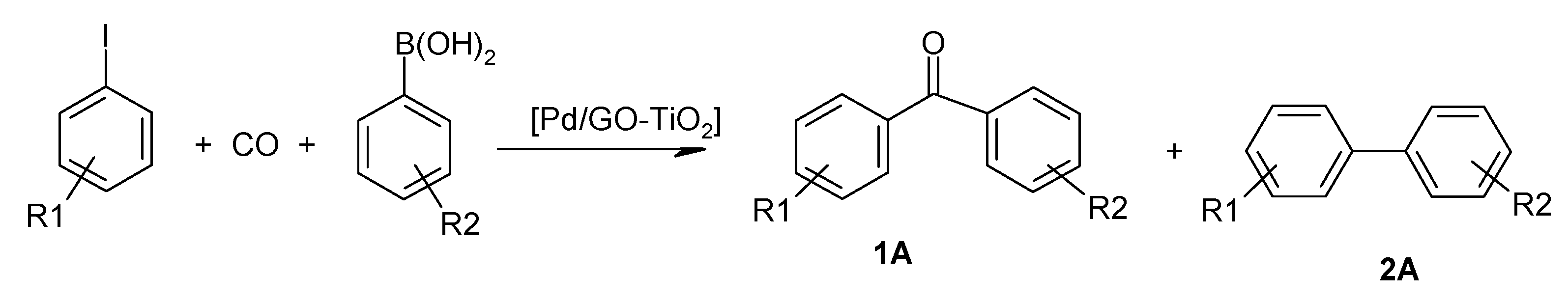
2.3. Substrate Screening in the Carbonylative Suzuki–Miyaura Coupling
2.4. Recycling of Pd/GO-B and Pd/GO-TiO2 Catalysts
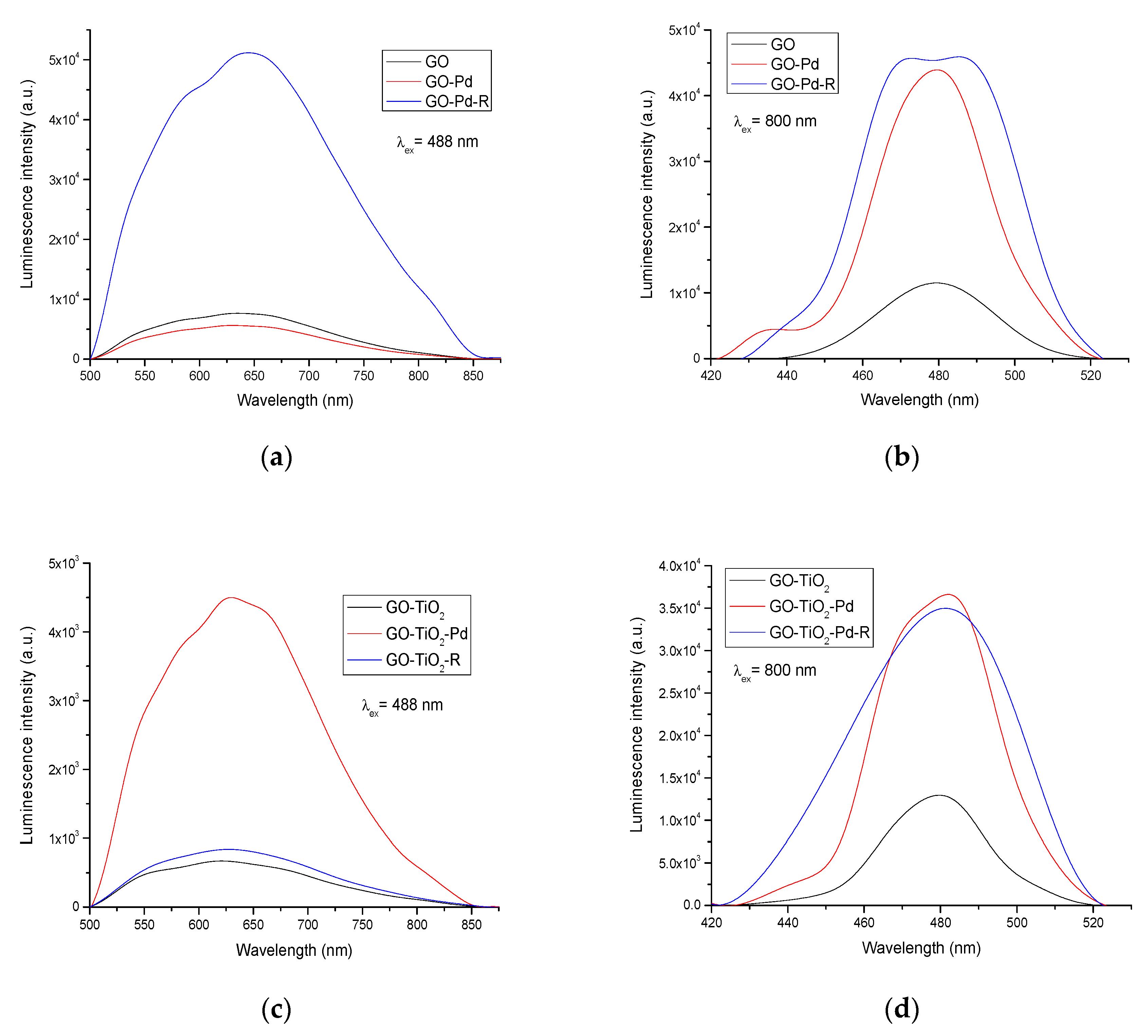
2.5. Luminescent Studies of Pd/GO Catalysts
3. Experimental
3.1. Synthesis of Pd/GO-A
3.2. Synthesis of Pd/GO-B
3.3. Synthesis of Pd/GO-TiO2
3.4. Electron Absorption and Emission Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Castro, M.; Lu, J.; Bruzaud, S.; Kumar, B.; Feller, J.F. Carbon nanotubes/poly (ε-caprolactone) composite vapour sensors. Carbon 2009, 47, 1930–1942. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhang, X.; Xu, Y.; Chen, Y.; Tian, J. Nonlinear optical properties of graphene oxide in nanosecond and picosecond regimes. Appl. Phys. Lett. 2009, 94, 021902. [Google Scholar] [CrossRef]
- Eda, G.; Mattevi, C.; Yamaguchi, H.; Kim, H.K.; Chhowalla, M. Insulator to Semimetal Transition in Graphene Oxide. Materials Science and Engineering, Rutgers University, Piscataway, New Jersey 08854. J. Phys. Chem. C 2009, 113, 15768–15771. [Google Scholar] [CrossRef]
- Omidvar, A.; Rashidian Vaziri, M.R.; Jaleh, B. Enhancing the nonlinear optical properties of graphene oxide by repairing with palladium nanoparticles. Phys. E Low Diment. Syst. Nanostruct. 2018, 103, 239–245. [Google Scholar] [CrossRef]
- Narayanam, P.K.; Sankaran, K. Optical behaviour of functional groups of graphene oxide. Mater. Res. Express 2016, 3, 105604. [Google Scholar] [CrossRef]
- Rumi, L.; Scheuermann, G.M.; Mülhaupt, R.; Bannwarth, W. Palladium Nanoparticles on Graphite Oxide as Catalyst for Suzuki-Miyaura, Mizoroki-Heck, and Sonogashira Reactions. Helv. Chim. Acta 2011, 94, 966–976. [Google Scholar] [CrossRef]
- Veisi, H.; Mirzaee, N. Ligand-free Mizoroki–Heck reaction using reusable modified graphene oxide-supported Pd(0) nanoparticles. Appl. Organomet. Chem. 2018, 32, e4067. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, G.; Du, J.; Tang, L.; Xu, J.; Li, J. New role of graphene oxide as active hydrogen donor in the recyclable palladium nanoparticles catalyzed Ullmann reaction in environmental friendly ionic liquid/supercritical carbon dioxide system. J. Mater. Chem. 2011, 21, 3485–3494. [Google Scholar] [CrossRef]
- Hemant Joshi, H.; Sharma, K.N.; Sharma, A.K.; Singh, A.K. Palladium–phosphorus/sulfur nanoparticles (NPs) decorated on graphene oxide: Synthesis using the same precursor for NPs and catalytic applications in Suzuki–Miyaura coupling. Nanoscale 2014, 6, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Shang, N.; Feng, C.; Wang, C.; Wang, Z. Graphene oxide–palladium modified Ag–AgBr: A visible-light-responsive photocatalyst for the Suzuki coupling reaction. RSC Adv. 2014, 4, 39242–39247. [Google Scholar] [CrossRef]
- Singh, V.V.; Kumar, U.; Tripathi, S.N.; Singh, A.K. Shape dependent catalytic activity of nanoflowers and nanospheres of Pd4S generated via one pot synthesis and grafted on graphene oxide for Suzuki coupling. Dalton Trans. 2014, 43, 12555–12563. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Yan, L. Three-dimensional reduced graphene oxide architecture embedded palladium nanoparticles as highly active catalyst for the Suzuki–Miyaura coupling reaction. Mater. Chem. Phys. 2014, 148, 103–109. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Khozestan, H.G.; Fath, R.H. Covalent attachment of 3-(aminomethyl)pyridine to graphene oxide: A new stabilizer for the synthesis of a palladium thin film at the oil–water interface as an effective catalyst for the Suzuki–Miyaura reaction. RSC Adv. 2015, 5, 47701–47708. [Google Scholar] [CrossRef]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Rolf Mülhaupt, R. Palladium Nanoparticles on Graphite Oxide and Its Functionalized Graphene Derivatives as Highly Active Catalysts for the Suzuki−Miyaura Coupling Reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Siamaki, A.R.; Khder, A.E.R.S.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-assisted synthesis of palladium nanoparticles supported on graphene: A highly active and recyclable catalyst for carbon–carbon cross-coupling reactions. J. Catal. 2011, 279, 1–11. [Google Scholar] [CrossRef]
- Moussa, S.; Siamaki, A.R.; Gupton, B.F.; El-Shall, M.S. Pd-Partially Reduced Graphene Oxide Catalysts (Pd/PRGO): Laser Synthesis of Pd Nanoparticles Supported on PRGO Nanosheets for Carbon–Carbon Cross Coupling Reactions. ACS Catal. 2012, 2, 145–154. [Google Scholar] [CrossRef]
- Nishina, Y.; Miyata, J.; Kawai, R.; Gotoh, K. Recyclable Pd–graphene catalyst: Mechanistic insights into heterogeneous and homogeneous catalysis. RSC Adv. 2012, 2, 9380–9382. [Google Scholar] [CrossRef]
- Sayedi, N.; Saidi, K.; Sheibani, H. Green Synthesis of Pd nanoparticles supported on magnetic graphene oxide by Origanum vilgae leaf plant extract: Catalytic activity in the reduction of organic dyes and Suzuki-Miyaura cross-coupling reaction. Catal. Lett. 2018, 148, 277–288. [Google Scholar] [CrossRef]
- Surjyakanta Rana, S.; Maddila, S.; Yalagala, K.; Jonnalagadda, S.B. Organo functionalized graphene with Pd nanoparticles and its excellent catalytic activity for Suzuki coupling reaction. Appl. Catal. A Gen. 2015, 505, 539–547. [Google Scholar] [CrossRef]
- Shang, N.; Gao, S.; Feng, C.; Zhang, H.; Wang, C.; Wang, Z.; Gao, S.; Feng, C.; Zhang, H.; Wang, C.; Wang, Z. Graphene oxide supported N-heterocyclic carbene-palladium as a novel catalyst for the Suzuki–Miyaura reaction. RSC Adv. 2013, 3, 21863–21868. [Google Scholar] [CrossRef]
- Santra, S.; Hota, P.K.; Bhattacharyya, R.; Bera, P.; Ghosh, P.; Mandal, S.K. Palladium Nanoparticles on Graphite Oxide: A Recyclable Catalyst for the Synthesis of Biaryl Cores. ACS Catal. 2013, 3, 2776–2789. [Google Scholar] [CrossRef]
- Gómez-Martínez, M.; Baeza, A.; Alonso, D.A. Graphene Oxide-Supported Oxime Palladacycles as Efficient Catalysts for the Suzuki–Miyaura Cross-Coupling Reaction of Aryl Bromides at Room Temperature under Aqueous Conditions. Catalysts 2017, 7, 94. [Google Scholar] [CrossRef]
- Biying, A.O.; Vangala, V.R.; Chen, C.S.; Stubs, L.P.; Hosmane, N.S.; Yinghuai, Z. Cross-coupling reaction between arylboronic acids and carboranyl iodides catalyzed by graphene oxide (GO)-supported Pd(0) recyclable nanoparticles for the synthesis of carboranylaryl ketones. Dalton Trans. 2014, 43, 5014–5020. [Google Scholar] [CrossRef]
- Rani, J.R.; Oh, J.; Park, J.; Lim, J.; Park, B.; Kim, K.; Kim, S.-J.; Jun, S.C. Controlling the luminescence emission from palladium grafted graphene oxide thin films via reduction. Nanoscale 2013, 5, 5620–5627. [Google Scholar] [CrossRef]
- Min, Y.L.; Zhang, K.; Zhao, W.; Zheng, F.C.; Zhang, Y.G. Enhanced chemical interaction between TiO2 and graphene oxide for photocatalytic decolorization of methylene blue. Chem. Eng. J. 2012, 193–194, 203–210. [Google Scholar] [CrossRef]
- Huang, Q.; Tian, S.; Zeng, D.; Wang, X.; Song, W.; Li, Y.; Xiao, W.; Xie, C. Enhanced Photocatalytic Activity of Chemically Bonded TiO2/Graphene Composites Based on the Effective Interfacial Charge Transfer through the C–Ti Bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Phan, N.T.S.; van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–680. [Google Scholar] [CrossRef]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]


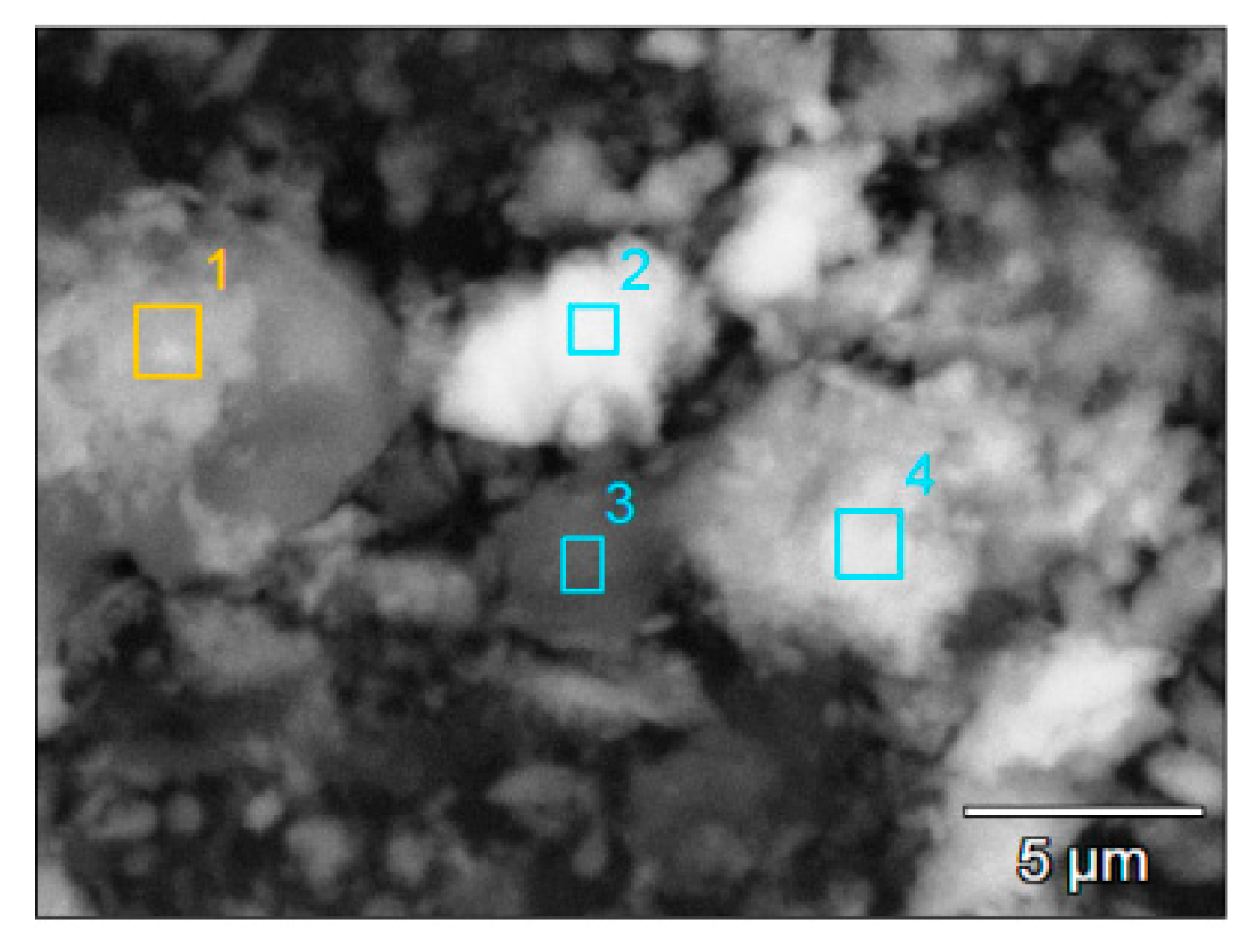
| Entry | Catalyst | Conversion (%) | Yield 1 (%) | Yield 2 (%) | Selectivity to 1 (%) |
|---|---|---|---|---|---|
| 1 | Pd/GO-A | 78 | 70 | 7 | 91 |
| 2 | Pd/GO-B | 82 | 74 | 7 | 90 |
| 3 | Pd/GO-TiO2 | 95 | 89 | 6 | 94 |
| 4 | Pd/C (10%) | 88 | 82 | 6 | 93 |
| Entry | Catalyst Loading (mol%) | K2CO3 (mmol) | Solvent | Temp. (°C) | Time (h) | Conversion (%) | Yield 1 (%) | Yield 2 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.23 | 3 | Anisole | 100 | 5 | 96 | 91 | 5 |
| 2 | 0.2 | 3 | Anisole | 100 | 4 | 95 | 89 | 6 |
| 3 | 0.2 | 2 | Anisole | 100 | 4 | 92 | 85 | 7 |
| 4 | 0.2 | 1 | Anisole | 100 | 4 | 77 | 72 | 5 |
| 5 | 0.2 | 3 | Anisole | 100 | 3 | 81 | 79 | 2 |
| 6 | 0.2 | 3 | Dioxane | 100 | 3 | 60 | 55 | 5 |
| 7 | 0.2 | 3 | Anisole | 80 | 3 | 51 | 51 | 0 |
| 8 | 0.2 | 3 | Water | 80 | 3 | 26 | 5 | 21 |
| Entry | Iodoarene | Arylboronic Acid | Conversion (%) | Yield 1A (%) | Yield 2A (%) | Selectivity to 1A (%) |
|---|---|---|---|---|---|---|
| 1 |  | 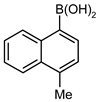 | 95 | 66 (57 b) | 27 | 72 |
| 2 | 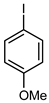 |  | 56 | 52 | 4 | 93 |
| 3 | 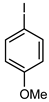 | 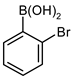 | 73 | 55 (42 b) | 18 | 75 |
| 4 |  | 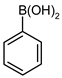 | 95 | 89 (80 b) | 6 | 94 |
| 5 | 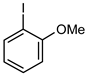 |  | 87 | 62 (50 b) | 25 | 71 |
| 6 |  | 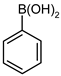 | 97 | 85 (77 b) | 12 | 88 |
| 7 |  | 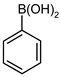 | 97 | 69 (58 b) | 28 | 71 |
| Entry | Recycling Run | Conversion (%) | Yield 1 (%) | Yield 2 (%) | Selectivity to 1 (%) |
|---|---|---|---|---|---|
| 1 | 0 | 97 | 94 | 3 | 97 |
| 2 | 1 | 98 | 82 | 16 | 84 |
| 3 | 2 | 84 | 66 | 18 | 78 |
| 4 | 3 | 80 | 63 | 17 | 79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzeciak, A.M.; Wojcik, P.; Lisiecki, R.; Gerasymchuk, Y.; Strek, W.; Legendziewicz, J. Palladium Nanoparticles Supported on Graphene Oxide as Catalysts for the Synthesis of Diarylketones. Catalysts 2019, 9, 319. https://doi.org/10.3390/catal9040319
Trzeciak AM, Wojcik P, Lisiecki R, Gerasymchuk Y, Strek W, Legendziewicz J. Palladium Nanoparticles Supported on Graphene Oxide as Catalysts for the Synthesis of Diarylketones. Catalysts. 2019; 9(4):319. https://doi.org/10.3390/catal9040319
Chicago/Turabian StyleTrzeciak, Anna M., Przemyslaw Wojcik, Radoslaw Lisiecki, Yuriy Gerasymchuk, Wieslaw Strek, and Janina Legendziewicz. 2019. "Palladium Nanoparticles Supported on Graphene Oxide as Catalysts for the Synthesis of Diarylketones" Catalysts 9, no. 4: 319. https://doi.org/10.3390/catal9040319
APA StyleTrzeciak, A. M., Wojcik, P., Lisiecki, R., Gerasymchuk, Y., Strek, W., & Legendziewicz, J. (2019). Palladium Nanoparticles Supported on Graphene Oxide as Catalysts for the Synthesis of Diarylketones. Catalysts, 9(4), 319. https://doi.org/10.3390/catal9040319







