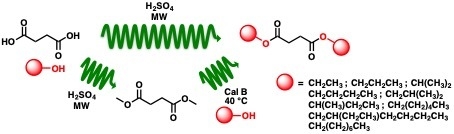
Microwave-Assisted Homogeneous Acid Catalysis and Chemoenzymatic Synthesis of Dialkyl Succinate in a Flow Reactor
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Methods
3.1. Materials
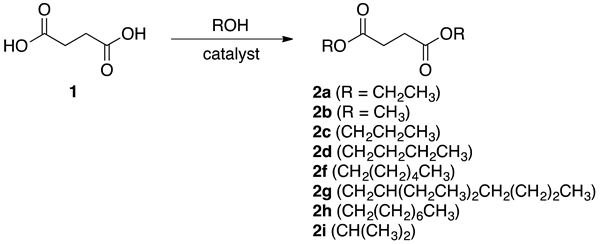
3.2. Microwave-Assisted Continuous Chemical Esterification
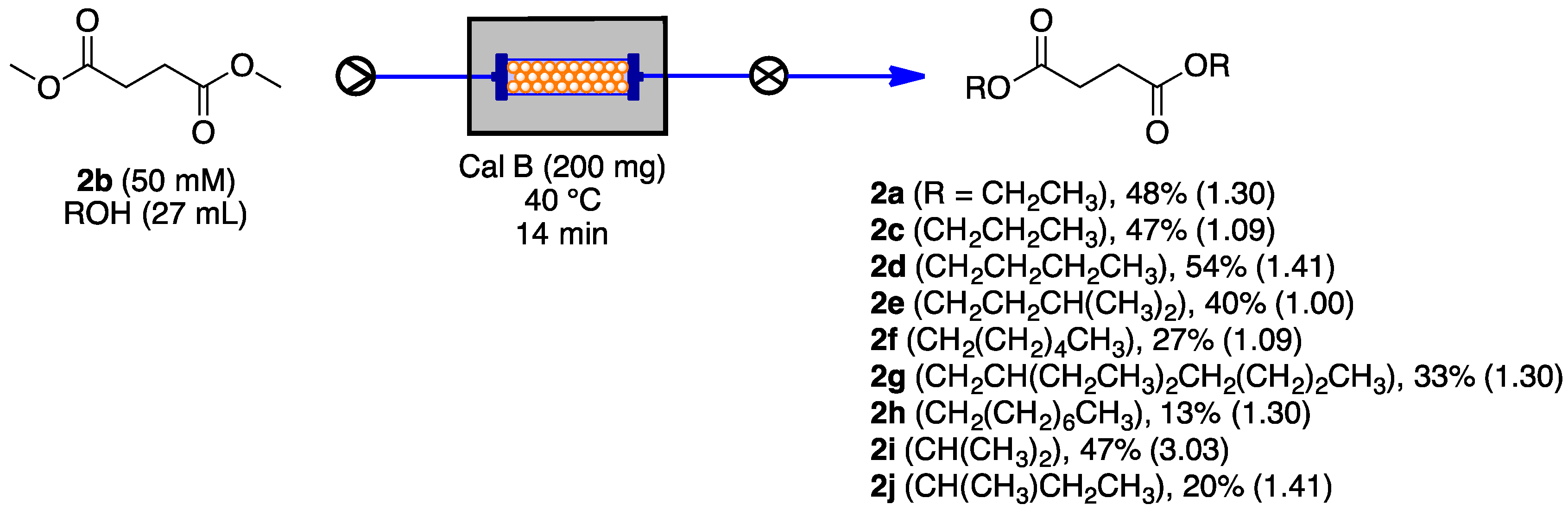
3.3. Continuous Biochemical Trans-Esterification
3.4. Gas Chromatography (GC) Analysis
3.5. High-Performance Liquid Chromatography (HPLC) Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazière, A.; Prinsen, P.; Garcia, A.; Luque, R.; Len, C. A review of progress in (bio)catalytic routes from/to renewable succinic acid. Biofuels Bioprod. Bioref. 2017, 11, 908–931. [Google Scholar] [CrossRef]
- Delhomme, C.; Weuster-Botz, D.; Kuhn, F.E. Succinic acid from renewable ressources as a C-4 building-block chemical—A review of the catalytic possibilities in aqueous media. Green Chem. 2009, 11, 13–26. [Google Scholar] [CrossRef]
- Hong, U.G.; Kim, J.K.; Lee, J.; Lee, J.K.; Song, J.H.; Yi, J.; Song, I.K. Hydrogenation of succinic acid to tetrahydrofuran (THF) over rhenium catalyst supported on H2SO4-treated mesoporous carbon. J. Ind. Eng. Chem. 2014, 20, 3834–3840. [Google Scholar] [CrossRef]
- Shao, Z.; Li, C.; Di, X.; Xiao, Z.; Liang, C. Aqueous-phase hydrogenation of succinic acid to g-butyrolactone and tetrahydrofuran over Pd/C, Re/C, and Pd-Re/C catalysts. Ind. Eng. Chem. Res. 2014, 53, 9638–9645. [Google Scholar] [CrossRef]
- Kang, K.H.; Hong, U.G.; Bang, Y.; Choi, J.H.; Kim, J.K.; Lee, K.L.; Han, S.J.; Song, I.K. Hydrogenation of succinic acid to 1,4-butanediol over Re-Ru bimetallic catalysts supported on mesoporous carbon. Appl. Catal. A 2015, 490, 153–162. [Google Scholar] [CrossRef]
- Delhomme, C. Process Integration of Fermentation and Catalysis for the Production of Succinic Acid Derivatives. Ph.D. Thesis, Technische Universitat München, Institute of Biochemical Engineering, Munich, Germany, 2011. [Google Scholar]
- Sattenapally, N.; Wang, W.; Liu, H.; Gao, Y. i-Butyl-2,4-dinitro-anilinium p-toluenesulfonate as a highly active and selective esterification catalyst. Tetrahedron Lett. 2013, 54, 6665–6668. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.; LeCaptain, D.J.; Lee, C.Y.; Mohanty, D.K. Poly(vinyl chloride)plasticized with mixtures of succinate di-esters—Synthesis and characterization. Eur. Polym. J. 2013, 49, 2785–2791. [Google Scholar] [CrossRef]
- Stuart, A.; McCallum, M.M.; Fan, D.; LeCaptain, D.J.; Lee, C.Y.; Mohanty, D.K. Poly(vinyl chloride) plasticized with succinate esters: Synthesis and characterization. Polym. Bull. 2010, 65, 589–598. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, M.; Zhang, J.; Li, J.; Ren, P. Synthesis, characterization, and properties of imidazole dicationic ionic liquids and their application in esterification. Chem. Eng. J. 2013, 221, 99–104. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Y.; Shen, Z. Nano-SO2-/TiO2 catalyzed eco-friendly esterification of dicarboxylic acids. Asian J. Chem. 2014, 26, 5769–5772. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J. Versatile mesoporous carbonaceous materials for acid catalysis. Chem. Commun. 2007, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Budarin, V.; Dugmore, T.; Luque, R.; Macquarrie, D.J.; Strelko, V. Catalytic performance of carbonaceous materials in the esterification of succinic acid. Catal. Commun. 2008, 9, 1709–1714. [Google Scholar] [CrossRef]
- Fabian, L.; Gomez, M.; Kuran, J.A.C.; Moltrasio, G.; Moglioni, A. Efficient microwave-assisted esterification reaction employing methanesulfonic acid supported on alumina as catalyst. Synth. Commun. 2014, 44, 2386–2392. [Google Scholar] [CrossRef]
- Varyambath, A.; Kim, M.R.; Kim, I. Sulfonic acid-functionalized organic knitted porous polyaromatic microspheres as heterogeneous catalysts for biodiesel production. New J. Chem. 2018, 42, 12745–12753. [Google Scholar] [CrossRef]
- Yang, Z.W.; Niu, L.Y.; Jia, X.J.; Kang, Q.X.; Ma, Z.H.; Lei, Z.Q. Preparation of silica-supported sulfate and its application as a stable and highly active solid acid catalyst. Catal. Commun. 2011, 12, 798–802. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, J.; Liu, X.; Guo, Y.; Guo, Y.; Lu, G.; Wang, Y. Novel sulfonated carbonaceous materials from p-toluenesulfonic acid/glucose as a high-performance solid-acid catalyst. Catal. Commun. 2010, 11, 629–632. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. Synthesis and characterization of 12-tungstosilicic acid anchored to MCM-41 as well as its use as environmentally benign catalyst for synthesis of succinate and malonate diesters. Ind. Eng. Chem. Res. 2011, 50, 13693–13702. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. Esterification of bioplatform molecules over 12-tungstophosphoric acid anchored to MCM-41. J. Porous Mater. 2013, 20, 209–217. [Google Scholar] [CrossRef]
- Santacrose, V.; Bigi, F.; Casnati, A.; Maggi, R.; Storaro, L.; Moretti, E.; Vaccaro, L.; Maestri, G. Selective monomethyl esterification of linear dicarboxylic acids with bifunctional alumina catalysts. Green Chem. 2016, 18, 5764–5768. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Han, J.; Hu, Y.; Yan, R. Uniform acid poly ionic liquid based large particle and its catalytic application in esterification reaction. Chem. Eng. J. 2015, 271, 269–275. [Google Scholar] [CrossRef]
- Delhomme, C.; Goh, S.L.M.; Kuhn, F.E.; Weuster-Botz, D. Esterification of bio-based succinic acid in biphasic systems: Comparison of chemical and biological catalysts. J. Mol. Catal. B Enz. 2012, 80, 39–47. [Google Scholar] [CrossRef]
- Orjuela, A.; Kolah, A.; Lira, C.T.; Miller, D.J. Mixed succinic acid/acetic acid esterification with ethanol by reactive distillation. Ind. Eng. Chem. Res. 2011, 50, 9209–9220. [Google Scholar] [CrossRef]
- Gerardy, R.; Emmanuel, N.; Toupy, T.; Kassin, V.E.; Tshibalonza, N.N.; Schmitz, M.; Monbaliu, J.C.M. Continuous flow organic chemistry: Successes and pitfalls at the interface with current societal challenges. Eur. J. Org. Chem. 2018, 20–21, 2301–2351. [Google Scholar] [CrossRef]
- Gerardy, R.; Morodo, R.; Estager, J.; Luis, P.; Debecker, D.P.; Monbaliu, J.C.M. Sustaining the transition from petro- to biobased chemical industry with flow chemistry. Top. Curr. Chem. 2019, 377, 1. [Google Scholar] [CrossRef]
- Cherkasov, N.; Bao, Y.; Rebrov, E. Process intensification of alkynol semihydrogenation in a tube reactor coated with a Pd/ZnO catalyst. Catalysts 2017, 7, 358. [Google Scholar] [CrossRef]
- Bai, Y.; Cherkasov, N.; Huband, S.; Walker, D.; Walton, R.I.; Rebrov, E. Highly selective continuous flow hydrogenation of cinnamaldehyde to cinnamyl alcohol in a Pt/SiO2 coated tube reactor. Catalysts 2018, 8, 58. [Google Scholar] [CrossRef]
- Kovalenko, G.A.; Perminova, L.V.; Beklemishev, A.B.; Parmon, V.N. Heterogeneous biocatalysts prepared by immuring enzymatic active components inside silica Xerogel and nanocarbons-in-silica composites. Catalysts 2018, 8, 177. [Google Scholar] [CrossRef]
- Carvalho, F.; Marques, M.P.C.; Fernandes, P. Sucrose hydrolysis in a bespoke capillary wall-coated microreactor. Catalysts 2017, 7, 42. [Google Scholar] [CrossRef]
- Sotto, N.; Cazorla, C.; Villette, C.; Billamboz, M.; Len, C. Toward the sustainable synthesis of biosourced divinylglycol from glycerol. ACS Sustain. Chem. Eng. 2016, 4, 6996–7003. [Google Scholar] [CrossRef]
- Garcia-Olmo, A.J.; Yepez, A.; Balu, A.M.; Prinsen, P.; Garcia, A.; Mazière, A.; Len, C.; Luque, R. Activity of continuous flow synthesized Pd-based nanocatalysts in the flow hydroconversion of furfural. Tetrahedron 2017, 73, 5599–5604. [Google Scholar] [CrossRef]
- Galy, N.; Nguyen, R.; Blach, P.; Sambou, S.; Luart, D.; Len, C. Glycerol oligomerization in continuous flow reactor. J. Ind. Eng. Chem. 2017, 51, 312–318. [Google Scholar] [CrossRef]
- Len, C.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-catalyzed Suzuki-Miyaura cross-coupling in continuous flow. Catalysts 2017, 7, 146. [Google Scholar] [CrossRef]
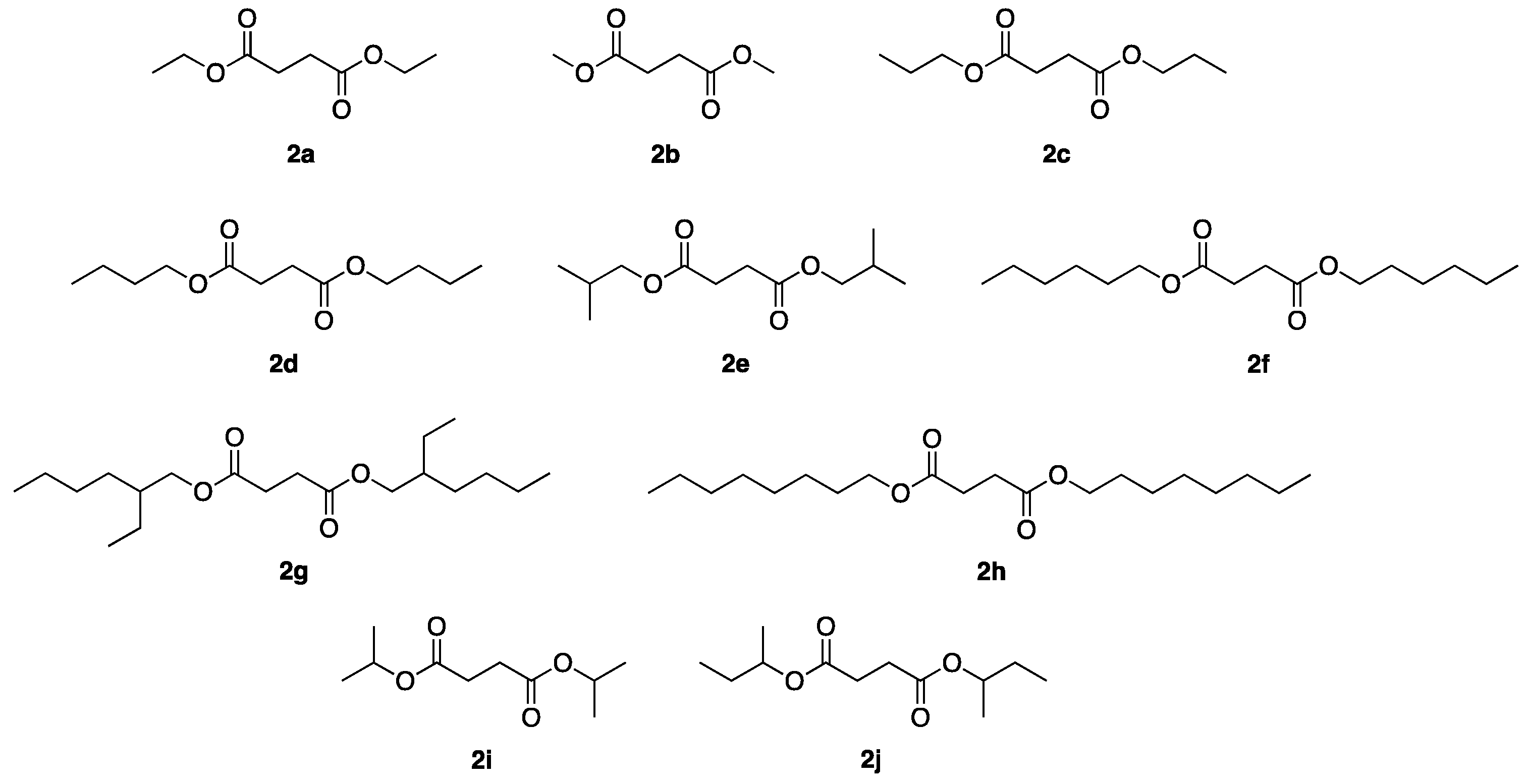
| Entry | Reactor | Catalyst | Reaction Conditions a | 2 | Yield of 2 (%) | Ref |
|---|---|---|---|---|---|---|
| 1 | batch | H2SO4 | nd:2:110:18 | 2g | 69 | [8] |
| 2 | batch | H2SO4 | nd:2.3:110:18 | 2f | 78 | [9] |
| 3 | batch | H2SO4 | nd:2.3:110:18 | 2h | 70 | [9] |
| 4 | batch | OPP-SO3H-1 | 10:50:70:6 | 2b | 88 | [15] |
| 5 | batch | SS-0.010 | 10:2:100:6.5 | 2a | 94 | [16] |
| 6 | batch | Glu-TsOH | 100:80:80:4 | 2a | 100 | [17] |
| 7 | batch b | CH3SO3H@Al2O3 | 332,000:2:80:8 | 2b | 97 | [14] |
| 8 | batch b | CH3SO3H@Al2O3 | 332,000:2:80:8 | 2a | 97 | [14] |
| 9 | batch b | CH3SO3H@Al2O3 | 332,000:2:80:8 | 2c | 97 | [14] |
| 10 | batch b | CH3SO3H@Al2O3 | 332,000:2:80:8 | 2i | 97 | [14] |
| 11 | batch | C2(Mim)2HSO4 | 2:3:60:3 | 2b | 76 | [10] |
| 12 | batch | C3(Mim)2HSO4 | 2:3:60:3.5 | 2a | 68 | [10] |
| 13 | batch | C4(Mim)2HSO4 | 2:4:60:4 | 2c | 74 | [10] |
| 14 | batch | N-Butyl-2,4-dinitro-anilinium p-toluenesulfonate | 1:2:99:25 | 2h | 93 | [7] |
| 15 | batch | nano-SO4 2-/TiO2 | 5:3:160:2 | 2g | 97 | [11] |
| 16 | batch | TSA3/MCM-41 | 0.1:3:80:14 | 2a | 66 | [18] |
| 17 | batch | TSA3/MCM-41 | 0.1:3:80:14 | 2d | 90 | [18] |
| 18 | batch | TPA2/MCM-41 | 100:3:80:8 | 2d | 68 | [19] |
| 19 | batch | TPA2/MCM-41 | 100:3:80:8 | 2f | 68 | [19] |
| 20 | batch | TPA2/MCM-41 | 100:3:80:8 | 2h | 73 | [19] |
| 21 | batch | Al2O3 | 50:1.6:25:48 | 2b | 70 | [20] |
| 22 | flow | PIL-A | 5:1.2:85:5 | 2b | 100 | [21] |
| 23 | flow | PIL-A | 5:1.2:87:4 | 2a | 100 | [21] |
| 24 | flow | PIL-A | 5:1.2:100:3.5 | 2d | 100 | [21] |
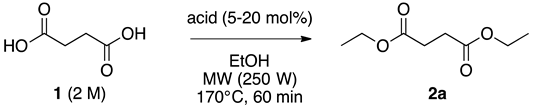
| Entry | Acid | [Acid] (mol %) | Yield of 2a (%) a | Error Bar |
|---|---|---|---|---|
| 1 | PTSA | 10 | 77 | 1.48 |
| 2 | CSA | 10 | 84 | 1.09 |
| 3 | H3PO4 | 10 | 50 | 1.52 |
| 4 | H2SO4 | 10 | 84 | 0.55 |
| 5 | H2SO4 | 20 | 87 | 0.84 |
| 6 | H2SO4 | 30 | 82 | 1.14 |
| 7 | H2SO4 | 5 | 70 | 3.36 |
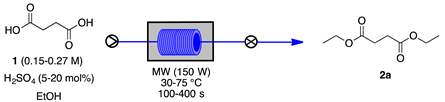
| Entry | 1 (mol L−1) | H2SO4 (mol %) | Temperature (°C) | Residence Time (s) | Conversion (%) a | Yield of 2a (%) a | Error Bar |
|---|---|---|---|---|---|---|---|
| 1 | 0.22 | 20 | 75 | 100 | 45 | 32 | 0.71 |
| 2 | 0.22 | 20 | 75 | 180 | 60 | 48 | 1.30 |
| 3 | 0.22 | 20 | 75 | 320 | 100 | 99 | 0.45 |
| 4 | 0.22 | 20 | 75 | 400 | 100 | 99 | 0.55 |
| 5 | 0.22 | 10 | 75 | 320 | 85 | 82 | 0.89 |
| 6 | 0.22 | 5 | 75 | 320 | 75 | 68 | 0.89 |
| 7 | 0.27 | 20 | 75 | 320 | 95 | 90 | 2.17 |
| 8 | 0.15 | 20 | 75 | 320 | 82 | 78 | 1.52 |
| 9 | 0.22 | 20 | 50 | 320 | 73 | 68 | 1.52 |
| 10 | 0.22 | 20 | 30 | 320 | 35 | 16 | 2.41 |

| Entry | 1 (mol L−1) | Temperature (°C) | Conversion (%) a | Diesters 2 | Yield of 2a–j (%) a | Error Bar |
|---|---|---|---|---|---|---|
| 1 | 0.22 | 65 | 100 | 2b | 100 | 0.89 |
| 2 | 0.22 | 75 | 100 | 2a | 99 | 0.55 |
| 3 | 0.22 | 95 | 95 | 2c | 92 | 0.89 |
| 4 | 0.18 | 115 | 98 | 2d | 89 | 0.55 |
| 5 | 0.18 | 115 | 98 | 2e | 88 | 1.30 |
| 6 | 0.18 | 115 | 97 | 2f | 78 | 1.30 |
| 7 | 0.18 | 115 | 98 | 2g | 80 | 4.55 |
| 8 | 0.18 | 115 | 96 | 2h | 65 | 0.84 |
| 9 | 0.22 | 80 | 98 | 2i | 89 | 1.64 |
| 10 | 0.18 | 96 | 98 | 2j | 36 | 1.95 |
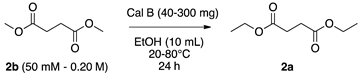
| Entry | 2b (M) | Cal B (mg) | Temperature (°C) | Yield of 2a (%) a | Error Bar |
|---|---|---|---|---|---|
| 1 | 0.050 | 270 | 20 | 60 | 0.89 |
| 2 | 0.050 | 270 | 40 | 60 | 1.22 |
| 3 | 0.050 | 270 | 60 | 60 | 0.55 |
| 4 | 0.050 | 270 | 80 | 20 | 2.51 |
| 5 | 0.050 | 40 | 20 | 30 | 1.30 |
| 6 | 0.050 | 130 | 20 | 55 | 1.73 |
| 7 | 0.050 | 200 | 20 | 60 | 1.09 |
| 8 | 0.10 | 200 | 20 | 50 | 1.30 |
| 9 | 0.20 | 200 | 20 | 45 | 1.30 |

| Entry | 2b (M) | Cal B (mg) | Residence Time (min) | Temperature (°C) | Conversion of 2b (%) a | Yield of 2a (%) a | Error Bar |
|---|---|---|---|---|---|---|---|
| 1 | 0.050 | 200 | 7 | 20 | 90 | 7 | 1.00 |
| 2 | 0.050 | 200 | 2.3 | 20 | 79 | 1 | 0.55 |
| 3 | 0.050 | 200 | 1.2 | 20 | 76 | 1 | 0.45 |
| 4 | 0.050 | 200 | 7 | 40 | 99 | 23 | 1.30 |
| 5 | 0.050 | 200 | 2.3 | 40 | 73 | 3 | 0.89 |
| 6 | 0.050 | 200 | 1.2 | 40 | 73 | 2 | 0.84 |
| 7 | 0.050 | 200 | 7 | 60 | 95 | 18 | 1.22 |
| 8 | 0.050 | 200 | 2.3 | 60 | 78 | 5 | 1.41 |
| 9 | 0.500 | 200 | 1.2 | 60 | 73 | 2 | 1.00 |
| 10 | 0.050 | 100 | 7 | 40 | 60 | 14 | 1.09 |
| 11 | 0.050 | 100 | 2.3 | 40 | 68 | 6 | 0.89 |
| 12 | 0.050 | 100 | 1.2 | 40 | 63 | traces | 0.09 |
| 13 | 0.050 | 400 | 7 | 40 | 100 | 24 | 0.89 |
| 14 | 0.050 | 400 | 2.3 | 40 | 97 | 12 | 1.30 |
| 15 | 0.050 | 400 | 1.2 | 40 | 91 | 5 | 0.89 |
| 16 | 0.025 | 200 | 7 | 40 | 89 | 10 | 1.30 |
| 17 | 0.025 | 200 | 2.3 | 40 | 72 | 3 | 0.89 |
| 18 | 0.025 | 200 | 1.2 | 40 | 75 | 1 | 0.27 |
| 19 | 0.100 | 200 | 7 | 40 | 94 | 14 | 1.41 |
| 20 | 0.100 | 200 | 2.3 | 40 | 85 | 5 | 0.89 |
| 21 | 0.100 | 200 | 1.2 | 40 | 78 | 3 | 0.27 |
| 22 | 0.050 | 200 | 28 | 40 | 95 | 18 | 1.30 |
| 23 | 0.050 | 200 | 14 | 40 | 96 | 48 | 1.52 |
| 24 | 0.050 | 100 | 14 | 40 | 100 | 34 | 1.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daviot, L.; Len, T.; Lin, C.S.K.; Len, C. Microwave-Assisted Homogeneous Acid Catalysis and Chemoenzymatic Synthesis of Dialkyl Succinate in a Flow Reactor. Catalysts 2019, 9, 272. https://doi.org/10.3390/catal9030272
Daviot L, Len T, Lin CSK, Len C. Microwave-Assisted Homogeneous Acid Catalysis and Chemoenzymatic Synthesis of Dialkyl Succinate in a Flow Reactor. Catalysts. 2019; 9(3):272. https://doi.org/10.3390/catal9030272
Chicago/Turabian StyleDaviot, Laura, Thomas Len, Carol Sze Ki Lin, and Christophe Len. 2019. "Microwave-Assisted Homogeneous Acid Catalysis and Chemoenzymatic Synthesis of Dialkyl Succinate in a Flow Reactor" Catalysts 9, no. 3: 272. https://doi.org/10.3390/catal9030272
APA StyleDaviot, L., Len, T., Lin, C. S. K., & Len, C. (2019). Microwave-Assisted Homogeneous Acid Catalysis and Chemoenzymatic Synthesis of Dialkyl Succinate in a Flow Reactor. Catalysts, 9(3), 272. https://doi.org/10.3390/catal9030272