Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Tests
2.2.1. Glucose Hydrogenation
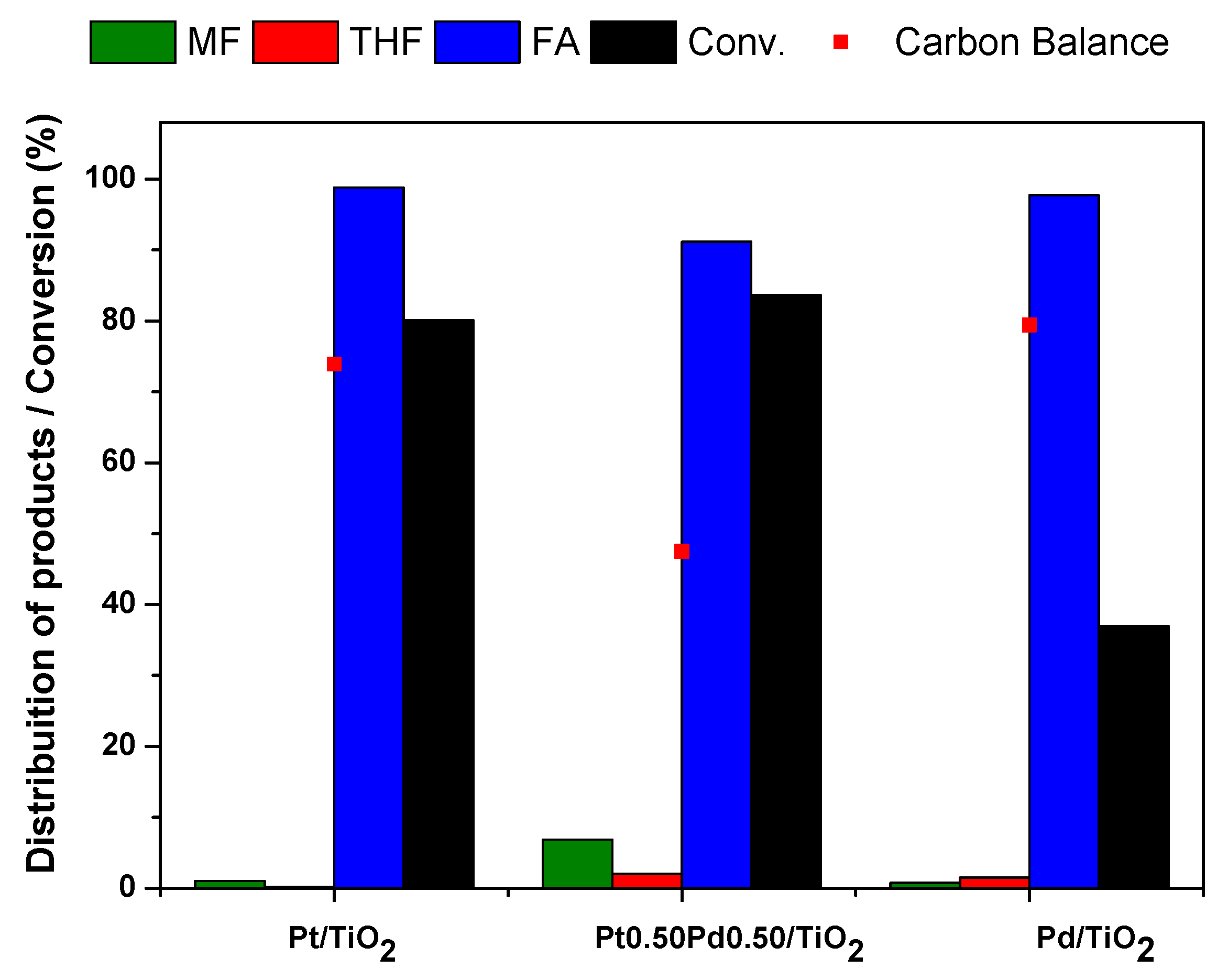
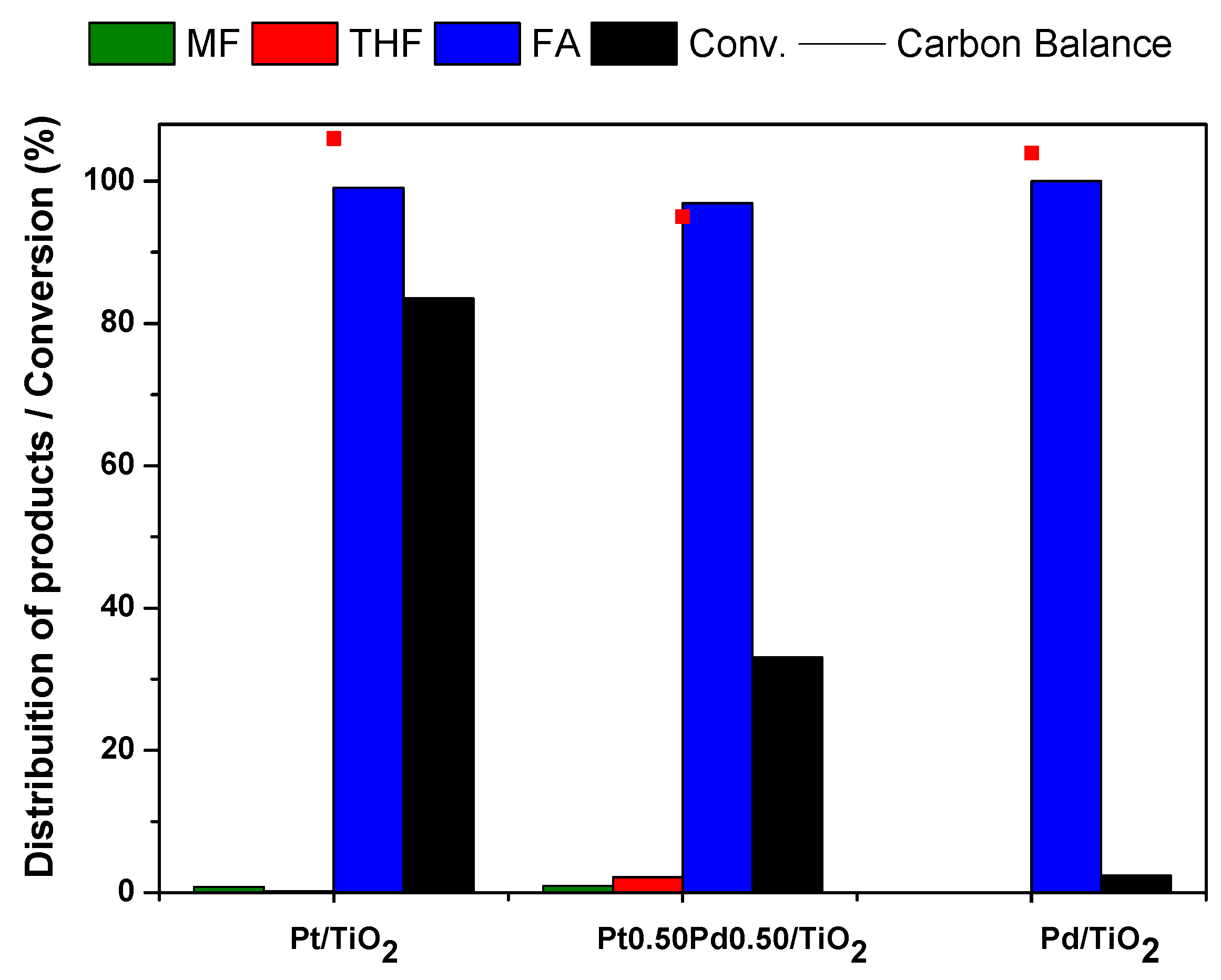
2.2.2. Furfural Hydrogenation Test
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Climent, M.J.; Corma, A.; Iborraa, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydro conversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborraa, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Hydrogenation of glucose and fructose into hexitols over heterogeneous catalysts: A review. J. Taiwan Inst. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Marques, C.; Tarek, R.; Sara, M.; Brar, S.K. Sorbitol Production from Biomass and Its Global Market. In Platform Chemical Biorefinery; Brar, S.K., Sarma, S.J., Pakshirajan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–227. ISBN 9780128029800. [Google Scholar]
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Takagaki, A.; Nishimura, S.; Ebitani, K. Catalytic Transformations of Biomass-Derived Materials into Value-Added Chemicals. Catal. Surv. Asia 2012, 16, 164–182. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal Catalysts for the Efficient Transformation of Biomass-derived HMF and Furfural to Value Added Chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Gorp, K.; Boerman, E.; Cavenaghi, C.V.; Berben, P.H. Catalytic hydrogenation of fine chemicals: Sorbitol production. Catal. Today 1999, 52, 349–361. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Romero, A.; Nieto-Márquez, A.; Alonso, E. Bimetallic Ru:Ni/MCM-48 catalysts for the effective hydrogenation of d-glucose into sorbitol. Appl. Catal. A Gen. 2017, 529, 49–59. [Google Scholar] [CrossRef]
- Romero, A.; Alonso, E.; Sastre, A.; Nieto-Márquez, A. Conversion of biomass into sorbitol: Cellulose hydrolysis on MCM-48 and d-Glucose hydrogenation on Ru/MCM-48. Microporous Mesoporous Mater. 2016, 224, 1–8. [Google Scholar] [CrossRef]
- Mishra, D.K.; Lee, J.-M.; Chang, J.-S.; Hwang, J.S. Liquid phase hydrogenation of d-glucose to d-sorbitol over the catalyst (Ru/NiO–TiO2) of ruthenium on a NiO-modified TiO2 support. Catal. Today 2012, 185, 104–108. [Google Scholar] [CrossRef]
- Bizhanov, F.B.; Sokolskiy, D.V.; Popov, N.I.; Malkina, N.Y.; Khisametdinov, A.M. Hydrogenation of glucose on Raney nickel. I. J. Catal. 1968, 10, 206–207. [Google Scholar] [CrossRef]
- Negoi, A.; Triantafyllidis, K.; Parvulescu, V.I.; Coman, S.M. The hydrolytic hydrogenation of cellulose to sorbitol over M (Ru, Ir, Pd, Rh)-BEA-zeolite catalysts. Catal. Today 2014, 223, 122–128. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Karakoulia, S.; Delimitis, A.; Coman, S.M.; Parvulescu, V.I.; Triantafyllidis, K. D-Glucose hydrogenation/hydrogenolysis reactions on noble metal (Ru, Pt)/activated carbon supported catalysts. Catal. Today 2015, 257, 281–290. [Google Scholar] [CrossRef]
- Zhang, X.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Platinum-Catalyzed Aqueous-Phase Hydrogenation of D-Glucose to D-Sorbitol. ACS Catal. 2016, 6, 7409–7417. [Google Scholar] [CrossRef]
- Baijun, L.; Lianhai, L.; Bingchun, W.; Tianxi, C.; Iwatani, K. Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl. Catal. A Gen. 1998, 171, 117–122. [Google Scholar] [CrossRef]
- Merat, N.; Godawa, C.; Gaset, A. High Selective Production of Tetrahydrofurfuryl Alcohol: Catalytic Hydrogenation of Furfural and Furfuryl Alcohol. J. Chem. Technol. Biotechnol. 1990, 48, 145–159. [Google Scholar] [CrossRef]
- Li, H.; Luo, H.; Zhuang, L.; Dai, W.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over the Fe-promoted Ni-B amorphous alloy catalysts. J. Mol. Catal. A Chem. 2003, 203, 267–275. [Google Scholar] [CrossRef]
- Neill, B.J.O.; Jackson, D.H.K.; Crisci, A.J.; Farberow, C.A.; Shi, F.; Alba-Rubio, A.C.; Lu, J.; Dietrich, P.J.; Gu, X.; Marshall, C.L.; et al. Stabilization of Copper Catalysts for Liquid-Phase Reactions by Atomic Layer Deposition. Angew. Chem. Int. Ed. 2013, 52, 13808–13812. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, D.; Rubio-Caballero, J.M.; Santamaria-Gonzalez, J.; Moreno-Tost, R.; Merida-Robles, J.M.; Perez-Cruz, M.A.; Jimenez-Lopez, A.; Hernandez-Huesca, R.; Maireles-Torres, P. Furfuryl alcohol from furfural hydrogenation over copper supported on SBA-15 silica catalysts. J. Mol. Catal. A Chem. 2014, 383–384, 106–113. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Huang, R.; Cui, Q.; Yuan, Q.; Wu, H.; Guan, Y.; Wu, P. Total hydrogenation of furfural over Pd/Al2O3 and Ru/ZrO2 mixture under mild conditions: Essential role of tetrahydrofurfural as an intermediate and support effect. ACS Sustain. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobroˇcka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd–Cu catalysts. Appl. Catal. A Gen. 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 580–585. [Google Scholar] [CrossRef]
- An, K.; Musselwhite, N.; Kennedy, G.; Pushkarev, V.V.; Baker, L.R.; Somorjai, G.A. Preparation of mesoporous oxides and their support effects on Pt nanoparticle catalysts in catalytic hydrogenation of furfural. J. Colloid Interface Sci. 2013, 392, 122–128. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multi walled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 5–10. [Google Scholar] [CrossRef]
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Lett. 2009, 10, 1665–1669. [Google Scholar] [CrossRef]
- Arena, B.J. Deactivation of ruthenium catalysts in continuous glucose hydrogenation. Appl. Catal. A Gen. 1992, 87, 219–229. [Google Scholar] [CrossRef]
- Kusserow, B.; Schimpf, S.; Claus, P. Hydrogenation of Glucose to Sorbitol over Nickel and Ruthenium Catalysts. Adv. Synth. Catal. 2003, 345, 289–299. [Google Scholar] [CrossRef]
- Liu, D.; Zemlynov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Rodiansono; Khairi, S.; Hara, T.; Ichikuni, N.; Shimazu, S. Highly efficient and selective hydrogenation of unsaturated carbonyl compounds using Ni–Sn alloy catalysts. Catal. Sci. Technol. 2012, 2, 2139–2145. [Google Scholar]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A Gen. 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Coupling Noble Metals and Carbon Supports in the Development of Combustion Catalysts for the Abatement of BTX Compounds in Air Streams. Catalysts 2015, 5, 774–799. [Google Scholar] [CrossRef]
- Muroi, T. Role of Precious Metal Catalysts. In Noble Metals; Su, Y.-H., Ed.; InTechOpen Limited: London, UK, 2012; ISBN 978-953-307-898-4. [Google Scholar]
- Basile, F.; Basini, L.; Fornasari, G.; Gazzano, M.; Trifiro, F.; Vaccari, A. Anionic Clays as Precursors of Noble Metal Based Catalysts for Methane Activation. Stud. Surf. Sci. Catal. 1998, 118, 31–40. [Google Scholar]
- Sitthisa, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Takada, K.; Tamura, M.; Tomishige, K. Total Hydrogenation of Furfural and 5-Hydroxymethylfurfural over Supported Pd−Ir Alloy Catalyst. ACS Catal. 2014, 4, 2718–2726. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Liu, K.; Wang, Y.; Fan, G.; Sun, S.; Xu, J.; Zhu, Y.; Li, Y. Aqueous-phase hydrogenolysis of glucose to value-added chemicals and biofuels: A comparative study of active metals. Biomass Bioenergy 2015, 72, 189–199. [Google Scholar] [CrossRef]
- Pino, N.; Sitthisa, S.; Tan, Q.; Souza, T.; López, D.; Resasco, D.E. Structure, activity, and selectivity of bimetallic Pd-Fe/SiO2 and Pd-Fe/γ-Al2O3 catalysts for the conversion of furfural. J. Catal. 2017, 350, 30–40. [Google Scholar] [CrossRef]
- Albertazzi, S.; Busca, G.; Finocchio, E.; Glöckler, R.; Vaccari, A. New Pd/Pt on Mg/Al basic mixed oxides for the hydrogenation and hydrogenolysis of naphthalene. J. Catal. 2004, 223, 372–381. [Google Scholar] [CrossRef]
- Gomez, R.; Angel, G.D.; Damian, C.; Corro, G. Platinum-palladium catalysts in hydrogenation of methylbenzenes. React. Kinet. Catal. Lett. 1979, 11, 137–142. [Google Scholar] [CrossRef]
- Rousset, J.L.; Stievano, L.; Aires, F.J.C.S.; Geantet, C.; Renouprez, A.J.; Pellarin, M. Hydrogenation of Toluene over γ-Al2O3-Supported Pt, Pd, and Pd–Pt Model Catalysts Obtained by Laser Vaporization of Bulk Metals. J. Catal. 2001, 197, 335–343. [Google Scholar] [CrossRef]
- Shafii, S.; Lihua, W.; Nordin, M.R.; Yong, L.K. Synthesis of Palladium-Platinum Bimetallic Nanoparticles and their Catalytic Activity towards the Hydrogenation Reaction of Palm Olein. J. Chem. Eng. Process Technol. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Pollefeyt, G.; Bliznuk, V.; Van Driessche, I.; Gaigneaux, E.M.; Ruiz, P.; Wojcieszak, R. Synergetic Behavior of TiO2-Supported Pd(z)Pt(1-z)Catalysts in the Green Synthesis of Methyl Formate. ChemCatChem 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Albilali, R.; Douthwaite, M.; He, Q.; Taylor, S.H. The selective hydrogenation of furfural over supported palladium nanoparticle catalysts prepared by sol-immobilisation: Effect of catalyst support and reaction conditions. Catal. Sci. Technol. 2018, 8, 252–267. [Google Scholar] [CrossRef]
- Geyer, R.; Kraak, P.; Pachulski, A.; Schodel, R. New Catalysts for the Hydrogenation of Glucose to Sorbitol. Chem. Ing. Tech. 2012, 84, 513–516. [Google Scholar] [CrossRef]
- Baker, L.R.; Kennedy, G.; Spronsen, M.V.; Hervier, A.; Cai, X.; Chen, S.; Wang, L.-W.; Somorjai, G.A. Furfuraldehyde Hydrogenation on Titanium Oxide-Supported Platinum Nanoparticles Studied by Sum Frequency Generation Vibrational Spectroscopy: Acid−Base Catalysis Explains the Molecular Origin of Strong Metal−Support Interactions. J. Am. Chem. Soc. 2012, 134, 14208–14216. [Google Scholar] [CrossRef]
- Fuentes Hernandez, A.; Lee, R.; Beland, N.; Zamboni, I.; Lavoie, J.M. Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst. Energies 2017, 10, 286. [Google Scholar] [CrossRef]
- Shi, D.; Yang, Q.; Peterson, C.; Lamic-Humblot, A.-F.; Girardon, J.-S.; Griboval-Constant, A.; Stievano, L.; Sougrati, M.T.; Briois, V.; Bagot, P.; et al. Bimetallic Fe-Ni/SiO2 catalysts for furfural hydrogenation: Identification of the interplay between Fe and Ni during deposition-precipitation and thermal treatments. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Zielinski, M.; Monteverdi, S.; Bettahar, M. Study of nickel nanoparticles on activated carbon prepared by aqueous hydrazine reduction. J. Coll. Inter. Sci. 2006, 299, 238. [Google Scholar] [CrossRef] [PubMed]
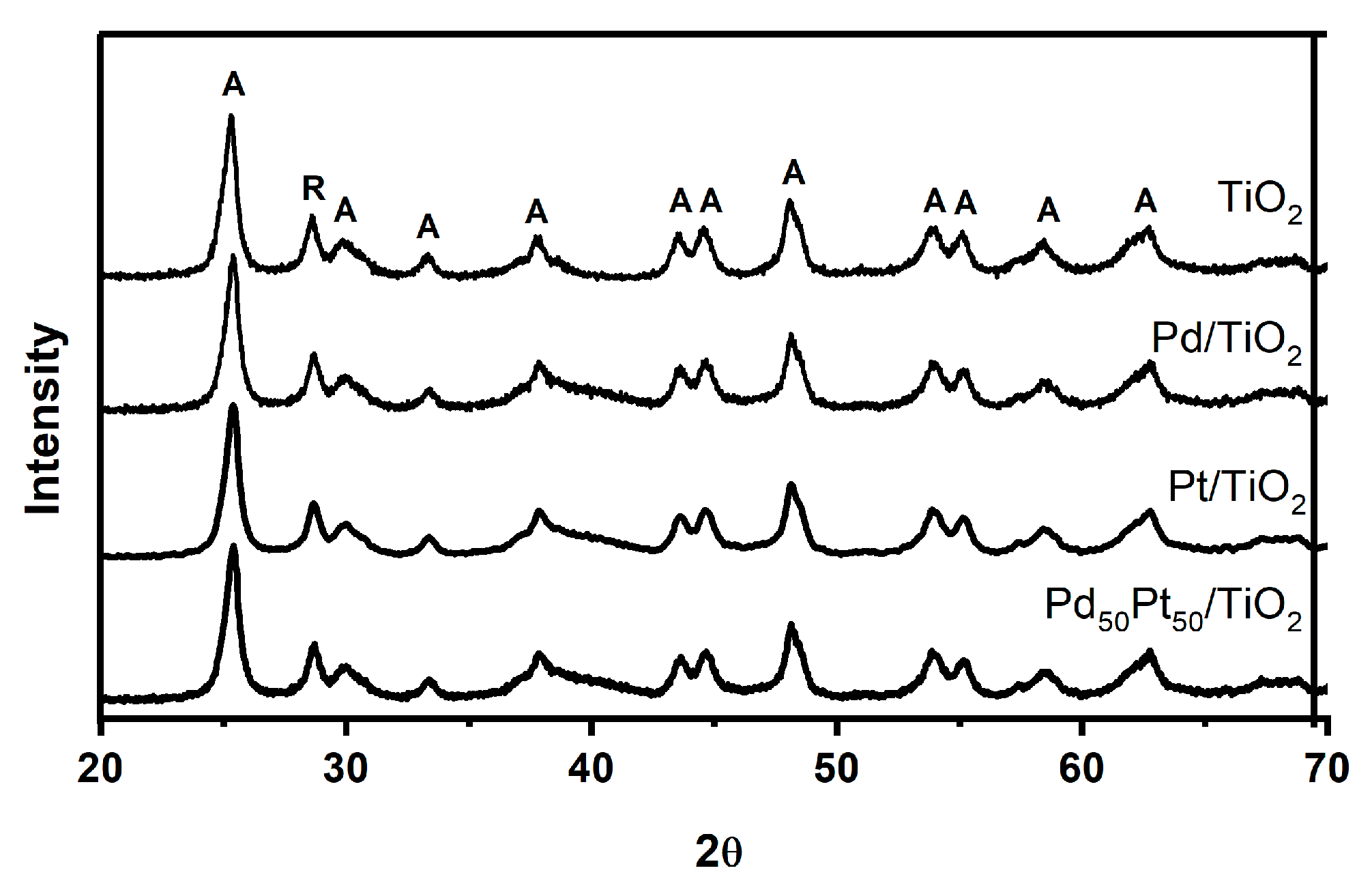


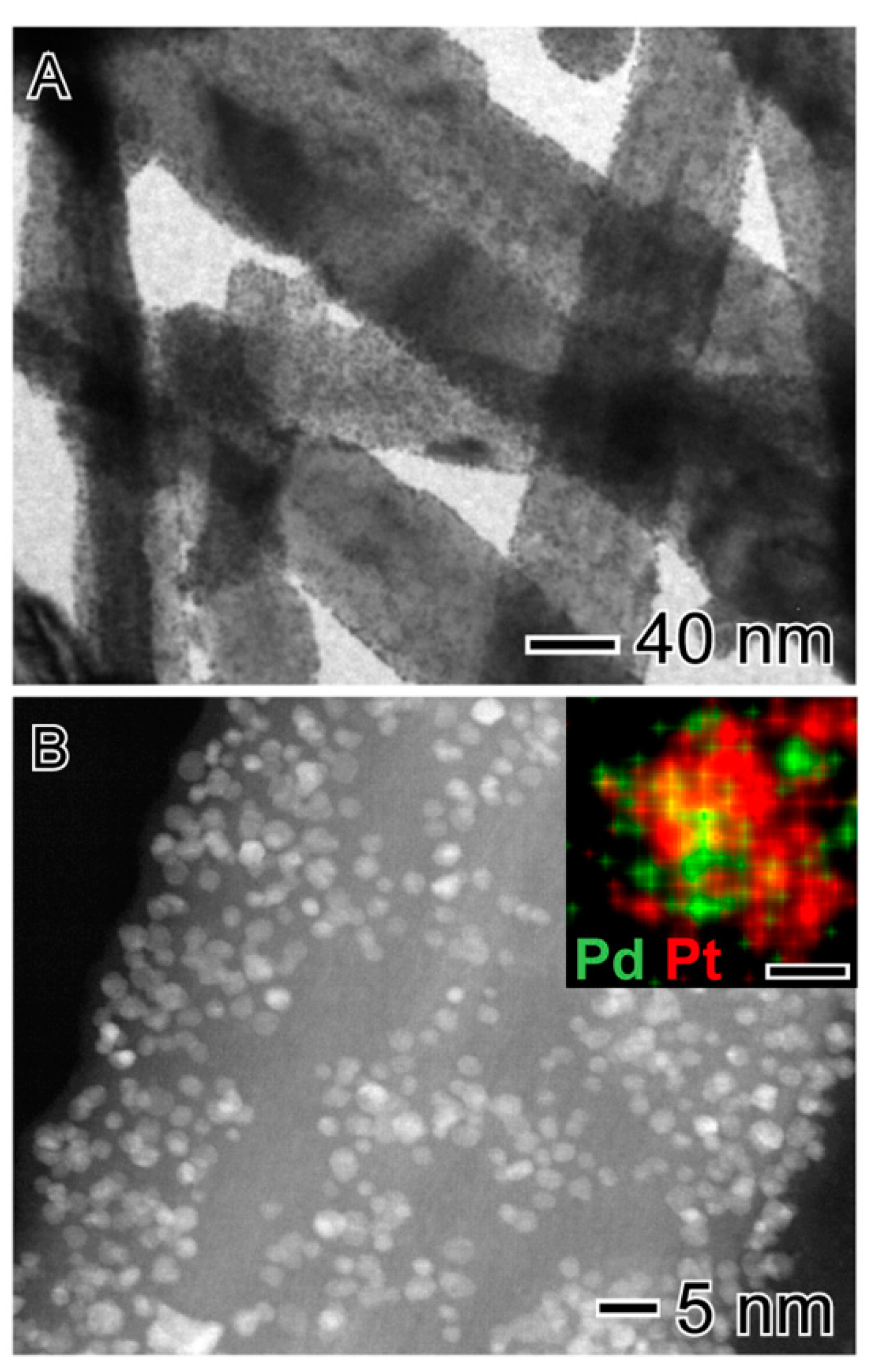
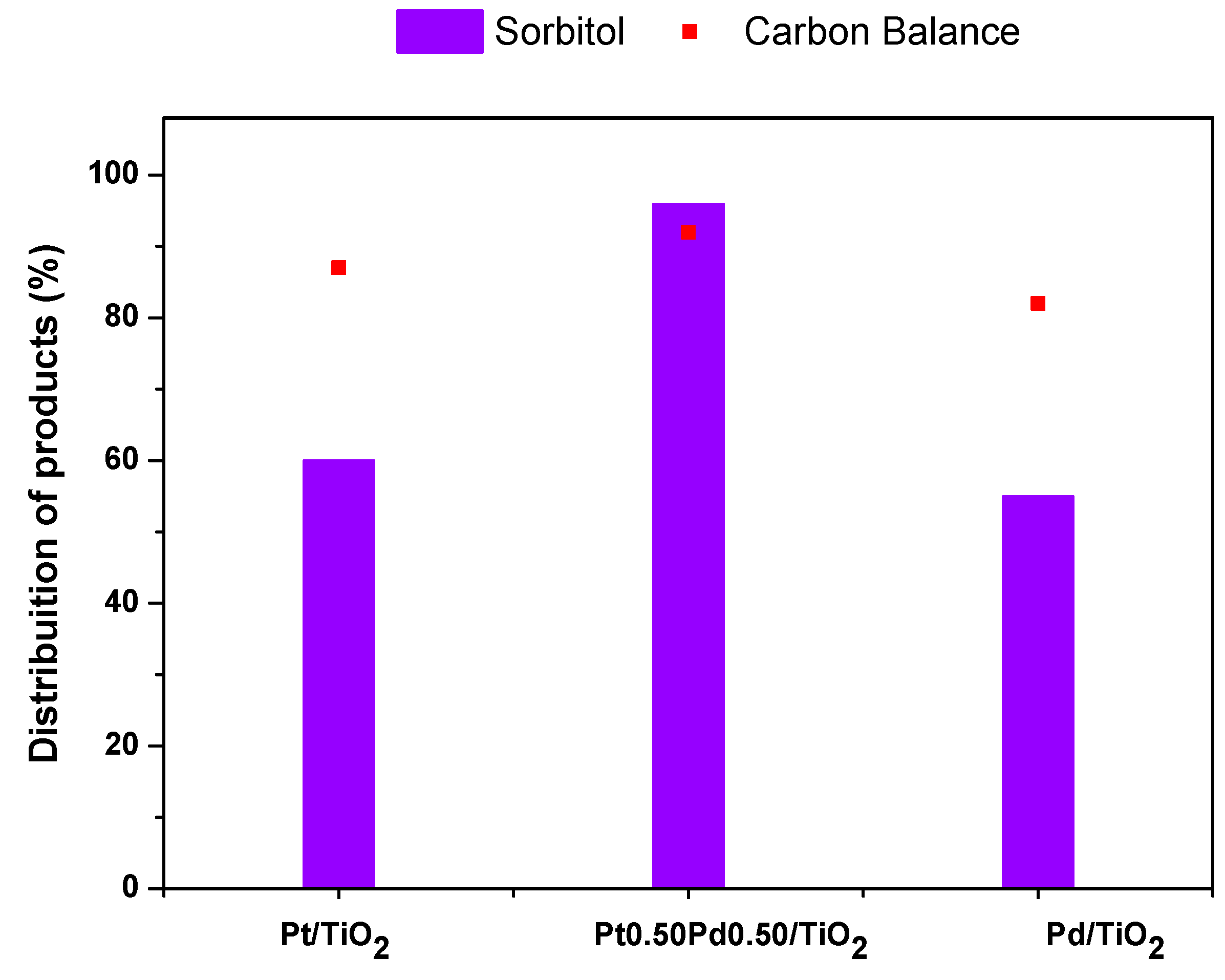
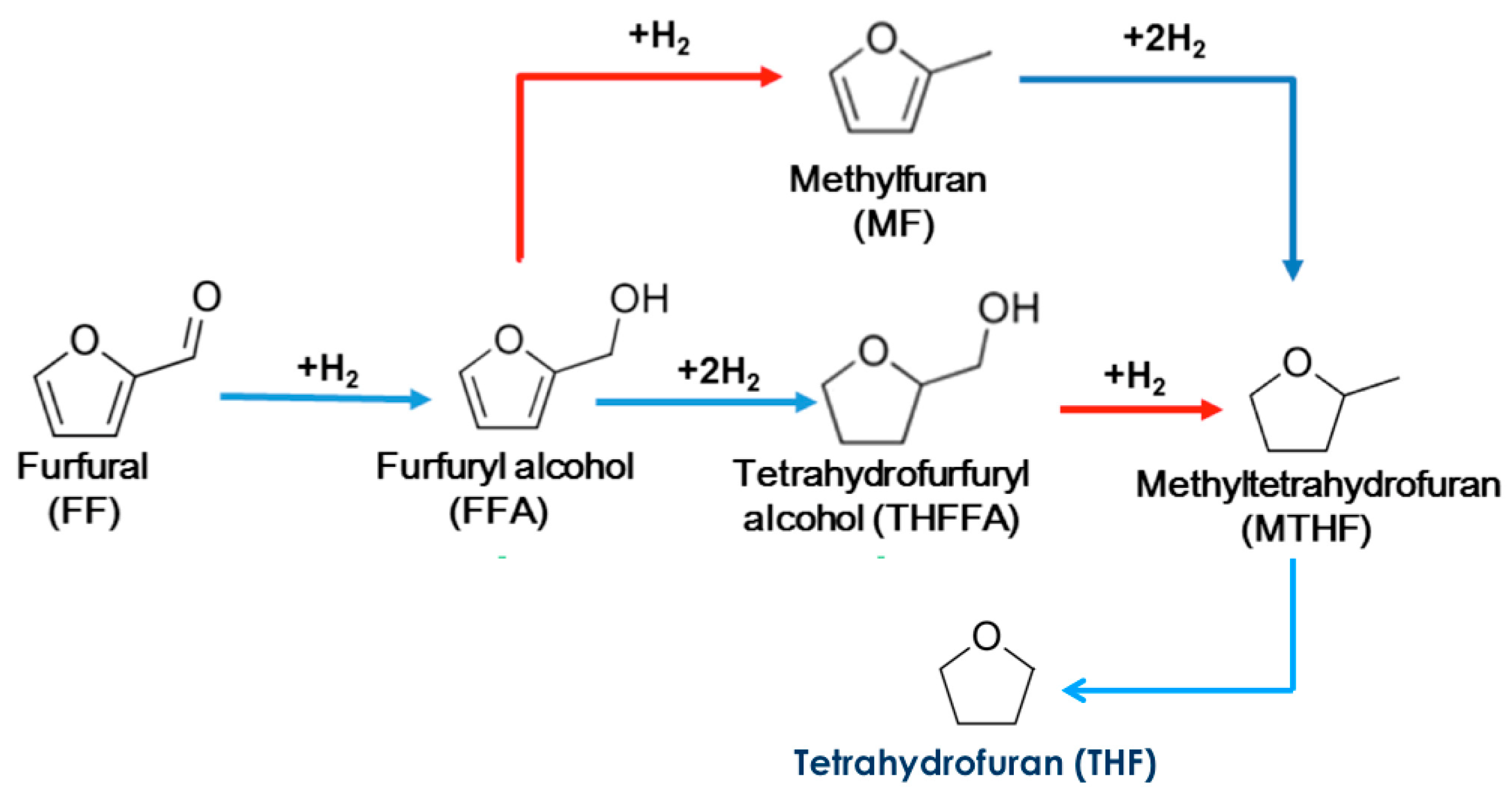
| Catalyst | (wt.%) | Surface Area (m2 g−1) | Average Pore Diameter (Å) | Pore Volume (cm3 g−1) | |
|---|---|---|---|---|---|
| Pt | Pd | ||||
| Pt/TiO2 | 2.0 | 0.0 | 49 | 55.9 | 0.0902 |
| Pt0.50Pd0.50/TiO2 | 1.3 | 0.9 | 44 | 59.4 | 0.0804 |
| Pd/TiO2 | 0.0 | 2.3 | 33 | 64.4 | 0.0691 |
| TiO2 | - | - | 40 | ||
| Sample | BE of Pd 3d3/2 (eV) | BE of Pt 4f7/2 (eV) | BE of O 1s (eV) | Pt 4f/Pd 3d | ||||
|---|---|---|---|---|---|---|---|---|
| Pd(0) | Pd(∂+) | Pt(0) | Pt(∂+) | OL | OS | OW | ||
| Pt/TiO2 | 71.1 (68) * | 73.9 (32) * | 530.8 (65) * | 532.4 (27) * | 534.0 (8) | - | ||
| Pt0.50Pd0.50/TiO2 | 335.8 (77) * | 337.4 (23) * | 72.4 (85) * | 74.3 (15) * | 530.8 (63) * | 532.8 (30) * | 534.9 (7) | 1.01 |
| Pd/TiO2 | 334.2 (83) * | 336.5 (17) * | - | - | 530.5 (63) * | 532.6 (29) * | 534.8 (8) | - |
| TiO2 | - | - | - | - | 530.3 (69) * | 532.2 (24) * | 534.0 (7) | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, P.M.; Silvester, L.; da Silva, A.G.M.; Fernandes, C.G.; Rodrigues, T.S.; Paul, S.; Camargo, P.H.C.; Wojcieszak, R. Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural. Catalysts 2019, 9, 132. https://doi.org/10.3390/catal9020132
de Souza PM, Silvester L, da Silva AGM, Fernandes CG, Rodrigues TS, Paul S, Camargo PHC, Wojcieszak R. Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural. Catalysts. 2019; 9(2):132. https://doi.org/10.3390/catal9020132
Chicago/Turabian Stylede Souza, Priscilla M., Lishil Silvester, Anderson G. M. da Silva, Cibele G. Fernandes, Thenner S. Rodrigues, Sebastien Paul, Pedro H. C. Camargo, and Robert Wojcieszak. 2019. "Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural" Catalysts 9, no. 2: 132. https://doi.org/10.3390/catal9020132
APA Stylede Souza, P. M., Silvester, L., da Silva, A. G. M., Fernandes, C. G., Rodrigues, T. S., Paul, S., Camargo, P. H. C., & Wojcieszak, R. (2019). Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural. Catalysts, 9(2), 132. https://doi.org/10.3390/catal9020132







