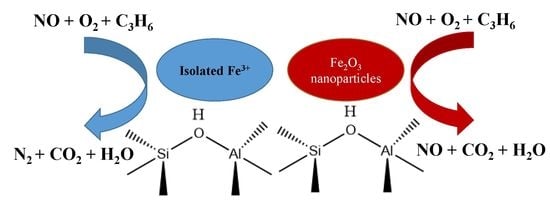
Selective Catalytic Reduction of Nitric Oxide with Propylene over Fe/Beta Catalysts Under Lean-Burn Conditions
Abstract
1. Introduction
2. Results
2.1. Physical Properties
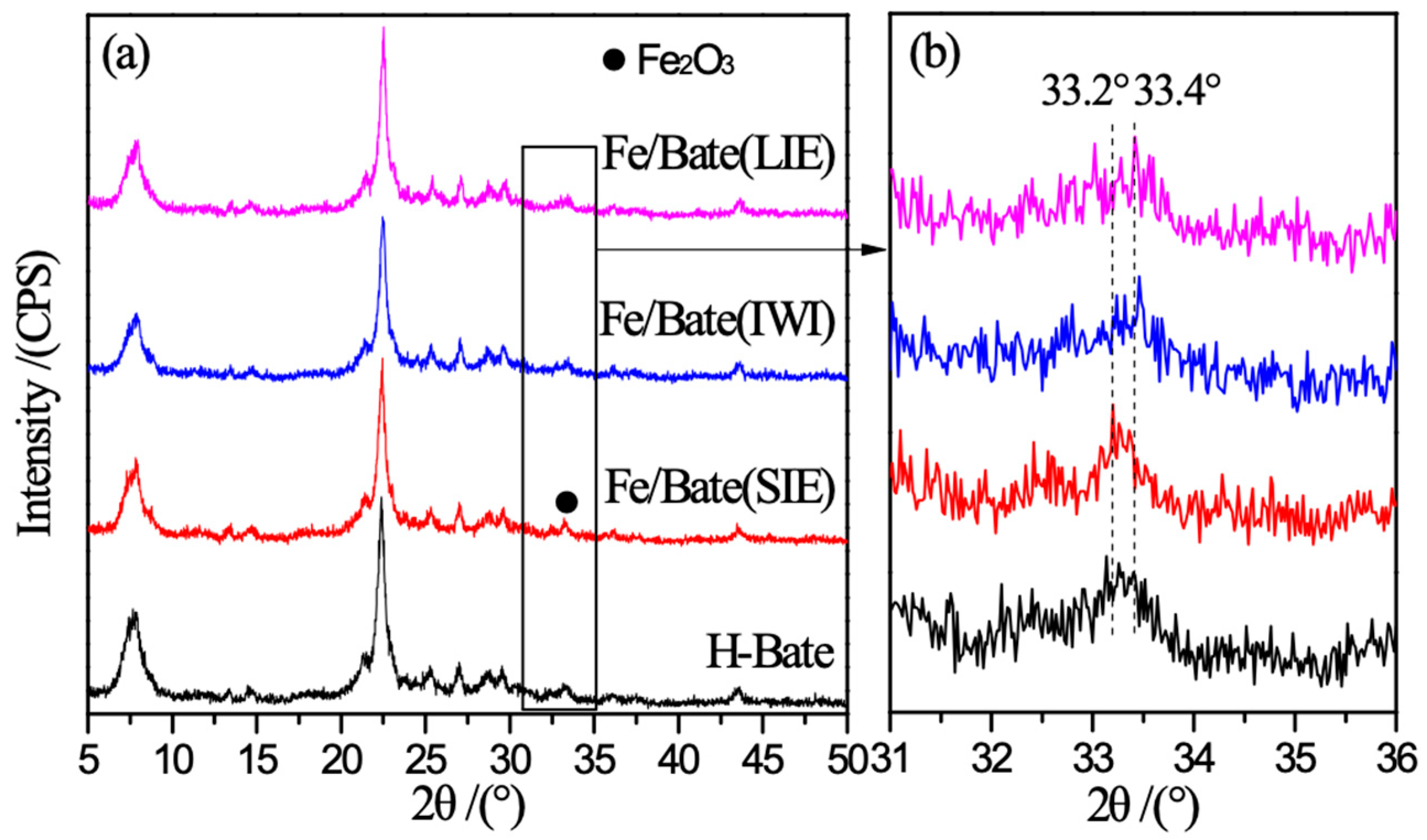
2.2. Structural Characteristics
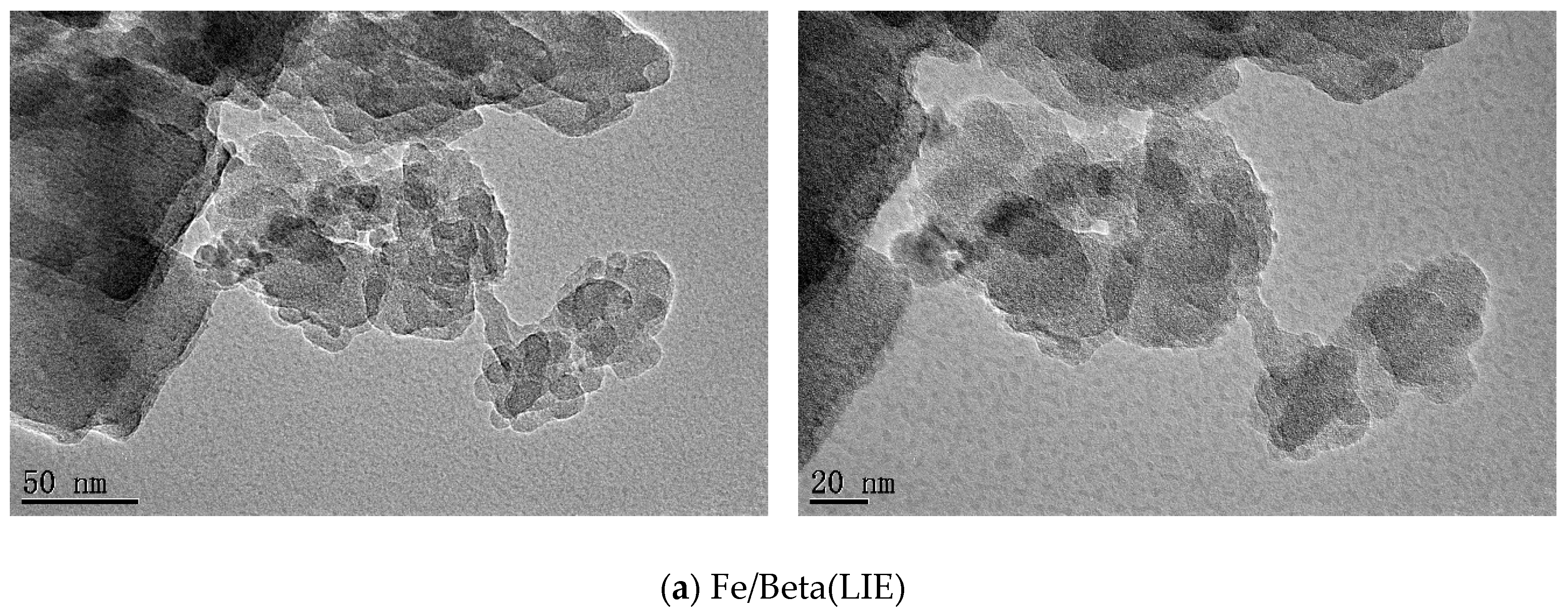
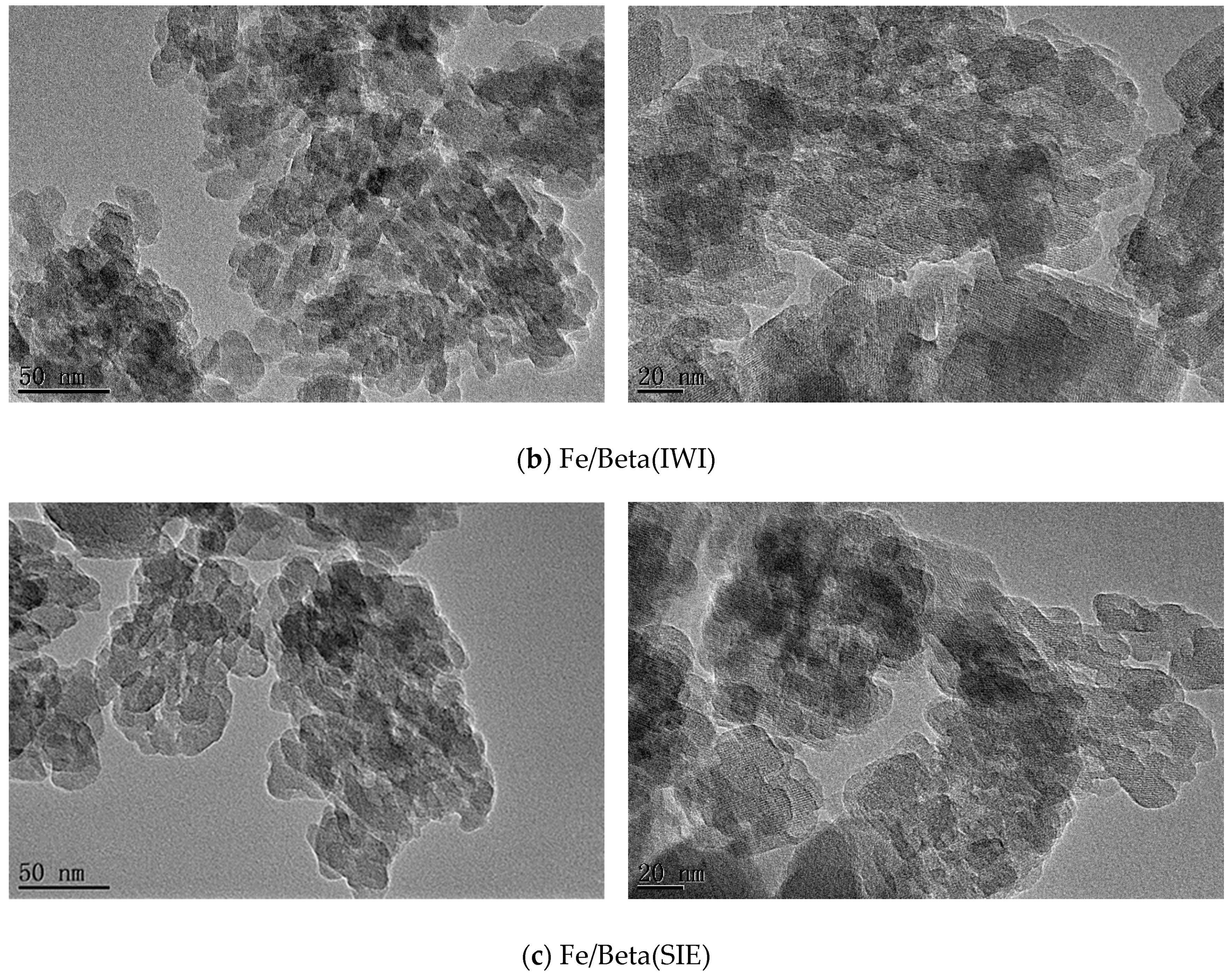
2.3. Morphologies
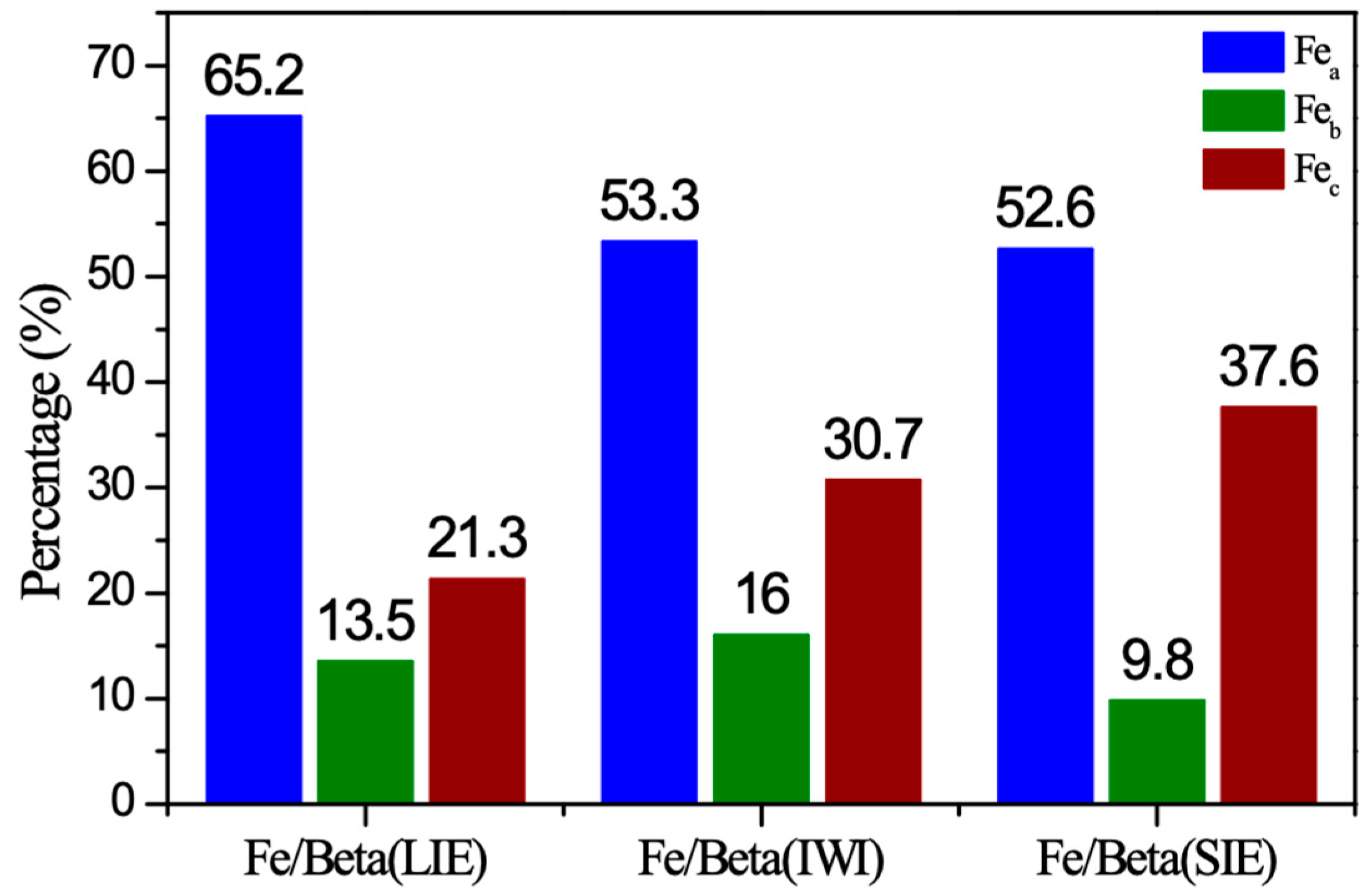
2.4. XPS
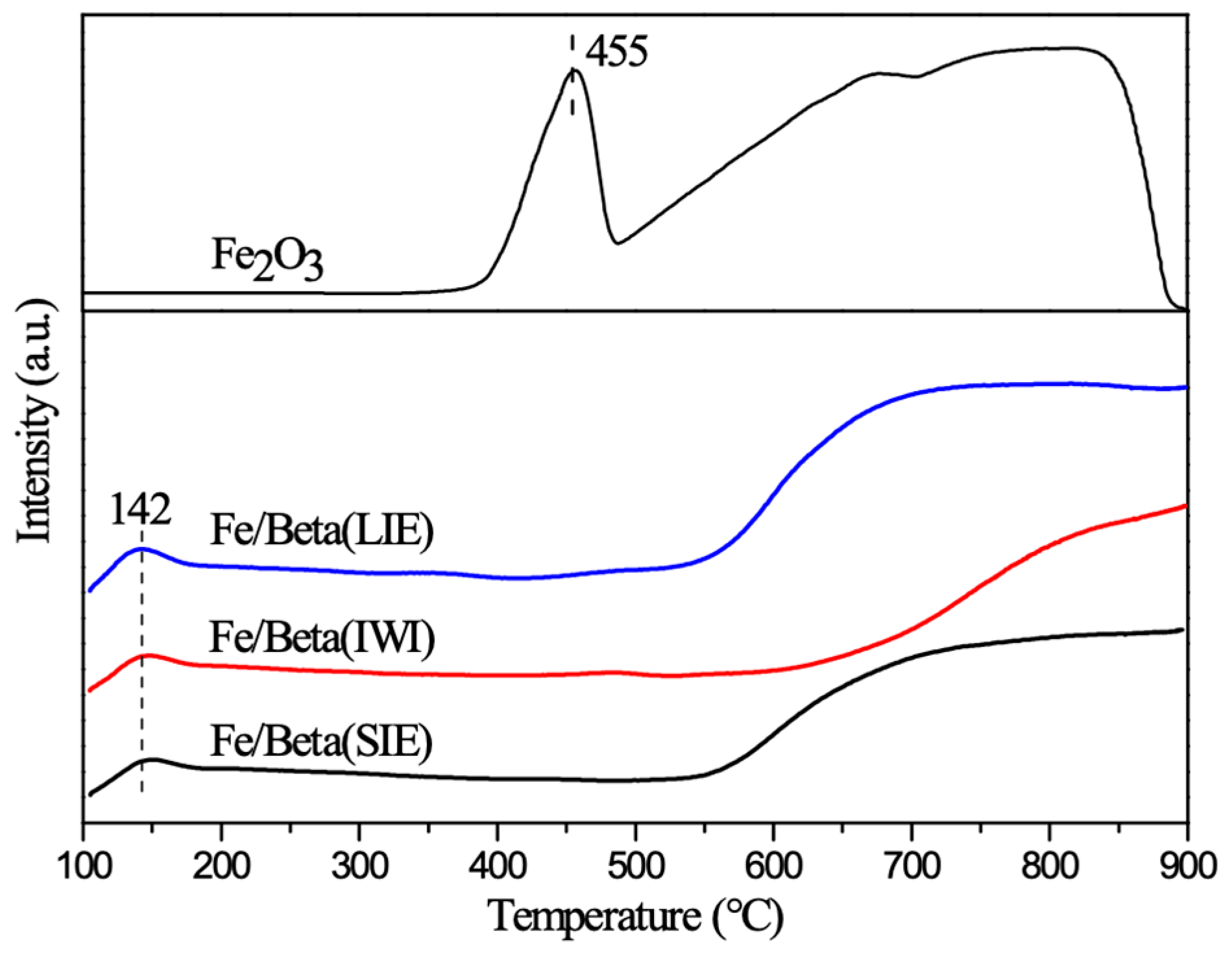
2.5. H2-TPR
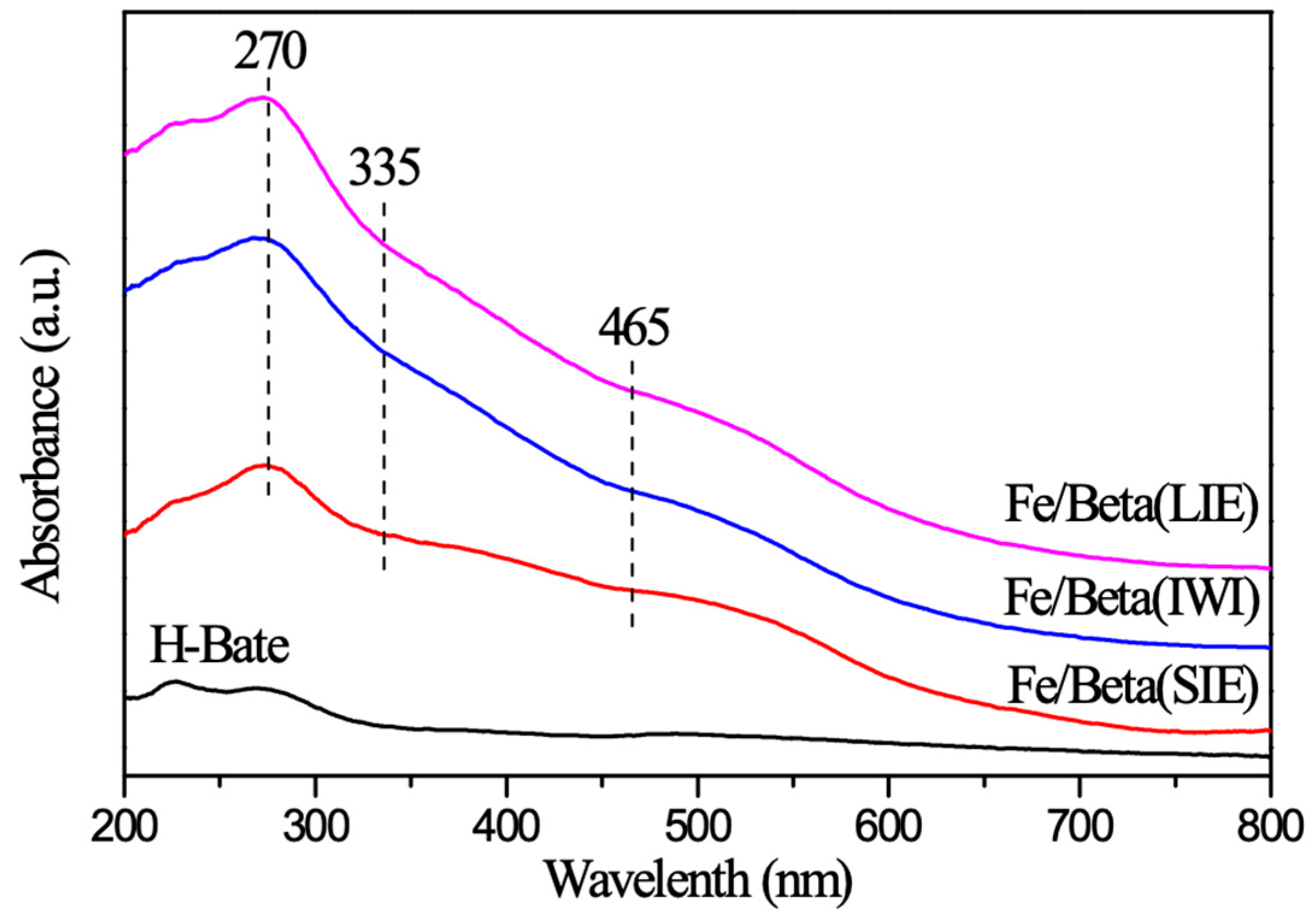
2.6. UV-Vis
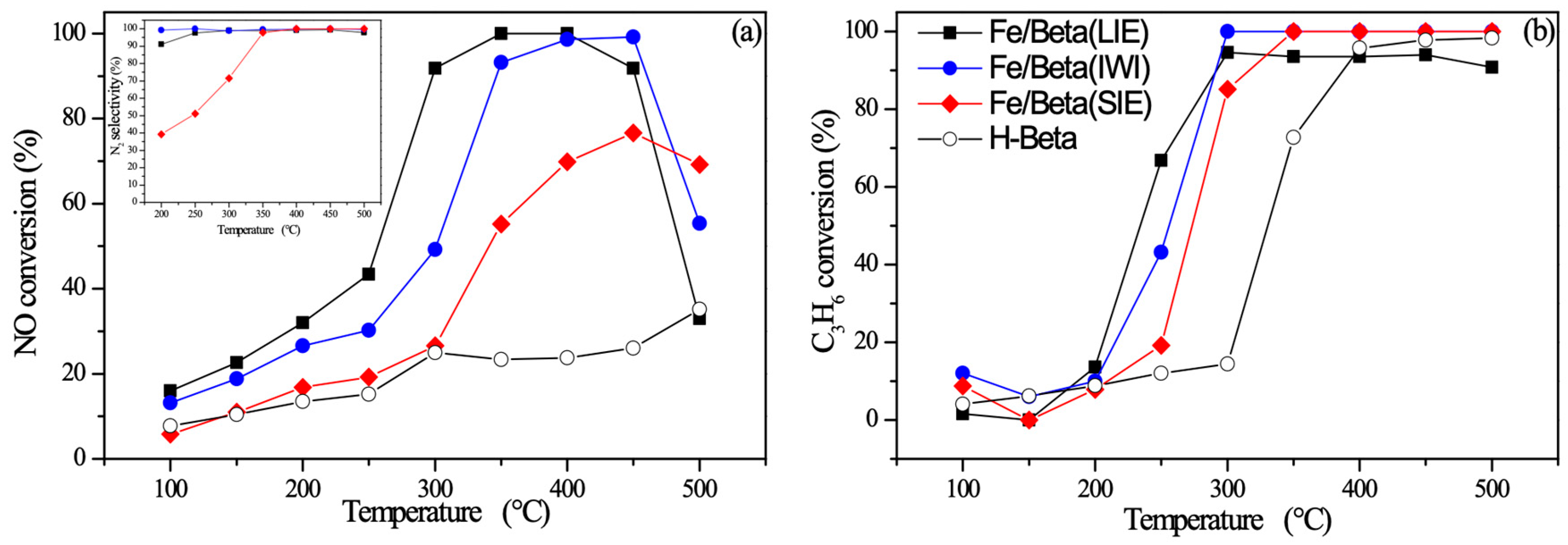
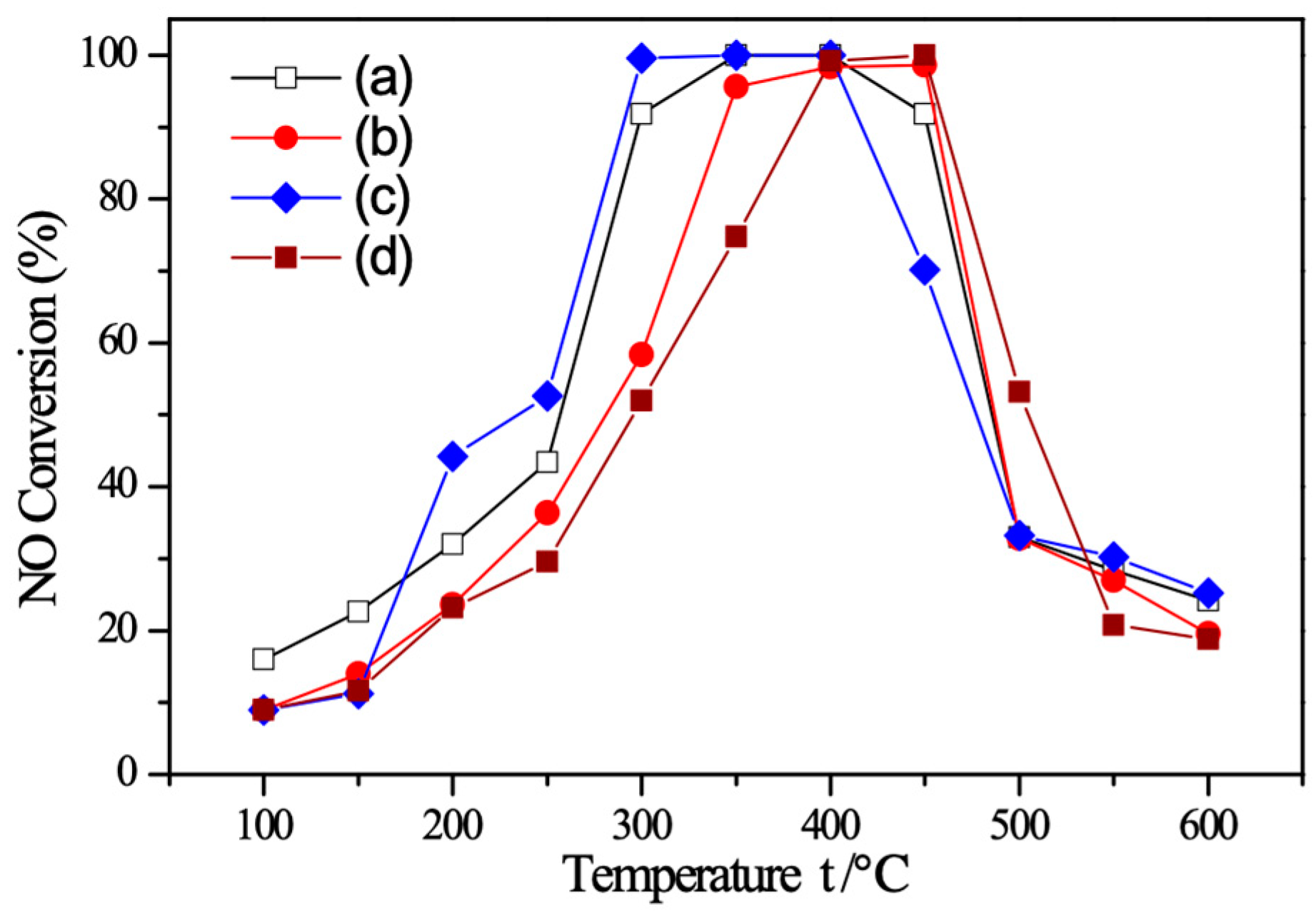
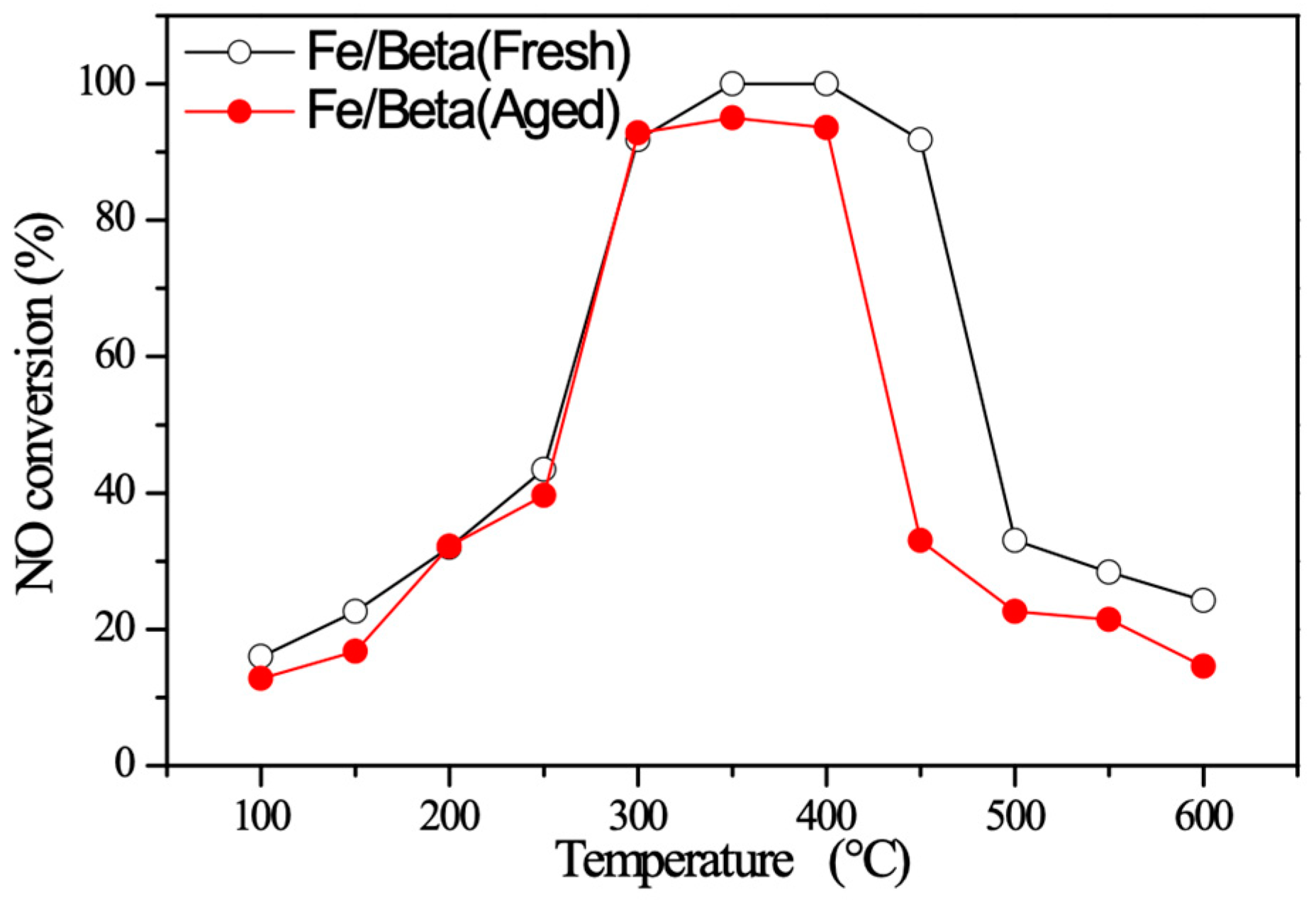
2.7. C3H6-SCR Performance
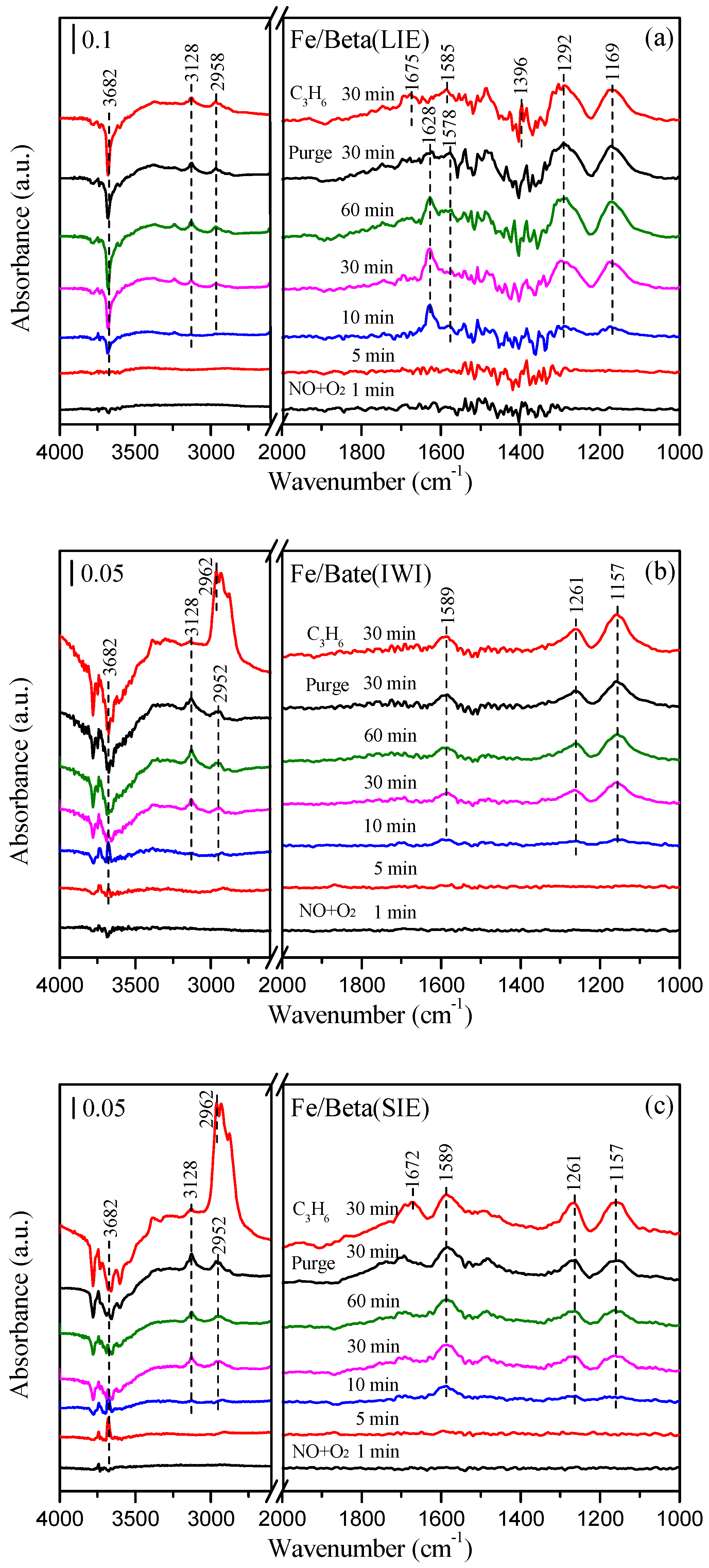
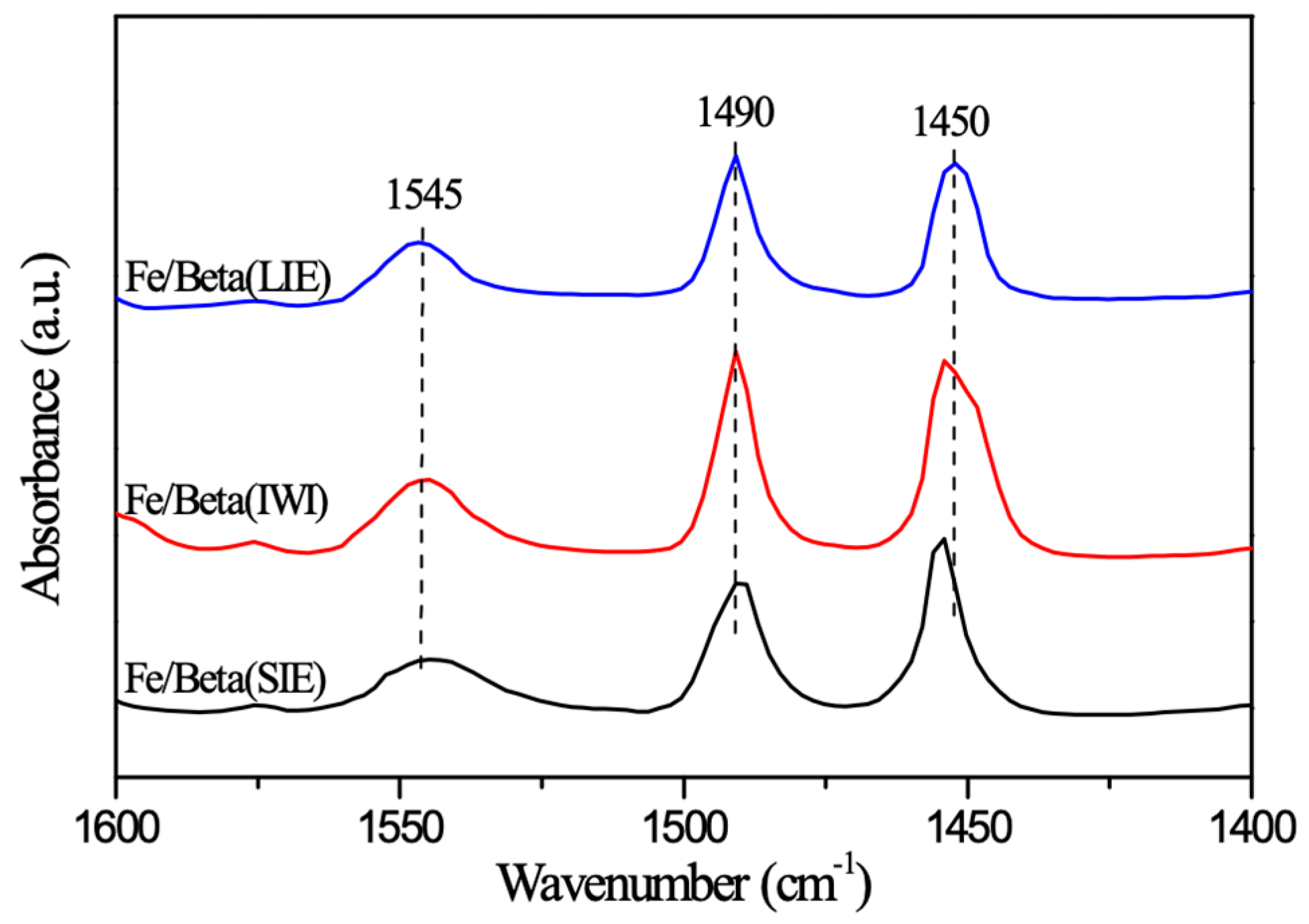
2.8. In Situ DRIFTS
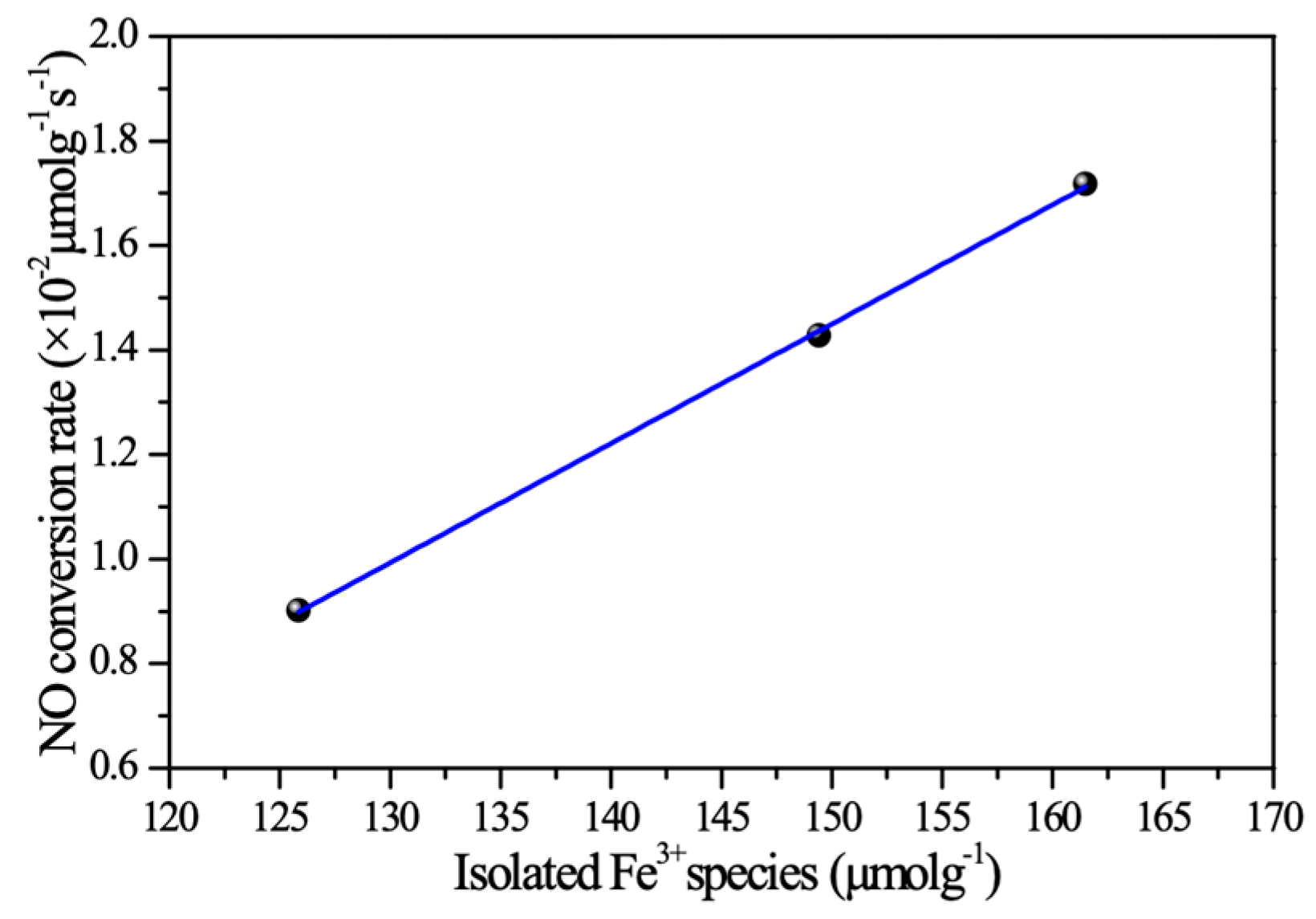
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalysts Characterizations
4.3. Catalytic Activity Tests
4.4. In Situ DRIFTS Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Liu, Z.; Wang, Y.; Yin, Y.; Pan, J.; Zhang, J.; Wang, Q. Simultaneous absorption of SO2 and NO from flue gas using ultrasound/Fe2+/heat coactivated persulfate system. J. Hazard. Mater. 2017, 342, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ge, M.; Wu, S.; Ye, B.; Su, Y. Iron based monolithic catalysts supported on Al2O3, SiO2, and TiO2: A comparison for NO reduction with propane. Fuel 2018, 220, 330–338. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Miridonov, S.; Chávez-Rivas, F.; Fuentes, S.; Petranovskii, V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts 2019, 9, 58. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sachtler, W.M.H. Activity and durability of Fe/ZSM-5 catalysts for lean burn NOx reduction in the presence of water vapor. Catal. Today 1998, 42, 73–83. [Google Scholar] [CrossRef]
- Janas, J.; Rojek, W.; Shishido, T.; Dzwigaj, S. Selective catalytic reduction of NO on single site FeSiBEA zeolite catalyst: Influence of the C1 and C2 reducing agents on the catalytic properties. Appl. Catal. B 2012, 123, 134–140. [Google Scholar] [CrossRef]
- Pan, H.; Guo, Y.; Bi, H.T. NOx adsorption and reduction with C3H6 over Fe/zeolite catalysts: Effect of catalyst support. Chem. Eng. J. 2015, 280, 66–73. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Liu, Z.; Ihl Woo, S. Recent advances in catalytic DeNOx science and technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, L.; Yang, C.; Chen, Y.; Sun, Q.; Zhang, H.; Li, C.; Nawaz, F.; Meng, X.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, X.; Weng, D.; Si, Z.; Ran, R. Evolution of copper species on Cu/SAPO-34 SCR catalysts upon hydrothermal aging. Catal. Today 2017, 281, 596–604. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Metkar, P.S.; Salazar, N.; Muncrief, R.; Balakotaiah, V.; Harold, M.P. Selective catalytic reduction of NO with NH3 on iron zeolite monolithic catalysts: Steady-state and transient kinetics. Appl. Catal. B 2011, 104, 110–126. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.; Cheng, Y.; Lambert, C.K.; Yang, R.T. Activity, stability and hydrocarbon deactivation of Fe/Beta catalyst for SCR of NO with ammonia. Appl. Catal. A 2009, 368, 121–126. [Google Scholar] [CrossRef]
- Fierro, G.; Moretti, G.; Ferraris, G.; Andreozzi, G.B. A Mössbauer and structural investigation of Fe-ZSM-5 catalysts: Influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8. Appl. Catal. B 2011, 102, 215–223. [Google Scholar] [CrossRef]
- Yuan, E.; Wu, G.; Dai, W.; Guan, N.; Li, L. One-pot construction of Fe/ZSM-5 zeolites for the selective catalytic reduction of nitrogen oxides by ammonia. Catal. Sci. Technol. 2017, 7, 3036–3044. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yamazaki, K.; Banno, K.; Shinjoh, H. Characterization of Fe/ZSM-5 DeNOx catalysts prepared by different methods: Relationships between active Fe sites and NH3-SCR performance. J. Catal. 2008, 260, 205–216. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; An, D.; Wang, Y. Catalytic behavior and kinetic features of FeOx /SBA-15 catalyst for selective oxidation of methane by oxygen. Appl. Catal. A 2009, 356, 103–111. [Google Scholar] [CrossRef]
- Ma, L.; Chang, H.; Yang, S.; Chen, L.; Fu, L.; Li, J. Relations between iron sites and performance of Fe/HBEA catalysts prepared by two different methods for NH3-SCR. Chem. Eng. J. 2012, 209, 652–660. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Qu, Z.; Chen, Y.; Zhou, Q.; Jia, J. Nanosized Cation-Deficient Fe-Ti Spinel: A Novel Magnetic Sorbent for Elemental Mercury Capture from Flue Gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, G.; Jin, C.; Liu, X.; Wang, J. One-step synthesis of nanorod-aggregated functional hierarchical iron-containing MFI zeolite microspheres. J. Mater. Chem A 2015, 3, 14786–14793. [Google Scholar] [CrossRef]
- Devaiah, D.; Smirniotis, P.G. Effects of the Ce and Cr Contents in Fe−Ce−Cr Ferrite Spinels on the High-Temperature Water−Gas Shift Reaction. Ind. Eng. Chem. Res. 2017, 56, 1772–1781. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Smirniotis, P.G. High-temperature water-gas shift over Fe/Ce/Co spinel catalysts: Study of the promotional effect of Ce and Co. Mol. Catal. 2018, 451, 20–32. [Google Scholar] [CrossRef]
- Zhou, H.; Su, Y.; Liao, W.; Deng, W.; Zhong, F. Preparation, characterization, and properties of monolithic Fe/Al2O3/cordierite catalysts for NO reduction with C2H6. Appl. Catal. A 2015, 505, 402–409. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.; Xu, L.; Chang, F.; He, Y.; Meng, S.; Su, B.L.; Liu, Z. Synthesis, characterization and catalytic performance of metal-incorporated SAPO-34 for chloromethane transformation to light olefins. Catal. Today 2008, 131, 262–269. [Google Scholar] [CrossRef]
- Dědeček, J.; Čapek, L.; Kaucký, D.; SobalíK, Z.; Wichterlová, B. Siting and Distribution of the Co Ions in Beta Zeolite: A UV–Vis–NIR and FTIR Study. J. Catal. 2002, 211, 198–207. [Google Scholar] [CrossRef]
- Čapek, L.; Kreibich, V.; Dědeček, J.; Grygar, T.; Wichterlová, B.; Sobalík, Z.; Martens, J.A.; Brosius, R.; Tokarová, V. Analysis of Fe species in zeolites by UV–VIS–NIR, IR spectra and voltammetry. Effect of preparation, Fe loading and zeolite type. Microporous Mesoporous Mater. 2005, 78, 279–289. [Google Scholar]
- Schwidder, M.; Kumar, M.S.; Klementiev, K.; Pohl, M.M.; Brückner, A.; Grünert, W. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: I. Relations between active site structure and catalytic performance. J. Catal. 2005, 231, 314–330. [Google Scholar] [CrossRef]
- Feng, X.; Cao, Y.; Lan, L.; Lin, C.; Li, Y.; Xu, H.; Gong, M.; Chen, Y. The promotional effect of Ce on CuFe/beta monolith catalyst for selective catalytic reduction of NOx by ammonia. Chem. Eng. J. 2016, 302, 697–706. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: Solid-state ion exchange and one-pot synthesis. Appl. Catal. B 2015, 162, 501–514. [Google Scholar] [CrossRef]
- Imai, H.; Ogawa, T.; Sugimoto, K.; Kataoka, M.; Tanaka, Y.; Ono, T. Comparison of activities in selective catalytic reduction of NOx by C3H8 over Co/MFI, Fe/MFI, and H/MFI zeolite catalysts. Appl. Catal. B 2005, 55, 259–265. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, D.; Zhao, Y.; Chen, B.; Xue, J.; Liang, X.; Lei, Z. The reaction of NO+C3H6 +O2 over the mesoporous SBA-15 supported transition metal catalysts. Catal. Today 2011, 175, 26–33. [Google Scholar] [CrossRef]
- Nagayama, T.; Iizuka, H.; Katougi, K.; Mukai, T. Hydrocarbon Desorption Characteristics of Metal-impregnated Zeolite and Its Evaluation in Vehicle Test. J. Jpn. Pet. Inst. 2009, 52, 205–210. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Montreuil, C.N.; Kucherova, T.N.; Shelef, M. In situ high-temperature ESR characterization of FeZSM-5 and FeSAPO-34 catalysts in flowing mixtures of NO, C3H6, and O2. Catal. Lett. 1998, 56, 173–181. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; He, H. Synergistic Effect between NO2 and SO2 in Their Adsorption and Reaction on γ-Alumina. J. Phys. Chem. A 2008, 112, 6630–6635. [Google Scholar] [CrossRef] [PubMed]
- Satsuma, A.; Shimizu, K.I. In situ FT/IR study of selective catalytic reduction of NO over alumina-based catalysts. Prog. Energy Combust. Sci. 2003, 29, 71–84. [Google Scholar] [CrossRef]
- Venkov, T.; Hadjiivanov, K.; Klissurski, D. IR spectroscopy study of NO adsorption and NO + O2 co-adsorption on Al2O3. Phys. Chem. Chem. Phys. 2002, 4, 2443–2448. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y.; Haiqiang Wang, A.; Jin, R. DRIFT Study of Manganese/Titania-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Yang, R. FTIR and kinetic studies of the mechanism of Fe3+-exchanged TiO2-pillared clay catalyst for selective catalytic reduction of NO with ammonia. J. Catal. 2000, 190, 22–31. [Google Scholar] [CrossRef]
- Kumar, P.A.; Reddy, M.P.; Ju, L.K.; Hyun-Sook, B.; Phil, H.H. Low temperature propylene SCR of NO by copper alumina catalyst. J. Mol. Catal. A 2008, 291, 66–74. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Z.; Ding, Z.; Ruan, R.; Li, Z.; Wang, X. Infrared study of the NO reduction by hydrocarbons over iron sites with low nuclearity: Some new insight into the reaction pathway. J. Phys. Chem. C 2010, 114, 15713–15727. [Google Scholar] [CrossRef]
- Jie, L.; Xinyong, L.; Qidong, Z.; Ce, H.; Dongke, Z. Insight into the mechanism of selective catalytic reduction of NO(x) by propene over the Cu/Ti(0.7)Zr(0.3)O2 catalyst by Fourier transform infrared spectroscopy and density functional theory calculations. Environ. Sci. Technol. 2013, 47, 4528–4535. [Google Scholar]
- Zhou, H.; Li, K.; Zhao, B.; Deng, W.; Su, Y.; Zhong, F. Surface properties and reactivity of Fe/Al2O3/cordierite catalysts for NO reduction by C2H6: Effects of calcination temperature. Chem. Eng. J. 2017, 326, 737–744. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grünert, W.; Bentrup, U.; Brückner, A. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: Part II. Assessing the function of different Fe sites by spectroscopic in situ studies. J. Catal. 2006, 239, 173–186. [Google Scholar]
- Zhou, H.; Su, Y.; Liao, W.; Deng, W.; Zhong, F. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst: Reaction mechanism and effect of H2O/SO2. Fuel 2016, 182, 352–360. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Zhang, D.; Ndokoye, P. The selective catalytic reduction of NO with propene over Cu-supported Ti–Ce mixed oxide catalysts: Promotional effect of ceria. J. Mol. Catal. A Chem. 2013, 378, 115–123. [Google Scholar] [CrossRef]
- Yuan, D.; Li, X.; Zhao, Q.; Zhao, J.; Tadé, M.; Liu, S. A novel CuTi-containing catalyst derived from hydrotalcite-like compounds for selective catalytic reduction of NO with C3H6 under lean-burn conditions. J. Catal. 2014, 309, 268–279. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A.; Althoff, R. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
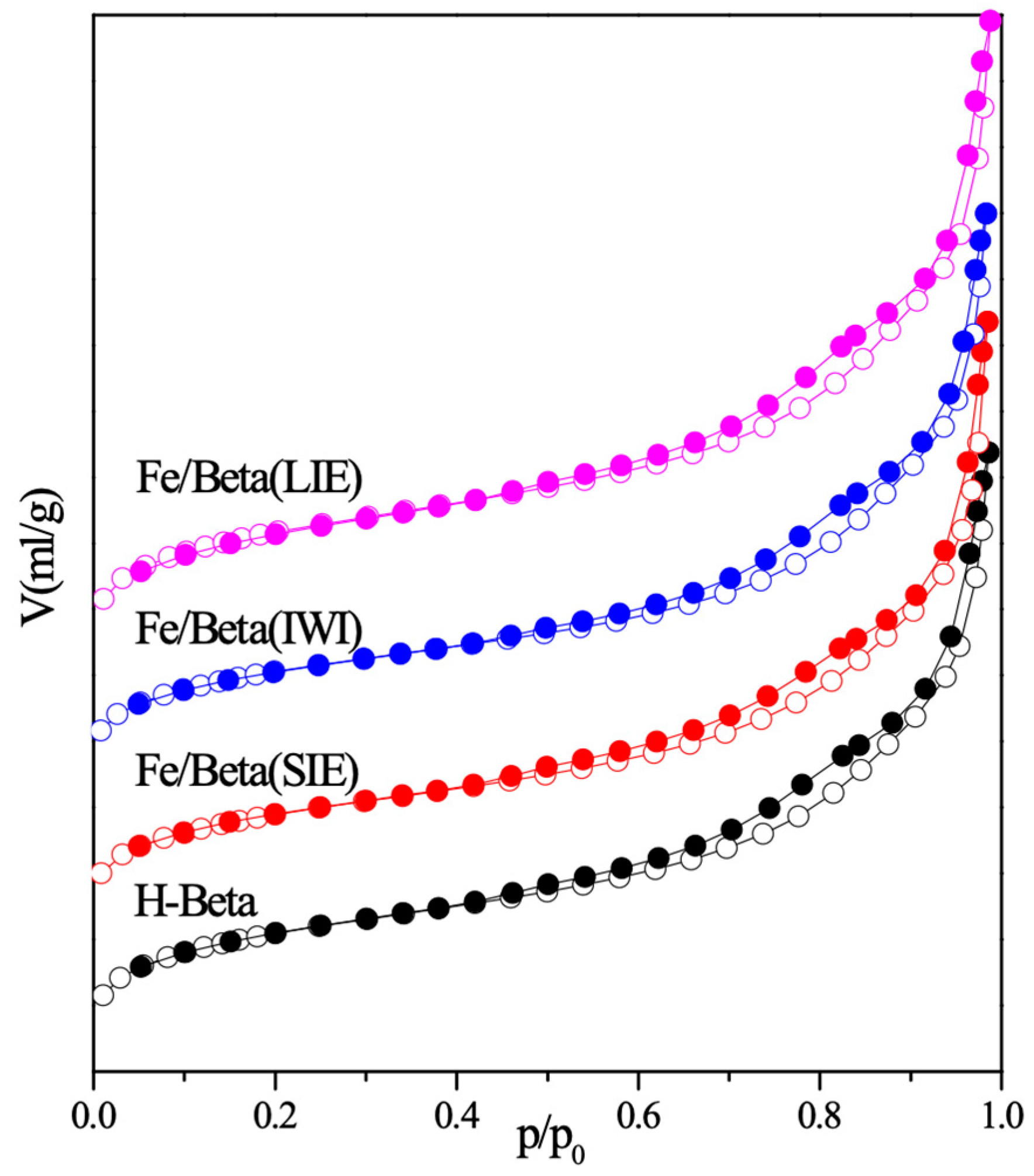
| Sample | Specific Surface Area (m2/g) | Pore Volume (mL/g) | Fe Loading a (mg/g) |
|---|---|---|---|
| H-Beta | 519.9 | 0.517 | - |
| Fe/Beta(LIE) | 512.1 | 0.541 | 13.87 |
| Fe/Beta(IWI) | 480.5 | 0.495 | 15.70 |
| Fe/Beta(SIE) | 486.5 | 0.506 | 13.40 |
| Sample | 150 °C Desorption (μmol/g) | 300 °C Desorption (μmol/g) | ||
|---|---|---|---|---|
| Brønsted Acidity | Lewis Acidity | Brønsted Acidity | Lewis Acidity | |
| Fe/Beta(LIE) | 229.23 | 555.66 | 197.24 | 251.65 |
| Fe/Beta(IWI) | 335.01 | 706.06 | 264.77 | 515.47 |
| Fe/Beta(SIE) | 288.63 | 631.22 | 194.10 | 346.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ge, M.; Zhao, H.; Wu, S.; Li, M.; Su, Y. Selective Catalytic Reduction of Nitric Oxide with Propylene over Fe/Beta Catalysts Under Lean-Burn Conditions. Catalysts 2019, 9, 205. https://doi.org/10.3390/catal9020205
Zhou H, Ge M, Zhao H, Wu S, Li M, Su Y. Selective Catalytic Reduction of Nitric Oxide with Propylene over Fe/Beta Catalysts Under Lean-Burn Conditions. Catalysts. 2019; 9(2):205. https://doi.org/10.3390/catal9020205
Chicago/Turabian StyleZhou, Hao, Mengyao Ge, Huishuang Zhao, Shiguo Wu, Mengyu Li, and Yaxin Su. 2019. "Selective Catalytic Reduction of Nitric Oxide with Propylene over Fe/Beta Catalysts Under Lean-Burn Conditions" Catalysts 9, no. 2: 205. https://doi.org/10.3390/catal9020205
APA StyleZhou, H., Ge, M., Zhao, H., Wu, S., Li, M., & Su, Y. (2019). Selective Catalytic Reduction of Nitric Oxide with Propylene over Fe/Beta Catalysts Under Lean-Burn Conditions. Catalysts, 9(2), 205. https://doi.org/10.3390/catal9020205