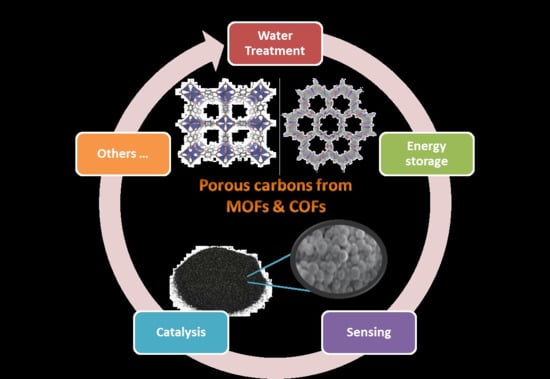
New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons
Abstract
:1. Porous carbons
1.1. Conventional Precursors and Classical Synthetic Strategies
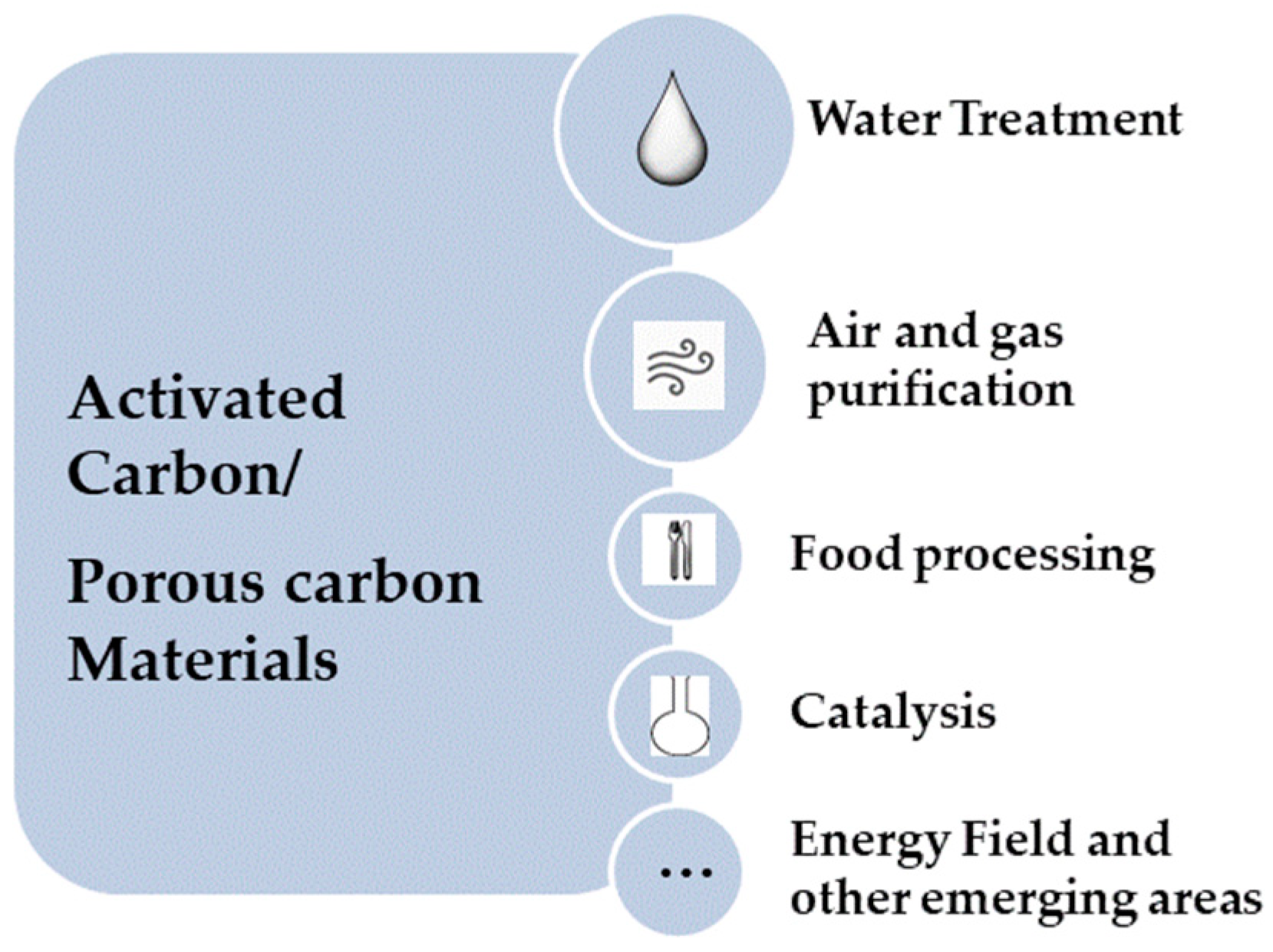
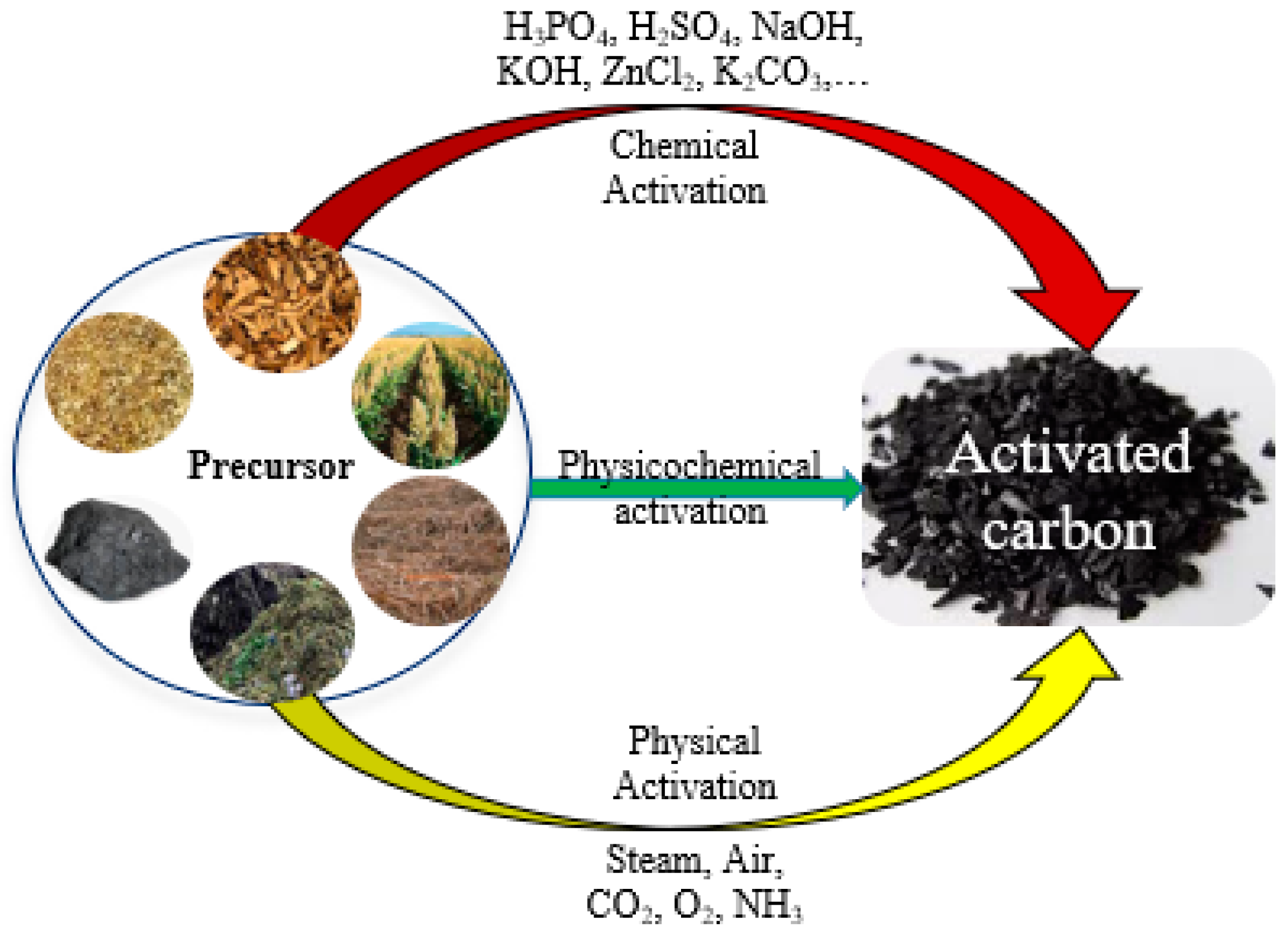
1.1.1. Activated Carbons—Physical and/or Chemical Activations
| C + CO2 → 2CO | (Boudouard reaction) | (1) |
| C + H2O → CO + H2 | (Water steam gasification) | (2) |
| CO + H2O → CO2 + H2 | (3) |
1.1.2. Hydrothermal Carbonization
1.1.3. Microwave-Assisted Carbonization/Activation
1.1.4. Porous Carbon Gels
1.1.5. Templating Strategies
2. Covalent Organic Frameworks (COFs) and Metal-Organic-Frameworks (MOFs) in the Synthesis of Porous Carbons. Advanced Synthetic Strategies and Applications
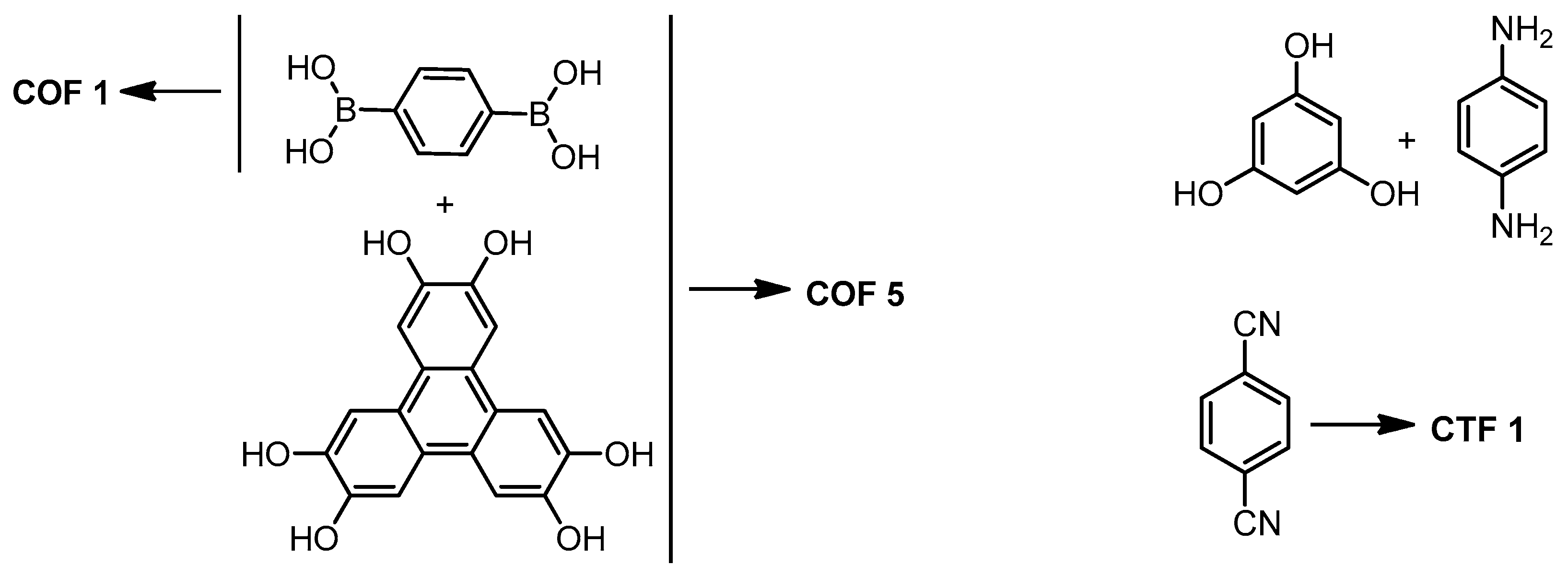
2.1. Porous Carbon from COFs
2.2. Porous Carbons from MOFs
2.2.1. MOFs as Precursors for Metal-Free Porous Carbon Synthesis
2.2.2. MOFs as Precursors for Carbon Hybrids
3. Porous Carbons from MOFs in Fine Chemical Synthesis
4. Porous Carbon from COFs in Fine Chemical Synthesis
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Functionalized activated carbon derived from biomass for photocatalysis applications perspective. Int. J. Photoenergy 2015, 2015, 218743. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Borchardt, L.; Zhu, Q.L.; Casco, M.E.; Berger, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.; DeGuzman, M. Activated Carbon, Chemical Economics Handbook; IHS Markit: London, UK, 2017. [Google Scholar]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Houshamnd, A.H. Textural characteristics, surface chemistry and oxidation of activated carbon. J. Nat. Gas Chem. 2010, 19, 267–279. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Oxidation of activated carbon by dry and wet methods surface chemistry and textural modifications. Fuel Process. Technol. 2010, 91, 1768–1775. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Characterization of Activated Carbon. In Activated Carbon; Elsevier: Kidlington, UK, 2006; Volume 18, pp. 143–242. ISBN 9780080444635. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Group, F. Adsorption; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780824753443. [Google Scholar]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; Van Der Voort, P.; De Clercq, J. Soft templated mesoporous carbons: Tuning the porosity for the adsorption of large organic pollutants. Carbon N. Y. 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon N. Y. 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351. [Google Scholar] [CrossRef]
- Matos, I.; Neves, P.D.; Castanheiro, J.E.; Perez-Mayoral, E.; Martin-Aranda, R.; Duran-Valle, C.; Vital, J.; Botelho Do Rego, A.M.; Fonseca, I.M. Mesoporous carbon as an efficient catalyst for alcoholysis and aminolysis of epoxides. Appl. Catal. A Gen. 2012, 439–440, 24–30. [Google Scholar] [CrossRef]
- Matos, I.; Fernandes, S.; Guerreiro, L.; Barata, S.; Ramos, A.M.; Vital, J.; Fonseca, I.M. The effect of surfactants on the porosity of carbon xerogels. Microporous Mesoporous Mater. 2006, 92, 38–46. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface modification and characterisation of a coal-based activated carbon. Carbon N. Y. 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Gorgulho, H.F.; Mesquita, J.P.; Gonçalves, F.; Pereira, M.F.R.; Figueiredo, J.L. Characterization of the surface chemistry of carbon materials by potentiometric titrations and temperature-programmed desorption. Carbon N. Y. 2008, 46, 1544–1555. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Kidlington, UK, 2006; ISBN 9780080444635. [Google Scholar]
- Activated Carbon Producers Association: Brussels, Belgium. ACPA Activated Carbon Producers Association, Brussels, Belgium. Available online: http://acpa.cefic.org/index.php (accessed on 28 November 2018).
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Canales-Flores, R.A.; Prieto-García, F. Activation Methods of Carbonaceous Materials Obtained from Agricultural Waste. Chem. Biodivers. 2016, 13, 261–268. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Ning, C.; Sze Ki Lin, C.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Adv. Colloid Interface Sci. 2014, 211, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kang, M.S.; Yoo, W.C. Highly Enhanced Gas Sorption Capacities of N-Doped Porous Carbon Spheres by Hot NH3 and CO2 Treatments. J. Phys. Chem. C 2015, 119, 28512–28522. [Google Scholar] [CrossRef]
- Xiong, Z.; Shihong, Z.; Haiping, Y.; Tao, S.; Yingquan, C.; Hanping, C. Influence of NH3/CO2 Modification on the Characteristic of Biochar and the CO2 Capture. BioEnergy Res. 2013, 6, 1147–1153. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. Nitrogen enriched biochar modified by high temperature CO2–ammonia treatment: Characterization and adsorption of CO2. Chem. Eng. J. 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Wachowski, L.; Hofman, M. Thermogravimetric and textural studies of modified carbonaceous materials. Thermochim. Acta 2005, 437, 82–86. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon N. Y. 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Montané, D.; Fierro, V.; Marêché, J.-F.; Aranda, L.; Celzard, A. Activation of biomass-derived charcoal with supercritical water. Microporous Mesoporous Mater. 2009, 119, 53–59. [Google Scholar] [CrossRef]
- Fujino, T. Phase and structural change of carbonized wood materials by hydrothermal treatment. Solid State Ion. 2002, 151, 197–203. [Google Scholar] [CrossRef]
- Salvador, F.; Sánchez-Montero, M.J.; Izquierdo, C. C/H2O Reaction under Supercritical Conditions and Their Repercussions in the Preparation of Activated Carbon. J. Phys. Chem. C 2007, 111, 14011–14020. [Google Scholar] [CrossRef]
- Yan, H.; Li, Y.; Guo, X.; Zhou, M.; Wang, H.-Q.; Dai, Y.; Zheng, J.-C. Synergistic Supercritical Water ‘Wet’ Activated Biomass Carbon as High Performances Electrode Materials for Supercapacitor. J. Electrochem. Soc. 2018, 165, A2075–A2083. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Brown, A.B.; Mudhoo, A.; Timko, M.T.; Rostagno, M.A.; Forster-Carneiro, T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: A critical review. Biofuel Res. J. 2017, 4, 611–626. [Google Scholar] [CrossRef]
- Inagaki, M.; Park, K.C.; Endo, M. Carbonization under pressure. New Carbon Mater. 2010, 25, 409–420. [Google Scholar] [CrossRef]
- Quesada-Plata, F.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Activated Carbons Prepared through H3PO4 -Assisted Hydrothermal Carbonisation from Biomass Wastes: Porous Texture and Electrochemical Performance. ChemPlusChem 2016, 81, 1349–1359. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Tuning hydrochar properties for enhanced mesopore development in activated carbon by hydrothermal carbonization. Microporous Mesoporous Mater. 2015, 203, 178–185. [Google Scholar] [CrossRef]
- Fechler, N.; Wohlgemuth, S.-A.; Jäker, P.; Antonietti, M. Salt and sugar: Direct synthesis of high surface area carbon materials at low temperatures via hydrothermal carbonization of glucose under hypersaline conditions. J. Mater. Chem. A 2013, 1, 9418. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Yuen, F.K.; Hameed, B.H. Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv. Colloid Interface Sci. 2009, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Cervantes, M.L. Some strategies to lower the production cost of carbon gels. J. Mater. Sci. 2015, 50, 1017–1040. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Arenillas, A.; Menéndez, J.A. Carbon Gels and Their Applications: A Review of Patents. In Submicron Porous Materials; Springer: Cham, Switzerland, 2017; pp. 25–52. [Google Scholar]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.M. Always look on the “light” side of life: Sustainable carbon aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; García-González, C.A.; Smirnova, I.; Titirici, M.M. Hydrothermal synthesis of highly porous carbon monoliths from carbohydrates and phloroglucinol. RSC Adv. 2013, 3, 17088. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent Progress in the Synthesis of Porous Carbon Materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Lu, A. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, J.; Xu, Q.; Wu, X.T.; Zhu, Q.L. Pore surface engineering of metal–organic frameworks for heterogeneous catalysis. Coord. Chem. Rev. 2018, 376, 248–276. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, Y.; Zheng, M.; Yang, P.; Asiri, A.M.; Wang, X. Metal–organic frameworks for solar energy conversion by photoredox catalysis. Coord. Chem. Rev. 2018, 373, 83–115. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Beyond pristine metal-organic frameworks: Preparation and application of nanostructured, nanosized, and analogous MOFs. Coord. Chem. Rev. 2018, 376, 20–45. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered Covalent Organic Frameworks, COFs and PAFs. From Preparation to Application; Elsevier: New York, NY, USA, 2016; Volume 311, pp. 85–124. [Google Scholar]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef] [PubMed]
- Lucovsky, G.; Rayner, B.; Zhang, Y.; Whitten, J. Experimental determination of band offset energies between Zr silicate alloy dielectrics and crystalline Si substrates by XAS, XPS and AES and ab initio theory: A new approach to the compositional dependence of direct tunneling currents. In Proceedings of the Digest. International Electron Devices Meeting, San Francisco, CA, USA, 8–11 December 2002; Volume 227, pp. 617–620. [Google Scholar] [CrossRef]
- Hu, H.; Yan, Q.; Ge, R.; Gao, Y. Covalent organic frameworks as heterogeneous catalysts. Chin. J. Catal. 2018, 39, 1167–1179. [Google Scholar] [CrossRef]
- Sun, J.K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Matsuyama, K. Supercritical fluid processing for metal–organic frameworks, porous coordination polymers, and covalent organic frameworks. J. Supercrit. Fluids 2018, 134, 197–203. [Google Scholar] [CrossRef]
- Xu, F.; Wu, D.; Fu, R.; Wei, B. Design and preparation of porous carbons from conjugated polymer precursors. Mater. Today 2017, 20, 629–656. [Google Scholar] [CrossRef]
- Yao, C.; Li, G.; Wang, J.; Xu, Y.; Chang, L. Template-free synthesis of porous carbon from triazine based polymers and their use in iodine adsorption and CO2 capture. Sci. Rep. 2018, 8, 1867. [Google Scholar] [CrossRef]
- Feng, X.; Liang, Y.; Zhi, L.; Thomas, A.; Wu, D.; Lieberwirth, I.; Kolb, U.; Müllen, K. Synthesis of Microporous Carbon Nanofibers and Nanotubes from Conjugated Polymer Network and Evaluation in Electrochemical Capacitor. Adv. Funct. Mater. 2009, 19, 2125–2129. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D.; Huang, L.; Shui, J.; Wang, M.; Dai, L. Nitrogen-doped holey graphitic carbon from 2D covalent organic polymers for oxygen reduction. Adv. Mater. 2014, 26, 3315–3320. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Schmidt, J. Microporous sulfur-doped carbon from thienyl-based polymer network precursors. Chem. Commun. 2011, 47, 8283. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, M.E.; Illathvalappil, R.; Kurungot, S.; Krishnamoorthy, K. Conjugated porous polymers as precursors for electrocatalysts and storage electrode materials. Chem. Commun. 2016, 52, 316–318. [Google Scholar] [CrossRef]
- Wang, J.; Senkovska, I.; Oschatz, M.; Lohe, M.R.; Borchardt, L.; Heerwig, A.; Liu, Q.; Kaskel, S. Imine-linked polymer-derived nitrogen-doped microporous carbons with excellent CO2 capture properties. ACS Appl. Mater. Interfaces 2013, 5, 3160–3167. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Sang, Y.; Huang, J.; Liu, Y.-N. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture. Chem. Eng. J. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Zhao, Y.; Bian, L.; Feng, X.; Pu, Q. Hollow, Spherical Nitrogen-Rich Porous Carbon Shells Obtained from a Porous Organic Framework for the Supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 10280–10287. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Yang, J.; Nakashima, N.; Shiraki, T. Highly Microporous Nitrogen-doped Carbon Synthesized from Azine-linked Covalent Organic Framework and its Supercapacitor Function. Chem. A Eur. J. 2017, 23, 17504–17510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Chai, S.; Nelson, K.; Han, K.S.; Hagaman, E.W.; Veith, G.M.; Mahurin, S.M.; Liu, H.; Dai, S. New tricks for old molecules: Development and application of porous N-doped, carbonaceous membranes for CO2 separation. Adv. Mater. 2013, 25, 4152–4158. [Google Scholar] [CrossRef]
- Zhu, X.; Chai, S.; Tian, C.; Fulvio, P.F.; Han, K.S.; Hagaman, E.W.; Veith, G.M.; Mahurin, S.M.; Brown, S.; Liu, H.; et al. Synthesis of Porous, Nitrogen-Doped Adsorption/Diffusion Carbonaceous Membranes for Efficient CO2 Separation. Macromol. Rapid Commun. 2013, 34, 452–459. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, J.; Zhang, J.; Xu, D.Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Rational Design of Fe/N/S-Doped Nanoporous Carbon Catalysts from Covalent Triazine Frameworks for Efficient Oxygen Reduction. ChemSusChem 2018, 11, 2402–2409. [Google Scholar] [CrossRef]
- Lee, Y.J.; Talapaneni, S.N.; Coskun, A. Chemically Activated Covalent Triazine Frameworks with Enhanced Textural Properties for High Capacity Gas Storage. ACS Appl. Mater. Interfaces 2017, 9, 30679–30685. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Ahn, W.S. Facile synthesis of microporous carbonaceous materials derived from a covalent triazine polymer for CO2 capture. J. Energy Chem. 2017, 26, 965–971. [Google Scholar] [CrossRef]
- Kim, M.; Puthiaraj, P.; Qian, Y.; Kim, Y.; Jang, S.; Hwang, S.; Na, E.; Ahn, W.-S.; Shim, S.E. High performance carbon supercapacitor electrodes derived from a triazine-based covalent organic polymer with regular porosity. Electrochim. Acta 2018, 284, 98–107. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Z.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Solovyeva, V.A.; Shekhah, O.; Eddaoudi, M. Carbonization of covalent triazine-based frameworks via ionic liquid induction. J. Mater. Chem. A 2018, 6, 15564–15568. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-B.; Pachfule, P.; Sun, J.-K.; Xu, Q. From covalent–organic frameworks to hierarchically porous B-doped carbons: A molten-salt approach. J. Mater. Chem. A 2016, 4, 4273–4279. [Google Scholar] [CrossRef]
- Bai, C.; Li, J.; Liu, S.; Yang, X.; Yang, X.; Tian, Y.; Cao, K.; Huang, Y.; Ma, L.; Li, S. In situ preparation of nitrogen-rich and functional ultramicroporous carbonaceous COFs by “segregated” microwave irradiation. Microporous Mesoporous Mater. 2014, 197, 148–155. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X. Recent Advances in Porous Carbon Materials for Electrochemical Energy Storage. Chem. Asian J. 2018, 13, 1518–1529. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, Y.; Zhang, X.; Oshima, Y.; Chen, Q.; Jiang, D. Template Conversion of Covalent Organic Frameworks into 2D Conducting Nanocarbons for Catalyzing Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1706330. [Google Scholar] [CrossRef]
- Kim, D.J.; Yoon, J.W.; Lee, C.S.; Bae, Y.S.; Kim, J.H. Covalent organic framework-derived microporous carbon nanoparticles coated with conducting polypyrrole as an electrochemical capacitor. Appl. Surf. Sci. 2018, 439, 833–838. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Yao, L.; Mai, Y.; Liu, J.; Hua, X.; Wei, H. Synthesis of core-shell covalent organic frameworks/multi-walled carbon nanotubes nanocomposite and application in lithium-sulfur batteries. Mater. Lett. 2018, 213, 143–147. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Chen, Z.; Liu, H.; Luque, R.; Li, Y. A covalent organic framework-based route to the: In situ encapsulation of metal nanoparticles in N-rich hollow carbon spheres. Chem. Sci. 2016, 7, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Zhu, Q.L.; Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.H. A systematic investigation of decomposition of nano Zn4O(C8H4O4)3 metal-organic framework. J. Phys. Chem. C 2010, 114, 2566–2572. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Gao, Q.; Guo, H. Porous carbons prepared by using metal-organic framework as the precursor for supercapacitors. Carbon N. Y. 2010, 48, 3599–3606. [Google Scholar] [CrossRef]
- Jin, S.L.; Deng, H.G.; Zhan, L.; Qiao, W.M.; Ling, L.C. Synthesis of 3D hierarchical porous carbon as an electrode material for electric double layer capacitors. Xinxing Tan Cailiao/New Carbon Mater. 2012, 27, 87–92. [Google Scholar] [CrossRef]
- Mo, S.; Sun, Z.; Huang, X.; Zou, W.; Chen, J.; Yuan, D. Synthesis, characterization and supercapacitive properties of hierarchical porous carbons. Synth. Met. 2012, 162, 85–88. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, J.; Tan, S.; Xia, N.; Liu, Y. Worm-like mesoporous carbon synthesized from metal-organic coordination polymers for supercapacitors. Electrochem. Commun. 2009, 11, 1191–1194. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, T.; Im, J.H.; Kim, Y.S.; Lee, K.; Jung, H.; Park, C.R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 2012, 24, 464–470. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Liu, Y.; Li, Y.; Lu, T.; Pan, L. From metal-organic frameworks to porous carbons: A promising strategy to prepare high-performance electrode materials for capacitive deionization. Carbon N. Y. 2016, 108, 433–439. [Google Scholar] [CrossRef]
- Ding, M.; Shi, W.; Guo, L.; Leong, Z.Y.; Baji, A.; Yang, H.Y. Bimetallic metal-organic framework derived porous carbon nanostructures for high performance membrane capacitive desalination. J. Mater. Chem. A 2017, 5, 6113–6121. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Bian, Z.; Shi, L.; Chen, X.; Zhou, J. ZnO nanosheet/squeezebox-like porous carbon composites synthesized by in situ pyrolysis of a mixed-ligand metal-organic framework. J. Mater. Chem. A 2017, 5, 5934–5942. [Google Scholar] [CrossRef]
- Indra, A.; Song, T.; Paik, U. Metal Organic Framework Derived Materials: Progress and Prospects for the Energy Conversion and Storage. Adv. Mater. 2018, 30, 1705146. [Google Scholar] [CrossRef]
- Zhu, D.; Li, H.; Su, Y.; Jiang, M. Pyridine-containing metal-organic frameworks as precursor for nitrogen-doped porous carbons with high-performance capacitive behavior. J. Solid State Electrochem. 2017, 21, 2037–2045. [Google Scholar] [CrossRef]
- Xu, J.; Xia, J.; Zhang, F.; Wang, Z. An electrochemical sensor based on metal-organic framework-derived porous carbon with high degree of graphitization for electroanalysis of various substances. Electrochim. Acta 2017, 251, 71–80. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Mu, S.; Wang, Y.; Jiang, C.; Liu, Q.; Fang, Q.; Xue, M.; Qiu, S. Cation exchanged MOF-derived nitrogen-doped porous carbons for CO2 capture and supercapacitor electrode materials. J. Mater. Chem. A 2017, 5, 9544–9552. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, Y.; Quan, G.; Han, X.; Yan, J. Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal. Nanomaterials 2018, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Yasin, G.; Bhatti, M.H.; Mehmood, M.; Arif, M.; Dai, L. Pt-M bimetallic nanoparticles (M = Ni, Cu, Er) supported on metal organic framework-derived N-doped nanostructured carbon for hydrogen evolution and oxygen evolution reaction. J. Power Sources 2018, 402, 34–42. [Google Scholar] [CrossRef]
- Dhawa, T.; Chattopadhyay, S.; De, G.; Mahanty, S. In Situ Mg/MgO-Embedded Mesoporous Carbon Derived from Magnesium 1,4-Benzenedicarboxylate Metal Organic Framework as Sustainable Li–S Battery Cathode Support. ACS Omega 2017, 2, 6481–6491. [Google Scholar] [CrossRef]
- Ulrich, H. Raw Materials for Industrial Polymers; Hanser Publishers: Munich, Germany, 1988; ISBN 3446150994. [Google Scholar]
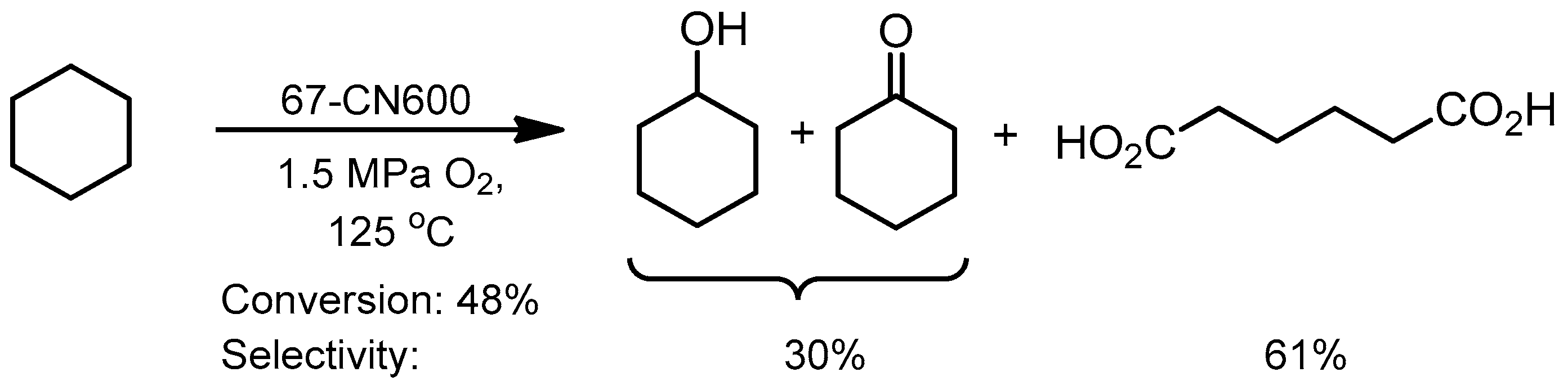
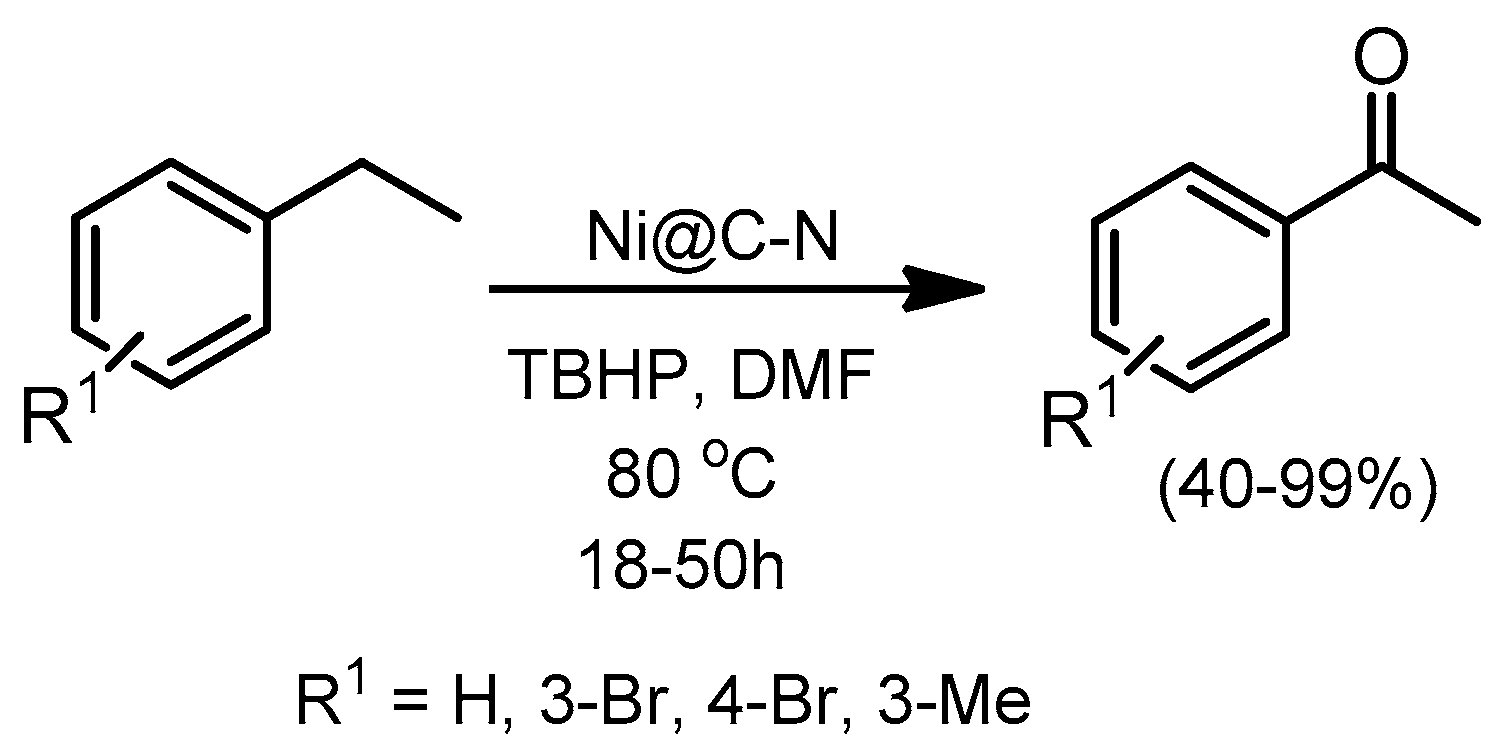
- Wang, X.; Li, Y. Nanoporous carbons derived from MOFs as metal-free catalysts for selective aerobic oxidations. J. Mater. Chem. A 2016, 4, 5247–5257. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, J.; Li, Y. Ni-based catalysts derived from a metal-organic framework for selective oxidation of alkanes. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 955–962. [Google Scholar] [CrossRef]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Ketones; Basic Reactions in Organic Synthesis; Springer: New York, NY, USA, 2006; ISBN 0-387-23607-4. [Google Scholar]
- Bai, C.; Li, A.; Yao, X.; Liu, H.; Li, Y. Efficient and selective aerobic oxidation of alcohols catalysed by MOF-derived Co catalysts. In Green Chemistry; The Royal Society of Chemistry: London, UK, 2016; Volume 18, pp. 1061–1069. [Google Scholar]
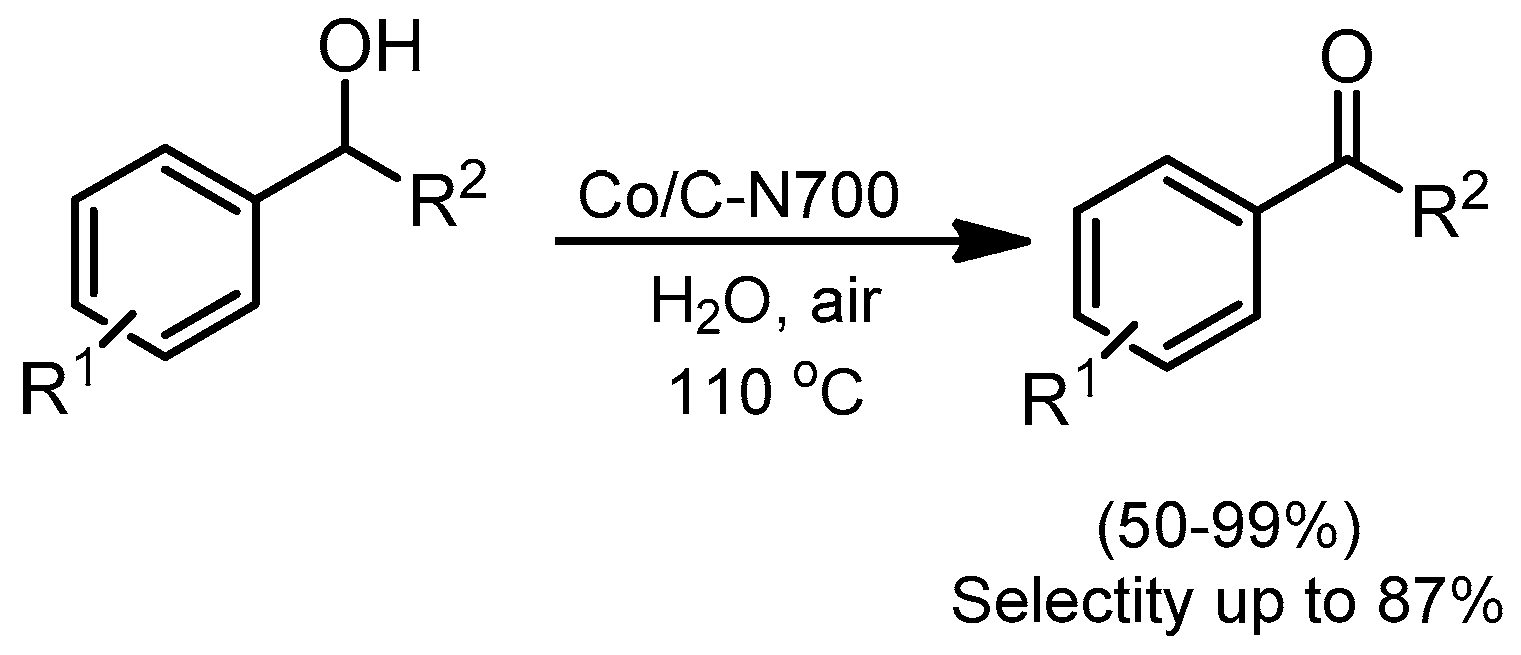
- Kim, B.R.; Oh, J.S.; Kim, J.; Lee, C.Y. Robust Aerobic Alcohol Oxidation Catalyst Derived from Metal-Organic Frameworks. Catal. Lett. 2016, 146, 734–743. [Google Scholar] [CrossRef]
- Otera, J. Esterification: Methods, Reactions and Applications; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3527304908. [Google Scholar]
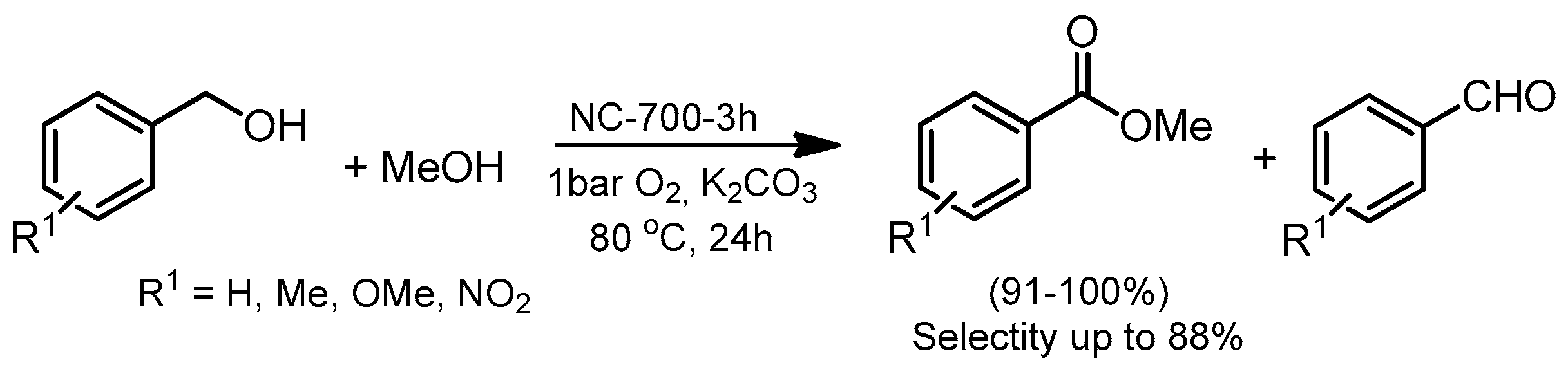
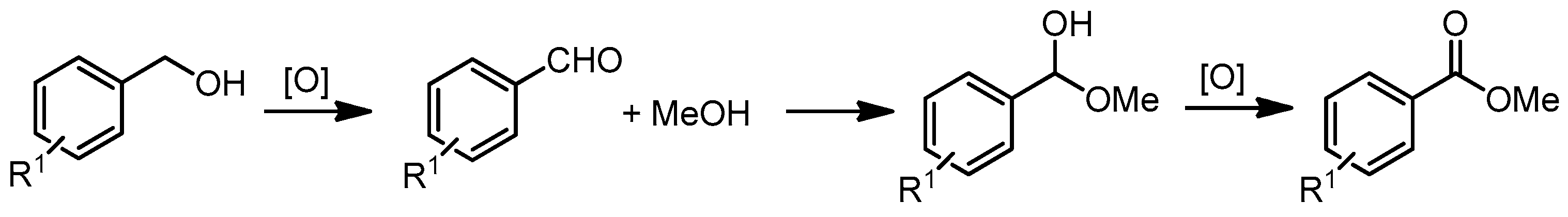
- Zhou, Y.-X.; Chen, Y.-Z.; Cao, L.; Lu, J.; Jiang, H.-L. Conversion of a metal–organic framework to N-doped porous carbon incorporating Co and CoO nanoparticles: Direct oxidation of alcohols to esters. Chem. Commun. 2015, 51, 8292–8295. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-free oxidation of alcohols to esters at room temperature and atmospheric conditions using nanoscale Co-based catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
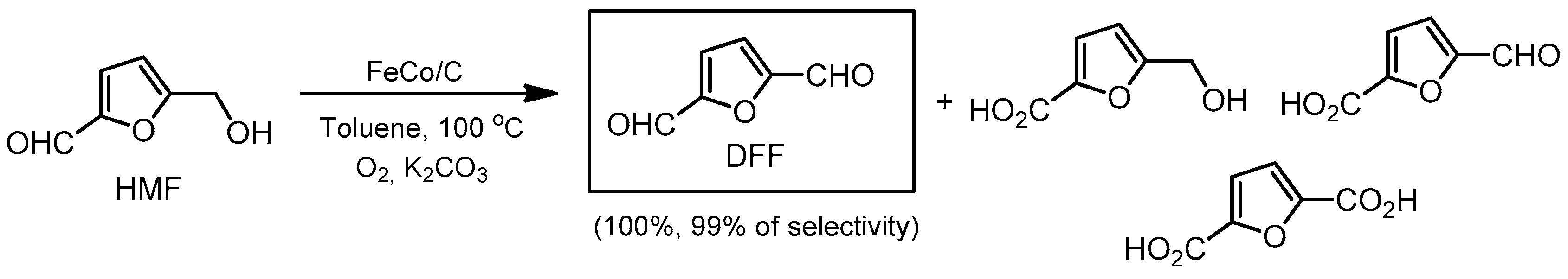
- Fang, R.; Luque, R.; Li, Y. Selective aerobic oxidation of biomass-derived HMF to 2,5-diformylfuran using a MOF-derived magnetic hollow Fe–Co nanocatalyst. Green Chem. 2016, 18, 3152–3157. [Google Scholar] [CrossRef]
- Liao, Y.T.; Matsagar, B.M.; Wu, K.C.-W. Metal-Organic Framework (MOF)-Derived Effective Solid Catalysts for Valorization of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2018, 6, 13628–13643. [Google Scholar] [CrossRef]
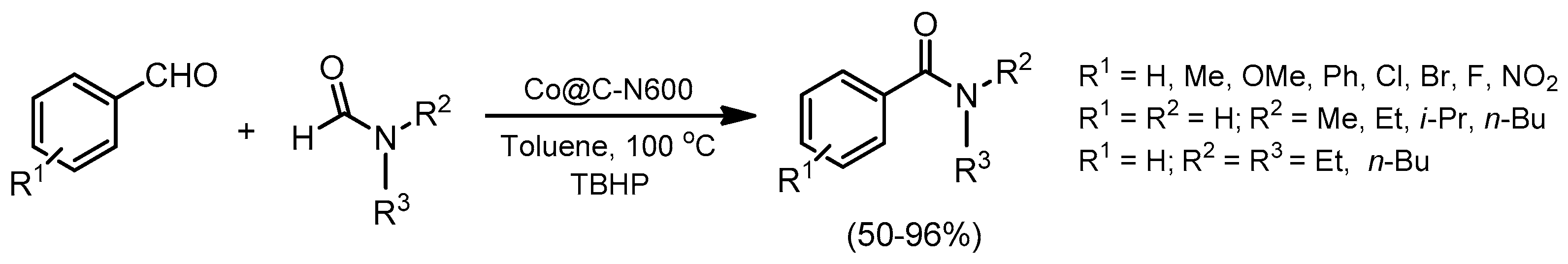
- Bai, C.; Yao, X.; Li, Y. Easy access to amides through aldehydic C-H bond functionalization catalyzed by heterogeneous Co-based catalysts. ACS Catal. 2015, 5, 884–891. [Google Scholar] [CrossRef]
- Long, J.; Zhou, Y.; Li, Y. Transfer hydrogenation of unsaturated bonds in the absence of base additives catalyzed by a cobalt-based heterogeneous catalyst. Chem. Commun. 2015, 51, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Long, J.; Zhang, W.; Liu, X.; Wei, D. MOFs-Derived Co@CN bi-functional catalysts for selective transfer hydrogenation of α,β-unsaturated aldehydes without use of base additives. Mater. Chem. Front. 2017, 1, 2005–2012. [Google Scholar] [CrossRef]
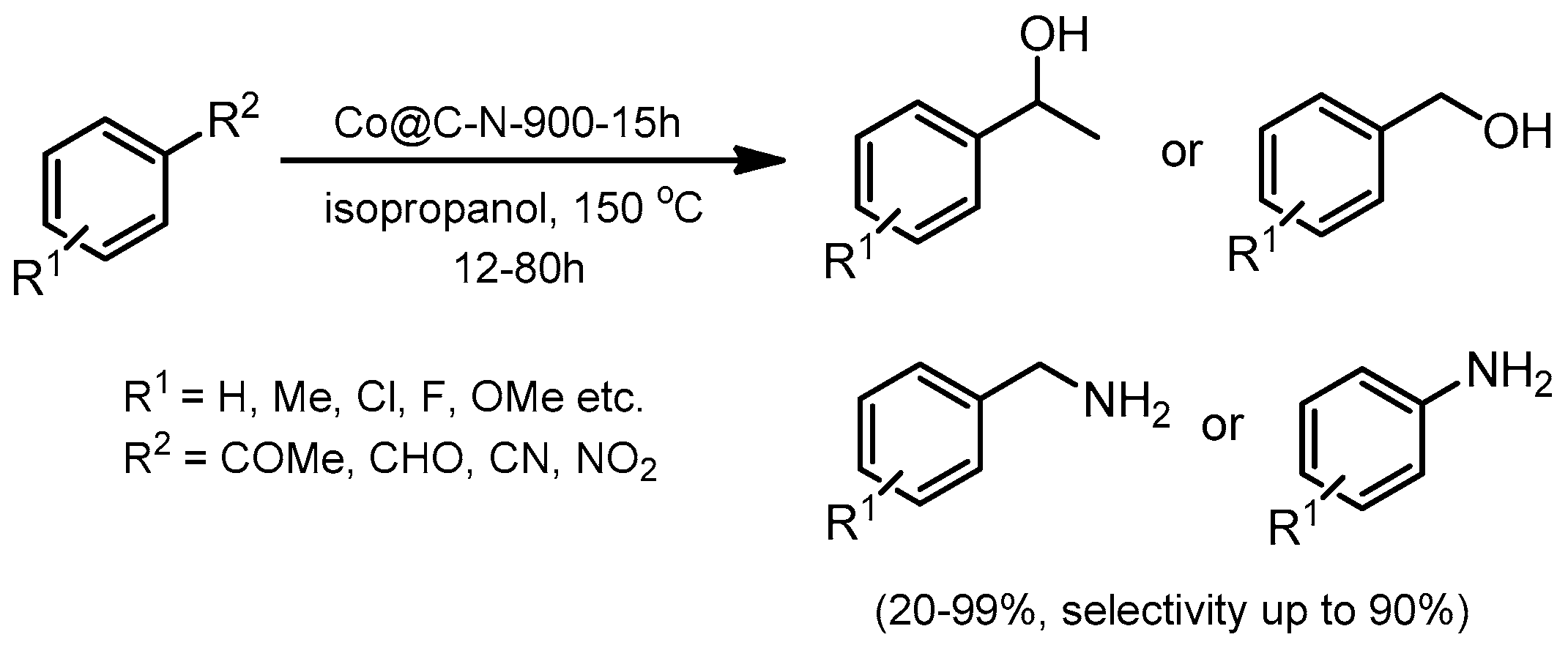
- Li, Y.; Zhou, Y.X.; Ma, X.; Jiang, H.L. A metal-organic framework-templated synthesis of γ-Fe2O3 nanoparticles encapsulated in porous carbon for efficient and chemoselective hydrogenation of nitro compounds. Chem. Commun. 2016, 52, 4199–4202. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Liu, S.; Cai, Y.; Wu, F.; Zhao, X. MOF derived porous carbon supported Cu/Cu2O composite as high performance non-noble catalyst. Microporous Mesoporous Mater. 2016, 219, 48–53. [Google Scholar] [CrossRef]
- Tang, B.; Song, W.C.; Yang, E.C.; Zhao, X.J. MOF-derived Ni-based nanocomposites as robust catalysts for chemoselective hydrogenation of functionalized nitro compounds. RSC Adv. 2017, 7, 1531–1539. [Google Scholar] [CrossRef]
- Shen, K.; Chen, L.; Long, J.; Zhong, W.; Li, Y. MOFs-Templated Co@Pd Core-Shell NPs Embedded in N-Doped Carbon Matrix with Superior Hydrogenation Activities. ACS Catal. 2015, 5, 5264–5271. [Google Scholar] [CrossRef]
- Yang, H.; Bradley, S.J.; Chan, A.; Waterhouse, G.I.N.; Nann, T.; Kruger, P.E.; Telfer, S.G. Catalytically Active Bimetallic Nanoparticles Supported on Porous Carbon Capsules Derived from Metal-Organic Framework Composites. J. Am. Chem. Soc. 2016, 138, 11872–11881. [Google Scholar] [CrossRef]
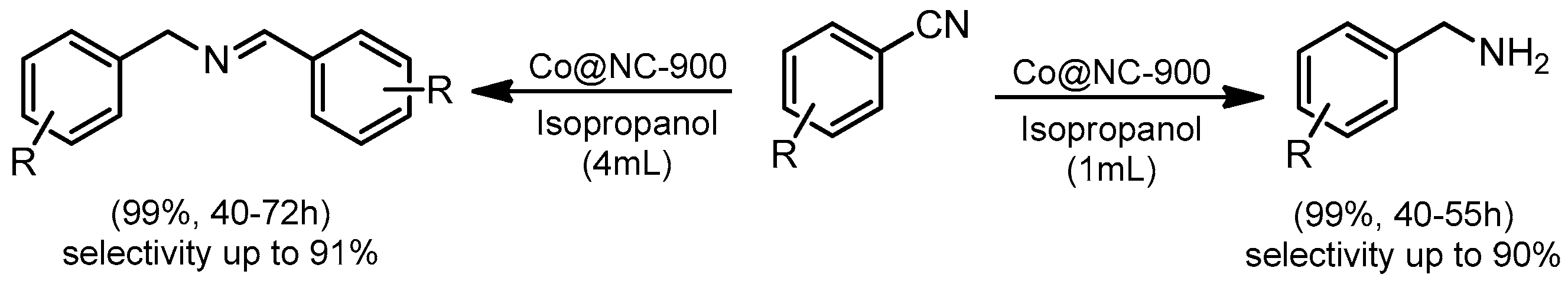
- Long, J.; Shen, K.; Li, Y. Bifunctional N-Doped Co@C Catalysts for Base-Free Transfer Hydrogenations of Nitriles: Controllable Selectivity to Primary Amines vs. Imines. ACS Catal. 2017, 7, 275–284. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Chen, L.; Li, Y. Multimetal-MOF-derived transition metal alloy NPs embedded in an N-doped carbon matrix: Highly active catalysts for hydrogenation reactions. J. Mater. Chem. A 2016, 4, 10254–10262. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Zhang, L.; Yao, T.; Liu, W.; Lin, Y.; Ju, H.; Dong, J.; Zheng, L.; Yan, W.; et al. Uncoordinated Amine Groups of Metal-Organic Frameworks to Anchor Single Ru Sites as Chemoselective Catalysts toward the Hydrogenation of Quinoline. J. Am. Chem. Soc. 2017, 139, 9419–9422. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, C.; Liu, Y.; Jiang, H.; Xing, W.; Chen, R. Pd nanoparticles supported on N-doped porous carbons derived from ZIF-67: Enhanced catalytic performance in phenol hydrogenation. J. Ind. Eng. Chem. 2017, 46, 258–265. [Google Scholar] [CrossRef]
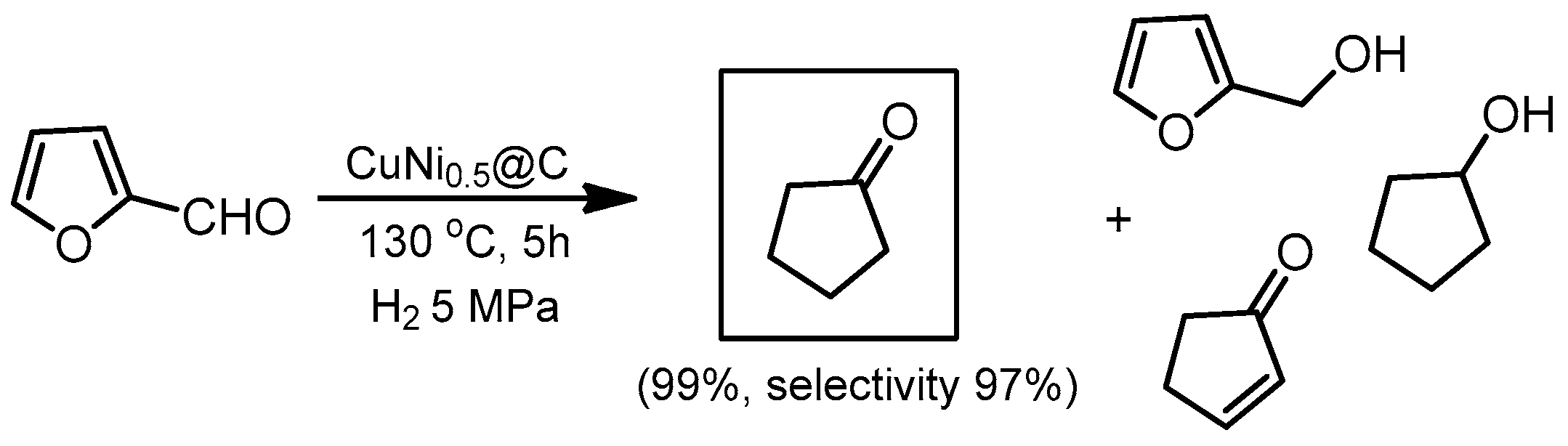
- Wang, Y.; Sang, S.; Zhu, W.; Gao, L.; Xiao, G. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
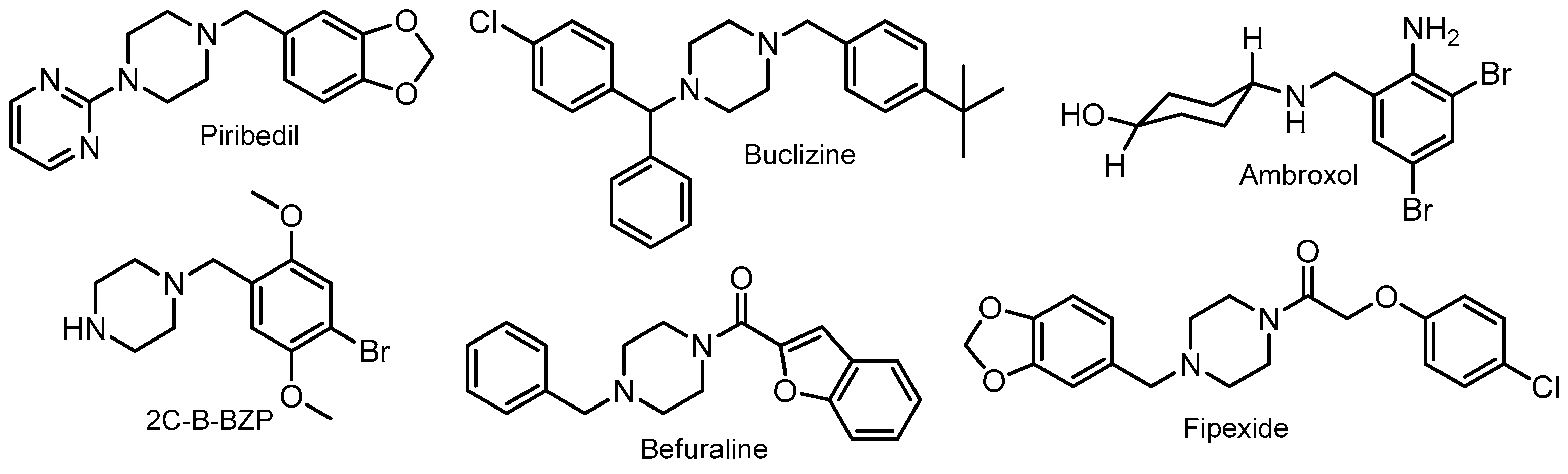
- Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences; Ricci, A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527317417. [Google Scholar]
- Dewick, P.M. Medicinal Natural Product: A Biosynthetic Approach; Wiley: New York, NY, USA, 2002; Volume 0471496405, ISBN 978-0-470-74168-9. [Google Scholar]
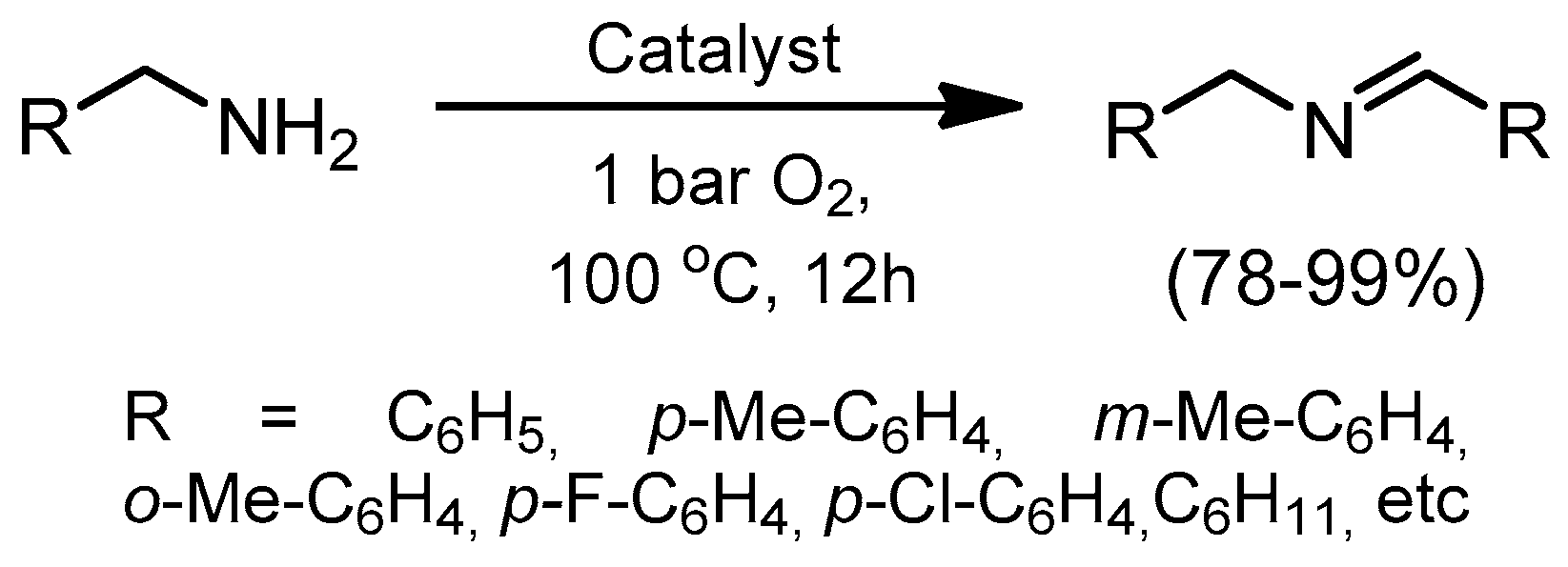
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Godino, M.; López-Peinado, A.J.; Martín-Aranda, R.M. Green Synthetic Approaches for Biologically Relevant Heterocycles: An Overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: New York, NY, USA, 2015; pp. 378–403. ISBN 9780128005903. [Google Scholar]
- Li, X.; Zhang, B.; Fang, Y.; Sun, W.; Qi, Z.; Pei, Y.; Qi, S.; Yuan, P.; Luan, X.; Goh, T.W.; et al. Metal–Organic-Framework-Derived Carbons: Applications as Solid-Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis. Chem. Eur. J. 2017, 23, 4266–4270. [Google Scholar] [CrossRef] [PubMed]
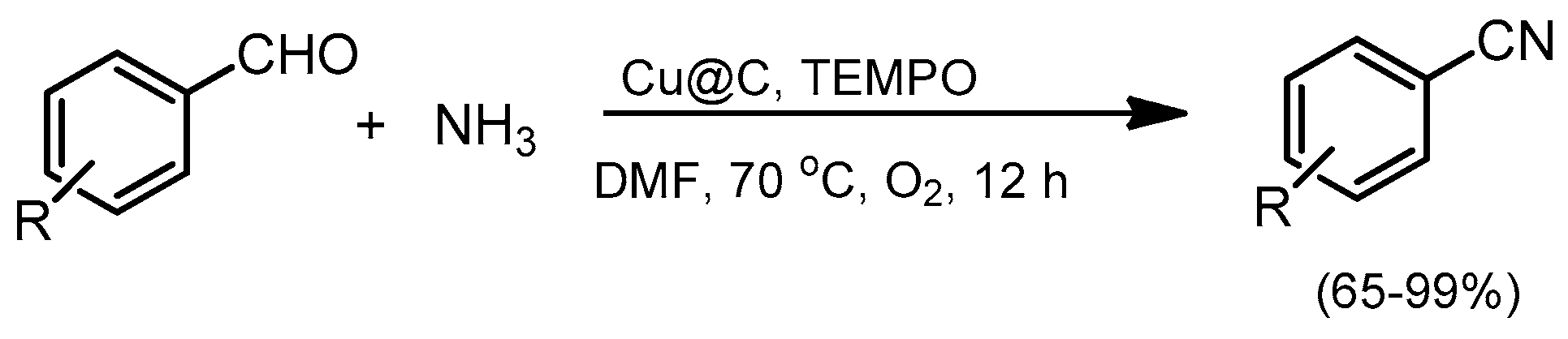
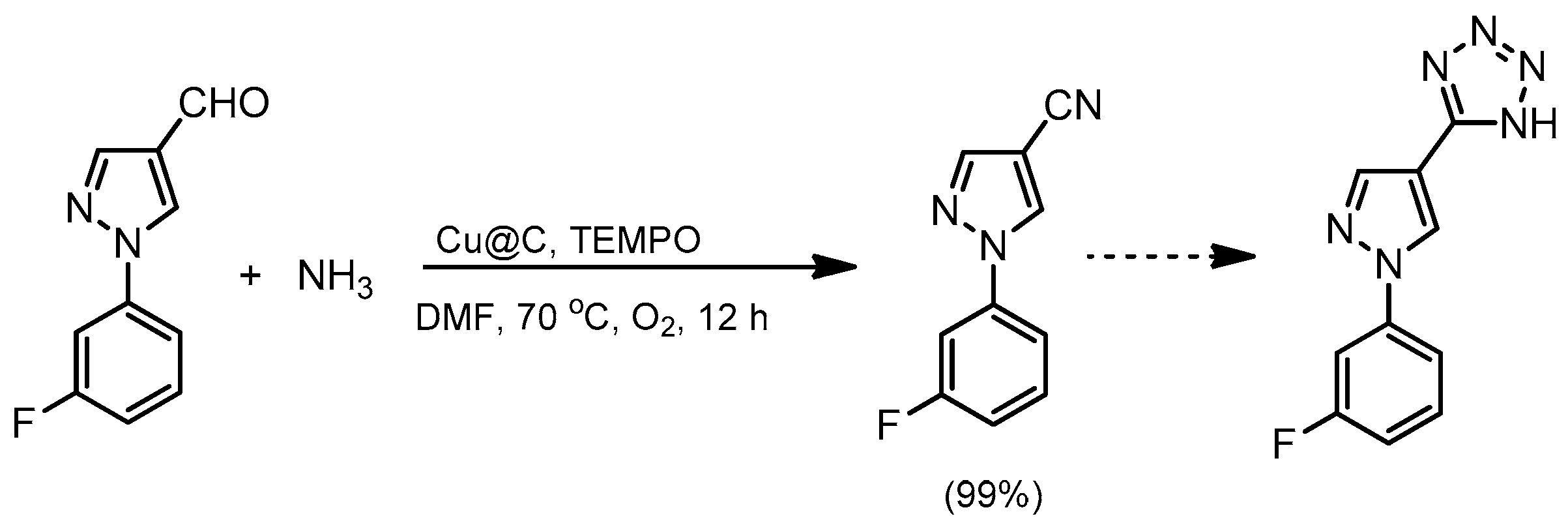
- Yoon, Y.; Kim, B.R.; Lee, C.Y.; Kim, J. Heterogeneous Copper-Catalyzed Aerobic Oxidative Conversions of Benzaldehydes with Aqueous Ammonia to Give Benzonitriles. Asian J. Org. Chem. 2016, 5, 746–749. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials: Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Li, W. MOF-derived nitrogen-doped porous carbon as metal-free catalysts for acetylene hydrochlorination. J. Ind. Eng. Chem. 2016, 44, 146–154. [Google Scholar] [CrossRef]
- Chao, S.; Zou, F.; Wan, F.; Dong, X.; Wang, Y.; Wang, Y.; Guan, Q.; Wang, G.; Li, W. Nitrogen-doped Carbon Derived from ZIF-8 as a High-performance Metal-free Catalyst for Acetylene Hydrochlorination. Sci. Rep. 2017, 7, 39789. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, B.R.; Lee, C.Y.; Kim, J. N-Sulfonyl amidine synthesis via three-component coupling reaction using heterogeneous copper catalyst derived from metal-organic frameworks. Tetrahedron Lett. 2016, 57, 4070–4073. [Google Scholar] [CrossRef]
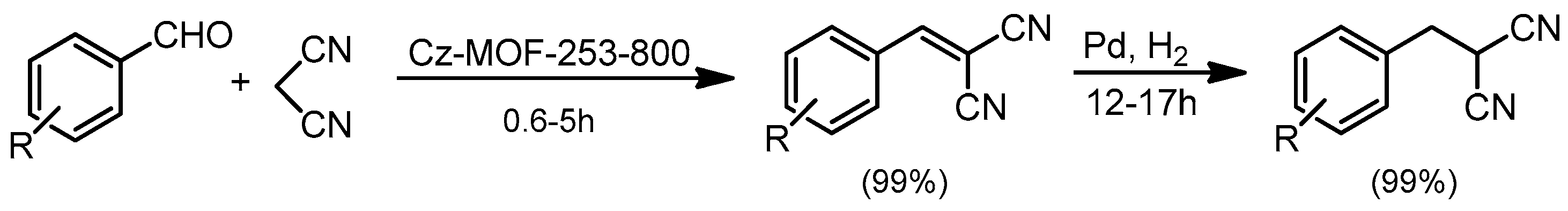
- Li, L.; Li, L.; Cui, C.; Fan, H.; Wang, R. Heteroatom-doped Carbon Spheres from Hierarchical Hollow Covalent Organic Framework Precursors for Metal-Free Catalysis. ChemSusChem 2017, 10, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis. Appl. Catal. B Environ. 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Friščić, T.; Julien, P.A.; Mottillo, C. Environmentally-Friendly Designs and Syntheses of Metal-Organic Frameworks (MOFs). In Green Technologies for the Environment; American Chemical Society: Washington, DC, USA, 2014; pp. 161–183. ISBN 9780841230187. [Google Scholar]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal-organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; López-Peinado, A.J.; Maldonado-Hódar, F.J.; Pérez-Mayoral, E. Highly Efficient and Selective Catalytic Synthesis of Quinolines Involving Transition-Metal-Doped Carbon Aerogels. ChemCatChem 2017, 9, 1422–1428. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Soriano, E.; Martín-Aranda, R.M. Maldonado-Hódar, F.J. Mesoporous Catalytic Materials and Fine Chemistry. In Comprehensive Guide for Mesoporous Materials. Volume 1: Synthesis and Characterization; Aliofkhazrae, M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 83–118. ISBN 978-1-63463-990-3. [Google Scholar]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.T.; Li, B.H.; Pei, T.; Lin, C.H.; Lee, S. Raman investigation on carbonization process of metal–organic frameworks. J. Raman Spectrosc. 2016, 47, 1271–1275. [Google Scholar] [CrossRef]





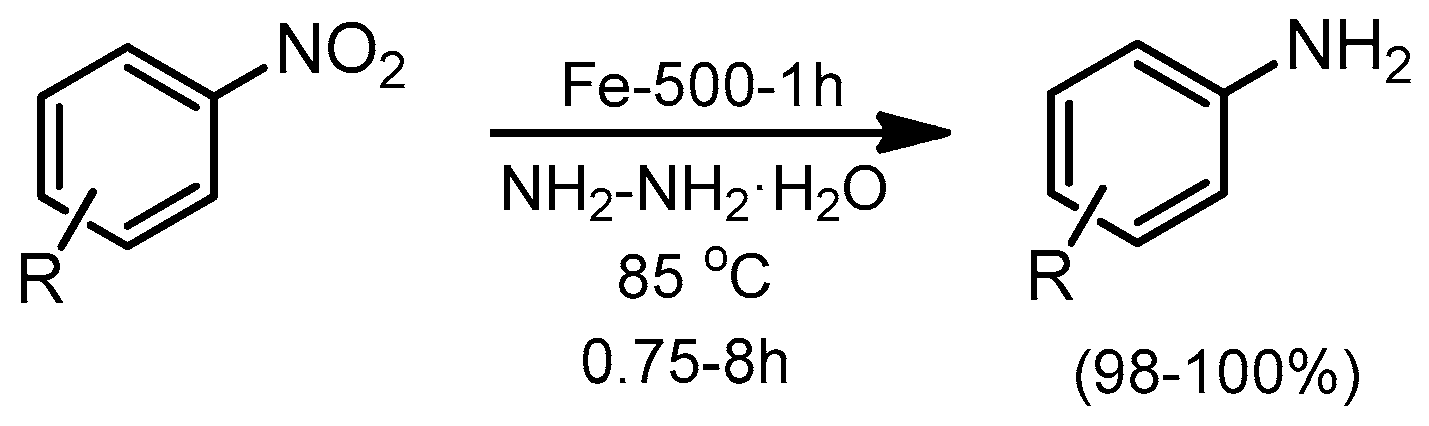


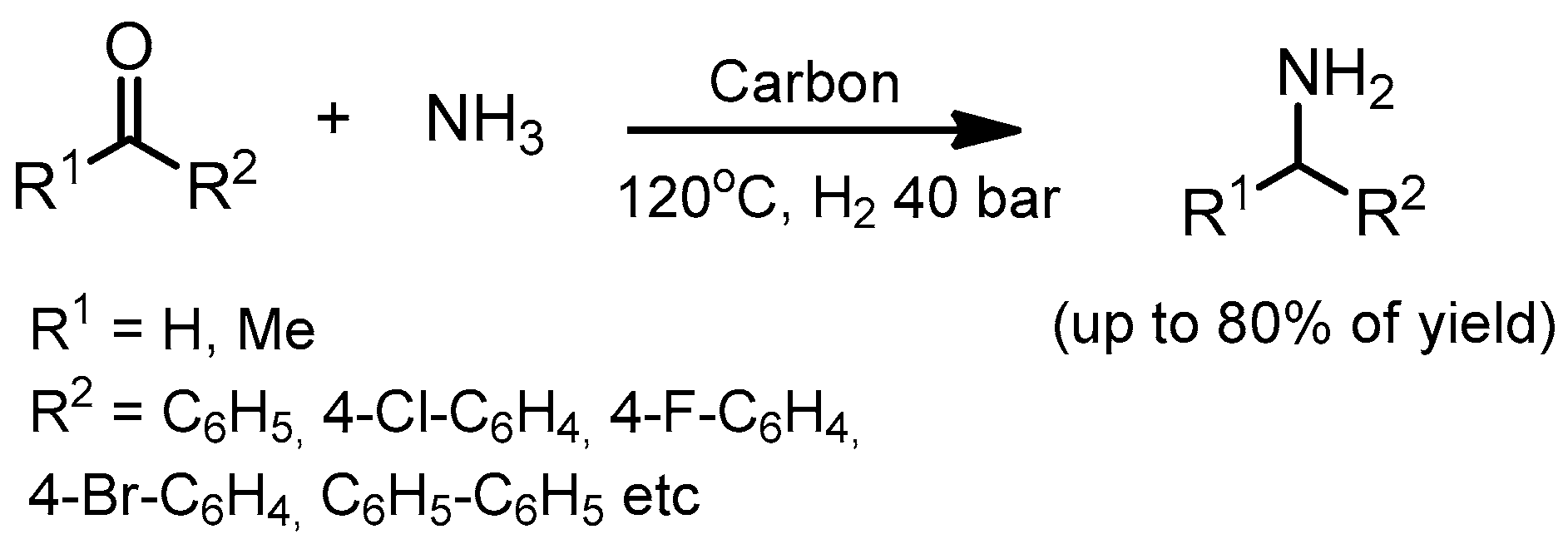


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Fonseca, I.M. New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons. Catalysts 2019, 9, 133. https://doi.org/10.3390/catal9020133
Pérez-Mayoral E, Matos I, Bernardo M, Fonseca IM. New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons. Catalysts. 2019; 9(2):133. https://doi.org/10.3390/catal9020133
Chicago/Turabian StylePérez-Mayoral, Elena, Inês Matos, Maria Bernardo, and Isabel M. Fonseca. 2019. "New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons" Catalysts 9, no. 2: 133. https://doi.org/10.3390/catal9020133
APA StylePérez-Mayoral, E., Matos, I., Bernardo, M., & Fonseca, I. M. (2019). New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons. Catalysts, 9(2), 133. https://doi.org/10.3390/catal9020133