Abstract
Glycerol is one of the most crucial by-products in the production of biodiesel, and owing to its oversaturation in the market, several synthetic strategies have been developed to transform it into other higher value-added products such as glycerol carbonate, epichlorohydrin, 1,3-propanediol, etc. Amongst them, glycerol carbonate is considered to be the most valuable product. Considering the facile separation and reusability of catalyst, heterogeneous base catalysts have attracted considerable attention due to the obvious advantages over Brϕnsted acid and homogeneous base catalysts in the transesterification of glycerol. Herein, we will give a short overview on the recent development of the heterogeneous catalysis in the transesterification of glycerol with dialkyl carbonate. Focus will be concentrated on the heterogeneous base catalysts including alkaline-earth metal oxides (MgO, CaO, and mixed oxides), hydrotalcites, zeolites, clinoptilolites, organic bases, etc. Their catalytic mechanisms during the heterogeneous process will be elucidated in detail.
1. Introduction
With the increasing demand for energy sources and environmental safety, biodiesel as a clean and renewable energy is considered to be a potentially high alternative energy of traditional fuels. The classical transesterification of vegetable oil and animal oil are the most widely used methods in large-scale production of biodiesel (Figure 1) [1,2,3,4]. Accordingly, during the production of biodiesel, glycerol is also generated simultaneously as a crucial by-product. According to the statistics [5], every nine kilograms of biodiesel can produce one kilogram of glycerol. However, with the rapid growing demand for biodiesel, glycerol is confronted with oversaturation in the market. Therefore, seeking solutions for the full use of this excess of glycerol is highly urgent and desirable.
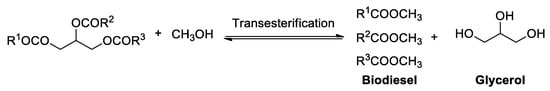
Figure 1.
Biodiesel syntheses via transesterification.
Since only a small proportion of glycerol goes directly into applications, it becomes highly desirable to seek for strategies to transform it into higher value-added products [6,7,8], such as glycerol carbonate, 1,3-dihydroxypropan-2-one, 1,3-propanediol, glycidol, epichlorohydrin, etc. (Figure 2). As depicted in Figure 2, glycerol can easily and efficiently be transformed into various products via direct oxidation [9,10,11], hydrogenolysis [12,13], dehydration [14], acetalization [15], transesterification [16,17,18,19], etc. Amongst these products, glycerol carbonate is considered to be the most valuable product [20,21,22,23,24,25]. As a high value-added glycerol derivative, 4-hydroxymethyl-1,3-dioxolan-2-one (also named glycerol carbonate, GC) is widely used as a solvent, coating material, lubricant, and personal care product attributing to its special physical properties in boiling point, melting point, and water solubility [19]. In addition, due to its specific structural characteristic and reactivity, glycerol carbonate, as a versatile building block, also has valuable applications in carbon dioxide separation [20,21] and organic syntheses [22,23,24,25], such as ring opening reaction, decarboxylation, esterification, polymerization, etc.
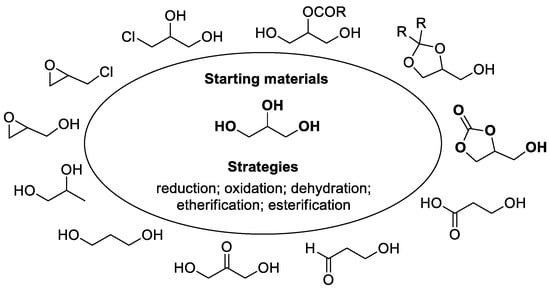
Figure 2.
Diversity in glycerol transformations.
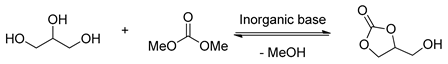
The diverse applications of glycerol carbonate has attracted much attention from chemists, and synthetic strategies for glycerol carbonate have developed rapidly in the past few decades. Traditional chemical syntheses are the most widely used methods in the large-scale preparation of glycerol carbonate directly from glycerol. With glycerol used as starting material, glycerol carbonate is obtained via chemical processes, such as carbonation with carbon dioxide [26,27,28] or carbon monoxide in the presence of oxidants [29,30], trans-carbonation with phosgene [21] or urea and its derivatives [31,32,33,34,35], and transesterification with simple carbonate esters (Figure 3) [15,16,17,18,19,20]. Transesterification of glycerol has significant advantages over other methodologies in the following aspects: (1) avoiding the employment and release of poisonous gases and toxic reagents; (2) higher activity and selectivity; (3) shorter reaction time and higher conversion; (4) free of solvent and simple separation of catalyst; and (5) environmentally friendly and intrinsically safe.
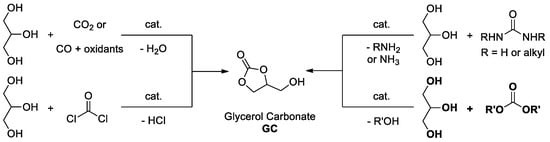
Figure 3.
Diverse syntheses of glycerol carbonate.
The activity and selectivity of transesterification rely heavily on the utility of catalyst systems. And catalysts often play crucial roles in the reaction, because they can promote the reactions in reducing activation energy of reaction, increasing the reactivity of substrates, and shortening reaction time. Various catalytic systems were developed over the past decades [36,37,38,39,40,41], and catalyst was concentrated on Brϕnsted acid [37], ionic liquid [38], organic base [39,40], enzyme [41], and heterogeneous base [15,16,17,18]. The traditional Brϕnsted acid catalyzed transesterification of glycerol was one of the most widely used methods for a long time, but it is usually limited by the drawbacks of equipment corrosion, the difficulty in catalyst recovery, and low conversion and selectivity [36]. Base catalysts have been extensively employed in the transesterification of glycerol carbonate in recent years. Although homogeneous base catalyst conducted well in the transesterification, it was still confronted with the similar matter of separation, recovery, and reusability of catalyst [36]. Therefore, heterogeneous base catalysts have attracted much more considerable attention because of their obvious advantages over homogeneous base catalysts in the transformation of glycerol especially in industry. Due to the reversibility of transesterification of glycerol with dialkyl carbonate, the conversion of glycerol is heavily dependent on the reaction temperature and molar ratio of starting materials [42]. This review aims at providing a short overview on the recent development of heterogeneous catalysis in the transesterification of glycerol to glycerol carbonate (Figure 4).

Figure 4.
Heterogeneous catalysis in the transesterification of glycerol.
2. Heterogeneous Catalysis in the Transesterification of Glycerol to Glycerol Carbonate
Heterogeneous catalysis in the transesterification of glycerol to glycerol carbonate has attracted much attention in recent years. Mechanisms for heterogeneous base catalysis in the transesterification of glycerol were completely different from the widely researched mechanism of traditional homogeneous Brϕnsted acid catalysis [43,44]. The general procedure for heterogeneous base catalysis in the transesterification of glycerol is depicted as follows according to the literature [36,43,44] (Figure 5). The base catalyst abstracts proton from the glycerol molecule, and the in situ generated glyceroxide anion then attacks dimethyl carbonate (DMC) as a nucleophile, along with the loss of a methanol molecule. Subsequently, followed by an intramolecular nucleophilic substitution, the target product glycerol carbonate is formed.
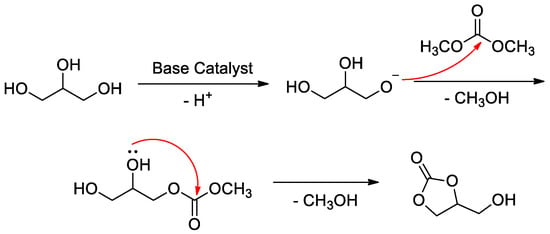
Figure 5.
General procedure for the transesterification of glycerol.
2.1. Alkaline Earth Metal Oxides Catalyzed Transesterification of Glycerol
Heterogeneous base catalysts possess strong basic sites at the solid surface which can effectively increase the reactivity of glycerol. Metal oxides, as the most important heterogeneous bases, tend to exhibit excellent catalytic performance in the transesterification of glycerol with dialkyl carbonate to glycerol carbonate, especially for alkaline earth metal oxides. Compared to other metal oxides, alkaline earth metal oxides not only possess strong basicity, but also have better stability.
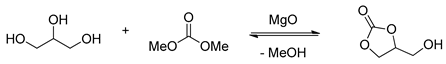
2.1.1. MgO Catalyzed Transesterification of Glycerol
Magnesium oxide with different sorts of morphologies is conventionally prepared from the thermal decomposition of various magnesium precursors (Figure 6) [45]. The magnesium salt, precipitant, additive, preparation condition, and calcination temperature all have significant effects on the physical and chemical properties of MgO [46,47,48,49,50]. More importantly, the catalytic performance of MgO is proved to have a close relationship with the morphology, surface area, particle size, crystallinity, and the concentration of basic sites of MgO catalyst.
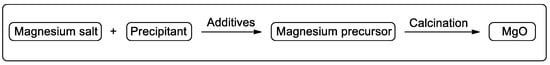
Figure 6.
General method for preparation of magnesium oxide.
With commercially available MgO selected as a catalyst, transesterification of glycerol and dimethyl carbonate was studied (Table 1, entries 1–3). Research shows that the transesterification of glycerol can conduct DMF in the presence of sub-equivalent commercial MgO catalyst, providing glycerol carbonate with a low yield [51]. However, in Wang and Yu’s [52] research, transesterification can be conducted much more smoothly with the commercial MgO free of solvent, producing the product in a moderate yield. In order to further enhance the performance of the catalytic activity, a series of preparation methodologies of MgO were developed. In 2014, Lee and co-workers [53] disclosed the surfactant-assisted syntheses of MgO catalyst and the applications in the transesterification of glycerol to glycerol carbonate. The catalyst was prepared from the reaction of Mg(NO3)2·6H2O and the surfactant Pluronic F127 (a triblock copolymer of ethylene oxide/propylene oxide/ethylene oxide) in the presence of nitric acid (Table 1, entry 6). The results indicated that a much higher yield of glycerol carbonate was obtained in 75.4% yield than the ones without any modification of catalysts with surfactant. According to the titrating tests and CO2-TPD experiments of the catalyst, the high catalytic activity of MgO is closely related with the higher basic site concentration of the surfactant-assisted MgO catalyst. Besides, the catalyst could be easily recovered after centrifuging and reused after activating the catalyst at 400 °C in the nitrogen atmosphere and calcining to remove glycerol carbonate residue and avoid the decrease of catalytic activity. The yield of glycerol carbonate could be maintained at 68% in the 5th reuse.

Table 1.
MgO catalyzed transesterification of glycerol 1.
Inspired by the previous work, Zhang and co-workers developed a series of micro-sized MgO catalysts with different morphologies and explored their catalytic performances in various organic syntheses in the past few years [48,49,50,54]. As expected, MgO catalysts with rod-like, spherical, flower-like, nest-like, and trapezoidal morphologies were prepared via the precipitation of Mg(NO3)2·6H2O and precipitants Na2CO3 or Na2C2O4 varying from 30–80 °C within 3 min of stirring and further calcination (Table 1, entries 7–11). Recently, the trapezoidal MgO catalyst was successfully applied to the transesterification of glycerol and dimethyl carbonate, providing glycerol carbonate in more than a 99% yield. However, MgO with other morphologies could not perform well in the transesterification. Compared to other morphologies of MgO, trapezoidal MgO possesses a bigger crystallite size, lower specific surface area, weaker surface basicity, and less Mg atom vacancies. Therefore, the perfect catalytic performance of MgO was attributed to the unique physicochemical property and morphology of the catalyst.
In the past few years, metal–organic frameworks (MOFs), as new microporous materials, have made great progress in heterogeneous catalysis [55,56,57]. Very recently, Wang and Yu [52] synthesized MgO-loaded zeolitic imidazolate framework-8 (designated as MgO@ZIF-8) catalysts with various MgO loadings through wet-impregnation and calcination (Table 1, entry 12). This MgO material maintained the ZIF-8 structure and possessed physical property of high surface area and regular porosity, and decent thermal/chemical stability. The catalytic performance of MgO@ZIF-8 in transesterification had a certain enhancement compared to MgO, ZIF-8, and the physically mixed counterparts. An acid–base bifunctional catalytic process was proposed by Wang and Yu et al. (Figure 7). The higher catalytic activity could be attributed to the bifunctional sites on the surface of MgO catalyst. The low-coordinated zinc atom and NH groups of imidazole provided acidic sites to activate dimethyl carbonate, meanwhile MgO and nitrogen atoms in imidazole provided basic sites to activate glycerol by abstracting proton to generate glyceroxide ion. Results also showed that more basic sites were generated on the ZIF-8 surface with the incorporation of MgO nanoparticles and the basic sites play much more important roles in the catalytic transesterification.
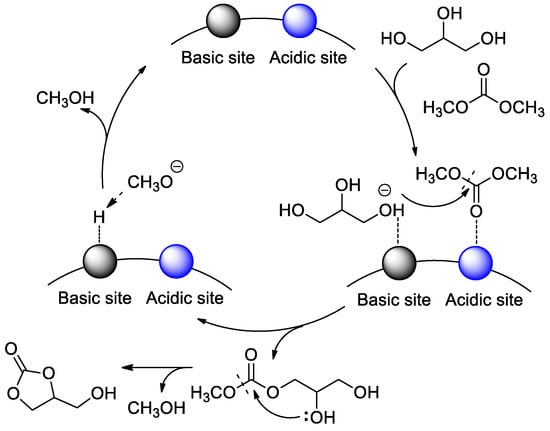
Figure 7.
Plausible reaction mechanism for bifunctional catalysis in the transesterification of glycerol, (reprinted with permission from Reference [52], Elsevier).
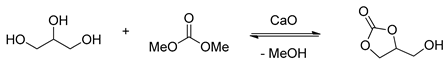
2.1.2. CaO-Catalyzed Transesterification of Glycerol
As an alkaline earth metal oxide, calcium oxide as an active catalyst has been extensively researched in the transesterification of glycerol with DMC in the past few years. In 2009, Ochoa–Gómez and co-workers [37] gained insight in to a series of CaO catalysts and explored the relationships between reaction parameters and reactivity (Table 2, entries 1–5). The calcination of CaO could increase the reactivity of catalyst dramatically due to the removal of Ca(OH)2 from the surface. In addition, in Li and Wang’s [58] work, owing to the formation of basic calcium carbonate Cax(OH)y(CO3)z, the activity of CaO catalysts decreased in the transesterification of glycerol. Considering the increased cost from calcination in terms of industrial feasibility, the uncalcined CaO was then studied in detail through a factorial design of experiment and a response surface methodology [37]. It revealed that uncalcined CaO catalyzed transesterification of glycerol performed smoothly and effectively under the optimized reaction conditions, giving the product glycerol carbonate in 95.3% yield [37]. Unfortunately, the recycling and reusing experiments could not be conducted well, and the yield of products decreased rapidly [58,59,60].

Table 2.
CaO-catalyzed transesterification of glycerol.
In order to gain further insight into the mechanism, a series of experiments were conducted with CaO employed as a catalyst by Lee and Kim’s group (Table 2, entries 7–15) [61]. They have successfully isolated an active homogeneous Ca species in the CaO-catalyzed transesterification of glycerol with DMC and characterized it as Ca(C3H7O3)(OCO2CH3) (II). The Ca species II was generated from the interaction of CaO with glycerol and DMC as depicted in Figure 8 and proved to be an active species of transesterification. With another glycerol molecule attacking the in situ generated Ca species II and loss of methanol, Ca species III was then generated. Followed by an intermolecular nucleophilic substitution, the target product glycerol carbonate was obtained and the catalyst precursor I was also regenerated simultaneously. Therefore, the catalytic activity of recycled CaO decreased sharply and the reusability of the catalyst was poor.
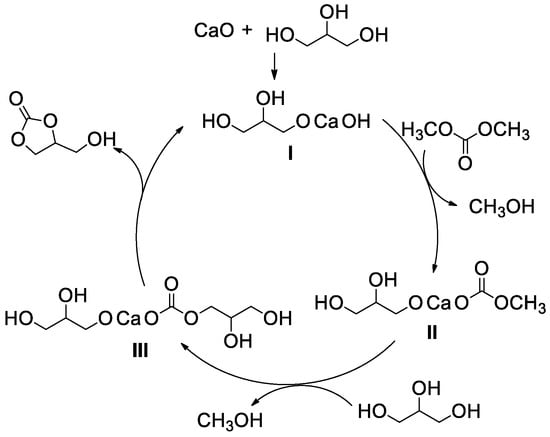
Figure 8.
Plausible reaction mechanism for CaO-catalyzed transesterification of glycerol, (reprinted with permission from Reference [57], Elsevier, 2011).
In order to increase the stability of CaO catalyst, various inorganic salts were loaded on CaO through dry impregnation or wet impregnation methods. In the catalytic system, neutral salts as key guest species always generated strong basic sites on various porous materials. Wang and co-workers disclosed that the KNO3 loaded CaO catalyst was prepared by a dry impregnation method using an aqueous solution of KNO3 and CaO powder (Table 2, entry 12) [62]. The research showed that the KNO3/CaO catalysts displayed much better stability than that of CaO. A 94.95% conversion of glycerol was obtained after the fifth reuse of KNO3/CaO catalyst, and the leaching test also showed that both the basic strength of CaO and the stability of the Ca2+ species were significantly improved. Recently, the Tang group reported the LiCl/CaO-catalyzed transesterification of glycerol, providing glycerol carbonate in a 94.2% yield (Table 2, entry 13) [63]. Pleasantly, the activity of the LiCl-modified catalyst was much more stable compared with the commercial CaO catalyst obviously. At the same time, with ethanol as the azeotropic agent, the best results were achieved at the ratio of DMC/glycerol 1:1.
In addition, Wang and co-workers proposed a new process for the synthesis of glycerol carbonate via coupling reaction and azeotropic distillation [64]. The product could be obtained in a yield of 98% at a low molar ratio of DMC/glycerol (1:1) with benzene as the azeotropic agent for both uncalcined CaO and calcined CaO (Table 2, entries 6, 14). The in situ removal of methanol from the system was favorable to the movement of chemical equilibrium of transesterification. However, the utility of toxicity benzene is not environmental. Then, in 2015, Wang and co-workers reported the production of glycerol carbonate via reactive distillation and extractive distillation processes (Table 2, entry 15) [65]. In the reactive distillation process, DMC was also used as an azeotropic agent to remove methanol from the reaction without introducing new impurities, and in the extractive distillation process, DMC could be effectively recovered. The dual function of the two processes reduced the cost and also saved raw materials and energy resource.
With the increasing demand for green and environmentally friendly resources, researchers gained insight into catalysts that derived from natural organisms. Recently, Roschat and co-workers first disclosed a green and economical CaO catalyst which was applied to the catalytic transesterification of glycerol with DMC [66]. The CaO catalysts were derived from natural sources, such as eggshells, golden apple snail shells, and cockle shells, after cleaned, air-dried, crushed, sieved, and calcined at 800 °C (Table 2, entries 16–18). The corresponding three types of catalysts obtained were named CaO_egg, CaO_gol, and CaO_coc and they all exhibited excellent catalytic activity and performed well in the transesterification of glycerol, and CaO derived from cockle shells could furnish glycerol carbonate in a 92.1% yield within 2 h. The results also showed that the higher yield of product was attributed to the higher total basic sites and the Brunauer–Emmett–Teller (SBET) surface area of the catalyst. It provides a concise, green, and environmentally friendly methodology for CaO preparation and can also be used as an inspiration to various applications of CaO catalyst.
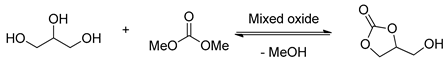
2.1.3. Mixed Oxides Catalyzed Transesterification of Glycerol
Mixed oxide catalysts have shown great potential for heterogeneous catalysis in the transesterification of glycerol with dialkyl carbonate and has attracted growing attention in this field (Table 3). The mixed oxide catalysts exhibited excellent catalytic performance due to strong basic sites and the high density of these basic sites. Besides, mixed oxides also show advantages over single metal oxide in catalytic activity. The catalytic activity of mixed oxides is associated with the ratio of metals, precipitating agents, and calcination temperatures. Catalysts with different molar ratios are generally synthesized via a co-precipitation methodology followed by decomposition and calcination at different temperatures.

Table 3.
Mixed oxides catalyzed transesterification of glycerol.
Both MgO and CaO exhibited good catalytic activity in the transesterification of glycerol with dialkyl carbonate (Table 3, entries 1–2). In 2013, Hameed and co-workers [67] reported a reusable and highly active heterogeneous catalyst in Mg1+xCa1−xO2 type (Table 3, entry 1). The results showed that, when Mg1.2Ca0.8O2 mixed oxide was used as a catalyst, glycerol carbonate could be obtained in a 100% yield. The catalytic activity of a catalyst mainly depends on the molar ratio of Mg/Ca and calcination temperature which are closely related with strong basic sites. Compared with CaO, the catalytic efficiency of mixed oxides increases due to the increased base amount and improves the stability of the Ca2+ species. Dolomite, as a mineral material that consists of MgCa(CO3)2, was calcined to provide a CaO–MgO catalyst for transesterification with excellent results by the Hameed group [68].
A series of Mg–Al mixed oxides were developed for the transesterification of glycerol with dialkyl carbonate (Table 3, entries 3–6). In 2013, Liu and Hensen group reported the Mg–Al mixed oxides derived from hydrotalcite-type layered double hydroxides for transesterification [69]. X-ray diffraction patterns show that mixed oxides have MgO-like structures and Al3+ cations are dissolved in the lattices. The catalytic activity was increased with the increase of the Al/Mg ratio which is identical to the trend of the surface basic site density. Transesterification of glycerol over Mg–Al mixed oxides supported on the mesoporous crystalline material (MCM-41) was reported by Wang et al. [70]. There are three types of basic sites on the catalyst: (1) the weak basic sites of the OH− group on the surface of MCM-41; (2) moderate basic sites ascribed to Mg–O and Al–O pairs; and (3) strong basic sites related to coordinatively unsaturated O2– ions. The catalytic results showed that the high catalytic activity was associated with the dispersed effect of MCM-41, which resulted in the basic sites being drastically exposed. In addition, the transesterification of glycerol conducted with Mg/Al/Zr mixed oxides was reported by Lingaiah et al., giving glycerol carbonate in a 94.0% yield [71]. The addition of Cu to Mg/Al mixed oxides also exhibited excellent activity and stability in the transesterification of glycerol [72]. The incorporation of Cu2+ cations into the MgAl(O) periclase was responsible for the strong basic sites and high activity. Furthermore, Ca–Al mixed oxide (Ca/Al = 2) was introduced to the transesterification to give glycerol carbonate in a 90.2% yield (Table 3, entry 7) [73,74]. The XRD patterns of fresh calcined catalyst and the 6 times recycled catalyst showed that the Ca12Al14O33 phase of the calcined catalysts was stable, but CaO was lost due to the leaching of the catalyst in the recycled experiments, which was attributed to the deactivation of the catalyst. The Sr/Al mixed oxides (Sr/Al = 0.5) catalyzed the transesterification of glycerol with full conversion and could be reused for five cycles without serious deactivation (Table 3, entry 8) [75].
Several groups reported the alkali metal modified mixed oxides catalyzed transesterification of glycerol (Table 3, entries 9–13, 20). Alkali oxides are not stable, but possess strong basic sites which can initiate the reaction by abstracting proton from the primary hydroxyl group of glycerol. The LiNO3 modified Mg–Al oxide catalyzed transesterification of glycerol performed well, resulting in a 96.3% yield, according Wang and Kang’s work [76]. Aluminium ion was well dispersed in the MgO lattice and LiAlO2 was formed at the same time. In addition, LiNO3-modified ZnO catalyst was also proved to be an efficient catalyst for the synthesis of glycerol carbonate [77]. Lithium was doped into the lattice of ZnO to form the strong basic sites of (Li+O−) species. The basic strength and basicity of the catalyst was both enhanced by the addition of LiNO3. Recently, He and co-workers disclosed that the catalytic performance can be increased with Li-doped La2O3 catalyst [78]. The doped Li can enter into the lattice of La2O3 to enhance the interaction of Li and La2O3 and provide strong and abundant basic sites. Furthermore, NaOH-modified γ-Al2O3 and KF-modified α-Al2O3 catalysts were also applied to the transesterification with excellent performance, respectively [79,80,81].
Mixed oxides of alkaline-earth metals with transition metals were developed for the transesterification of glycerol with moderate to good yields (Table 3, entries 14–16). Van Zyl et al. [82] reported the nanocrystalline-ordered mesoporous MgO–ZrO2 catalyst for transesterification. Mg2+ was incorporated into the ZrO2 lattice to form the highly dispersed MgO species which is attributed to the high catalytic performance of the catalyst. However, the Mg/Zr/Sr mixed oxide did not perform in the transesterification and only a moderate yield of glycerol carbonate was achieved [83]. Besides, a novel Ti-SBA-15 (Ti–Si mixed oxide: Si/Ti = 4) catalyst was developed for the transesterification [84]. Transesterification results showed that the reaction rate for the Ti-SBA-15 catalyst was more than 10 times faster than the SBA-15 catalyst. Therefore, the activation of the carbonyl group of DMC via Lewis acidic site Ti4+ was the major driving force toward the product.
Although Lanthanide metal oxides possess strong basic sites, the catalytic performance for transesterification of glycerol was relative lower due to the low surface area (Table 3, entries 17–23) [91]. Lanthanide metal oxides are usually supported with various metal oxides to increase the surface area and the catalytic activity. In 2013, various Mg–La mixed oxides were prepared for the transesterification of glycerol with DMC by Kim and Lee et al. [85]. With the increasing loadings of Mg, the surface area was increased along with the catalytic activity. When the Mg content was increased to a certain amount, the concentration of basic sites decreased. With the optimized conditions, glycerol carbonate was achieved in a 83.1% yield at a molar ratio of Mg/La = 3. Porous Ca–La mixed oxides with a hierarchical structure were synthesized via an exo- and endo-templating method by Kumar et al. [86]. A yield of 74% was achieved with Ca3La catalyst and the catalyst also showed good reusability. The ZnO/La2O3 mixed oxides were proved to be an effective and efficient catalyst for the transesterification [87]. With 0.5 wt. % loadings of ZnO/La2O3 catalyst, the product could be obtained in 95.7% yield at a ratio of Zn/La = 4:1. The ZnO/La2O3 oxide exhibited much higher catalytic activity compared to the single metal oxides. Cerium oxide, as one of the lanthanide metal oxides, was also explored in the transesterification of glycerol. Mixed oxides, such as MgO–CeO2, Ce0.7Cd0.3O, Ce–Ni, exhibited good to excellent catalytic activity in the transesterification of glycerol with DMC or DEC [88,89,90]. Ce4+ doped to the CdO lattice or Ni lattice resulted in more oxygen atoms adsorbed on the surface of catalyst and high reactivity.
A new hypothesized reaction mechanism for the transesterification of glycerol with DMC was proposed by Rode et al., as depicted in Figure 9 [92]. It is believed that the higher catalytic activity of mixed oxides than single metal oxides can be attributed to the higher number of acidic sites and basic sites. The transesterification was promoted via two activation modes. Firstly, the Lewis basic site BaCeO3 phase (Oδ−) of the Ba−Ce catalyst abstracted the proton of the primary hydroxyl group of glycerol to enhance the nucleophilicity of glycerol. Then, the Lewis acidic site (Ce4+) activated the carbonyl carbon of DMC simultaneously, followed by an intramolecular nucleophilic substitution, after which the target product glycerol carbonate was achieved.
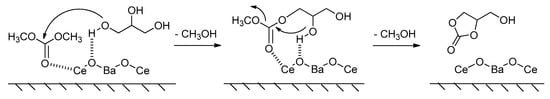
Figure 9.
Heterogeneous catalysis in the transesterification of glycerol, (reprinted with permission from Reference [92], American Chemical Society, 2017).
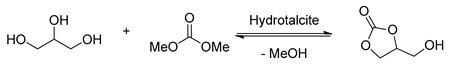
2.2. Hydrotalcite Catalyzed Transesterification of Glycerol
Hydrotalcite (HT) and hydrotalcite-like compounds have been widely investigated in the transesterification of glycerol (Table 4) [51,93,94,95,96,97,98,99,100,101,102]. Hydrotalcite is a double-layered anionic clay with the formula of (M2+1-xM3+x(OH)2)x+An−x/n·mH2O, Where M2+ and M3+ stand for divalent and trivalent cations, An− is the layered anion [51]. Calcination of hydrotalcite could simultaneously provide the corresponding mixed-oxide form product and the hydroxide form product. Therefore, both the Lewis basic sites and Brϕnsted basic sites were produced and promoted the transesterification of glycerol with a synergistic effect.

Table 4.
Hydrotalcite catalyzed transesterification of glycerol 1.
In 2010, an uncalcined Mg–Al hydrotalcite catalyst involving hydromagnesite was researched for the synthesis of glycerol carbonate from transesterification of glycerol with DMC [51]. With an Mg/Al molar ratio of five and DMF as a solvent, the product was obtained in the 98% yield (Table 4, entry 1). However, hydromagnesite often showed poor catalytic activity in the transesterification of glycerol. Therefore, the high catalytic activity of catalyst should be attributed to the hydrotalcite structure and the relatively high surface areas. The reactivity dramatically decreased using the calcined hydrotalcite catalyst. It is suggested that the reaction was promoted by moderate basic hydrogen carbonate ions (HCO3−). Hydrotalcite-like compounds activated by calcination and rehydration under ultrasound were investigated as catalysts (named as HTr4: prepared via calcination and rehydration of Mg(OH)2 and Al(OH)3 with the molar ratio of Mg/Al 4) for transesterification by Medina et al. [93] (Table 4, entry 3). With the prolonged reaction time, glycerol dicarbonate was, unfortunately, generated. The high catalytic activity of the HTr4 catalyst was probably associated with the Brϕnsted basic sites of catalyst due to the better abstraction hydrogen activity than catalyst HTO4 (HTO4 was prepared via the calcination of Mg(OH)2 and Al(OH)3 in air at 450 °C). Employing methanol as solvent, only a moderate yield could be achieved with a Mg–Al hydrotalcite catalyst (Mg/Al = 2) [94]. Medina et al. then reported a bulk and carbon nanofiber supported MgAlHTs (HT-CNFc) catalyst (Table 4, entry 5) [95]. However, the transesterification could not be improved effectively. With calcined hydrotalcite supported on hexagonal silica (CHT-HMS), the reaction performed smoothly with an 84.3% yield and the catalytic activity of transesterification had a slight increase compared with a Mg–Al CHT catalyst (Table 4, entry 6) [96]. The lower reaction activity was attributed to the employment of methanol or ethanol as a solvent. The excess of methanol would prevent the transesterification toward to product.
Various modified HT catalysts have been studied over the past few years (Table 4, entries 7–10) [99,100,101,102]. In 2014, Liu and Hensen [99] prepared a series of transition metal-doped HTs. Amongst them, Ni-modified HT gave the best results in the synthesis of glycerol carbonate with a 95% yield and 100% selectivity. Hong et al. [100] reported a KF loaded Ca–Mg–Al hydrotalcite catalyst and it was proved to be an excellent catalyst for transesterification of glycerol in 99% yield. Th eCa-HT was applied to the transesterification of glycerol and propylene carbonate by Rode and co-workers [101]. The reaction was conducted with a carbonate/glycerol molar ratio of 1:1 at 160 °C, giving the product an 84% yield. However, the reduction of the carbonate/glycerol ratio resulted in the increase of temperature. In 2017, Zhang and Wang’s group [102] reported the fluorinated Mg–Al HT-like compounds catalyzed transesterification of glycerol with a glycerol carbonate yield of 95.3%. With the introduction of an appropriate amount of (AlF6)3− into the hydrotalcite structure, the catalytic activity of transesterification was increased, and the side reaction was also inhibited effectively. Fortunately, the modified HT catalysts all have excellent stability and reusability in the transesterification of glycerol.
2.3. Other Inorganic Base Catalyzed Transesterification of Glycerol
2.3.1. NaAlO2 Catalyzed Transesterification of Glycerol
A series of inorganic base catalysts for the synthesis of glycerol carbonate via transesterification was summarized in Table 5. NaAlO2 as a heterogeneous base catalyst is considered to be the best choice for transesterification of glycerol (Table 5, entries 1–3) [103,104,105]. In 2017, Debecker and co-workers prepared a spray-dried nanostructured NaAlO2 microsphere catalyst and applied it to the catalytic transesterification of glycerol [103]. The catalysis performed well at room temperature with a 94% yield (30 °C). Due to the highly hygroscopic and corrosiveness of pure NaAlO2, Debecker et al. [104] then reported a new type of basic hydrotalcite catalyst promoted by NaAlO2. Compared to the calcined hydrotalcite catalyst, the transesterification performed more smoothly with NaAlO2 as an additive and the product was achieved with 92% conversion and 100% selectivity after 30 min. This compatible combination of hydrotalcites and NaAlO2 enables an efficient methodology for the transesterification of glycerol to glycerol carbonate, and the catalyst also shows excellent stability and reusability. Besides, waste red mud calcined at 500 °C (RM-500) possessed abundant active NaAlO2 and Ca2SiO4 sites and also exhibited excellent catalytic performance in the transesterification with a 92.0% yield of glycerol carbonate [105]. Therefore, red mud would be a potential alternative to traditional metal oxides for the synthesis of glycerol carbonate via transesterification.

Table 5.
Other inorganic base catalyzed transesterification of glycerol.
2.3.2. Na2SiO3-Catalyzed Transesterification of Glycerol
Calcined sodium silicate exhibited excellent catalytic performance for transesterification of glycerol (Table 4, entries 4–6) [106,107]. Li et al. reported the Na2SiO3-catalyzed transesterification of glycerol, giving glycerol carbonate in a 95.5% yield at 75 °C [106]. Increasing the reaction temperature to 95 °C, the reaction time could be shortened within 15 min. Subsequently, Wang et al. disclosed a microwave-assisted transesterification of glycerol over Na2SiO3 catalyst in the sealed reaction system [107]. The reaction was performed smoothly in 50 s in the power constant mode with microwave irradiation input power of 175 W, providing the corresponding product in a 94.3% yield. The transesterification rate was much faster than results reported previously. Besides, trisodium phosphate with strong basic sites showed great catalytic activity, stability, and reusability in the synthesis of glycerol carbonate. The catalyst could be reused for nine cycles without deactivation [108].
2.3.3. Zeolite/Clinoptilolite-Catalyzed Transesterification of Glycerol
Natural zeolite with the structure of A(x/q)((AlO2)x(SiO2)y)·n(H2O) was considered to be an appropriate base catalyst for transesterification of glycerol in terms of environmentally friendly and green chemistry (Table 4, entries 8–10) [43,109,110,111,112,113,114,115]. In 2012, Hou et al. [43] reported a series of Na-based zeolites for transesterification. The glycerol carbonate achieved an 80% yield with commercial Na1.88(Al2Si4.8O13.5) used as catalyst. Subsequently, Hameed and co-workers reported the synthesis of glycerol carbonate by transesterification of glycerol with DMC over K-zeolite derived from coal fly ash in 96% yield [109]. Then, they employed lithium-oil palm ash zeolite as catalyst in the transesterification in a 98.1% yield. The strong basicity of catalyst was attributed to the increased lithium impregnation [110].
Compared to natural clinoptilolite, dealuminated clinoptilolite has lower activation energy for the transesterification of glycerol with sodium bicarbonate (Table 4, entry 11) [111]. With sodium bicarbonate used as reactant and water as solvent, glycerol carbonate was achieved with 28% conversion in 15 min. Recently, Li et al. reported that the main composition of calcinated oil palmempty fruit bunch ash was K2Mg(SiO4) which was proved to be excellent catalyst for transesterification (Table 4, entry 12) [112]. K+ was incorporated into the Mg2+O4 connected tetrahedral framework of SiO2, and the concentration of basic sites increased and the basic strength enhanced simultaneously. A series of transition metal modified hierarchical ETS-10 (ETS-10: 1.0TiO2/5.5SiO2/3.5Na2O/1.6K2O/181.0H2O) zeolite catalysts were prepared for the transesterification of glycerol by Wu et al. [113] (Table 4, entry 13). Glycerol carbonate could be obtained using a nickel modified METS-10 (Ni/METS-10) catalyst in a 97.1% yield. Results revealed the catalytic activities of the catalysts in the following order: Ni/METS-10 > Zn/METS-10 > Mn/METS-10 > Fe/METS-10 > Co/METS-10 > Cu/METS-10 > METS-10 > ETS-10. The catalytic activities of catalysts were not only related to the nature of the metal, but also the zeolite structure. The mechanism showed that Ni0 species could accelerate the abstraction of proton from the primary hydroxyl group of glycerol towards to the basic sites’ TiO62−. Meanwhile, the carbonyl group could be activated by the in situ generated TiO61-H. Therefore, the transesterification performed smoothly with the Ni/METS-10 catalyst.
2.3.4. Others
Ordered mesoporous BaCO3/C using phenolic resol as a carbon source and triblock copolymer Pluronic F127 as a template has also been researched for the transesterification of glycerol to glycerol carbonate with a conversion of 97.8% and selectivity of 98.5% (Table 4, entry 14) [114]. The high catalytic activity was attributed to the well dispersed BaCO3 in the carbon framework. Unfortunately, the catalyst was deactivated due to the decomposition of carbon.
2.4. Heterogeneous Organocatalysis for Transesterification of Glycerol
Organocatalysis has been rapidly and widely developed in the past few decades. With the exception of inorganic base, amines as organic bases are often chosen as catalysts in transesterification, and DABCO (1,4-diazabicyclo(2.2.2)octane) has proved to be a highly active and efficient homogeneous organic catalyst for the transesterification of glycerol with DMC to afford glycerol carbonate [115]. As a homogeneous catalyst, DABCO is still confronted with problems of catalyst recovery and reusability. Therefore, Kim and Lee et al. synthesized a series of heterogeneous polyamine-anchored Merrifield resin catalysts and the DABCO-anchored catalyst exhibited excellent activity and selectivity [116] (Figure 10). The resin catalyst can easily be recovered after simple manipulation and directly reused without any loss in yield and selectivity. Subsequently, Lei and co-workers reported an organocatalytic transesterification of glycerol with DMC using a bifunctional and robust catalyst with a DABCO-embedded porous organic polymer structure [117]. With this excellent solubilization capacity polymer catalyst, comparative results were achieved in both activity and selectivity. Besides, the improvement of catalyst can easily be achieved in chemistry through the modification of polymer precursors.
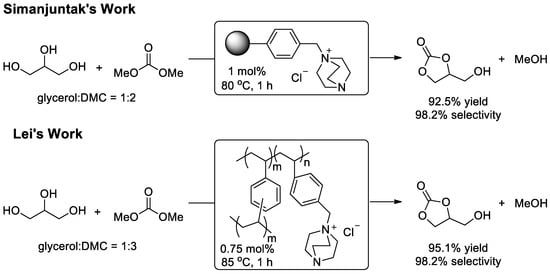
Figure 10.
DABCO-derived heterogeneous catalysis in the transesterification of glycerol.
According to the results of the computational calculation and experiments, Simanjuntak and co-workers proposed a plausible mechanism for the DABCO-anchored Merrifield resin-catalyzed transesterification of glycerol [116] (Figure 11). The high activity and selectivity of the reaction could be attributed to the following respects: 1) glycerol could be activated via the formation of strong hydrogen bonds with the chloride anion of catalyst (I); 2) hydrogen bonding interactions of DMC with the nitrogen atom and the remaining hydroxyl group of glycerol (II); 3) electrostatic interaction of contact counter ions in catalyst (III) which would effectively promote the intramolecular cyclization in the activity and selectivity of the reaction.
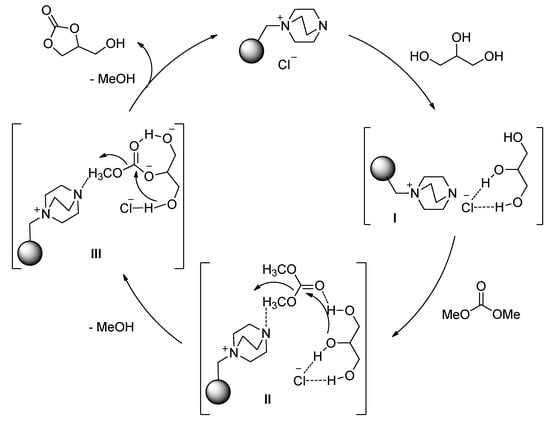
Figure 11.
Plausible mechanism of DABCO-anchored Merrifield resin-catalyzed transesterification of glycerol, (reprinted with permission from Reference [116], Elsevier, 2015).
N-heterocyclic carbene (NHC), as an active organic base catalyst, has been used as an efficient homogeneous organocatalyst for the transesterification of glycerol with DMC for a long time [118,119]. Considering the facile recovery and reusability of catalyst, NHC was immobilized on silica-supported mesostructured cellular foam (MCF) with hydrogen carbonate as protecting group by Bruijnincx and co-workers [120]. This “masked” NHC pre-catalyst performed smoothly in the transesterification of glycerol with 75% yield and 91% selectivity, and could be easily recovered and reused up to three times (Figure 12). Based on studies with 13C-labelled dimethyl carbonate, the catalyst could be regenerated after the reaction between carbene and DMC. These organic methodologies provide novel and concise access to glycerol carbonate from glycerol with heterogenized masked organic base and also give inspiration for various organocatalyst for transesterification.
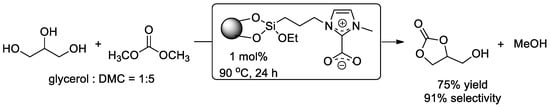
Figure 12.
NHC-catalyzed transesterification of glycerol.
3. Future Prospects of Heterogeneous Catalysis in the Transesterification of Glycerol
Heterogeneous bases catalyzed transesterification of glycerol has achieved great progress in the past decades. The heterogeneous base catalysts were focused on the alkaline-earth metal oxides, hydrotalcites, zeolites, clinoptilolites, and organic base catalysts. Despite much progress in heterogeneous catalysis, there still remain great opportunities for progress and developments in catalysis. In order to acquire the catalyst with high reactivity and stability, the catalyst always needs to be calcined at high temperature. Future efforts may focus on the following aspects: 1) exploring cheaper metal oxide catalysts for the catalysis, such as Fe, Cu, etc.; 2) reducing the molar ratio of DMC/glycerol and the loading of catalysts to avoid the waste of resources, such as the addition of environmentally friendly azeotropic agents; 3) research on various new types of organocatalysts with bifunctional groups filled with acidic and basic sites to activate glycerol and dialkyl carbonate for the transesterification; and 4) thorough understanding of mechanistic details of transesterification which will eventually lead to the next generation of general transesterification methods. In view of the development of catalysts, we believe that there will be more breakthroughs in this area.
4. Conclusions
With the increasing demand for the clean energy of biodiesel, glycerol as the main byproduct is confronted with a saturated state of the market. Glycerol carbonate, as one of the promising downstream products, is gaining widespread concern. This review has documented recent advances in heterogeneous catalysis in the transesterification of glycerol to glycerol carbonate. The heterogeneous catalysts are focused on alkaline-earth metal oxides, hydrotalcites, zeolites, clinoptilolites, organic base catalysts, etc. Heterogeneous base catalysts, especially for alkaline-earth metal oxides, possess strong basic sites and exhibit excellent catalytic performance in transesterification. Mixed oxides are particularly preferred for transesterification due to their higher contents of acidic and basic sites, which are essential for the reaction. Hydrotalcite with a double-layered anionic clay structure contains abundant Lewis basic sites and Brϕnsted basic sites. With the synergistic effect of Lewis basic sites and Brϕnsted basic sites between hydrotalcite and substrates, the transesterification of glycerol was performed smoothly. Althoughheterogeneous organocatalytic transesterification of glycerol is a challenging process for the synthesis of glycerol carbonate, it also raises concerns from chemists withprogress due to the prospects in synthetic strategy. In brief, the catalytic performance of catalyst in transesterification is closely related with the basicity, stability, and concentration of basic sites of catalyst. Moreover, from the perspective of the reaction mechanism, both the acidic sites and basic sites of catalysts could promote the transesterification. Catalysts, especially for heterogeneous catalysts, as essential factors for the transesterification of glycerol, will be the key focus of future research, and future efforts will be centered on the development of novel heterogeneous catalytic systems that are effective and efficient.
Funding
This research was funded by the National Natural Science Foundation of China (No. 21801204) and Science and Technology Program of Xi’an, China (No. 201805038YD16CG22(4)).
Conflicts of Interest
The author declares no conflict of interest.
References
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Engin. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Babajide, O. Sustaining biodiesel production via value-added applications of glycerol. J. Energy 2013, 2013. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Q. Novel design for simultaneous production of biodiesel and glycerol carbonate from soybean oil. Ind. Eng. Chem. Res. 2018, 57, 16809–16816. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversionof glycerol into biofuels, fuel additives and otherbio-based chemicals. Catalysts 2019, 9, 15–61. [Google Scholar] [CrossRef]
- Veluturla, S.; Archna, N.; Rao, D.S.; Hezil, N.; Indraja, I.S.; Spoorthi, S. Catalytic valorization of raw glycerol derived from biodiesel: A review. Biofuels 2018, 9, 305–314. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhao, H.; Tong, D.S.; Wu, L.M.; Yu, W.H. Recent advances in catalytic conversion of glycerol. Catal. Rev. 2013, 55, 369–453. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Campisi, S.; Wang, D.; Prati, L.; Villa, A. Selective oxidation of raw glycerol using supported AuPd nanoparticles. Catalysts 2015, 5, 131–144. [Google Scholar] [CrossRef]
- Huang, L.; Sun, J.-Y.; Cao, S.-H.; Zhan, M.; Ni, Z.-R.; Sun, H.-J.; Chen, Z.; Zhou, Z.-Y.; Sorte, E.G.; Tong, Y.J.; et al. Combined EC-NMR and in situ FTIR spectroscopic studies of glycerol electrooxidation on Pt/C, PtRu/C, and PtRh/C. ACS Catal. 2016, 6, 7686–7695. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Xiong, W.; Ding, H.; He, B. Hydrogenolysis of glycerol to 1,2-propanediol and ethylene glycol over Ru-Co/ZrO2 catalysts. Catalysts 2016, 6, 51–63. [Google Scholar] [CrossRef]
- Rosas, I.P.; Contreras, J.L.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vázquez, T.; García, D.C. Catalytic dehydration of glycerol to acrolein over a catalyst of Pd/LaY zeolite and comparison with the chemical equilibrium. Catalysts 2017, 7, 73–101. [Google Scholar] [CrossRef]
- Sun, S.; He, M.; Dai, Y.; Li, X.; Liu, Z.; Yao, L. Catalytic acetalization: An efficient strategy for high-value utilization of biodiesel-derived glycerol. Catalysts 2017, 7, 184–194. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A brief review on industrial alternatives for the manufacturing of glycerol carbonate, a green chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Sustainable production of glycerol carbonate from by-product in biodiesel plant. Waste Biom. Valor 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Shaikh, A.-A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Sirkar, K.K. Carbon dioxide separation with novel solvents as liquid membranes. Ind. Eng. Chem. Res. 2002, 41, 2287–2295. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; de Givenchy, E.P.T.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building blockfor tomorrow: Synthesis, reactivity, propertiesand applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for asustainable valorization of renewable. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Szőri, M.; Giri, B.R.; Wang, Z.; Dawood, A.E.; Viskolcz, B.; Farooq, A. Glycerol carbonate as fuel additive for sustainable future. Sustain. Energy Fuels 2018, 2, 2171–2178. [Google Scholar] [CrossRef]
- Parzuchowski, P.G.; Świderska, A.; Roguszewska, M.; Frączkowski, T.; Tryznowski, M. Amine functionalized polyglycerols obtained by copolymerization of cyclic carbonate monomers. Polymer 2018, 151, 250–260. [Google Scholar] [CrossRef]
- Vieville, C.; Yoo, J.W.; Pelet, S.; Mouloungui, Z. Synthesis of glycerol carbonate by direct carbonatation of glycerolin supercritical CO2 in the presence of zeolites and ion exchangeresins. Catal. Lett. 1998, 56, 245–247. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Gao, P.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal. Sci. Technol. 2013, 3, 2801–2809. [Google Scholar] [CrossRef]
- Su, X.; Lin, W.; Cheng, H.; Zhang, C.; Wang, Y.; Yu, X.; Wu, Z.; Zhao, F. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate. Green Chem. 2017, 19, 1775–1781. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Gu, Y.; Guan, Z.; Mo, W.; Ni, Y.; Li, T.; Li, G. Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2(phen)/KI. Appl. Catal. A Gen. 2010, 386, 188–193. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakai, T.; Mihara, M. New synthesis of glycerol carbonate from glycerol using sulfur-assisted carbonylation with carbon monoxide. Heteroatom Chem. 2010, 21, 99–102. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.S.; Loridant, S.; Lingaiah, N. Understanding the surface and structural characteristics of tungsten oxide supported on tin oxide catalysts for the conversion of glycerol. J. Chem. Sci. 2015, 127, 897–908. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Ferragin, C. Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J. Catal. 2009, 268, 106–114. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; De Frutos, P.; Iborra, S.; Noy, M.; Velty, A.; Concepción, P. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. the role of acid–base pairs. J. Catal. 2010, 269, 140–149. [Google Scholar] [CrossRef]
- Chaves, D.M.; Da Silva, M.J. A selective synthesis of glycerol carbonate from glycerol and urea over Sn(OH)2: A solid and recyclable in situ generated catalyst. New J. Chem. 2019, 43, 3698–3706. [Google Scholar] [CrossRef]
- Kumar, C.R.; Jagadeeswaraiah, K.; Prasad, P.S.S.; Lingaiah, N. Samarium-exchanged heteropoly tungstate: An efficient solid acid catalyst for the synthesis of glycerol carbonate from glycerol and benzylation of anisole. ChemCatChem 2012, 4, 1360–1367. [Google Scholar] [CrossRef]
- Guidi, S.; Calmanti, R.; Noè, M.; Perosa, A.; Selva, M. Thermal (catalyst-free) transesterification of diols and glycerol withdimethyl carbonate: A flexible reaction for batch and continuous-flow applications. ACS Sustain. Chem. Eng. 2016, 4, 6144–6151. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesisof glycerol carbonate from glycerol and dimethyl carbonate bytransesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Maestro-Madurga, B. Synthesis of glycerol 1,2-carbonate by transesterification of glycerol with dimethyl carbonate using triethylamine as a facile separable homogeneous catalyst. Green Chem. 2012, 14, 3368–3376. [Google Scholar] [CrossRef]
- Nogueira, D.O.; de Souza, S.P.; Leão, R.A.C.; Miranda, L.S.M.; de Souza, R.O.M.A. Process intensification for tertiary amine catalyzed glycerol carbonate production: Translating microwave irradiation to a continuous-flow process. RSC Adv. 2015, 5, 20945–20950. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.; Kong, W.; Zhou, L.; Ma, L.; He, Y.; Huang, Z.; Jiang, Y. Enzymatic synthesis of glycerol carbonate using a lipaseimmobilized on magnetic organosilica nanoflowers as a catalyst. ACS Omega 2018, 3, 6642–6650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Pan, S.; Zheng, L.; Nie, R.; Xia, S.; Chen, P.; Hou, Z. Transesterification of glycerol with dimethyl carbonate to glycerol carbonate over Na–based zeolites. Chin. J. Catal. 2012, 33, 1772–1777. [Google Scholar] [CrossRef]
- Bancquart, S.; Vanhove, C.; Pouilloux, Y.; Barrault, J. Glycerol transesterification with methyl stearate over solid basic catalysts: I. relationship between activity and basicity. Appl. Catal. A Gen. 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, G.; Wu, H.; Hai, B.; Wang, L.; Qian, Y. Nanoscale magnesium hydroxide and magnesium oxide powders: Control over size, shape, and structure via hydrothermal synthesis. Chem. Mater. 2001, 13, 435–440. [Google Scholar] [CrossRef]
- Bian, S.-W.; Baltrusaitis, J.; Galhotra, P.; Grassian, V.H. A template-free, thermal decomposition method to synthesize mesoporous MgO with a nanocrystalline framework and its application in carbon dioxide adsorption. J. Mater. Chem. 2010, 20, 8705–8710. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Yang, H.; Wang, Q.; Zhang, Z. Controlled synthesis of mesocrystal magnesiumoxide parallelogram and its catalytic performance. CrystEngComm 2015, 17, 2642–2650. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Yang, H.; Wang, Q.; Zhang, Z. Shape evolution of parallelogrammic magnesium oxalate controlled by phosphate species. RSC Adv. 2015, 5, 63034–63043. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Wang, X.; Wang, Q.; Bai, Z.; Zhang, Z. Morphological and surface structural evolutions of MgO particles from parallelograms to rods. CrystEngComm 2016, 18, 2612–2616. [Google Scholar] [CrossRef]
- Takagaki, A.; Iwatani, K.; Nishimura, S.; Ebitani, K. Synthesis of glycerol carbonate from glycerol and dialkyl carbonates using hydrotalcite as a reusable heterogeneous base catalyst. Green Chem. 2010, 12, 578–581. [Google Scholar] [CrossRef]
- Chang, C.-W.; Gong, Z.-J.; Huang, N.-C.; Wang, C.-Y.; Yu, W.-Y. MgO nanoparticles confined in ZIF-8 as acid-base bifunctional catalysts for enhanced glycerol carbonate production from transesterification of glycerol and dimethyl carbonate. Catal. Today 2019. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Lim, S.R.; Ahn, B.S.; Kim, H.S.; Lee, H. Surfactant-assisted synthesis of MgO: Characterization and catalyticactivity on the transesterification of dimethyl carbonate with glycerol. Appl. Catal. A Gen. 2014, 484, 33–38. [Google Scholar] [CrossRef]
- Bai, Z.; Zheng, Y.; Han, W.; Ji, Y.; Yan, T.; Tang, Y.; Chen, G.; Zhang, Z. Development of trapezoidal MgO catalyst for high-efficient transesterification of glycerol and dimethyl carbonate. CrystEngComm 2018, 20, 4090–4098. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal−organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.P.; Wang, G.Y.; Yao, Z.G.; Tang, Y.R.; Zheng, S.S. Zeolitic imidazolate framework as efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate. J. Mol. Catal. A Chem. 2013, 366, 43–47. [Google Scholar] [CrossRef]
- Mc Guire, C.V.; Forgan, R.S. The surface chemistry of metal–organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. On the deactivation of alkali solid catalysts for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Reac. Kinet. Mech. Cat. 2011, 102, 113–126. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Yamanaka, S.; Hidaka, J. Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing methanol. Appl. Catal. A Gen. 2008, 334, 357–365. [Google Scholar] [CrossRef]
- Granados, M.L.; Alonso, D.M.; Sádaba, I.; Mariscal, R.; Ocón, P. Leaching and homogeneous contribution in liquid phase reaction catalysed by solids: The case of triglycerides methanolysis using CaO. Appl. Catal. B Environ. 2009, 89, 265–272. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Kim, T.K.S.; Lee, D.; Ahn, B.S.; Kim, H.S.; Lee, H. CaO-catalyzed synthesis of glycerol carbonate from glycerol and dimethylcarbonate: Isolation and characterization of an active Ca species. Appl. Catal. A Gen. 2011, 401, 220–225. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Liu, Y.; Yang, C. KNO3/CaO as cost-effective heterogeneous catalyst for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. J. Ind. Eng. Chem. 2015, 28, 334–343. [Google Scholar] [CrossRef]
- Tang, Y.; Xue, Y.; Li, Z.; Yan, T.; Zhou, R.; Zhang, Z. Heterogeneous synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by LiCl/CaO. J. Saudi Chem. Soc. 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Coupling reaction and azeotropic distillation for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Chem. Eng. Process. 2010, 49, 530–535. [Google Scholar] [CrossRef]
- Wang, H.; Pang, L.; Yang, C.; Liu, Y. Production of glycerol carbonate via reactive distillation and extractive distillation: An experimental study. Chin. J. Chem. Eng. 2015, 23, 1469–1474. [Google Scholar] [CrossRef]
- Roschat, W.; Phewphong, S.; Kaewpuang, T.; Promarak, V. Synthesis of glycerol carbonate from transesterification of glycerolwith dimethyl carbonate catalyzed by CaO from natural sources asgreen and economical catalyst. Mater. Today Proc. 2018, 5, 13909–13915. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Mg1+xCa1−xO2 as reusable and efficient heterogeneous catalyst for the synthesis of glycerol carbonate via the transesterification of glycerol with dimethyl carbonate. Appl. Catal. A Gen. 2013, 466, 272–281. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Kabir, G.; Hameed, B.H. Synthesis of glycerol carbonate from biodiesel by-product glycerol over calcined dolomite. J. Taiwan Inst. Chem. Eng. 2017, 70, 179–187. [Google Scholar] [CrossRef]
- Liu, P.; Derchi, M.; Hensen, E.J.M. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over MgAl mixed oxide catalysts. Appl. Catal. A Gen. 2013, 467, 124–131. [Google Scholar] [CrossRef]
- Fan, H.; Liu, X.; Chen, S. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over Mg-Al mixed oxides supported on MCM-41. Adv. Mater. Res. 2014, 1008–1009, 319–322. [Google Scholar] [CrossRef]
- Malyaadri, M.; Jagadeeswaraiah, K.; Prasad, P.S.S.; Lingaiah, N. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over Mg/Al/Zr catalysts. Appl. Catal. A Gen. 2011, 401, 153–157. [Google Scholar] [CrossRef]
- Manikandan, M.; Prabu, M.; Kumar, S.K.A.; Sangeetha, P.; Vijayaraghavan, R. Tuning the basicity of Cu-based mixed oxide catalysts towards the efficient conversion of glycerol to glycerol carbonate. Mol. Catal. 2018, 460, 53–62. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Lu, X.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over calcined Ca-Al hydrocalumite. Chin. J. Catal. 2015, 36, 1759–1765. [Google Scholar] [CrossRef]
- Lu, P.; Wang, H.; Hu, K. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over the extruded CaO-based catalyst. Chem. Eng. J. 2013, 228, 147–154. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Akpan, U.G.; Kabir, G.; Asif, M.; Hameed, B.H. Upgrading of glycerol from biodiesel synthesis with dimethyl carbonate on reusable Sr–Al mixed oxide catalysts. Energy Convs. Manag. 2017, 138, 183–189. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Kang, M.; Yin, N.; Wang, X.; Tan, Y.; Zhu, Y. Structure-activity correlations of LiNO3/Mg4AlO5.5 catalysts for glycerol carbonate synthesis from glycerol and dimethyl carbonate. J. Ind. Eng. Chem. 2015, 21, 394–399. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Cai, F.; Pan, D.; Xiao, G. High-efficiency and low-cost Li/ZnO catalysts for synthesis of glycerol carbonate from glycerol transesterification: The role of Li and ZnO interaction. Appl. Catal. A Gen. 2017, 532, 77–85. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; He, D. Catalytic synthesis of glycerol carbonate from biomass-based glycerol and dimethyl carbonate over Li-La2O3 Catalysts. Appl. Catal. A Gen. 2018, 564, 234–242. [Google Scholar] [CrossRef]
- Bai, R.; Wang, Y.; Wang, S.; Mei, F.; Li, T.; Li, G. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by NaOH/γ-Al2O3. Fuel Process. Technol. 2013, 106, 209–214. [Google Scholar] [CrossRef]
- Sandesh, S.; Shanbhag, G.V.; Halgeri, A.B. Transesterification of glycerol to glycerol carbonate using KF/Al2O3 catalyst: The role of support and basicity. Catal. Lett. 2013, 143, 1226–1234. [Google Scholar] [CrossRef]
- Song, X.; Pan, D.; Wu, Y.; Cheng, P.; Wei, R.; Gao, L.; Zhang, J.; Xiao, G. Synthesis of glycerol carbonate over porous La-Zr based catalysts: The role of strong and super basic sites. J. Alloys Compd. 2018, 750, 828–837. [Google Scholar] [CrossRef]
- Varkolu, M.; Burri, D.R.; Kamaraju, S.R.R.; Jonnalagadda, S.B.; Van Zyl, W.E. Transesterification of glycerol with dimethyl carbonate over nanocrystalline ordered mesoporous MgO–ZrO2 solid base catalyst. J. Porous Mater. 2016, 23, 185–193. [Google Scholar] [CrossRef]
- Parameswaram, G.; Srinivas, M.; Babu, B.H.; Prasad, P.S.S.; Lingaiah, N. Transesterification of glycerol with dimethyl carbonate for the synthesis of glycerol carbonate over Mg/Zr/Sr mixed oxide base catalysts. Catal. Sci. Technol. 2013, 3, 3242–3249. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. Production of glycerol carbonate using a novel Ti-SBA-15 catalyst. Chem. Eng. J. 2018, 346, 477–488. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Widyaya, V.T.; Kim, C.S.; Ahn, B.S.; Kim, Y.J.; Lee, H. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate using magnesium–lanthanum mixed oxide catalyst. Chem. Eng. Sci. 2013, 94, 265–270. [Google Scholar] [CrossRef]
- Kumar, P.; With, P.; Srivastava, V.C.; Gläser, R.; Mishra, I.M. Glycerol carbonate synthesis by hierarchically structured catalysts: Catalytic activity and characterization. Ind. Eng. Chem. Res. 2015, 54, 12543–12552. [Google Scholar] [CrossRef]
- Singh, D.; Reddy, B.; Ganesh, A.; Mahajani, S. Zinc/lanthanum mixed-oxide catalyst for the synthesis of glycerol carbonate by transesterification of glycerol. Ind. Eng. Chem. Res. 2014, 53, 18786–18795. [Google Scholar] [CrossRef]
- Parameswaram, G.; Rao, P.S.N.; Srivani, A.; Rao, G.N.; Lingaiah, N. Magnesia-ceria mixed oxide catalysts for the selective transesterification of glycerol to glycerol carbonate. Mol. Catal. 2018, 451, 135–142. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Zhang, J.; Li, S.; Yang, X.; Wang, H.; Wei, R.; Gao, L.; Zhang, J.; Xiao, G. Synthesis of glycerol carbonate from glycerol and diethyl carbonate over CeO2-CdO catalyst: The role of Ce4+ doped into CdO lattice. J. Taiwan Inst. Chem. Eng. 2018, 87, 131–139. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Cai, F.; Xiao, G. Synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce-NiO catalyst: The role of multiphase Ni. J. Alloys Compd. 2017, 720, 360–368. [Google Scholar] [CrossRef]
- Lim, S.R.; Lee, S.D.; Kim, H.S.; Simanjuntak, F.S.H.; Lee, H. Lanthanum oxide-catalyzed transesterification of dimethyl carbonate with glycerol: Effect of surfactant. Bull. Korean Chem. Soc. 2014, 35, 3163–3168. [Google Scholar] [CrossRef][Green Version]
- Kondawar, S.E.; Patil, C.R.; Rode, C.V. Tandem synthesis of glycidol via transesterification of glycerol with DMC over Ba-mixed metal oxide catalysts. ACS Sustain. Chem. Eng. 2017, 5, 1763–1774. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Segarra, A.M.; Contreras, S.; Sueiras, J.E.; Medina, F.; Figueras, F. Enhanced use of renewable resources: Transesterification of glycerol catalyzed by hydrotalcite-like compounds. Chem. Eng. J. 2010, 161, 340–345. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Hou, Z.; Zhang, M.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over Mg-Al hydrotalcites. Chin. J. Catal. 2014, 35, 310–318. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Frey, A.M.; Bitter, J.H.; Segarra, A.M.; de Jong, K.P.; Medin, F. On the role of the activation procedure of supported hydrotalcites for base catalyzed reactions: Glycerol to glycerol carbonate and self-condensation of acetone. Appl. Catal. B Environ. 2013, 134–135, 231–237. [Google Scholar] [CrossRef]
- Yadav, G.D.; Chandan, P.A. A green process for glycerol valorization to glycerol carbonate over heterogeneous hydrotalcite catalyst. Catal. Today 2014, 237, 47–53. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Chimentão, R.J.; Figueras, F.; Medin, F. Tunable basic and textural properties of hydrotalcite derived materials for transesterification of glycerol. Appl. Clay Sci. 2012, 58, 16–24. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Plíšková, M.; Segarra, A.M.; Medina, F.; Figueras, F. Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Appl. Catal. B Environ. 2012, 113–114, 212–220. [Google Scholar] [CrossRef]
- Liu, P.; Derchi, M.; Hensen, E.J.M. Promotional effect of transition metal doping on the basicity and activity of calcined hydrotalcite catalysts for glycerol carbonate synthesis. Appl. Catal. B Environ. 2014, 144, 135–143. [Google Scholar] [CrossRef]
- Hong, M.; Gao, L.; Xiao, G. An efficient and green transesterification of glycols into cyclic carbonates catalysed by KF/Ca–Mg–Al hydrotalcite. J. Chem. Res. 2014, 38, 679–681. [Google Scholar] [CrossRef]
- Kondawar, S.; Rode, C. Solvent-free glycerol transesterification with propylene carbonate to glycerol carbonate over a solid base catalyst. Energy Fuels 2017, 31, 4361–4371. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Ma, J.; Wei, W. Fluorinated Mg–Al hydrotalcites derived basic catalysts for transesterification of glycerol with dimethyl carbonate. Catal. Lett. 2017, 147, 1181–1196. [Google Scholar] [CrossRef]
- Ramesh, S.; Debecker, D.P. Room temperature synthesis of glycerol carbonatecatalyzed by spray dried sodium aluminate microspheres. Catal. Commun. 2017, 97, 102–105. [Google Scholar] [CrossRef]
- Ramesh, S.; Devred, F.; van den Biggelaar, L.; Debecker, D.P. Hydrotalcite promoted by NaAlO2 as strongly basic catalysts withrecord activity in glycerol carbonate synthesis. ChemCatChem 2018, 10, 1398–1405. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. A green and facile production of catalysts from waste red mud for the one-pot synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2019, 7, 102888–102898. [Google Scholar] [CrossRef]
- Wang, S.; Hao, P.; Li, S.; Zhang, A.; Guan, Y.; Zhang, L. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by calcined silicates. Appl. Catal. A Gen. 2017, 542, 174–181. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Okoye, P.U.; Li, S.; Tian, C. Microwave-assisted transesterification of glycerol with dimethyl carbonate over sodium silicate catalyst in the sealed reaction system. Energy Convers. Manag. 2018, 164, 543–551. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using trisodium phosphate. J. Taiwan Inst. Chem. Eng. 2016, 68, 51–58. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Hameed, B.H. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over K-zeolite derived from coal fly ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Khanday, W.A.; Okoye, P.U.; Hameed, B.H. Biodiesel byproduct glycerol upgrading to glycerol carbonate over lithium–oil palm ash zeolite. Energy Convers. Manag. 2017, 151, 472–480. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Irawan, E.; Nuryoto, N.; Jayanudin, J.; Sulistyo, H.; Sediawan, W.B.; Muraza, O. Glycerol carbonate production from biodiesel waste over modified natural clinoptilolite. Waste Biomass Valor. 2016, 7, 1349–1356. [Google Scholar] [CrossRef]
- Okoye, P.U.; Wang, S.; Xu, L.; Li, S.; Wang, J.; Zhang, L. Promotional effect of calcination temperature on structural evolution, basicity, and activity of oil palm empty fruit bunch derived catalyst for glycerol carbonate synthesis. Energy Convers. Manag. 2019, 179, 192–200. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, D. Transition metal-promoted hierarchical ETS-10 solid base for glycerol transesterification. RSC Adv. 2018, 8, 33473–33486. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Sun, J.; Yang, R.; Dong, W. Ordered mesoporous BaCO3/C-catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Sci. China Chem. 2015, 58, 708–715. [Google Scholar] [CrossRef]
- Qing, Y.; Lu, H.; Liu, Y.; Liu, C.; Liang, B.; Jiang, W. Production of glycerol carbonate using crude glycerol frombiodiesel production with DBU as a catalyst. Chin. Chem. Eng. 2018, 26, 1912–1919. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Choi, J.S.; Lee, G.; Lee, H.J.; Lee, S.D.; Cheong, M.; Kim, H.S.; Lee, H. Synthesis of glycerol carbonate from the transesterification of dimethyl carbonate with glycerol using DABCO and DABCO-anchored Merrifield resin. Appl. Catal. B Environ. 2015, 165, 642–650. [Google Scholar] [CrossRef]
- Wan, Y.; Lei, Y.; Lan, G.; Liu, D.; Li, G.; Bai, R. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over DABCO embedded porous organic polymer as a bifunctional and robust catalyst. Appl. Catal. A Gen. 2018, 562, 267–275. [Google Scholar] [CrossRef]
- Naik, P.U.; Petitjean, L.; Refes, K.; Picquet, M.; Plasseraud, L. Imidazolium-2-carboxylate as an efficient, expeditious and eco-friendly organocatalyst for glycerol carbonate synthesis. Adv. Synth. Catal. 2009, 351, 1753–1756. [Google Scholar] [CrossRef]
- Hervert, B.; McCarthy, P.D.; Palencia, H. Room temperature synthesis of glycerol carbonate catalyzed by N-heterocyclic carbenes. Tetrahedron Lett. 2014, 55, 133–136. [Google Scholar] [CrossRef]
- Stewart, J.A.; Drexel, R.; Arstad, B.; Reubsaet, E.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Homogeneous and heterogenised masked N-heterocyclic carbenes for bio-based cyclic carbonate synthesis. Green Chem. 2016, 18, 1605–1618. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).