Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Substrates
2.2. Inoculum Selection with Thermal and Acid Pretreatments in Hydrogen Production
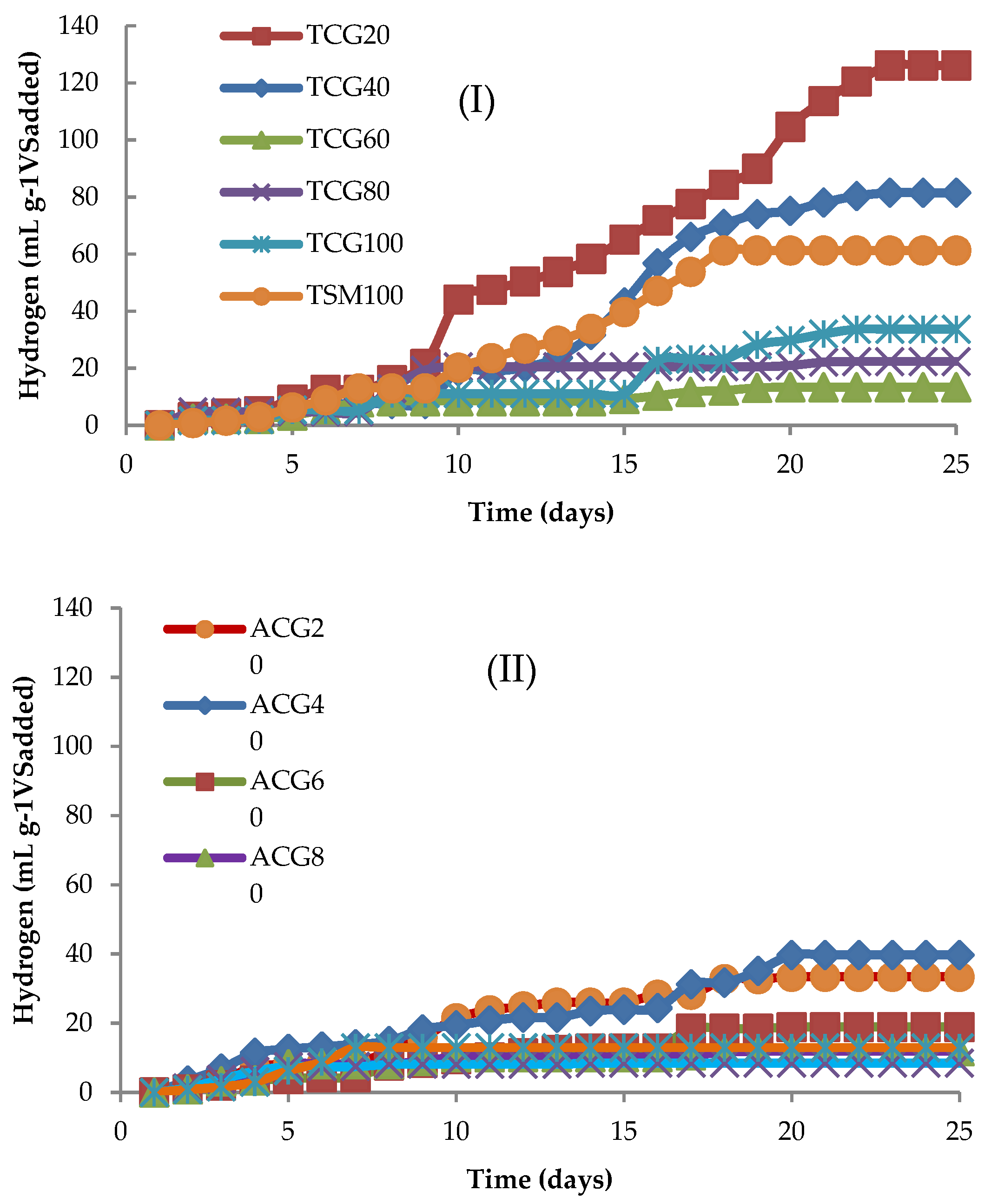
2.2.1. Kinetics of Hydrogen Yield
2.2.2. Effect of CG/SM Ratios on Reactors with Thermal and Acid Inocula
2.3. Increases in the Organic Load of the CG/SM Ratio with Thermal Inoculum
2.3.1. Characterization of the CG/SM Combination
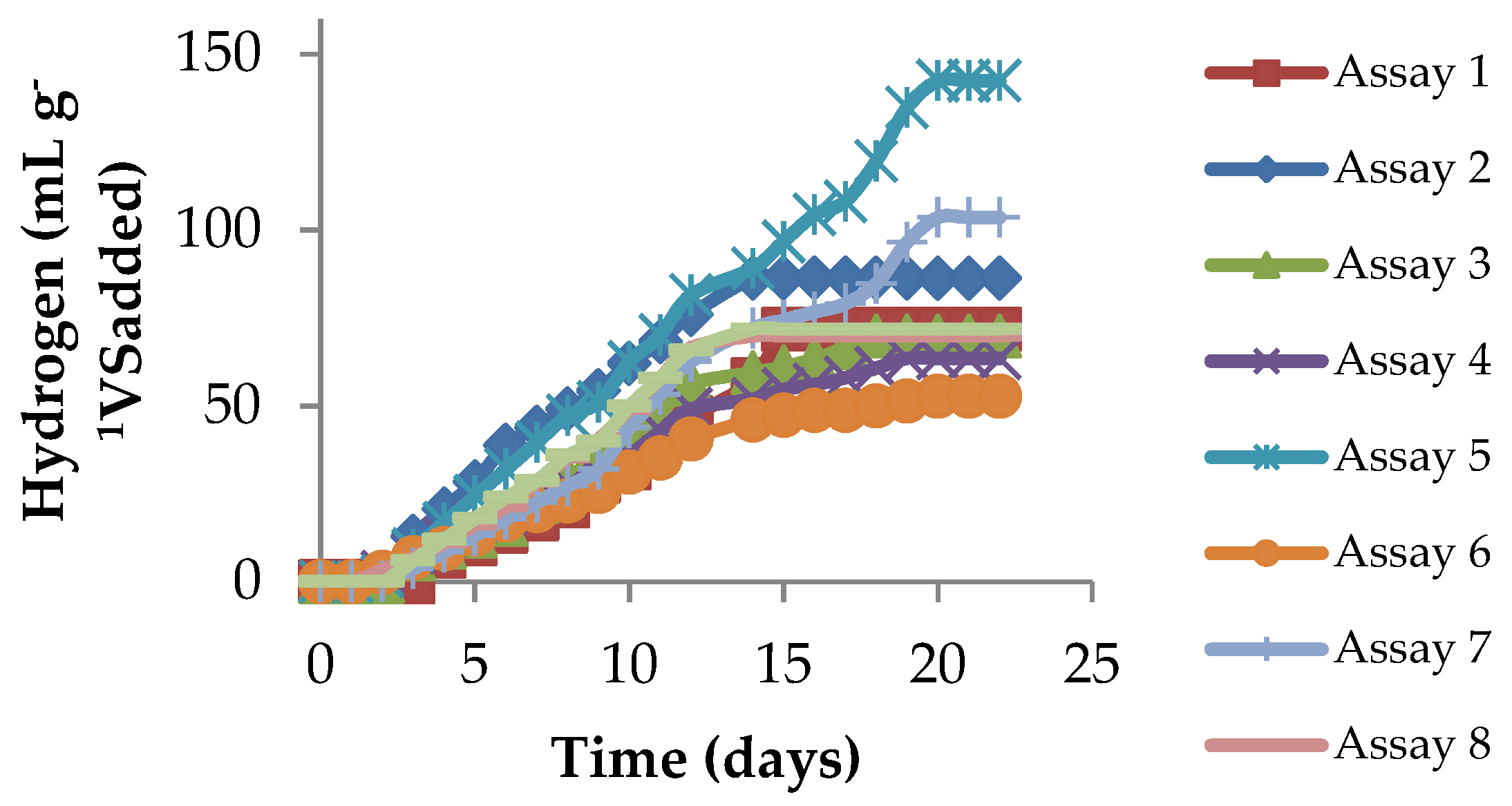
2.3.2. Kinetics of the Hydrogen Yield for Different Concentrations of the CG/SM Ratio
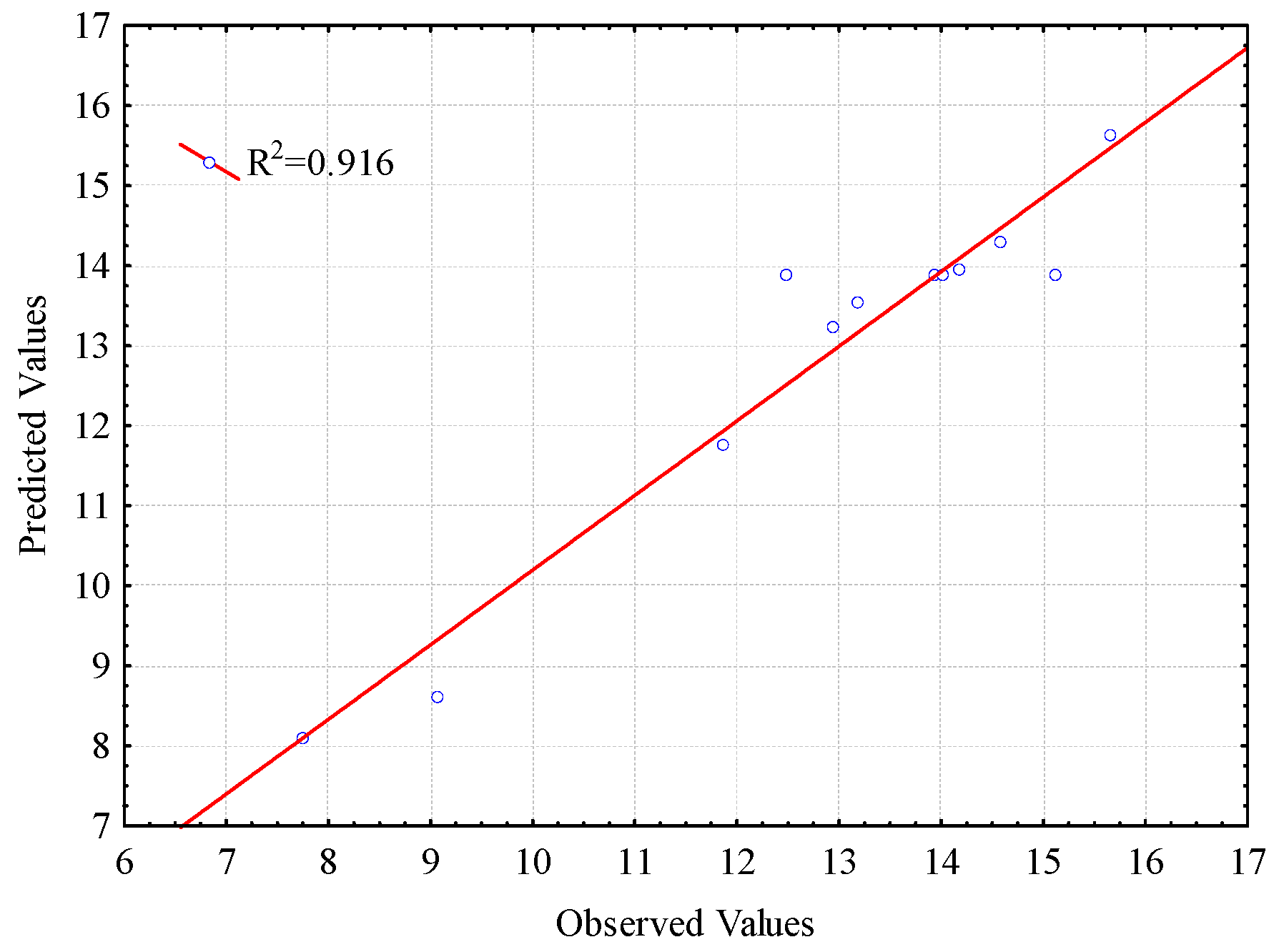
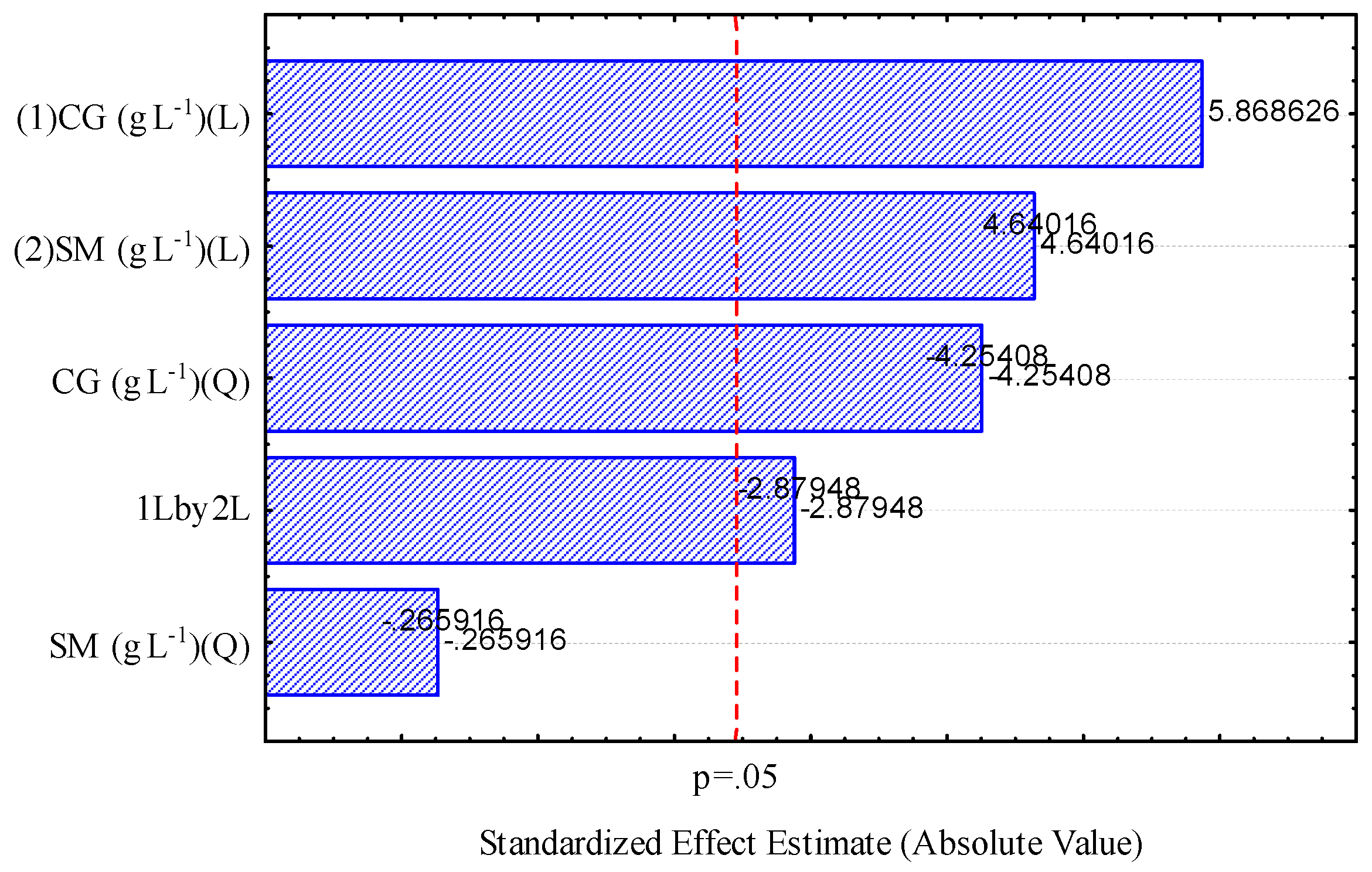
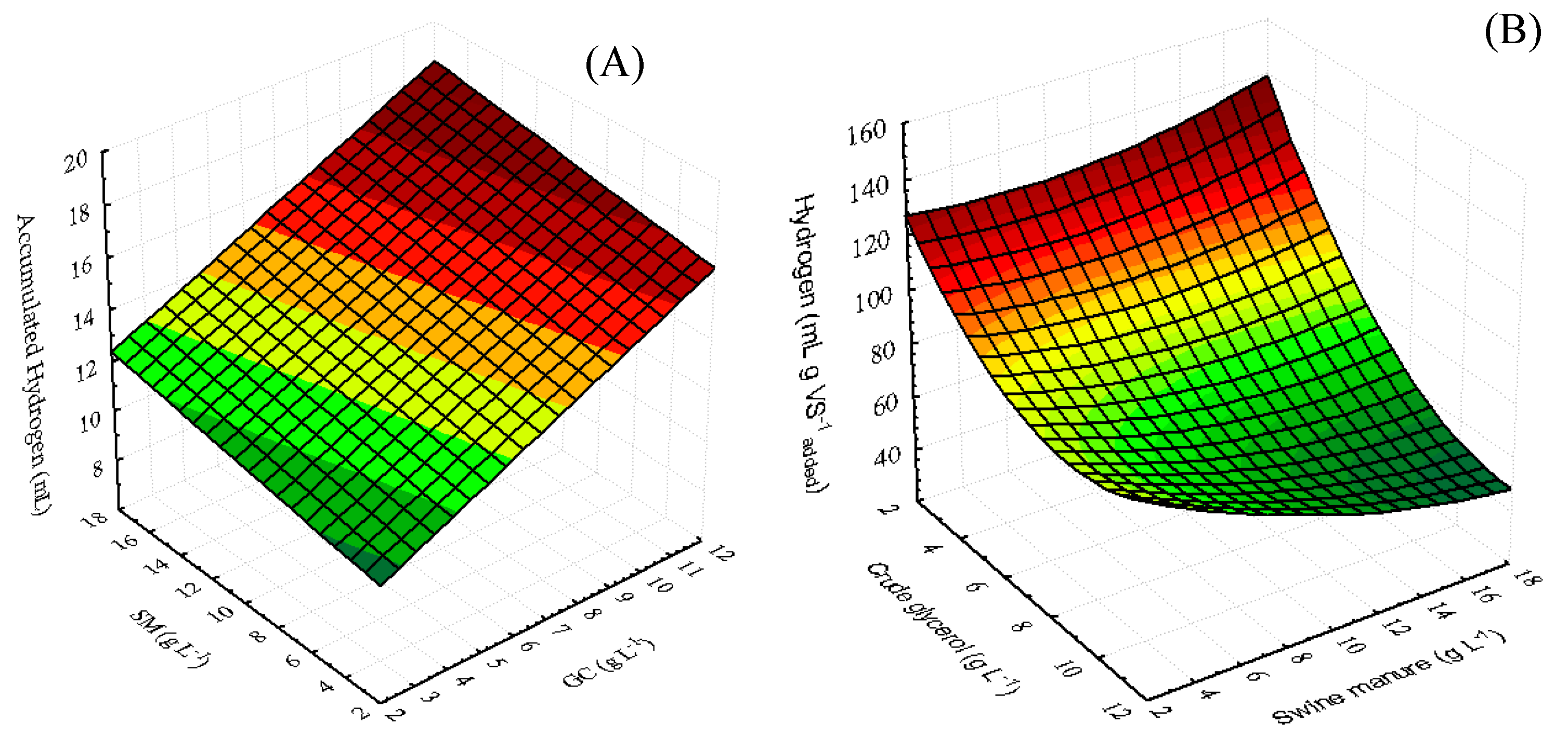
2.3.3. Analysis of the Response Surface and the Analytical Model
2.3.4. Volatile Fatty Acids
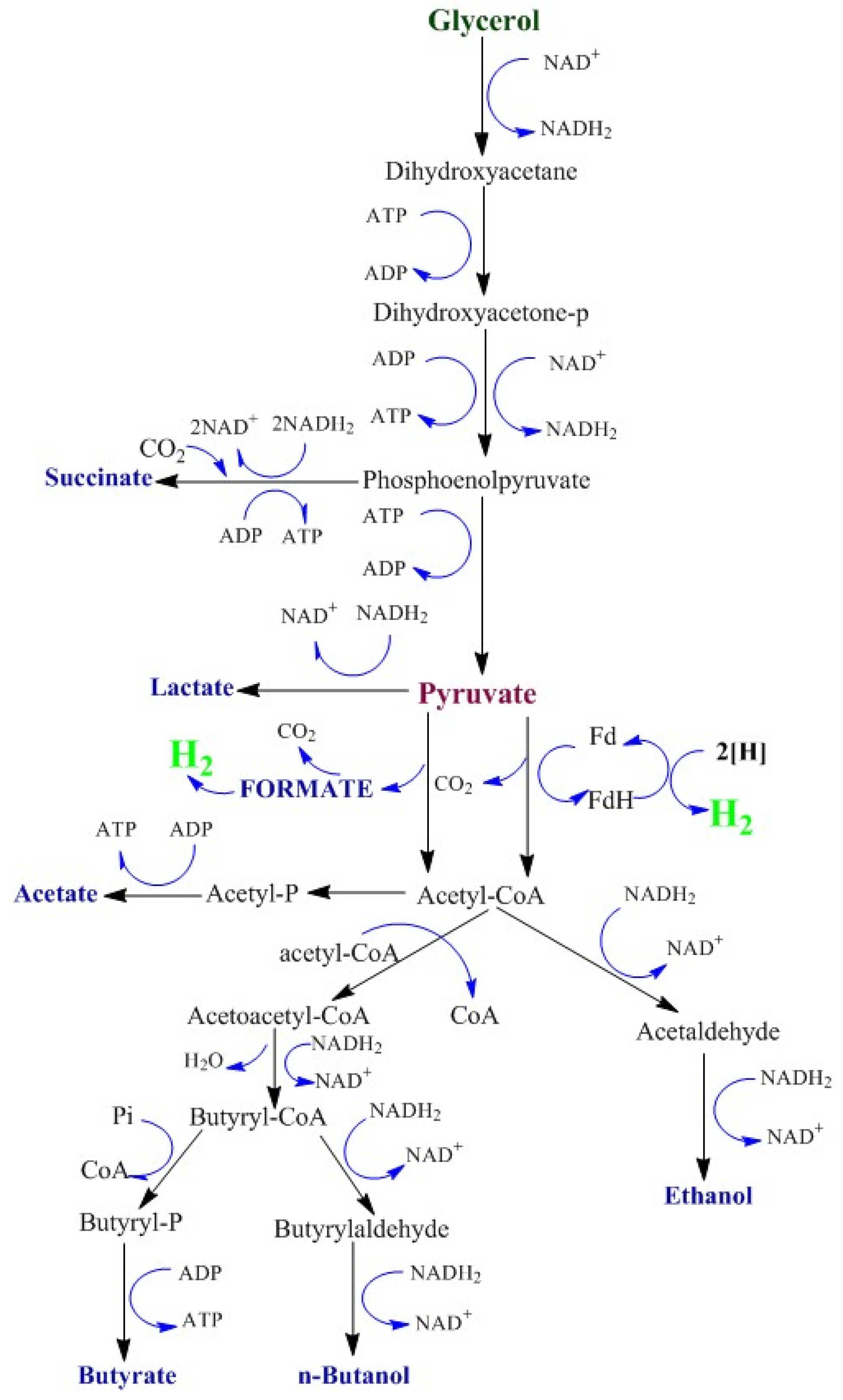
3. Discussion
4. Materials and Methods
4.1. Feedstock and Enrichment of Inoculum
4.2. Physicochemical Characterization of Residues
4.3. Comparison of Hydrogen-Producing Bacteria
4.4. Experimental Planning for Hydrogen Production
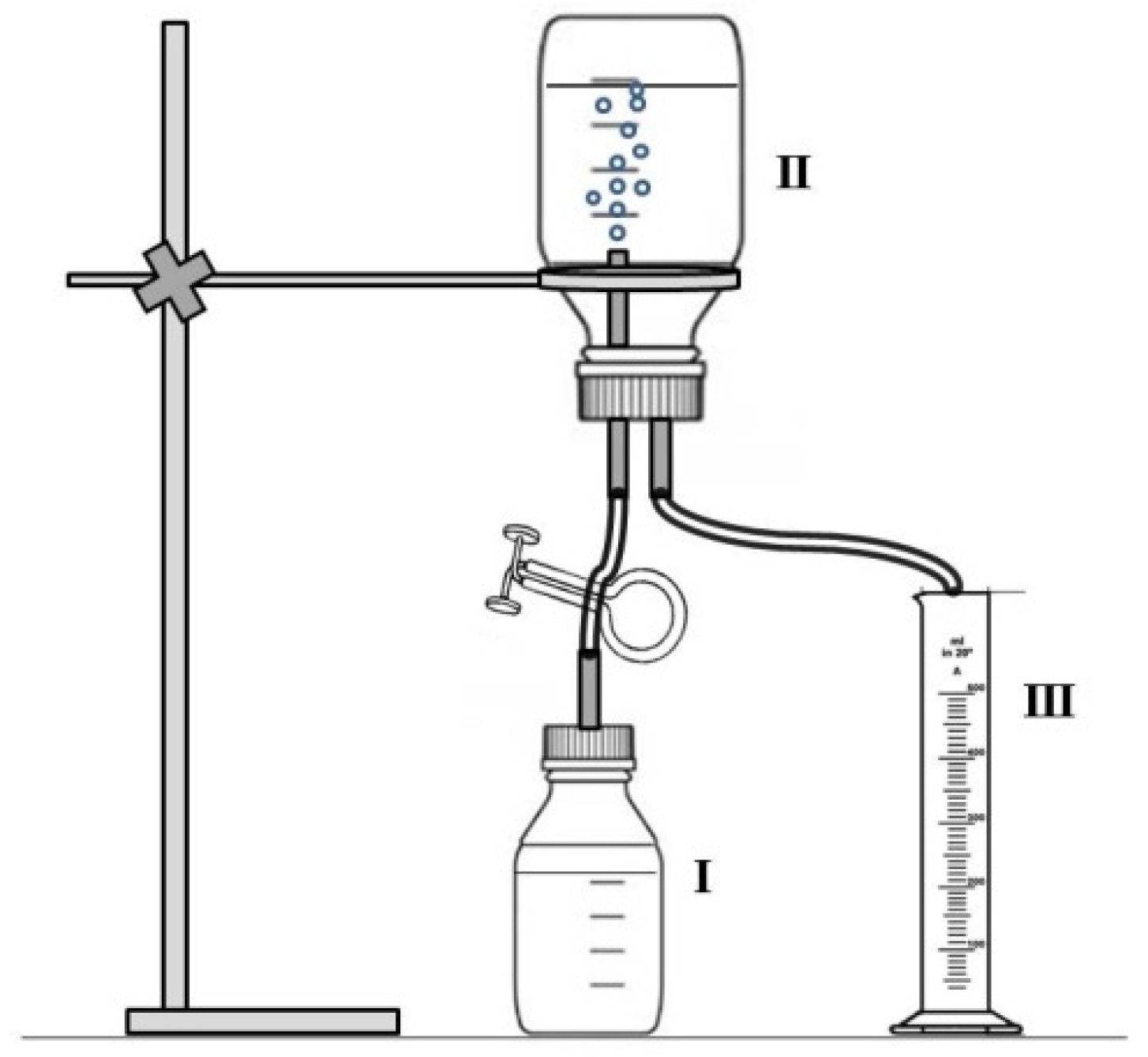
4.5. Quantification of the Volume of Hydrogen
4.6. Quantification of Glycerol and Organic Acids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Singh, L.; Wahid, Z.A. Methods for enhancing bio-hydrogen production from biological process: A review. J. Ind. Eng. Chem. 2015, 21, 70–80. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas – An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Improvements in fermentative biological hydrogen production through metabolic engineering. J. Environ. Manag. 2012, 95, S360–S364. [Google Scholar] [CrossRef] [PubMed]
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Kotsopoulos, T.A.; Fotidis, I.A.; Tsolakis, N.; Martzopoulos, G.G. Biohydrogen production from pig slurry in a CSTR reactor system with mixed cultures under hyper-thermophilic temperature (70 °C). Biomass and Bioenergy 2009, 33, 1168–1174. [Google Scholar] [CrossRef]
- Aguilar-Aguilar, F.A.; Nelson, D.L.; Pantoja, L.A.; Santos, A.S. Study of Anaerobic Co-digestion of Crude Glycerol and Swine Manure for the Production of Biogas Study of Anaerobic Co-digestion of Crude Glycerol and Swine Manure for the Production of Biogas. Rev. Virtual Química 2017, 9. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Anaerobic Thermophilic Digestion of Manure At Different Ammonia Loads: Effect of temperature. 1994, 28, 727–731. [Google Scholar]
- Zhu, J.; Li, Y.; Wu, X.; Miller, C.; Chen, P.; Ruan, R. Swine manure fermentation for hydrogen production. Bioresour. Technol. 2009, 100, 5472–5477. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.B.; Park, W.; Zuo, Y.; Logan, B.E. Inhibition of biohydrogen production by ammonia. Water Res. 2006, 40, 1167–1172. [Google Scholar] [CrossRef]
- Viana, M.B.; Freitas, A.V.; Leitão, R.C.; Pinto, G.A.S.; Santaella, S.T. Anaerobic digestion of crude glycerol: A review. Environ. Technol. Rev. 2012, 1, 81–92. [Google Scholar] [CrossRef]
- Mangayil, R.; Karp, M.; Santala, V. Bioconversion of crude glycerol from biodiesel production to hydrogen. Int. J. Hydrogen Energy 2012, 37, 12198–12204. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Guo, Q.; Qian, X.; Niu, D. Bio-hydrogen production from food waste and sewage sludge in the presence of aged refuse excavated from refuse landfill. Renew. Energy 2008, 33, 2573–2579. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Lazaro, C.Z.; Hallenbeck, P.C. Hydrogen production by co-cultures of Clostridium butyricum and Rhodospeudomonas palustris: Optimization of yield using response surface methodology. Int. J. Hydrogen Energy 2017, 42, 6578–6589. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Andres, Y.; Blel, W.; Gad, A. Methane Production By Anaerobic Co-Digestion Of Sewage Sludge And Wheat Straw Under Mesophilic Conditions. Int. J. Sci. Technol. Res. 2015, 4, 1–6. [Google Scholar]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Tian, H.; Duan, N.; Lin, C.; Li, X.; Zhong, M. Anaerobic co-digestion of kitchen waste and pig manure with different mixing ratios. J. Biosci. Bioeng. 2015, 120, 51–57. [Google Scholar] [CrossRef]
- Dennehy, C.; Lawlor, P.G.; Croize, T.; Jiang, Y.; Morrison, L.; Gardiner, G.E.; Zhan, X. Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag. 2016, 56, 173–180. [Google Scholar] [CrossRef]
- Ren, N.Q.; Guo, W.Q.; Wang, X.J.; Xiang, W.S.; Liu, B.F.; Wang, X.Z.; Ding, J.; Chen, Z.B. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ai, P.; Hu, C.X.; Zhang, Y.L. Effects of various pretreatment methods of anaerobic mixed microflora on biohydrogen production and the fermentation pathway of glucose. Int. J. Hydrogen Energy 2011, 36, 390–396. [Google Scholar] [CrossRef]
- Li, C.; Champagne, P.; Anderson, B.C. Evaluating and modeling biogas production from municipal fat, oil, and grease and synthetic kitchen waste in anaerobic co-digestions. Bioresour. Technol. 2011, 102, 9471–9480. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yu, H.Q.; Wang, G. Evaluation of three methods for enriching H2-producing cultures from anaerobic sludge. Enzyme Microb. Technol. 2007, 40, 947–953. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Optimization of fermentative hydrogen production process using genetic algorithm based on neural network and response surface methodology. Int. J. Hydrogen Energy 2009, 34, 255–261. [Google Scholar] [CrossRef]
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-treatment technologies for dark fermentative hydrogen production: Current advances and future directions. Waste Manag. 2018, 71, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Béland, M. Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int. J. Hydrogen Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Dong, L.; Zhenhong, Y.; Yongming, S.; Longlong, M. Evaluation of pretreatment methods on harvesting hydrogen producing seeds from anaerobic digested organic fraction of municipal solid waste (OFMSW). Int. J. Hydrogen Energy 2010, 35, 8234–8240. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2017, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Tenca, A.; Schievano, A.; Perazzolo, F.; Adani, F.; Oberti, R. Biohydrogen from thermophilic co-fermentation of swine manure with fruit and vegetable waste: Maximizing stable production without pH control. Bioresour. Technol. 2011, 102, 8582–8588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas Production by Co-Digestion of Goat Manure with Three Crop Residues. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Marone, A.; Varrone, C.; Fiocchetti, F.; Giussani, B.; Izzo, G.; Mentuccia, L.; Rosa, S.; Signorini, A. Optimization of substrate composition for biohydrogen production from buffalo slurry co-fermented with cheese whey and crude glycerol, using microbial mixed culture. Int. J. Hydrogen Energy 2015, 40, 209–218. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Peixoto, G.; Albrecht, F.R.; Saavedra del Aguila, N.K.; Zaiat, M. Potential to produce biohydrogen from various wastewaters. Energy Sustain. Dev. 2010, 14, 143–148. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Ferreira, T.F.; Fontes, G.C.; Coelho, M.A.Z. Glycerol valorization: New biotechnological routes. Food Bioprod. Process. 2009, 87, 179–186. [Google Scholar] [CrossRef]
- Drozdzyńska, A.; Leja, K.; Czaczyk, K. Biotechnological production of 1,3-propanediol from crude glycerol. Biotechnologia 2011, 92, 92–100. [Google Scholar] [CrossRef]
- Sarma, S.J.; Brar, S.K.; Sydney, E.B.; Le Bihan, Y.; Buelna, G.; Soccol, C.R. Microbial hydrogen production by bioconversion of crude glycerol: A review. Int. J. Hydrogen Energy 2012, 37, 6473–6490. [Google Scholar] [CrossRef]
- Varrone, C.; Giussani, B.; Izzo, G.; Massini, G.; Marone, A.; Signorini, A.; Wang, A. Statistical optimization of biohydrogen and ethanol production from crude glycerol by microbial mixed culture. Int. J. Hydrogen Energy 2012, 37, 16479–16488. [Google Scholar] [CrossRef]
- Guwy, A.J.; Dinsdale, R.M.; Kim, J.R.; Massanet-Nicolau, J.; Premier, G. Fermentative biohydrogen production systems integration. Bioresour. Technol. 2011, 102, 8534–8542. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yang, Q.; Yu, J.; Li, X.; Yang, G.; Xie, B.; Yang, F.; Zheng, W.; Zeng, G. Combined effect of sodium dodecyl sulfate and enzyme on waste activated sludge hydrolysis and acidification. Bioresour. Technol. 2011, 102, 7103–7110. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Akutsu, Y.; Lee, D.Y.; Li, Y.Y.; Noike, T. Hydrogen production potentials and fermentative characteristics of various substrates with different heat-pretreated natural microflora. Int. J. Hydrogen Energy 2009, 34, 5365–5372. [Google Scholar] [CrossRef]
- Bouallagui, H.; Lahdheb, H.; Ben Romdan, E.; Rachdi, B.; Hamdi, M. Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J. Environ. Manag. 2009, 90, 1844–1849. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater (APHA), 21st ed.; American Public Health Association Water Works Association, Ed.; American Water Environment Federation: Washington, DC, USA, 1995. [Google Scholar]
- Carlos Augusto de Lemos Chernicharo. Biological Wastewater Treatment Vol. 4: Anaerobic Reactors; Iwa publishing, Ed.: London, UK, 2007; Vol. 4, ISBN 9781843391647. [Google Scholar]
- Aquino, F.S.; Chernicharo, C.A.L.; Foresti, E.; Florêncio, M.L.S.; Monteggia, L.O. Metodologias para determinação da Atividade Metanogênica Específica (AME) em Lodos Anaeróbios. Eng. Sanit. e Ambient. 2007, 12, 192–201. [Google Scholar] [CrossRef]
| Parameters | CG | SM | Inoculum |
|---|---|---|---|
| pH | 10.35 ± 0.00 | 6.513 ± 0.53 | 6.73 ± 0.15 |
| TS (g L−1) | 870.34 ± 0.00 | 199.86 ± 0.01 | 6.77 ± 0.60 |
| VS (g L−1) | 870.09 ± 0.00 | 167.55 ± 0.01 | 5.04 ± 0.69 |
| VS/TS (% m m−1) | 99.97 | 83.83 | 74.39 |
| COD (g L−1) | 1974.40 | 137.83 | 5.47 |
| BOD (g L−1) | 1934.91 | 83.41 | n/d |
| BOD/COD | 0.98 | 0.61 | n/d |
| Carbon (% w w−1) | 88.04 | 49.62 | n/d |
| Nitrogen (% w w−1) | <0.05 | 4.08 | n/d |
| Oxygen (% w w−1) | 11.08 | 6.50 | n/d |
| Protein (% w w−1) | <0.05 | 25.55 | n/d |
| C/N Ratio | 1760.80 | 12.14 | n/d |
| SM(%) | CG(%) | C/N Ratio | Hydrogen (mL g−1VSadded) | ||
|---|---|---|---|---|---|
| pHinitial | Thermal Pretreatment | Acid Pretreatment | |||
| 80 | 20 | 16.24 | 7.38 ± 0.08 | c 126.14 ± 12.3 | a 33.44 ± 10.1 |
| 60 | 40 | 23.04 | 7.29 ± 0.20 | d 81.51 ± 9.9 | a 39.75 ± 4.9 |
| 40 | 60 | 36.48 | 7.33 ± 0.15 | e 13.38 ± 7.4 | b 18.92 ± 5.6 |
| 20 | 80 | 75.57 | 7.50 ± 0.11 | e 22.39 ± 10.2 | b 11.89 ± 3.1 |
| 0 | 100 | 1760.80 | 7.26 ± 0.23 | d 33.70 ± 6.98 | b 8.46 ± 4.4 |
| 100 | 0 | 12.14 | 7.00 ± 0.04 | d 61.25 ± 15.4 | b12.94 ± 2.7 |
| Assays | Concentration of the Combined Substrates (g L−1) | CODl (g L−1) | TS (g L−1) | VS (g L−1) | C/N Ratio (g g−1) | |
|---|---|---|---|---|---|---|
| CG | SM | |||||
| 1 | 4.00 | 5.00 | 7.17 ± 0.35 | 4.48 ± 0.13 | 4.32 ± 0.08 | 29.37 |
| 2 | 4.00 | 15.00 | 8.98 ± 0.79 | 6.48 ± 0.29 | 5.99 ± 0.13 | 17.88 |
| 3 | 10.00 | 5.00 | 16.56 ± 0.35 | 9.70 ± 0.39 | 9.54 ± 0.37 | 55.22 |
| 4 | 10.00 | 15.00 | 18.37 ± 0.26 | 11.70 ± 0.80 | 11.21 ± 0.67 | 26.50 |
| 5 | 2.75 | 10.00 | 6.12 ± 0.18 | 4.39 ± 0.37 | 4.07 ± 0.36 | 18.06 |
| 6 | 11.24 | 10.00 | 19.41 ± 0.35 | 11.78 ± 1.15 | 11.45 ± 1.07 | 36.35 |
| 7 | 7.00 | 2.93 | 11.49 ± 0.78 | 6.68 ± 0.70 | 6.58 ± 0.74 | 63.63 |
| 8 | 7.00 | 17.07 | 14.05 ± 0.10 | 9.50 ± 1.07 | 8.95 ± 0.97 | 20.97 |
| 9 | 7.00 | 10.00 | 12.77 ± 0.47 | 8.09 ± 0.27 | 7.77 ± 0.27 | 27.22 |
| 10 | 7.00 | 10.00 | 12.77 ± 0.47 | 8.09 ± 0.27 | 7.77 ± 0.27 | 27.22 |
| 11 | 7.00 | 10.00 | 12.77 ± 0.47 | 8.09 ± 0.27 | 7.77 ± 0.27 | 27.22 |
| 12 | 7.00 | 10.00 | 12.77 ± 0.47 | 8.09 ± 0.27 | 7.77 ± 0.27 | 27.22 |
| Assays | Concentration of Combined Substrates (%) | Hydrogen (mL g−1 VSadded) | Accumulated Hydrogen (mL) | |
|---|---|---|---|---|
| CG | SM | |||
| 1 | 44.44 | 55.56 | 71.78 ± 5.90 | 7.75 ± 0.37 |
| 2 | 21.05 | 78.95 | 86.36 ± 6.68 | 12.94 ± 0.33 |
| 3 | 66.67 | 33.33 | 69.86 ± 9.88 | 16.66 ± 1.37 |
| 4 | 40.00 | 60.00 | 63.57 ± 2.85 | 17.82 ± 0.55 |
| 5 | 21.56 | 78.44 | 142.46 ± 3.24 | 14.49 ± 0.63 |
| 6 | 52.92 | 47.08 | 52.76 ± 4.84 | 15.11 ± 0.94 |
| 7 | 70.51 | 29.49 | 103.61 ± 6.71 | 17.05 ± 0.91 |
| 8 | 29.08 | 70.92 | 69.95 ± 4.97 | 15.65 ± 0.99 |
| 9 | 41.18 | 58.82 | 71.84 ± 4.69 | 13.95 ± 0.81 |
| 10 | 41.18 | 58.82 | 77.82 ± 5.33 | 15.11 ± 1.04 |
| 11 | 41.18 | 58.82 | 72.23 ± 5.05 | 14.02 ± 0.93 |
| 12 | 41.18 | 58.82 | 64.25 ± 5.57 | 13.89 ± 1.08 |
| Assay | Acetic Acid (g L−1) | Butyric Acid (g L−1) | Propionate Acid (g L−1) | Final pH |
|---|---|---|---|---|
| 1 | 48.69 | 0.00 | 0.00 | 5.17 |
| 2 | 48.70 | 0.00 | 0.08 | 5.17 |
| 3 | 0.00 | 0.00 | 0.00 | 5.89 |
| 4 | 48.69 | 0.00 | 0.10 | 5.92 |
| 5 | 48.68 | 0.00 | 0.07 | 5.88 |
| 6 | 48.70 | 68.15 | 0.05 | 4.77 |
| 7 | 48.69 | 0.00 | 0.00 | 5.89 |
| 8 | 48.67 | 68.08 | 0.07 | 5.14 |
| 9 | 48.67 | 0.00 | 0.03 | 4.93 |
| 10 | 48.67 | 68.15 | 0.05 | 4.72 |
| 11 | 48.67 | 68.10 | 0.03 | 5.02 |
| 12 | 48.67 | 0.00 | 0.03 | 5.03 |
| Assay with Inoculum Pretreated with Acid | Assay with Thermally Pretreated Inoculum | CG (%) | SM (%) | COD (g L−1) |
|---|---|---|---|---|
| ACG100 | TCG100 | 0 | 100 | 4.59 ± 0.18 |
| ASM100 | TSM100 | 100 | 0 | 4.54 ± 0.18 |
| ACG80 | TCG80 | 80 | 20 | 4.49 ± 0.17 |
| ACG60 | TCG60 | 60 | 40 | 4.45 ± 0.17 |
| ACG40 | TCG40 | 40 | 60 | 4.65 ± 0.18 |
| ACG20 | TCG20 | 20 | 80 | 3.20 ± 0.12 |
| Factors | Axial (−α) | Minimum (−1) | Central Point (0) | Maximum (+1) | Axial (+α) |
|---|---|---|---|---|---|
| Crude Glycerol (g L−1) | 2.75 | 4.00 | 7.00 | 10.00 | 11.24 |
| Swine Manure (g L−1) | 2.93 | 5.00 | 10.00 | 15.00 | 17.07 |
| Assays | CG (g L−1) | SM (g L−1) | (I/S) Ratio (g VS g VS−1) |
|---|---|---|---|
| 1 | 4.00 | 5.00 | 1.16 |
| 2 | 4.00 | 15.00 | 0.83 |
| 3 | 10.00 | 5.00 | 0.52 |
| 4 | 10.00 | 15.00 | 0.45 |
| 5 | 2.75 | 10.00 | 1.23 |
| 6 | 11.24 | 10.00 | 0.44 |
| 7 | 7.00 | 2.93 | 0.76 |
| 8 | 7.00 | 17.07 | 0.56 |
| 9 | 7.00 | 10.00 | 0.64 |
| 10 | 7.00 | 10.00 | 0.64 |
| 11 | 7.00 | 10.00 | 0.64 |
| 12 | 7.00 | 10.00 | 0.64 |
| Macro Nutrients | Concentration (mg L−1) | Micro Nutrients | Concentration (mg L−1) |
|---|---|---|---|
| NH4Cl | 1112 | FeCl3·6H2O | 5 |
| (NH4)H2PO4 | 132.5 | ZnCl2 | 0.13 |
| (NH4)2HPO4 | 44.5 | MnCl2·4H2O | 1.25 |
| MgCl2·6H2O | 250 | (NH4)6MO7O24·4H2O | 1.6 |
| CaCl2·2H2O | 189 | AlCl3·6H2O | 0.13 |
| NaHCO3 | 2500 | CoCl2·6H2O | 5 |
| - | - | NICl2·6H2O | 13 |
| - | - | H3BO3 | 3 |
| - | - | CuCl2·2H2O | 8 |
| - | - | HCl | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
F. A., A.-A.; Longoria, A.; A. U., J.; A. S., S.; L. A., P.; Sebastian, P.J. Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure. Catalysts 2019, 9, 316. https://doi.org/10.3390/catal9040316
F. A. A-A, Longoria A, A. U. J, A. S. S, L. A. P, Sebastian PJ. Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure. Catalysts. 2019; 9(4):316. https://doi.org/10.3390/catal9040316
Chicago/Turabian StyleF. A., Aguilar-Aguilar, Adriana Longoria, Juantorena A. U., Santos A. S., Pantoja L. A., and P. J. Sebastian. 2019. "Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure" Catalysts 9, no. 4: 316. https://doi.org/10.3390/catal9040316
APA StyleF. A., A.-A., Longoria, A., A. U., J., A. S., S., L. A., P., & Sebastian, P. J. (2019). Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure. Catalysts, 9(4), 316. https://doi.org/10.3390/catal9040316





