A Photo-Enzymatic Cascade to Transform Racemic Alcohols into Enantiomerically Pure Amines
Abstract
:1. Introduction
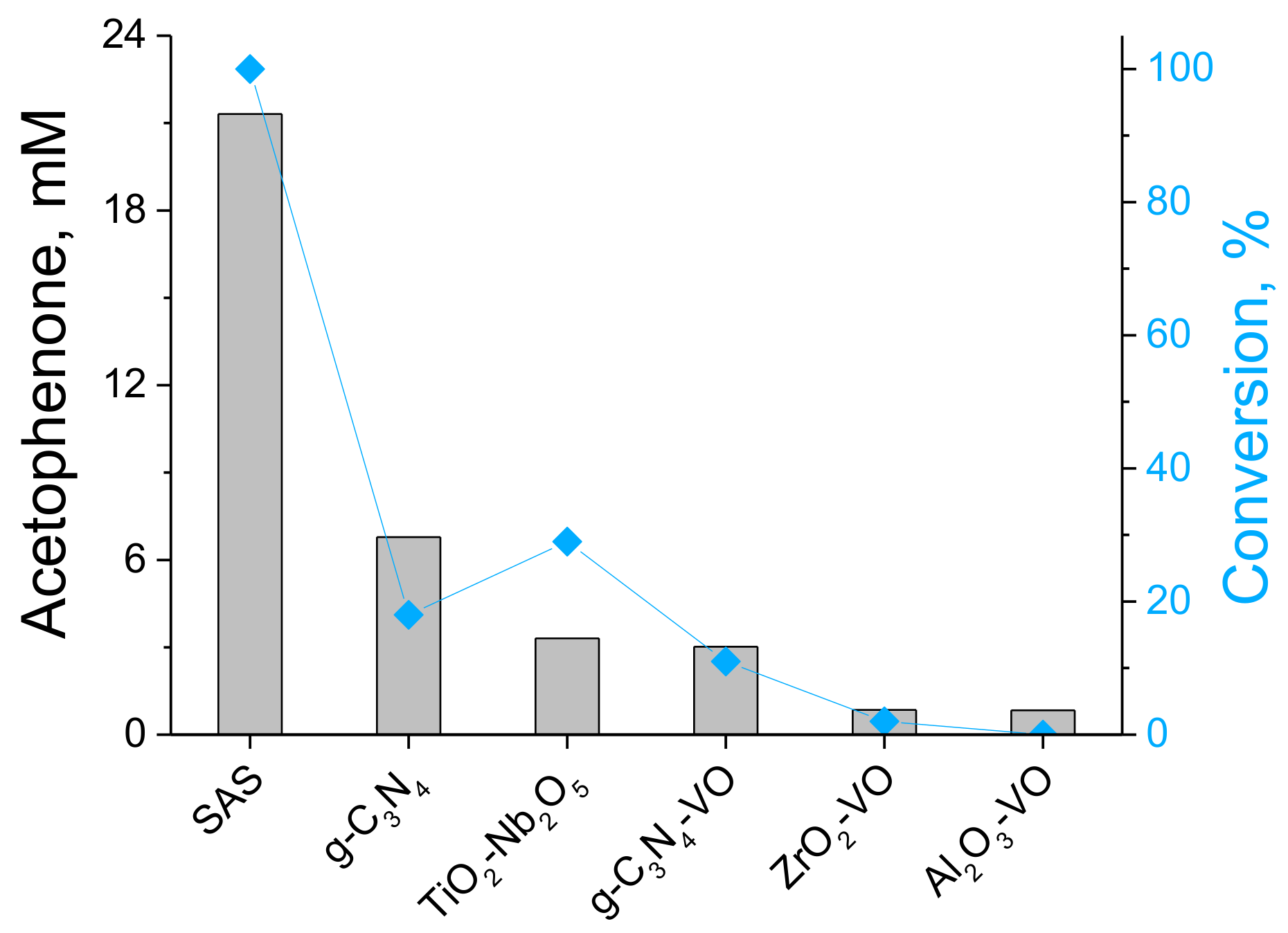
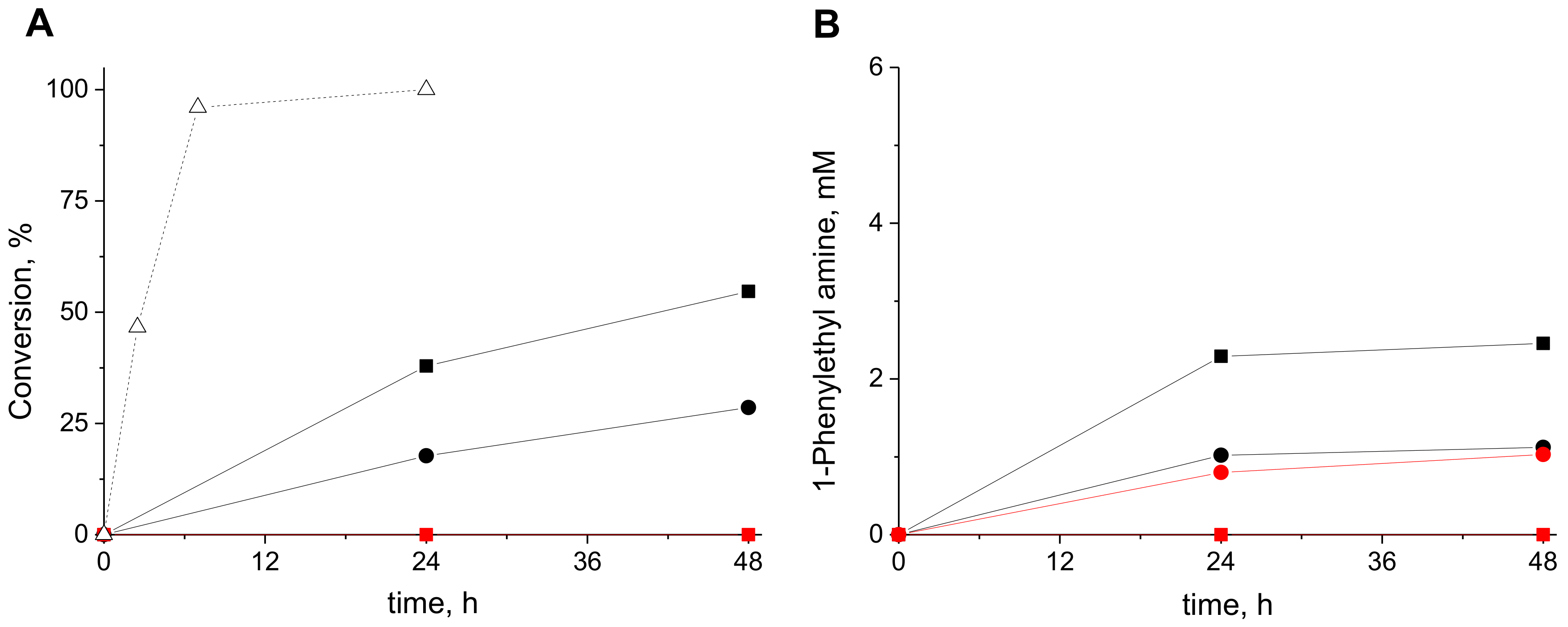
2. Results and Discussion
3. Materials and Methods
3.1. Reaction Conditions for the One-Pot One-Step Cascade
3.2. Reaction Conditions for the One-Pot Two-Step Cascade
3.3. Derivatization of GC Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nugent, T.C.; El-Shazly, M. Chiral amine synthesis—Recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 2010, 352, 753–819. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Green, A.P.; Turner, N.J.; O’Reilly, E. Chiral amine synthesis using omega-transaminases: An amine donor that displaces equilibria and enables high-throughput screening. Angew. Chem. Int. Ed. 2014, 53, 10714–10717. [Google Scholar] [CrossRef]
- Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The catalytic amination of alcohols. ChemCatChem 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Imm, S.; Bähn, S.; Zhang, M.; Neubert, L.; Neumann, H.; Klasovsky, F.; Pfeffer, J.; Haas, T.; Beller, M. Improved ruthenium-catalyzed amination of alcohols with ammonia: Synthesis of diamines and amino esters. Angew. Chem. Int. Ed. 2011, 50, 7599–7603. [Google Scholar] [CrossRef] [PubMed]
- Mutti, F.G.; Knaus, T.; Scrutton, N.S.; Breuer, M.; Turner, N.J. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science 2015, 349, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Fabiano, E.; Golding, B.T.; Sadeghi, M.M. A simple conversion of alcohols into amines. Synthesis 1987, 1987, 190–192. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, C.H.; Li, H.; Zhao, X.Y.; Song, L.; Li, X.B. An overview of selective oxidation of alcohols: Catalysts, oxidants and reaction mechanisms. Catal. Surv. Asia 2016, 20, 13–22. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Schultz, M.J.; Sigman, M.S. Recent advances in homogeneous transition metal-catalyzed aerobic alcohol oxidations. Tetrahedron 2006, 62, 8227–8241. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Sheldon, R.A. Modern oxidation of alcohols using environmentally benign oxidants. In Modern Oxidation Method; Bäckvall, J.-E., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Volume 2, pp. 147–185. [Google Scholar]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO(2) and WO(3) photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Shishido, T.; Miyatake, T.; Teramura, K.; Hitomi, Y.; Yamashita, H.; Tanaka, T. Mechanism of photooxidation of alcohol over nb2o5. J. Phys. Chem. C 2009, 113, 18713–18718. [Google Scholar] [CrossRef]
- Furukawa, S.; Shishido, T.; Teramura, K.; Tanaka, T. Photocatalytic oxidation of alcohols over TiO2 covered with Nb2O5. ACS Catal. 2012, 2, 175–179. [Google Scholar] [CrossRef]
- Chen, J.; Cen, J.; Xu, X.L.; Li, X.N. The application of heterogeneous visible light photocatalysts in organic synthesis. Catal. Sci. Technol. 2016, 6, 349–362. [Google Scholar] [CrossRef]
- Su, F.Z.; Mathew, S.C.; Lipner, G.; Fu, X.Z.; Antonietti, M.; Blechert, S.; Wang, X.C. Mpg-C3N4-catalyzed selective oxidation of alcohols using O-2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef]
- Zhang, L.G.; Liu, D.; Guan, J.; Chen, X.F.; Guo, X.C.; Zhao, F.H.; Hou, T.G.; Mu, X.D. Metal-free g-C3N4 photocatalyst by sulfuric acid activation for selective aerobic oxidation of benzyl alcohol under visible light. Mater. Res. Bull. 2014, 59, 84–92. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Selective oxidation of alcohols using photoactive vo@g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Zavahir, S.; Xiao, Q.; Sarina, S.; Zhao, J.; Bottle, S.; Wellard, M.; Jia, J.F.; Jing, L.Q.; Huang, Y.M.; Blinco, J.P.; et al. Selective oxidation of aliphatic alcohols using molecular oxygen at ambient temperature: Mixed-valence vanadium oxide photocatalysts. ACS Catal. 2016, 6, 3580–3588. [Google Scholar] [CrossRef]
- Clark, K.P.; Stonehil, H.I. Photochemistry and radiation-chemistry of anthraquinone-2-sodium-sulphonate in aqueous-solution Part 1. Photochemical kinetics in aerobic solution. J. Chem. Soc. Faraday Trans. I 1972, 68, 577–590. [Google Scholar] [CrossRef]
- Clark, K.P.; Stonehil, H.I. Photochemistry and radiation-chemistry of 9,10-anthraquinone-2-sodium sulfonate in aqueous-solution Part 2. Photochemical products. J. Chem. Soc. Faraday Trans. I 1972, 68, 1676–1686. [Google Scholar] [CrossRef]
- Wells, C.F. Hydrogen transfer to quinones Part 2. Reactivities of alcohols, ethers and ketones. Trans. Faraday Soc. 1961, 57, 1719–1731. [Google Scholar] [CrossRef]
- Wells, C.F. Hydrogen transfer to quinones Part 1. Kinetics of deactivation of photo-excited quinone. Trans. Faraday Soc. 1961, 57, 1703–1718. [Google Scholar] [CrossRef]
- Zhang, W.; Gacs, J.; Arends, I.W.C.E.; Hollmann, F. Selective photooxidation reactions using water soluble anthraquinone photocatalysts. ChemCatChem 2017, 9, 3821–3826. [Google Scholar] [CrossRef]
- Zhang, W.; Fernandez Fueyo, E.; Hollmann, F.; Leemans Martin, L.; Pesic, M.; Wardenga, R.; Höhne, M.; Schmidt, S. Combining photo-organo redox- and enzyme catalysis facilitates asymmetric C-H bond functionalization. Eur. J. Org. Chem. 2019, 10, 80–84. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Farnberger, J.E.; Steinkellner, G.; Sattler, J.H.; Pickl, M.; Simon, R.C.; Zepeck, F.; Gruber, K.; Kroutil, W. Asymmetric amination of α-chiral aliphatic aldehydes via dynamic kinetic resolution to access stereocomplementary brivaracetam and pregabalin precursors. Adv. Synth. Catal. 2018, 360, 768–778. [Google Scholar] [CrossRef]
- Hohne, M.; Bornscheuer, U.T. Biocatalytic routes to optically active amines. ChemCatChem 2009, 1, 42–51. [Google Scholar] [CrossRef]
- Ward, J.; Wohlgemuth, R. High-yield biocatalytic amination reactions in organic synthesis. Curr. Org. Chem. 2010, 14, 1914–1927. [Google Scholar] [CrossRef]
- Slabu, I.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. Discovery, engineering, and synthetic application of transaminase biocatalysts. ACS Catal. 2017, 7, 8263–8284. [Google Scholar] [CrossRef]
- Fuchs, M.; Farnberger, J.E.; Kroutil, W. The industrial age of biocatalytic transamination. Eur. J. Org. Chem. 2015, 2015, 6965–6982. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of omega-transaminases in the pharmaceutical industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.M.; Crismaru, C.G.; Bartsch, S.; Navickas, V.; Ditrich, K.; Breuer, M.; Abu, R.; Woodley, J.M.; Baldenius, K.; Wu, B.; et al. Enzymatic network for production of ether amines from alcohols. Biotechnol. Bioeng. 2016, 113, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yun, H. Ω-transaminases for the production of optically pure amines and unnatural amino acids. ACS Catal. 2012, 2, 993–1001. [Google Scholar] [CrossRef]
- Sattler, J.H.; Fuchs, M.; Mutti, F.G.; Grischek, B.; Engel, P.; Pfeffer, J.; Woodley, J.M.; Kroutil, W. Introducing an in situ capping strategy in systems biocatalysis to access 6-aminohexanoic acid. Angew. Chem. Int. Ed. 2014, 53, 14153–14157. [Google Scholar] [CrossRef]
- Wang, B.; Land, H.; Berglund, P. An efficient single-enzymatic cascade for asymmetric synthesis of chiral amines catalyzed by [small omega]-transaminase. Chem. Comm. 2013, 49, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montero, L.; Gotor, V.; Gotor-Fernández, V.; Lavandera, I. But-2-ene-1,4-diamine and but-2-ene-1,4-diol as donors for thermodynamically favored transaminase- and alcohol dehydrogenase-catalyzed processes. Adv. Synth. Catal. 2016, 358, 1618–1624. [Google Scholar] [CrossRef]
- Gomm, A.; Lewis, W.; Green, A.P.; O’Reilly, E. A new generation of smart amine donors for transaminase-mediated biotransformations. Chem. Eur. J. 2016, 22, 12692–12695. [Google Scholar] [CrossRef]
- Tufvesson, P.; Jensen, J.S.; Kroutil, W.; Woodley, J.M. Experimental determination of thermodynamic equilibrium in biocatalytic transamination. Biotechnol. Bioeng. 2012, 109, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Montero, L.; Gotor, V.; Gotor-Fernandez, V.; Lavandera, I. Stereoselective amination of racemic sec-alcohols through sequential application of laccases and transaminases. Green Chem. 2017, 19, 474–480. [Google Scholar] [CrossRef]
- Sattler, J.H.; Fuchs, M.; Tauber, K.; Mutti, F.G.; Faber, K.; Pfeffer, J.; Haas, T.; Kroutil, W. Redox self-sufficient biocatalyst network for the amination of primary alcohols. Angew. Chem. Int. Ed. 2012, 51, 9156–9159. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Sattler, J.; Resch, V.; Mutti, F.G.; Kroutil, W. Recent biocatalytic oxidation-reduction cascades. Curr. Opin. Chem. Biol. 2011, 15, 249–256. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Mutti, F.G.; Fuchs, C.S.; Pressnitz, D.; Sattler, J.H.; Kroutil, W. Stereoselectivity of four (R)-selective transaminases for the asymmetric amination of ketones. Adv. Synth. Catal. 2011, 353, 3227–3233. [Google Scholar] [CrossRef]
- Mutti, F.G.; Kroutil, W. Asymmetric bio-amination of ketones in organic solvents. Adv. Synth. Catal. 2012, 354, 3409–3413. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Humble, M.S.; Miceli, V.; Colomina, C.G.; Berglund, P. Active site quantification of an ω-transaminase by performing a half transamination reaction. ACS Catal. 2011, 1, 1051–1055. [Google Scholar] [CrossRef]
- Fesko, K.; Steiner, K.; Breinbauer, R.; Schwab, H.; Schurmann, M.; Strohmeier, G.A. Investigation of one-enzyme systems in the omega-transaminase-catalyzed synthesis of chiral amines. J. Mol. Catal. B Enzym. 2013, 96, 103–110. [Google Scholar] [CrossRef]
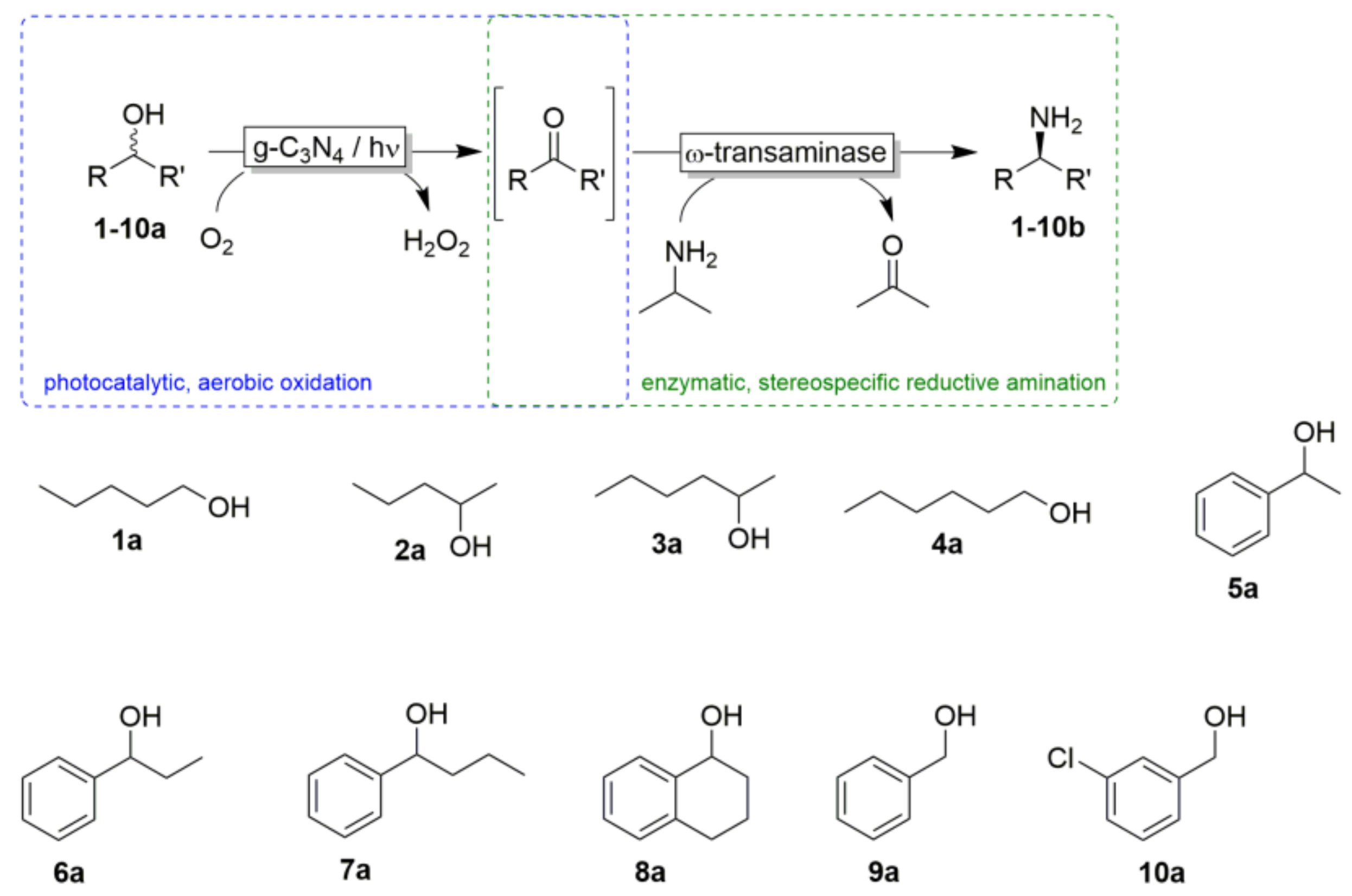

| Substrate | Conversion a (%) | Yield b (%) |
|---|---|---|
| 1ac | 23 | 74 |
| 2ac | 26 | 51 |
| 3ac | 29 | 27 |
| 4ac | 28 | 27 |
| 5a | 99 | 94 |
| 6a | >99 | 74 |
| 7a | 98 | 59 |
| 8a | 88 | 28 |
| 9a | 98 | 88 |
| 10a | >99 | 100 |
| 2b | 3b | 5b | 6b | 7b | 8b | ||
|---|---|---|---|---|---|---|---|
| ATωTA | camine (mM) a | 0.66 (R) | n.d. | 2.8 (R) | n.d. | n.d. | n.d. |
| Conversion (%) b | 36 | n.d. | 72 | n.d. | n.d. | n.d. | |
| ee (%) c | >99 | n.d. | >99 | n.d. | n.d. | n.d. | |
| BMωTA | camine (mM) | n.d. | n.d. | 4.93 (S) | 10.75 (S) | n.c | 0.42 (S) |
| Conversion (%) | n.d. | n.d. | 95 | 89 | n.d. | 20 | |
| ee (%) | n.d. | n.d. | >99 | >99% | n.d. | >99% | |
| CVωTA | camine (mM) | n.d. | n.d. | 1.29 (S) | n.d. | n.d. | n.d. |
| Conversion (%) | n.d. | n.d. | 34 | n.d. | n.d. | n.d. | |
| ee (%) | n.d. | n.d. | >99 | n.d. | n.d. | n.d. | |
| PFωTA | camine (mM) | n.d. | n.d. | 1.95 (S) | 2.40 | n.d. | n.d. |
| Conversion (%) | n.d. | n.d. | 43 | 35 | n.d. | n.d. | |
| ee (%) | n.d. | n.d. | >99 | >99 | n.d. | n.d. | |
| VFωTA | camine (mM) | n.d. | 2.25 (S) | 3.73 (S) | 2.44 | 1.20 (S) | n.d. |
| Conversion (%) | n.d. | 100 | 72 | 35 | 35 | n.d. | |
| ee (%) | n.d. | >99 | >99 | >99 % | >99 | n.d. |
| 4b | 9b | 10b | ||
|---|---|---|---|---|
| ATωTA | camine (mM) a | 2.21 | 6.03 | 8.20 |
| Conversion (%) b | 100 | 100 | 95 | |
| BMωTA | camine (mM) | 2.21 | 6.86 | 11.97 |
| Conversion (%) | 100 | 100 | 98 | |
| CVωTA | camine (mM) | 2.21 | 7.00 | 12.82 |
| Conversion (%) | 100 | 100 | 91 | |
| PFωTA | camine (mM) | 2.21 | 6.84 | 11.79 |
| Conversion (%) | 100 | 100.00 | 91 | |
| VFωTA | camine (mM) | n.d. c | 7.19 | 11.83 |
| Conversion (%) | n.d. | 100.00 | 98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacs, J.; Zhang, W.; Knaus, T.; Mutti, F.G.; Arends, I.W.C.E.; Hollmann, F. A Photo-Enzymatic Cascade to Transform Racemic Alcohols into Enantiomerically Pure Amines. Catalysts 2019, 9, 305. https://doi.org/10.3390/catal9040305
Gacs J, Zhang W, Knaus T, Mutti FG, Arends IWCE, Hollmann F. A Photo-Enzymatic Cascade to Transform Racemic Alcohols into Enantiomerically Pure Amines. Catalysts. 2019; 9(4):305. https://doi.org/10.3390/catal9040305
Chicago/Turabian StyleGacs, Jenő, Wuyuan Zhang, Tanja Knaus, Francesco G. Mutti, Isabel W.C.E. Arends, and Frank Hollmann. 2019. "A Photo-Enzymatic Cascade to Transform Racemic Alcohols into Enantiomerically Pure Amines" Catalysts 9, no. 4: 305. https://doi.org/10.3390/catal9040305
APA StyleGacs, J., Zhang, W., Knaus, T., Mutti, F. G., Arends, I. W. C. E., & Hollmann, F. (2019). A Photo-Enzymatic Cascade to Transform Racemic Alcohols into Enantiomerically Pure Amines. Catalysts, 9(4), 305. https://doi.org/10.3390/catal9040305







