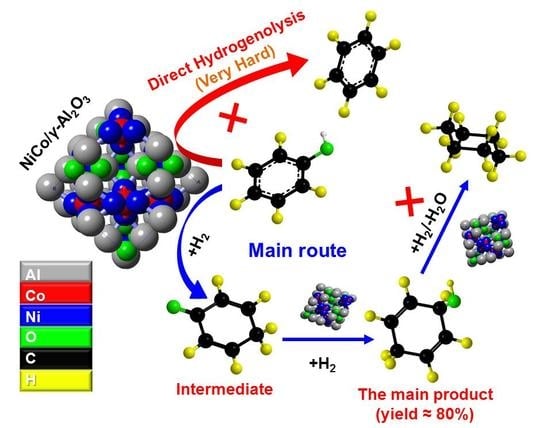
Selective Conversion of Phenol in a Subcritical Water Medium Using γ-Al2O3 Supported Ni–Co Bimetallic Catalyst
Abstract
1. Introduction
2. Results and Discussion
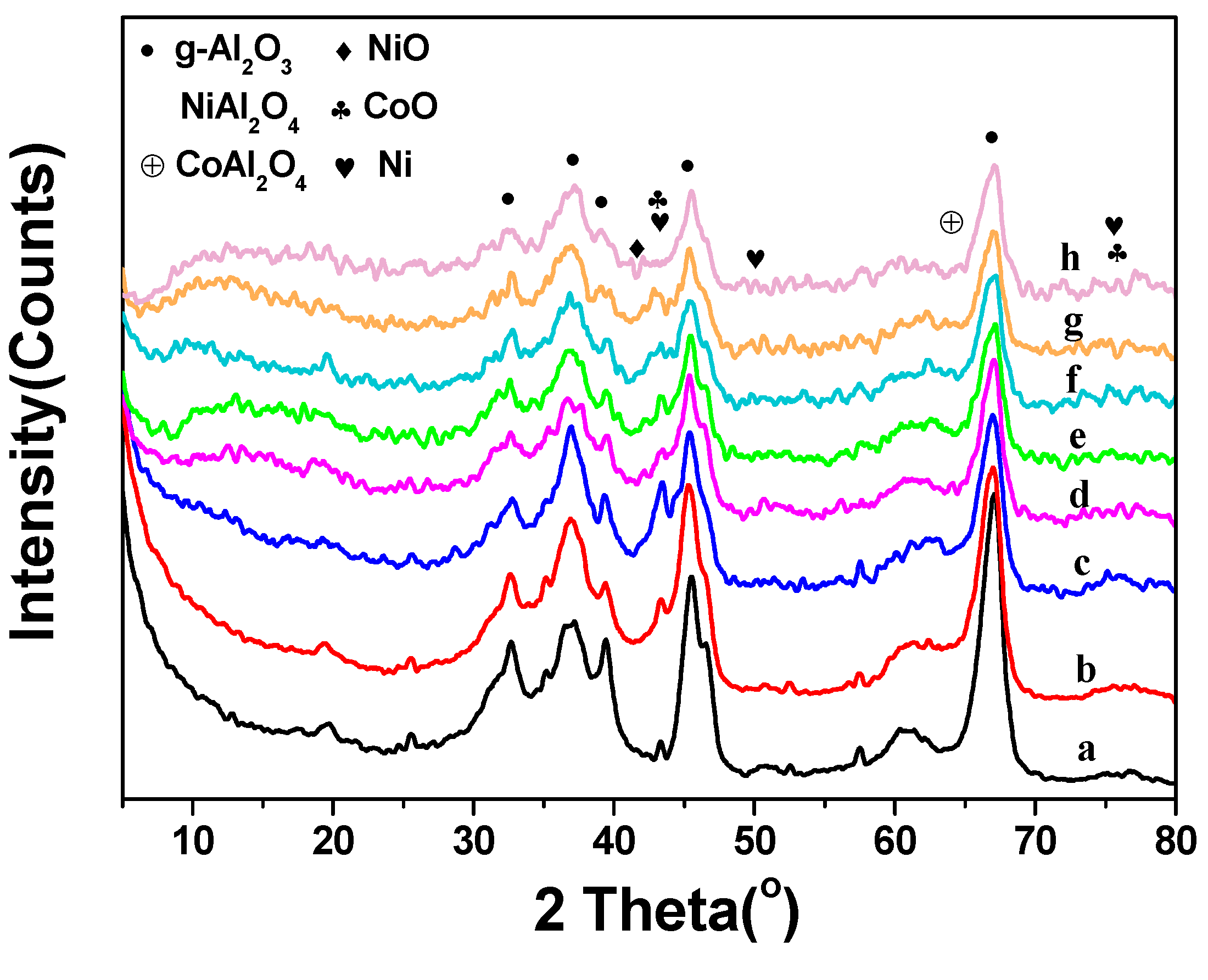
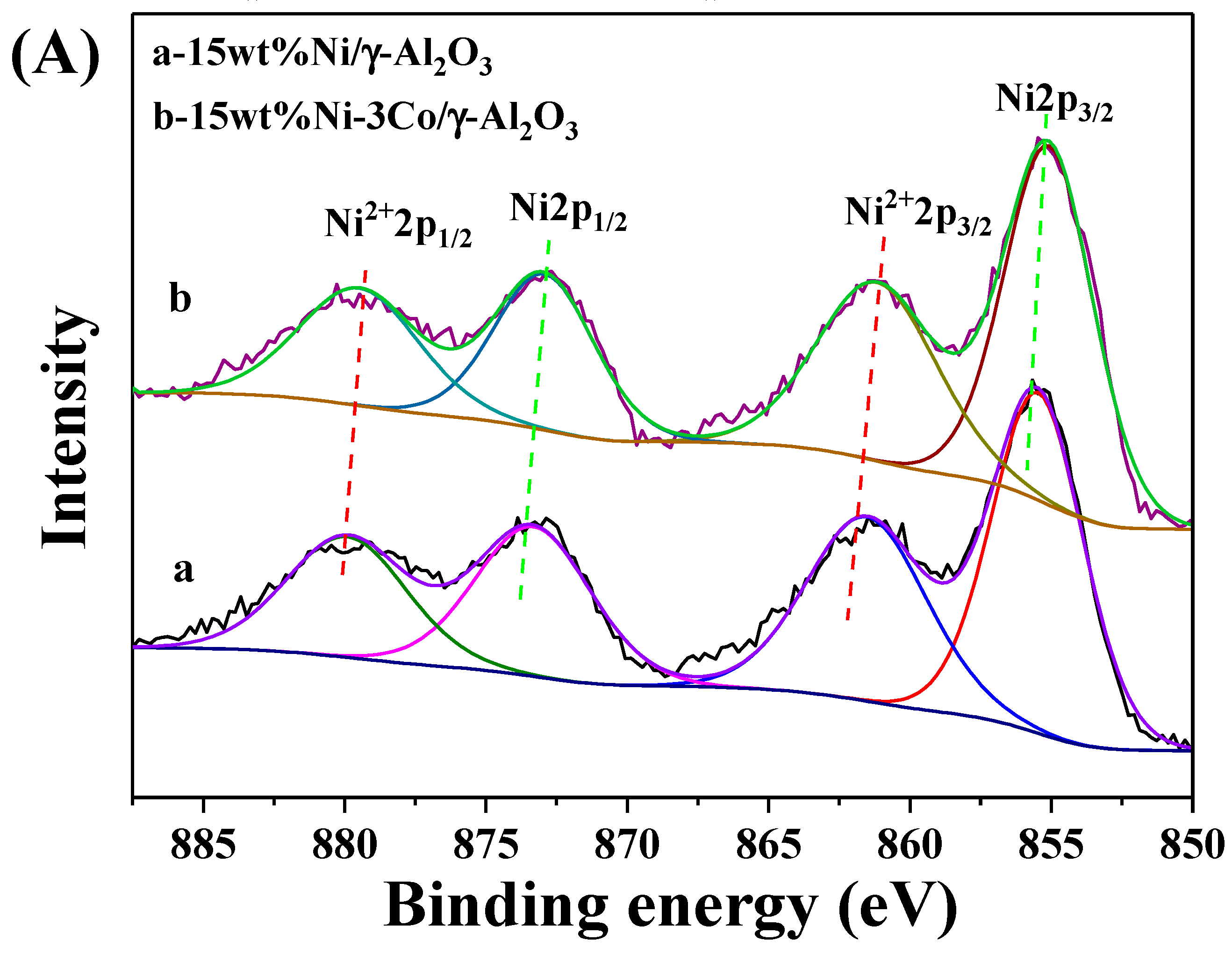
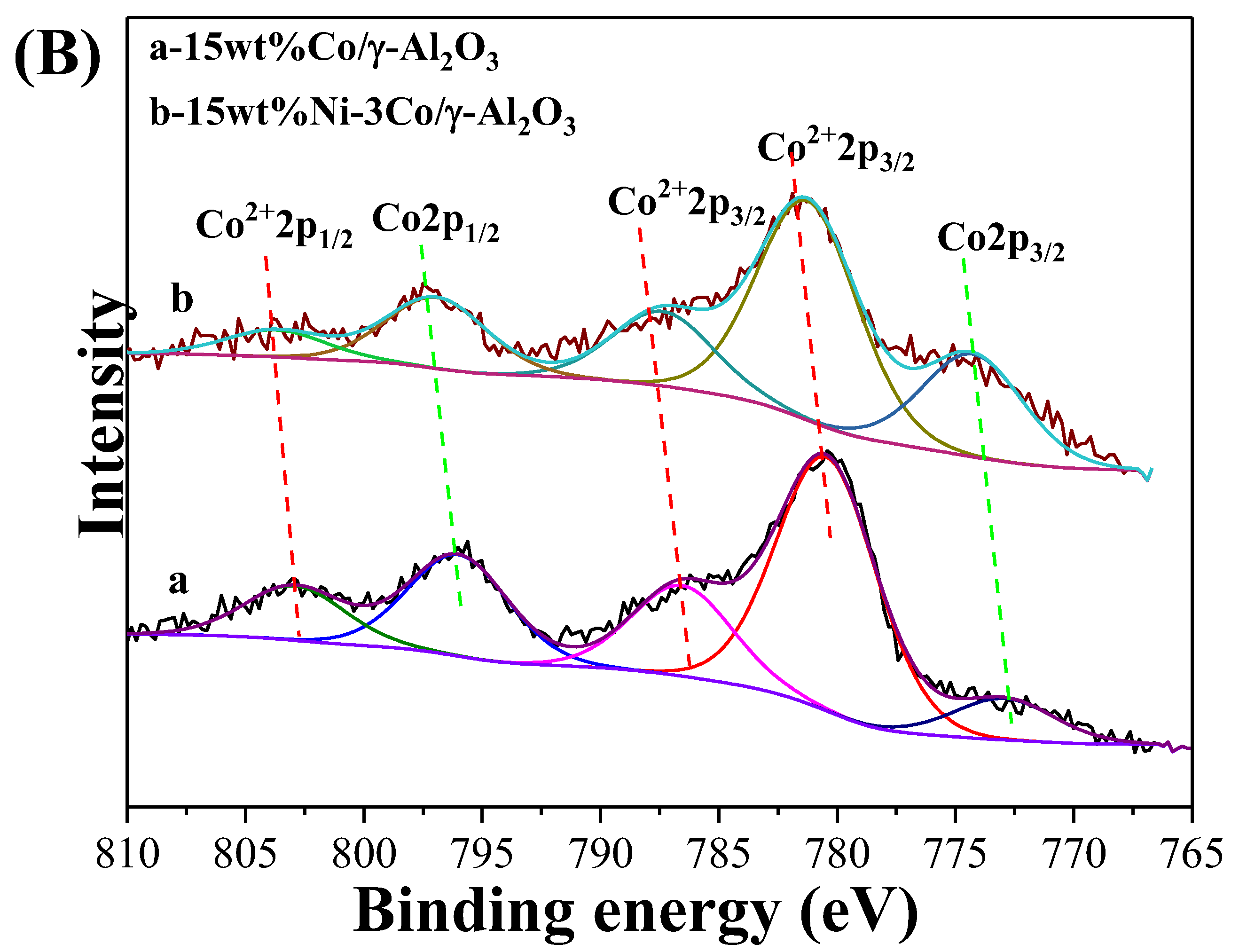
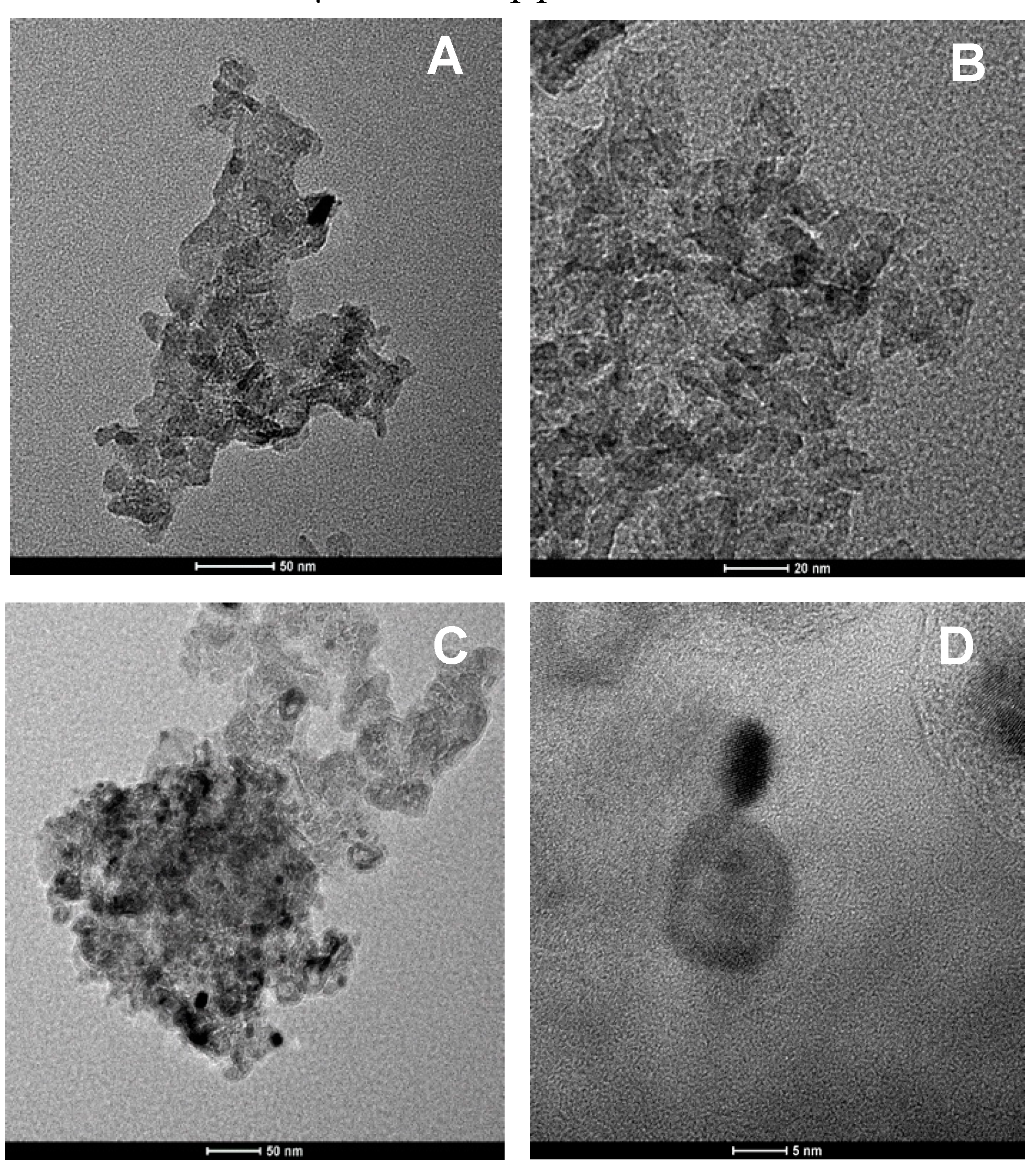
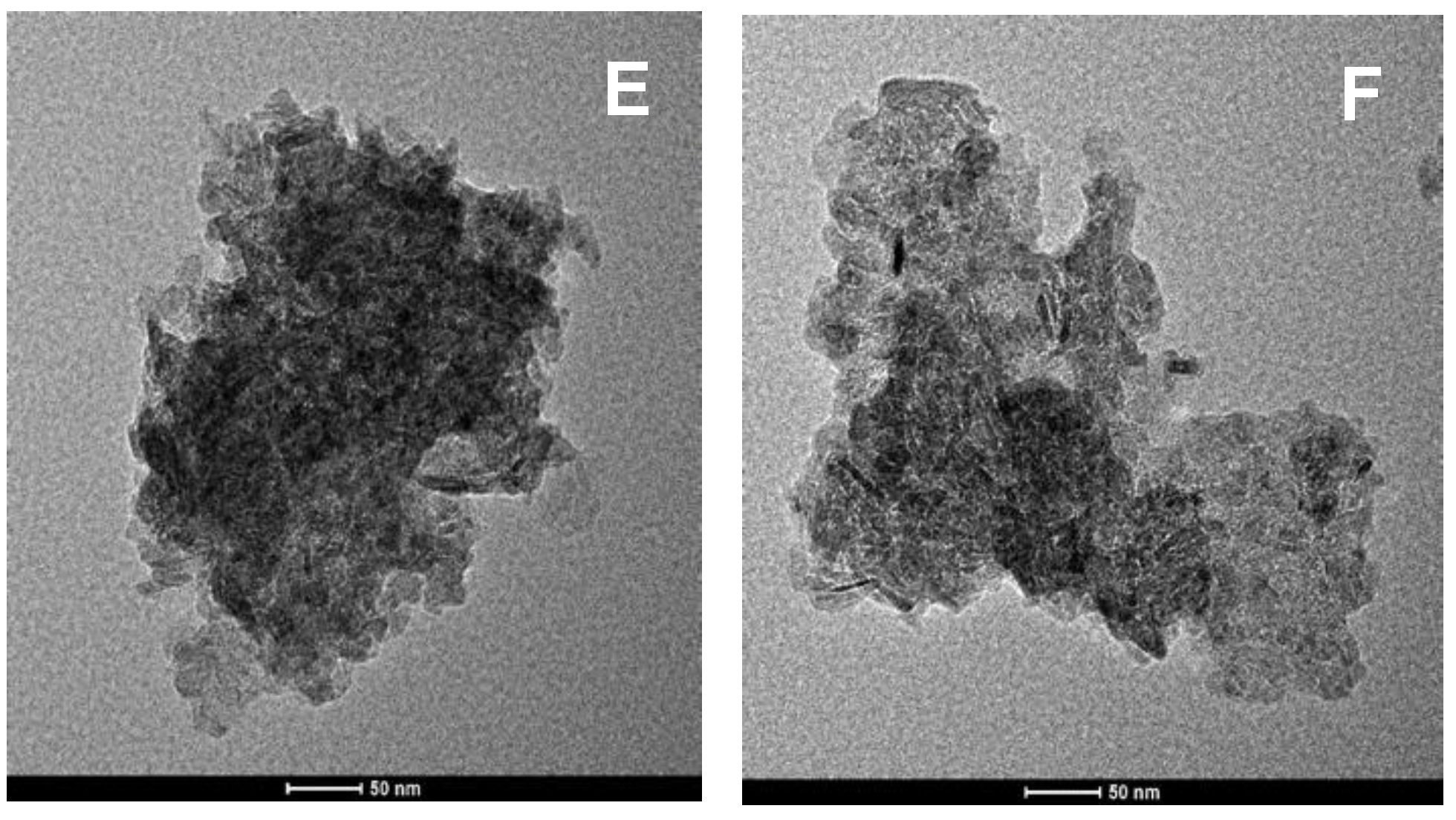
2.1. Catalyst Characterization
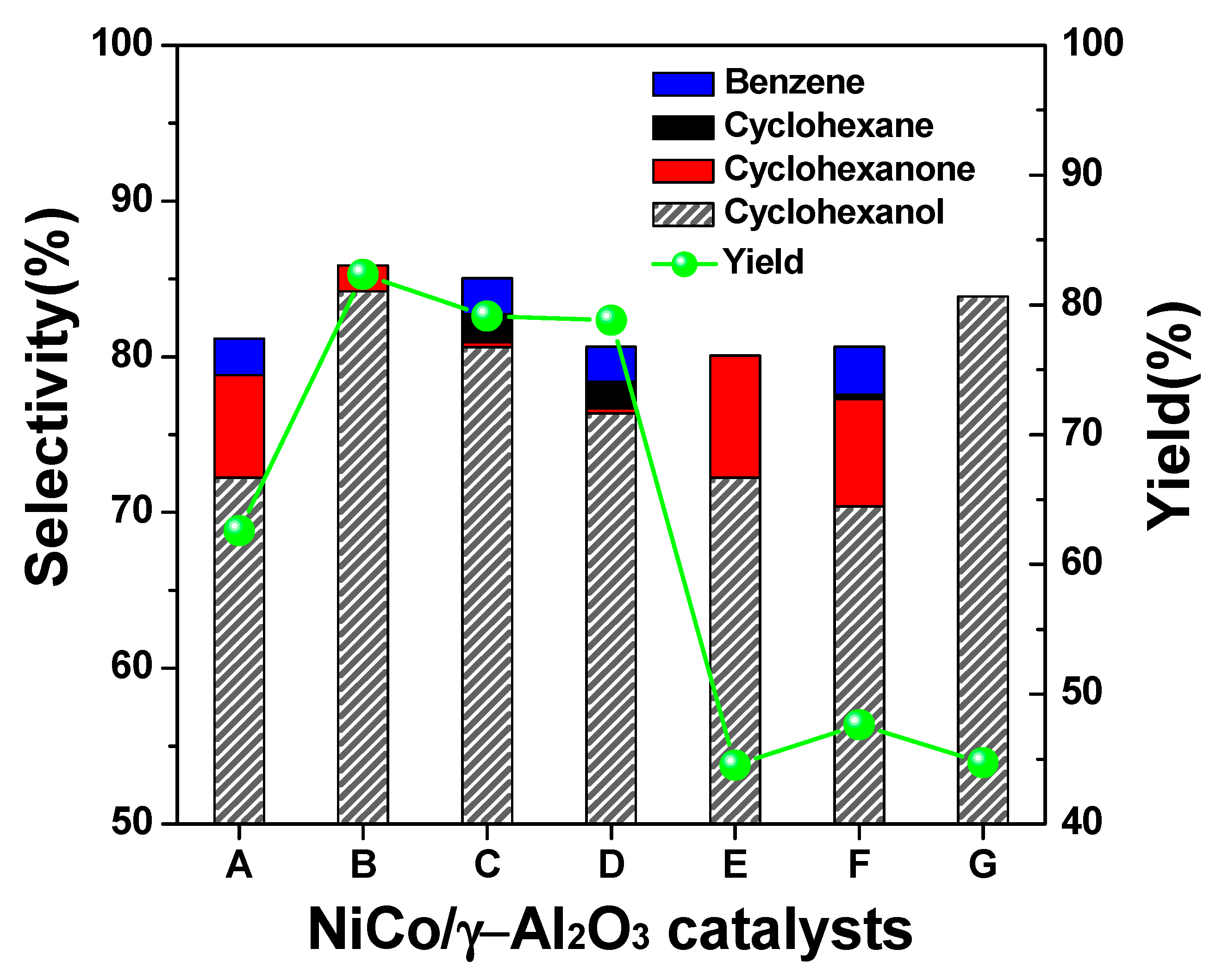
2.2. Selection of Catalyst for the Conversion of Phenol
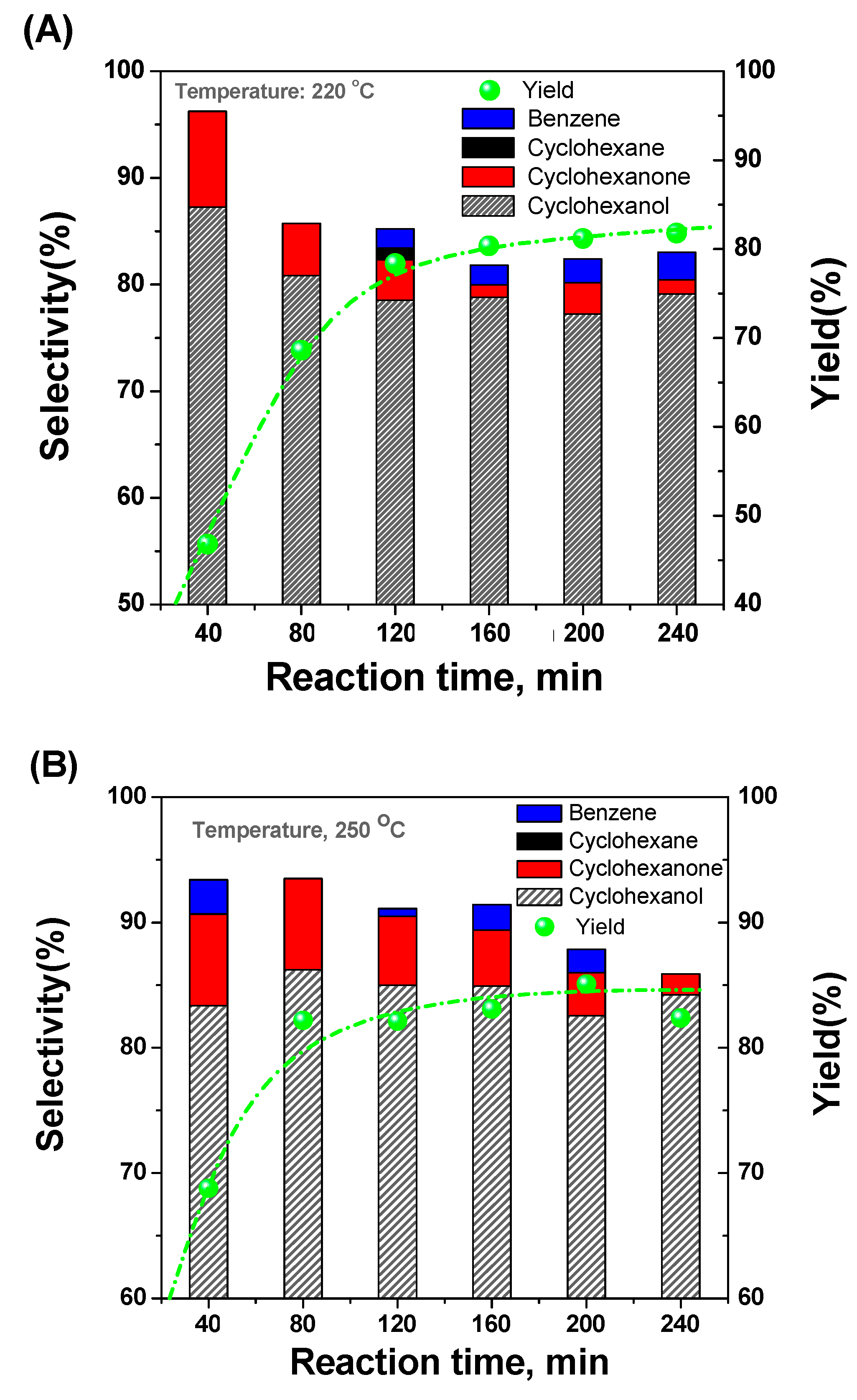
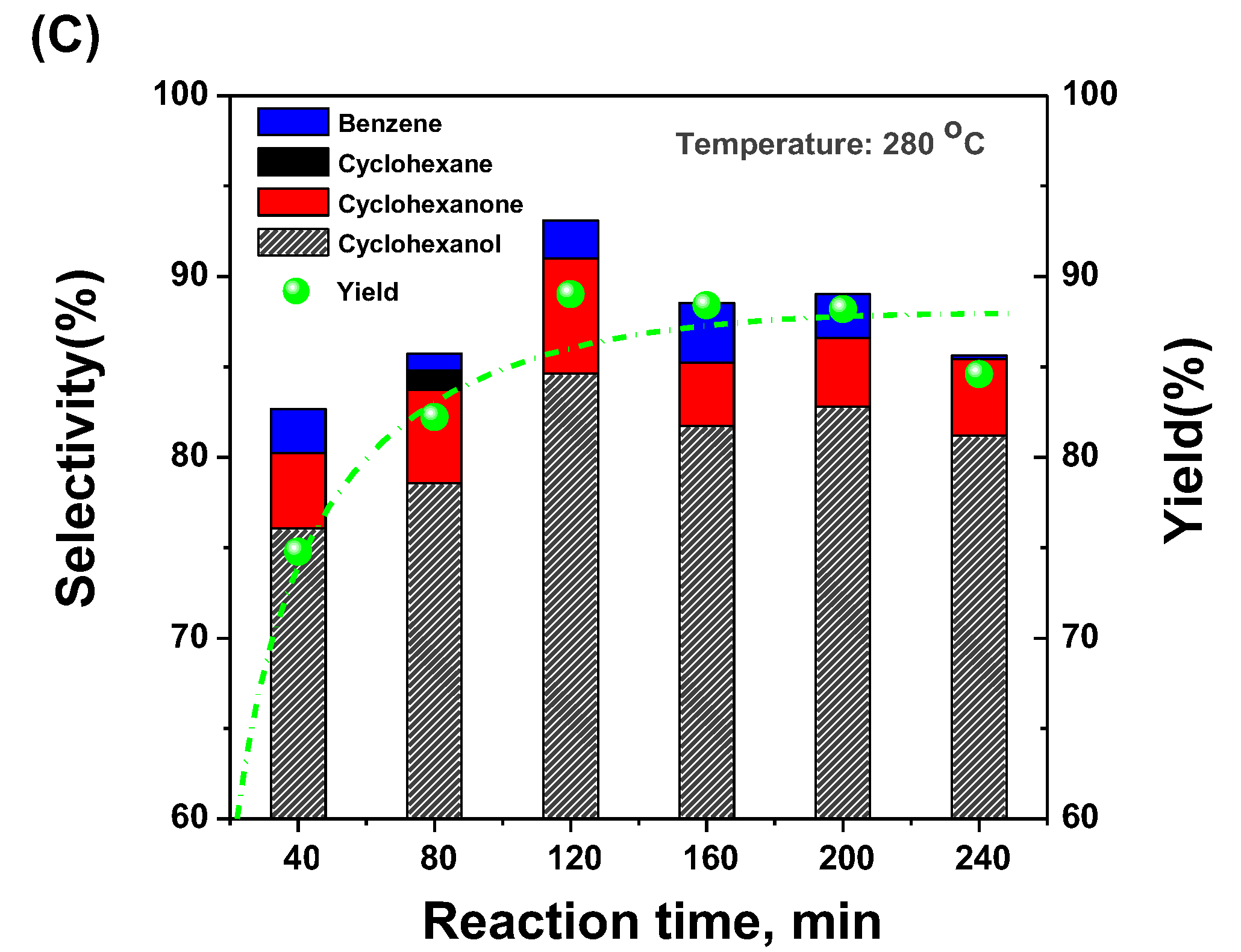
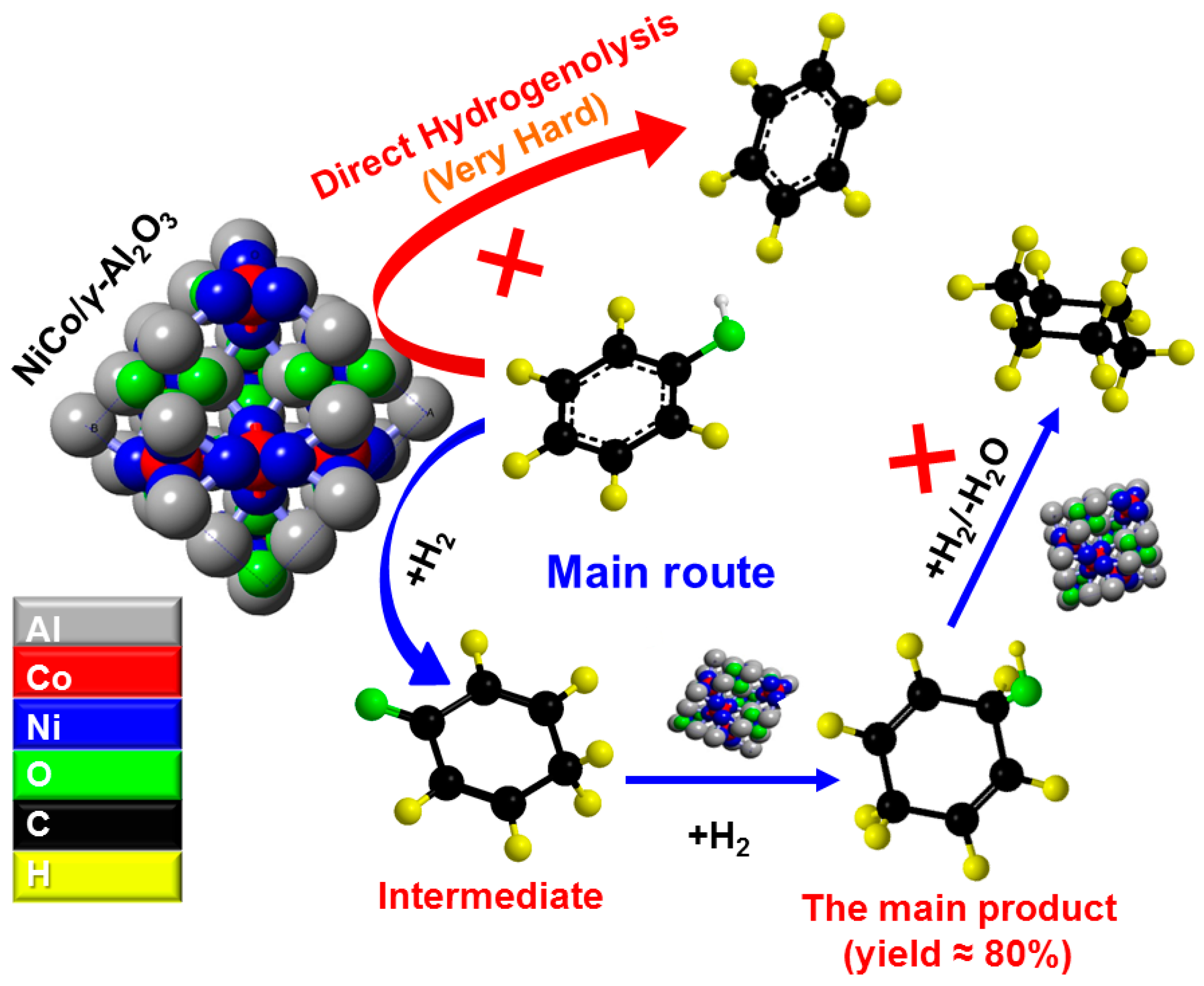
2.3. Selective Conversion Kinetic Behaviors of Phenol
2.4. Developing a Kinetic Model for the Conversion of Phenol
2.4.1. Model Development
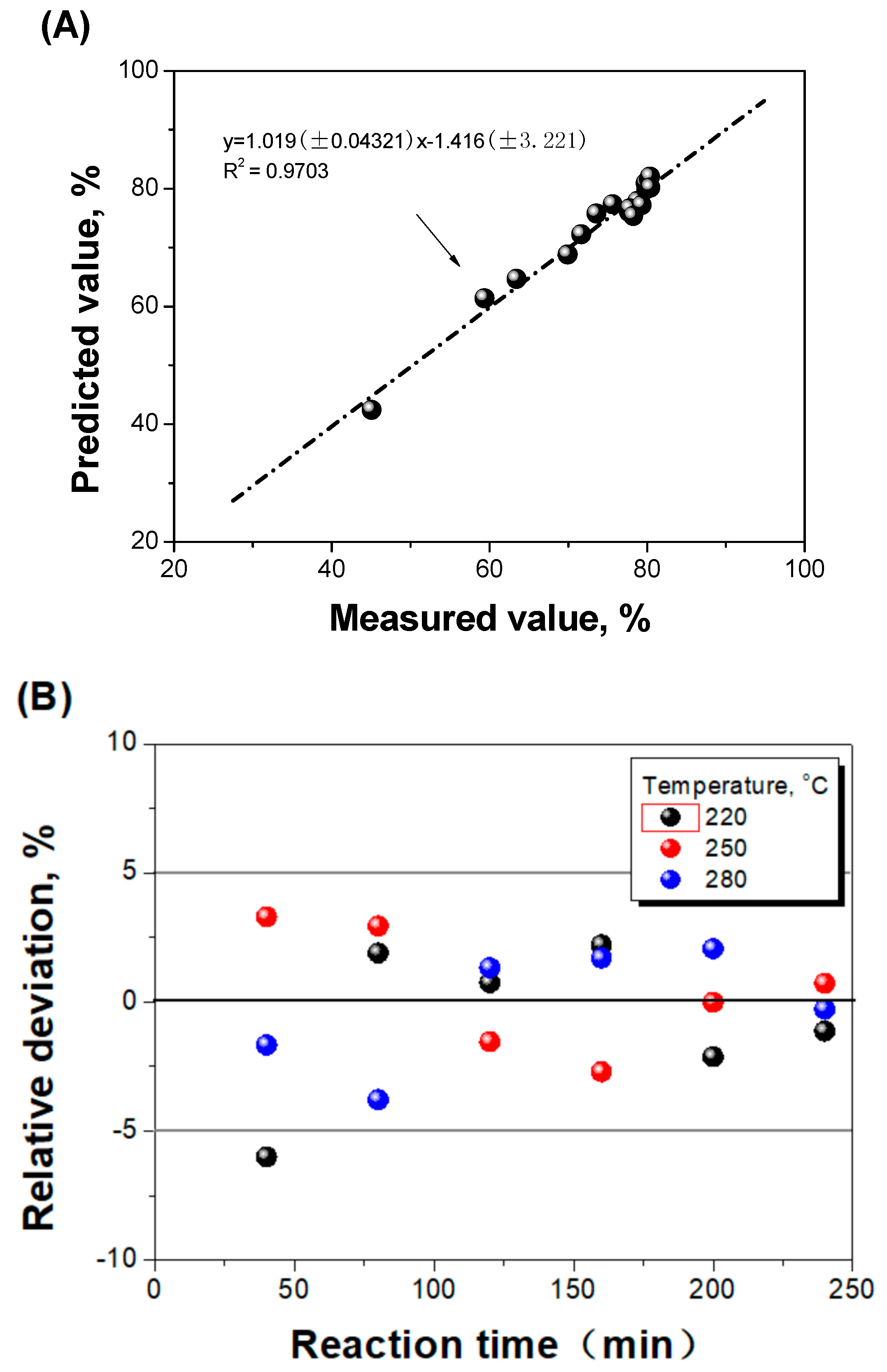
2.4.2. Parameters Determination and Model Evaluation
3. Experiments
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Phenol Hydrogenation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costa, S.C.S.; Diniz, A.S.A.C.; Kazmerski, L.L. Solar energy dust and soiling R&D progress: Literature review update for 2016. Renew. Sustain. Energy Rev. 2018, 82, 2504–2536. [Google Scholar]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Biofuels–Renewable Energy Sources: A Review. J. Dispers. Sci. Technol. 2010, 31, 409–425. [Google Scholar] [CrossRef]
- Ran, G.; Li, D.; Zheng, T.; Liu, X.; Chen, L.; Cao, Q.; Yan, Z. Hydrothermal pretreatment on the anaerobic digestion of washed vinegar residue. Bioresour. Technol. 2018, 248, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub—And supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Catalytic hydrotreatment of crude algal bio-oil in supercritical water. Appl. Catal. B 2011, 104, 136–143. [Google Scholar] [CrossRef]
- Ralph, J.; Mackay, J.J.; Hatfield, R.D.; O’Malley, D.M.; Whetten, R.W.; Sederoff, R.R. Abnormal lignin in a loblolly pine mutant. Science 1997, 277, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.; Ana, G.; Lidia, N.; Jiménez-Barbero, J.; Faulds, C.B.; Hoon, K.; John, R.; Martínez, A.T.; Del Río, J.C. Lignin composition and structure in young versus adult Eucalyptus globulus plants. Plant Physiol. 2011, 155, 667–682. [Google Scholar]
- Zhao, C.; He, J.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J. Catal. 2011, 280, 8–16. [Google Scholar] [CrossRef]
- Ning, Y.; Yuan, Y.; Ryan, D.; Yuan, K.; Dyson, P.J. Hydrodeoxygenation of lignin-derived phenols into alkanes by using nanoparticle catalysts combined with Bronsted acidic ionic liquids. Angew. Chem. 2010, 49, 5549–5553. [Google Scholar]
- Guan, Q.; Zeng, Y.; Shen, J.; Chai, X.S.; Gu, J.; Miao, R.; Li, B.; Ping, N. Selective hydrogenation of phenol by phosphotungstic acid modified Pd/Ce-AlO x catalyst in high-temperature water system. Chem. Eng. J. 2016, 299, 63–73. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Chen, B.; Wang, W. Synergetic catalysis of NiOx with oxygen vacancies and Ni2P for highly efficient hydrodeoxygenation of phenolic compounds. Chemcatchem 2018, 10, 2612–2619. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Ning, Y. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Pohl, M.M.; Schneider, M.; Radnik, J.; Hoang, D.L.; Phan, B.M.Q.; Nguyen, D.A.; Martin, A. Hydrodeoxygenation of Phenol as a Model Compound for Bio-oil on Non-noble Bimetallic Nickel-based Catalysts. Chemcatchem 2014, 6, 1940–1951. [Google Scholar] [CrossRef]
- Guo, Q.; Man, W.; Kai, W.; Liang, Z.; Xu, X. Catalytic Hydrodeoxygenation of Algae Bio-oil over Bimetallic Ni-Cu/ZrO 2 Catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Yang, G.; Wang, S.; Yeh, T.; Savage, P.E. Catalytic gasification of indole in supercritical water. Appl. Catal. B Environ. 2015, 166–167, 202–210. [Google Scholar]
- Sanjay Kumar, S.; Ashish Kumar, S.; Kengo, A.; Qiang, X. Noble-metal-free bimetallic nanoparticle-catalyzed selective hydrogen generation from hydrous hydrazine for chemical hydrogen storage. J. Am. Chem. Soc. 2011, 133, 19638–19641. [Google Scholar]
- Felix, S.; Frank, A.P.; Thomas, B.; S?Rensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 2008, 320, 1320–1322. [Google Scholar]
- Sabo, M.; Henschel, A.; Frode, H.; Klemm, E.; Kaskel, S. Solution infiltration of palladium into MOF-5: Synthesis, physisorption and catalytic properties. J. Mater. Chem. 2007, 17, 3827–3832. [Google Scholar] [CrossRef]
- Guan, Q.; Huang, X.; Jing, L.; Gu, J.; Miao, R.; Chen, Q.; Ping, N. Supercritical water gasification of phenol using a Ru/CeO2 catalyst. Chem. Eng. J. 2016, 283, 358–365. [Google Scholar] [CrossRef]
- Yong, W.; Jia, Y.; Haoran, L.; Dangsheng, S.; Markus, A. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. Cheminform 2011, 42, 2362–2365. [Google Scholar]
- Díaz, E.; Mohedano, A.F.; Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Rodríguez, J.J. Hydrogenation of phenol in aqueous phase with palladium on activated carbon catalysts. Chem. Eng. J. 2007, 131, 65–71. [Google Scholar] [CrossRef]
- Pérez, Y.; Fajardo, M.; Corma, A. Highly selective palladium supported catalyst for hydrogenation of phenol in aqueous phase. Catal. Commun. 2011, 12, 1071–1074. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Wang, Y.; Xiao, G. Catalytic conversion of guaiacol to alcohols for bio-oil upgrading. J. Energy Chem. 2015, 24, 425–431. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Effect of alkali additives over nanocrystalline Co-Cu-based perovskites as catalysts for higher-alcohol synthesis. J. Catal. 2007, 245, 348–357. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2016, 281, 250–258. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Zhang, F.; Ning, W.; Lu, Y.; Mao, L.; Huang, L. Ni-Co bimetallic MgO-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil. Int. J. Hydrogen Energy 2014, 39, 18688–18694. [Google Scholar] [CrossRef]
- Zhou, M.; Ye, J.; Peng, L.; Xu, J.; Jiang, J.; Zhou, M.; Ye, J.; Peng, L.; Xu, J.; Jiang, J. Water-assisted selective hydrodeoxygenation of guaiacol to cyclohexanol over supported Ni and Co bimetallic catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8824–8835. [Google Scholar] [CrossRef]
- Jia, L.; Yu, J.; Yuan, C.; Ping, N.; Guan, Q.; Gu, J.; Miao, R.; Chen, Q. Noble-metal-free bimetallic alloy nanoparticle-catalytic gasification of phenol in supercritical water. J. Supercrit. Fluids 2017, 126, 79–88. [Google Scholar] [CrossRef]
- Guan, Q.; Chen, S.; Chen, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. High performance noble-metal-free NiCo/AC bimetal for gasification in supercritical water. Int. J. Hydrogen Energy 2017, 42, 6511–6518. [Google Scholar] [CrossRef]
- Kordouli, E.; Kordulis, C.; Lycourghiotis, A.; Cole, R.; Vasudevan, P.T.; Pawelec, B.; Fierro, J.L.G. HDO activity of carbon-supported Rh, Ni and Mo-Ni catalysts. Mol. Catal. 2017, 441, 209–220. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst. China Basic Sci. 2010, 326, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wang, B.; Chai, X.; Liu, J.; Gu, J.; Ning, P. Comparison of Pd-UiO-66 and Pd-UiO-66-NH2 catalysts performance for phenol hydrogenation in aqueous medium. Fuel 2017, 205, 130–141. [Google Scholar] [CrossRef]
| Sample | SBET (m2.g−1) a | VP (cm3.g−1) b | DP (nm) c |
|---|---|---|---|
| γ-Al2O3 | 122.75 | 0.6627 | 22.90 |
| Ni/γ-Al2O3 | 109.50 | 0.5237 | 17.24 |
| Ni–3Co/γ-Al2O3 | 102.48 | 0.4496 | 13.76 |
| Ni–1.5Co/γ-Al2O3 | 113.18 | 0.4634 | 13.89 |
| Ni–1Co/γ-Al2O3 | 107.70 | 0.4922 | 15.39 |
| Ni–0.67Co/γ-Al2O3 | 105.30 | 0.5026 | 16.72 |
| Ni–0.33Co/γ-Al2O3 | 101.06 | 0.5149 | 17.52 |
| Co/γ-Al2O3 | 106.42 | 0.5061 | 16.28 |
| Parameter | Cp0 (mol/L) | k’ | m | t0 (min) | Ea (kJ/mol) |
|---|---|---|---|---|---|
| Value | 0.0864 | 317.13 | 1.15 | 0 | 37.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Chen, S.; He, L.; Ning, P.; Guan, Q. Selective Conversion of Phenol in a Subcritical Water Medium Using γ-Al2O3 Supported Ni–Co Bimetallic Catalyst. Catalysts 2019, 9, 212. https://doi.org/10.3390/catal9030212
Shi Y, Chen S, He L, Ning P, Guan Q. Selective Conversion of Phenol in a Subcritical Water Medium Using γ-Al2O3 Supported Ni–Co Bimetallic Catalyst. Catalysts. 2019; 9(3):212. https://doi.org/10.3390/catal9030212
Chicago/Turabian StyleShi, Yuzhen, Shanshuai Chen, Liang He, Ping Ning, and Qingqing Guan. 2019. "Selective Conversion of Phenol in a Subcritical Water Medium Using γ-Al2O3 Supported Ni–Co Bimetallic Catalyst" Catalysts 9, no. 3: 212. https://doi.org/10.3390/catal9030212
APA StyleShi, Y., Chen, S., He, L., Ning, P., & Guan, Q. (2019). Selective Conversion of Phenol in a Subcritical Water Medium Using γ-Al2O3 Supported Ni–Co Bimetallic Catalyst. Catalysts, 9(3), 212. https://doi.org/10.3390/catal9030212