Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures
Abstract
1. Introduction
2. Results and Discussion
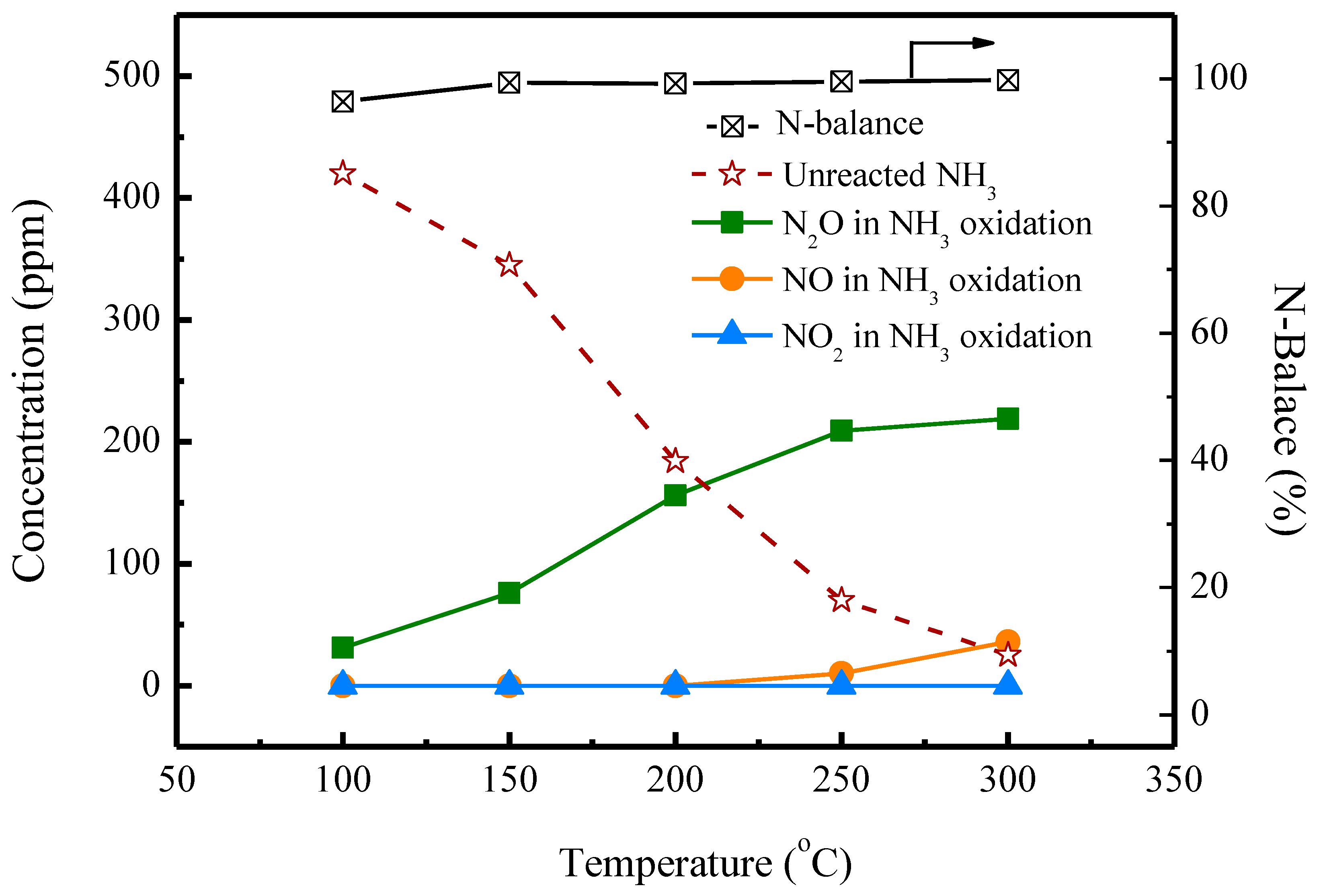
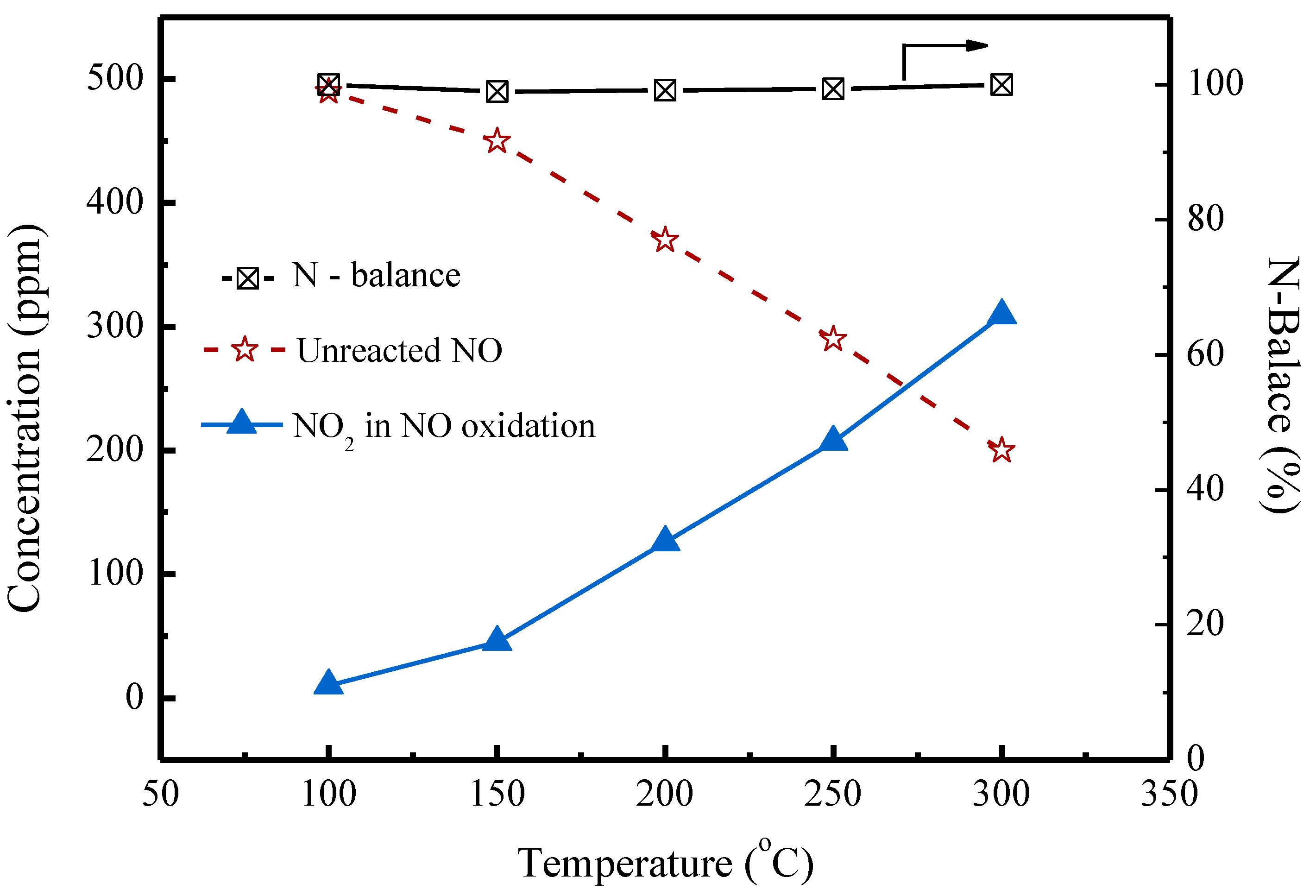
2.1. Oxidation Reactions of NH3 and NO without SO2
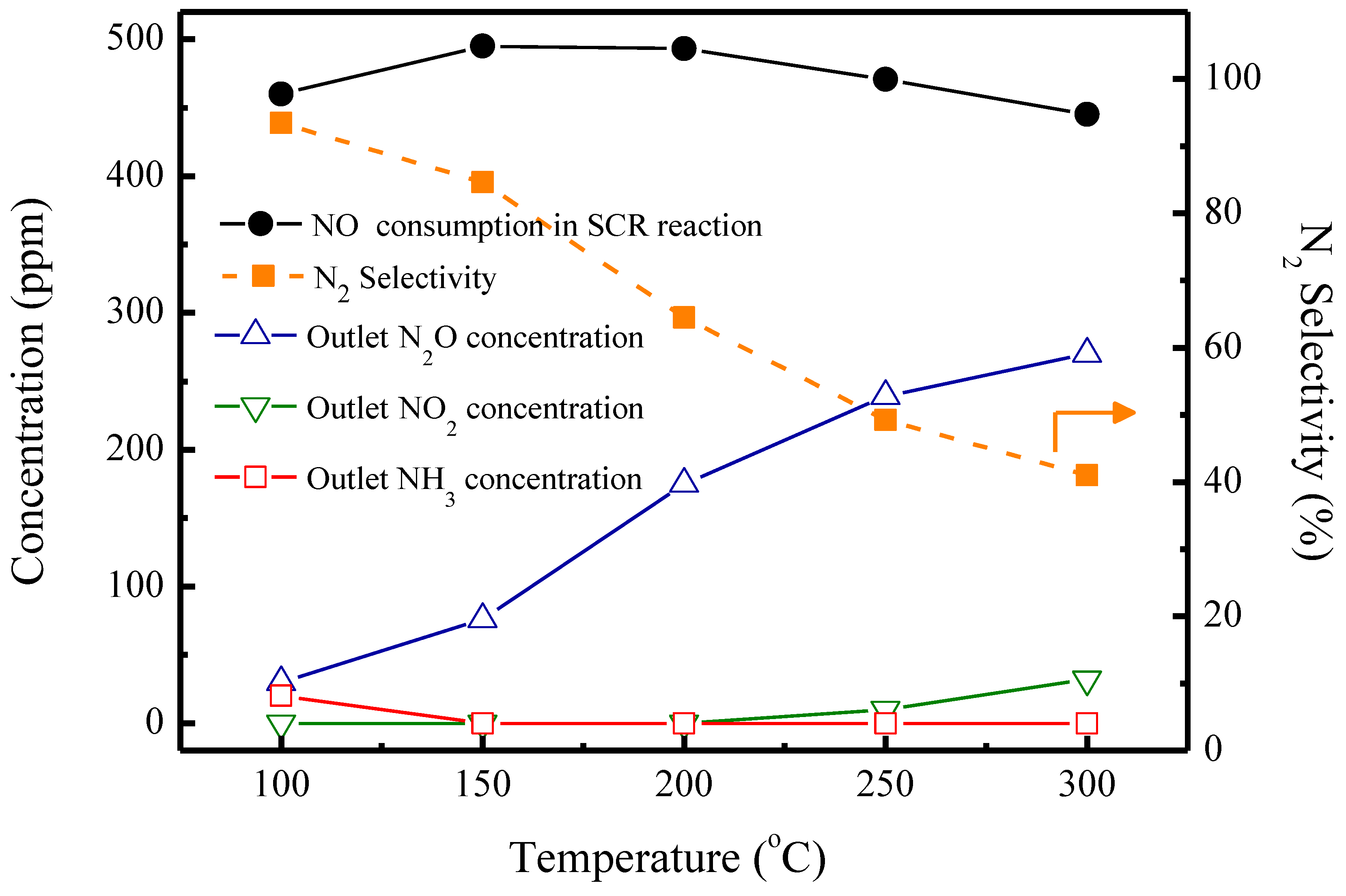
2.2. SCR Reactions without SO2
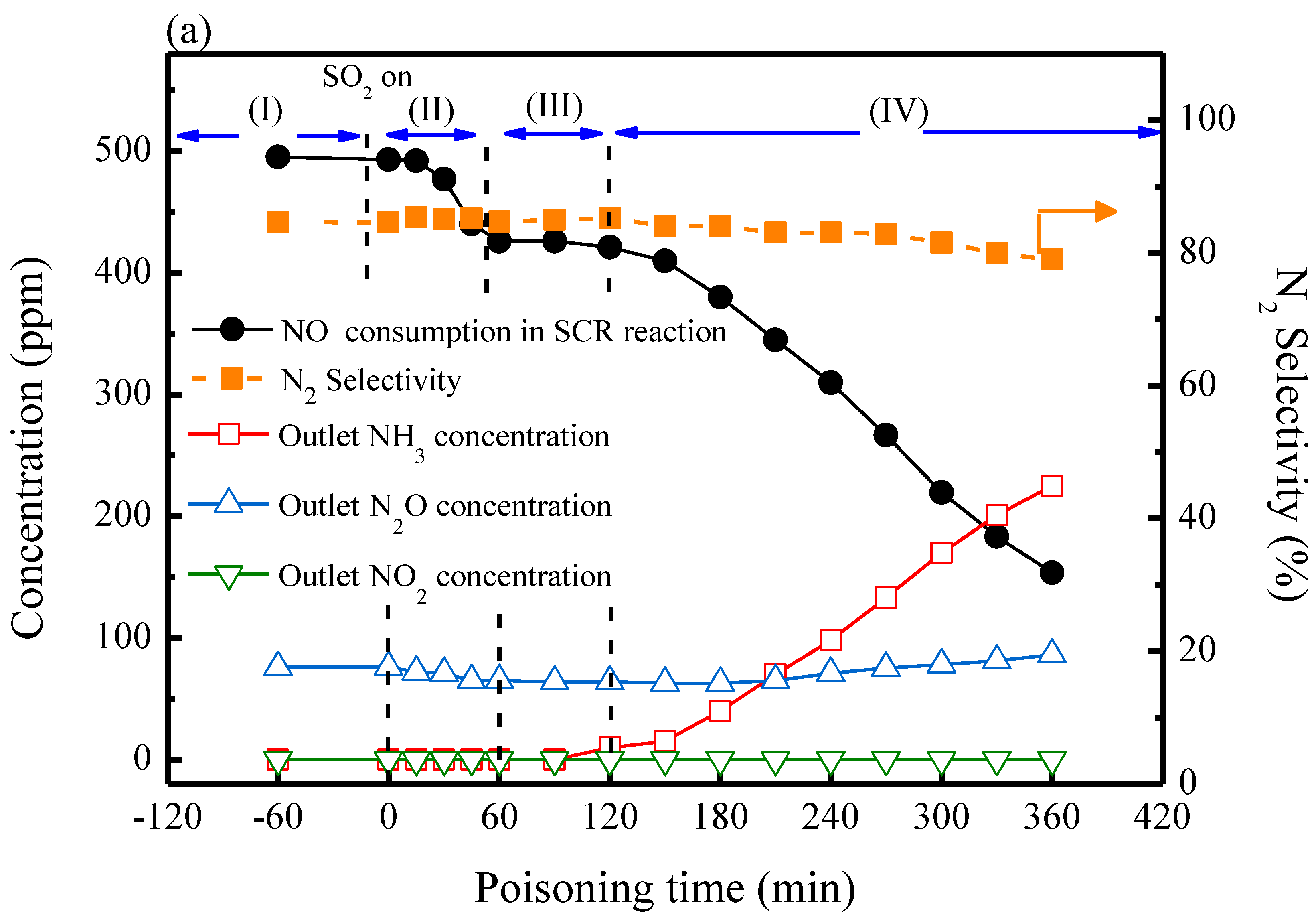
2.3. SCR Reactions with SO2
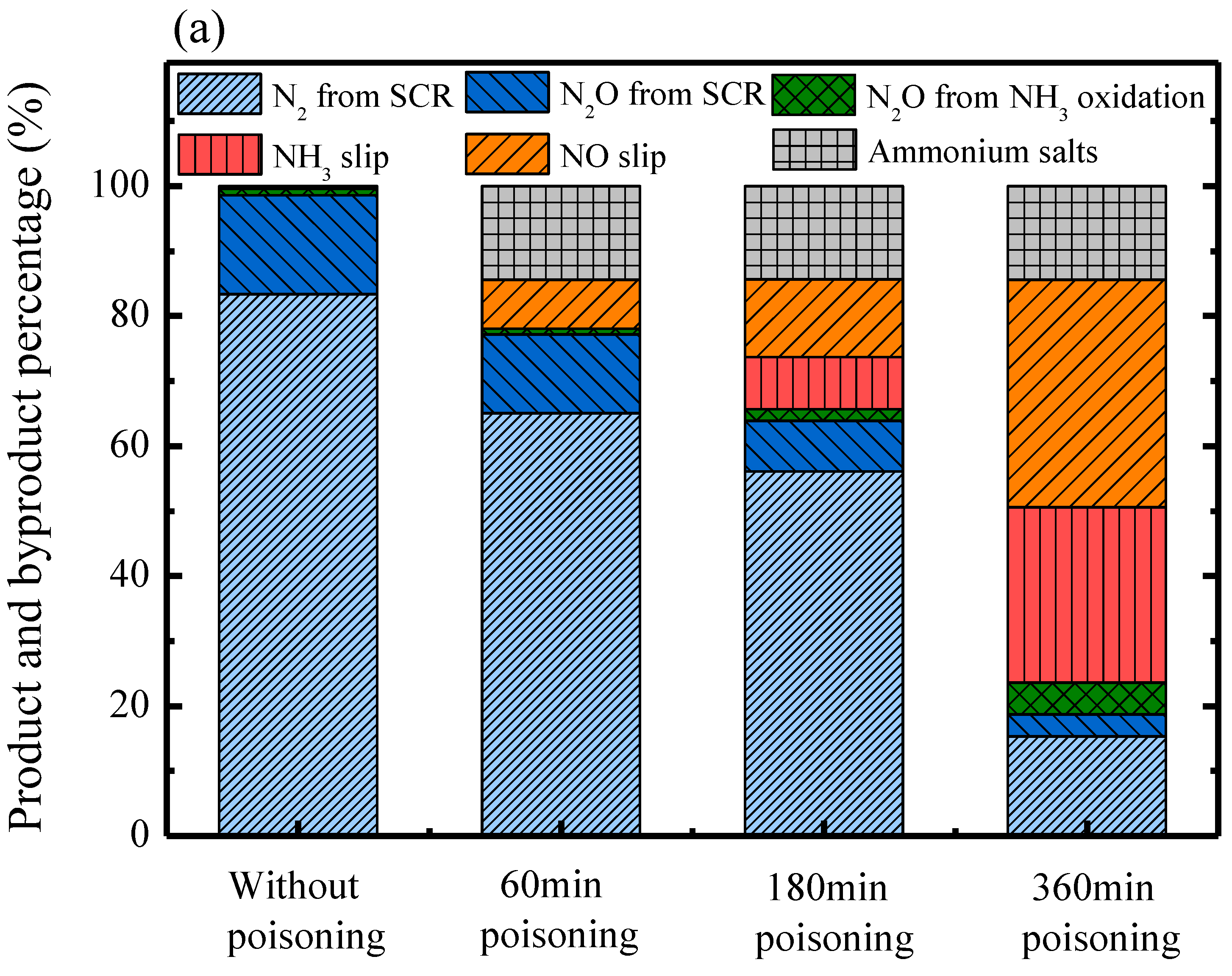
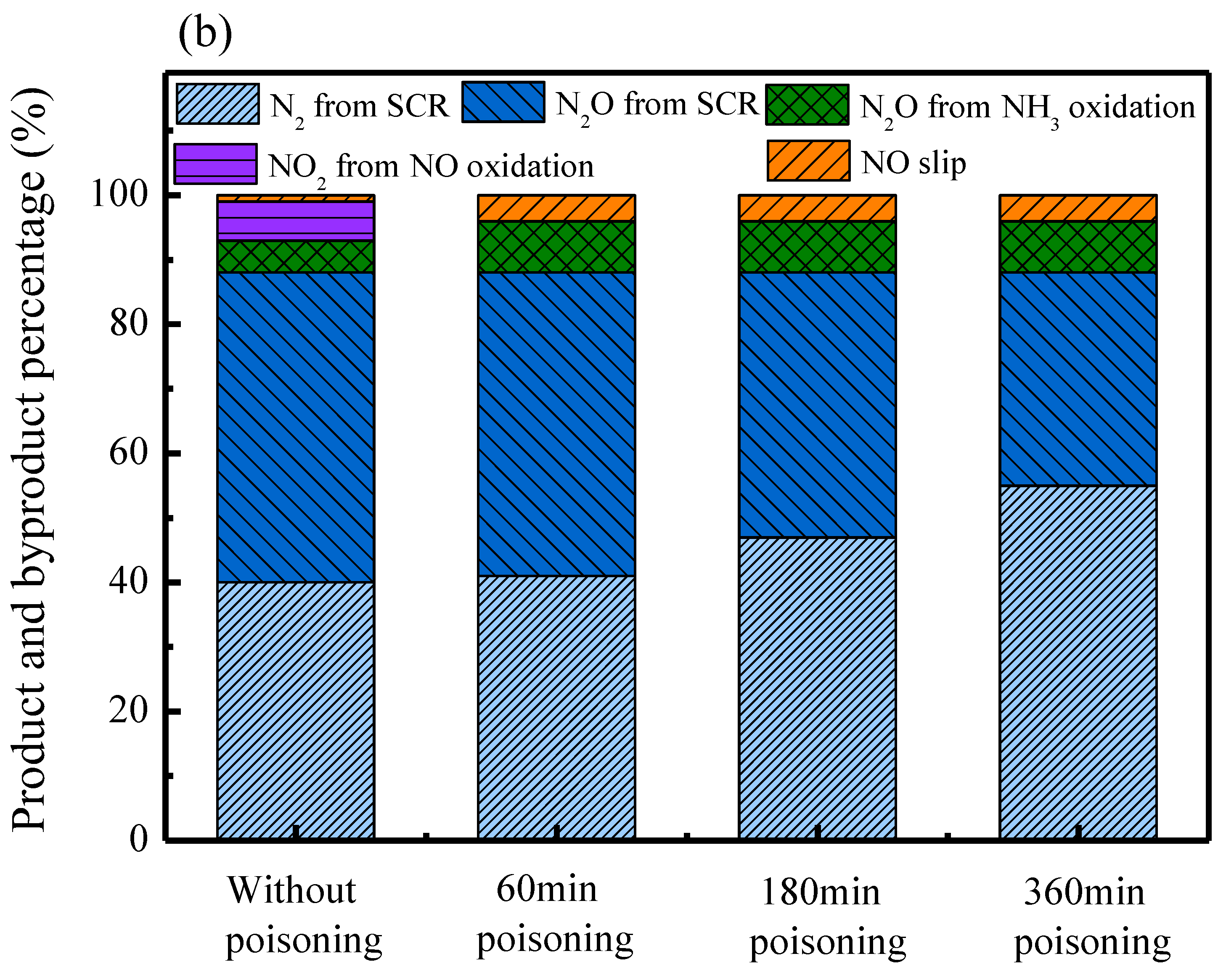
2.4. Product and Byproduct Analysis
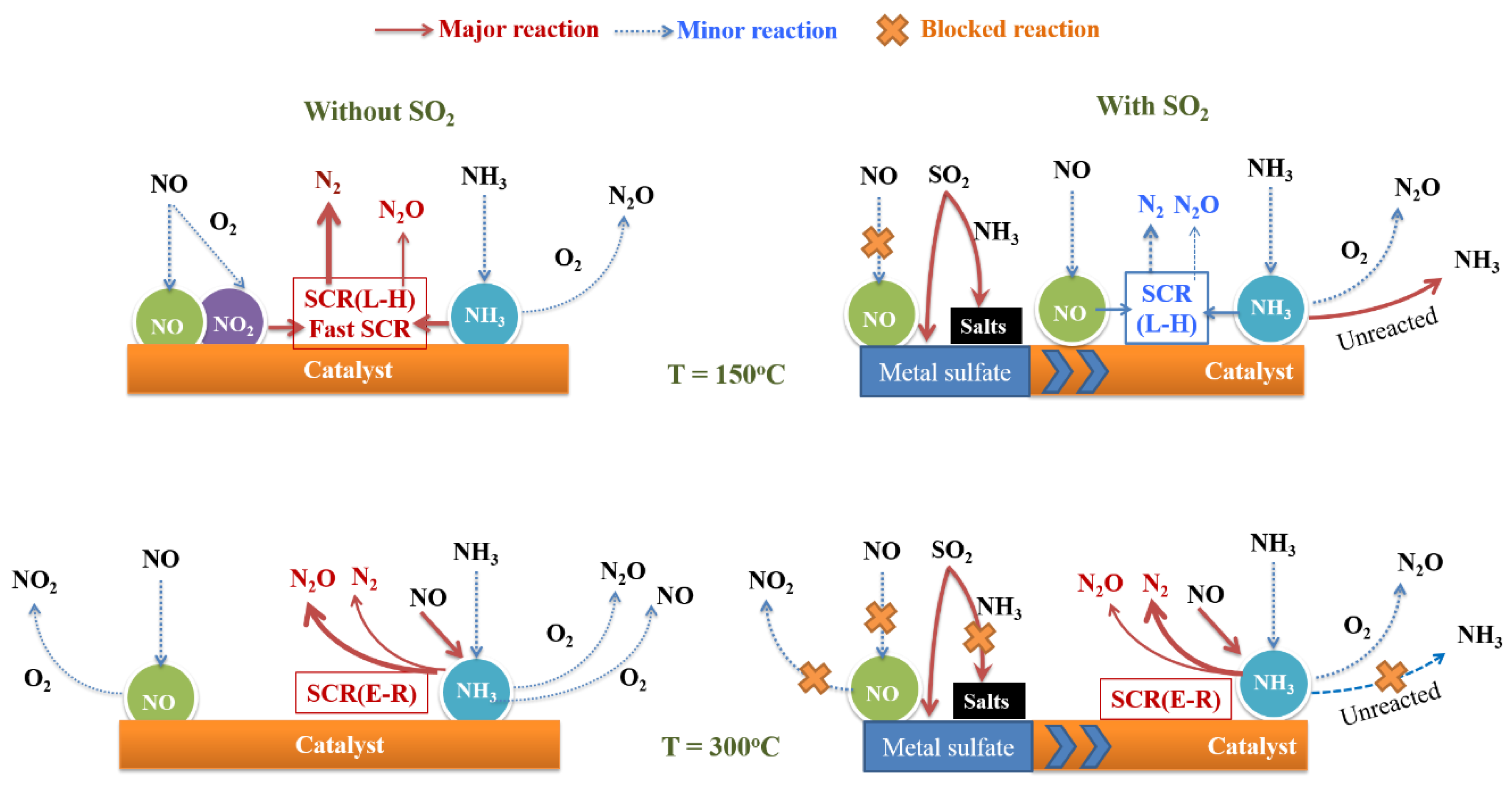
2.5. Reaction Pathways
3. Materials and Method
3.1. Reactions in SCR System
3.2. Synthesis of MnFe/TiO2 Catalysts
3.3. Catalyst Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Yan, Y.L.; Tseng, C.H.; Syu, J.Y.; Lin, W.Y.; Yuan, Y.C. Development of an Innovative Circulating Fluidized-Bed with Microwave System for Controlling NOx. Aerosol Air Qual. Res. 2012, 12, 379–386. [Google Scholar] [CrossRef]
- Garcia-Bordeje, E.; Pinilla, J.L.; Lazaro, M.J.; Moliner, R.; Fierro, J.L.G. Role of sulphates on the mechanism of NH3-SCR of NO at low temperatures over presulphated vanadium supported on carbon-coated monoliths. J. Catal. 2005, 233, 166–175. [Google Scholar] [CrossRef]
- Gan, L.N.; Guo, F.; Yu, J.; Xu, G.W. Improved Low-Temperature Activity of V2O5-WO3/TiO2 for Denitration Using Different Vanadium Precursors. Catalysts 2016, 6, 25. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Bruckner, A.; Hipler, F.; Auer, G.; Loffler, E.; Grunert, W. A new view on the relations between tungsten and vanadium in V2O5-WO3/TiO2 catalysts for the selective reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Krocher, O. Selective Catalytic Reduction of NOx. Catalysts 2018, 8, 459. [Google Scholar] [CrossRef]
- Balle, P.; Geiger, B.; Kureti, S. Selective catalytic reduction of NOx by NH3 on Fe/HBEA zeolite catalysts in oxygen-rich exhaust. Appl. Catal. B Environ. 2009, 85, 109–119. [Google Scholar] [CrossRef]
- Kim, M.H.; Yang, K.H. The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts. Catalysts 2018, 8, 134. [Google Scholar]
- Lee, S.M.; Kim, S.S.; Hong, S.C. Systematic mechanism study of the high temperature SCR of NOx by NH3 over a W/TiO2 catalyst. Chem. Eng. Sci. 2012, 79, 177–185. [Google Scholar]
- Ning, R.; Chen, L.; Li, E.; Liu, X.; Zhu, T. Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas. Catalysts 2019, 9, 220. [Google Scholar] [CrossRef]
- Gao, R.H.; Zhang, D.S.; Liu, X.G.; Shi, L.Y.; Maitarad, P.; Li, H.R.; Zhang, J.P.; Cao, W.G. Enhanced catalytic performance of V2O5-WO3/Fe2O3/TiO2 microspheres for selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2013, 3, 191–199. [Google Scholar] [CrossRef]
- Xu, W.Q.; He, H.; Yu, Y.B. Deactivation of a Ce/TiO2 Catalyst by SO2 in the Selective Catalytic Reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Yang, R.; Huang, H.F.; Chen, Y.J.; Zhang, X.X.; Lu, H.F. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2015, 36, 1256–1262. [Google Scholar] [CrossRef]
- Kong, Z.J.; Wang, C.; Ding, Z.N.; Chen, Y.F.; Zhang, Z.K. Enhanced activity of MnxW0.05Ti0.95−xO2−δ for selective catalytic reduction of NOx with ammonia by self-propagating high-temperature synthesis. Catal. Commun. 2015, 64, 27–31. [Google Scholar] [CrossRef]
- Lian, Z.H.; Liu, F.D.; He, H.; Shi, X.Y.; Mo, J.S.; Wu, Z.B. Manganese-niobium mixed oxide catalyst for the selective catalytic reduction of NOx with NH3 at low temperatures. Chem. Eng. J. 2014, 250, 390–398. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.W.; Fan, Z.Y.; Gao, G.; Niu, C.M. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts 2018, 8, 11. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Godiksen, A.; Poreddy, R.; Mossin, S.; Jensen, A.D.; Fehrmann, R. Promoted V2O5/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2016, 183, 282–290. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, J.M.; Liotta, L.F.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic Oxidation of Propene over Pd Catalysts Supported on CeO2, TiO2, Al2O3 and M/Al2O3 Oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Liu, L.; Gao, X.; Song, H.; Zheng, C.H.; Zhu, X.B.; Luo, Z.Y.; Ni, M.J.; Cen, K.F. Study of the Promotion Effect of Iron on Supported Manganese Catalysts for No Oxidation. Aerosol Air Qual. Res. 2014, 14, 1038–1046. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Z.H.; Chen, M.X.; Zhang, Z.X.; Shangguan, W.F. Promotion effect of tungsten and iron co-addition on the catalytic performance of MnOx/TiO2 for NH3-SCR of NOx. Fuel 2017, 210, 783–789. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.P.; Yang, H.; Wang, C.H. NH3-SCR Performance of Mn-Fe/TiO2 Catalysts at Low Temperature in the Absence and Presence of Water Vapor. Water Air Soil Poll. 2016, 227, 476. [Google Scholar] [CrossRef]
- Deng, S.C.; Zhuang, K.; Xu, B.L.; Ding, Y.H.; Yu, L.; Fan, Y.N. Promotional effect of iron oxide on the catalytic properties of Fe-MnOx/TiO2 (anatase) catalysts for the SCR reaction at low temperatures. Catal. Sci. Technol. 2016, 6, 1772–1778. [Google Scholar] [CrossRef]
- Dong, L.F.; Fan, Y.M.; Ling, W.; Yang, C.; Huang, B.C. Effect of Ce/Y Addition on Low-Temperature SCR Activity and SO2 and H2O Resistance of MnOx/ZrO2/MWCNTs Catalysts. Catalysts 2017, 7, 181. [Google Scholar] [CrossRef]
- Mu, W.T.; Zhu, J.; Zhang, S.; Guo, Y.Y.; Su, L.Q.; Li, X.Y.; Li, Z. Novel proposition on mechanism aspects over Fe-Mn/ZSM-5 catalyst for NH3-SCR of NOx at low temperature: Rate and direction of multifunctional electron-transfer-bridge and in situ DRIFTs analysis. Catal. Sci. Technol. 2016, 6, 7532–7548. [Google Scholar] [CrossRef]
- Chen, T.; Guan, B.; Lin, H.; Zhu, L. In situ DRIFTS study of the mechanism of low temperature selective catalytic reduction over manganese-iron oxides. Chin. J. Catal. 2014, 35, 294–301. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhong, B.C.; Wang, W.H.; Guan, X.J.; Huang, B.C.; Ye, D.Q.; Wu, H.J. In situ DRIFTS study of NO reduction by NH3 over Fe-Ce-Mn/ZSM-5 catalysts. Catal. Today 2011, 175, 157–163. [Google Scholar] [CrossRef]
- Lin, Q.C.; Li, J.H.; Ma, L.; Hao, J.M. Selective catalytic reduction of NO with NH3 over Mn-Fe/USY under lean burn conditions. Catal. Today 2010, 151, 251–256. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, F.; Zhu, M.Y.; Dan, J.M.; Wang, X.G.; Zhang, J.L.; Dai, B. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef]
- Liu, F.D.; He, H.; Ding, Y.; Zhang, C.B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2009, 93, 194–204. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S.O. Enhancement of Catalytic Activity Over the Iron-Modified Ce/TiO2 Catalyst for Selective Catalytic Reduction of NOx with Ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Yang, S.J.; Xiong, S.C.; Liao, Y.; Xiao, X.; Qi, F.H.; Peng, Y.; Fu, Y.W.; Shan, W.P.; Li, J.H. Mechanism of N2O Formation during the Low-Temperature Selective Catalytic Reduction of NO with NH3 over Mn-Fe Spinel. Environ. Sci. Technol. 2014, 48, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Liou, S.Y.; Bai, H.L. Comparison of titania nanotubes and titanium dioxide as supports of low-temperature selective catalytic reduction catalysts under sulfur dioxide poisoning. J. Air Waste Manag. 2017, 67, 292–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuenter, G.; Vanleeuwen, W.F.; Snepvangers, L.J.M. Kinetics and Mechanism of the NOx Reduction with NH3 on V2O5-WO3-TiO2 Catalyst. Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 633–636. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Grossale, A.; Nova, I.; Tronconi, E.; Jirglova, H.; Sobalik, Z. FTIR in situ mechanistic study of the NH3-NO/NO2 “Fast SCR” reaction over a commercial Fe-ZSM-5 catalyst. Catal. Today 2012, 184, 107–114. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, S.M.; Kim, S.S.; Kwon, D.W.; Hong, S.C. Reversibility of Mn Valence State in MnOx/TiO2 Catalysts for Low-temperature Selective Catalytic Reduction for NO with NH3. Catal. Lett. 2013, 143, 246–253. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.Y.; Hu, S.; Sun, L.S. Ag modified Mn-Ce/gamma-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature. Fuel Process. Technol. 2015, 135, 66–72. [Google Scholar] [CrossRef]
- Xu, H.D.; Fang, Z.T.; Cao, Y.; Kong, S.; Lin, T.; Gong, M.C.; Chen, Y.Q. Influence of Mn/(Mn plus Ce) Ratio of MnOx-CeO2/WO3-ZrO2 Monolith Catalyst on Selective Catalytic Reduction of NOx with Ammonia. Chin. J. Catal. 2012, 33, 1927–1937. [Google Scholar] [CrossRef]
- Liu, F.D.; Asakura, K.; He, H.; Shan, W.P.; Shi, X.Y.; Zhang, C.B. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H.T. Selective catalytic reaction of NOx with NH3 over Ce-Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B Environ. 2014, 150, 630–635. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhang, X.L.; Lv, S.S.; Wu, X.P.; Wang, P.M. Promoted performance of a MnOx/PG catalyst for low-temperature SCR against SO2 poisoning by addition of cerium oxide. RSC Adv. 2015, 5, 82952–82959. [Google Scholar] [CrossRef]
- Pourkhalil, M.; Moghaddam, A.Z.; Rashidi, A.; Towfighi, J.; Mortazavi, Y. Preparation of highly active manganese oxides supported on functionalized MWNTs for low temperature NOx reduction with NH3. Appl. Surf. Sci. 2013, 279, 250–259. [Google Scholar] [CrossRef]
- Wang, X.B.; Gui, K.T. Fe2O3 particles as superior catalysts for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2013, 25, 2469–2475. [Google Scholar] [CrossRef]
- Jin, R.B.; Liu, Y.; Wang, Y.; Cen, W.L.; Wu, Z.B.; Wang, H.Q.; Weng, X.L. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B Environ. 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Shen, B.X.; Zhang, X.P.; Ma, H.Q.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. China 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Metal Sulfate Poisoning Effects over MnFe/TiO2 for Selective Catalytic Reduction of NO by NH3 at Low Temperature. Ind. Eng. Chem. Res. 2018, 57, 4848–4858. [Google Scholar] [CrossRef]
- Busca, G.; Larrubia, M.A.; Arrighi, L.; Ramis, G. Catalytic abatement of NOx: Chemical and mechanistic aspects. Catal. Today 2005, 107–108, 139–148. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and Selectivity of Pure Manganese Oxides in the Selective Catalytic Reduction of Nitric-Oxide with Ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, X.X.; Zhan, L.; Li, C.; Qiao, W.M.; Ling, L.C. Effect of SO2 on Activated Carbon Honeycomb Supported CeO2-MnOx Catalyst for NO Removal at Low Temperature. Ind. Eng. Chem. Res. 2015, 54, 2274–2278. [Google Scholar] [CrossRef]
- Yang, W.W.; Liu, F.D.; Xie, L.J.; Lan, Z.H.; He, H. Effect of V2O5 Additive on the SO2 Resistance of a Fe2O3/AC Catalyst for NH3-SCR of NOx at Low Temperatures. Ind. Eng. Chem. Res. 2016, 55, 2677–2685. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Pang, D.D.; Ouyang, F.; Zhang, C.L.; Cao, G. Characterization and Catalytic Activity of Mn-Co/TiO2 Catalysts for NO Oxidation to NO2 at Low Temperature. Catalysts 2016, 6, 9. [Google Scholar] [CrossRef]
- Lippits, M.J.; Gluhoi, A.C.; Nieuwenhuys, B.E. A comparative study of the selective oxidation of NH3 to N2 over gold, silver and copper catalysts and the effect of addition of Li2O and CeOx. Catal. Today 2008, 137, 446–452. [Google Scholar] [CrossRef]
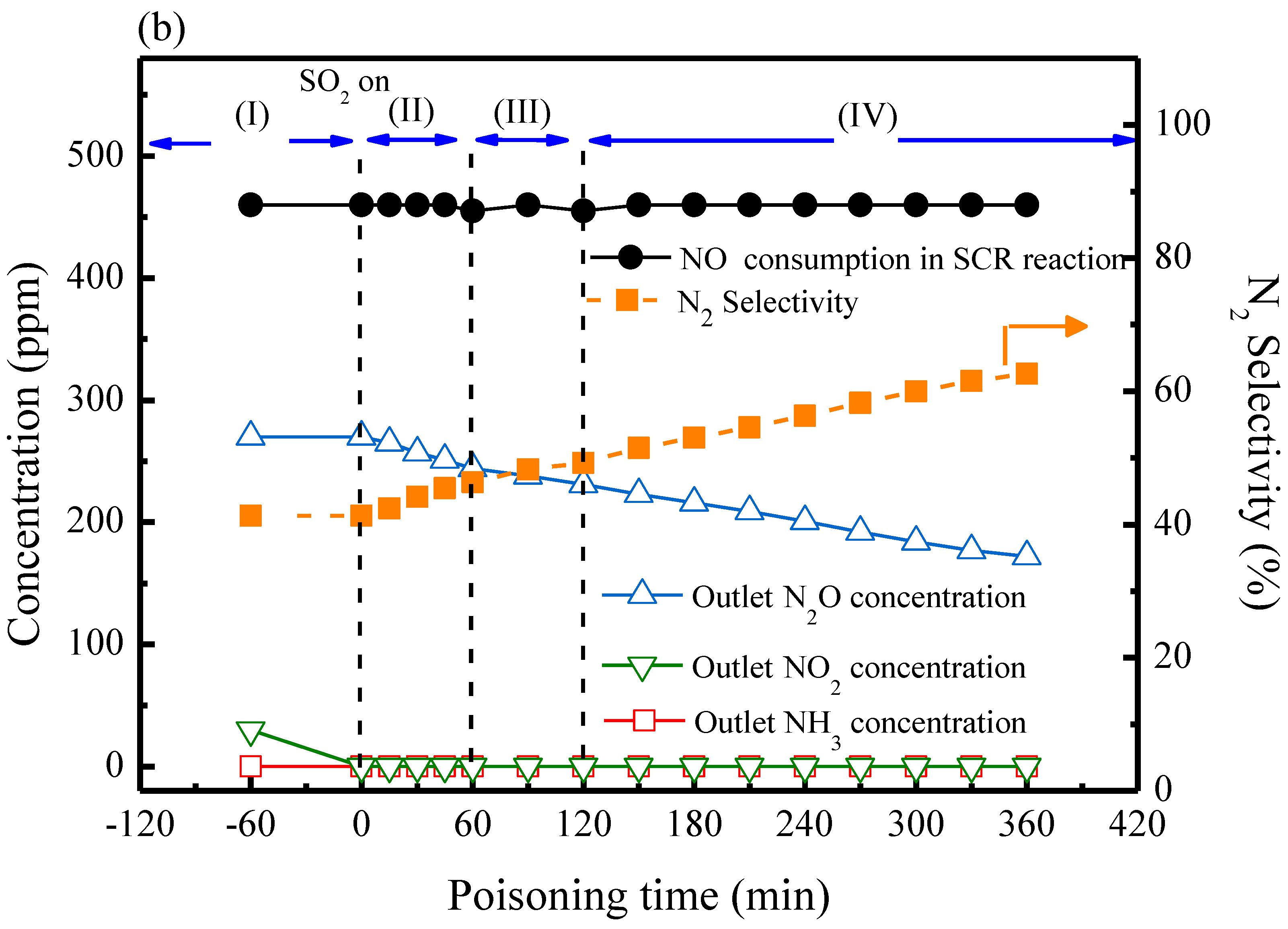
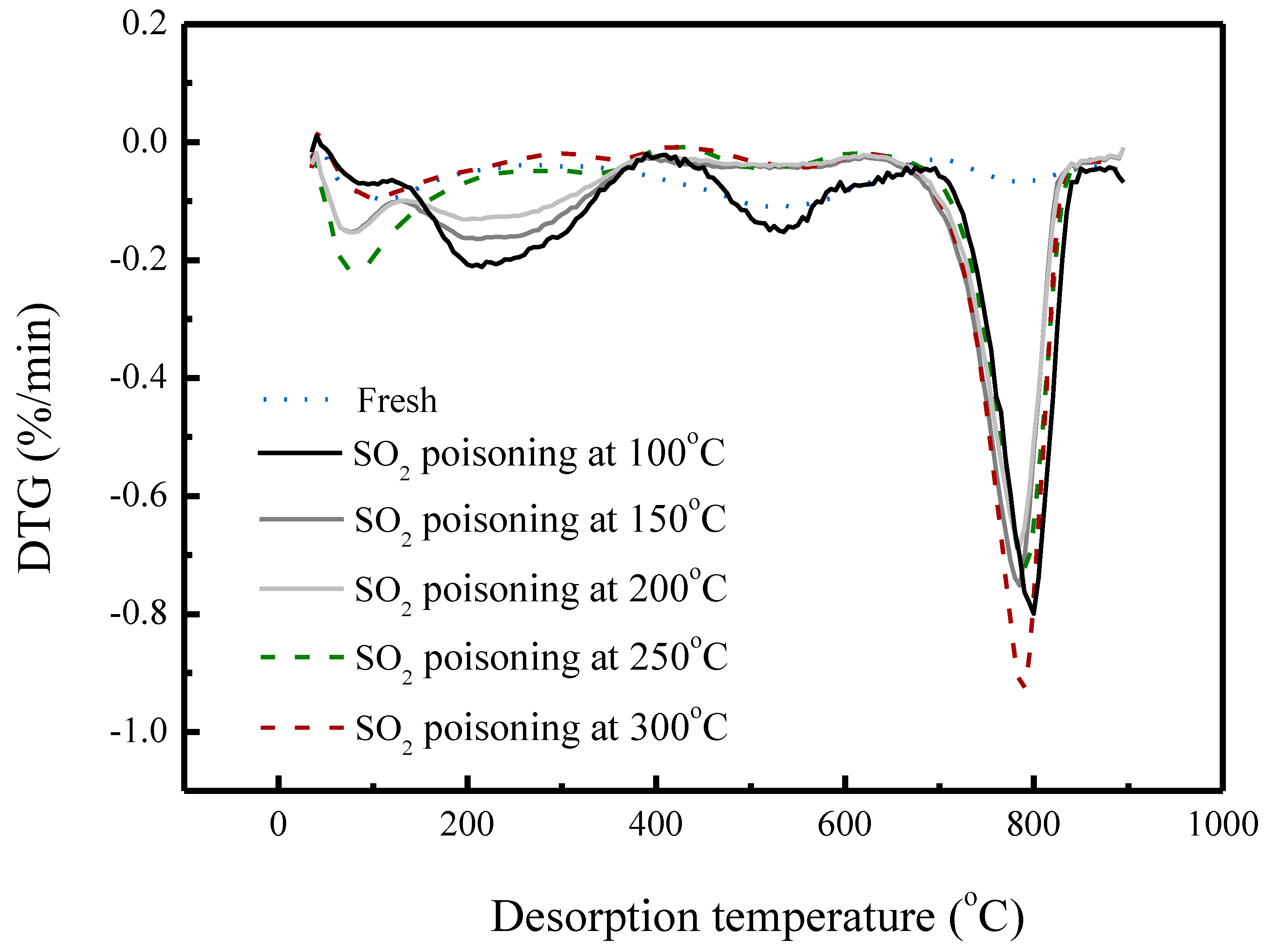
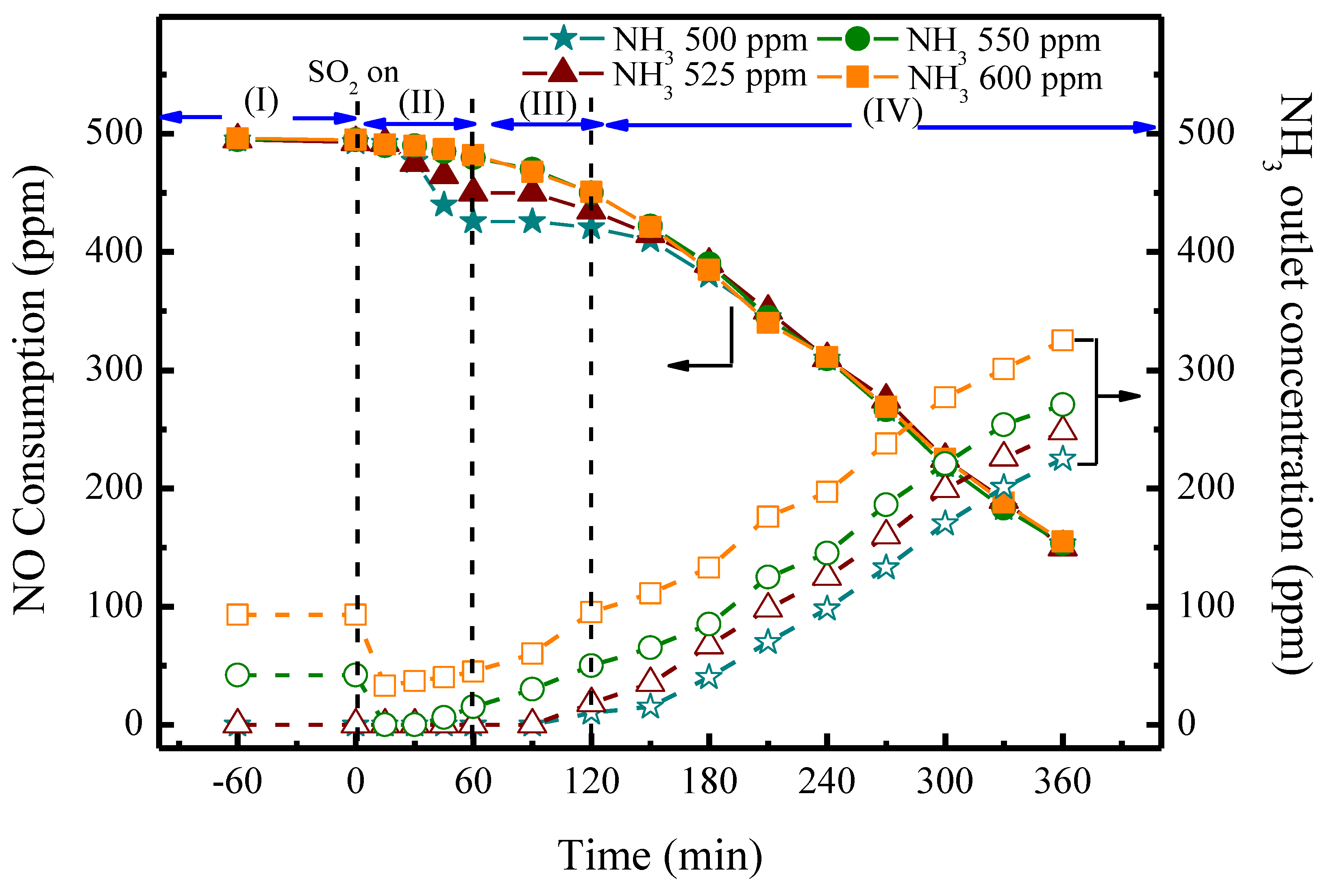
| MnFe/TiO2 Poisoned with Different Reaction Temperatures | Ammonium Salts a (by weight) % | Metal Sulfates b (by Weight) % |
|---|---|---|
| Fresh | 0.0 | 0.0 |
| 100 °C | 2.3 | 4.21 c |
| 150 °C | 1.8 | 4.11 c |
| 200 °C | 1.1 | 4.03 c |
| 250 °C | 0.1 | 4.08 c |
| 300 °C | 0(−0.1) | 4.52 c |
| Product and Byproduct | Reaction Equation | Percentage Calculation Equation |
|---|---|---|
| NO2 | NO oxidation to NO2 (Equation (5)): 2NO + O2 → 2NO2 | The percentage of NO2 from NO oxidation |
| Salts (NH4)2SO4 NH4HSO4 | Ammonium salts Equations (10) and (11): SO3 + 2NH3 + H2O (NH4)2SO4 SO3 + NH3 + H2O → NH4HSO4 | The percentage of salts #the NH3 consumption of salts reaction ( were indicated by result of Figure 6 |
| N2O | NH3 slip oxidation to form N2O [N2O] slip (%) | The percentage of N2O from NH3 slip oxidation *: from results of NH3 oxidation test (Figure 1) |
| NH3 oxidation to form N2O Equation (3): 2NH3 + 2O2 → N2O + 3H2O | The percentage of N2O from NH3 oxidation | |
| SCR reaction to form N2O Equation (7): 4NO + 4NH3 + 3O2 → 4N2O + 6H2O | The percentage of N2O from SCR reaction | |
| N2 | SCR reaction to form N2 Equation (6): 4NO + 4NH3 + O2 → 4N2 + 6H2O | The percentage of N2 from SCR reaction |
| NH3 (ppmv) | NO (ppmv) | SO2 (ppmv) | O2 (%) | Temperature (°C) | GHSV (hr−1) | |
|---|---|---|---|---|---|---|
| NH3 oxidation test | 500 | 0 | 0 | 10% | 100–300 | 50,000 |
| NO oxidation test | 0 | 500 | 0 | 10% | 100–300 | 50,000 |
| SCR test without SO2 | 500 | 500 | 0 | 10% | 100–300 | 50,000 |
| SCR test with SO2 | 500 | 500 | 150 | 10% | 150 & 300 | 50,000 |
| SCR test with SO2 at different NH3 amounts | 500–600 | 500 | 150 | 10% | 150 | 50,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Bai, H. Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures. Catalysts 2019, 9, 265. https://doi.org/10.3390/catal9030265
Lee T, Bai H. Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures. Catalysts. 2019; 9(3):265. https://doi.org/10.3390/catal9030265
Chicago/Turabian StyleLee, Tsungyu, and Hsunling Bai. 2019. "Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures" Catalysts 9, no. 3: 265. https://doi.org/10.3390/catal9030265
APA StyleLee, T., & Bai, H. (2019). Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures. Catalysts, 9(3), 265. https://doi.org/10.3390/catal9030265





