Abstract
Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are the largest selling class of drugs prescribed for the pharmacological treatment of hypercholesterolemia and dyslipidaemia. Statins also possess other therapeutic effects, called pleiotropic, because the blockade of the conversion of HMG-CoA to (R)-mevalonate produces a concomitant inhibition of the biosynthesis of numerous isoprenoid metabolites (e.g., geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP)). Thus, the prenylation of several cell signalling proteins (small GTPase family members: Ras, Rac, and Rho) is hampered, so that these molecular switches, controlling multiple pathways and cell functions (maintenance of cell shape, motility, factor secretion, differentiation, and proliferation) are regulated, leading to beneficial effects in cardiovascular health, regulation of the immune system, anti-inflammatory and immunosuppressive properties, prevention and treatment of sepsis, treatment of autoimmune diseases, osteoporosis, kidney and neurological disorders, or even in cancer therapy. Thus, there is a growing interest in developing more sustainable protocols for preparation of statins, and the introduction of biocatalyzed steps into the synthetic pathways is highly advantageous—synthetic routes are conducted under mild reaction conditions, at ambient temperature, and can use water as a reaction medium in many cases. Furthermore, their high selectivity avoids the need for functional group activation and protection/deprotection steps usually required in traditional organic synthesis. Therefore, biocatalysis provides shorter processes, produces less waste, and reduces manufacturing costs and environmental impact. In this review, we will comment on the pleiotropic effects of statins and will illustrate some biotransformations nowadays implemented for statin synthesis.
1. Introduction
It is very well known that raised cholesterol levels increase the risks of heart disease and stroke. Globally, a third of ischaemic heart disease is attributable to high cholesterol and, according to the World Health Organization, raised cholesterol is estimated to cause 2.6 million deaths (4.5% of total) and 29.7 million disability adjusted life years (DALYS) [1]. In this sense, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, commonly known as statins (Figure 1), are the largest selling class of drugs prescribed for the pharmacological treatment of hypercholesterolemia and dyslipidaemia [2,3], and it has been also reported that since their introduction in 1987, the lives of millions of people have been extended through statin therapy and, more importantly, quality of life has been drastically improved [4].
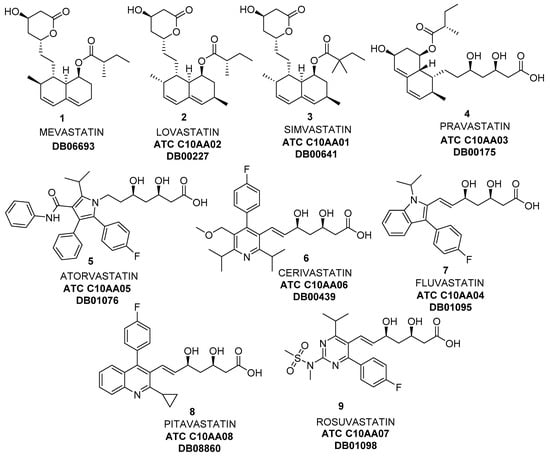
Figure 1.
Some Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Since the discovery of the first statins from natural sources, mevastatin (Figure 1, 1, also named compactin, from the fungi Penicillium citrinum [5] and Penicillium brevicompactum [6]), lovastatin (Figure 1, 2, Mevinolin, found in Aspergillus terreus [7] and food such as oyster mushrooms [8] or red yeast rice [9]), simvastatin (Figure 1, 3, Mevacor, also isolated from Aspergillus terreus [10]), and pravastatin (Figure 1, 4, initially known as CS-514, originally identified in the bacterium Nocardia autotrophica [11]), synthetic more potent compounds (Figure 1, 5–9), also known as superstatins, were introduced into the drug market [12,13]. As can be observed, the common structure of these compounds is formed by a central core of different heterocyclic aromatic rings containing nitrogen, and a lateral chain derived from (3R,5R)-3,5-dihydroxyheptanoic acid.
The economic impact of statins on the drug market is enormous. For instance, simvastatin was originally developed by Merck under the brand name Zocor™; in 2005, Zocor™ was Merck’s best-selling drug and the second-largest selling statin in the world (more than US$3 million only in USA, according to different reports [14,15,16,17,18,19,20,21]). In 2006, Zocor™ went off patent, and the annual sales drastically dropped; anyhow, from that moment, generic simvastatin became the most-prescribed statin in the world between 2010 and 2015 [19,20,22]. On the other hand, atorvastatin (Figure 1, ATC (Anatomical Therapeutic Chemical) classification system, according to World Health Organization) Code C10AA05, DrugBank Code DB01076, 5) is the greatest blockbuster drug in pharmaceutical history, and the best known representative of superstatins, receiving this name because of its pronounced ability to reduce low-density lipoprotein cholesterol levels and increase high-density lipoprotein cholesterol compared with other existing agents [13]. It was first synthesized in 1985 by Bruce Roth of Parke-Davis Warner-Lambert Company (now Pfizer), which commercialized it under the name of Lipitor™. Since it was approved in 1996, sales have exceeded US$125 billion, and the drug has topped the list of best-selling branded pharmaceuticals in the world for nearly a decade. When Pfizer’s patent on Lipitor™ expired in USA by the end of 2011 and in Europe in mid-2012, generic atorvastatin from other companies became available, and it is still being widely sold (US$2.16 billion in sales, standing as the year’s fourth-best-selling cardiovascular drug, with analysts predicting sales of US$1.85 billion in 2024 [23]). Finally, rosuvastatin (Figure 1, 9, ATC Code C10AA07, DrugBank Code DB01098) was marketed as calcium salt in 2003 by AstraZeneca under the name of Crestor™. Like atorvastatin, rosuvastatin is also a superstatin; the initial patent on rosuvastatin synthesis (purely chemical) was developed by Shionogi Research Laboratories [24] and later sold to AstraZeneca. This patent expired in June 2016, but anyhow, it still can be considered a blockbuster drug, by looking at the great volume of sales of Crestor™ (around U$2.7 billion in 2017, and US$727 million for the first half of 2018 [23]). A recent study [25] points toward global sales of statins of US$1 trillion by 2020, thus pharmaceutical companies are still interested in developing new synthetic strategies for putting these drugs on the market.
Thus, it is undoubtedly clear that the statin market involves a huge amount of money. Furthermore, the importance of this type of drug is even higher because of their new therapeutic uses that are recently becoming more and more recognized, which will be commented on in Section 2. Finally, as the absolute configuration of statins plays a crucial role in the activity of these compounds, the enormous potential of an enantioselective biocatalytic process for the sustainable synthesis of chiral building blocks involved in statin preparative procedures will further be commented on in Section 3.
2. New Therapeutic Effects of Statins
As mentioned before, these drugs act by reversibly and competitively inhibiting the bioreduction of S-3-hydroxy-3-methylglutaryl-coA (HMG-CoA), the rate-limiting step of the mevalonate pathway in cholesterol biosynthesis (Figure 2), because of the chemical similitude with mevalonyl-CoA, the intermediate obtained after the first reduction of HMG-CoA. Furthermore, there is extensive recent evidence suggesting that statins are more than simple lipid-lowering drugs [3,26]; in fact, a large amount of up-to-date experimental data have confirmed that statins may exert many different potentially beneficial therapeutic effects, by several mechanisms not essentially related to cholesterol metabolism. These so-called pleiotropic effects [27] could be attributed to their ability to prevent the conversion of HMG-CoA to R-mevalonate, which results in the concomitant inhibition of the downstream biosynthesis of cholesterol, as well as of numerous isoprenoid metabolites, such as geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP), as shown in Figure 2.
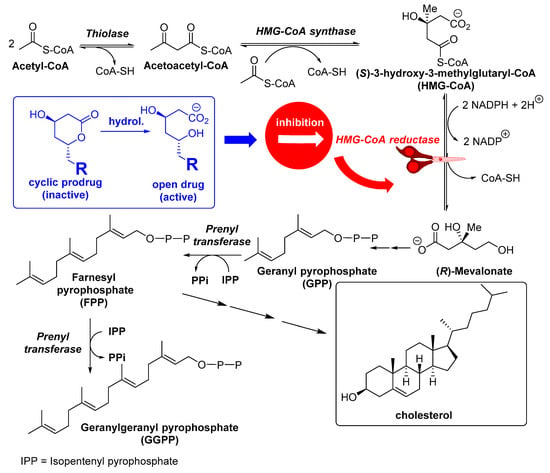
Figure 2.
Mevalonate pathway.
These molecules are well-known key intermediates for prenylation of several cell signalling proteins (such as small GTPase family members: Ras, Rac, Rho, Rab), which act as molecular switches controlling multiple pathways and cell functions (maintenance of cell shape, motility, factor secretion, differentiation, and proliferation), so that they can be inhibited by statin treatment [28]. For instance, when Ras and Rho isoprenylation is inhibited, there is a concurrent accumulation of inactive forms of both proteins in cytoplasm and an inhibition of these signalling molecules [29]. Certainly, it has been reported that small G-proteins like Rho and Rac influence endothelial nitric oxide synthase (eNOS) expression and nitric oxide (NO) availability [30]. Rho negatively regulates eNOS expression, while Rac activates nicotinamide dinucleotide phosphate (NADPH)-oxidase and the correspondent superoxide production, which in turn inactivates NO. If statins block both Rho and Rac GTPase activity via inhibition of geranylgeranylation, this leads to eNOS upregulation [31,32]. Remarkably, some beneficial effects of statins were displayed before cholesterol levels were reduced [30], and it can be assumed that those effects, dependent on the enhancement of eNOS expression and/or activity, result in a decline of platelet activation, attenuation of adhesion molecules expression, decrease of inflammatory cytokine production, and increase of reactive oxygen species (ROS) [33]. Therefore, pleiotropic effects of statins include the reduction of haemostasis by reducing platelet activation and the pro-coagulation cascade; the increase of fibrinolysis and the anticoagulation cascade; the improvement of endothelial function; the increase of NO bioavailability; as well as antioxidant, immune modulatory, and anti-inflammatory activities and stabilization of atherosclerotic plaques [27,29,34,35,36,37]. Thus, the therapeutic effects of statins are nowadays present in areas such as cardiovascular health, regulation of the immune system, anti-inflammatory and immunosuppressive properties, prevention and treatment of sepsis, treatment of autoimmune diseases, osteoporosis, kidney and neurological disorders, and even in cancer therapy; some of these therapeutic areas will be commented on.
2.1. Cardiovascular Effects
Aside from the main mechanism of action of lowering cholesterol levels, statins are also useful in the treatment of some other cardiovascular disorders, including acute coronary syndrome, heart failure, cardiac arrhythmias, aortic stenosis, peripheral arterial disease, cerebrovascular disease, and essential hypertension, as recently reviewed (see papers by Mihos et al. [38], Oesterle et al. [39], and references therein). In fact, chronic administration of statins is believed to produce what is known as PIC (“pre-ischemic conditioning”), protecting the myocardium during ischemic insult and injury [40], as a consequence of an increase in nitric oxide availability and immunomodulation; thus, statins increase the production of nitric oxide and blunt the formation of superoxide radicals via the upregulation of eNOS and stabilization of its mRNA, leading to an improved vascular function and a reduction in vascular inflammation [34]. In this sense, recent studies show the effectiveness of statins’ cardiovascular primary prevention [41], also for elderly people [42], and point towards the special benefits of fluvastatin [43].
2.2. Immunomodulatory Effects
The main objectives of autoimmune therapies are to re-establish immunological homeostasis and reduce autoimmune damages. Different studies are increasingly suggesting that an imbalance between Th17 and Treg cells, as well as the incorrect release of potent pro-inflammatory mediators by Th17 cells, are crucial for the pathogenesis of a number of autoimmune disorders [44]. Thus, a new immunotherapeutic strategy could be based on increasing Treg or inhibiting Th17 differentiation/effector functions. In this respect, statins show an outstanding potential, especially considering the increasing evidence that they might inhibit Th17 differentiation/effector functions and conversely promote Treg differentiation/suppressive function selectively in the setting of autoimmune diseases [44]. Small GTPases have been centrally implicated in regulating the development and functions of T and B lymphocytes as well as of dendritic cells (DC) [45,46]. Thus, as a consequence of the inhibition of GTPases prenylation, statin-based therapy can be a potential alternative for the treatment of autoimmune diseases [44,47,48]. In fact, positive effects of statin treatment have been reported in numerous autoimmune diseases such as multiple sclerosis [49,50], systemic lupus erythematosus [51,52,53], autoimmune myocarditis [54,55,56], or rheumatoid arthritis [44,57,58,59].
2.3. Neurological Disorders
This is probably one of the most attractive therapeutic areas in which the use of statins introduces interesting advances. Pleiotropic effects of statins via GTPases inhibition might have potential therapeutic implications in many neurological disorders, as the current connection between neurodegenerative diseases and vascular risk factors is becoming more and more evident [30,60]; therefore, statin treatment could display beneficial effects in neurological disorders such as stroke, Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), primary brain tumours, or depression.
2.3.1. Stroke
The risk factors for cerebrovascular disease are well known and largely variable and, in this sense, reduction of serum cholesterol levels could be highly beneficial for reducing the hazard of suffering a cerebrovascular accident (CVA), also named stroke [30]. Anyhow, an indubitable link between high cholesterol level and stroke risk is difficult to establish, because controversial data from several clinical studies have been published in the literature, some of them finding no relationship between cholesterol and stroke [61,62], while in some other cases, the beneficial effects are indeed observed [63,64]. A possible explanation for these discrepancies could be based on the fact that stroke can be either ischemic or haemorrhagic, and there are evidences supporting an association between elevated cholesterol and increased risk of ischemic stroke, but also showing a relationship between low cholesterol levels and increased risk of haemorrhagic stroke [30]. So, while disagreements are still present on the usefulness of statins in the primary prevention of acute stroke, there is a wide consensus on the positive aspects of statins treatment in secondary prevention after stroke or transient ischemic attack for diminishing the menace of suffering a new stroke [65,66,67,68]. Even in haemorrhagic stroke, some data from recent studies suggest that statin therapy could improve the outcome after spontaneous intra-cerebral haemorrhage and statin therapy should be not discontinued [69,70,71]. In any case, the most feasible explanation for reduction in clinical events reported for patients treated with statins is the stabilization of atherosclerotic plaques, which are generated by lipids deposition and migration and proliferation of vascular smooth muscle cells (see report from Malfitano et al. [30] and references cited therein for a more detailed explanation).
2.3.2. Alzheimer’s Disease (AD)
AD is a chronic neurodegenerative syndrome caused by the appearance of brain senile plaques composed of aggregated forms of β-amyloid peptide (Aβ), and it is the most common cause of dementia in elderly people, with a new case globally occurring every seven seconds [72]. Emerging evidence suggests a link between cholesterol and AD [37,72,73,74,75,76], and extensive studies have been published stressing the therapeutic utility of pleiotropic effects of statins, showing a dose-dependent beneficial effect on cognition, memory, and neuroprotection [72] by different mechanisms, such as altering the properties of plasma membrane by a reduction in cholesterol levels and a modulation of secretase activities, thus decreasing amyloid precursor protein (APP) processing [77], or by altering neuronal activity via modification of GTPases prenylation [28,74,78]. On the other hand, a possible effect of statins in cholinergic neurotransmission has been also described; in fact, simvastatin inhibits acetyl cholinesterase (AChE) activity in rats [79] and prevents the blockade caused by AChE inhibitors at α 7-nicotinic AChE receptors [80], thus increasing cholinergic neurotransmission. In this sense, Ghodke et al. [81] reported that statins treatment for 4 months, but not for 15 days, showed noteworthy enhancement in mice memory function, whereas a high cholesterol diet showed significant diminishing of memory. However, long-term statin treatment showed a significant decrease in serum cholesterol level as well as brain AChE level. Moreover, a high cholesterol diet showed a significant decrease in memory function with an increase in serum cholesterol level as well as brain AChE level. Thus, they concluded that there was no direct correlation between brain cholesterol level, as well as HMG-CoA activity with memory function regulation, although there is tangible link between plasma cholesterol level and AChE level, and long-standing plasma cholesterol alteration may be essential to regulate memory function through the AChE modulated pathway. Finally, a simvastatin-related rise of butyryl cholinesterase (BuChE) activity in mice brain, which may be a potential adverse effect in patients with AD, has been recently reported [82].
Another feasible mechanism for explaining statins’ neuroprotective effect considers an activation of the heme oxygenase/biliverdin reductase (HO/BVR-A) system [37]. Statins can also be active in AD treatment because of their protecting effect against glutamate toxicity over primary cortical neurons [83,84]. Low-dose administration of statins avoids aberrant neuronal entry into mitosis [85], promotes anti-apoptotic pathways [86], and impairs inflammation [87], although higher doses of statins have been shown to induce toxic effects [88]. Recently, some studies point towards the utility of simvastatin administration in the improving of hippocampus-dependent spatial memory in mice, due to an activation of Akt (protein kinase B), via a depletion of FPP and inhibition of farnesylation [89,90]. This same group has recently shown how simvastatin administration potentiates the contribution of N-methyl D-aspartate receptor (NMDAR) to synaptic transmission, by increasing the surface distribution of the GluN2B subunit of the NMDAR without affecting cellular cholesterol content [91]. The influence of statins in these ionotropic glutamate-receptors, and the succeeding utility of these drugs on treatment of AD and other mental disorders, is undoubtedly a very attractive and innovative research field [91,92].
Lamentably, although most evidence consistently confirms how statins do afford neuroprotection and improve disease pathology in animal models [93,94], results are rather controversial or even disappointing in human trials [72,95,96,97,98], thus a very careful study design and analysis will be essential in the future [95].
2.3.3. Parkinson’s Desease (PD)
PD, the second most common chronic neurodegenerative disorder in adults over the age of 65 years [99], is a progressive neurodegenerative disorder characterised by the presence of intracellular protein aggregates (Lewy bodies) and the loss of dopaminergic neurons from the pars compacta component of the substantia nigra in the midbrain; PD-related clinical manifestations of dopamine deficiency (gait, tremor, rigidity, and bradykinesia) are the most archetypical symptoms of this disease. There are several studies showing that some statins (simvastatin, but neither atorvastatin nor lovastatin) may reduce the incidence of PD in patients aged over 65 years [100]. Compared with discontinuation of statins, continuation of lipophilic statin use has been associated with a reduced risk of PD, particularly in the elderly [101]; nevertheless, in patients with existing PD, 10-day treatment of simvastatin (40 mg/day) showed no significant effects on dyskinesia, functional impairment, or involuntary movement [102].
As inflammation is accepted to be a main contributor to the PD aetiology, the anti-inflammatory action of statins could be a rational explanation for their activity [30]; in fact, simvastatin has been reported useful for reversing the loss of striatal dopamine activity and the production of nitrosylated free radicals, thus inducing neuro-protection [103,104], by decreasing the release of inflammatory mediators from microglia. Also, some studies in rats have shown that simvastatin can protect against loss of NMDA receptors produced by 6-hydroxydopamine (6-OHDA) [105]; also using the 6-OHDA model in rats, Wang et al. [106] recently described the beneficial effect of simvastatin in reducing abnormal involuntary movements known as l-DOPA-induced dyskinesia, commonly observed in patients chronically treated with l-DOPA. Finally, simvastatin and pravastatin can decrease the dopaminergic neuronal loss induced by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) via inhibition of p21(Ras)-induced NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [107].
Anyhow, as mentioned in the previous paragraph, more definitive evidence from prospective and clinical studies is required before drawing any conclusions about statins efficacy for treatment of PD.
2.3.4. Depression
As well as for the previous neurological disorders discussed so far, there are reported discrepancies about statins’ effect in depression, with some studies reporting positive effects of statins in reducing depression and depression-like symptoms in animals [108,109,110,111,112,113,114,115] or humans [116,117], while some others stated no relationships [118,119,120]. These divergences require more detailed studies, also for elucidating the possible mechanism of the positive effects, which, in some cases, have been associated with a modulation of NMDA receptors [121] or peroxisome proliferator-activated receptor gamma (PPAR-γ) receptors, by NO inhibition [122].
2.3.5. Epilepsy
In several studies, a reduced risk of developing epilepsy after age 50 has been reported [123,124,125,126,127,128], and the mechanism for this neuroprotective effect has been associated with a decrease in the association of subunit 1 of NMDA receptors to lipid rafts [129], as well as inhibition of calcium-dependent calpain activation, ROCK inhibition, the activation of the PI3K pathway, and increased APP cleavage [124], or the increased expression level of eNOS [130]; a recent publication by Scicchitano et al. [131] summarises the currently available data concerning statin effects in modulating epileptic seizure activity (sometimes adversely) and epileptogenesis in different experimental models, as well as in clinical studies [123,132].
2.4. Cancer
There are many studies dealing with the potential antitumor efficacy of statins, reporting effects in different cancer cell lines, as well as the possible risks of cancer development caused by statins treatment and the results of different clinical trials [133,134,135,136,137,138]. Once again, the molecular mechanisms explaining statins’ effects are quite different, and clinical trials are not reporting conclusive results; in fact, although some large scale meta-analyses seem to indicate that statins do not have significant effects on cancer incidence [133,139,140], in some other cases, some beneficial effects associated with statins’ administration in the treatment of different cancers have been described [141,142,143,144].
What is really clear is that there is not just one mechanism explaining the anticancer activity of statins, because depending on the type and dosages of statin used, the type of cancer cells, and the time of exposure of cells to statins, different effects leading to cell-cycle arrest, induction of apoptosis, or changes in molecular pathways are reported [138]. Concerning cell modifications, a common scheme is followed, starting with an arrest of cells in the G1 [145,146] or S-phase [147], and this inhibition of cell-cycle progression is mediated by cyclins (such as cyclin D1 [148]), cyclin-dependent kinases (CDKs, such as p21WAF1/CIP1 [148], p27 [149], CD4 [148], or p53 [150,151]), and inhibitors of CDKs [145]. Simultaneously, inhibition of G-protein prenylation is produced, leading to the arrest of proliferation and/or induction of apoptosis in cancer cells [152,153], by an increase in caspases activity [147,154,155]; henceforward, inhibition of prenylation is a promising way to impede progression of cancer (see the recent review of Matusewicz et al. [138] and cites therein). On the other hand, it has been also reported that a substantial reduction in the amount of cholesterol leads to a reduction in the content of membrane lipid rafts in the cell membrane, altering cell signalling [156,157], and loosing membrane integrity; in this sense, it is known that the membrane of breast cancer and prostate cancer cells has higher level of cholesterol and lipid rafts, so these cells are more susceptible to apoptosis promoted by statins compared with normal cells [158,159]. In another feasible mechanism, statins are associated with inhibition of phosphorylation of caveolin-1 (Cav-1), the integral membrane protein that binds and transport cholesterol, which promotes tumour cell survival and resistance to chemotherapy by different mechanisms [160].
Anyhow, an exhaustive recompilation of all other mechanisms proposed to explain the action of statins in cancer treatment would be out of the scope of this manuscript and can be found in recent reviews [135,136,138,161]. However, once again, while the correlation between data obtained in vitro with those other ones reported in animal models is very high, clinical trials are not that irrefutable in their conclusions, and more detailed studies are demanded.
3. Biocatalyzed Synthesis of Statins
As previously indicated in the Introduction, the use of biocatalyzed steps for preparing homochiral synthons useful for the synthesis of statins is a smart strategy for gaining both efficiency and sustainability. In this section, we will present some examples.
3.1. Simvastatin
Lovastatin (Mevacor 2, ATC Code C10AA02, DrugBank Code DB00227, Figure 1) is a naturally-occurring fungal polyketide produced by Aspergillus terreus [162], while simvastatin (3, ATC Code C10AA01, DrugBank Code DB00641, Figure 1) is a semisynthetic analogue of 2 and is more effective in treating hypercholesterolemia, because of the fact that the substitution of the α-methylbutyrate side chain with α-dimethylbutyrate significantly increases the inhibitory properties of 2, while lowering undesirable side effects [10].
Because of the economic importance of simvastatin, as mentioned in the Introduction, various multistep syntheses of 3 starting from 2 have been described; thus, a widely used process (route #1) starts with the hydrolysis of the C8 ester in 2 to yield the triol Monacolin J 10, followed by selective silylation of the C13 alcohol to yield 11, esterification of C8 alcohol with dimethylbutyryl chloride to furnish 12, and deprotection of C13 alcohol to finally yield 3 [163] (Figure 3).
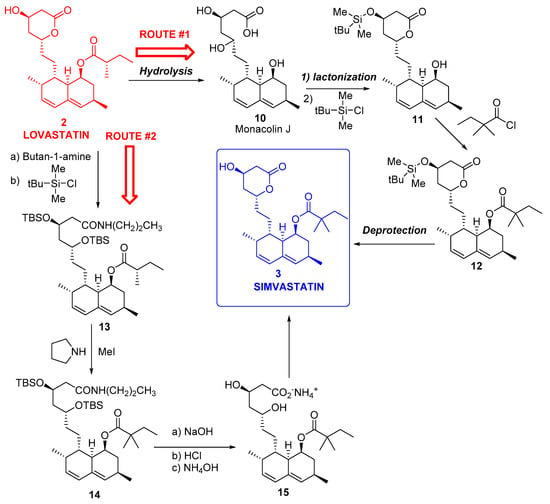
Figure 3.
Chemical transformations of lovastatin 2 into simvastatin 3.
In another option, namely route #2 [164], lovastatin 2 was treated with n-butylamine and TBSCl to obtain 13, which was alkylated with another methyl group to furnish 14, and finally transformed into 3 by hydrolysis and lactonization. Both multistep processes shown in Figure 3 were laborious, thus contributing to simvastatin being nearly five times more expensive than lovastatin [165].
Some enzymatic transformations using lipases and esterases were investigated as alternatives to chemical hydrolysis leading to Monacolin J 10 [166,167]. However, the requirement of regioselective esterification of the C8 alcohol invariably involves protection of other reactive alcohol groups in 10, and generally leads to lowered overall yield. Therefore, a specific reagent that is able to selectively acylate C8 of 10 is important for the efficient synthesis of simvastatin 3 and additional statin analogues.
In this sense, Tang and co-workers [22] described an acyltransferase (LovD) able to catalyse the last step of lovastatin biosynthesis, as shown in Figure 4, by transferring a 2,2-dimethylbutyryl acyl group from dimethylbutyryl-S-methylmercaptopropionate (DMB-SMMP, 16) regioselectively to the C8 hydroxyl of Monacolin J 10, the immediate biosynthetic precursor of simvastatin. The reaction proceeds via a ping-pong mechanism, and LovD is inhibited by Monacolin J at moderate substrate concentrations. LovD displayed broad substrate specificity toward the acyl carrier, the acyl substrate, and the decalin core of the acyl acceptor. This same group developed a one-step, whole-cell biocatalyzed process for the synthesis of Simvastatin from Monacolin J using an Escherichia coli strain overexpressing LovD, leading to >99% conversion of monacolin J to simvastatin without the use of any chemical protection steps [165]. The process was scaled up for gram-scale synthesis of simvastatin, also showing that simvastatin synthesized via this method could be readily purified from the fermentation broth with >90% recovery and >98% purity, as determined by high-performance liquid chromatography.
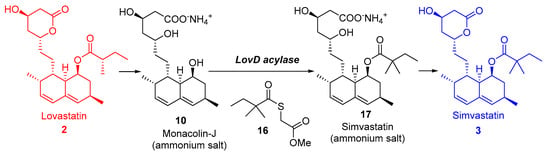
Figure 4.
Biocatalyzed transformations of lovastatin 2 into simvastatin 3.
Codexis improved not only the enzyme (previously modified used directed evolution at lab scale in an E. coli-based biocatalytic platform [168]) but also process chemistry to enable a large-scale simvastatin manufacturing process, by carrying out nine iterations of in vitro evolution, creating 216 libraries and screening 61,779 variants to develop a LovD variant with improved activity, in-process stability, and tolerance to product inhibition. The approximately 1000-fold improved enzyme and the new process pushed the reaction to completion at high substrate loading and minimized the amounts of acyl donor and of solvents for extraction and product separation. This process possesses many advantageous characteristics from a Green Chemistry point of view:
- Catalyst is produced efficiently from renewable feedstock.
- Reduced use of toxic and hazardous substances like tert-butyl dimethyl silane chloride, methyl iodide, and n-butyl lithium.
- Improved energy efficiency as the reaction is run at ambient temperature and at near atmospheric pressure.
- Reduction in solvent use because of the aqueous nature of the reaction conditions.
- The only by-product (methyl 3-mercaptopropionic acid) is recycled.
- The major waste streams generated are biodegraded in bio treatment facilities.
- Codexis’ process can produce simvastatin with yields of 97%, significant when compared with <70% with other manufacturing routes.
For these reasons, Codexis and Prof. Tang obtained the U.S. Environmental Protection Agency’s Green Chemistry Presidential Award in 2012 [169], inside the category of Greener Synthetic Pathway. Recently, identification of the complete biosynthetic pathway leading to monacolin J has been reported [170].
3.2. Biocatalyzed Synthesis of the Lateral Chain of Superstatins
Different biocatalytic routes have been proposed and implemented at industrial scale for the stereoselective preparation of the lateral chain (bearing the stereocentres) of superstatins. Thus, we would use the preparation of atorvastatin as a reference to illustrate how different biotransformations can be included in the overall protocol.
The chemical synthesis of atorvastatin originally described by researchers at Warner-Lambert Company [171], shown in Figure 5, started from a chiral building block, ethyl (R)-4-cyano-3-hydroxybutyrate 18, also known as “hydroxynitrile” (HN), and the second stereogenic centre of 20 was obtained by diastereomeric induction, using cryogenic borohydride reduction of a boronate derivative of the 5-hydroxy-3-keto intermediate 19 derived from HN.
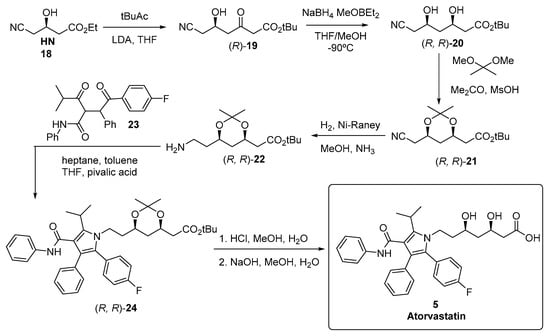
Figure 5.
Chemical synthesis of atorvastatin 5.
Taking this procedure as model, different strategies for generating the desired chirality can be envisaged from a biocatalytic retrosynthetic scheme [172], as depicted in Figure 6, in which purely chemical steps are denoted by a red C, while those syntheses feasible to be biocatalyzed are represented by a blue BT and a number, corresponding to the type of biocatalyst used. Thus, route #1 creates the desired chirality by a stereoselective desymmetrization of dinitrile 25 using a nitrilase (BT-1), while route #2 requires the preparation of HN 18 via a bioreduction of ketoester 27, so a ketoreductase (BT-2) is the biocatalyst required for that aim. Anyhow, in this synthetic path, another bioreduction should be used for avoiding the previously mentioned borohydride reduction of intermediate 19, using another ketoreductase (BT-3), so this can be considered route #3. Finally, if an aldolase (BT-4) is the enzyme selected, it is possible to envisage route #4 as an alternative through cyclic intermediate 28. These different routes will be discussed in the following sections.
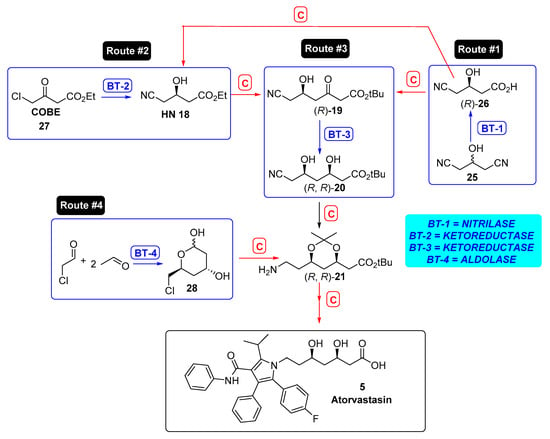
Figure 6.
Biocatalytic retrosynthetic routes to atorvastatin. BT represents biotransformation step, while C stands for chemical processes.
3.2.1. Hydrolases as Catalysts for the Preparation of the Lateral Chain of Atorvastatin and Other Superstatins
As shown in route #1, a nitrilase-catalyzed enzymatic desymmetrization of prochiral 3-hydroxyglutaronitrile 25 and subsequent esterification of the resulting (R)-3-hydroxy-4-cyanobutyric acid (R)-26 can lead to HN. The use of enzymatic protocols for hydrolysing nitriles is a green alternative compared with chemical methodologies [173], because of the harsh reaction conditions required, demanding either strong mineral acids (e.g., hydrochloric or phosphoric acid) or bases (e.g., potassium or sodium hydroxide) and relatively high reaction temperatures. Moreover, chemical procedures sometimes give low yields because of both unwanted by-product formation and the generation of concentrated contaminating waste salt streams (e.g., 6 mol L−1) when the acid or base is neutralized prior to disposal [174].
Thus, researchers at Diversa described a wild type nitrilase enzyme that catalysed the desymmetrization of 25 at high substrate concentration (3M) at lab-scale reaction, with an enantiomeric excess (ee) of 88%. [175]. A mutant nitrilase, obtained by directed evolution using gene site saturation mutagenesis (GSSM), and showing Ala190His single mutation, resulted in an excellent biocatalyst; hence, after a 15 h reaction at 20 °C, (R)-26 was isolated in 96% yield, with an excellent ee of 98.5% and a volumetric productivity of 619 g L−1 d−1 [176]. Subsequently, Dow Chirotech, a subsidiary of Dow Chemical Company, developed the Diversa nitrilase further into a biocatalysis process [177] and used the Pfenex expression system (a Pseudomonas fluorescens-based host expression system) to overproduce the enzyme. In this way, optimal reaction conditions for desymmetrization of 25 were as follows: 3 M (330 g L−1), pH 7.5, 27 °C, under 16 h reaction time. A conversion of 100% and 99% product ee was obtained, and the so-formed (R)-26 was consequently esterified to give HN. Overall, a highly efficient three-stage synthesis of HN starting from low-cost epichlorohydrin (required to produce 25) was achieved with an overall yield of 23%, 98% ee, and 97% purity [177]. Recently, an enzymatic method has been described for the synthesis of ethyl (R)-3-hydroxyglutarate from HN using free and immobilized recombinant Escherichia coli BL21(DE3)pLysS harbouring a nitrilase gene from Arabidopsis thaliana (AtNIT2) [178]. The hydrolysis of HN proceeded with the freely suspended cells of the biocatalyst under the optimized conditions of 1.5 mol L−1 (235.5 g L−1) substrate concentration and 6.0 wt % loading of wet cells at pH 8.0 and 25 °C, with 100% conversion obtained in 4.5 h. Furthermore, immobilization of the whole cells enhanced their substrate tolerance, stability, and reusability. Under the optimized conditions (100 mmol L−1 tris(hydroxymethyl)aminomethane hydrochloride buffer, pH 8.0, 25 °C), the immobilized biocatalyst could be reused for up to 16 batches, with a biocatalyst productivity of 55.6 g gwet cells−1 and a space–time productivity of 625.5 g L−1 d−1.
Hydrolases are also useful for preparing (S)-3-hydroxy butyrolactone (S)-32, another enantiopure intermediate to furnish HN (Figure 7). In fact, opening of (S)-32 with HBr/EtOH will yield the corresponding ethyl (S)-4-bromo-3-hydroxybutanoate ((S)-BHBE, (S)-33) [179], later transformed into HN via SN2 when treated with sodium (or potassium) bromide. Although (S)-32 can be produced from chiral pool raw materials (lactose or malic acid), it can be conveniently obtained by enzymatic hydrolysis of the racemic ethyl 4-chloro-3-hydroxybutanoate (CHBE, rac-29) in the aqueous phase [180]. The lipase stereoselectively hydrolysed only the (S)-enantiomer; however, the resulting acid (S)-30 is unstable, and it readily loses one HCl molecule to give the corresponding lactone of high enantiopurity (>99% ee). However, the enantiopurity of the lactone rapidly decreased when the process was operated at yields of more than 40%. The hydrolysis of the enantiopure benzoic ester of (S)-hydroxybutyrolactone (S)-31 has also been described using lipase from Candida rugosa (CRL) immobilized on amberlite XAD-7 as polymeric support, with ee of 99% [181]. This enzymatic hydrolysis was observed to be non-stereoselective in nature, because the enzymatic hydrolysis of the racemic benzoic ester yielded the racemic lactone, so that a chiral pool precursor (l-malic acid) for this process was necessary. Anyhow, this method has been scaled up to a ton scale, with an overall yield of over 80%, and a reaction time of 14 h [182]. Recently, a platform pathway for the production of 3-hydroxyacids has been described as an alternative biosynthetic route to generate the enantiopure lactone [183].
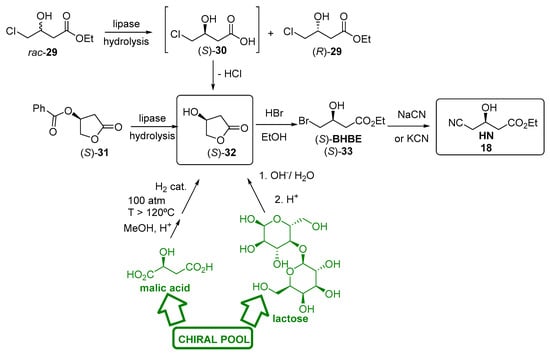
Figure 7.
Preparation of HN 18 starting from (S)-3-hydroxy butyrolactone (S)-32.
More recently, new biocatalytic approaches employing hydrolases have been described for furnishing the lateral chain of superstatins. Actually, Figure 8 shows a synthetic scheme for preparing rosuvastatin 9. As can be seen, conjugated ketoester 36 is subsequently transformed into final calcium rosuvastatin 9 by different steps (silyl ether cleavage, diastereoselective Narasaka-Prasad [184,185] syn-reduction using diethylmethoxy borane leading to ester 37, and finally ester hydrolysis and salt formation).
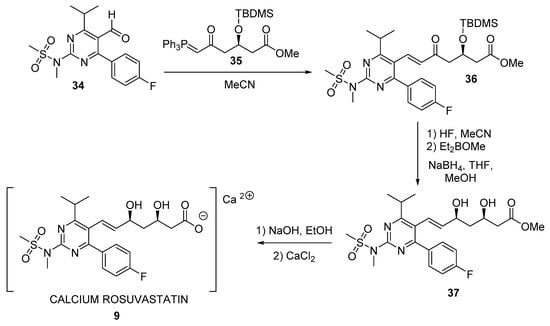
Figure 8.
Final steps in the chemical synthesis of rosuvastatin 9.
Aldehyde 34 can be easily obtained [186], while the preparation of enantiopure ylide 35 is much more complicated. Thus, several examples can be found in the literature starting from racemic diethyl 3-hydroxyglutarate, which had to be previously transformed in an activated derivative to react with the corresponding methyltriphenylphosphonium ylide to finally yield 35; although this route has been described using an enzymatic desymmetrization step [187,188], different side reactions were observed to decrease either the final yield or the enantiomeric excess. Recently, a bi-enzymatic process has been described for obtaining enantiopure monoester (R)-40 (Figure 9), combining a stereoselective hydrolysis of prochiral 38 to obtain (R)-39 with high yield and enantiopurity, and a subsequent removal of the acetyl group with cephalosporin acetyl esterase [189].
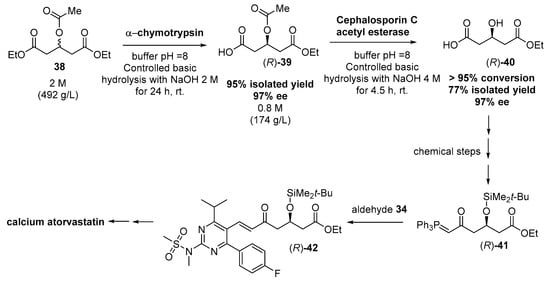
Figure 9.
Two-enzymatic system for synthesizing chiral intermediates for Rosuvastatin, as described by Metzner et al. [189].
Furthermore, these same authors have optimized the overall procedure, using a smart engineering approach with an enzyme recycling of chymotrypsin and immobilized cephalosporin C acetyl esterase, with excellent volumetric productivity, transferring this technology to Sandoz for its industrial implementation [190].
3.2.2. Ketoreductases as Catalysts for the Preparation of the Lateral Chain of Atorvastatin and Other Superstatins
As commented before, HN 18 was the starting point for the first synthesis of atorvastatin (Figure 5). For preparing HN, apart from the hydrolytic procedures described in Figure 7, some different purely chemical methodologies have been also described [191], and are depicted in Figure 10.
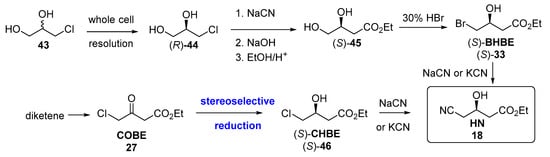
Figure 10.
Different chemical methodologies for the preparation of HN 18.
The first synthetic protocols involved kinetic resolutions of prochiral 1,3-dichloropropan-2-ol 43 using microbes, and transformation to dihidroxyester (S)-45 and subsequently to bromohydrine (S)-33 [179]. Later routes have involved asymmetric reduction of ethyl 4-chloroacetoacetate (COBE, 27), produced from diketene, to furnish ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE, (S)-46), using either chemical or biocatalytic reductions, as previously shown in route #2, Figure 6. Finally, the corresponding halohydrin ((S)-33 or (S)-46) could be converted to HN by treatment with cyanide.
In this sense, the enzymatic asymmetric reduction of 4-bromo-3-oxobutyrate esters has hardly been investigated compared with the corresponding chlorine analogue, because of the lower reactivity and enantioselectivity of enzymes towards brominated compounds, although (S)-4-bromo-3-hydroxybutanoate esters would be better substrates for the ulterior cyanide treatment; anyhow, some examples can be found in the literature, starting from methyl 4-bromo-3-oxobutyrate (BAM), using Escherichia coli engineered cells containing a mutant β-keto ester reductase (KER-L54Q) from Penicillium citrinum and a cofactor-regeneration enzyme such as glucose dehydrogenase (GDH) or Leifsonia sp. alcohol dehydrogenase (LSADH) [192,193].
Regarding chlorine containing oxoesters, the seminal paper of Patel et al. using glucose-, acetate-, or glycerol-grown cell (10% w/v) suspensions of Geotrichum candidum SC 5469 [194] to produce (S)-46 in reaction yield of 95% and optical purity of 96%, starting from 10 mg mL−1 of 27, showed how the bio-reduction could be an interesting alternative to asymmetrical chemical reduction. Furthermore, the optical purity of (S)-46 was increased to >99% by heat treatment of cell suspensions (55 °C for 30 min) prior to conducting bio-reduction at 28 °C.
Ye et al. [195] have reviewed a list of different yeast able to reduce 27 to furnish (S)-46, such as Candida etchellsii [196], Candida parapsilosis [197], Candida magnoliae [198], Saccharomycopsis lipolytica [196], or Candida macedoniensis [199], but in many cases, the stereoselectivity values obtained were not very high. Also, fungi as Aureobasidium pullulans CGMCC 1244 [200], Cylindrocarpon sclerotigenum IFO31855 [201], Penicillium oxalicum IFO 5748 [197], Botrytis allii IFO9430 [197], or Pichia stipitis CBS 6054 [202] can produce (S)-46 with a higher enantiomeric excess compared with yeasts. This same group, through genome database mining of this yeast Pichia stipitis, found two carbonyl reductases (PsCRI and PsCRII) leading to (S)-46 with >99% enantiomeric excess, which were subsequently characterized, cloned, and expressed in E. coli [195]. On the other hand, Cai et al. [203] described a substrate-coupled biocatalytic process based on the reactions catalyzed by an NADPH-dependent sorbose reductase (SOU1) from Candida albicans in which 27 was reduced to (S)-46, while NADPH was regenerated by the same enzyme via oxidation of sugar alcohols (sorbitol, mannitol, or xylitol). Optimization of COBE and sorbitol proportions yielded 2340 mM of (S)-46 starting from 2500 mM 27 with an enantiomeric excess was 99%. This substrate-coupled system maintained a stable pH and a robust intracellular NADPH circulation, so that pH adjustment and the addition of extra coenzymes were unnecessary, thus making this system very attractive. The bio-reduction of 27 and the scaling up of the process using Escherichia coli cells expressing a reductase (ScCR) from Streptomyces coelicolor to afford enantiopure (S)-46 has recently being described [204], at substrate loading of 100 g/L, while the concentration of coenzyme NAD+ was limited to 0.1 mM based on cost considerations, other reaction parameters were optimized as 25 °C and pH 6.5, with a biocatalyst dose of 10 kU/L in the presence of isopropanol (1.5 equiv of 27) as co-substrate for regenerating NADH. The reaction was performed in a toluene−aqueous biphasic system (1:1, v/v), with agitation at the maximal linear rate of 0.88 m/s. Finally, the bio-reaction was performed on a pilot scale using a 50 L thermostatised stirred-tank-reactor, affording (S)-46 in 85.4% yield and 99.9% ee, and a total turnover number (TTN) of 6060 for the cofactor NAD+. The specific production was calculated to be 36.8 g product/g dcw, which is the highest value reported to date among the whole-cell-mediated processes for producing (S)-46. Furthermore, from the point of view of sustainability, for this bio-reduction, the reaction and extraction solvent (toluene) was recycled with a loss of only 4.1%, so that the E factor (kg waste per kg product) for the process was determined as 1.8 if the process water was excluded, which was much lower than that value (2.3) obtained from the process using isolated ketoreductase, glucose dehydrogenase as the biocatalyst for cofactor regeneration, and glucose as the co-substrate [179]. The main contributors to the low E factor were the loss of the solvent toluene (46.1%), the use of excessive isopropanol, and the formation of coproduct acetone (combined ca. 35%). If water was also included, then the E factor would be 13.4.
Very recently, a recombinant Escherichia coli harbouring both the carbonyl reductase and glucose dehydrogenase has been described [205]. The recombinant E. coli was cultured in a 500-L fermenter, and the biocatalytic process for the synthesis of (S)-46 in an aqueous-organic solvent system was constructed and optimized with a substrate fed-batch strategy. Concentration of 27 reached to 1.7 M, and (S)-46 was obtained after a 4 h reaction in a 50-L reactor with yield of 97.2% and enantiomeric excess (ee) of 99%. Finally, (S)-46 was extracted from the reaction mixture with 82% yield and 95% purity.
Nevertheless, because of the great overall demand of HN required for atorvastin synthesis (estimated to be in excess of 100 mT [179]), it is highly desirable to reduce the wastes and hazards involved in its manufacture, while reducing its cost and maintaining or, preferably, improving its quality. This has been successfully carried out on a multiton scale by Codexis by means of a three-enzyme two-step process, the detailed description of which is depicted in Figure 11.
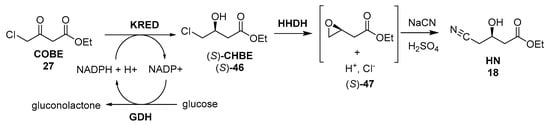
Figure 11.
Codexis synthesis of HN.
Hence, the first step involves the biocatalytic reduction of 27, using a ketoreductase (KRED) in combination with glucose and an NADP-dependent glucose dehydrogenase (GDH) for cofactor regeneration, leading to (S)-46 in 96% isolated yield and >99.5% ee. In the second step, a halohydrin dehalogenase (HHDH), an enzyme capable of catalysing the elimination of halides from vicinal haloalcohols, resulting in epoxide ring formation [206], was employed to catalyse a nucleophilic substitution of chloride by cyanide, using HCN at neutral pH and ambient temperature. The efficiency and greenness of this protocol (Codexis was awarded the U.S. Environmental Protection Agency’s Presidential Green Chemistry Challenge Award in 2006 for this work [207]) is based on the fact that all previous manufacturing routes to HN shown in Figure 10 involved, as the final step, a standard but troublesome SN2 substitution of halide with cyanide ion in alkaline solution (pH = 10) at high temperatures (80 °C), being this reaction substituted in the Codexis protocol. In fact, in the SN2 chlorine substitution, both (S)-46 and HN are base-sensitive molecules, and extensive by-product formation is observed, leading to high E values [179]. Moreover, the product is a high-boiling oil, and a troublesome high-vacuum fractional distillation is required to recover HN, resulting in further yield losses and waste, and clearly contravening the first and sixth principles of Green Chemistry [208]. Thus, conducting the cyanation reaction under milder conditions at neutral pH, by employing the enzyme, HHDH, is the key step for increasing the greenness of the overall process.
Coming back to the Codexis protocol, awkwardly, both the wild-type KRED and GDH as well as HHDH displayed very low activities, so that in the first experiments, huge enzyme loadings were required to obtain an economically feasible reaction rate, thus leading to troublesome emulsions, which hampered the subsequent downstream processing. Additionally, severe product inhibition and poor stability under operating conditions were observed. To enable a practical large-scale process, the three enzymes were optimized by in vitro enzyme evolution using gene shuffling technologies according to predefined criteria and process parameters, resulting in an overall process in which the volumetric productivity per mass catalyst load of the cyanation process was improved ~2500-fold, comprising a 14-fold reduction in reaction time, a 7-fold increase in substrate loading, a 25-fold reduction in enzyme use, and a 50% improvement in isolated yield [179].
Also using bio-reductions, some other strategies have been developed for the preparation of chiral building blocks for statins synthesis. Thus, Figure 12 illustrates route #3, previously shown in Figure 6, depicting bioreduction of the corresponding 6-substitued-3,5-dioxohexanoates 48 to furnish (R or S)-49 (similar to (R)-19, Figure 6). As depicted in Figure 5, the homochiral intermediate (3R,5R)-20 was originally prepared by diastereoselective chemical reduction of (R)-19, using NaBH4 and MeOBEt2 and, so as to obtain a high diastereoselectivity (>99.5% de), an extremely low temperature (−90 °C) and pyrophoric triethyl borane were demanded [209], with a concomitant extensive energy consumption and substantial amount of waste formation. Another alternative chemical route using chlororuthenium(II) arene/β-amino alcohol as the catalyst for the reduction was described [210], although the diastereoselectivity was insufficient (80% de).
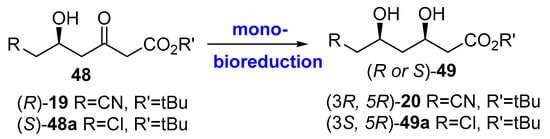
Figure 12.
Bio-reductions to produce chiral building blocks for statins.
Therefore, the use of a ketoreductase is highly desirable to develop green and sustainable bioreduction. This process has been described [211,212] using NADP(H)-dependent alcohol dehydrogenase of Lactobacillus brevis. This enzyme was overexpressed in a recombinant E. coli and the cell extracts were then employed for carrying out the biocatalytic reactions on a gram scale, to reduce (S)-48a to give the corresponding (3S, 5R)-49a in >99.5% de and isolated yield of 72%, at 24 h. Alcohol dehydrogenase itself recycles its cofactor by a substrate coupled methodology, by oxidation of 2-propanol to acetone. This process was scaled up to 100 g [213] using a fed-batch reactor, with the conversion of more than 90% attained in a total reaction time of 24 h. For the same substrate, Liu and co-workers have reported the use of a ketoreductase from Rhodosporidium toruloides, wild-type and genetically evolved, under different reaction conditions [214,215,216,217], while Xu et al. used a ketoreductase from Klebsiella oxytoca [218]. On the other hand, for reducing (R)-19 (up to 300 g L−1), the ketoreductase from L. brevis overexpressed in E. coli cells has also been employed [219], coupled to glucose-GDH for cofactor recycling, yielding (R,R)-20 in >99.5% de and 351 g L−1 d−1 space–time yield under the optimized conditions. Very recently, the same group has evolved the ketoreductase in order to improve the activity and thermostability of the enzyme [220]; thus, by coexpressing both the mutant ketoreductase and GDH, they describe the bioreduction of (R)-19 to (R,R)-20 at 40 °C in only 6 h, leading to values of >99.5% de and 1050 g L−1 d−1 space–time yield. Other similar bioreductions have also been reported using ketoreductases from other sources, such as Rhodotorula glutinis (whole cells [221]); engineered cells containing overexpressed NADPH-dependant ketoreductase from Saccharomyces cerevisiae and GDH [222,223]; a wild-type ketoreductase from Kluyveromuces lactis XP1461 (NADH-dependant) expressed in E. coli [224], subsequently improved by site-saturation mutagenesis [225]; or the ketoreductase from Candida albicans XP1463, also expressed in E. coli cells [226].
In a similar way, the double reduction of dioxoesters 50 (Figure 13) would directly lead to the target dihydroxyester 51. For this purpose, whole cells of Lactobacillus kefir, which contain two different types of alcohol dehydrogenase, are able to convert 50b into the dihydroxy ester (3R, 5S)-51a (99% ee in a total yield of 47.5% after 22 h, [227]) and the cofactor NADP(H) was regenerated by the usual glucose metabolism of the cell.
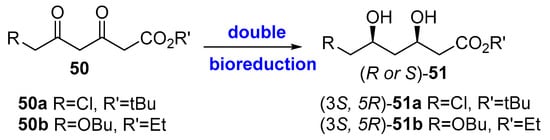
Figure 13.
Bio-reductions of dioxoesters to produce chiral building blocks for statins.
The double bio-reduction has been also described using isolated enzymes, from Acinetobacter species; in fact, Patel et al. originally described the bio-reduction of 50b using both whole cells and cell extracts from Acinetobacter calcoaceticus [228], and some years later, they also cloned and overexpressed [229] the diketoreductase responsible for the double reduction, which was efficiently carried out with the engineered enzyme [230]. Similarly, a diketoreductase from Acinetobacter baylyi ATCC 33305 was cloned and heterogeneously expressed in Escherichia coli by Wu et al. [231], showing an excellent biocatalytic performance at substrate concentration around 100 g L−1 [232] for the double reduction of 50a. Interestingly, the 3D structure of this enzyme was reported, and the details of the catalytic mechanism were explained [233,234,235].
3.2.3. Aldolases for the Preparation of the Lateral Chain of Atorvastatin and Other Superstatins
Aldolases can also be used in the preparation of chiral building blocks for statin synthesis. This would correspond to route #4 in Figure 6. In fact, Gijsen and Wong [236,237] first described the use of 2-deoxy-d-ribose 5-phosphate aldolase (DERA) from E. coli in the preparation of intermediate 28, in a reaction mixture consisting of 133 mg of chloroacetaldehyde and 264 mg of acetaldehyde in a total reaction volume of 20 mL (Figure 14). The atorvastatin intermediate lactone (4R, 6S)-54 can be easily formed by oxidation of lactol 28. However, aldolase showed low affinity to chloroacetaldehyde and was promptly inactivated at required aldehyde concentrations, so that a huge amount of aldolase was required. Furthermore, a very long reaction time of 6 days was required because of the reversible nature of aldol reactions, making this process unpractical for scaling up.
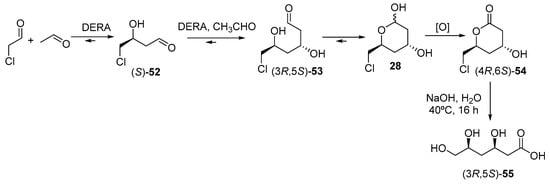
Figure 14.
Aldolase-catalysed synthesis of chiral building blocks for statins.
Subsequent studies by Liu et al. [238] described a mutant aldolase, leading to an increased yield of (4R, 6S)-54 to 43%, in comparison with 25% for the wild type aldolase, although the other reaction drawbacks were not overpassed. The process was markedly improved and scaled up by Greenberg et al. [239] of Diversa Corporation, by genetically modifying DERA by means of high throughput screenings of environmental DNA libraries, focussing on chloroacetaldehyde resistance and higher productivity; in a second step, the process was further improved by using a fed-batch bioreactor, in order to avoid significant substrate inhibition. Thus, the final synthesis of (4R, 6S)-54 on a 100 g scale in a total reaction time of 3 h with an ee of >99.9% and a 10-fold reduction in catalyst load over the previous method [240]. More recently, the use of whole cells systems is being evaluated for this process [241,242], as well as new strategies for improving DERA by genetic engineering [243]. Finally, a simple basic hydrolysis of lactone (4R, 6S)-54 leads to the trihydroxyacid (3R, 5S)-55, which is the precursor [244] of the lateral chain of superstatins. On the other hand, scientists from Lek Pharmaceutical (a Sandoz company) have described the use of whole cells of Escherichia coli BL21 (DE3) overexpressing the native E. coli deoC DERA gene for production of chiral lactols such as 28 [241], with excellent volumetric productivity (up to 50 g L−1 h−1), >80% yield, and >80% chromatographic purity with titers reaching 100 g L−1. This process is highly cost effective and environmentally friendly, and its sustainability is even improved if the oxidation of 28 to (4R, 6S)-54 is also catalysed with an enzyme, as this same group has reported using PQQ-dependent glucose dehydrogenases [245]. Ohshima and co-workers described the sequential aldol reactions depicted in Figure 14 using DERA isolated from thermophilic organisms, describing a relatively lower activity compared with the enzyme from E. coli, although this fact was compensated by a better synthetic yield caused by the increased acetaldehyde resistance shown by the thermophilic enzyme [246]. Shen and co-workers reported higher conversions when chloroacetaldehyde was used as the acceptor substrate, as compared with acetaldehyde [243], and thus the development of new DERAs from different microorganisms is an open research area, as reported in recent revisions [247,248].
In any case, compared with other chemical protocols, most pharmaceutical processes are performed on a smaller scale, with the production volume of 1000 to 10,000 tons per year and product concentration ranging between 50 and 100 g/L; hence, the main drawback is the transfer of the biocatalytic process from laboratory to a larger scale, especially with respect to retention times, which are greater on a larger scale (compared with those in the laboratory). A good example of industrial scale-up has been described by Ručigaj and Krajnc [242], who used acetoxyacetaldehyde and acetaldehyde as substrates, which are presented in an aldol reaction catalyzed by a crude DERA expressing culture lysate. By optimizing addition regimes of both reactants into a reaction mixture, the corresponding lactol was produced at near 77 g/L. The complete process was designed in a practical and economical manner and could be used further on an industrial scale. Another industrial scale, low temperature process was developed by DSM, leading to a final product concentration of 100 g L−1 [249].
4. Prognosis and Conclusions
It is easily foreseen that because our diet habits are becoming progressively unhealthier, with an increased uptake of fats and abandoning the traditional “Mediterranean diet”, hypercholesterolemia and dyslipidaemia will be typical maladies in Western society. Thus, statins would be gradually more present in our lives, being a very important piece of the global pharmaceutical market, either as branded or generic drugs. This fact, combined with the plethora of other pharmacological activities, called pleiotropic effects, that are being ascribed to statins, as revised in Section 2, makes us predict an ever-growing market for this type of drug. Anyway, more detailed and careful studies are demanded in order to be sure about the real efficiency of pleiotropic therapeutic effects of statins, by clearly identifying those patients who could be the best ones for responding to the desired effect of statins, and by establishing the most effective dose, duration of use, and statin drug entity required. Besides, more accurate clinical trials have to be conducted in order to evaluate the real effect upon the desired target, by designing more effective and truthful biomarkers.
For statins’ preparation, new and more sustainable protocols would be demanded; in this context, the substitution of chemical by biocatalyzed processes will certainly help to gain sustainability, because of the well-known green features of biocatalysis—synthetic routes conducted under mild reaction conditions; at ambient temperature; using water as reaction medium in many cases; and, last but not least, avoiding functional group activation and protection/deprotection steps usually required in traditional organic synthesis. Thus, we also foresee a growing increase in the use of biocatalysis and biotransformations for the preparation of statins, mainly promoted by the enhancement of biocatalysts’ performance through chemical modification and genetic engineering.
In another context, very recently, a new type of drug has emerged for dealing with those patients already using statins, but not reaching low-density lipoprotein cholesterol levels, rather by genetic and environmental factors or by pathological states—known as statin-resistance [250,251]. These drugs are the inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), a hepatic protease that becomes attached to low-density lipoproteins receptors (LDLRs), causing them to remain inside liposomes and get destroyed [252]. Nowadays, there are two PCSK9 inhibitors commercialized, both of them approved in 2015: alirocumab (Praluent®, from Sanofi) and evolucomab (Repatha®, from Amgen), both of them are used not as monotherapy, but are rather combined with a low cholesterol diet as well as with statins at maximally tolerated doses [253]. These two drugs are monoclonal antibodies, and their high price hampers their prior authorization practices and reduces their long-term adherence, so that the search for small molecules active as PCSK9 inhibitors is a “Holy Grail” in medicinal chemistry [254]. This situation leads us to think that (a) the statin market is not going to decrease, because they are going to be complemented (not substituted) with new drugs; and (b) as most of the new small molecules tested as PCSK9 inhibitors contain stereocenters in their structures [254], surely biocatalysis would become a very useful tool to facilitate more sustainable synthetic routes for their preparation.
Author Contributions
All authors (P.H., V.P., and A.R.A.) contributed equally in the preparation of this manuscript.
Funding
This research was partially funded by the Spanish Ministerio de Economia, Industria y Competitividad (MINECO), Project CTQ2015-66206-C2-1-R.
Acknowledgments
Authors thank Pablo Domínguez de María (CEO of Sustainable Momentum) for the critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Raised Cholesterol. Situation and Trends. Available online: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 15 February 2019).
- Stein, E.A. The power of statins: Aggressive lipid lowering. Clin. Cardiol. 2003, 26, 25–31. [Google Scholar] [CrossRef]
- Bifulco, M.; Endo, A. Statin: New life for an old drug. Pharmacol. Res. 2014, 88, 1–2. [Google Scholar] [CrossRef]
- Stossel, T.P. The discovery of statins. Cell 2008, 134, 903–905. [Google Scholar] [CrossRef]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef]
- Brown, A.G.; Smale, T.C.; King, T.J.; Hasenkamp, R.; Thompson, R.H. Crystal and molecular-structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1 1976, 1165–1173. [Google Scholar] [CrossRef]
- Moore, R.N.; Bigam, G.; Chan, J.K.; Hogg, A.M.; Nakashima, T.T.; Vederas, J.C. Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen, and oxygen-atoms by 13C NMR and Mass Spectrometry. J. Am. Chem. Soc. 1985, 107, 3694–3701. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Cimerman, A. Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase—Lovastatin. Exp. Mycol. 1995, 19, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fonnebo, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: A meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef]
- Mol, M.; Erkelens, D.W.; Leuven, J.A.G.; Schouten, J.A.; Stalenhoef, A.F.H. Simvastatin (MK-733)—A potent cholesterol-synthesis inhibitor in heterozygous familial hypercholesterolemia. Atherosclerosis 1988, 69, 131–137. [Google Scholar] [CrossRef]
- Yoshino, G.; Kazumi, T.; Kasama, T.; Iwatani, I.; Iwai, M.; Inui, A.; Otsuki, M.; Baba, S. Effect of CS-514, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on lipoprotein and apolipoprotein in plasma of hypercholesterolemic diabetics. Diabetes Res. Clin. Pract. 1986, 2, 179–181. [Google Scholar] [CrossRef]
- Li, J.J. Triumph of the Heart: The Story of Statins; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Casar, Z. Historic Overview and Recent Advances in the Synthesis of Super-statins. Curr. Org. Chem. 2010, 14, 816–845. [Google Scholar] [CrossRef]
- Lindsley, C.W. The top prescription drugs of 2010 in the United States: Antipsychotics show strong growth. ACS Chem. Neurosci. 2011, 2, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W. The top prescription drugs of 2011 in the United States: Antipsychotics and antidepressants once again lead CNS therapeutics. ACS Chem. Neurosci. 2012, 3, 630–631. [Google Scholar] [CrossRef]
- Lindsley, C.W. 2012 Trends and statistics for prescription medications in the United States: CNS therapeutics continue to hold leading positions. ACS Chem. Neurosci. 2013, 4, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W. The top prescription drugs of 2012 globally: Biologics dominate, but small molecule CNS drugs hold on to top spots. ACS Chem. Neurosci. 2013, 4, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W. 2013 Trends and statistics for prescription medications in the United States: CNS highest ranked and record number of prescriptions dispensed. ACS Chem. Neurosci. 2015, 6, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.; Kleinrock, M.; Lyle, J.; Nass, D.; Caskey, L. Medicines Use and Spending Shifts: A Review of the Use of Medicines in the U.S. in 2014; IMS Institute for Healthcare Informatics: Parsippany, NJ, USA, 2015. [Google Scholar]
- Lindsley, C.W. 2014 Prescription medications in the United States: Tremendous growth, specialty/orphan drug expansion, and dispensed prescriptions continue to increase. ACS Chem. Neurosci. 2015, 6, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W. 2014 Global prescription medication statistics: Strong growth and CNS well represented. ACS Chem. Neurosci. 2015, 6, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.K.; Watanabe, K.; Wojcicki, W.A.; Wang, C.C.C.; Tang, Y. Biosynthesis of lovastatin analogs with a broadly specific acyltransferase. Chem. Biol. 2006, 13, 1161–1169. [Google Scholar] [CrossRef]
- FiercePharma. The Cardiovascular Scene to Shift by 2024 as Next-Generation Drugs like Eliquis and Xarelto Eclipse Stalwarts. Available online: https://www.fiercepharma.com/pharma/cardiovascular-landscape-from-2017-to-2024-featuring-eliquis-xarelto-and-more (accessed on 14 February 2019).
- Watanabe, M.; Koike, H.; Ishiba, T.; Okada, T.; Seo, S.; Hirai, K. Synthesis and biological activity of methanesulfonamide pyrimidine- and N-methanesulfonyl pyrrole-substituted 3,5-dihydroxy-6-heptenoates, a novel series of HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 1997, 5, 437–444. [Google Scholar] [CrossRef]
- Demasi, M. Statin wars: Have we been misled about the evidence? A narrative review. Br. J. Sports Med. 2018, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Corsini, A. Clinical evidence of statin therapy in non-dyslipidemic disorders. Pharmacol. Res. 2014, 88, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cordle, A.; Koenigsknecht-Talboo, J.; Wilkinson, B.; Limpert, A.; Landreth, G. Mechanisms of statin-mediated inhibition of small G-protein function. J. Biol. Chem. 2005, 280, 34202–34209. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Marasco, G.; Proto, M.C.; Laezza, C.; Gazzerro, P.; Bifulco, M. Statins in neurological disorders: An overview and update. Pharmacol. Res. 2014, 88, 74–83. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Delles, C.; Jacobi, J.; Schlaich, M.P.; Schneider, M.; Schmitz, G.; Schmieder, R.E. Rapid improvement of nitric oxide bioavailability after lipid-lowering therapy with cerivastatin within two weeks. J. Am. Coll. Cardiol. 2001, 37, 1351–1358. [Google Scholar] [CrossRef]
- Cheng, W.H.; Ho, W.Y.; Chang, C.F.; Lu, P.J.; Cheng, P.W.; Yeh, T.C.; Hong, L.Z.; Sun, G.C.; Hsiao, M.; Tseng, C.J. Simvastatin induces a central hypotensive effect via Ras-mediated signalling to cause eNOS up-regulation. Br. J. Pharmacol. 2013, 170, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Walter, M.F.; Jacob, R.F. Effects of HMG-CoA reductase inhibitors on endothelial function—Role of microdomains and oxidative stress. Circulation 2004, 109, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mihos, C.G.; Salas, M.J.; Santana, O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in cardiovascular disease a comprehensive review. Cardiol. Rev. 2010, 18, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Yanuck, D.; Mihos, C.G.; Santana, O. Mechanisms and clinical evidence of the pleiotropic effects of the Hydroxy-Methyl-Glutaryl-CoA Reductase inhibitors in Central Nervous System disorders: A comprehensive review. Int. J. Neurosci. 2012, 122, 619–629. [Google Scholar] [CrossRef]
- Mihos, C.G.; Artola, R.T.; Santana, O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in rheumatologic disorders: A comprehensive review. Rheumatol. Int. 2012, 32, 287–294. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Butterfield, D.A. Statins more than cholesterol lowering agents in Alzheimer disease: Their pleiotropic functions as potential therapeutic targets. Biochem. Pharmacol. 2014, 88, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Mihos, C.G.; Pineda, A.M.; Santana, O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol. Res. 2014, 88, 12–19. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Cohen, M.V.; Yang, X.M.; Downey, J.M. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc. Res. 2006, 70, 231–239. [Google Scholar] [CrossRef]
- Mills, E.J.; Wu, P.; Chong, G.; Ghement, I.; Singh, S.; Akl, E.A.; Eyawo, O.; Guyatt, G.; Berwanger, O.; Briel, M. Efficacy and safety of statin treatment for cardiovascular disease: A network meta-analysis of 170 255 patients from 76 randomized trials. QJM-Int. J. Med. 2011, 104, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Lin, L.; Zhao, Y.J.; Khoo, A.L.; Davis, B.R.; Yong, Q.W.; Yeo, T.C.; Lim, B.P. Statins for primary prevention of cardiovascular disease in elderly patients: Systematic review and meta-analysis. Drugs Aging 2015, 32, 649–661. [Google Scholar] [CrossRef]
- Tervonen, T.; Naci, H.; van Valkenhoef, G.; Ades, A.E.; Angelis, A.; Hillege, H.L.; Postmus, D. Applying multiple criteria decision analysis to comparative benefit-risk assessment: Choosing among statins in primary prevention. Med. Decis. Mak. 2015, 35, 859–871. [Google Scholar] [CrossRef]
- Ulivieri, C.; Baldari, C.T. Statins: From cholesterol-lowering drugs to novel immunomodulators for the treatment of Th17-mediated autoimmune diseases. Pharmacol. Res. 2014, 88, 41–52. [Google Scholar] [CrossRef]
- Scheele, J.S.; Marks, R.E.; Boss, G.R. Signaling by small GTPases in the immune system. Immunol. Rev. 2007, 218, 92–101. [Google Scholar] [CrossRef]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006, 6, 358–370. [Google Scholar] [CrossRef]
- Chow, S.C. Immunomodulation by statins: Mechanisms and potential impact on autoimmune diseases. Arch. Immunol. Ther. Exp. (Warsz.) 2009, 57, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Khattri, S.; Zandman-Goddard, G. Statins and autoimmunity. Immunol. Res. 2013, 56, 348–357. [Google Scholar] [CrossRef]
- Ciurleo, R.; Bramanti, P.; Marino, S. Role of statins in the treatment of multiple sclerosis. Pharmacol. Res. 2014, 87, 133–143. [Google Scholar] [CrossRef]
- Pihl-Jensen, G.; Tsakiri, A.; Frederiksen, J.L. Statin treatment in multiple sclerosis: A systematic review and meta-analysis. CNS Drugs 2015, 29, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, M.; Mathieu, S.; Hermet, M.; Makarawiez, C.; Bruckert, E. Do all lupus patients need statins? Jt. Bone Spine 2013, 80, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Chen, P.-C.; Yang, Y.-H.; Wang, L.-C.; Lee, J.-H.; Lin, Y.-T.; Chiang, B.-L. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: A nationwide population-based cohort study. Atherosclerosis 2015, 243, 11–18. [Google Scholar] [CrossRef]
- Ruiz-Limon, P.; Barbarroja, N.; Perez-Sanchez, C.; Aguirre, M.A.; Bertolaccini, M.L.; Khamashta, M.A.; Rodriguez-Ariza, A.; Almaden, Y.; Segui, P.; Khraiwesh, H.; et al. Atherosclerosis and cardiovascular disease in systemic lupus erythematosus: Effects of in vivo statin treatment. Ann. Rheum. Dis. 2015, 74, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, B.; Wang, W.; Zhang, C.; Zhang, M.; Zhang, Y.; Xia, Y.; Dong, Z.; Guo, Y.; An, F. Effects of HMG-CoA Reductase inhibitor on experimental autoimmune myocarditis. Cardiovasc. Drugs Ther. 2012, 26, 121–130. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Statins as a new therapeutic perspective in myocarditis and postmyocarditis dilated cardiomyopathy. Cardiovasc. Drugs Ther. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimojo, N.; Sakai, S.; Machino-Ohtsuka, T.; Imanaka-Yoshida, K.; Hiroe, M.; Tsujimura, Y.; Kimura, T.; Sato, A.; Yasutomi, Y.; et al. Pitavastatin regulates helper T-cell differentiation and ameliorates autoimmune myocarditis in mice. Cardiovasc. Drugs Ther. 2013, 27, 413–424. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Y.; Zou, Z.; Li, F.; Zhao, S.; Shi, R.; Bian, R.; Tian, H. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: A meta-analysis. Clin. Exp. Rheumatol. 2015, 33, 69–76. [Google Scholar]
- Tascilar, K.; Dell’Aniello, S.; Hudson, M.; Suissa, S. Statins and risk of rheumatoid arthritis: A nested case-control study. Arthritis Rheumatol. 2016, 68, 2603–2611. [Google Scholar] [CrossRef]
- de Jong, H.J.I.; Tervaert, J.W.C.; Lalmohamed, A.; de Vries, F.; Vandebriel, R.J.; van Loveren, H.; Klungel, O.H.; van Staa, T.P. Pattern of risks of rheumatoid arthritis among patients using statins: A cohort study with the clinical practice research datalink. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef]
- McFarland, A.J.; Anoopkumar-Dukie, S.; Arora, D.S.; Grant, G.D.; McDermott, C.M.; Perkins, A.V.; Davey, A.K. Molecular mechanisms underlying the effects of statins in the central nervous system. Int. J. Mol. Sci. 2014, 15, 20607–20637. [Google Scholar] [CrossRef]
- Qizilbash, N.; Lewington, S.; Duffy, S.; Peto, R.; Smith, T.; Spiegelhalter, D.; Iso, H.; Shimamoto, T.; Komachi, Y.; Iida, M.; et al. Cholesterol, diastolic blood pressure, and stroke: 13000 strokes in 450000 people in 45 prospective cohorts. Lancet 1995, 346, 1647–1653. [Google Scholar]
- O’Brien, E.C.; Greiner, M.A.; Xian, Y.; Fonarow, G.C.; Olson, D.M.; Schwamm, L.H.; Bhatt, D.L.; Smith, E.E.; Maisch, L.; Hannah, D.; et al. Clinical effectiveness of statin therapy after ischemic stroke: Primary results from the statin therapeutic area of the Patient-centered Research into Outcomes Stroke Patients prefer and Effectiveness Research (PROSPER) study. Circulation 2015, 132, 1404–1413. [Google Scholar] [CrossRef]
- Naci, H.; Brugts, J.J.; Fleurence, R.; Ades, A.E. Comparative effects of statins on major cerebrovascular events: A multiple-treatments meta-analysis of placebo-controlled and active-comparator trials. QJM-Int. J. Med. 2013, 106, 299–306. [Google Scholar] [CrossRef]
- Markel, A. Statins and peripheral arterial disease. Int. Angiol. 2015, 34, 416–427. [Google Scholar]
- Colivicchi, F.; Bassi, A.; Santini, M.; Caltagirone, C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke 2007, 38, 2652–2657. [Google Scholar] [CrossRef]
- Laloux, P. Risk and benefit of statins in stroke secondary prevention. Curr. Vasc. Pharmacol. 2013, 11, 812–816. [Google Scholar] [CrossRef]
- Song, B.; Wang, Y.L.; Zhao, X.Q.; Liu, L.P.; Wang, C.X.; Wang, A.X.; Du, W.L.; Wang, Y.J. Association between statin use and short-term outcome based on severity of ischemic stroke: A cohort study. PLoS ONE 2014, 9, 7. [Google Scholar] [CrossRef]
- Gutierrez-Vargas, J.; Cespedes-Rubio, A.; Cardona-Gomez, G. Perspective of synaptic protection after post-infarction treatment with statins. J. Transl. Med. 2015, 13, 118. [Google Scholar] [CrossRef]
- Bustamante, A.; Montaner, J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke 2013, 44, 2060–2061. [Google Scholar] [CrossRef]
- Molina, C.A.; Selim, M.H. Continued statin treatment after acute intracranial hemorrhage fighting fire with fire. Stroke 2013, 44, 2062–2063. [Google Scholar] [CrossRef]
- Pan, Y.S.; Jing, J.; Wang, Y.L.; Zhao, X.Q.; Song, B.; Wang, W.J.; Wang, D.; Liu, G.F.; Liu, L.P.; Wang, C.X.; et al. Use of statin during hospitalization improves the outcome after intracerebral hemorrhage. CNS Neurosci. Ther. 2014, 20, 548–555. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Silva, T.; Teixeira, J.; Remiao, F.; Borges, F. Alzheimer’s disease, cholesterol, and statins: The junctions of important metabolic pathways. Angew. Chem. Int. Ed. 2013, 52, 1110–1121. [Google Scholar] [CrossRef]
- Hottman, D.A.; Li, L. Protein prenylation and synaptic plasticity: Implications for Alzheimer’s disease. Mol. Neurobiol. 2014, 50, 177–185. [Google Scholar] [CrossRef]
- Mendoza-Oliva, A.; Zepeda, A.; Arias, C. The complex actions of statins in brain and their relevance for Alzheimer’s disease treatment: An analytical review. Curr. Alzheimer Res. 2014, 11, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Wanamaker, B.L.; Swiger, K.J.; Blumenthal, R.S.; Martin, S.S. Cholesterol, statins, and dementia: What the cardiologist should know. Clin. Cardiol. 2015, 38, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.D.; Cullen, E.I.; Friedhoff, L.T. Pharmacological concentrations of the HMG-CoA reductase inhibitor lovastatin decrease the formation of the Alzheimer beta-amyloid peptide in vitro and in patients. Front. Biosci. 2002, 7, A50–A59. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Cheng, S.W.; Cao, D.F.; Parent, M. Isoprenoids and related pharmacological interventions: Potential application in Alzheimer’s disease. Mol. Neurobiol. 2012, 46, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Cibickova, L.; Palicka, V.; Cibicek, N.; Cermakova, E.; Micuda, S.; Bartosova, L.; Jun, D. Differential effects of statins and alendronate on cholinesterases in serum and brain of rats. Physiol. Res. 2007, 56, 765–770. [Google Scholar]
- Mozayan, M.; Lee, T.J.F. Statins prevent cholinesterase inhibitor blockade of sympathetic alpha 7-nAChR-mediated currents in rat superior cervical ganglion neurons. Am. J. Physiol.-Heart Circul. Physiol. 2007, 293, H1737–H1744. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, R.M.; Tour, N.; Devi, K. Effects of statins and cholesterol on memory functions in mice. Metab. Brain Dis. 2012, 27, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Macan, M.; Vuksic, A.; Zunec, S.; Konjevoda, P.; Lovric, J.; Kelava, M.; Stambuk, N.; Vrkic, N.; Bradamante, V. Effects of simvastatin on malondialdehyde level and esterase activity in plasma and tissue of normolipidemic rats. Pharmacol. Rep. 2015, 67, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Bosel, J.; Gandor, F.; Harms, C.; Synowitz, M.; Harms, U.; Djoufack, P.C.; Megow, D.; Dirnagl, U.; Hortnagl, H.; Fink, K.B.; et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J. Neurochem. 2005, 92, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Krisanova, N.; Sivko, R.; Kasatkina, L.; Borisova, T. Neuroprotection by lowering cholesterol: A decrease in membrane cholesterol content reduces transporter-mediated glutamate release from brain nerve terminals. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 1553–1561. [Google Scholar] [CrossRef]
- Sala, S.G.; Munoz, U.; Bartolome, F.; Bermejo, F.; Martin-Requero, A. HMG-CoA reductase inhibitor simvastatin inhibits cell cycle progression at the G(1)/S checkpoint in immortalized lymphocytes from Alzheimer’s disease patients independently of cholesterol-lowering effects. J. Pharmacol. Exp. Ther. 2008, 324, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Merla, R.; Ye, Y.; Lin, Y.; Manickavasagam, S.; Huang, M.-H.; Perez-Polo, R.J.; Uretsky, B.F.; Birnbaum, Y. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am. J. Physiol.-Heart Circul. Physiol. 2007, 293, H1918–H1928. [Google Scholar] [CrossRef] [PubMed]
- Cordle, A.; Landreth, G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J. Neurosci. 2005, 25, 299–307. [Google Scholar] [CrossRef]
- Fonseca, A.C.R.G.; Resende, R.; Oliveira, C.R.; Pereira, C.M.F. Cholesterol and statins in Alzheimer’s disease: Current controversies. Exp. Neurol. 2010, 223, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Mans, R.A.; McMahon, L.L.; Li, L. Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience 2012, 202, 1–9. [Google Scholar] [CrossRef]
- Mans, R.A.; Chowdhury, N.; Cao, D.; McMahon, L.L.; Li, L. Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience 2010, 166, 435–444. [Google Scholar] [CrossRef]
- Parent, M.; Hottman, D.A.; Cheng, S.W.; Zhang, W.; McMahon, L.L.; Yuan, L.L.; Li, L. Simvastatin treatment enhances NMDAR-mediated synaptic transmission by upregulating the surface distribution of the GluN2B subunit. Cell. Mol. Neurobiol. 2014, 34, 693–705. [Google Scholar] [CrossRef]
- Wollmuth, L.P. Is cholesterol good or bad for your brain?—NMDARs have a say. J. Physiol.-Lond. 2015, 593, 2251–2252. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, M.; Kundu, M.; Corbett, G.T.; Rangaswamy, S.B.; Mishra, R.K.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. HMG-CoA Reductase Inhibitors bind to PPAR alpha to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. 2015, 22, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Yum, K.S.; Chang, J.Y.; Kim, M.; Ahn, J.Y.; Kim, S.; Lapchak, P.A.; Han, M.K. Dose-specific effect of simvastatin on hypoxia-induced HIF-1 alpha and BACE expression in Alzheimer’s disease cybrid cells. BMC Neurol. 2015, 15, 7. [Google Scholar] [CrossRef]
- Power, M.C.; Weuve, J.; Sharrett, A.R.; Blacker, D.; Gottesman, R.F. Statins, cognition, and dementia-systematic review and methodological commentary. Nat. Rev. Neurol. 2015, 11, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Karlawish, J.; Hennessy, S.; Teal, V.; Bilker, W.B. Statin therapy and risk of acute memory impairment. JAMA Intern. Med. 2015, 175, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Oliva, A.; Ferrera, P.; Fragoso-Medina, J.; Arias, C. Lovastatin differentially affects neuronal cholesterol and amyloid- production invivo and invitro. CNS Neurosci. Ther. 2015, 21, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.S.; Lin, C.L.; Lin, M.C.; Sung, F.C.; Kao, C.H. Decreased prevalence of dementia associated with statins: A national population-based study. Eur. J. Neurol. 2015, 22, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Phani, S.; Loike, J.D.; Przedborski, S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S207–S209. [Google Scholar] [CrossRef]
- Wolozin, B.; Kellman, W.; Ruosseau, P.; Celesia, G.G.; Siegel, G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000, 57, 1439–1443. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lin, C.-H.; Wu, R.-M.; Lin, M.-S.; Lin, J.-W.; Chang, C.-H.; Lai, M.-S. Discontinuation of statin therapy associates with Parkinson disease. A population-based study. Neurology 2013, 81, 410–416. [Google Scholar] [CrossRef]
- Tison, F.; Negre-Pages, L.; Meissner, W.G.; Dupouy, S.; Li, Q.; Thiolat, M.-L.; Thiollier, T.; Galitzky, M.; Ory-Magne, F.; Milhet, A.; et al. Simvastatin decreases levodopa-induced dyskinesia in monkeys, but not in a randomized, placebo-controlled, multiple cross-over (“n-of-1”) exploratory trial of simvastatin against levodopa-induced dyskinesia in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2013, 19, 416–421. [Google Scholar] [CrossRef]
- Selley, M.L. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005, 1037, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Long, L.; Yan, J.Q.; Wei, L.; Pan, M.Q.; Gao, H.M.; Zhou, P.; Liu, M.; Zhu, C.S.; Tang, B.S.; et al. Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci. Ther. 2013, 19, 170–177. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Zhu, C.; Zhang, L.; Wu, A.; Yang, Y.; Xiong, Z.; Deng, C.; Huang, X.-F.; Yenari, M.A.; et al. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: The association with anti-inflammatory responses. PLoS ONE 2011, 6, e20945. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, X.B.; Zhang, T.; Shi, Q.Q.; Chen, Z.B.; Tang, B.S. Effect of simvastatin on L-DOPA-induced abnormal involuntary movements of hemiparkinsonian rats. Neurol. Sci. 2015, 36, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Roy, A.; Matras, J.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Simvastatin inhibits the activation of p21(Ras) and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009, 29, 13543–13556. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Jick, S.S.; Jick, H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch. Intern. Med. 2003, 163, 1926–1932. [Google Scholar] [CrossRef]
- Feng, L.; Tan, C.-H.; Merchant, R.A.; Ng, T.-P. Association between depressive symptoms and use of HMG-CoA reductase inhibitors (statins), corticosteroids and histamine H-2 receptor antagonists in community-dwelling older persons—Cross-sectional analysis of a population-based cohort. Drugs Aging 2008, 25, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.; Berk, M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: Proof of concept for the inflammatory and oxidative hypotheses of depression? J. Clin. Psychiatry 2011, 72, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Zhao, S.; Whooley, M.A. Statin use and risk of depression in patients with coronary heart disease: Longitudinal data from the heart and soul study. J. Clin. Psychiatry 2012, 73, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Stewart, R.; Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Kim, J.-T.; Park, M.-S.; Cho, K.-H.; Yoon, J.-S. A prospective study of statin use and poststroke depression. J. Clin. Psychopharmacol. 2014, 34, 72–79. [Google Scholar] [CrossRef]
- Chuang, C.-S.; Yang, T.-Y.; Muo, C.-H.; Su, H.-L.; Sung, F.-C.; Kao, C.-H. Hyperlipidemia, statin use and the risk of developing depression: A nationwide retrospective cohort study. Gen. Hosp. Psych. 2014, 36, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Chang, A.Y.W.; Lin, T.-K. Simvastatin treatment exerts antidepressant-like effect in rats exposed to chronic mild stress. Pharmacol. Biochem. Behav. 2014, 124, 174–179. [Google Scholar] [CrossRef]
- ElBatsh, M.M. Antidepressant-like effect of simvastatin in diabetic rats. Can. J. Physiol. Pharmacol. 2015, 93, 649–656. [Google Scholar] [CrossRef]
- Abbasi, S.H.; Mohammadinejad, P.; Shahmansouri, N.; Salehiomran, A.; Beglar, A.A.; Zeinoddini, A.; Forghani, S.; Akhondzadeh, S. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: A double-blind, placebo-controlled, randomized trial. J. Affect. Disord. 2015, 183, 149–155. [Google Scholar] [CrossRef]
- Gougol, A.; Zareh-Mohammadi, N.; Raheb, S.; Farokhnia, M.; Salimi, S.; Iranpour, N.; Yekehtaz, H.; Akhondzadeh, S. Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: A double-blind placebo-controlled trial. J. Psychopharmacol. 2015, 29, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Agostini, J.V.; Tinetti, M.E.; Han, L.; McAvay, G.; Foody, J.M.; Concato, J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J. Am. Geriatr. Soc. 2007, 55, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yap, K.B.; Kua, E.H.; Ng, T.P. Statin use and depressive symptoms in a prospective study of community-living older persons. Pharmacoepidemiol. Drug Saf. 2010, 19, 942–948. [Google Scholar] [CrossRef]
- Al Badarin, F.J.; Spertus, J.A.; Gosch, K.L.; Buchanan, D.M.; Chan, P.S. Initiation of statin therapy after acute myocardial infarction is not associated with worsening depressive symptoms: Insights from the Prospective Registry Evaluating Outcomes After Myocardial Infarctions: Events and Recovery (PREMIER) and Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) registries. Am. Heart J. 2013, 166, 879–886. [Google Scholar] [PubMed]
- Ludka, F.K.; Zomkowski, A.D.E.; Cunha, M.P.; Dal-Cim, T.; Zeni, A.L.B.; Rodrigues, A.L.S.; Tasca, C.I. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur. Neuropsychopharmacol. 2013, 23, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarian, A.; Javadi, S.; Jahanabadi, S.; Khoshnoodi, M.; Shamsaee, J.; Shafaroodi, H.; Mehr, S.E.; Dehpour, A. Antidepressant-like effect of atorvastatin in the forced swimming test in mice: The role of PPAR-gamma receptor and nitric oxide pathway. Eur. J. Pharmacol. 2014, 745, 52–58. [Google Scholar] [CrossRef]
- Citraro, R.; Chimirri, S.; Aiello, R.; Gallelli, L.; Trimboli, F.; Britti, D.; De Sarro, G.; Russo, E. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia 2014, 55, 1284–1291. [Google Scholar] [CrossRef]
- Etminan, M.; Samii, A.; Brophy, J.M. Statin use and risk of epilepsy A nested case-control study. Neurology 2010, 75, 1496–1500. [Google Scholar] [CrossRef]
- Piermartiri, T.C.B.; Vandresen-Filho, S.; Herculano, B.d.A.; Martins, W.C.; Dal’Agnolo, D.; Stroeh, E.; Carqueja, C.L.; Boeck, C.R.; Tasca, C.I. Atorvastatin prevents hippocampal cell death due to quinolinic acid-induced seizures in mice by increasing Akt phosphorylation and glutamate uptake. Neurotox. Res. 2009, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhao, Y.; Kwak, Y.-D.; Yang, Z.; Thompson, R.; Luo, Z.; Xu, H.; Liao, F.-F. Statin’s excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via Rho-ROCK signaling. J. Neurosci. 2009, 29, 11226–11236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Won, J.-S.; Singh, A.K.; Singh, I. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci. Lett. 2008, 440, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, A.; Mintzer, S. Statins for poststroke seizures The first antiepileptogenic agent? Neurology 2015, 85, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; de la Ossa, N.P.; Hurtado, O.; Millan, M.; Arenillas, J.F.; Davalos, A.; Gasull, T. Simvastatin reduces the association of NMDA receptors to lipid rafts: A cholesterol-mediated effect in neuroprotection. Stroke 2008, 39, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Seker, F.B.; Kilic, U.; Caglayan, B.; Ethemoglu, M.S.; Caglayan, A.B.; Ekimci, N.; Demirci, S.; Dogan, A.; Oztezcan, S.; Sahin, F.; et al. HMG-CoA reductase inhibitor rosuvastatin improves abnormal brain electrical activity via mechanisms involving eNOs. Neuroscience 2015, 284, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, F.; Constanti, A.; Citraro, R.; De Sarro, G.; Russo, E. Statins and epilepsy: Preclinical studies, clinical trials and statin-anticonvulsant drug interactions. Curr. Drug Targets 2015, 16, 747–756. [Google Scholar] [CrossRef]
- Sierra-Marcos, A.; Alvarez, V.; Faouzi, M.; Burnand, B.; Rossetti, A.O. Statins are associated with decreased mortality risk after status epilepticus. Eur. J. Neurol. 2015, 22, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and cancer risk—A meta-analysis. JAMA-J. Am. Med. Assoc. 2006, 295, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.E. Statins and Cancer-Related Mortality—Let’s Work Together. N. Engl. J. Med. 2012, 367, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Osmak, M. Statins and cancer: Current and future prospects. Cancer Lett. 2012, 324, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Picardi, P.; Ciaglia, E.; D’Alessandro, A.; Bifulco, M. Novel prospects of statins as therapeutic agents in cancer. Pharmacol. Res. 2014, 88, 84–98. [Google Scholar] [CrossRef]
- Kubatka, P.; Kruzliak, P.; Rotrekl, V.; Jelinkova, S.; Mladosievicova, B. Statins in oncological research: From experimental studies to clinical practice. Crit. Rev. Oncol. Hematol. 2014, 92, 296–311. [Google Scholar] [CrossRef]
- Matusewicz, L.; Meissner, J.; Toporkiewicz, M.; Sikorski, A.F. The effect of statins on cancer cells-review. Tumor Biol. 2015, 36, 4889–4904. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.C.; Lin, C.L.; Hsu, W.Y.; Chang, C.S.; Yeh, H.Z.; Tung, C.F.; Wu, Y.L.; Sung, F.C.; Kao, C.H. Statins are associated with a reduced risk of cholangiocarcinoma: A population-based case-control study. Br. J. Clin. Pharmacol. 2015, 80, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Archibugi, L.; Maisonneuve, P.; Piciucchi, M.; Valente, R.; Delle Fave, G.; Capurso, G. Statins but not aspirin nor their combination have a chemopreventive effect on pancreatic cancer occurrence. Pancreas 2015, 44, 1359. [Google Scholar]
- Sivaprasad, U.; Abbas, T.; Dutta, A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol. Cancer Ther. 2006, 5, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, B.-C.; Lu, X.-Y.; Yang, L.-L.; Zhai, Y.-J.; Eaton, A.F.; Thai, T.L.; Eaton, D.C.; Ma, H.-P.; Shen, B.-Z. Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-S.; Kang, X.-L.; Zhou, J.-G.; Lv, X.-F.; Tang, Y.-B.; Guan, Y.-Y. Involvement of Chk1-Cdc25A-cyclin A/CDk2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells. Eur. J. Pharmacol. 2011, 670, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Luo, Y.; Zhou, Y.; Zhang, Q.; Wang, J.; Wei, N.; Mi, M.; Zhu, J.; Wang, B.; Chang, H.; et al. BRCA1 overexpression sensitizes cancer cells to lovastatin via regulation of cyclin D1-CDK4-p21(WAF1/CIP1) pathway: Analyses using a breast cancer cell line and tumoral xenograft model. Int. J. Oncol. 2008, 33, 555–563. [Google Scholar] [PubMed]
- Rao, S.; Porter, D.C.; Chen, X.M.; Herliczek, T.; Lowe, M.; Keyomarsi, K. Lovastatin-mediated G(1) arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc. Natl. Acad. Sci. USA 1999, 96, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Sumitomo, M.; Asakuma, J.; Asano, T.; Asano, T.; Hayakawa, M. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin. Cancer Res. 2004, 10, 8648–8655. [Google Scholar] [CrossRef]
- Denoyelle, C.; Vasse, M.; Korner, M.; Mishal, Z.; Ganne, F.; Vannier, J.P.; Soria, J.; Soria, C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: An in vitro study. Carcinogenesis 2001, 22, 1139–1148. [Google Scholar] [CrossRef]
- Spampanato, C.; De Maria, S.; Sarnataro, M.; Giordano, E.; Zanfardino, M.; Baiano, S.; Carteni, M.; Morelli, F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 2012, 40, 935–941. [Google Scholar] [CrossRef]
- Herrero-Martin, G.; Lopez-Rivas, A. Statins activate a mitochondria-operated pathway of apoptosis in breast tumor cells by a mechanism regulated by ErbB2 and dependent on the prenylation of proteins. FEBS Lett. 2008, 582, 2589–2594. [Google Scholar] [CrossRef]
- Zhu, Y.; Casey, P.J.; Kumar, A.P.; Pervaiz, S. Deciphering the signaling networks underlying simvastatin-induced apoptosis in human cancer cells: Evidence for non-canonical activation of RhoA and Rac1 GTPases. Cell Death Dis. 2013, 4, e568. [Google Scholar] [CrossRef]
- Miller, T.; Yang, F.; Wise, C.E.; Meng, F.; Priester, S.; Munshi, M.K.; Guerrier, M.; Dostal, D.E.; Glaser, S.S. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig. Liver Dis. 2011, 43, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem. Biophys. Res. Commun. 2004, 320, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.Y.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Park, M.J.; Ye, S.K.; Kim, C.W.; Kim, Y.N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006, 168, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Boscher, C.; Nabi, I.R. CAVEOLIN-1: Role in Cell Signaling. In Caveolins and Caveolae: Roles in Signaling and Disease Mechanisms; Jasmin, J.F., Frank, P.G., Lisanti, M.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 729, pp. 29–50. [Google Scholar]
- Altwairgi, A.K. Statins are potential anticancerous agents (Review). Oncol. Rep. 2015, 33, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Gonzalez, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.F.; Alberts, A.W.; Anderson, P.S.; Chen, J.S.; Smith, R.L.; Willard, A.K. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. 4. Side-chain ester derivatives of mevinolin. J. Med. Chem. 1986, 29, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Askin, D.; Verhoeven, T.R.; Liu, T.M.H.; Shinkai, I. Synthesis of synvinolin—Extremely high conversion alkylation of an ester enolate. J. Org. Chem. 1991, 56, 4929–4932. [Google Scholar] [CrossRef]
- Xie, X.K.; Tang, Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysis. Appl. Environ. Microbiol. 2007, 73, 2054–2060. [Google Scholar] [CrossRef]
- Schimmel, T.G.; Borneman, W.S.; Conder, M.J. Purification and characterization of a lovastatin esterase from Clonostachys compactiuscula. Appl. Environ. Microbiol. 1997, 63, 1307–1311. [Google Scholar] [PubMed]
- Chen, L.C.; Lai, Y.K.; Wu, S.C.; Lin, C.C.; Guo, J.H. Production by Clonostachys compactiuscula of a lovastatin esterase that converts lovastatin to monacolin J. Enzyme Microb. Technol. 2006, 39, 1051–1059. [Google Scholar] [CrossRef]
- Gao, X.; Xie, X.K.; Pashkov, I.; Sawaya, M.R.; Laidman, J.; Zhang, W.J.; Cacho, R.; Yeates, T.O.; Tang, Y. Directed Evolution and Structural Characterization of a Simvastatin Synthase. Chem. Biol. 2009, 16, 1064–1074. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Presidential Green Chemistry Challenge: 2012 Greener Synthetic Pathways Award. Available online: http://www2.epa.gov/green-chemistry/2012-greener-synthetic-pathways-award (accessed on 14 February 2019).
- Xu, W.; Chooi, Y.H.; Choi, J.W.; Li, S.; Vederas, J.C.; Da Silva, N.A.; Tang, Y. LovG: The thioesterase required for dihydromonacolin L release and lovastatin nonaketide synthase turnover in lovastatin biosynthesis. Angew. Chem. Int. Ed. 2013, 52, 6472–6475. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.E.; Le, T.V.; Millar, A.; Nanninga, T.N. Process for the synthesis of (5R)-1,1-dimethylethyl-6-cyano-5-hydroxy-3-oxo-hexanoate. US Patent 5155251, 13 October 1992. [Google Scholar]
- Turner, N.J.; O’Reilly, E. Biocatalytic retrosynthesis. Nat. Chem. Biol. 2013, 9, 285–288. [Google Scholar] [CrossRef]
- Brady, D. Biocatalytic Hydrolysis of Nitriles. In Handbook of Green Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2010; Volume 3, (Biocatalysis). [Google Scholar]
- Faber, K. Biotransformations of non-natural compounds: State of the art and future development. Pure Appl. Chem. 1997, 69, 1613–1632. [Google Scholar] [CrossRef]
- DeSantis, G.; Zhu, Z.L.; Greenberg, W.A.; Wong, K.V.; Chaplin, J.; Hanson, S.R.; Farwell, B.; Nicholson, L.W.; Rand, C.L.; Weiner, D.P.; et al. An enzyme library approach to biocatalysis: Development of nitrilases for enantioselective production of carboxylic acid derivatives. J. Am. Chem. Soc. 2002, 124, 9024–9025. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, G.; Wong, K.; Farwell, B.; Chatman, K.; Zhu, Z.L.; Tomlinson, G.; Huang, H.J.; Tan, X.Q.; Bibbs, L.; Chen, P.; et al. Creation of a productive, highly enantioselective nitrilase through gene site saturation mutagenesis (GSSM). J. Am. Chem. Soc. 2003, 125, 11476–11477. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, S.; Chaplin, D.A.; Edwards, J.H.; Ellis, B.S.W.; Hill, C.L.; Holt-Tiffin, K.; Knight, J.R.; Mahoney, T.; Osborne, A.P.; Ruecroft, G. Nitrilase-catalysed desymmetrisation of 3-hydroxyglutaronitrile: Preparation of a statin side-chain intermediate. Org. Process Res. Dev. 2006, 10, 661–665. [Google Scholar] [CrossRef]
- Yao, P.; Li, J.; Yuan, J.; Han, C.; Liu, X.; Feng, J.; Wu, Q.; Zhu, D. Enzymatic synthesis of a key intermediate for rosuvastatin by nitrilase-catalyzed hydrolysis of ethyl (R)-4-cyano-3-hydroxybutyate at high substrate concentration. ChemCatChem 2015, 7, 271–275. [Google Scholar] [CrossRef]
- Ma, S.K.; Gruber, J.; Davis, C.; Newman, L.; Gray, D.; Wang, A.; Grate, J.; Huisman, G.W.; Sheldon, R.A. A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem. 2010, 12, 81–86. [Google Scholar] [CrossRef]
- Chung, S.; Hwang, Y. Stereoselective hydrolysis of racemic ethyl 4-chloro-3-hydroxybutyrate by a lipase. Biocatal. Biotransform. 2008, 26, 327–330. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, O.J.; Uh, H.S. A chemoenzymatic approach to the synthesis of enantiomerically pure (S)-3-hydroxy-gamma-butyrolactone. Appl. Microbiol. Biotechnol. 2008, 79, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M. Biocatalytic synthesis of atorvastatin intermediates. J. Mol. Catal. B-Enzym. 2009, 61, 123–128. [Google Scholar] [CrossRef]
- Martin, C.H.; Dhamankar, H.; Tseng, H.C.; Sheppard, M.J.; Reisch, C.R.; Prather, K.L.J. A platform pathway for production of 3-hydroxyacids provides a biosynthetic route to 3-hydroxy-gamma-butyrolactone. Nat. Commun. 2013, 4, 1414. [Google Scholar] [CrossRef] [PubMed]
- Narasaka, K.; Pai, F.C. Stereoselective reduction of beta-hydroxyketones to 1,3-diols highly selective 1,3-asymmetric induction via boron chelates. Tetrahedron 1984, 40, 2233–2238. [Google Scholar] [CrossRef]
- Chen, K.M.; Hardtmann, G.E.; Prasad, K.; Repic, O.; Shapiro, M.J. 1,3-syn diastereoselective reduction of beta-hydroxyketones utilizing alkoxydialkylboranes. Tetrahedron Lett. 1987, 28, 155–158. [Google Scholar] [CrossRef]
- Sterk, D.; Casar, Z.; Jukic, M.; Kosmrlj, J. Concise and highly efficient approach to three key pyrimidine precursors for rosuvastatin synthesis. Tetrahedron 2012, 68, 2155–2160. [Google Scholar] [CrossRef]
- Ohrlein, R.; Baisch, G. Chemo-enzymatic approach to statin side-chain building blocks. Adv. Synth. Catal. 2003, 345, 713–715. [Google Scholar] [CrossRef]
- Santaniello, E.; Chiari, M.; Ferraboschi, P.; Trave, S. Enhanced and reversed enantioselectivity of enzymatic-hydrolysis by simple substrate modifications—The case of 3-hydroxyglutarate diesters. J. Org. Chem. 1988, 53, 1567–1569. [Google Scholar] [CrossRef]
- Metzner, R.; Hummel, W.; Wetterich, F.; Konig, B.; Groger, H. Integrated biocatalysis in multistep drug synthesis without intermediate isolation: A de novo approach toward a rosuvastatin key building block. Org. Process Res. Dev. 2015, 19, 635–638. [Google Scholar] [CrossRef]
- König, B.; Wetterich, F.; Gröger, H.; Metzner, R. Process for enantioselective synthesis of 3-hydroxy-glutaric acid monoesters and use thereof. WO 2014/140006, 18 September 2014. [Google Scholar]
- You, Z.Y.; Liu, Z.Q.; Zheng, Y.G. Chemical and enzymatic approaches to the synthesis of optically pure ethyl (R)-4-cyano-3-hydroxybutanoate. Appl. Microbiol. Biotechnol. 2014, 98, 11–21. [Google Scholar] [CrossRef]
- Asako, H.; Shimizu, M.; Itoh, N. Biocatalytic production of (S)-4-bromo-3-hydroxybutyrate and structurally related chemicals and their applications. Appl. Microbiol. Biotechnol. 2009, 84, 397–405. [Google Scholar] [CrossRef]
- Asako, H.; Shimizu, M.; Makino, Y.; Itoh, N. Biocatalytic reduction system for the production of chiral methyl (R)/(S)-4-bromo-3-hydroxybutyrate. Tetrahedron Lett. 2010, 51, 2664–2666. [Google Scholar] [CrossRef]
- Patel, R.N.; McNamee, C.G.; Banerjee, A.; Howell, J.M.; Robison, R.S.; Szarka, L.J. Stereoselective reduction of beta-keto-esters by Geotrichum candidum. Enzyme Microb. Technol. 1992, 14, 731–738. [Google Scholar] [CrossRef]
- Ye, Q.; Ouyang, P.K.; Ying, H.J. A review-biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: Recent advances and future perspectives. Appl. Microbiol. Biotechnol. 2011, 89, 513–522. [Google Scholar] [CrossRef]
- Yasohara, Y.; Kizaki, N.; Hasegawa, J.; Takahashi, S.; Wada, M.; Kataoka, M.; Shimizu, S. Synthesis of optically active ethyl 4-chloro-3-hydroxybutanoate by microbial reduction. Appl. Microbiol. Biotechnol. 1999, 51, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Kita, K.; Kataoka, M.; Shimizu, S. Diversity of 4-chloroacetoacetate ethyl ester-reducing enzymes in yeasts and their application to chiral alcohol synthesis. J. Biosci. Bioeng. 1999, 88, 591–598. [Google Scholar] [CrossRef]
- Wada, M.; Kataoka, M.; Kawabata, H.; Yasohara, Y.; Kizaki, N.; Hasegawa, J.; Shimizu, S. Purification and characterization of NADPH-dependent carbonyl reductase, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate, from Candida magnoliae. Biosci. Biotechnol. Biochem. 1998, 62, 280–285. [Google Scholar] [CrossRef]
- Kataoka, M.; Doi, Y.; Sim, T.S.; Shimizu, S.; Yamada, H. A novel NADPH-dependent carbonyl reductase of Candida macedoniensis. Purification and characterization. Arch. Biochem. Biophys. 1992, 294, 469–474. [Google Scholar] [CrossRef]
- He, J.Y.; Sun, Z.H.; Ruan, W.Q.; Xu, Y. Biocatalytic synthesis of ethyl (S)-4-chloro-3-hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem. 2006, 41, 244–249. [Google Scholar] [CrossRef]
- Saratani, Y.; Uheda, E.; Yamamoto, H.; Nishimura, A.; Yoshizako, F. Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by fungi. Biosci. Biochem. Biophys. 2001, 65, 1676–1679. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, M.; Xu, L.; Cao, H.; Li, Z.; Chen, Y.; Li, S.; Ying, H. A novel carbonyl reductase from Pichia stipitis for the production of ethyl (S)-4-chloro-3-hydroxybutanoate. Biotechnol. Lett. 2009, 31, 537–542. [Google Scholar] [CrossRef]
- Cai, P.; An, M.D.; Xu, L.; Xu, S.; Hao, N.; Li, Y.; Guo, K.; Yan, M. Development of a substrate-coupled biocatalytic process driven by an NADPH-dependent sorbose reductase from Candida albicans for the asymmetric reduction of ethyl 4-chloro-3-oxobutanoate. Biotechnol. Lett. 2012, 34, 2223–2227. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, G.-W.; Ye, Q.; Xu, J.-H. Optimization and scale-up of a bioreduction process for preparation of ethyl (S)-4-chloro-3-hydroxybutanoate. Org. Process Res. Dev. 2014, 18, 739–743. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Ye, J.-J.; Shen, Z.-Y.; Hong, H.-B.; Yan, J.-B.; Lin, Y.; Chen, Z.-X.; Zheng, Y.-G.; Shen, Y.-C. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl. Microbiol. Biotechnol. 2015, 99, 2119–2129. [Google Scholar] [CrossRef]
- Schallmey, M.; Floor, R.J.; Hauer, B.; Breuer, M.; Jekel, P.A.; Wijma, H.J.; Dijkstra, B.W.; Janssen, D.B. Biocatalytic and structural properties of a highly engineered halohydrin dehalogenase. ChemBioChem 2013, 14, 870–881. [Google Scholar] [CrossRef]
- Ritter, S.K. Going green keeps getting easier. Chem. Eng. News 2006, 84, 24–27. [Google Scholar] [CrossRef]
- Li, C.-J.; Anastas, P.T. Green Chemistry: Present and future. Chem. Soc. Rev. 2012, 41, 1413–1414. [Google Scholar] [CrossRef]
- Chen, X.F.; Xiong, F.J.; Chen, W.X.; He, Q.Q.; Chen, F.E. Asymmetric Synthesis of the HMG-CoA Reductase Inhibitor Atorvastatin Calcium: An Organocatalytic Anhydride Desymmetrization and Cyanide-Free Side Chain Elongation Approach. J. Org. Chem. 2014, 79, 2723–2728. [Google Scholar] [CrossRef]
- Everaere, K.; Franceschini, N.; Mortreux, A.; Carpentier, J.F. Diastereoselective synthesis of syn-3,5-dihydroxyesters via ruthenium-catalyzed asymmetric transfer hydrogenation. Tetrahedron Lett. 2002, 43, 2569–2571. [Google Scholar] [CrossRef]
- Wolberg, M.; Hummel, W.; Muller, M. Biocatalytic reduction of beta, delta-diketo esters: A highly stereoselective approach to all four stereoisomers of a chlorinated beta, delta-dihydroxy hexanoate. Chem. Eur. J. 2001, 7, 4562–4571. [Google Scholar] [CrossRef]
- Wolberg, M.; Hummel, W.; Wandrey, C.; Muller, M. Highly regio- and enantioselective reduction of 3,5-dioxocarboxylates. Angew. Chem. Int. Ed. 2000, 39, 4306–4308. [Google Scholar] [CrossRef]
- Wolberg, M.; Villela, M.; Bode, S.; Geilenkirchen, P.; Feldmann, R.; Liese, A.; Hummel, W.; Muller, M. Chemoenzymatic synthesis of the chiral side-chain of statins: Application of an alcohol dehydrogenase catalysed ketone reduction on a large scale. Bioproc. Biosyst. Eng. 2008, 31, 183–191. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Hu, Z.L.; Zhang, X.J.; Tang, X.L.; Cheng, F.; Xue, Y.P.; Wang, Y.J.; Wu, L.; Yao, D.K.; Zhou, Y.T.; et al. Large-scale synthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by a stereoselective carbonyl reductase with high substrate concentration and product yield. Biotechnol. Progr. 2017, 33, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wu, L.; Zhang, X.J.; Xue, Y.P.; Zheng, Y.G. Directed evolution of carbonyl reductase from Rhodosporidium toruloides and its application in stereoselective synthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate. J. Agric. Food Chem. 2017, 65, 3721–3729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Yin, H.H.; Zhang, X.J.; Zhou, R.; Wang, Y.M.; Zheng, Y.G. Improvement of carbonyl reductase activity for the bioproduction of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate. Bioorg. Chem. 2018, 80, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wu, L.; Zheng, L.; Wang, W.Z.; Zhang, X.J.; Jin, L.Q.; Zheng, Y.G. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media. Bioresour. Technol. 2018, 249, 161–167. [Google Scholar] [CrossRef]
- Xu, T.T.; Wang, C.; Zhu, S.Z.; Zheng, G.J. Enzymatic preparation of optically pure t-butyl 6-chloro-(3R,5S)-dihydroxyhexanoate by a novel alcohol dehydrogenase discovered from Klebsiella oxytoca. Process Biochem. 2017, 57, 72–79. [Google Scholar] [CrossRef]
- Gong, X.M.; Zheng, G.W.; Liu, Y.Y.; Xu, J.H. Identification of a robust carbonyl reductase for diastereoselectively building syn-3,5-dihydroxy hexanoate: A bulky side chain of atorvastatin. Org. Process Res. Dev. 2017, 21, 1349–1354. [Google Scholar] [CrossRef]
- Gong, X.M.; Qin, Z.; Li, F.L.; Zeng, B.B.; Zheng, G.W.; Xu, J.H. Development of an engineered ketoreductase with simultaneously improved thermostability and activity for making a bulky atorvastatin precursor. ACS Catal. 2019, 9, 147–153. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.-J.; Zheng, Y.-G. Improved stereoselective bioreduction of t-butyl 6-cyano-(5R)-hydroxy-3-oxohexanoate by Rhodotorula glutinis through heat treatment. Biotechnol. Appl. Biochem. 2016, 63, 795–804. [Google Scholar] [CrossRef]
- Wu, X.; Gou, X.; Chen, Y. Enzymatic preparation of t-butyl-6-cyano-(3R,5R)-dihydroxyhexanoate by a whole-cell biocatalyst co-expressing carbonyl reductase and glucose dehydrogenase. Process Biochem. 2015, 50, 104–110. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, X.P.; Shen, W.; Liu, Z.Q.; Zheng, Y.G. Chiral diol t-butyl 6-cyano-(3R,5R)-dihydroxylhexanoate synthesis catalyzed by immobilized cells of carbonyl reductase and glucose dehydrogenase co-expression E. coli. Biochem. Eng. J. 2017, 128, 54–62. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.-J.; Zheng, Y.-G. Cloning and characterization of a NADH-dependent aldo-keto reductase from a newly isolated Kluyveromyces lactis XP1461. Enzyme Microb. Technol. 2015, 77, 68–77. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.-J.; Shen, W.; Zheng, Y.-G. Activity improvement of a Kluyveromyces lactis aldo-keto reductase KlAKR via rational design. J. Biotechnol. 2016, 224, 20–26. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Liu, X.-Q.; Luo, X.; Liu, Z.-Q.; Zheng, Y.-G. Cloning, expression and enzymatic characterization of an aldo-keto reductase from Candida albicans XP1463. J. Mol. Catal. B-Enzym. 2015, 122, 44–50. [Google Scholar] [CrossRef]
- Pfruender, H.; Amidjojo, M.; Hang, F.; Weuster-Botz, D. Production of Lactobacillus kefir cells for asymmetric synthesis of a 3,5-dihydroxycarboxylate. Appl. Microbiol. Biotechnol. 2005, 67, 619–622. [Google Scholar] [CrossRef]
- Patel, R.N.; Banerjee, A.; McNamee, C.G.; Brzozowski, D.; Hanson, R.L.; Szarka, L.J. Enantioselective microbial reduction of 3,5-dioxo-6-(benzyloxy) hexanoic acid, ethyl-ester. Enzyme Microb. Technol. 1993, 15, 1014–1021. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Goswami, A.; Hanson, R.L.; Patel, R.N. Synthesis of ethyl and t-butyl (3R,5S)-dihydroxy-6-benzyloxy hexanoates via diastereo- and enantioselective microbial reduction. Tetrahedron Asymm. 2006, 17, 1589–1602. [Google Scholar] [CrossRef]
- Goldberg, S.; Guo, Z.; Chen, S.; Goswami, A.; Patel, R.N. Synthesis of ethyl-(3R,5S)-dihydroxy-6-benzyloxyhexanoates via diastereo- and enantioselective microbial reduction: Cloning and expression of ketoreductase III from Acinetobacter sp. SC 13874. Enzyme Microb. Technol. 2008, 43, 544–549. [Google Scholar] [CrossRef]
- Wu, X.; Liu, N.; He, Y.; Chen, Y. Cloning, expression, and characterization of a novel diketoreductase from Acinetobacter baylyi. Acta Biochim. Biophys. Sin. 2009, 41, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Wu, X. Dicarbonyl reduction by single enzyme for the preparation of chiral diols. Chem. Soc. Rev. 2012, 41, 1742–1753. [Google Scholar] [CrossRef]
- Lu, M.; Huang, Y.; White, M.A.; Wu, X.; Liu, N.; Cheng, X.; Chen, Y. Dual catalysis mode for the dicarbonyl reduction catalyzed by diketoreductase. Chem. Commun. 2012, 48, 11352–11354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Z.; Ma, M.; Liu, N.; Chen, Y. Functional roles of tryptophan residues in diketoreductase from Acinetobacter baylyi. BMB Rep. 2012, 45, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Z.; Liu, N.; Chen, Y. Identification of important residues in diketoreductase from Acinetobacter baylyi by molecular modeling and site-directed mutagenesis. Biochimie 2012, 94, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, H.J.M.; Wong, C.H. Sequential 3-substrate and 4-substrate aldol reactions catalyzed by aldolases. J. Am. Chem. Soc. 1995, 117, 7585–7591. [Google Scholar] [CrossRef]
- Gijsen, H.J.M.; Wong, C.H. Unprecedented asymmetric aldol reactions with 3 aldehyde substrates catalyzed by 2-deoxyribose-5-phosphate aldolase. J. Am. Chem. Soc. 1994, 116, 8422–8423. [Google Scholar] [CrossRef]
- Liu, J.J.; Hsu, C.C.; Wong, C.H. Sequential aldol condensation catalyzed by DERA mutant Ser238Asp and a formal total synthesis of atorvastatin. Tetrahedron Lett. 2004, 45, 2439–2441. [Google Scholar] [CrossRef]
- Greenberg, W.A.; Varvak, A.; Hanson, S.R.; Wong, K.; Huang, H.J.; Chen, P.; Burk, M.J. Development of an efficient, scalable, aldolase-catalyzed process for enantioselective synthesis of statin intermediates. Proc. Natl. Acad. Sci. USA 2004, 101, 5788–5793. [Google Scholar] [CrossRef]
- Jennewein, S.; Schurmann, M.; Wolberg, M.; Hilker, I.; Luiten, R.; Wubbolts, M.; Mink, D. Directed evolution of an industrial biocatalyst: 2-deoxy-d-ribose 5-phosphate aldolase. Biotechnol. J. 2006, 1, 537–548. [Google Scholar] [CrossRef]
- Oslaj, M.; Cluzeau, J.; Orkic, D.; Kopitar, G.; Mrak, P.; Casar, Z. A highly productive, whole-cell DERA chemoenzymatic process for production of key lactonized side-chain intermediates in statin synthesis. PLoS ONE 2013, 8, e62250. [Google Scholar] [CrossRef]
- Ručigaj, A.; Krajnc, M. Optimization of a crude deoxyribose-5-phosphate aldolase lyzate-catalyzed process in synthesis of statin intermediates. Org. Process Res. Dev. 2013, 17, 854–862. [Google Scholar] [CrossRef]
- You, Z.Y.; Liu, Z.Q.; Zheng, Y.G.; Shen, Y.C. Characterization and application of a newly synthesized 2-deoxyribose-5-phosphate aldolase. J. Ind. Microbiol. Biotechnol. 2013, 40, 29–39. [Google Scholar] [CrossRef]
- Casar, Z.; Steinbucher, M.; Kosmrlj, J. Lactone pathway to statins utilizing the Wittig reaction. The synthesis of rosuvastatin. J. Org. Chem. 2010, 75, 6681–6684. [Google Scholar] [CrossRef]
- Vajdic, T.; Oslaj, M.; Kopitar, G.; Mrak, P. Engineered, highly productive biosynthesis of artificial, lactonized statin side-chain building blocks: The hidden potential of Escherichia coli unleashed. Metab. Eng. 2014, 24, 160–172. [Google Scholar] [CrossRef]
- Sakuraba, H.; Yoneda, K.; Yoshihara, K.; Satoh, K.; Kawakami, R.; Uto, Y.; Tsuge, H.; Takahashi, K.; Hori, H.; Ohshima, T. Sequential aldol condensation catalyzed by hyperthermophilic 2-deoxy-d-ribose-5-phosphate aldolase. Appl. Environ. Microbiol. 2007, 73, 7427–7434. [Google Scholar] [CrossRef]
- Fei, H.; Zheng, C.C.; Liu, X.Y.; Li, Q. An industrially applied biocatalyst: 2-Deoxy-d-ribose-5-phosphate aldolase. Process Biochem. 2017, 63, 55–59. [Google Scholar] [CrossRef]
- Haridas, M.; Abdelraheem, E.M.M.; Hanefeld, U. 2-Deoxy-d-ribose-5-phosphate aldolase (DERA): Applications and modifications. Appl. Microbiol. Biotechnol. 2018, 102, 9959–9971. [Google Scholar] [CrossRef]
- Muller, M. Chemoenzymatic synthesis of building blocks for statin side chains. Angew. Chem. Int. Ed. 2005, 44, 362–365. [Google Scholar] [CrossRef]
- Garattini, L.; Padula, A. Cholesterol-lowering drugs: Science and marketing. J. R. Soc. Med. 2017, 110, 57–64. [Google Scholar] [CrossRef]
- Reiner, Z. Resistance and intolerance to statins. Nutr. Metab. Carbiovasc. Dis. 2014, 24, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Garg, J.; Shah, N.; Sumner, A. PCSK9 inhibitors: A new era of lipid lowering therapy. World J. Cardiol. 2017, 9, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C. New directions in managing dyslipidemia. J. Nurse Pract. 2019, 15, 73–79. [Google Scholar] [CrossRef]
- Xu, S.T.; Luo, S.S.; Zhu, Z.Y.; Xu, J.Y. Small molecules as inhibitors of PCSK9: Current status and future challenges. Eur. J. Med. Chem. 2019, 162, 212–233. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).